Abstract
BACKGROUND
Various mechanisms in cardiac remodeling related to atrial fibrillation (AF) lead to elevated circulating cardiac troponin levels, but little is known about such elevations upstream to AF onset.
OBJECTIVE
The purpose of this study was to study the association between circulating troponin levels as assessed by a highly sensitive cardiac troponin T (hs-cTnT) assay and incident atrial fibrillation (AF).
METHODS
In a large prospective cohort of ambulatory older adults [the Cardiovascular Health Study (CHS)], hs-cTnT levels were measured in sera that were collected at enrollment from 4262 participants without AF (2871 with follow-up measurements). Incident AF was identified by electrocardiograms during CHS visits, hospital discharge diagnoses, and Medicare files, including outpatient and physician claims diagnoses.
RESULTS
Over median follow-up of 11.2 years (interquartile range 6.1–16.5), 1363 participants (32.0%) developed AF. Higher baseline levels of hs-cTnT were associated with incident AF in covariate-adjusted analyses accounting for demographics, traditional risk factors, and incident heart failure in time-dependent analyzes (hazard ratio for 3rd tertile vs undetectable 1.75, 95% confidence interval 1.48–2.08). This association was statistically significant in analyses that additionally adjusted for biomarkers of inflammation and hemodynamic strain (hazard ratio for 3rd tertile vs undetectable 1.38, 95% confidence interval 1.16–1.65). Significant associations were also found when hs-cTnT levels were treated as a continuous variable and when examining change from baseline of hs-cTnT levels and incident AF.
CONCLUSION
The findings show a significant association of circulating troponin levels in ambulatory older adults with incident AF beyond that of traditional risk factors, incident heart failure, and biomarkers of inflammation and hemodynamic strain.
Keywords: Atrial fibrillation, Biomarker, Cardiac remodeling Aging
Introduction
Atrial fibrillation (AF), the most common cardiac arrhythmia encountered in clinical practice, is associated with increased morbidity and mortality, particularly in older adults.1,2 The arrhythmia has become a major public health problem, one that is expected to grow as greater longevity expands the population of older adults in modern societies.1
It stands to a reason that there has been significant interest in better understanding AF and noninvasive modalities that could identify key components of cardiac remodeling that predispose to and promote the arrhythmia.3 Importantly, there is a paucity of data on serum biomarkers of pathways involved in structural remodeling in AF and particularly on alterations of such biomarkers upstream to AF onset.
In animal models4–7 and in patients with AF,8–11 cardiac remodeling processes related to the arrhythmia lead to release of troponin into the circulation. A highly sensitive cardiac troponin T assay (hs-cTnT) that allows assessment of such processes showed that hs-cTnT levels are elevated in a significant proportion of adults aged 65 or older and are associated with incident heart failure.12 This patient population is of particular interest because it is at highest risk for AF in the community. In younger populations, baseline troponin I levels by a highly sensitive assay have been recently associated with incident AF.13,14
We hypothesized that baseline and serial measures of hs-cTnT levels in the general population of older adults are associated with incident AF beyond that of traditional risk factors and incident heart failure. The study hypothesis was tested in a large community-based prospective cohort of older adults: the Cardiovascular Health Study (CHS).
Methods
Study population
The CHS is a longitudinal study of adults aged 65 years or older at recruitment. The rationale, design, and methods of CHS, including information on data collection and definition of comorbid conditions, have been previously published.15,16 In brief, the CHS population consisted of 5888 men and women recruited from Medicare files from 4 communities in the United States (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Pittsburgh, PA). The original cohort of participants included 5201 subjects who were enrolled from 1989 to 1990. A supplemental cohort was enrolled between 1992 and 1993, which included 687 African Americans. The cohort for current analysis included 4262 participants without AF who had baseline levels of hs-cTnT from sera collected at enrollment and were not missing covariate information (n = 31). Of them, 2871 participants had follow-up measures (2–3 years after the original assay). One individual was excluded because of extreme change in hs-cTnT; thus, 2870 were included in analyses of the association between change in hs-cTnT levels and incident AF. The CHS was approved by the institutional review boards at University of Washington (Seattle, WA) and participating sites. All subjects gave written informed consent at time of enrollment.
Initial assessment, follow-up, and cardiovascular events
At enrollment, study participants were assessed by a standardized questionnaire that addressed various health and behavioral risk factors along with a physical examination.17 For each cardiovascular condition, self-reports were confirmed by components of the baseline examination or, if necessary, by a validation protocol that included either the review of medical records or surveys of treating physicians. Prevalent AF was identified by electrocardiograms (ECGs) obtained at baseline. Self-reported heart failure was confirmed by symptoms, physical signs, and the use of both diuretics and either digitalis or a vasodilator. Further confirmation of prevalent AF or heart failure was sought from treating physicians by questionnaires or from hospitals by discharge summaries, as well as by review of medical records. After the initial assessment, enrolled subjects were contacted every 6 months for follow-up, alternating between telephone interviews and clinic visits through 1998–1999, thereafter, except for a follow-up clinic visit in 2005–2006 in a subset, contacts were by telephone interviews only. In all participants, resting 12-lead electrocardiograms were recorded at baseline and repeated annually until the last clinic visit. Echocardiograms were obtained at baseline for the original cohort and at the 1994–1995 study visit for both cohorts. In addition, discharge diagnoses for all hospitalizations were collected. New cardiovascular events, reported during a clinical visit, telephone encounter, or hospital stay, were confirmed by obtaining medical records and adjudicated by a centralized events committee.17 The details on ascertainment and adjudication of death and cardiovascular events in CHS have been previously published.17 Incident AF was identified by electrocardiograms during CHS visits, hospital discharge diagnoses, and Medicare files, including outpatient and physician claims diagnoses. For Medicare data, diagnosis of AF was based on a single inpatient claim or on 2 outpatient or carrier claims within 365 days of each other. For outpatient/carrier claim AF diagnoses, qualifying claims had to specify different dates of service, and carrier claims were restricted to those received from an office, home, skilled nursing facility, nursing facility, or custodial care facility to avoid double-counting inpatient and outpatient claims. Electrocardiograms from CHS visits were read by the CHS Electrocardiography Reading Center. Post open heart surgery was not counted as incident AF. For these participants, when a subsequent hospitalization or study examination revealed AF unrelated to heart surgery, the date of the subsequent AF occurrence was used as the date of incident AF. Incident heart failure was confirmed by documentation in the medical record of a constellation of symptoms and physical signs with supporting clinical findings or a record of medical therapy for heart failure.
Cardiac troponin T assays
Details on blood sample acquisition as well as analytical and quality assurance methods in CHS were previously published.18 All measurements of troponin T levels were performed in a central blood analysis laboratory. Baseline measures were obtained from sera collected at enrollment. Follow-up measures were performed on blood samples collected 2 to 3 years later. Blood samples were stored at −70°C to −80°C and thawed just before laboratory assays (maximum of 3 freeze–thaw cycles) in April 2010. All cardiac troponin T concentrations were measured using highly sensitive reagents on an Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, IN). The analytical measurement range of the assay was 3 to 10,000 ng/L with an analytical coefficient of variation of 10%.19 Values of hs-cTnT that were below the threshold of detection were set to 2.99 for continuous analyses. Analytical sensitivity, specificity, interferences, and precision of the assay were previously validated.19 The hs-cTnT measurements for this study are from reagent lots not affected by the technical bulletin from Roche Diagnostics regarding calibration curves of the assay for some prior reagent lots.20 All technologists performing and recording the biomarker assay results were blinded to participants’ outcomes, including incident AF.
Statistical analysis
The distributions of baseline characteristics are summarized by categories of hs-cTnT based on undetectable and tertiles of detectable levels of hs-cTnT. Data are summarized as mean and standard deviation or median and interquartile range for continuous variables and number (percentage) for categorical variables. Kaplan–Meier curves are used to present the survival free from AF based on undetectable and tertiles of detectable levels of hs-cTnT.
Covariate adjusted Cox proportional hazards models were used to study the association of baseline hs-cTnT levels and incident AF. The linearity of the association between log-transformed hs-cTnT and incident AF was assessed using penalized cubic splines. Based on this graph, we modeled the continuous association using linear splines with a knot at log hs-cTnT value of 3.25. We also categorized hs-cTnT as defined previously. Individuals were censored due to death, loss to follow-up, or end of event ascertainment (June 30, 2009). The first model adjusted for age, race, and gender. The second model additionally adjusted for traditional risk factors for AF, based on prior publications from large-scale epidemiologic studies,21–23 and the use of cardiac medications. These baseline covariates included body mass index, known prevalent coronary disease (including a prior diagnosis of myocardial infarction [MI]), prevalent heart failure, smoking status (current vs not), systolic blood pressure, fasting glucose levels, and use of an antihypertensive drug (beta-blocker, calcium channel blocker, diuretic, vasodilator, angiotensin-converting enzyme inhibitor, angiotensin type 2 antagonist), an antiarrhythmic agent (any class IA, IB, IC or III agent), or digoxin.
The third model additionally included updated heart failure status in a time-dependent analysis to account for the known association between heart failure and AF and the fact that hs-cTnT levels have been associated with incident heart failure (HF).12 The fourth model (n = 4233) additionally adjusted model 2 for biomarkers of inflammation (C-reactive protein [CRP]) and hemodynamic strain (N-terminal pro–B-type natriuretic peptide [NT–pro-BNP]) in sera collected at baseline. This analysis was conducted to assess the potential influence of these 2 important pathways of cardiac structural remodeling on the associations of interest. As a sensitivity analysis, further adjustment was made for valvular disease defined by echocardiogram at baseline for the first cohort and at 2 years after baseline for the second cohort. A second sensitivity analysis was also conducted and excluded participants who were taking any antiarrhythmic medications or digitalis at baseline, with follow-up starting 2 years after the initial troponin measures to identify incident AF cases. This sensitivity analysis aimed to account for potentially missing prevalent asymptomatic paroxysmal AF, which may have preceded troponin measures, as a potential confounder of the association between circulating troponin levels and incident AF.
The association between change in hs-cTnT levels and incident AF was evaluated in covariate-adjusted Cox models as detailed earlier. The change in hs-cTnT levels was analyzed as a continuous variable of change per year from baseline and adjusted for baseline levels in multivariable models. Hazard ratio (HR) and 95% confidence interval (CI) are reported from the proportional hazards models. Statistical analyses were performed using the statistical software STATA (version 12.1, StataCorp, College Station, TX). P < .05 was considered significant.
Results
The study population consisted of 4262 CHS participants without AF who had hs-cTnT levels measured in sera collected at enrollment and non-missing covariate information. Of those patients, 1419 (33.3%) had undetectable hs-cTnT levels (<3.00 ng/L) and 2843 had detectable levels (≥3.00 ng/L). Baseline demographics and clinical characteristics of the study population, categorized into groups of undetectable and tertiles of detectable hs-cTnT levels, are summarized in Table 1. Overall, participants with higher hs-cTnT levels at baseline were more likely to have known prevalent cardiovascular disease or risk factors.
Table 1.
Baseline demographics and clinical characteristics of the study population (4262 Cardiovascular Health Study participants [CHS] without atrial fibrillation at enrollment) categorized according to baseline troponin T levels by a highly sensitive assay (hs-cTnT)
| hs-cTnT levels (ng/L) | ||||
|---|---|---|---|---|
| <3.00 (undetectable) | 3.00–6.29 (1st tertile) | 6.30–11.21 (2nd tertile) |
>11.21 (3rd tertile) | |
| N | 1419 | 949 | 947 | 947 |
| Age (years) | 70.40 ± 4.03 | 72.23 ± 4.69 | 73.52 ± 5.52 | 75.88 ± 6.52 |
| Race (African-American) | 245 (17.3%) | 142 (15.0%) | 127 (13.4%) | 181 (19.1%) |
| Gender (female) | 322 (22.7%) | 323 (34.0%) | 478 (50.5%) | 599 (63.3%) |
| Body mass index (kg/m2) | 26.74 ± 4.72 | 26.70 ± 4.93 | 26.87 ± 4.76 | 26.81 ± 4.74 |
| Systolic blood pressure (mm Hg) | 132.61 ± 20.11 | 135.40 ± 20.57 | 139.51 ± 21.65 | 142.29 ± 24.33 |
| Diabetes | 161 (11.3%) | 145 (15.3%) | 197 (20.8%) | 268 (28.3%) |
| Fasting glucose (mg/dL) | 106.64 ± 32.69 | 109.81 ± 31.66 | 113.27 ± 34.25 | 121.87 ± 50.70 |
| Albumin (mg/dL) | 4.02 ± 0.28 | 3.99 ± 0.29 | 4.01 ± 0.30 | 3.96 ± 0.30 |
| Heart rate (bpm) | 64.26 ± 10.10 | 64.20 ± 10.58 | 65.04 ± 11.98 | 67.17 ± 13.95 |
| Coronary disease | 174 (12.3%) | 171 (18.0%) | 202 (21.3%) | 292 (30.8%) |
| Prior myocardial infarction | 83 (5.8%) | 67 (7.1%) | 99 (10.5%) | 158 (16.7%) |
| Heart failure | 15 (1.1%) | 30 (3.2%) | 34 (3.6%) | 98 (10.3%) |
| Valvular disease | 81 (5.9%) | 85 (9.2%) | 86 (9.3%) | 104 (11.8%) |
| Current smoker | 198 (14.0%) | 88 (9.3%) | 90 (9.5%) | 97 (10.2%) |
| Cardiac medication | ||||
| Aspirin | 446 (31.4%) | 303 (31.9%) | 349 (36.9%) | 356 (37.6%) |
| Beta blocker | 165 (11.6%) | 127 (13.4%) | 134 (14.1%) | 130 (13.7%) |
| Angiotensin-converting enzyme inhibitor | 72 (5.1%) | 65 (6.8%) | 77 (8.1%) | 110 (11.6%) |
| Antiarrhythmic drug | 25 (1.8%) | 26 (2.7%) | 29 (3.1%) | 47 (5.0%) |
| Diuretic | 323 (22.8%) | 242 (25.5%) | 279 (29.5%) | 375 (39.6%) |
| Digitalis | 39 (2.7%) | 46 (4.8%) | 68 (7.2%) | 133 (14.0%) |
| N-terminal pro–B-type natriuretic peptide (ng/L)* | 79.26 (43.50, 141.70) | 97.21 (54.66, 184.80) | 126.60 (62.70, 257.20) | 219.60 (104.30, 531.50) |
| C-reactive protein(mg/dL)* | 2.51 (1.31, 4.28) | 2.31 (1.08, 4.34) | 2.33 (1.23, 4.22) | 3.04 (1.55, 6.21) |
Values are given as mean ± SD or number (percentage) unless otherwise specified.
Median (interquartile range).
During median follow-up of 11.2 years (interquartile range 6.1–16.5), 1363 participants (32.0%) developed new-onset AF. Of these participants, 73.1% were identified by hospital records or inpatient claims, 18.2% were identified by outpatient or physician claims, and the remaining 8.7% were identified by electrocardiograms obtained during CHS visits.
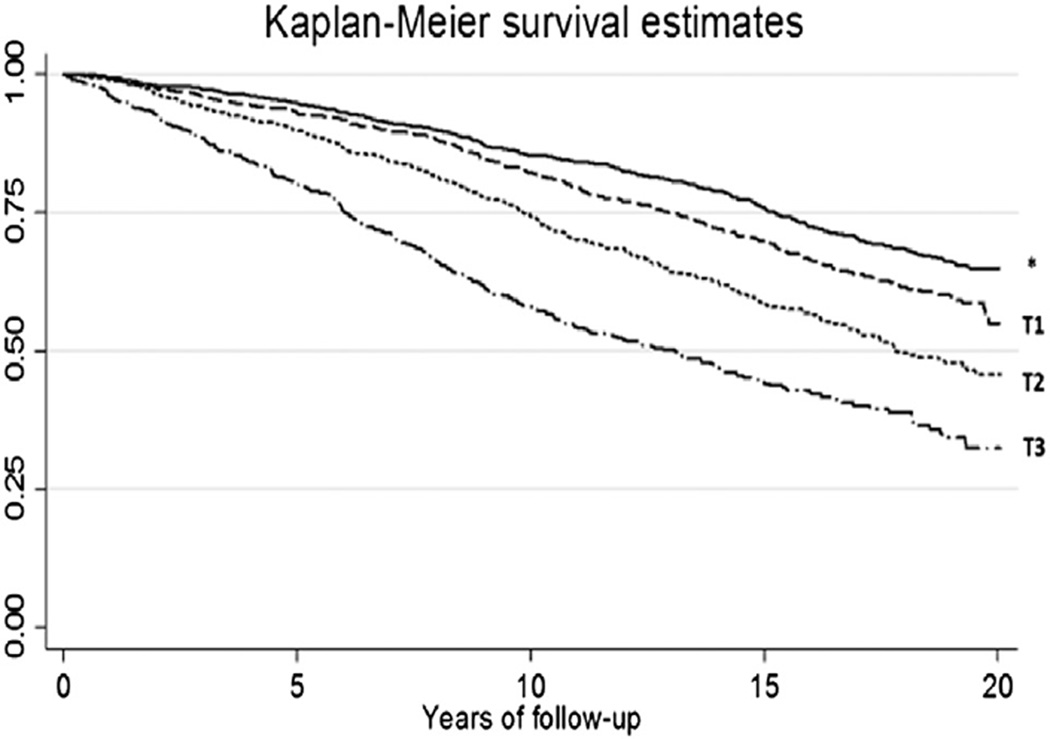
Participants with higher hs-cTnT levels at baseline were more likely to have incident AF during follow-up (Figure 1). The relationship between log hs-cTnT was mostly linear and leveled off in a plateau pattern after a cutoff of 3.25 log-ng/L, which corresponds to a baseline hs-cTnT level of 24.53 ng/L. The hs-cTnT levels of most of the study participants (95.4%) were within the first spline (≤24.53 ng/L), in which a linear relationship was observed.
Figure 1.
Kaplan–Meier estimates of survival free from atrial fibrillation in participants of the Cardiovascular Health Study (CHS) according to baseline levels of troponin T levels measured by a highly sensitive assay (hs-cTnT). Log-rank P < .0001. *Undetectable levels. T1, T2, T3 = first, second, and third tertile of detectable hs-cTnT levels, respectively.
In Cox proportional hazards analyses adjusting for age, race, and gender, baseline levels of hs-cTnT were found to be associated with incident AF up to 24.53 ng/L (HR 1.67 per log unit hs-cTnT, 95% CI 1.52–1.83), with no additional increased risk above 24.53 ng/L (HR 0.97 per log unit hs-cTnT, 95% CI 0.67–1.39). This association persisted when hs-cTnT was modeled categorically (3rd tertile of detectable vs undetectable levels HR 2.39, CI 2.03–2.82). This association was attenuated on further adjustment but remained statistically significant (Table 2). In the third model adjusting AF risk factors and heart failure status updated over time, the association of hs-cTnT up to 24.53 ng/L and incident AF had an HR of 1.39 per log unit hs-cTnT (95% CI 1.26–1.53). The association of hs-cTnT above 24.53 ng/L and incident AF remained insignificant (HR 0.79 per log unit hs-cTnT, CI 0.52–1.18). Further attenuation was observed in models that adjusted for CRP and NT–pro-BNP in addition to baseline risk factors, but the association remained statistically significant below 24.53 ng/L hs-cTnT (Table 2). Results were similar in models adjusting for valvular disease, as well as in those that excluded participants who were taking antiarrhythmic medications or digitalis at baseline, with a follow-up starting 2 years after the initial troponin measures (sensitivity analyses data not shown). Similar findings were observed in a sensitivity analysis in which only subjects with ECG-validated or inpatient AF diagnoses were counted as incident AF.
Table 2.
Covariate-adjusted Cox analyses of the association between cardiac troponin T levels by a highly sensitive assay (hs-cTnT) and incident atrial fibrillation (AF) in the Cardiovascular Health Study (CHS)
| Model 1 HR (95%CI) |
Model 2 HR (95%CI) |
Model 3 HR (95%CI) |
Model 4 HR (95%CI) |
|
|---|---|---|---|---|
| Log-transformed hs-cTnT values | ||||
| Spline model, knot at log (hs-cTnT) = 3.25 | ||||
| Spline 1*, hs-cTnT ≤24.53 | 1.67 (1.52,1.83) | 1.45 (1.32,1.59) | 1.39 (1.26,1.53) | 1.21 (1.10,1.34) |
| Spline 2*, hs-cTnT >24.53 | 0.97 (0.67,1.39) | 0.88 (0.60,1.29) | 0.79 (0.52,1.18) | 0.66 (0.44,1.00) |
| hs-cTnT levels (ng/L) | ||||
| <3.00 (undetectable) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 3.00–6.23 (1st tertile) | 1.16 (0.99,1.36) | 1.09 (0.93,1.28) | 1.08 (0.93,1.27) | 1.02 (0.88,1.20) |
| 6.24–10.97 (2nd tertile) | 1.50 (1.29,1.75) | 1.32 (1.13,1.54) | 1.27 (1.09,1.49) | 1.12 (0.96,1.32) |
| >10.97 (3rd tertile) | 2.39 (2.03,2.82) | 1.89 (1.60,2.24) | 1.75 (1.48,2.08) | 1.38 (1.16,1.65) |
Model 1 adjusted for age, race, and gender.
Model 2 additionally adjusted for body mass index, known prevalent coronary disease or myocardial infarction, prevalent heart failure, smoking status (current vs not), systolic blood pressure, fasting glucose levels, and use of antihypertensive (beta-blocker, calcium channel blocker, diuretic, vasodilator, angiotensin-converting enzyme inhibitor, angiotensin type 2 antagonist), antiarrhythmic (any class IA, IB, IC or III agents), or digoxin.
Model 3 adjusted for model 2 variables and incident heart failure.
Model 4 adjusted for model 2 variables and baseline levels of N-terminal pro–B-type natriuretic peptide and C-reactive protein (both log transformed).
CI = confidence interval.
Hazard ratio (HR) per log(hs-cTnT) increase.
In the analysis on change in hs-cTnT levels (n = 2870), an increase from baseline of hs-cTnT levels was significantly associated with incident AF (Table 3). After adjustment for age, race, gender, and log hs-cTnT, the HR for incident AF for 1 ng/L per year increment in hs-cTnT was 1.03 (95% CI 1.01–1.04). The association between change in hs-cTnT and incident AF was also observed in covariate-adjusted analyses accounting for baseline demographics, risk factors, cardiac medications, prevalent and incident heart failure, as well as baseline CRP and NT–pro-BNP levels (Table 3).
Table 3.
Covariate-adjusted Cox analyses of the association between change per year in cardiac troponin T levels by a highly sensitive assay (hs-cTnT) and incident atrial fibrillation (AF) in the Cardiovascular Health Study (CHS)
| Covariates | Hazard ratio* |
95% Confidence interval |
|
|---|---|---|---|
| Model 1 | Age, race, gender | 1.03 | 1.01–1.04 |
| Model 2 | Model 1 + baseline risk factors | 1.03 | 1.01–1.04 |
| Model 3 | Model 2 + incident HF | 1.02 | 1.01–1.04 |
| Model 4 | Model 2 + CRP, NT–pro-BNP | 1.02 | 1.01–1.03 |
Covariates in models are those in Table 2 legends with additional adjustment for baseline hs-cTnT.
CRP = C-reactive protein; HF = heart failure; NT–pro-BNP = N-terminal pro–B-type natriuretic peptide.
Hazard ratio for every 1 ng/L increase from baseline.
Discussion
In a large community-based prospective cohort of older adults with long-term follow-up, there was a significant association between measures of cardiac troponin T by a highly sensitive assay and incident AF. This association was significant in covariate-adjusted analyses accounting for baseline demographics, traditional AF risk factors, and use of cardiac medications. Importantly, these associations persisted even after adjustment for incident HF in time-dependent analyses as well as adjustment for biomarkers of inflammation and hemodynamic strain. Another important finding is that the change in hs-cTnT levels with serial measures was associated with incident AF, which denotes a dynamic change in risk. The findings suggest an association between processes that involve release of troponins into the circulation in ambulatory older subjects and incident AF beyond that of traditional risk factors and potential confounders or mediators such as inflammation, hemodynamic strain, and incident HF. Such processes involve but are not limited to cardiomyocyte injury, myocardial damage, proteolysis, or myocardial contractile protein turnover in heart failure.
The findings provide important mechanistic insights about pathophysiologic processes occurring before the onset of AF in older adults. In the past few decades, significant research has focused on alterations of multiple pathways of cardiac structural remodeling in patients with AF,24 but little is known about the alterations of these pathways upstream to AF onset. The close association of AF and remodeling processes that release cardiac troponins into the circulation has been suggested in animal models of HF4–7 as well as in patients with AF.8–11 Our study suggests that such processes in the aging heart can occur prior to, and predisposes to, AF independent of traditional risk factors and heart failure. The findings are important because they identify a key component of cardiac remodeling before AF onset.
The measures of very low concentrations of cardiac troponins have become possible with the introduction of highly sensitive assays.19 Traditionally, troponin measures have been used for the diagnosis of MI, but the new assays have suggested that troponin measures may provide important information regarding cardiovascular risk in various populations, beyond that of MI diagnosis.12,25–27 In fact, circulating hs-cTnT levels have been linked to cardiovascular mortality in patients with stable coronary disease or HF.26,27 In the general population, cardiac troponin levels by a highly sensitive assay were found to be associated with structural heart disease and traditional cardiovascular risk factors,12,25 but provided independent prognostic information regarding incident heart failure and cardiovascular and all-cause mortality.12,25 More recently, we found a significant association between hs-cTnT levels and the risk of potentially arrhythmic sudden cardiac death in older adults, which was independent of HF, MI, and other cardiovascular risk factors.28 Based on prior publications, it appears that elevated hs-cTnT levels cosegregate with other cardiovascular conditions and risk factors but are independently associated with incident AF, as suggested by our observations.
The pathophysiologic mechanisms underlying the associations in the current study deserve further investigation. It is possible that hs-cTnT levels reflect ongoing cardiomyocyte injury, myocardial damage, inflammation, proteolysis, myocardial contractile protein turnover in heart failure, or cardiac strain in the aging heart, which would predispose to AF.24 However, the observation of an association between hs-cTnT and AF after adjusting for prevalent and incident HF and biomarkers of inflammation and hemodynamic strain suggests the involvement of hs-cTnT in an independent pathway upstream to AF onset. The adjustment for incident HF had only a mild effect on the association of interest, which could be related to the adjustment for a number of other risk factors that predispose to both HF and AF.
The findings are concordant with recent observations in younger populations of an association between cardiac troponin levels and incident AF.13,14 The Framingham offspring study did not adjust for the interval development of HF, which is a potential mediator and confounder in analyses addressing pathways of cardiac remodeling and AF.14 The current study addressed this potential effect of incident HF and showed only minimal confounding or mediating effect. Another novel finding is a dynamic change in risk, which is reflected by serial measures of hs-cTnT. The associations seem to level off with higher concentrations, which was not reported in the prior reports. Importantly, the current study addresses the association of hs-cTnT and AF in an older population, which is the group at highest risk for AF in the community and in whom circulating hs-cTnT levels are detectable in most subjects. This could potentially explain the much higher incidence of AF in our report compared to the prior data.
Study limitations
The study has the inherent limitations of observational studies; therefore, the findings may have been affected by residual confounding. However, the associations persisted after adjustment for a range of clinical risk factors and biomarkers, which makes the link between hs-cTnT and AF compelling. We note that the study aimed to assess and highlight a key element in cardiac remodeling and the biologic alterations upstream to AF onset in older adults and not to build a risk prediction model. In the prior publications, risk prediction was not improved with the use of biomarkers. Given the multifactorial and complex pathophysiology of AF, no predictive diagnostic test exists. However, the findings suggest that troponin release into the circulation in the aging heart is associated with AF in a process that has yet to be identified. Another limitation was that the study could have missed asymptomatic AF. To address the possibility that such asymptomatic paroxysmal AF could have preceded hs-cTnT elevations, we conducted a sensitivity analysis that showed the association to persist even when participants who were taking any antiarrhythmic medication or digitalis at baseline were excluded and follow-up started 2 years after baseline (sensitivity analysis data not shown). Furthermore, prior publications from CHS showed the validity of AF ascertainment and adjudication compared to 24-hour Holter monitoring data and missed only 0.1% of sustained or intermittent AF.29,30 Finally, it is important to note that the observations were made in a population of older adults and might not be generalizable to younger populations in whom other risk factors or pathophysiologic processes could predispose to AF.
In addition to novel observations, the study has several strengths, including the prospective design, large cohort, long-term follow-up, measures of hs-cTnT in a central laboratory, and adjudication of cardiovascular events by a central committee.
Conclusion
In a large prospective cohort of community-dwelling older adults, we found an association between baseline and serial measures of cardiac troponin T by a highly sensitive assay and incident AF. The findings suggest an association of processes that leads to release of troponins from myocytes into the circulation in ambulatory older adults with incident AF beyond that of traditional risk factors, incident heart failure, inflammation, and hemodynamic strain.
CLINICAL PERSPECTIVES.
In the past few decades, significant research has focused on better understanding atrial fibrillation (AF) and noninvasive modalities that could identify key components of cardiac remodeling that predispose to and promote the arrhythmia. This is especially true for older adults who are at highest risk for AF in the community. Various mechanisms in cardiac remodeling related to AF lead to elevated circulating cardiac troponin levels, but little is known about such elevations upstream to AF onset. In this study, the association between circulating troponin T levels by a highly sensitive assay (hs-cTnT) and incident AF was studied in a large community-based prospective cohort of older adults: The Cardiovascular Health Study. The main observation was a significant association between baseline and serial measures of hs-cTnT and incident AF beyond that of traditional risk factors, incident heart failure, inflammation, and hemodynamic strain. The findings suggest an association between processes that involve release of troponins into the circulation in ambulatory older subjects and incident AF. Such processes involve but are not limited to cardiomyocyte injury, myocardial damage, proteolysis, or myocardial contractile protein turnover in heart failure. The findings provide important mechanistic insights into pathophysiologic processes occurring upstream to AF in older adults.
Acknowledgments
This research was supported by Contracts HHSN268201200036C, HHSN268200800007C, N01 HC55222, N01HC85079, N01HC85080, N01HC85081, N01HC85082, N01HC85083, and N01HC85086, and Grants HL080295 and HL102214 from the National Heart, Lung, and Blood Institute (NHLBI), with additional contribution from the National Institute of Neurological Disorders and Stroke (NINDS). Additional support was provided by AG023629 from the National Institute on Aging (NIA). A full list of principal Cardiovascular Health Study (CHS) investigators and institutions can be found at CHS-NHLBI.org. Additional funding was provided by Roche Diagnostics. Roche Diagnostics provided funding and laboratory reagents for the highly sensitive cardiac troponin T assay. Dr. deFilippi receives honoraria, consulting, and grant support from Roche Diagnostics and Siemens Healthcare Diagnostics; and consulting and grant support from Critical Diagnostics and BG Medicine.
ABBREVIATIONS
- AF
atrial fibrillation
- CHS
Cardiovascular Health Study
- CI
confidence interval
- CRP
C-reactive protein
- ECG
electrocardiogram
- HF
heart failure
- HR
hazard ratio
- hs-cTnT
highly sensitive cardiac troponin T (assay)
- IQR
interquartile range
- MI
myocardial infarction
- NT–pro-BNP
N-terminal pro–B-type natriuretic peptide
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Fuster V, Ryden LE, Cannom DS, et al. ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation. Circulation. 2006;114:e257–e354. doi: 10.1161/CIRCULATIONAHA.106.177292. [DOI] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Chen PS, Bild DE, et al. Prevention of atrial fibrillation: report from a national heart, lung, and blood institute workshop. Circulation. 2009;119:606–618. doi: 10.1161/CIRCULATIONAHA.108.825380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li D, Fareh S, Leung TK, Nattel S. Promotion of atrial fibrillation by heart failure in dogs: atrial remodeling of a different sort. Circulation. 1999;100:87–95. doi: 10.1161/01.cir.100.1.87. [DOI] [PubMed] [Google Scholar]
- 5.Bauer A, McDonald AD, Donahue JK. Pathophysiological findings in a model of persistent atrial fibrillation and severe congestive heart failure. Cardiovasc Res. 2004;61:764–770. doi: 10.1016/j.cardiores.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Cardin S, Li D, Thorin-Trescases N, Leung TK, Thorin E, Nattel S. Evolution of the atrial fibrillation substrate in experimental congestive heart failure: angiotensin-dependent and -independent pathways. Cardiovasc Res. 2003;60:315–325. doi: 10.1016/j.cardiores.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 7.Schoonderwoerd BA, Ausma J, Crijns HJ, Van Veldhuisen DJ, Blaauw EH, Van Gelder IC. Atrial ultrastructural changes during experimental atrial tachycardia depend on high ventricular rate. J Cardiovasc Electrophysiol. 2004;15:1167–1174. doi: 10.1046/j.1540-8167.2004.03693.x. [DOI] [PubMed] [Google Scholar]
- 8.Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A. Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation. 1997;96:1180–1184. doi: 10.1161/01.cir.96.4.1180. [DOI] [PubMed] [Google Scholar]
- 9.Han W, Fu S, Wei N, Xie B, Li W, Yang S, Li Y, Liang Z, Huo H. Nitric oxide overproduction derived from inducible nitric oxide synthase increases cardiomyocyte apoptosis in human atrial fibrillation. Int J Cardiol. 2008;130:165–173. doi: 10.1016/j.ijcard.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Aime-Sempe C, Folliguet T, Rucker-Martin C, Krajewska M, Krajewska S, Heimburger M, Aubier M, Mercadier JJ, Reed JC, Hatem SN. Myocardial cell death in fibrillating and dilated human right atria. J Am Coll Cardiol. 1999;34:1577–1586. doi: 10.1016/s0735-1097(99)00382-4. [DOI] [PubMed] [Google Scholar]
- 11.Thiedemann KU, Ferrans VJ. Left atrial ultrastructure in mitral valvular disease. Am J Pathol. 1977;89:575–604. [PMC free article] [PubMed] [Google Scholar]
- 12.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filion KB, Agarwal SK, Ballantyne CM, Eberg M, Hoogeveen RC, Huxley RR, Loehr LR, Nambi V, Soliman EZ, Alonso A. High-sensitivity cardiac troponin T and the risk of incident atrial fibrillation: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2015;169:31–38. doi: 10.1016/j.ahj.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rienstra M, Yin X, Larson MG, et al. Relation between soluble ST2, growth differentiation factor-15, and high-sensitivity troponin I and incident atrial fibrillation. Am Heart J. 2014;167:109–115. e2. doi: 10.1016/j.ahj.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 16.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 17.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 18.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 19.Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, Katus HA. Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem. 2010;56:254–261. doi: 10.1373/clinchem.2009.132654. [DOI] [PubMed] [Google Scholar]
- 20.Apple FS, Jaffe AS. Clinical implications of a recent adjustment to the high-sensitivity cardiac troponin T assay: user beware. Clin Chem. 2012;58:1599–1600. doi: 10.1373/clinchem.2012.194985. [DOI] [PubMed] [Google Scholar]
- 21.Alonso A, Krijthe B, Aspelund T, et al. Simple risk model predicts incidence of atrial fibrillation in a racially and geographically diverse population: the CHARGE-AF consortium. J Am Heart Assoc. 2013;2:e000102. doi: 10.1161/JAHA.112.000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schnabel RB, Sullivan LM, Levy D, et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): a community-based cohort study. Lancet. 2009;373:739–745. doi: 10.1016/S0140-6736(09)60443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnabel RB, Aspelund T, Li G, et al. Validation of an atrial fibrillation risk algorithmin whites and African Americans. Arch Intern Med. 2010;170:1909–1917. doi: 10.1001/archinternmed.2010.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goudis CA, Kallergis EM, Vardas PE. Extracellular matrix alterations in the atria: insights into the mechanisms and perpetuation of atrial fibrillation. Europace. 2012;14:623–630. doi: 10.1093/europace/eur398. [DOI] [PubMed] [Google Scholar]
- 25.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Latini R, Masson S, Anand IS, et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–1249. doi: 10.1161/CIRCULATIONAHA.106.655076. [DOI] [PubMed] [Google Scholar]
- 27.Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. Prevention of Events with Angiotensin Converting Enzyme Inhibition (PEACE) Trial Investigators. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. doi: 10.1056/NEJMoa0805299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hussein AA, Gottdiener JS, Bartz TM, Sotoodehnia N, deFilippi C, Dickfeld T, Deo R, Siscovick D, Stein PK, Lloyd-Jones D. Cardiomyocyte injury assessed by a highly sensitive troponin assay and sudden cardiac death in the community: the Cardiovascular Health Study. J Am Coll Cardiol. 2013;62:2112–2120. doi: 10.1016/j.jacc.2013.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. doi: 10.1161/01.cir.96.7.2455. [DOI] [PubMed] [Google Scholar]
- 30.Mozaffarian D, Psaty BM, Rimm EB, Lemaitre RN, Burke GL, Lyles MF, Lefkowitz D, Siscovick DS. Fish intake and risk of incident atrial fibrillation. Circulation. 2004;110:368–373. doi: 10.1161/01.CIR.0000138154.00779.A5. [DOI] [PMC free article] [PubMed] [Google Scholar]