Abstract
Crystallins in the retina may serve a chaperone-like protective function. In this study we measured mRNA levels for alpha-, beta- and gamma-crystallins in rat retinas following treatment with potentially damaging levels of light. We also determined crystallin protein patterns in photoreceptor cell rod outer segments isolated from rats exposed to intense light. Weanling albino rats were maintained in a dim cyclic light environment or in darkness for 40 days. At P60 animals were treated with intense visible light, for as long as 8 hours, beginning at various times of the day or night. Retinas were excised immediately after light treatment and used for quantitative RT-PCR, or to prepare rod outer segments for western analysis. Some eyes were frozen in OCT for crystallin immunohistochemistry. Intense light exposure led to increases in mRNA expression for all retinal crystallins and to changes in rod outer segment crystallin immunoreactivity. These light-induced changes were found to depend on the time of day that exposure started, duration of light treatment and previous light rearing history. We suggest that crystallin synthesis in retina exhibits a dependence on both light stress and circadian rhythm and that within photoreceptor cells crystallins appear to migrate in a light-independent, circadian fashion.
INTRODUCTION
Crystallins serve a well known structural role in the lens, where they help to maintain tissue transparency. In non-lenticular post mitotic tissues crystallins may serve as low molecular weight chaperons which help to prevent protein denaturation (1). They may also prevent apoptosis, by binding to pro-apoptotic members of the BCL family of proteins or by inhibiting caspase activation (2, 3). In retina, three classes of crystallins (α, β, and γ) have been found in a wide variety of species (4–10). Among these, α-crystallins are members of the family of small heat shock proteins (11) that may become modified by oxidative processes during normal aging (12, 13). In age related macular degeneration (AMD) oxidatively modified crystallins accumulate in drusen and in Bruch’s membrane, possibly as part of a stress response (14–16). In animal models, α-crystallin, as well as β- and γ-crystallin, protein expression is induced during inherited retinal degenerations (8–10, 17), following retinal trauma (18) and as part of the injury response to intense visible light exposure (19). Following trauma, enhanced crystallin immunoreactivity is seen in all retinal layers (18), while in photoreceptor cells crystallins are normally present in the nuclear region, post-Golgi membranes (5) and in the rod outer segment (ROS) organelle (9, 10, 19).
Photoreceptor ROS also contain the visual pigment rhodopsin and the enzymatic machinery necessary for transducing light energy into an electrical signal. This visual transduction process functions over a wide range of ambient light intensities, primarily because of reactions that quench the photoresponse at high light levels. Quenching involves rhodopsin phosphorylation, the light driven movement of retinal S-antigen (arrestin) into ROS and its binding to rhodopsin (20), and the simultaneous translocation of G-proteins (21), and other proteins (22) out of ROS (see 23 for a review). The synthesis of rhodopsin and α-transducin occurs in a circadian manner, leading to higher levels of these proteins early in the morning, while arrestin mRNA levels are high later in the day (24, 25). Longer term changes in the levels of rhodopsin and other visual cell transduction proteins can also impact the photoreceptor’s ability to adapt to light. Rats maintained in a dim cyclic light environment have decreased mRNA and protein levels for rhodopsin and α-transducin and higher arrestin levels than found in retinas from animals reared in continuous darkness (26, 27).
In addition to initiating visual transduction, intense or prolonged visible light can trigger photoreceptor cell damage and death, a process that also begins with rhodopsin bleaching (28). A number of environmental factors are known to influence the extent of light-induced photoreceptor cell damage (for recent reviews see 29, 30). For example, rats shifted to a dim cyclic light environment from a long term dark environment exhibit reduced retinal light damage compared to their littermates kept in darkness (27). Susceptibility to retinal light damage also depends on a number of endogenous factors, including age, genetic predisposition (31) and circadian rhythms (32). Cyclic light reared rats are protected against retinal light damage when exposed to light during normal daylight hours, but incur extensive visual cell loss when light exposure begins in the dark phase of the circadian cycle (32). Herein we describe differential expression patterns for retinal crystallins induced in rats by exposure to intense visible light starting at different times of the day or night. In addition, we show that retinal crystallin synthesis normally exhibits a modest circadian expression pattern and present evidence suggesting that crystallins migrate into and out of photoreceptor ROS in a light independent-circadian manner.
MATERIALS AND METHODS
Animals and rearing conditions
Male Sprague Dawley rats were obtained from Harlan Inc., Indianapolis, IN as weanlings and kept in a 12 hour dim cyclic light environment, consisting of 20–40 lux incandescent light (on at 8 am and off at 8 pm), or in darkness. Dark rearing conditions were interrupted for less than one-half hour each day by dim red light (< 600 nm), for routine animal maintenance. Rats were fed rat chow ad libitum (Teklad, Madison, WI) and had continuous access to water. The use of animals in this investigation conformed to the ARVO Statement for The Use of Animals in Ophthalmic and Vision Research and with laboratory animal resource committee guidelines at Wright State University.
Intense light treatment
At P60 rats were dark adapted for 16 hours and then exposed to intense visible light (490–580nm) for as long as 8 hours. Light treatments were in green Plexiglas (# 2092) cylinders (28), surrounded by 7 circular fluorescent lights, beginning at 1 am, 9 am, or 5 pm (31). Light intensity inside the chambers was 1200–1400 lux, approximately 200 µW per cm2 at the corneal surface (31). During light exposure the rats were unanaesthetized and had food and water available. Normal body temperature was maintained by a gentle air flow through the light treatment cylinders. Immediately after light exposure all rats were euthanized in a CO2 atmosphere. Retinas were excised in dim red light and flash frozen for storage in liquid N2 or processed for ROS isolation.
Isolation of ROS
Sucrose density ultracentrifugation was used for the isolation of photoreceptor ROS (27). Briefly, the retinas from 4 rats were homogenized in a loose fitting glass/Teflon homogenizer, in 47% sucrose in phosphate buffered saline (PBS) containing proteolysis inhibitors. The homogenate was then filtered, through 50 and 500 mesh screens, into a 5 ml ultracentrifuge tube and overlaid with PBS buffer solutions containing 42, 37 and 32% sucrose and proteolysis inhibitors. Following centrifugation at 105,000×g for 1 hour at 4 C°, ROS from the 32/37% sucrose interface (band 1) and the 37/42% interface (band 2) were collected. Band 1 represents a relatively pure fraction of ROS and contains over 75% of the rhodopsin in the gradient (27). The band 2 ROS fraction has less than 6% rhodopsin, but contains a number of other subcellular organelles. For example, the mitochondrial marker prohibitin (reviewed in 33) is not found in band 1 ROS isolated from dark adapted rats and is practically absent in band 1 ROS from rats exposed to intense light, but is present in band 2 ROS under all conditions.
Quantitative RT-PCR (qRT-PCR)
Retinas were excised from 3 separate groups of 3 rats each at 1am, 9am, and 5pm. The animals either remained in darkness for 8 hours or were exposed to intense light for 8 hours before sacrifice. The experiment was repeated 3 times on different days. Immediately after light/dark treatment total retinal RNA was extracted and quantified (OD 260) from each group of rats. RNA (0.5 µg) from each sample was then pooled and reverse transcribed with Multiscribe Reverse Transcriptase (Applied Biosystems, Foster City, CA). The resulting cDNA (10 ng) was used for RT-PCR, along with Amplitaq Gold, dNTP mixture, SYBR Green PCR buffer (Applied Biosystems) and appropriate primers (Table 1). Applied Biosystem’s Gene Amp 5700 Sequence Detection System was used to quantify target gene mRNA expression levels, normalized to the expression level of the house keeping gene GAPDH. RT-PCR for each target gene was done in triplicate. Fold change was then calculated by the delta-delta method (Applied Biosystems) for comparing relative expression results for target genes with the expression of a reference gene (34).
Table 1.
Real-Time PCR Primers
| name | forward primer | reverse primer |
|---|---|---|
| α A crystallin | 5’CCCCCTTTGCCCACACTT3’ | 5’GCATGAGAGAAGCGGACTGTT3’ |
| α B crystallin | 5’TCCGGCGTCCCTTCTTTC3’ | 5’CAGGTGCTCTCCGAAGAACTG3’ |
| β A3/A1 crystallin | 5’CCTTACAAGCCATGGGTTGGT3’ | 5’CAAACCCAAGCTCCACACTGT3’ |
| β A4 crystallin | 5’TGGCGCGTGGGTTTGT3’ | 5’TGATCGCTCTCCAGCACGTA3 |
| β B1 crystallin | 5’GCTACAGGCTGGTCGTCTTTG3’ | 5’CACTCCCCCGAGAATTCCA3’ |
| β B2 crystallin | 5’GACAATCCATGGGAGAGATCCTT3’ | 5’CATCTGCGCTCTGGCTTCA3’ |
| β B3 crystallin | 5’CAGCAGCCGGCGTAGTG3’, | 5’TGGGCCATCAATGTGCAA3’ |
| γ A crystallin | 5’CCTGCCGTTCCATTCCCTAT3’ | 5’AAGGCCCCGGTAGTCATCTC3’ |
| γ B crystallin | 5’GCTCCTGCCGCCTCATC3’ | 5’TGTCCTCTGAAGTCATCTCTTTCG3’ |
| γ C crystallin | 5’CGAGGCCGGCAGTATCTG3’ | 5’AAAGAGCCTGCCTTAGCATCTACA3’ |
| γ D crystallin | 5’GGCCAGATGGTAGAGTTCACTGA3’ | 5’CATTGAGGGAGTAGATCTCATTGAAG3’ |
| γ E/F crystallin | 5’CTTCAGTGACTTCCACTCTTTCCA3’ | 5’CCGGTAGTTGGGCATCTCA3’ |
| GAPDH | 5’TGAGAATGGGAAGCTGGTCAT3’ | 5’TCTCGCTCCTGGAAGATGGT3’ |
| β-actin | 5’AGGGAAATCGTGCGTGAC3’ | 5’CGCTCATTGCCGATAGTG3’ |
Gel electrophoresis and western analysis
ROS proteins were extracted with 5× SDS-PAGE buffer (27) and then separated by 1D gel electrophoresis (10 µg per lane). Band 1 and band 2 ROS samples were prepared from 2 or 3 separate groups of 4 rats and combined to provide average crystallin profiles. Following electrophoresis, the proteins were transferred to PVDF membranes and then probed with antibodies against α, β or γ-crystallins (9). Gels were stripped and re-probed as needed to detect other proteins. Western blots were visualized by using chemiluminescence with audioradiographic film (9). Some gels were fixed in 50% methanol/10% acetic acid and stained with Coomassie blue to visualize total ROS proteins (9). Anti-GAPDH or anti-β-actin immunostaining was used to verify equivalent protein loading on the gels. Goat-anti rabbit HRP antibody was from BioRad Laboratories (Hercules, CA). Rabbit anti αA- and αB- crystallin polyclonal antibodies were obtained from Stressgen Biotechnologies (Victoria, B C, Canada), anti β- and anti γ-crystallin antibodies were kindly provided by Dr. Sam Zigler (Department of Ophthalmology, Johns Hopkins University, Baltimore, MD).
Immunohistochemistry
Enucleated eyes were fixed in 4% paraformaldehyde (p-CHO; Sigma-Aldrich, St. Louis, MO) in PBS and cryopreserved in 30% sucrose-PBS (w/v). Eyes were frozen on dry ice in Optimal Cutting Temperature (OCT) compound (Sakura Finetek USA, Torrance, CA) in a tissue holder and stored at −70°C until needed. Six µm sections of the eye were cut using a TissueTek II Microtome/Cryostat 4551 (Lab-Tek Division Miles Laboratories, Naperville, IL) and transferred to SuperFrost Plus microscope slides (Fisher Scientific Ltd., Nepean, Ontario). Slides were fixed in 4% p-CHO-PBS for 5 minutes, dehydrated sequentially for 2 minutes in 30%, 80% and 100% EtOH, and stored at −70°C.
For immunodetection of specific proteins, frozen sections were first immersed in hot (95°C) citrate buffer (10 mM citric acid monohydrate, pH 6.5), denatured in a steam bath for 15 minutes, cooled to room temperature, blocked with 0.1% Triton X-100 and 5% skim milk in Tris-buffered saline (TBS), and rinsed in 0.1% Triton X-100 in TBS (TBS/T). The primary antibody was diluted with 5% skim milk in TBS/T. A 1:200 dilution of anti αB-crystallin, or anti γS-crystallin (a generous gift from Dr. Graeme Wistow, NEI, NIH, Bethesda, MD), was then applied to the retinal sections for 12 hours at 4°C, and washed 3× for 5 minutes in TBS/T. Secondary antibody (anti-rabbit IgG F(ab’)2 Cy3 conjugate (Jackson Immunoresearch)) was diluted to 2 µg/ml with 5% skim milk in TBS/T, applied to sections for 1 hour at 4°C in the dark and followed by three 5 minute washes in TBS/T under the same conditions. A solution of 1 µg/ml 4', 6-diamidinophenyl-indole (DAPI; Sigma-Aldrich) was prepared in 5% skim milk in TBS/T, applied to the sections and incubated for 10 minutes at 4°C, and slides were again submitted to three 5 minute washes in TBS/T at 4°C. The fluorescence of the Cy3-DAPI labeled sections was digitally microphotographed using a 40× oil immersion objective with a Leica DMR multiphoton microscope and the accompanying software (Leica Microsystems, Wetzlar, Germany). Bright field micrographs were also taken of each tissue section. The Cy3 fluorophore is excited at 550 nm and emits at 615 nm, while the DAPI fluorophore is excited at 350 nm and emits at 470 nm. The multichannel capacity of the microscope allowed overlapping Cy3, DAPI and bright field images to be taken. To create a heat map of Cy3 labeling, the Cy3 signal intensity was translated into a red (100% of maximum) to blue (31% of maximum) spectrum using Adobe Photoshop. All experiments were done in triplicate.
RESULTS
Time course of crystallin mRNA expression
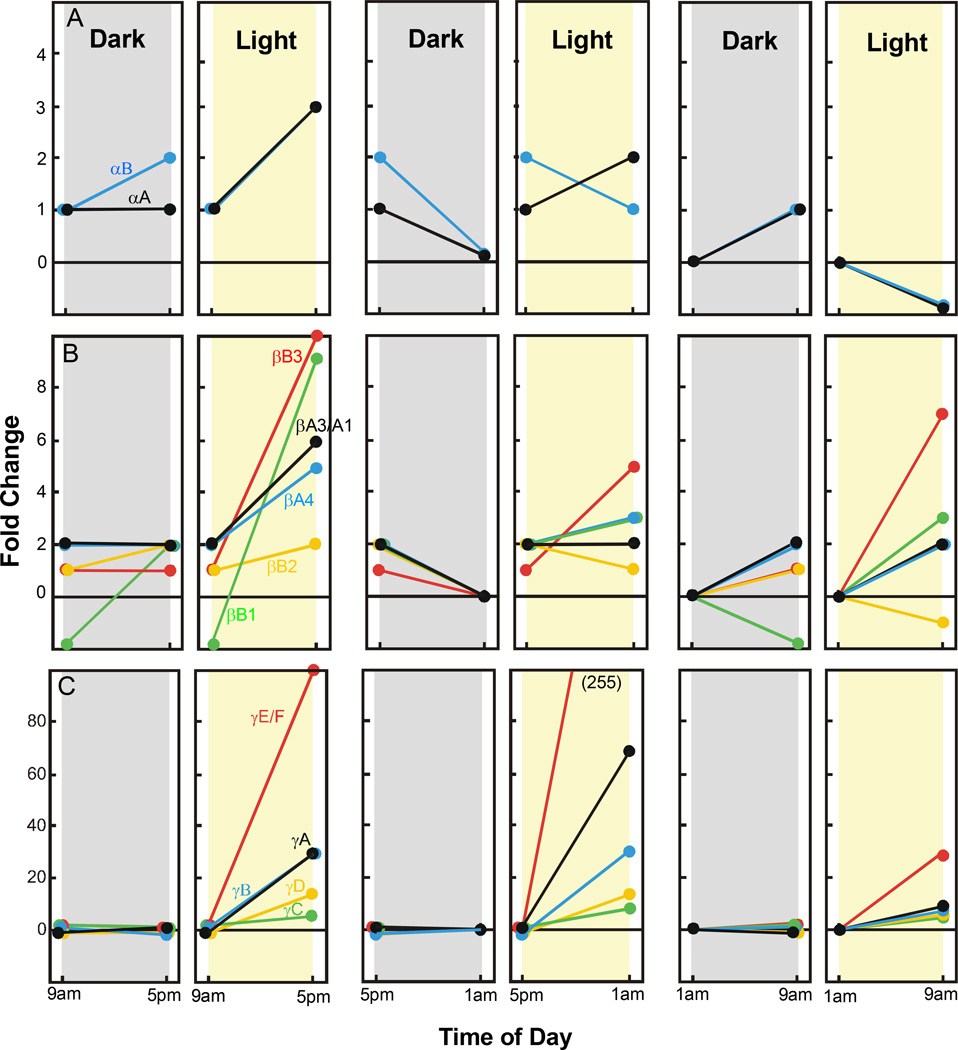
To determine light and circadian effects on the expression of retinal crystallins we used qRT-PCR and retinal mRNA extracts from groups of rats sacrificed at various times of the day or night. All animals were dark adapted for 16 hours and then either treated with intense visible light for 8 hours beginning at 9 am, 5 pm, or 1 am, or retained in darkness for the same 8 hour period of time. As shown in Figure 1A, the expression of retinal α-crystallin mRNA was little affected during an 8 hour period of darkness beginning at 9 am. There was no change in αA-crystallin mRNA while the level of αB-crystallin mRNA increased only 1 fold between 9 am and 5 pm. Intense light treatment over the same 8 hour time frame resulted in about a 2 fold increase in α-crystallin mRNAs. A modest down regulation of retinal αB-crystallin mRNA occurred in both light and dark treated rats between 5 pm and 1 am (1–2 fold), whereas, αA-crystallin levels decreased 1 fold in darkness and increased 1 fold in light. Likewise, α-crystallin mRNA levels were only modestly affected (−1 to +1 fold) by dark or light treatment during the period from 1 am to 9 am. More substantial changes in β-crystallin mRNA levels were found in light exposed rat retinas (Figure 1B). Increases of between 2–10 fold were measured for the five β-crystallin mRNA’s during 8 hours of light, beginning at 9 am. For light exposure beginning at 5 pm the changes in mRNA levels were more modest (−1 to +5 fold), while light treatment starting at 1 am resulted in 2–7 fold increases for most species of β-crystallin. The changes in β-crystallin mRNA for rats kept in darkness at either 9 am or 5 pm were in the range of –2 to +2 fold, relative to the 1 am time point. Whereas darkness had little affect on the 5 species of γ-crystallin mRNA (−1 to +2 fold), light treatment caused dramatic increases in their expression. Intense light exposure starting at either 9 am or 5 pm resulted in increases of 5 fold for γC-crystallin, to over 100 fold for γE/F-crystallin. The increases in retinal γ-crystallin mRNA gene expression following light treatment at 1 am were also substantial (5 to 30 fold) relative to unexposed rats.
Figure 1.
Time dependent changes in retinal crystallin gene expression in darkness and in light. Results are presented as the average fold change in mRNA levels relative to retinal crystallin mRNA measured by qRT-PCR for dark adapted rats at 1 am. Crystallin mRNA expression was measured in a combined total of 9 retinal extracts from 9 different cyclic light reared rats, obtained in groups of 3 retinas per experiment. Panel A. Expression levels of mRNA for αA- and αB-crystallins. Panel B. mRNA levels for 5 different species of β-crystallin (βB1, βB2, βB3, βA4 and βA3/A1). Panel C. Crystallin mRNA levels for γA, γB, γC, γD and γE/F. Standard deviations (not shown) were 10–15% of the mean.
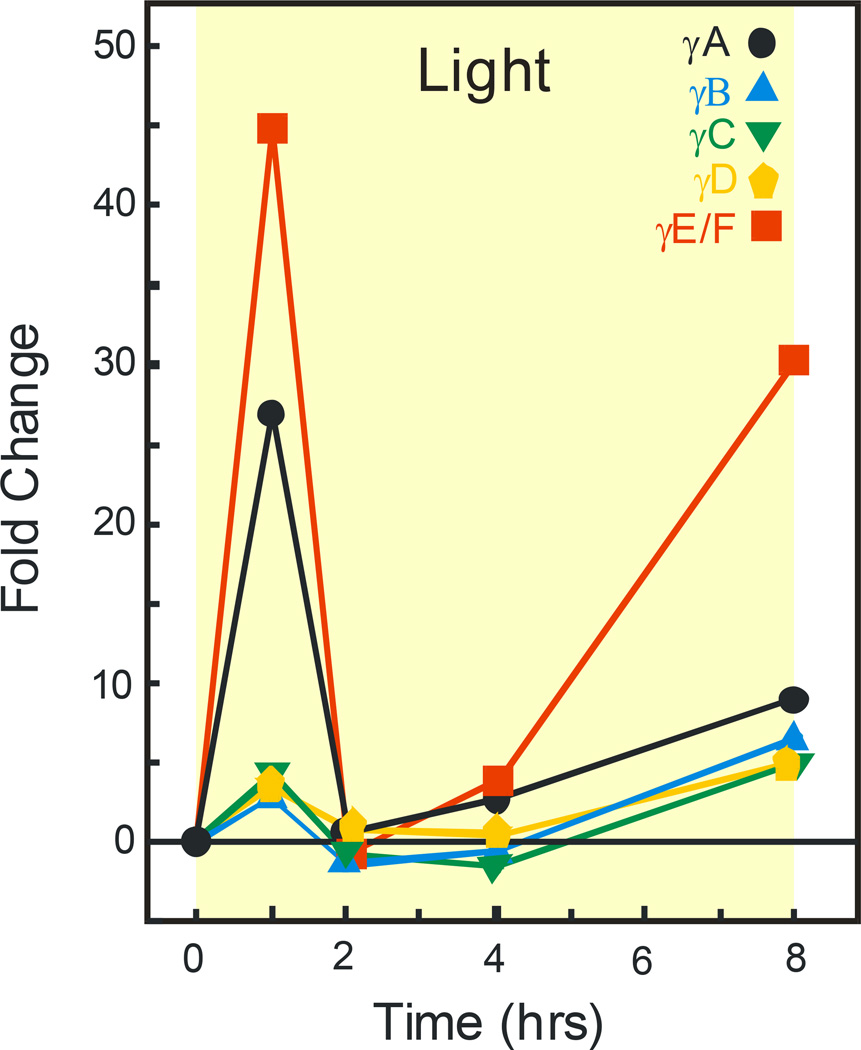
To better assess the time course of light’s effects on retinal γ-crystallins, we measured mRNA levels at various times during an 8 hour period of light exposure. Figure 2 shows that intense light treatment starting at 1 am resulted in dramatic increases in γA and γE/F-crystallin mRNA. After only 1 hour of light, the level of γA-crystallin had increased about 25 fold from the level present in unexposed dark controls. The increase in expression of γE/F-crystallin mRNA was even greater, approximately 45 fold, at the 1 hour time point. The mRNA expression levels for both γA- and γE/F-crystallins decreased thereafter. They were less than 5 fold higher from 2–4 hours and 8–30 fold higher after 8 hours of light. During the same 8 hour light period the other γ-crystallins followed the same general pattern as for γA- and γ-E/F-crystallin, but they never exhibited mRNA expression changes that were more than 5 fold higher than control.
Figure 2.
Relative γ-crystallin gene expression in retina after increasing periods of time in light. Results are average fold changes in various γ-crystallin mRNA levels. Retinal extracts (n=9) were from 3 separate experiments as described in figure 1. The data for 8 hours light exposure is from Figure 1, panel C. Standard deviations (not shown) were 10–15% of the mean.
Light exposure and ROS crystallin protein levels
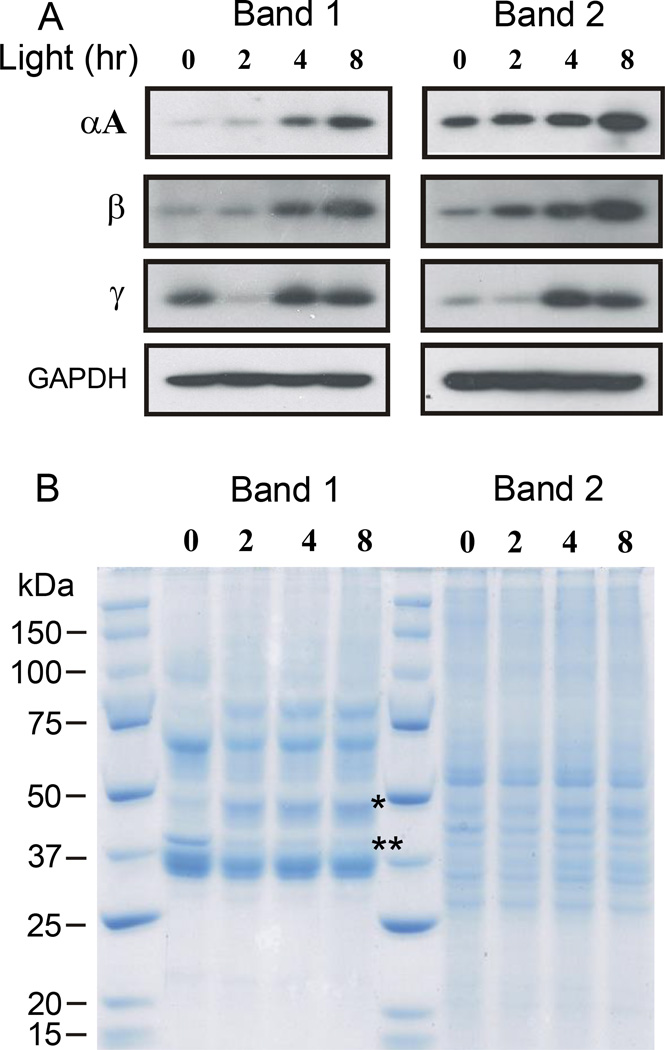
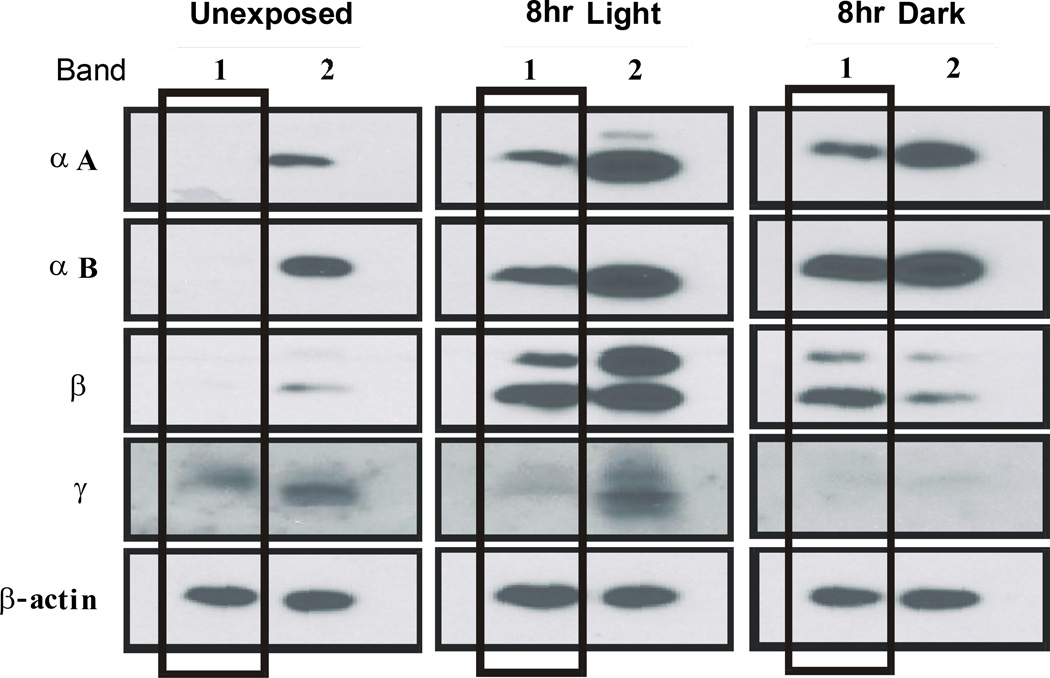
Light-induced visual cell damage begins in the photoreceptor ROS and progresses by overwhelming endogenous protective mechanisms within visual cells. Because crystallins may provide a chaperone-like protective effect, we determined the effects of potentially damaging light on their levels in ROS. Figure 3 contains a western blot (panel A) and Coomassie stained gel (panel B) for band 1 and band 2 ROS isolated from 3 separate groups of cyclic reared rats exposed to light beginning at 9 am. As shown in panel A, crystallin immunoreactivity increased with time in light in both the band 1 and band 2 ROS fractions. The increase in immunostaining was most noticeable for αA-crystallin and for the β-crystallins after 8 hours of light. Gamma crystallin immunostaining also increased in both band 1 and band 2 ROS after 4 or 8 hours of light treatment, but was unexpectedly lower in band 1 ROS after 2 hours of light. Thus, light-induced increases in crystallin gene expression, which would be expected to translate into increased crystallin protein levels in retina (19), also result in higher crystallin levels in rat ROS. Figure 3B shows that there are different protein patterns in band 1 and band 2 ROS. As expected, band 1 ROS contain an intense band of staining for rhodopsin, whereas band 2 ROS lack the same staining intensity at about 36 kDa. Band 1 ROS also exhibit an increase in staining at about 48 kDa, associated with arrestin uptake during light (20), and a loss of staining (~37 kDa), associated with the light driven translocation of transducin out of ROS (21).
Figure 3.
Western analysis of ROS crystallins following light exposure of increasing duration. Band 1 and band 2 ROS were prepared from the retinas of rats reared in dim cyclic light. Light exposures began at 9 am and lasted for as long as 8 hours. Three groups of 4 rats each were used to prepare ROS, providing an average response from a combined total of 24 retinas for each time point. Both band 1 and 2 ROS fractions were run on the same electrophoretic gels at the same time (10 µg/lane). Panel A, western analysis of α- β- and γ-crystallin immunoreactivity. GAPDH immunostaining was used to assess protein loading on the gels. Panel B, Coomassie staining of band 1 and 2 ROS fractions. The staining of arrestin (*) increases in Band 1 ROS during light exposure, whereas the intensity of transducin (**) staining decreases in light exposed rat ROS. Faint crystallin protein staining is seen between 16 and 25 kDA.
Potential crystallin migration within photoreceptors
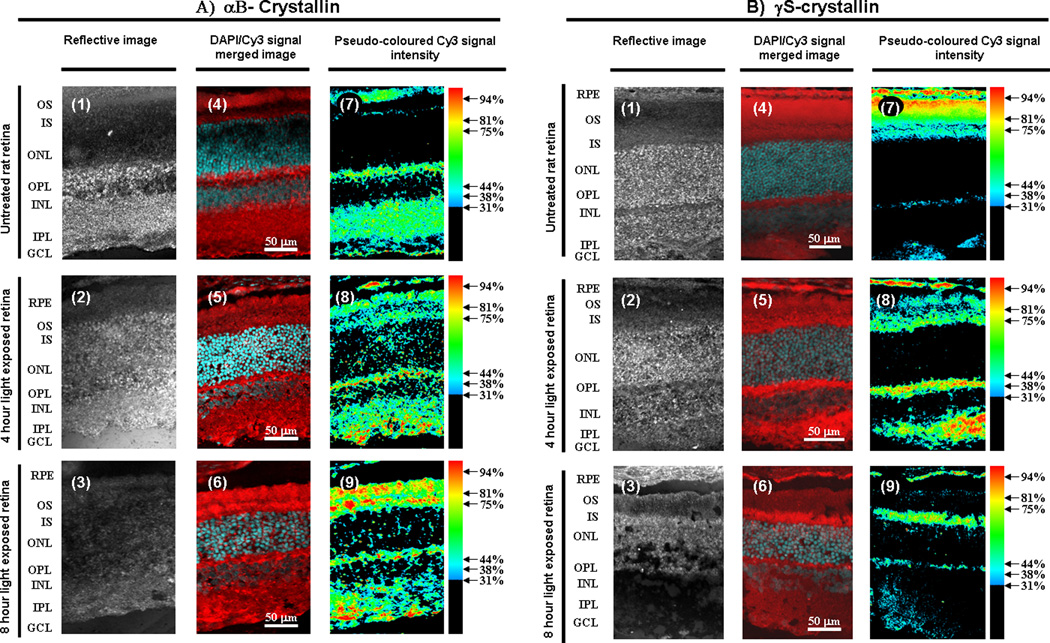
Immunohistochemistry was used to visualize the effects of intense light on retinal crystallins. Figure 4 contains retinal sections taken from rats also treated with damaging light beginning at 9 am. The rats were previously reared in darkness and then exposed to light for either 4 or 8 hours. Retinal sections were immunostained for αB- or γS-crystallin and counterstained with DAPI. In the dark reared rat retina αB-crystallin is present at the tips of the ROS, in the inner segment (IS) region, and in all other regions of the retina except the outer and inner nuclear layers (ONL and INL) and the portion of the ROS closest to the IS (Figure 4A, panels 1,4,7). The peak detection of αB-crystallin occurs within discrete foci in the outer plexiform (OPL) and ganglion cell layers (GCL). After a 4 hour light exposure the general localization of αB-crystallin is similar to unexposed retina except that significant levels are also detected in the IPL and discrete areas in the ONL and INL (Figure 4A, panels 2, 5,8). In comparison to unexposed retina the relative levels of αB-crystallin increases in the OPL and the GCL after a 4 hour light exposure. After an 8 hour light exposure the general localization of αB-crystallin is similar to the 4 hour light exposure profile except that its relative levels are higher in the IS and the ROS (Figure 4A, panels 3, 6, 9).
Figure 4.
Immunodetection of αB- and γS-crystallin on cross-sections of light-exposed and unexposed rat retina. Animals were treated with intense light 4 or 8 hours beginning at 9 am. Representative retinal sections from untreated (panels 1, 4, 7), 4 hour light-treated (panels 2, 5, 8), and 8 hour light-treated retina (panels 3, 6, 9) immunostained for αB-crystallin (A) or γS-crystallin(B) and counter stained with DAPI are shown. In each case a reflective image (panels 1–3), a DAPI/Cy3 signal merged image (panels 4–6), and a pseudo-colored Cy3 signal intensity image (panels 7–9) of the same section is provided. Retinal layers are indicated on the left: RPE = retinal pigment epithelium, ROS = outer segments, IS = inner segments, ONL = outer nuclear layer, OPL = outer plexiform layer, INL = inner nuclear layer, IPL = inner plexiform layer, GCL = ganglion cell layer. Cy3 signal is red, while DAPI signal is cyan. Cy3 signal intensity was translated into a red (100% of maximum) to blue (31% of maximum) spectrum. Signal intensity below 31% of maximum was colored black for clarity. Experiments were done in triplicate.
In the dark-reared rat retina peak levels of γS-crystallin were observed in the apical tips of the ROS, high levels were detected in the remainder of the ROS, modest levels (30–50% of peak intensities) were found in the IS, OPL, and the IPL, and protein levels were undetectable in the ONL and INL (Figure 4B, panels 1, 4, 7). After a 4 hour light exposure γS-crystallin levels decreased (relative to the dark reared control retina) in the ROS and increased in the IS (Figure 4B, panels 2, 5, 8). In contrast to the dark reared retina, peak levels of γS-crystallin were detected in the OPL, IPL, and minor levels within scattered regions in the ONL and INL. After an 8 hour light exposure peak γS-crystallin levels appear in the IS, diffuse levels are present in the ONL and INL, and γS-crystallin levels decreased (relative to the 4 hour light exposed retina) in the ROS, OPL, and IPL (Figure 4B, panels 3, 6, 9).
With respect to retinal photoreceptor cells, there is a general trend for an increase in αB-crystallin levels after light exposure. More specifically there is an increase in αB-crystallin levels after light exposure in the ROS that appears to subsequently spread into the IS and then the ONL and OPL. In contrast to αB-crystallin, there is a general trend for a decrease in γS-crystallin levels in photoreceptors after light exposure, with an apparent relocalization of γS-crystallin from the ROS to the IS and OPL and a subsequent reconcentration of peak levels to the IS. These results suggest that crystallin migration may occur in photoreceptors during light exposure.
Western analysis/crystallin migration
In an attempt to confirm the retinal immunohistochemistry, we used western analysis to examine ROS crystallin levels in rats exposed to intense light. Figure 5 contains results from dark reared rats treated with light for 8 hours, also beginning at 9 am. In unexposed control rats, the levels of α- and β-crystallins were very low in band 1 ROS (the band more highly enriched in ROS), but readily detectable in the band 2 fraction. Gamma crystallin immunoreactivity was found in both ROS fractions. As expected, ROS from rats exposed to light for 8 hours exhibited increased levels of crystallin immunoreactivty. Alpha- and β-crystallins were detected in both the band 1 and band 2 fractions, and the staining intensity was noticeably greater than at 9 am. Immunoreactivity for the γ-crystallins was also higher in band 2 ROS, while it was relatively low in the band 1 fraction.
Figure 5.
Crystallin immunoreactivity in ROS fractions from dark reared rats after light treatment. ROS fractions were prepared by density gradient ultracentrifugation using the retinas (24) from 3 separate groups of 4 rats each. The animals were treated with intense light or not for 8 hours beginning at 9 am. The results are from a series of gels run concurrently and then simultaneously probed with anti crystallin antibodies. Band 1 (vertical boxes) and band 2 ROS fractions were run on the same electrophoretic gels at a concentration of 10 µg protein/lane. Anti actin staining was used to assess the uniformity of protein loading on the gels.
Surprisingly, ROS isolated from rats maintained in darkness for the same 8 hour period, as for intense light treatment, exhibited a similar crystallin profile. Alpha A- and αB-crystallin immunoreactivity was present in both band 1 and band 2 ROS and the staining intensities resembled those for light exposed rat ROS. Beta-crystallin staining was elevated in band 1 ROS and, based on relative actin staining, similar to the staining intensity found in band 1 ROS from light exposed rats. Beta-crystallin staining was markedly lower in the band 2 fraction, while γ-crystallins were barely detectable in either ROS fraction after 8 hours of darkness.
Additional evidence for crystallin migration
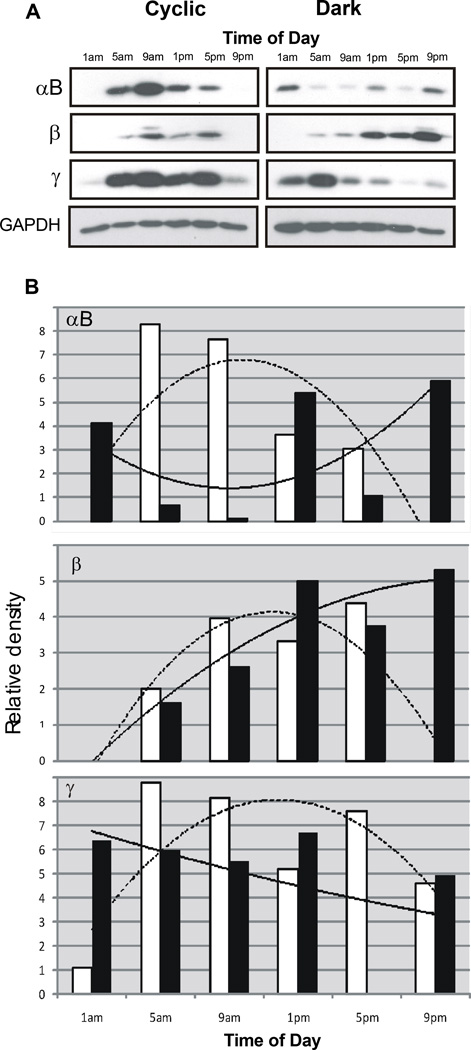
The similar pattern of crystallin immunoreactivity in ROS following light treatment or darkness suggests the possibility of a time dependent, or circadian, migration process in photoreceptors. To determine if light environment could impact these patterns, we compared crystallin immunoreactivity in ROS isolated from rats reared under dim cyclic light conditions with that of rats reared in darkness. In each case, ROS were obtained from 3 separate groups of 4 animals, sacrificed at 4 hour intervals around the clock. All were dark adapted for 16 hours before sacrifice. Figure 6 contains results from western analysis for the combined band 1 ROS samples. As shown in Figure 6A, ROS crystallin protein patterns are different in cyclic light reared- and dark reared- rats and those differences appear to depend on time of day. In ROS from cyclic light reared animals, αB-, β- and γ-crystallins are readily detected between the 5 am and 5 pm time points. Crystallin immunoreactivity is either undetectable or very much reduced in band 1 ROS at the 1 am and 9 pm time points. In ROS from dark reared rats αB-crystallin staining is present, but variable, at all times. ROS β-crystallin immunoreactivity also appears to be present most of the day, but the staining intensity is noticeably greater from 1 pm to 9 pm. Conversely, ROS γ-crystallin levels are highest between 1 am and 9 am, with lower levels thereafter.
Figure 6.
Time dependence of ROS crystallin immunoreactivity. Band 1 ROS were prepared from rats reared in dim cyclic light or in darkness and used for SDS gel electrophoresis. Three different sets of 4 rats each were sacrificed at 4 hour intervals, providing a pool of 24 retinas for each ROS sample. Panel A. ROS protein from 3 groups of cyclic light and 3 groups of dark reared rats simultaneously run on the same electrophoretic gels and then probed with anti-crystallin antibodies. To assess the uniformity of protein loading (10 µg/lane) GAPDH immunoreactivity was determined. Panel B. Graphic analysis of ROS crystallin levels relative to GAPDH staining intensity. Analysis was done with GelScape, an on-line viewing and annotation program from the University of Alberta, Canada. Relative staining intensity for cyclic light reared rats (open bars) and dark reared rats (solid bars). Computer generated average responses for cyclic reared rats (dashed line) and dark reared rats (solid line) are superimposed.
Figure 6 B is a graphic representation of α-, β- and γ-crystallin immunoreactivity in ROS relative to GAPDH staining and contains computer generated average response curves for the 24 hour period. For cyclic light raised rats, the results confirm the near absence of αB- and β-crystallins at 1 am and 9 pm, with a more modest decrease in γ-crystallin levels at those time points. The relative staining intensities for β-crystallins in dark reared rats exhibit a marked increase over the course of the day while γ-crystallin staining decreases about 50% from 1 am to 9 pm. ROS αB-crystallin immunoreactivity is high at 9 pm and, as expected, is much lower 9 am. However, the relative intensity of αB-crystallin staining at 5 pm is also lower than expected. Using a computer generated averaging program to assess the changes anticipated in staining intensity, the data suggests that αB-crystallin levels increase in ROS from about 1 pm onward and remain high through 1 am the next day.
DISCUSSION
This study shows that retinal crystallin synthesis is influenced by the stress of intense light exposure and the time of day that light exposure begins. Whether mRNA expression was used as an indicator of synthesis, or crystallin immunoreactivity was used, light stress led to an increase in crystallin levels in rats reared in a normal cyclic light environment. The changes were modest, but expected, for the α-crystallins (8, 10, 19), which are members of the family of small heat shock proteins (2, 3). However, the increases in mRNA expression found for both the β- and γ-crystallins were much larger and they varied with time of day (Figure 1). The upregulation of γ-crystallin expression also occurred rapidly (Figure 2), suggesting an early response to light stress. Previous studies suggest a protective role for γ-crystallins in retina (6, 10), and recent phylogenetic work has identified a common ancestral crystallin, linking together the evolution of β- and γ-crystallins (35). Our study confirms these earlier reports (6, 10, 35), and provides additional evidence that an upregulation of all 3 crystallin classes may be a general response to cellular stress. Long term environmental or genetic stress is known to alter the expression of retinal crystallins and chronic retinal disease can lead to a variety of crystallin modifications (9–17). This report identifies acute light-induced stress coupled with time of day as an additional effector of crystallin synthesis.
The increase in crystallin synthesis was also found to depend on the duration of light exposure and the previous environmental light history of the animals. Rats reared in darkness are susceptible to retinal damage by light beginning at 9 am while cyclic light rats are resistant to light damage at that time (32). Although measurements of retinal mRNA levels are useful, they provide limited direct information about ROS crystallins. By western analysis, the crystallin protein patterns in ROS were found to be different in the two types of rats. Cyclic reared animals exhibited a light-induced increase for all 3 classes of crystallins in band 1 and 2 ROS (Figure 3). Alpha B-crystallin immunoreactivity was previously shown to be higher in ROS from dark reared rats after 4 hours of light exposure (19). Dark reared rats exposed to light for 8 hours also had higher levels of α- and β-crystallins in band 1 ROS, but lower γ-crystallin levels (Figure 5). At the same time, immunostaining for the γ-crystallin class was higher in band 2 ROS, suggesting a light induced increase in retina. Immunohistochemistry of αB- and γS-crystallin showed the same relative changes during light, suggesting that crystallin migration into or out of ROS occurred during light treatment (Figure 4). A similar pattern of immunostaining was found in ROS isolated from rats kept in darkness for the same 8 hour period (Figure 5), indicating that crystallin migration within photoreceptors may also depend on time of day. Further evidence for a potential circadian protein migration process was found in ROS from dark adapted rats raised in either dim cyclic light or in darkness. The patterns of ROS crystallin immunoreactivity were different over the 24 hour period studied (Figure 6). These differences do not appear to arise from retinal pigment epithelial (RPE) contamination, because RPE crystallin levels were consistent over time (data not shown). It is also worth noting that these results are average responses for ROS isolated from a large number of animals, sacrificed at different times of the day and night. As reported previously (8), retinal crystallins vary considerably among individual animals, suggesting that individual variations in the retinal stress response can also occur.
If there is a circadian protein migration process in photoreceptors it is likely driven by a mechanism different from the well described light driven translocation of visual cell proteins such as arrestin and α-transducin (20–22). One possible signaling mechanism involves the family of Toll-like Receptors (TLR) which initiates the upregulation of retinal αA-crystallin during autoimmune uveitis (36). Although oxidative stress may be directly involved in TLR mediated (36) and intense light-induced (19) retinal crystallin synthesis, our work suggests that anticipation of oxidative stress in advance of light onset can elicit a similar response. It is known that rhodopsin synthesis and the formation of several other visual transduction proteins occurs in a circadian manner (24, 25), indicating that the daily process of protein insertion into nascent ROS disks has a well regulated time dependence. Additional work will be required to determine whether the initial appearance of crystallins in ROS is also as well regulated. Likewise, it will be important to verify that photoreceptor cell crystallins actually migrate in a circadian fashion and to determine the types of proteins that might accompany crystallin migration and the machinery involved in the process. To this end, quantitative approaches involving isotopic labeling of ROS protein proteolytic fragments followed by mass spectroscopy (37) should be effective in determining circadian changes in crystallin levels.
Acknowledgements
Thanks to Drs. Wistow and Zigler for supplying some of the crystallin antibodies used in this study. This study was supported by funding from a State of Ohio BRTT grant 05-064, the Ohio Lions Eye Research Foundation and M. Petticrew, Springfield, OH (DTO) and NSERC, RPB, NIH (NEI) P30-EY006360 and the Knights of Templar of Georgia (PW).
REFERENCES
- 1.Horwitz J. Alpha crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. 1992;89:10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao Y-W, Liu J-P, Xiang H, Wu DW-C. Human αA- and αB- crystallins bind to Bax and Bcl-Xs to sequester their translocation during staurosporine-induced apoptosis. Cell Death and Differentiation. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- 3.Kamradt MC, Chen F, Cryns VL. The small heat shock protein αB-crystallin negatively regulates cytochrome c- and caspase-8-dependent activation of caspase-3 by inhibiting its autoproteolytic maturation. J. Biol. Chem. 2001;276:16059–16063. doi: 10.1074/jbc.C100107200. [DOI] [PubMed] [Google Scholar]
- 4.Bhat SP, Nagineni CN. αB subunit of lens specific protein α-crystallin is present in other ocular and non-ocular tissues. Biochem. Biophys. Res. Comm. 1989;58:319–325. doi: 10.1016/s0006-291x(89)80215-3. [DOI] [PubMed] [Google Scholar]
- 5.Deretic D, Aebersold RH, Morrison HD, Papermaster DS. αA- and αB-crystallin in the retina. Association with the post-Golgi compartment of frog retinal photoreceptors. J. Biol. Chem. 1994;269:16853–16861. [PubMed] [Google Scholar]
- 6.Jones SE, Jomary C, Grist J, Makwana J, Neal MJ. Retinal expression of γ-crystallins in the mouse. Invest. Ophthalmol. Vis. Sci. 1999;40:3017–3020. [PubMed] [Google Scholar]
- 7.Magabo KS, Horwitz J, Piatigorsky J, Kantorow M. Expression of βB2-crystallin mRNA and protein in retina, brain and testis. Invest. Ophthalmol. Vis. Sci. 2000;41:3056–3060. [PMC free article] [PubMed] [Google Scholar]
- 8.Xi J, Farjo R, Yoshida S, Kern TS, Swaroop A, Andley UP. A comprehensive analysis of the expression of crystallins in mouse retina. Mol. Vis. 2003;9:410–419. [PubMed] [Google Scholar]
- 9.Organisciak D, Darrow R, Gu X, Barsalou L, Crabb JW. Genetic, age and light mediated effects on crystallin protein expression in the retina. Photochem. Photobiol. 2006;82:1088–1096. doi: 10.1562/2005-06-30-RA-599. [DOI] [PubMed] [Google Scholar]
- 10.Andley UP. Crystallins in the eye: function and pathology. Prog, Retinal Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Ganea E. Chaperone-like activity of α-crystallin and other small heat shock proteins. Curr. Protein Peptide Sci. 2001;2:205–225. doi: 10.2174/1389203013381107. [DOI] [PubMed] [Google Scholar]
- 12.Kapphahn RJ, Ethen CM, Peters EA, Higgins L, Ferrington DA. Modified αA crystallin in the retina: altered expression and truncation with aging. Biochemistry. 2003;42:15310–15325. doi: 10.1021/bi034774e. [DOI] [PubMed] [Google Scholar]
- 13.Kapphahn RJ, Giwa BM, Berg KM, Roehrich H, Feng X, Olsen TW, Ferrington DA. Retinal proteins modified by 4-hydroxynonenal: identification of molecular targets. Exp. Eye Res. 2006;83:165–175. doi: 10.1016/j.exer.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 14.Crabb JW, Miyagi M, Gu X, Shadrach K, West K, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Solomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakata K, Crabb JW, Hollyfield JG. Crystallin distribution in Bruch’s membrane-choroid complex from AMD and age-matched donor eyes. Exp. Eye Res. 2005;80:821–826. doi: 10.1016/j.exer.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Johnson PT, Brown MN, Pulliam BC, Anderson DH, Johnson LV. Synaptic pathology, altered gene expression, and degeneration in photoreceptors impacted by drusen. Invest. Ophthalmol. Vis. Sci. 2005;46:4788–4795. doi: 10.1167/iovs.05-0767. [DOI] [PubMed] [Google Scholar]
- 17.Jones SE, Jomary C, Grist J, Thomas MR, Neal MJ. Retinal expression of alpha B-crystallin in a mouse model of inherited retinal degeneration. Neuroreport. 1998;9:4161–4165. doi: 10.1097/00001756-199812210-00030. [DOI] [PubMed] [Google Scholar]
- 18.Vazquez-Chona F, Song BK, Geisert EE. Temporal changes in gene expression after injury in the rat retina. Invest. Ophthalmol. Vis. Sci. 2004;45:2737–2746. doi: 10.1167/iovs.03-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakaguchi H, Miyagi M, Darrow RM, Crabb JS, Hollyfield JG, Organisciak DT, Crabb JW. Intense light exposure changes the crystallin content in retina. Exp. Eye Res. 2003;76:131–133. doi: 10.1016/s0014-4835(02)00249-x. [DOI] [PubMed] [Google Scholar]
- 20.Broekhuyse RM, Tolhuizen EFJ, Janssen APM, Winkes HJ. Light induced shift and binding of S-antigen in retinal rods. Curr. Eye Res. 1985;4:613–618. doi: 10.3109/02713688508999993. [DOI] [PubMed] [Google Scholar]
- 21.Sokolov M, Lyubarsky AL, Strissel KJ, Savchenko AB, Govardovskii VI, Pugh EN, Arshavsky VY. Massive light-driven translocation of transducin between the two major compartments of rod cells: a novel mechanism of light adaptation. Neuron. 2002;35:95–106. doi: 10.1016/s0896-6273(02)00636-0. [DOI] [PubMed] [Google Scholar]
- 22.Whelan JP, McGinnis JF. Light-dependent subcellular movement of photoreceptor proteins. J. Neurosci. Res. 1988;20:263–270. doi: 10.1002/jnr.490200216. [DOI] [PubMed] [Google Scholar]
- 23.Arshavsky VY, Lamb TD, Pugh EN. G proteins and visual transduction. Annu. Rev. Physiol. 2002;64:153–187. doi: 10.1146/annurev.physiol.64.082701.102229. [DOI] [PubMed] [Google Scholar]
- 24.Bowes C, van Veen T, Farber DB. Opsin, G-protein and 48- kDa protein in normal and rd mouse retinas: developmental expression of mRNAs and proteins and light-dark cycling of mRNAs. Exp. Eye Res. 1988;47:369–390. doi: 10.1016/0014-4835(88)90049-8. [DOI] [PubMed] [Google Scholar]
- 25.Korenbrot JI, Fernald RD. Circadian rhythm and light regulate opsin mRNA in rod photoreceptors. Nature. 1989;337:454–457. doi: 10.1038/337454a0. [DOI] [PubMed] [Google Scholar]
- 26.Farber DB, Seager Danciger J, Orgnanisciak DT. Levels of mRNA encoding proteins of the cGMP cascade as a function of light environment. Exp. Eye Res. 1991;53:781–786. doi: 10.1016/0014-4835(91)90114-t. [DOI] [PubMed] [Google Scholar]
- 27.Organisciak DT, Xie A, Wang H-M, Jiang Y-L, Darrow RM, Donoso LA. Adaptive changes in visual cell transduction protein levels: effect of light. Exp. Eye Res. 1991;53:773–779. doi: 10.1016/0014-4835(91)90113-s. [DOI] [PubMed] [Google Scholar]
- 28.Noell WK, Walker VS, Kang BS, Berman S. Retinal damage by light in rats. Invest. Ophthalmol. 1966;5:450–473. [PubMed] [Google Scholar]
- 29.Rozanowska M, Rosanowski B, Boulton M. Light-induced damage to the retina. Photobiological Sciences Online, ED. K.C. Smith, Photobiology of the Retina. 2009 http://www.photobiology.info/. [Google Scholar]
- 30.Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog. Retinal Eye Res. 2010;29:113–134. doi: 10.1016/j.preteyeres.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LaVail MM, Gorrin GM, Rapaci MA, Yasurmura D. Light-induced retinal degeneration in albino mice and rats: strain and species differences. In: Hollyfield JG, Anderson RE, LaVail MM, editors. Degenerative Retinal Disorders: Clinical and Laboratory Investigations. New York: Alan R. Liss; 1987. pp. 439–454. [PubMed] [Google Scholar]
- 32.Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Circadian-dependent retinal light damage in rats. Invest. Ophthalmol. Vis. Sci. 2000;41:3694–3701. [PubMed] [Google Scholar]
- 33.McClung JK, Jupe ER, Liu X-T, Dell’Orco RT. Prohibitin: potential role in senescence, development, and tumor suppression. Exp. Gerontology. 1995;30:99–124. doi: 10.1016/0531-5565(94)00069-7. [DOI] [PubMed] [Google Scholar]
- 34.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:2002–2007. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wistow G, Wyatt K, David L, Gao C, Bateman O, Bernstein S, Tomarev S, Segovia L, Slingsby C, Vihtelic T. γN-crystallin and the evolution of the βγ-crystallin superfamily in vertebrates. FEBS J. 2005;272:2276–2291. doi: 10.1111/j.1742-4658.2005.04655.x. [DOI] [PubMed] [Google Scholar]
- 36.Saraswathy S, Nguyen AM, Rao NA. The role of TLR4 in photoreceptor αA crystallin upregulation during early experimental autoimmune uveitis. Invest. Ophthalmol. Vis. Sci. 2010;51:3680–3686. doi: 10.1167/iovs.09-4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyagi M, Rao KC. Proteolytic 18O labeling strategies for quantitative proteomics. Mass Spectrom. Rev. 2007;26:121–136. doi: 10.1002/mas.20116. [DOI] [PubMed] [Google Scholar]