Abstract
Objective
Determine if oral pretreatment with a vasoconstrictor decreases the blood to middle-ear exchange-rate of the perfusion-limited gas, Nitrous Oxide (N2O).
Study Design
Randomized, double-blind, crossover study.
Methods
Ten adult subjects with and 10 without past middle-ear disease completed paired experimental sessions, identical but for oral pretreatment with either pseudoephedrine HCL or lactose placebo. At each session, subjects were fitted with a non-rebreathing mask and breathed room air for 20 minutes (acclimation period), 50% N2O:50% O2 for 20 minutes (experimental period) and 100% O2 for 10 minutes (recovery period). Throughout, heart-rate, blood-pressure and O2 saturation were monitored and bilateral middle-ear pressures were recorded by tympanometry every minute. The primary outcome was the slope of the middle-ear pressure-time function for the experimental period which estimates the volume N2O exchange-rate. Using repeated measures ANOVA, the effects of Group (disease history), Treatment (active vs. placebo) and Period (1 vs. 2) on the recorded vital signs, and of Group, Treatment and Ear (left/right) on the middle-ear pressure-time slope were evaluated for statistical significance.
Results
Statistically significant effects of Period on O2 saturation (Period 2>Period 1) and of Treatment on heart-rate (Active>Placebo) were documented. Only Treatment was statistically significant for the middle-ear pressure-time slope with a shallower slope characterizing the active treatment session.
Conclusion
The volume exchange-rate across the middle-ear mucosa of perfusion-limited gases can be modulated pharmacologically. Theoretically, similar drugs can be used to reduce the requisite Eustachian tube opening efficiency for adequate middle-ear pressure regulation.
Keywords: adults, N2O breathing, middle ear, pressure-regulation, decongestants, modeling
Introduction
Middle-ear (ME) pressure-regulation (MEPR) is a homeostatic mechanism that maintains the ME-ambient pressure-gradient within a narrow, zero-centered, range that maximizes hearing and preserves ME health1. Hearing acuity is diminished as the absolute value of that gradient increases and, because the ME mucosal tissue-pressure tracks local ambient pressure, large negative ME-ambient pressure-gradients cause vascular disruption with leakage, mucosal inflammation and fluid accumulation within the normally gas-filled ME2,3, a pathology not different from the clinical expression of otitis media with effusion (OME)4. Thus, the “risk” for certain types of hearing losses and ME pathologies is indirectly related to MEPR efficiency1.
The ME is a usually closed, relatively fixed-volume, temperature-stable, gas-pocket. ME pressure is governed by the ideal gas law, PME= RTME(VME)-1ΣsnMEs, and the rate of pressure change by the time-derivate of that equation, δPME/δt=RTME(VME)-1ΣsδnMEs/δt, where PME, TME and VME are the ME pressure, temperature and volume, R is a gas constant, nMEs is species-moles, t is time, Σs indicates summing over all represented species and δ denotes change1. Physiologically, ME pressure is changed by processes that change its species-moles and, specifically, by passive gas-transfers between the ME and adjacent compartments2.
The ME simultaneously exchanges gas-species with its local blood by diffusion across the ME mucosa (transMEM exchange), the ambient environment by diffusion across the tympanic membrane (transTM exchange) and the cochlear fluids by diffusion across the round window membrane (transRWM exchange)2. The rates for these exchanges is given by Fick's law of diffusion, Qbarriers=CKbarriersΔPbarriers, where Qbarriers is trans-barrier volume gas-flow, C is a “moles to volume” scaling-constant, Kbarriers is the species-conductance for the barrier, and ΔPbarriers is the trans-barrier species pressure-gradient. Measurement shows that the transTM and transRWM species-conductances are negligibly small compared to the respective transMEM conductances5,6. Moreover, the only physiologically extant species-gradient to drive transMEM gas-exchange is the ME-blood N2 gradient of approximately 600 daPa since the ME-blood gradients for the other represented species, O2, CO2 and H2O, are well-buffered at close to 0 daPa7,8. Therefore, of the ME exchange pathways, only transMEM N2 exchange satisfies the conditions for inter-compartment gas-exchange prescribed by Fick's diffusion equation and the net direction, magnitude and rate of pressure-change for the closed ME under physiologic conditions are solely determined by the transMEM N2 conductance and pressure-gradient. For N2 and other inert-gases (e.g. N2O), the transMEM exchange-rate is perfusion-limited9-12 such that species-conductance is proportional to the volume-blood flow-rate per tissue-volume (i.e. the perfusion-rate), Qblood, and species-solubility in the mucosa, Smucsas, or: Kmucsas=QbloodSmucsas. Consequently, the equilibrium pressure for a closed ME is equal to the ambient pressure minus the ME-blood N2 gradient (≈Pambient-600 daPa) and the rate of ME pressure decrease to equilibrium is equal to the product of a scaling constant, the extant ME-blood N2 gradient, the mucosal perfusion-rate and the transMEM N2 conductance1. Therefore, the rate, but not magnitude, of pressure-change for a closed-ME can be modulated by effecting changes in the mucosal blood perfusion-rate13,14.
Periodic and transient, muscle-assisted Eustachian tube (ET) openings establish a gas-phase communication between the ME and nasopharynx (NP). Non-zero ME-NP (=ME-ambient) pressure-gradients at the time of ET openings drive a bolus gas-flow between the compartments according to the Hagen-Poiseuille equation, QETg=ҚETgΔPC1-C2g, which relates the transET volume-gas flow-rate, QETg, to the product of the transET gas-conductance, Қ′cg, and pressure-gradient, ΔPETg2. These gas-flows quickly change ME, but not ambient, pressure and, thereby, decrease the pre-opening, absolute value of the ME-ambient pressure-gradient. The efficiency of this ET pressure-equilibrating function can be expressed as the fractional ME-ambient pressure-gradient equilibrated per swallow (FGE), which is proportional to transET gas-conductance and ET opening duration, but independent of pressure-gradient15.
From these considerations, MEPR represents a time-limited balance in the ME volume-gas influx (ViMEg) associated with ET openings and the volume-N2 efflux (VeMEN2) attributable to gradient-driven N2 transfer from the ME to local blood1. For periods of stable ambient pressure, MEPR efficiency is directly related to the ViMEg/VeMEN2 ratio. Experiments show that disrupting that ME gas-balance by decreasing ET efficiency (i.e. lowering ViMEg)3,16,17, increasing ME to blood volume gas-transfer (i.e. raising VeMEg)18, or both causes ME pressure-dysregulation (MEPD) expressed as the sequential development of negative ME-ambient pressure-gradients, conductive hearing-loss and, at a ME-ambient pressure-gradient of about -250 daPa, ME mucosal inflammation and OME17,18.
In persons with MEPD not caused by conditions that abolish transET gas-exchange (i.e. ViMEg=0), the “risk” for these pathologies can be lessened by interventions that increase the ViMEg/VeMEN2 ratio. To that end, one relatively simple strategy is to effect a decrease in ME gas-efflux by pharmacologically lowering the ME mucosal perfusion-rate. This would effectively decrease the requisite volume-gas influx (i.e. the frequency of ET openings and constitutive FGE) needed to stabilize the ME-ambient pressure-gradient at near 0 daPa (i.e. establish a ViMEg/VeMEN2 ratio of 1). Here, the feasibility of that strategy is explored by creating a significant transMEM inert-gas pressure-gradient and determining the effect, if any, of a vasoconstrictive drug on the rate of ME pressure-change. The hypothesis tested is that the rate of ME pressure-increase in response to a positive transMEM blood-ME N2O pressure-gradient is reduced by pretreatment with pseudoephedrine HCL (PDE)19.
Materials and Methods
Study Population
The protocol was approved by the IRB at the University of Pittsburgh. Healthy adults, recruited by advertisement, presented to the laboratory and signed an informed-consent for study participation. Consented subjects provided medical and surgical histories and a comprehensive history for ME diseases, comorbidities and predisposing conditions. They had an Ear Nose and Throat (ENT) examination with bilateral tympanometry (Titan, Eden Prairie, MN) and women had a urine pregnancy test (Consult Diagnostics hCG Dipstick, Jacksonville, FL). Pregnant women and persons with lactose intolerance or a past adverse reaction to breathing gas-mixtures containing N2O, with chronic illness, who had taken prescription medication within the previous month (with the exception of birth control) or oral/nasal decongestants within the 2 weeks before either test session or with extant unilateral or bilateral OME or low tympanic membrane compliance or symptoms/signs of extant nasopharyngeal inflammation were excluded. The target population was 20 otherwise healthy adults, 10 with and 10 without past ME disease.
Protocol
The design was a randomized, double-blind, placebo-controlled, cross-over study requiring that participants complete paired N2O breathing sessions at a minimum 1-week interval. At each session, the subject reported to the research clinic, provided an interval history for medication use, ME disease and upper respiratory diseases and had a brief ENT examination to verify continued eligibility, was pretreated with a double-blinded, oral medication (identical appearing capsules containing either 60 mg of immediate-release PDE or lactose) and, then, escorted to the Dental Clinic. There, the subject was seated in a reclined examination chair and fitted with: the finger probe of a pulse oximeter (Massimo RDS1), the cuff of an automated blood-pressure monitor (Critikon Dynamap 1846SX) and an appropriately sized Rudolph Nasal & Mouth Breathing Silicone Face Mask (Model 8900, Kansas, USA) with a two-way non-rebreathing valve. The delivery system supplying the mask was configured such that three gas sources could be placed “on-line”; room-air, a N20 gas-mixture (50%O2, 50% N20) or 100% O2.
The experiment was begun 1 to 1.5 hours after dosing and consisted of a 20-minute acclimation period (Period 1, room-air breathing), a 20-minute experimental period (Period 2, gas-mixture breathing) and a 10-minute recovery period (Period 3, 100% O2). Throughout, bilateral ME pressures were recorded by tympanometry at 1-minute intervals, blood O2 saturation and heart-rate were monitored continuously and recorded every 5 minutes, and blood-pressure was measured and recorded every 5 minutes. At the end of Period 3, the subject was observed for 20 minutes, given a brief physical examination and was free to leave the clinic when recovered from breathing the N2O gas-mixture. At least 1-week later, these procedures were repeated and the subject was dosed with the crossover capsule.
Pharmacology
PDE is a member of the phenethylamine and amphetamine chemical classes with direct and indirect sympathomimetic actions20. Peak plasma concentrations are achieved between 1 and 2 hours21,22 peak salivary concentrations between 2 and 4 hours23 and vasoconstrictive effects from 30 minutes to 4 hours after a single oral dose of 60 mg immediate-release PDE19. N2O is a colorless, odorless gas that is highly soluble in blood and other tissues. On breathing, N2O substitutes for N2, rapidly achieves blood-alveolar equilibrium pressure24 and exhibits a perfusion-limited trans-mucosal exchange-rate25.
Data Analysis
A randomization code was submitted to a pharmacist who prepared the 2 pretreatments as identical looking capsules supplied to the investigators in sealed bottles labeled by session and subject number. Personnel were blinded to the code until all recordings were double-entered into a computer data-file, reconciled and locked to change.
For each session, the average blood O2 saturation, heart-rate and systolic and diastolic blood-pressures were calculated for Periods 1 and 2 and the rate of increase in ME pressure was calculated for Period 225,26. There, ME pressure-time functions were analyzed independently by 2 blinded-investigators to calculate the slope of a linear segment representative of the change in ME pressure per change in time (δPME/δt) during the last 15 minutes of Period 2. Paired slope-estimates were compared, discrepancies reconciled and the average slopes used in the analysis.
The effects of Group, Treatment, Period and the Treatment by Group Interaction on blood O2 saturation, heart-rate and systolic and diastolic blood-pressure, and the effects of Treatment, Group, Ear (right/left) and the Treatment by Group Interaction on the ME pressure-time slope were evaluated for significance using repeated measures ANOVAs (NCSS 2007 statistical package, Kaysville, Utah).
Results
Twenty-six subjects were screened, 3 disqualified and 23 enrolled. Twenty subjects (average age=26.7±8.8 years; 11 Males; 13 white, 6 black and 1 mixed race) completed the 2 test-sessions. For Group-1, 6 of the 10 subjects reported repeated episodes of ME effusion, 8 reported 3 or more episodes of “ear-ache” treated with antibiotics and 8 reported ventilation tube insertion(s) during childhood. Most (8/10) had outgrown their disease by late childhood, but 1 had ear problems into adolescence (13 years) and 1 (white female, aged 30.1 years) reported episodes of OME continuing into adulthood. No Group-2 subject reported past OME or other ME diseases. Four subjects in each group had nasal allergy, none asthma and 1, in Group-1, gastro-esophageal reflux disease.
There was a significant difference among individuals in all 4 vital signs measures (P<0.001) and a significant, but clinically irrelevant, effect of Group on systolic blood-pressure (Group-1=116 vs. Group-2=107 mmHg; P=0.040) and heart-rate (Group-1=68.5 vs. Group-2=60.6 BPM; P=0.047), of Period on O2 saturation (Period 1=99.3% vs. Period 2=99.9%; P<0.001), and of Treatment on heart-rate (Placebo=62.3 vs. Active=67.1 BPM; P=0.001).
The Table reports the results for the ANOVA operating on the slopes of the ME pressure-time function. Those slopes were significantly different among individuals and between Treatments. The adjusted average slopes were 6.21±2.21 and 4.81±2.21 daPa/min for the placebo and PDE pretreatments. There was no effect of Group, Ear or the Interaction on this variable.
Table.
ANOVA for the ME Pressure-Time Slopes listing the sources of variation and, for each source, the associated degrees of freedom (df), sum of squares (SSQ), mean square (MSQ), F-Ratio and probability level (P-Value).
| Source | df | SSQ | MSQ | F-Ratio | P-Value |
|---|---|---|---|---|---|
| Group | 1 | 9.86 | 9.86 | 0.28 | 0.606 |
| Individual | 18 | 645.54 | 35.86 | 7.17 | 0.000 |
| Ear | 1 | 0.15 | 0.15 | 0.03 | 0.865 |
| Treatment | 1 | 39.49 | 39.49 | 7.90 | 0.007 |
| Group × Treatment | 1 | 0.14 | 0.14 | 0.03 | 0.867 |
| Error | 57 | 284.93 | 5.00 | ||
| Total (Adjusted) | 79 | 980.11 | |||
| Total | 80 |
Figure 1 shows a scatterplot of the ME pressure-time slopes for the PDE pretreatment as a function of the paired-slopes for the placebo pretreatment. The majority of points lay below the identity line for the placebo pretreatment documenting lesser values for the PDE pretreatment. As shown in Figure 2, the ME pressure-time slopes for the right and left ears of individuals were related linearly. The fit of the regression line between right and left slopes was significantly better for the placebo pretreatment (Right Slope = 0.12 ×Left Slope + 0.78; r2=0.72) when compared to the PDE pretreatment (Right Slope = 0.71 ×Left Slope + 1.76; r2=0.26).
Figure 1.
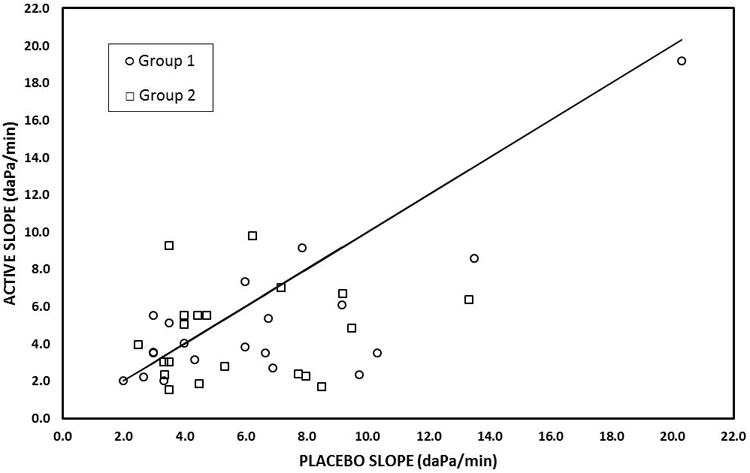
Scatterplot of the ME Pressure-Time slopes recorded after active pretreatment as a function of the paired slopes recorded after the placebo pretreatment for Group-1 (history of middle ear disease - open circles) and Group-2 (controls - open squares). The solid line is the line of identity for the placebo slopes.
Figure 2.
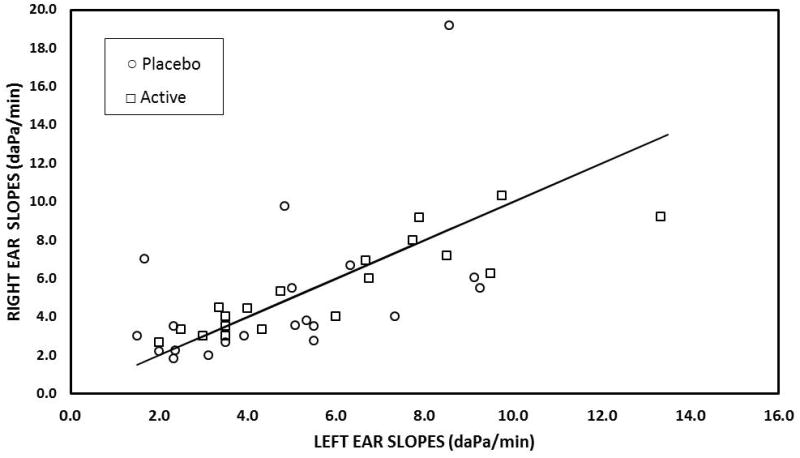
Scatterplot of ME Pressure-Time slopes recorded for the right ears as a function of the paired slopes recorded for the left ears after the placebo (open circles) and active (open squares) pretreatments. The solid line is the line of identity for the left ear slopes.
Discussion
For normal auditory function, ME gas-pressure needs to be maintained at near-ambient levels. However, isolated, biological gas-pockets such as the ME are not stable at ambient pressure but exhibit either a progressive volume-collapse or, if rigid, an exponential pressure decrease to approach the tissue gas-pressure of about ambient-600 daPa27,28. The anatomical construction of the ME allows for both of these behaviors, wherein ME pressure first exhibits a progressive decrease to a mucosa-ME hydrostatic pressure-gradient of about -250 daPa and, then, the ME gas-space shows a volume-collapse as vascular fluids accumulate to displace the ME gas-volume1. In healthy MEs, this fate is avoided by periodic ET openings which establish short-duration, ME-NP gas-phase communications and permit gradient-driven ME-NP gas-flow which resets ME pressure at near-ambient levels. This process represents a homeostatic mechanism to preserve a near-zero ME-ambient pressure-gradient, MEPR, with ET opening being the sole pressure-regulator and diffusive ME to blood N2 transfer being the primary driver of gradient instability.
The opposing processes of MEPR operate over different time-scales. Specifically, ME volume gas-efflux is continuous for relatively long time-periods between ET openings (measured in minutes to hours) and ME volume gas-influx is a discrete event occurring during a tubal opening (average duration≈400 msec)29. Thus, the ViMEg/VeMEN2 ratio exhibits a temporal pattern characterized by successive values of 1 bounding variable-length periods during which the ratio decreases. Because ME pressure is governed by the ideal gas law, these changes are reflected as changes in ME pressure and, at stable environmental pressure, changes in the ME-ambient pressure-gradient. Adequate MEPR requires that the sum of accumulated ME volume-gas gain and loss is 0 ul (i.e. ViMEg/VeMEN2=1) at a time, Δte, within all time-intervals, Δtp, where Δte is the time between each occurrence of ViMEg/VeMEN2=1 and Δtp is the time required for ME gas-volume efflux to drive the ME-ambient pressure-gradient to the critical-level that causes pathology in a specified ME. Thus, MEPR efficiency can be quantified as the Δtp/Δte time-ratio with higher ratios reflecting greater efficiencies and ratios less than 1 defining MEPD. Because an individual's “risk” for conductive hearing-loss and certain ME pathologies is inversely related to MEPR efficiency30, interventions that effect an increase in the Δtp/Δte time-ratio will decrease those risks and, perhaps, promote resolution of existing pathologies.
The Δtp/Δte time-ratio can be increased by increasing Δtp, decreasing Δte or both. Past approaches to modulating MEPR efficiency focused almost exclusively on decreasing Δte by pharmacological/surgical interventions intended to improve ET efficiency, but with limited success31. Here, the feasibility of the alternative strategy to increase the Δtp/Δte time-ratio was explored. Because Δtp measures the time required for the extant rate of ME volume gas-loss to precipitate pathology, it is inversely related to the transMEM N2 exchange-rate that, in turn, depends on the mucosal blood perfusion-rate. Consequently, it was hypothesized that pharmacological interventions that decrease the perfusion-rate would decrease the transMEM N2 exchange-rate, extend Δte, and increase the Δtp/Δte time-ratio
That hypothesis was tested using a previously developed25 and field-tested26,32 experimental protocol capable of estimating the transMEM N2 exchange-rate from measured values of the transMEM N2O exchange-rate. A formal mathematical description of the blood-ME species-exchanges under this protocol documented the correspondence between the measured variable (i.e. slope of the ME pressure-time function) and the parameter to be estimated (i.e. transMEM N2 conductance) as well as the validity of using the surrogate gas, N2O, to estimate the transMEM N2 exchange-rate1,25,26,32. N2O was used as the test gas because it exhibits a perfusion-limited transMEM exchange-rate that is approximately 33 times faster than the N2 rate33 which allows the required measurements to be made within a “reasonable” time-period. The active treatment, PDE, was chosen for its known mucosal vasoconstrictive effects19. Because the transMEM N2O exchange-rate depends on mastoid geometry26,32, a cross-over design was used and the trans-MEM N2O exchange-rate was compared between placebo and active treatments within ears/persons.
The results support the tested hypothesis that the rate of ME pressure-increase in response to a positive transMEM blood-ME N2O pressure-gradient is decreased by pretreatment with PDE and, by implication, support the validity of the underlying assumptions applicable to this setting. The latter include: 1) oral PDE at the specified dose causes a significant ME mucosal vasoconstriction for a duration sufficient to include the time-period when the measurements were made, and 2) this vasoconstriction is expressed as a significant decrease in the ME mucosal blood perfusion-rate. Because the transMEM exchange-rates for both N2O and N2 are governed by the same perfusion-limited exchange-equation, it is expected that PDE and other pharmacological modulators of blood perfusion will have predictable effects on the transMEM N2 exchange-rate and, consequently, MEPR efficiency. From the effect size for this experiment, it is expected that dose of PGE used will increase MEPR efficiency by about 33%. Experiments are ongoing in our laboratory to test these expectations.
A few studies reported that ET efficiency was poorer for otherwise healthy MEs with past ME disease when compared to ears without past disease34,35. Extant inflammation with/without ME fluid is associated with an increased transMEM inert-gas exchange-rate presumably due to an increased volume-blood perfusion-rate for inflamed mucosa12,36,37. However, the possible effect of disease history on transMEM inert-gas exchange-rates has not been explored. In this study, persons with and without past ME disease were enrolled but the analysis identified no significant effect of disease history on the measured trans-MEM N2O exchange-rate. However, the power to detect expected differences was low given the sample size of 10 subjects/group. Thus, this remains an unresolved question to be evaluated more rigorously in future studies.
As an aside, a significant left-right correlation in the magnitudes of the trans-MEM N2O exchange rates was documented. Given the results of previous studies showing rate dependence on mastoid geometry26, this correlation may reflect a bilateral symmetry in ME geometry for those enrolled. If supported by future experiments, there may be a basal level of trans-MEM N2 exchange-rate fixed by ME geometry and other factors. In turn, variations in this could affect the degree of achievable pharmacological modulation of the trans-MEM N2 exchange-rate and thereby restrict the expected benefit of using vasoconstrictors as a “treatment” to improve MEPR efficiency to subgroups pre-selected on the basis of their basal exchange-rate.
Conclusion
Oral administration of a vasoconstrictive drug decreased the transMEM N2O exchange-rate. Because all inert-gases are characterized by a perfusion-limited transMEM exchange-rate, this effect is expected to extend to the transMEM N2 exchange-rate which is the primary determinant of the requisite ET efficiency for adequate MEPR. Modulating ME mucosal blood-perfusion can increase MEPR efficiency but, alone, cannot stabilize ME pressure at near-ambient levels.
Acknowledgments
Funding: a Grant DC007667
This study was supported in part by a grant from the National Institutes of Health (P50 DC007667) and by the Hamburg and Eberly Endowments to the Division of Pediatric Otolaryngology, University of Pittsburgh. These sources provided funding for the study, but did not have input into the design, analyses or interpretation of the data. The authors thank Dr. J. Douglas Swarts for his input into the study design and data interpretation, Dr. Ellen Mandel for preparing the necessary IRB submissions and related correspondence and Mr. James T. Seroky, Ms. Julianne Banks and Ms. Jenna El-Wagaa for assistance with subject recruitment, data acquisition and data entry.
Footnotes
Financial Disclosure Information: The authors have no financial disclosures.
Conflict of Interest Statement: None of the listed authors have any real or apparent conflicts of interest with respect to the material presented in this manuscript.
References
- 1.Doyle W. Middle ear pressure regulation. In: Rosowski J, Merchant S, editors. The Function and Mechanics of Normal, Diseased and Reconstructed Middle Ears. The Hague, The Netherlands: Kugler Publications; 2000. [Google Scholar]
- 2.Kanick SC, Doyle WJ. Barotrauma during air travel: predictions of a mathematical model. J Appl Physiol (1985) 2005 May;98(5):1592–1602. doi: 10.1152/japplphysiol.00974.2004. [DOI] [PubMed] [Google Scholar]
- 3.Casselbrant ML, Cantekin EI, Dirkmaat DC, Doyle WJ, Bluestone CD. Experimental paralysis of tensor veli palatini muscle. Acta Otolaryngol. 1988 Sep-Oct;106(3-4):178–185. doi: 10.3109/00016488809106423. [DOI] [PubMed] [Google Scholar]
- 4.Bluestone CD, Doyle WJ. Anatomy and physiology of eustachian tube and middle ear related to otitis media. J Allergy Clin Immunol. 1988 May;81(5 Pt 2):997–1003. doi: 10.1016/0091-6749(88)90168-6. [DOI] [PubMed] [Google Scholar]
- 5.Yuksel S, Douglas Swarts J, Banks J, Doyle WJ. CO(2) gas exchange across the human tympanic membrane is not appreciably affected by pathology. Eur Arch Otorhinolaryngol. 2011 Feb;268(2):203–206. doi: 10.1007/s00405-010-1368-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuksel S, Swarts JD, Banks J, Seroky JT, Doyle WJ. In vivo measurement of O(2) and CO(2) gas exchange across the human tympanic membrane. Acta Otolaryngol. 2009 Jul;129(7):716–725. doi: 10.1080/00016480802360657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hergils L, Magnuson B. Human middle ear gas composition studied by mass spectrometry. Acta Otolaryngol. 1990 Jul-Aug;110(1-2):92–99. doi: 10.3109/00016489009122520. [DOI] [PubMed] [Google Scholar]
- 8.Hergils L, Magnuson B. Middle ear gas composition in pathologic conditions: mass spectrometry in otitis media with effusion and atelectasis. Ann Otol Rhinol Laryngol. 1997 Sep;106(9):743–745. doi: 10.1177/000348949710600905. [DOI] [PubMed] [Google Scholar]
- 9.Doyle WJ, Alper CM, Seroky JT. Trans-mucosal inert gas exchange constants for the monkey middle ear. Auris Nasus Larynx. 1999 Jan;26(1):5–12. doi: 10.1016/s0385-8146(98)00060-1. [DOI] [PubMed] [Google Scholar]
- 10.Doyle WJ, Alper CM, Seroky JT, Karnavas WJ. Exchange rates of gases across the tympanic membrane in rhesus monkeys. Acta Otolaryngol. 1998 Jul;118(4):567–573. doi: 10.1080/00016489850154748. [DOI] [PubMed] [Google Scholar]
- 11.Doyle WJ, Seroky JT. Middle ear gas exchange in rhesus monkeys. Ann Otol Rhinol Laryngol. 1994 Aug;103(8 Pt 1):636–645. doi: 10.1177/000348949410300811. [DOI] [PubMed] [Google Scholar]
- 12.Doyle WJ, Seroky JT, Alper CM. Gas exchange across the middle ear mucosa in monkeys. Estimation of exchange rate. Arch Otolaryngol Head Neck Surg. 1995 Aug;121(8):887–892. doi: 10.1001/archotol.1995.01890080055011. [DOI] [PubMed] [Google Scholar]
- 13.Seroky JT, Alper CM, Tabari R, Doyle WJ. Effects of intranasal challenge with histamine, bradykinin and prostaglandin on middle ear pressure and blood flow in cynomolgus monkeys. Acta Otolaryngol. 1995 Jan;115(1):83–87. doi: 10.3109/00016489509133352. [DOI] [PubMed] [Google Scholar]
- 14.Yuksel S, Doyle WJ, Banks J, Seroky JT, Alper CM. Nasal prostaglandin challenge increases N2O exchange from blood to middle ear. Auris Nasus Larynx. 2005 Mar;32(1):29–32. doi: 10.1016/j.anl.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Swarts JD, Casselbrant ML, Teixeira MS, et al. Eustachian tube function in young children without a history of otitis media evaluated using a pressure chamber protocol. Acta Otolaryngol. 2014 Jun;134(6):579–587. doi: 10.3109/00016489.2014.882017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casselbrant ML, Doyle WJ, Cantekin EI, Ingraham AS. Eustachian tube function in the rhesus monkey model of cleft palate. Cleft Palate J. 1985 Jul;22(3):185–191. [PubMed] [Google Scholar]
- 17.Alper CM, Tabari R, Seroky JT, Doyle WJ. Magnetic resonance imaging of the development of otitis media with effusion caused by functional obstruction of the eustachian tube. Ann Otol Rhinol Laryngol. 1997 May;106(5):422–431. doi: 10.1177/000348949710600511. [DOI] [PubMed] [Google Scholar]
- 18.Swarts JD, Alper CM, Seroky JT, Chan KH, Doyle WJ. In vivo observation with magnetic resonance imaging of middle ear effusion in response to experimental underpressures. Ann Otol Rhinol Laryngol. 1995 Jul;104(7):522–528. doi: 10.1177/000348949510400704. [DOI] [PubMed] [Google Scholar]
- 19.Stubner UP, Toth J, Marks B, Berger UE, Burtin B, Horak F. Efficacy and safety of an oral formulation of cetirizine and prolonged-release pseudoephedrine versus xylometazoline nasal spray in nasal congestion. Arzneimittelforschung. 2001;51(11):904–910. doi: 10.1055/s-0031-1300135. [DOI] [PubMed] [Google Scholar]
- 20.Johnson DA, Hricik JG. The pharmacology of alpha-adrenergic decongestants. Pharmacotherapy. 1993;13(6 Pt 2):110S–115S. [PubMed] [Google Scholar]
- 21.Simons FE, Gu X, Watson WT, Simons KJ. Pharmacokinetics of the orally administered decongestants pseudoephedrine and phenylpropanolamine in children. J Pediatr. 1996;129(5):729–34. doi: 10.1016/s0022-3476(96)70157-9. [DOI] [PubMed] [Google Scholar]
- 22.Kanfer I, Dowse R, Vuma V. Pharmacokinetics of oral decongestants. Pharmacotherapy. 1993;13(6 Pt 2):116S–128S. [PubMed] [Google Scholar]
- 23.Strano-Rossi S, Leone D, de la Torre X, Botrè F. Analysis of stimulants in oral fluid and urine by gas chromatography-mass spectrometry II: pseudophedrine. J Anal Toxicol. 2010;34(4):210–5. doi: 10.1093/jat/34.4.210. [DOI] [PubMed] [Google Scholar]
- 24.Evers AS, Crowder CM. General anesthetics. In: Hardman JG, Limbird LE, Gilman AG, editors. The pharmacological basis of therapeutics. New York, NY: McGraw-Hill; 2001. pp. 337–365. [Google Scholar]
- 25.Doyle WJ, Banks JM. Middle ear pressure change during controlled breathing with gas mixtures containing nitrous oxide. J Appl Physiol (1985) 2003 Jan;94(1):199–204. doi: 10.1152/japplphysiol.00634.2002. [DOI] [PubMed] [Google Scholar]
- 26.Alper CM, Kitsko DJ, Swarts JD, et al. Role of the mastoid in middle ear pressure regulation. Laryngoscope. 2011 Feb;121(2):404–408. doi: 10.1002/lary.21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Liew HD, Schoenfisch WH, Goldberg MM. Diffusion of oxygen from gas pockets to capillaries. Microvasc Res. 1969 Apr;1(3):257–265. doi: 10.1016/0026-2862(69)90027-2. [DOI] [PubMed] [Google Scholar]
- 28.Burkard ME, Van Liew HD. Simulation of exchanges of multiple gases in bubbles in the body. Respir Physiol. 1994 Feb;95(2):131–145. doi: 10.1016/0034-5687(94)90111-2. [DOI] [PubMed] [Google Scholar]
- 29.Alper CM, Swarts JD, Singla A, Banks J, Doyle WJ. Relationship between the electromyographic activity of the paratubal muscles and eustachian tube opening assessed by sonotubometry and videoendoscopy. Arch Otolaryngol Head Neck Surg. 2012 Aug;138(8):741–746. doi: 10.1001/archoto.2012.1293. [DOI] [PubMed] [Google Scholar]
- 30.Bluestone CD. Eustachian Tube Structure, Function, Role in Otitis Media. Hamilton, Ontario: BC Decker Inc; 2005. [Google Scholar]
- 31.Swarts JD, Alper CM, Luntz M, et al. Panel 2: Eustachian tube, middle ear, and mastoid--anatomy, physiology, pathophysiology, and pathogenesis. Otolaryngol Head Neck Surg. 2013 Apr;148(4 Suppl):E26–36. doi: 10.1177/0194599812472631. [DOI] [PubMed] [Google Scholar]
- 32.Doyle WJ. The mastoid as a functional rate-limiter of middle ear pressure change. Int J Pediatr Otorhinolaryngol. 2007 Mar;71(3):393–402. doi: 10.1016/j.ijporl.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Doyle WJ, Alper CM, Banks JM, Swarts JD. Rate of nitrous oxide exchange across the middle ear mucosa in monkeys before and after blockage of the mastoid antrum. Otolaryngol Head Neck Surg. 2003 May;128(5):732–741. doi: 10.1016/S0194-59980223309-4. [DOI] [PubMed] [Google Scholar]
- 34.Teixeira MS, Banks J, Swarts JD, Alper CM, Doyle WJ. Eustachian tube opening measured by sonotubometry is poorer in adults with a history of past middle ear disease. Int J Pediatr Otorhinolaryngol. 2014 Apr;78(4):593–598. doi: 10.1016/j.ijporl.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swarts JD, Alper CM, Mandel EM, Villardo R, Doyle WJ. Eustachian tube function in adults without middle ear disease. Ann Otol Rhinol Laryngol. 2011 Apr;120(4):220–225. doi: 10.1177/000348941112000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alper CM, Doyle WJ, Seroky JT. Higher rates of pressure decrease in inflamed compared with noninflamed middle ears. Otolaryngol Head Neck Surg. 1999 Jul;121(1):98–102. doi: 10.1016/S0194-5998(99)70133-6. [DOI] [PubMed] [Google Scholar]
- 37.Doyle WJ, Alper CM. A model to explain the rapid pressure decrease after air-inflation of diseased middle ears. Laryngoscope. 1999 Jan;109(1):70–78. doi: 10.1097/00005537-199901000-00015. [DOI] [PubMed] [Google Scholar]


