Sickle cell diseases are inborn blood disorders caused by the presence of an abnormal form of hemoglobin, hemoglobin S (HbS). Mortality from sickle cell disease during the first 3 to 4 years of life can be virtually eliminated by newborn screening and appropriate follow-up and treatment [1]. Three sickle cell diseases (sickle cell anemia, sickle hemoglobin C disease and sickle β thalassemia) are included in the United States' recommended uniform newborn screening panel [2]. HbS is the newborn screening marker for all three disorders.
The Newborn Screening Quality Assurance Program of the Centers for Disease Control and Prevention (CDC) prepares and validates dried-blood spot (DBS) proficiency testing materials to assist laboratories with monitoring the performance of their newborn screening tests [3]. As part of routine evaluations of marker stabilities in DBS samples, we used measurements by high performance liquid chromatography (HPLC) and liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) methods to compare the stabilities of HbA and HbS in DBSs stored for predetermined intervals at elevated temperature (37°C) and low (<30%) or high (>50%) humidity. The objectives of these studies were to measure separately the contributions of heat and humidity to the degradation of HbS and HbA in DBS samples and to evaluate the level of concordance between results from the two analysis methods used.
The DBS materials for this study were made from the blood of an anonymous adult HbS carrier. Their use in this study was determined not to be human subject research. The accelerated degradation studies of Hbs A and S were performed according to a previously described design [4]. Identical 11-member sets of the DBS materials were stored in zip-closure specimen bags containing humidity indicator cards. One sample set was stored with relative humidity controlled below 30% by enclosing desiccant packets with the DBSs and zip-sealing the bags before storage. The other set was stored without desiccant in open bags in a high-humidity chamber. The Day 0 member of each sample set (in a zip-closed bag with humidity controlled below 30%) remained in −70°C storage throughout the study to serve as the stability control for its sample set. At predetermined intervals, DBSs from each sample set were removed from 37°C storage and transferred to storage at −70°C. Immediately before transfer, desiccant packets were added to the bags containing DBSs that had been stored at high humidity and those bags were zip-sealed. The marker levels of all samples in each complete sample set were analyzed in triplicate in a single run of each analytic method.
A Trinity Biotech Gensys Ultra 2 Variant HPLC system was used to measure the levels of intact hemoglobin tetramer molecules [5], and a LC-ESI-MS/MS method [6] was used to measure levels of hemoglobin α and β chain peptides in trypsin digests of DBS eluates.
Geometric means of triplicate measurements of initial (Day 0) marker levels and marker levels remaining on the last day of each degradation study were used to determine the percentage of each marker's initial level that was lost during the study. Marker losses from sample sets stored at low humidity were attributed to the effects of the elevated storage temperature. The percentage loss of each marker from samples stored at low humidity was subtracted from the percentage loss sustained by paired samples stored at high humidity, and the difference was attributed to the effects of elevated storage humidity.
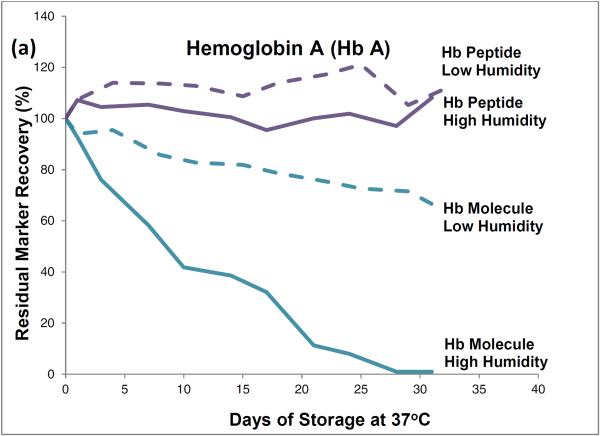
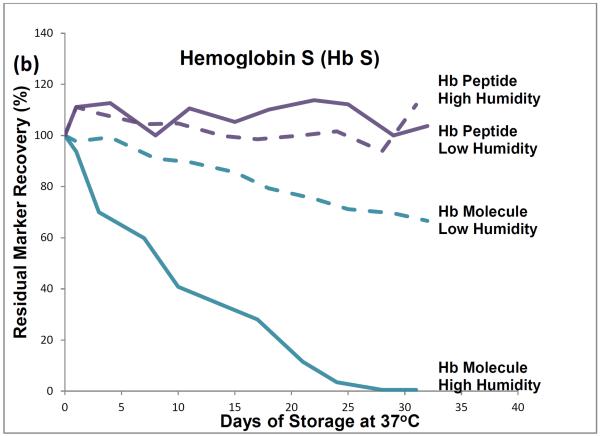
HPLC results showed that, during one month of storage at 37°C, initial levels of intact hemoglobin molecules declined by about 35% in the low-humidity environment and by almost 100% in the high humidity environment (Figure 1). The approximately 35% degradation that occurred during low temperature storage (and was attributed to effects of the elevated storage temperature) was subtracted from the almost 100% degradation that occurred during high humidity storage to find that about 65% of the degradation of hemoglobin tetramers was attributable to the effects of high storage humidity.
Figure 1.
Recoveries of hemoglobin A peptides and molecules (a) and hemoglobin S peptides and molecules (b) from dried-blood spots stored for pre-determined intervals at 37°C in low-humidity and high-humidity environments and analyzed by liquid chromatography tandem mass spectrometry (peptide measurements) and high performance liquid chromatography (molecule measurements).
LC-ESI-MS/MS results showed no statistically significant decline in levels of hemoglobin peptide-markers during one month of 37°C storage at either humidity.
The HPLC method measured intact hemoglobin tetramers only, whereas the LC-ESI-MS/MS method measured the marker peptides generated from the tryptic digestion of both intact hemoglobin tetramers and polypeptide monomers from denatured tetramers.
HPLC results from these accelerated degradation studies show that degradations of HbA and HbS are similar and that molecules of both hemoglobins are highly susceptible to the adverse effects of high temperature and humidity in the DBS storage environment. Results from the comparison of hemoglobin tetramers in DBSs stored at low and high humidities indicate that most of their degradation was attributable to the adverse effects of high humidity.
The HPLC results show that elevated temperature or humidity can disrupt the non-covalent bonds among the hemoglobin tetramer subunits. The LC-ESI-MS/MS results suggest that the covalent bonds of the polypeptide hemoglobin subunits are resistant to moderate heat-related and humidity-related environmental stress and suggest that marker-peptide analyses may be useful for identifying and measuring hemoglobin species in DBSs in which hemoglobin tetramers have been denatured.
Many newborn screening programs routinely use HPLC as a primary screening test for sickle cell disease and other hemoglobinopathies. Existing LC-ESI-MS/MS methods do not offer rapid enough throughput to be used as a primary population screening tool, but are needed for second tier/confirmatory testing of challenging DBS samples.
Newborn screening programs routinely request a second DBS specimen if laboratory findings indicate compromised integrity of the initial newborn screening specimen [7]. In applications such as forensic chemistry, collection of a second specimen may not be possible. LC-ESI-MS/MS analysis may provide a mechanism for salvaging valid hemoglobin variant identifications from denatured dried-blood samples.
Acknowledgement
D.L. Chafin was funded by the Research Participation Program at the Centers for Disease Control and Prevention, an interagency agreement with the U.S. Department of Energy administered by the Oak Ridge Institute for Science and Education. The finding and conclusions in this letter are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Kaye CI. Newborn screening fact sheets. Pediatrics. 2006;118:e934–63. doi: 10.1542/peds.2006-1783. [DOI] [PubMed] [Google Scholar]
- 2.Watson MS, Mann MY, Lloyd-Puryear MA, Rinaldo P, Howell RR. Newborn screening: toward a uniform screening panel and system—executive summary. Pediatrics. 2006;117S:S296–307. doi: 10.1542/peds.2005-2633I. [DOI] [PubMed] [Google Scholar]
- 3.Zobel S. In: Newborn Screening Quality Assurance Program 2012 annual summary report. Zobel S, editor. Centers for Disease Control and Prevention; Atlanta, GA: 2013. [Google Scholar]
- 4.Adam BW, Hall EM, Sternberg M, Lim TH, Flores SR, O'Brien S, et al. The stabilities of markers in dried-blood spots for recommended newborn screening disorders in the United States. Clin Biochem. 2011;44:1445–50. doi: 10.1016/j.clinbiochem.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adam BW, Chafin DL, De Jesus VR. Stabilities of hemoglobins A and S in dried blood spots stored under controlled conditions. Clin Biochem. 2013;46:1089–92. doi: 10.1016/j.clinbiochem.2013.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haynes CA, Guerra SL, Fontana JC, De Jesus VR. HPLC-ESI-MS/MS analysis of hemoglobin peptides in tryptic digests of dried-blood spot extracts detects HbS, HbC, HbD, HbE, HbO-Arab, and G-Philadelphia mutations. Clin Chim Acta. 2013;424:191–200. doi: 10.1016/j.cca.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Clinical and Laboratory Standards Institute . Blood collection on filter paper for newborn screening programs. Fifth edition. CLSI document LA4-A5; Wayne, PA: 2007. approved standard. [Google Scholar]