Abstract
Different from physiological axon growth during development, a major limiting factor for successful axon regeneration is the poor intrinsic regenerative capacity in mature neurons in the adult mammalian central nervous system (CNS). Recent studies identified several molecular pathways, including PTEN/mTOR, Jak/STAT, DLK/JNK, providing important probes in investigating the mechanisms by which the regenerative ability is regulated. This review will summarize these recent findings and speculate their implications.
Introduction
Similar to axon growth during development, axon regeneration requires the axonal extension guided by growth cone structures. This led to the hypothesis that similar principles and molecular players might operate these different processes. Ample evidence suggests that during development the extrinsic environmental cues largely determine the projection of axon growth, although the intrinsic states of responding neurons also modulate axonal responses (1). For axon regeneration, dissecting the relative contributions of such extrinsic and intrinsic mechanisms has been a major focus in the past decades (2–8). Early studies showed that reconstituting a permissive environment by transplanting peripheral nerve grafts allows the regrowth of some injured axons in the adult CNS, even though their numbers are limited (9, 10). These observations have been further supported by elegant in vivo imaging-based analysis studies (11**, 12). Canty et al employed a focused laser method to transect individual axons, with minimal glial scar formation, in the gray matter of the adult mouse brain. Ylera et al also demonstrated that central sensory axon lesion produced by two-photon laser in the spinal cord has minimal scar formation (12). Again, while some types of axons in the brain could regenerate, the majority of injured axons fail to regrow even when visualized for periods of up to a year (11**). These observations further substantiate the notion that the majority of mature neurons in the adult CNS have diminished intrinsic growth ability.
For a successful regeneration to occur, injured axonal terminals need to re-seal quickly and reform growth cone-like structure which will explore the extracellular environment, determine the direction of growth, and then guide the extension of the axon to the direction of their appropriate targets (13–15). Presumably, the ability of injured axons to regenerate their growth cone structures and extend in injury-disturbed environments should represent the rate-limiting steps of axon regeneration. Recent genetic studies indicated that manipulating several signaling pathways could allow certain populations of mature CNS neurons to mount regenerative growth after injury and provide valuable molecular probes to explore the inner mechanisms of axon regeneration control in mammalian CNS neurons.
PTEN/mTOR: a general pathway of regulating axon regeneration?
All cell types possess certain molecular mechanisms that prevent cellular overgrowth, and many of these pathways have been implicated as tumor suppressors. In an effort to assess whether these growth suppressors play a role in limiting the intrinsic axon regenerative ability, Park et al used an optic nerve injury model and discovered robust long-distance axon regenerations in adult mice with conditional deletion of the phosphatase and tensin homolog (PTEN) gene in retinal ganglion cells (RGCs) (16). Since no manipulation was made in the lesion site, the observed regeneration phenotypes are likely due to the altered intrinsic regenerative ability in the RGCs (16).
The PTEN protein is a phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase. It contains a tensin-like domain and a catalytic domain similar to that of the dual specificity protein tyrosine phosphatases. Unlike most of the protein tyrosine phosphatases, this protein preferentially dephosphorylates phosphoinositide substrates. It negatively regulates intracellular levels of phosphatidylinositol-3,4,5-trisphosphate and the activity of the Akt/PKB signaling pathway (17–19). Among multiple down-stream targets in this pathway, mTOR appears to be a critical effector, because administration of rapamycin, an mTOR inhibitor, abolishes the regeneration effect of PTEN deletion (16). Further, the mTOR activation by genetic deletion of TSC1, a specific negative regulator of the mTOR, also promotes axon regeneration (16).
In addition to RGCs, the activation of mTOR activity has been shown to promote the regenerative growth of other types of CNS axons. For example, the adult mice with PTEN inhibition, by either genetic deletion (20) or shRNA-mediated inhibition (21*), in the cortical neurons from the neonatal ages, showed robust regrowth of injured corticospinal tract axons, which are known to be refractory to regeneration (22–24). Similarly, over-expression of constitutively active forms of the kinase Akt and the GTPase Ras homolog enriched in brain (Rheb) induces the regrowth of axons from dopaminergic neurons to their target, the striatum (25). Moreover, a recent study showed that newly differentiated neurons from transplanted neuronal stem cells are able to extend their axons from the spinal cord lesion sites to both sides of host tissues and such axonal growth could be partially blocked by an mTOR inhibitor (*26). However, it remains unknown whether the manipulation of the PTEN/Akt/mTOR pathway could promote the regeneration of all types of axons in the adult CNS.
In contrast to the adult mammalian CNS, injured axons in the peripheral nervous system (PNS) are able to regenerate spontaneously. Activating PI3K or inhibiting PTEN in dorsal root ganglion (DRG) neurons increases the neurite growth (27–32). However, inhibiting mTOR activity by rapamycin fails to block the neurite growth of DRG neurons (28, *31), suggesting that mTOR-independent mechanisms mediate the regeneration of peripheral sensory axons. Instead, Saijilafu et al found that glycogen synthase kinase 3 (GSK3), another down-stream target of PI3K (32), mediates PI3K-dependent augmentation of the growth potential in the PNS (*31). Furthermore, instead of the known role of GSK-3 in regulating microtubule re-organization (32, 33), PI3K-GSK3 signal may induce a transcription factor Smad1, which has been suggested to enhance sensory axon regeneration (34–36). Thus, it appears that different mechanisms mediate the effects of the PTEN/PI3K pathway in axon regeneration of PNS and CNS neurons.
Interestingly, recent studies indicated that the regulation of axon regeneration by the PTEN/mTOR pathway is not limited to mammalian neurons. Inhibiting PTEN or activating Akt in Drosophila class IV da neurons enhances their regenerative responses to axotomy and dendriotomy, suggesting this as an evolutionally conserved growth program that regulates neuronal regenerative ability (37). Although little is known about the regeneration of dendrites in mammals, these observations suggested a possibility that common mechanisms regulate the regeneration of axons and dendrites. However, activation of the Akt pathway is insufficient to confer a regenerative ability in regeneration-incompetent Class I da neurons (38). Nevertheless, these results suggested that PTEN-dependent pathways might be evolutionally conserved in regulating neuronal regenerative responses in a neuron type-specific manner (37, 38).
mTOR as an indicator of regenerative competence?
The precise mechanisms by which PTEN/mTOR controls axon regeneration remain to be elucidated. Like other resting cells, intact mature neurons produce ATP mainly through catabolic processes to fuel the maintenance of energy-costly homoeostatic processes, such as cytoskeletal functions and ion and nutrient transport. However, for an injured neuron to initiate a regenerative growth, it has to shift towards anabolic processes, allowing for the de novo synthesis of macromolecules from available nutrients (39). For this, there must be molecular links between the pathways sensing cellular growth signals and those controlling the metabolic networks underlying cell growth. In this regard, mTOR might be a good candidate for such tasks. It is known that in all cell types, mTOR is able to alter cellular metabolism to drive the biosynthesis of building blocks and macromolecules essential for cell growth (17–19). In addition to its well-known effects on cap-dependent protein translation (19), mTOR has been shown to regulate the synthesis of other cellular building blocks such as lipids and nucleotides (40) and regulate mitochondrial oxidative function (41, 42).
On the other hand, mTOR is activated by nutrients, growth factors and certain hormones and is tightly regulated in vivo (17–19). In mammalian RGCs and cortical neurons, the mTOR activity undergoes a development-dependent down-regulation (16, 20). Axotomy further inhibits the neuronal mTOR activity, presumably as a result of injury-triggered stress responses (16, 20). The detailed molecular mechanisms for such mTOR regulations in neurons are still unknown, although many mTOR regulators have been identified in non-neuronal cells (17–19). A recent study showed that the microRNA (miRNA) bantam (ban) regulates neuronal Akt activity and regenerative ability in Drosophila (37). Instead of acting in the neurons, this miRNA appears to function in their target cells, resulting in a down-regulation of Akt expression in neurons during development (37). Such cell non-autonomous regulation by a miRNA is reminiscent of the role of miR-206, a skeletal muscle-specific miRNA, in regulating the regeneration of the neuromuscular synapses in mice (43). mTOR inactivation has also been documented under cellular stress conditions, such as hypoxia or DNA damage, and many molecules, such as REDD1 (43–45) and sestrin (46), have been implicated in such stress responses. But their relevance to axotomy-induced mTOR regulation remains to be studied. Elucidating the mechanisms of mTOR regulation during development and after injury should reveal new insights into the regulatory mechanisms of axon regeneration.
Although most of studies on PTEN have been focused on its cytosolic lipid phosphatase activity, recent studies suggested that PTEN might also function in the nucleus (47–49). Recently, an intriguing study by Zhang et al demonstrated that nuclear translocation of PTEN might be a step causatively leading to excitotoxic (in vitro) and ischemic (in vivo) neuronal loss (50). Since PTEN deletion impact both neuronal survival and axon regeneration, it would be interesting to assess the contribution of nuclear vs cytosolic PTEN after axotomy.
Injury signals from the lesioned axons to neuronal soma
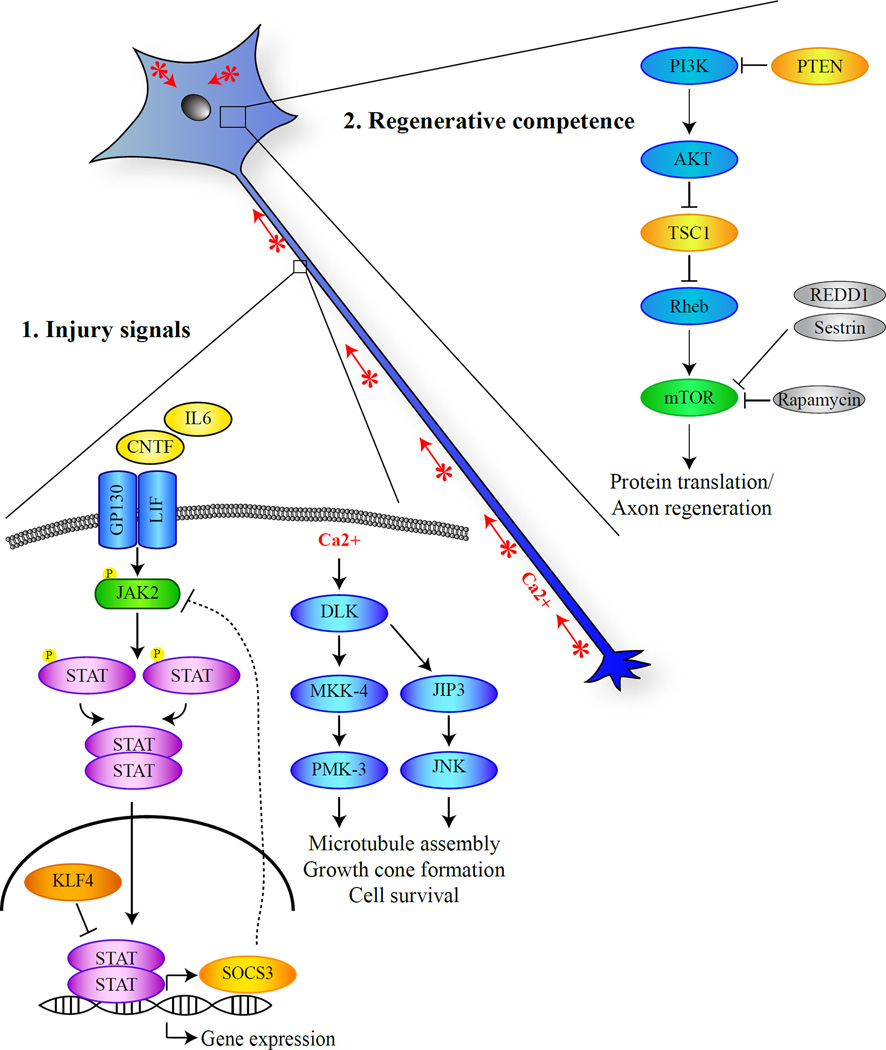
As neuronal networks are formed in the developing nervous system, axons progressively cease growing. Even in the adult CNS, transient sprouting could occur in the terminals of injured axons, likely as the result of local cytoskeleton rearrangements. To convert such abortive local events to sustained axon extension, a set of injury signals generated locally need to be retrogradely transported to the cell body and initiate injury responses (Fig. 1). An important topic in the field is to determine the molecular signatures of such injury signals.
Figure 1. A working model of the mechanisms that regulate the intrinsic regenerative ability of axon regeneration.
(1) To respond to axotomy, the neuronal cell bodies need to be informed by retrogradely transported injury signals. In addition to acute axotomy-induced changes such as ion influx, cytokines such as IL-6 and CNTF are up-regulated at the lesion site and/or around the cell body. As a result, DLK and Jak/STAT pathways are activated, resulting in the generation and transport of the injury signals. (2). How injured neurons respond to injury signals is dependent on their regenerative competence. An important determinant of the competence might be the mTOR activity but other molecular pathways likely exist.
As a drastic stress condition, axotomy leads to a variety of changes in axotomized neurons. For example, axotomy triggers a rapid depolarization and leads to an increase of local calcium concentrations that may further propagate towards other neuronal compartments (51). At least in Aplysia, such calcium increase is important for initiating axonal regrowth program (13–15). A recent study suggested that in DRG neurons such back-propagating calcium wave causes nuclear export of histone deacetylase 5(HDAC5), which subsequently activates a regenerative program (52, *53).
In addition, a number of other molecules have been implicated as possible carriers of injury signals. An emerging common theme is that following axotomy different transcription factors undergo certain modifications, which lead to the alteration of their subcellular localizations (*54–58). A well-documented example is that an injury in the peripheral axons of DRG neurons led to the nuclear accumulation of phosphorylated STAT3 (*54,50,60), presumably due to the activation of Jak2 kinase.
In cultured DRG neurons, an inhibitor of Jak2 blocks the neurite growth (61). In vivo perineural infusion of Jak2 inhibitor also attenuates dorsal column axonal regeneation (60). In addition, motorneurons from STAT3 conditional knockout mice showed decreased survival after nerve lesion but it can be rescued by addition neurotrophic factors, including CNTF (59). STAT3 is activated upon injury and retrogradely transported to modulate survival of the host neurons (54), suggesting a role of the Jak/STAT pathway in regulating axon regeneration and survival in these neurons. Cytokines such as CNTF have also been suggested to regulate the survival and axon regeneration of CNS neurons (62–64). However, several studies showed that exogenously delivered cytokines have only limited effects on promoting survival and regeneration following optic nerve injury (65, 66) or spinal cord injury (67). This might be due to the SOCS3-mediated negative feedback (68), thus limiting the activation of the Jak/STAT pathway in responding neurons, because increased neuronal survival and axon regeneration are seen in the adult mice with conditional deletion of SOCS3, a negative regulator of Jak/STAT pathway (69). The co-deletion of SOCS3 and STAT3 in RGCs abolishes the regeneration phenotypes, suggesting that the regeneration phenotype of SOCS3 deletion is dependent on the STAT3-activated gene expression program.
The next question is what are the endogenous sources of cytokines after injury. After peripheral nerve injury, the cells in the lesion, such as Schwann cells, up-regulate expression of interleukin 6 (IL-6) and perhaps other cytokines (70–73). After optic nerve injury, the cells in the retinal ganglion layers, likely to be astrocytes, also showed increased expression of CNTF and LIF (62–65). It is also known that inflammatory stimulations, such as lens injury, could result in enhanced optic nerve regeneration (75). Although multiple molecular players have been implicated (76), genetic studies suggested that neuronal STAT3 is essential for the axon regeneration after such inflammatory stimulations (64, 77).
Compelling evidence suggests the existence of other important molecular carriers of injury signals. For example, dual lencine zipper kinase (DLK) is a component of a conserved MAPK cascade that includes the MAP kinase kinase MKK-4 and the p38 MAP kinase PMK-3. Loss-of-function mutations of the dlk-1, mkk-4 or pmk-3 gene result in axon regeneration defects (78, 79), suggesting that this entire signaling pathway is required for axon regeneration in C. elegans. Importantly, the involvement of this pathway in axon regeneration control is conserved in other species (80–*82). Deletion of DLK in DRG neurons blocks their axon regeneration (81). After optic nerve injury, deletion of DLK increases the survival of injured RGCs but blocks the axon regeneration induced by PTEN deletion (*82). Mechanistically, DLK protein is present in axons, and protein levels are increased in response to axonal injury (80). In C. elegans DLK is activated by a Ca++-dependent switch from inactive heteromeric to active homomeric protein complexes (83). Further, it interacts with the scaffolding protein JNK-interacting protein 3 (JIP3), a component of axonal transport (84–85). As positive and negative retrograde signals have been proposed (86), it will be interesting to examine whether different transcription factors mediate the effects of DLK on neuronal survival and axon regeneration. DLK interacts with JNK1 and is implicated in regulation of microtubule stability (87). It has been demonstrated that microtubule stabilization is an important component of axon regeneration after spinal cord injury (88).
Signaling networks for integrated regenerative programs?
Differential injury responses in axotomized neurons should be the results of the interactions between the intrinsic neuronal state and the injury-triggered signals. As discussed above, JAK/STATs and DLK might participate in axotomy-triggered injury signal generation and delivery, and PTEN/mTOR might be a potential determinant of neuronal competence for axon regeneration (Fig. 1). Recent studies start to reveal interactive mechanisms of these and other molecular pathways.
Kruppel-like factors (KLFs), a subclass of the zinc-finger transcription factors, have been implicated in axon growth control (89–91). In zebrafish, KLF6 and KLF7 were identified among the group of up-regulated genes in regenerating RGCs (89). In mice, different KLFs differ in their expression levels over the course of development: while KLF6/7 are down-regulated, KLF4/9 are up-regulated in adult RGCs (90). Knockout of KLF4 in RGCs promotes axon regeneration after an optic nerve injury (90). Forced expression of KLF7 promotes the regenerative growth of injured corticospinal tract axons (91). In a recent study, Qin et al. showed that KLF4 physically interacts with STAT3 upon cytokine-induced phosphorylation of tyrosine 705 (Y705) on STAT3, resulting in the suppression of STAT3-dependent gene expression by blocking its DNA-binding activity (*92). These findings suggested a possible mechanism by which KLFs impact on the neuronal regenerative ability is by regulating the activity of STAT3.
The cross-talks between these pathways are also indicated from the synergistic effects of PTEN deletion and other treatments, such as inflammatory stimulation (*93) or SOCS3 deletion (**94), on promoting optic nerve regeneration. An obvious explanation is the coordination of increased protein translation and gene transcription under these treatments respectively, resulting in the higher and more sustained activation of regeneration-initiating programs. This could be important considering ample evidence for drastic changes of gene expression in injured neurons. In addition, the gene expression profiling studies indicated that injured RGCs with PTEN and SOCS3 double deletion showed increased expression of mTOR activators, such as small GTPase Rheb and Insulin-like growth factor 1 (IGF1), suggesting that such a positive feedback regulation of the mTOR activity may contribute to the enhanced and sustained axon regeneration (*94). Further studies are needed to dissect the contributions of individual pathways and develop optimized combinatorial treatments with other manipulations.
Perspectives
While these new studies demonstrated exciting possibilities of promoting the regeneration of injured axons in the adult CNS, many challenges remain towards translating these findings to therapeutic strategies. With dramatically increased body size in the adult, regenerating axons usually need to carry out de novo growth over relatively vast distances to reach their targets (95). Even with these newly developed strategies of promoting axon regeneration, it is unclear what might be maximal distances these regenerating axons can grow in the adult mammalian CNS. Another key question concerns whether these regenerating axons are able to follow their original projection paths and find their appropriate targets. A recent study suggested that after a combinatorial treatment of PTEN deletion, inflammatory stimulation and cAMP elevation, regenerating optic nerve axons could follow their original paths and resulted in functional recovery (96). However, others studies failed to reproduce these findings and instead showed that regenerating axons did not follow their original trajectories (97, 98). This might not be completely unexpected, since even in the PNS system, regenerating axons often project ectopically initially and activity-dependent processes later drive the refinement of such regenerating axons. On the other hand, it is not known whether it is necessary for regenerating axons to make direct connections with their original targets for functional recovery. Recent studies suggested that indirect relay connections, such as the axons from differentiated neurons from transplanted neuronal stem cells (*26, 99) or from the rearrangements of local circuits (100, 101), might allow some degree of functional recovery after spinal cord injury. Addressing these and other questions might pave the paths for developing strategies of rebuilding neuronal circuits for functional recovery after damage.
Highlights.
-
•
Differential intrinsic regenerative ability of adult cortical neurons revealed by In vivo imaging analysis
-
•
Evolutionarily conserved pathways in regulating axon regeneration
-
*
Functional interactions among different pathways in regulating axon regeneration
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- 1.Kolodkin AL, Tessier-Lavigne M. Mechanisms and molecules of neuronal wiring: a primer. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwab ME, Bartholdi D. Degeneration and regeneration of axons in the lesioned spinal cord. Physiol Rev. 1996;76:319–370. doi: 10.1152/physrev.1996.76.2.319. [DOI] [PubMed] [Google Scholar]
- 3.Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harel NY, Strittmatter SM. Can regenerating axons recapitulate developmental guidance during recovery from spinal cord injury? Nat Rev Neurosci. 2006;7:603–616. doi: 10.1038/nrn1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filbin MT. Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci. 2006;361:1565–1574. doi: 10.1098/rstb.2006.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fitch MT, Silver J. CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol. 2008;209:294–301. doi: 10.1016/j.expneurol.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JK, Zheng B. Role of myelin-associated inhibitors in axonal repair after spinal cord injury. Exp Neurol. 2012;235:33–342. doi: 10.1016/j.expneurol.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fawcett JW, Schwab ME, Montani L, Brazda N, Müller HW. Defeating inhibition of regeneration by scar and myelin components. Handb Clin Neurol. 2012;109:503–522. doi: 10.1016/B978-0-444-52137-8.00031-0. [DOI] [PubMed] [Google Scholar]
- 9.Aguayo AJ, David S, Bray GM. Influences of the glial environment on the elongation of axons after injury: transplantation studies in adult rodents. J Exp Biol. 1981;95:231–240. doi: 10.1242/jeb.95.1.231. [DOI] [PubMed] [Google Scholar]
- 10.David S, Aguayo AJ. Axonal elongation into peripheral nervous system "bridges" after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- 11. Canty AJ, Huang L, Jackson JS, Little GE, Knott G, Maco B, De Paola V. In-vivo single neuron axotomy triggers axon regeneration to restore synaptic density in specific cortical circuits. Nat Commun. 2013;4:2038. doi: 10.1038/ncomms3038. After laser-assisted focal axotomy in the adult mouse brain, the authors used two-photon imaging and focused ion beam-scanning electron microscopy to monitor regenerative responses of injured axons chronically. Time-lapse analysis of >100 individually ablated axons for periods of up to a year reveals an inability to regenerate even in a glial scar-free environment. However, some types of axons spontaneously regenerate at maximum speeds comparable to peripheral nerve regeneration.
- 12.Ylera B1, Ertürk A, Hellal F, Nadrigny F, Hurtado A, Tahirovic S, Oudega M, Kirchhoff F, Bradke F. Chronically CNS-injured adult sensory neurons gain regenerative competence upon a lesion of their peripheral axon. Curr Biol. 2009;19:930–936. doi: 10.1016/j.cub.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 13.Bradke F, Fawcett JW, Spira ME. Assembly of a new growth cone after axotomy: the precursor to axon regeneration. Nat Rev Neurosci. 2012;13:183–193. doi: 10.1038/nrn3176. [DOI] [PubMed] [Google Scholar]
- 14.Spira ME, Oren R, Dormann A, Gitler D. Critical calpain-dependent ultrastructural alterations underlie the transformation of an axonal segment into a growth cone after axotomy of cultured Aplysia neurons. J Comp Neurol. 2003;457:293–312. doi: 10.1002/cne.10569. [DOI] [PubMed] [Google Scholar]
- 15.Spira ME, Oren R, Dormann A, Ilouz N, Lev S. Calcium, protease activation, and cytoskeleton remodeling underlie growth cone formation and neuronal regeneration. Cell Mol Neurobiol. 2001;21:591–604. doi: 10.1023/a:1015135617557. [DOI] [PubMed] [Google Scholar]
- 16.Park KK, Hu Y, Muhling J, Pollett MA, Dallimore EJ, Turnley AM, Cui Q, Harvey AR. Cytokine-induced SOCS expression is inhibited by cAMP analogue: impact on regeneration in injured retina. Mol Cell Neurosci. 2009;41:313–324. doi: 10.1016/j.mcn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 18.Xie J, Proud CG. Crosstalk between mTOR complexes. Nat Cell Biol. 2013;15:1263–1265. doi: 10.1038/ncb2877. [DOI] [PubMed] [Google Scholar]
- 19.Proud CG. mTORC1 regulates the efficiency and cellular capacity for protein synthesis. Biochem Soc Trans. 2013;587:2623–2628. doi: 10.1042/BST20130036. [DOI] [PubMed] [Google Scholar]
- 20.Liu K, Lu Y, Lee JK, Samara R, Willenberg R, et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zukor K, Belin S, Wang C, Keelan N, Wang X, He Z. Short hairpin RNA against PTEN enhances regenerative growth of corticospinal tract axons after spinal cord injury. J Neurosci. 2013;33:15350–15361. doi: 10.1523/JNEUROSCI.2510-13.2013. By using virus-assisted delivery of short-hairpin RNA to suppress PTEN in the cortical neurons, the authors showed an increased regenerative growth of injured corticospinal tract axons after spinal cord injury, consistent with the results from previous genetic analysis (18). These results establish shRNA as a viable means to manipulate these regulators and translate findings into other mammalian models.
- 22.Bregman BS, Kunkel-Bagden E, McAtee M, O'Neill A. Extension of the critical period for developmental plasticity of the corticospinal pathway. J Comp Neurol. 1989;282:355–370. doi: 10.1002/cne.902820304. [DOI] [PubMed] [Google Scholar]
- 23.Tuszynski MH, Steward O. Concepts and methods for the study of axonal regeneration in the CNS. Neuron. 2012;74:777–791. doi: 10.1016/j.neuron.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng B, Lee JK, Xie F. Genetic mouse models for studying inhibitors of spinal axon regeneration. Trends Neurosci. 2006;29:640–646. doi: 10.1016/j.tins.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 25.Kim SR, Chen X, Oo TF, Kareva T, Yarygina O, Wang C, During M, Kholodilov N, Burke RE. Dopaminergic pathway reconstruction by Akt/Rheb-induced axon regeneration. Ann Neurol. 2011 Jul;70(1):110–120. doi: 10.1002/ana.22383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lu P, Wang Y, Graham L, McHale K, Gao M, Wu D, Brock J, Blesch A, Rosenzweig ES, Havton LA, Zheng B, Conner JM, Marsala M, Tuszynski MH. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012 Sep 14;150(6):1264–1273. doi: 10.1016/j.cell.2012.08.020. Using a refined method to transplant neuronal stem cells into the spinal cord lesion sites in adult rats, the authors showed that grafted cells differentiated into multiple cellular phenotypes, including neurons, which extended large numbers of axons over remarkable distances. Axonal growth was partially dependent on mammalian target of rapamycin (mTOR). Grafted neurons supported formation of electrophysiological relays across sites of complete spinal transection, resulting in partial functional recovery.
- 27.Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem. 2010;285:28034–28043. doi: 10.1074/jbc.M110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christie KJ, Webber CA, Martinez JA, Singh B, Zochodne DW. PTEN inhibition to facilitate intrinsic regenerative outgrowth of adult peripheral axons. J Neurosci. 2010;30:9306–9315. doi: 10.1523/JNEUROSCI.6271-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christie KJ, Martinez JA, Zochodne DW. Disruption of E3 ligase NEDD4 in peripheral neurons interrupts axon outgrowth: Linkage to PTEN. Mol Cell Neurosci. 2012 Jun;50(2):179–192. doi: 10.1016/j.mcn.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhou S, Shen D, Wang Y, Gong L, Tang X, Yu B, Gu X, Ding F. microRNA-222 targeting PTEN promotes neurite outgrowth from adult dorsal root ganglion neurons following sciatic nerve transection. PLoS One. 2012;7(9):e44768. doi: 10.1371/journal.pone.0044768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saijilafu, Hur EM, Liu CM, Jiao Z, Xu WL, Zhou FQ. PI3K-GSK3 signalling regulates mammalian axon regeneration by inducing the expression of Smad1. Nat Commun. 2013 Oct 28;4:2690. doi: 10.1038/ncomms3690. This study shows that PI3K pathway is required for sensory axon regeneration. However, glycogen synthase kinase 3 (GSK3), rather than mammalian target of rapamycin, mediates PI3K-dependent augmentation of the growth potential in the PNS. An implicated mediator is the induction of a transcription factor Smad1. Together with Christie (27), this study suggested an mTOR-independent axon regeneration in peripheral sensory axons.
- 32.Hur EM, Saijilafu, Lee BD, Kim SJ, Xu WL, Zhou FQ. GSK3 controls axon growth via CLASP-mediated regulation of growth cone microtubules. Genes Dev. 2011;25:1968–1981. doi: 10.1101/gad.17015911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou FQ, Snider WD. Intracellular control of developmental and regenerative axon growth. Philos Trans R Soc Lond B Biol Sci. 2006 Sep 29;361(1473):1575–1592. doi: 10.1098/rstb.2006.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009 Jun 3;29(22):7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parikh P, Hao Y, Hosseinkhani M, Patil SB, Huntley GW, Tessier-Lavigne M, Zou H. Regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc Natl Acad Sci U S A. 2011 May 10;108(19):E99–E107. doi: 10.1073/pnas.1100426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Finelli MJ, Murphy KJ, Chen L, Zou H. Differential phosphorylation of Smad1 integrates BMP and neurotrophin pathways through Erk/Dusp in axon development. Cell Rep. 2013 May 30;3(5):1592–1606. doi: 10.1016/j.celrep.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Song Y, Ori-McKenney KM, Zheng Y, Han C, Jan LY, Jan YN. Regeneration of Drosophila sensory neuron axons and dendrites is regulated by the Akt pathway involving Pten and microRNA bantam. Genes Dev. 2012 Jul 15;26(14):1612–1625. doi: 10.1101/gad.193243.112. In this study, the authors investigated axonal and dendritic regeneration in the Drosophila sensory neurons. With some mechanisms shared with mammals, such as PTEN/Akt, this study reveals surprisingly complicated regenerative responses in terms of cell type, developmental stage, and mechanism specificity.
- 38.Nawabi H, Zukor K, He Z. No simpler than mammals: axon and dendrite regeneration in Drosophila. Genes Dev. 2012;26:1509–1514. doi: 10.1101/gad.198150.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lunt SY, Vander Heiden MG. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 2011;27:441–464. doi: 10.1146/annurev-cellbio-092910-154237. [DOI] [PubMed] [Google Scholar]
- 40.Howell JJ, Ricoult SJ, Ben-Sahra I, Manning BD. A growing role for mTOR in promoting anabolic metabolism. Biochem Soc Trans. 2013;41:906–912. doi: 10.1042/BST20130041. [DOI] [PubMed] [Google Scholar]
- 41.Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P. mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex. Nature. 2007;450:736–740. doi: 10.1038/nature06322. [DOI] [PubMed] [Google Scholar]
- 42.Blättler SM, Cunningham JT, Verdeguer F, Chim H, Haas W, Liu H, Romanino K, Rüegg MA, Gygi SP, Shi Y, Puigserver P. Yin Yang 1 deficiency in skeletal muscle protects against rapamycin-induced diabetic-like symptoms through activation of insulin/IGF signaling. Cell Metab. 2012;15:505–517. doi: 10.1016/j.cmet.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem. 2005;280:9769–9772. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- 45.Reiling JH, Hafen E. The hypoxia-induced paralogs Scylla and Charybdis inhibit growth by down-regulating S6K activity upstream of TSC in Drosophila. Genes Dev. 2004;18:2879–2892. doi: 10.1101/gad.322704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H. Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res. 2000;60:7033–7038. [PubMed] [Google Scholar]
- 48.Walker SM, Leslie NR, Perera NM, Batty IH, Downes CP. The tumour-suppressor function of PTEN requires an N-terminal lipid-binding motif. Biochem J. 2004;379:301–307. doi: 10.1042/BJ20031839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trotman LC, Wang X, Alimonti A, Chen Z, Teruya-Feldstein J, Yang H, Pavletich NP, Carver BS, Cordon-Cardo C, Erdjument-Bromage H, Tempst P, Chi SG, Kim HJ, Misteli T, Jiang X, Pandolfi PP. Ubiquitination regulates PTEN nuclear import and tumor suppression. Cell. 2007;128:141–156. doi: 10.1016/j.cell.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S, Taghibiglou C, Girling K, Dong Z, Lin SZ, Lee W, Shyu WC, Wang YT. Critical role of increased PTEN nuclear translocation in excitotoxic and ischemic neuronal injuries. J Neurosci. 2013 May 1;33(18):7997–8008. doi: 10.1523/JNEUROSCI.5661-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mandolesi G, Madeddu F, Bozzi Y, Maffei L, Ratto GM. Acute physiological response of mammalian central neurons to axotomy: ionic regulation and electrical activity. FASEB J. 2004;18:1934–1936. doi: 10.1096/fj.04-1805fje. [DOI] [PubMed] [Google Scholar]
- 52.Cho Y, Cavalli V. HDAC5 is a novel injury-regulated tubulin deacetylase controlling axon regeneration. EMBO J. 2012;31:3063–3078. doi: 10.1038/emboj.2012.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cho Y, Sloutsky R, Naegle KM, Cavalli V. Injury-Induced HDAC5 Nuclear Export Is Essential for Axon Regeneration. Cell. 2013;155:894–908. doi: 10.1016/j.cell.2013.10.004. In peripheral sensory neurons, reactivation of a silent regenerative program is needed for successful axon regeneration after injury. This study shows that in these neurons, axotomy elicits a back-propagating calcium wave that invades the soma and causes nuclear export of HDAC5. Injury-induced HDAC5 nuclear export enhances histone acetylation to activate a regenerative gene-expression program. HDAC5 nuclear export is required for axon regeneration in these neurons. In contrast, components of this HDAC5 pathway fail to be activated in a model of central nervous system injury.
- 54. Ben-Yaakov K, Dagan SY, Segal-Ruder Y, Shalem O, Vuppalanchi D, Willis DE, Yudin D, Rishal I, Rother F, Bader M, Blesch A, Pilpel Y, Twiss JL, Fainzilber M. Axonal transcription factors signal retrogradely in lesioned peripheral nerve. EMBO J. 2012;31:1350–1363. doi: 10.1038/emboj.2011.494. In this study, the authors show that multiple transcription factors are found in sensory axons and associate with dynein in axoplasm from injured nerve. Among others, axonal STAT3 is locally translated and activated upon injury, and is transported retrogradely with dynein and importin α5 to modulate survival of peripheral sensory neurons after injury. Hence, retrograde transport of TFs from axonal lesion sites provides a direct link between axon and nucleus.
- 55.Michaelevski I, Segal-Ruder Y, Rozenbaum M, Medzihradszky KF, Shalem O, Coppola G, Horn-Saban S, Ben-Yaakov K, Dagan SY, Rishal I, Geschwind DH, Pilpel Y, Burlingame AL, Fainzilber M. Signaling to transcription networks in the neuronal retrograde injury response. Sci. Signaling. 2010;3:ra53. doi: 10.1126/scisignal.2000952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Moore DL, Goldberg JL. Multiple transcription factor families regulate axon growth and regeneration. Dev Neurobiol. 2011;71:1186–1211. doi: 10.1002/dneu.20934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ji SJ, Jaffrey SR. Axonal transcription factors: Novel regulators of growth cone-to-nucleus signaling. Dev Neurobiol. 2013 Jul 29; doi: 10.1002/dneu.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Annu Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 59.Schweizer U, Gunnersen J, Karch C, Wiese S, Holtmann B, Takeda K, Akira S, Sendtner M. Conditional gene ablation of Stat3 reveals differential signaling requirements for survival of motoneurons during development and after nerve injury in the adult. J Cell Biol. 2002;156:287–297. doi: 10.1083/jcb.200107009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiu J, Cafferty WB, McMahon SB, Thompson SW. Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J Neurosci. 2005;25:1645–1653. doi: 10.1523/JNEUROSCI.3269-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu RY, Snider WD. Different signaling pathways mediate regenerative versus developmental sensory axon growth. J Neurosci. 2001;21:RC164. doi: 10.1523/JNEUROSCI.21-17-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park KK, Hu Y, Muhling J, Pollett MA, Dallimore EJ, Turnley AM, Cui Q, Harvey AR. Cytokine-induced SOCS expression is inhibited by cAMP analogue: impact on regeneration in injured retina. Mol Cell Neurosci. 2009;41:313–324. doi: 10.1016/j.mcn.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Muller A, Hauk TG, Fischer D. Astrocyte-derived CNTF switches mature RGCs to a regenerative state following inflammatory stimulation. Brain. 2007;130:3308–3320. doi: 10.1093/brain/awm257. [DOI] [PubMed] [Google Scholar]
- 64.Leibinger M, Muller A, Andreadaki A, Hauk TG, Kirsch M, Fischer D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009;29:14334–14341. doi: 10.1523/JNEUROSCI.2770-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cui Q, Lu Q, So KF, Yip HK. CNTF, not other trophic factors, promotes axonal regeneration of axotomized retinal ganglion cells in adult hamsters. Invest Ophthalmol Vis Sci. 1999;40:760–766. [PubMed] [Google Scholar]
- 66.Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–742. doi: 10.1016/s0306-4522(02)00562-6. [DOI] [PubMed] [Google Scholar]
- 67.Lacroix S, Chang L, Rose-John S, Tuszynski MH. Delivery of hyper-interleukin-6 to the injured spinal cord increases neutrophil and macrophage infiltration and inhibits axonal growth. J Comp Neurol. 2002;454:213–228. doi: 10.1002/cne.10407. [DOI] [PubMed] [Google Scholar]
- 68.Piessevaux J, Lavens D, Peelman F, Tavernier J. The many faces of the SOCS box. Cytokine Growth Factor Rev. 2008;19:371–381. doi: 10.1016/j.cytogfr.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 69.Smith PD, Sun F, Park KK, Cai B, Wang C, Kuwako K, Martinez-Carrasco I, Connolly L, He Z. SOCS3 deletion promotes optic nerve regeneration in vivo. Neuron. 2009;64:617–623. doi: 10.1016/j.neuron.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banner LR, Patterson PH. Major changes in the expression of the mRNAs for cholinergic differentiation factor/leukemia inhibitory factor and its receptor after injury to adult peripheral nerves and ganglia. Proc Natl Acad Sci U S A. 1994;91:7109–7113. doi: 10.1073/pnas.91.15.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, DiStefano PS. Retrograde axonal transport of LIF is increased by peripheral nerve injury: correlation with increased LIF expression in distal nerve. Neuron. 1994;12:191–204. doi: 10.1016/0896-6273(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 72.Sun Y, Zigmond RE. Leukaemia inhibitory factor induced in the sciatic nerve after axotomy is involved in the induction of galanin in sensory neurons. Eur J Neurosci. 1996;8:2213–2220. doi: 10.1111/j.1460-9568.1996.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 73.Hyatt Sachs H, Rohrer H, Zigmond RE. The conditioning lesion effect on sympathetic neurite outgrowth is dependent on gp130 cytokines. Exp Neurol. 2010;223:516–522. doi: 10.1016/j.expneurol.2010.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fischer D, Pavlidis M, Thanos S. Cataractogenic lens injury prevents traumatic ganglion cell death and promotes axonal regeneration both in vivo and in culture. Invest Ophthalmol Vis Sci. 2000;41:3943–3954. [PubMed] [Google Scholar]
- 75.Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000;20:4615–4626. doi: 10.1523/JNEUROSCI.20-12-04615.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yin Y, Cui Q, Gilbert HY, Yang Y, Yang Z, Berlinicke C, Li Z, Zaverucha-do-Valle C, He H, Petkova V, et al. : Oncomodulin links inflammation to optic nerve regeneration. Proc Natl Acad Sci U S A. 2009;106:19587–19592. doi: 10.1073/pnas.0907085106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leibinger M, Andreadaki A, Diekmann H, Fischer D. Neuronal STAT3 activation is essential for CNTF- and inflammatory stimulation-induced CNS axon regeneration. Cell Death Dis. 2013 Sep 19;4:e805. doi: 10.1038/cddis.2013.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hammarlund M, Nix P, Hauth L, Jorgensen EM, Bastiani M. Axon regeneration requires a conserved MAP kinase pathway. Science. 2009;323:802–806. doi: 10.1126/science.1165527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yan D, Wu Z, Chisholm AD, Jin Y. The DLK-1 kinase promotes mRNA stability and local translation in C. elegans synapses and axon regeneration. Cell. 2009;138:1005–1018. doi: 10.1016/j.cell.2009.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Xiong X, Wang X, Ewanek R, Bhat P, Diantonio A, Collins CA. Protein turnover of the Wallenda/DLK kinase regulates a retrograde response to axonal injury. J Cell Biol. 2010;191:211–223. doi: 10.1083/jcb.201006039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shin JE, Cho Y, Beirowski B, Milbrandt J, Cavalli V, DiAntonio A. Dual leucine zipper kinase is required for retrograde injury signaling and axonal regeneration. Neuron. 2012;74:1015–1022. doi: 10.1016/j.neuron.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Watkins TA, Wang B, Huntwork-Rodriguez S, Yang J, Jiang Z, Eastham-Anderson J, Modrusan Z, Kaminker JS, Tessier-Lavigne M, Lewcock JW. DLK initiates a transcriptional program that couples apoptotic and regenerative responses to axonal injury. Proc Natl Acad Sci U S A. 2013 Mar 5;110(10):4039–4044. doi: 10.1073/pnas.1211074110. In this study, the authors show that DLK is required for optic nerve regeneration even after PTEN deletion, suggesting that DLK is a required component in the generation or transport of the injury signal in injured retinal ganglion cells.
- 83.Yan D, Jin Y. Regulation of DLK-1 kinase activity by calcium-mediated dissociation from an inhibitory isoform. Neuron. 2012;76:534–548. doi: 10.1016/j.neuron.2012.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huntwork-Rodriguez S, Wang B, Watkins T, Ghosh AS, Pozniak CD, Bustos D, Newton K, Kirkpatrick DS, Lewcock JW. JNK-mediated phosphorylation of DLK suppresses its ubiquitination to promote neuronal apoptosis. J Cell Biol. 2013;202:747–763. doi: 10.1083/jcb.201303066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nix P, Hisamoto N, Matsumoto K, Bastiani M. Axon regeneration requires coordinate activation of p38 and JNK MAPK pathways. Proc Natl Acad Sci U S A. 2011;108:10738–10743. doi: 10.1073/pnas.1104830108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ambron RT, Walters ET. Priming events and retrograde injury signals. A new perspective on the cellular and molecular biology of nerve regeneration. Mol Neurobiol. 1996;13:61–79. doi: 10.1007/BF02740752. [DOI] [PubMed] [Google Scholar]
- 87.Hirai S, Banba Y, Satake T, Ohno S. Axon formation in neocortical neurons depends on stage-specific regulation of microtubule stability by the dual leucine zipper kinase-c-Jun N-terminal kinase pathway. J Neurosci. 2011;31:6468–6480. doi: 10.1523/JNEUROSCI.5038-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hellal F1, Hurtado A, Ruschel J, Flynn KC, Laskowski CJ, Umlauf M, Kapitein LC, Strikis D, Lemmon V, Bixby J, Hoogenraad CC, Bradke F. Microtubule stabilization reduces scarring and causes axon regeneration after spinal cord injury. Science. 2011;331:928–931. doi: 10.1126/science.1201148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Veldman MB, Bemben MA, Thompson RC, Goldman D. Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration. Dev Biol. 2007;312:596–612. doi: 10.1016/j.ydbio.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 90.Moore DL, Blackmore MG, Hu Y, Kaestner KH, Bixby JL, Lemmon VP, Goldberg JL. KLF family members regulate intrinsic axon regeneration ability. Science. 2009;326:298–301. doi: 10.1126/science.1175737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blackmore MG, Wang Z, Lerch JK, Motti D, Zhang YP, Shields CB, Lee JK, Goldberg JL, Lemmon VP, Bixby JL. Krüppel-like Factor 7 engineered for transcriptional activation promotes axon regeneration in the adult corticospinal tract. Proc Natl Acad Sci U S A. 2012;109:7517–7522. doi: 10.1073/pnas.1120684109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Qin S, Zou Y, Zhang CL. Cross-talk between KLF4 and STAT3 regulates axon regeneration. Nat Commun. 2013;4:2633. doi: 10.1038/ncomms3633. This study shows unexpected physical and functional interactions between KLF4 and STAT3. The deletion of KLF4 in vivo induces axon regeneration of RGCs via JAK/STAT3 signaling. This regeneration can be greatly enhanced by exogenous cytokine treatment, or removal of SOCS3
- 93.Kurimoto T, Yin Y, Omura K, Gilbert HY, Kim D, Cen LP, Moko L, Kügler S, Benowitz LI. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci. 2010 Nov 17;30(46):15654–15663. doi: 10.1523/JNEUROSCI.4340-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Sun F, Park KK, Belin S, Wang D, Lu T, Chen G, Zhang K, Yeung C, Feng G, Yankner BA, He Z. Sustained axon regeneration induced by co-deletion of PTEN and SOCS3. Nature. 2011;480:372–375. doi: 10.1038/nature10594. This study shows a synergistic effect of co-deletion of PTEN and SOCS3 on promoting the neuronal survival and axon regeneration after optic nerve injury models. Together with (*90), these results demonstrated the functional interactions between PTEN and other pathways. Further, the gene profiling analysis suggested a possible contribution of a positive feedback loop in the neurons with co-deletion of PTEN and SOCS3 to sustain mTOR activity.
- 95.Chew DJ, Fawcett JW, Andrews MR. The challenges of long-distance axon regeneration in the injured CNS. Prog Brain Res. 2012;201:253–294. doi: 10.1016/B978-0-444-59544-7.00013-5. [DOI] [PubMed] [Google Scholar]
- 96.de Lima S, Koriyama Y, Kurimoto T, Oliveira JT, Yin Y, Li Y, Gilbert HY, Fagiolini M, Martinez AM, Benowitz L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A. 2012 Jun 5;109(23):9149–9154. doi: 10.1073/pnas.1119449109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Luo X, Salgueiro Y, Beckerman SR, Lemmon VP, Tsoulfas P, Park KK. Three-dimensional evaluation of retinal ganglion cell axon regeneration and pathfinding in whole mouse tissue after injury. Exp Neurol. 2013;247:653–662. doi: 10.1016/j.expneurol.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diekmann H, Leibinger M, Fischer D. Do growth-stimulated retinal ganglion cell axons find their central targets after optic nerve injury? New insights by three-dimensional imaging of the visual pathway. Exp Neurol. 2013 Oct;248:254–257. doi: 10.1016/j.expneurol.2013.06.021. [DOI] [PubMed] [Google Scholar]
- 99.Bonner JF, Connors TM, Silverman WF, Kowalski DP, Lemay MA, Fischer I. Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci. 2011 Mar 23;31(12):4675–4686. doi: 10.1523/JNEUROSCI.4130-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.van den Brand R, Heutschi J, Barraud Q, DiGiovanna J, Bartholdi K, Huerlimann M, Friedli L, Vollenweider I, Moraud EM, Duis S, Dominici N, Micera S, Musienko P, Courtine G. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 101.Courtine G, Song B, Roy RR, Zhong H, Herrmann JE, Ao Y, Qi J, Edgerton VR, Sofroniew MV. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nature Med. 2008;14:69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]