Abstract
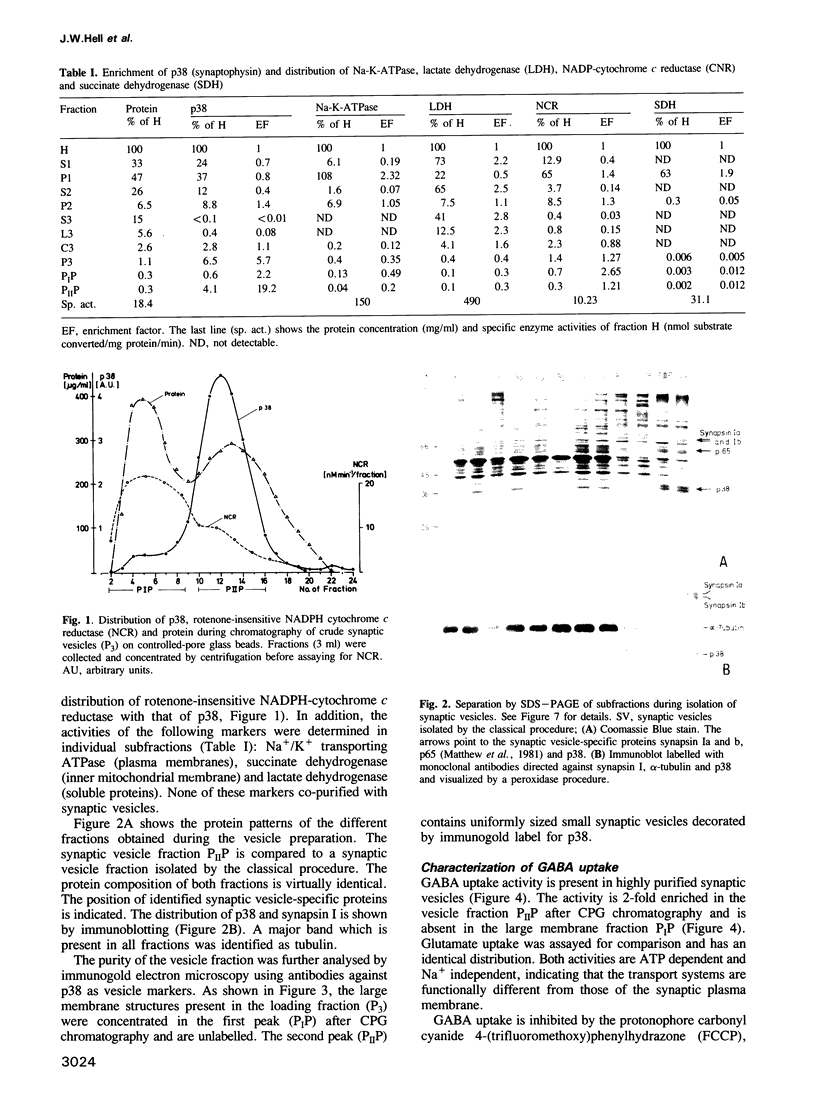
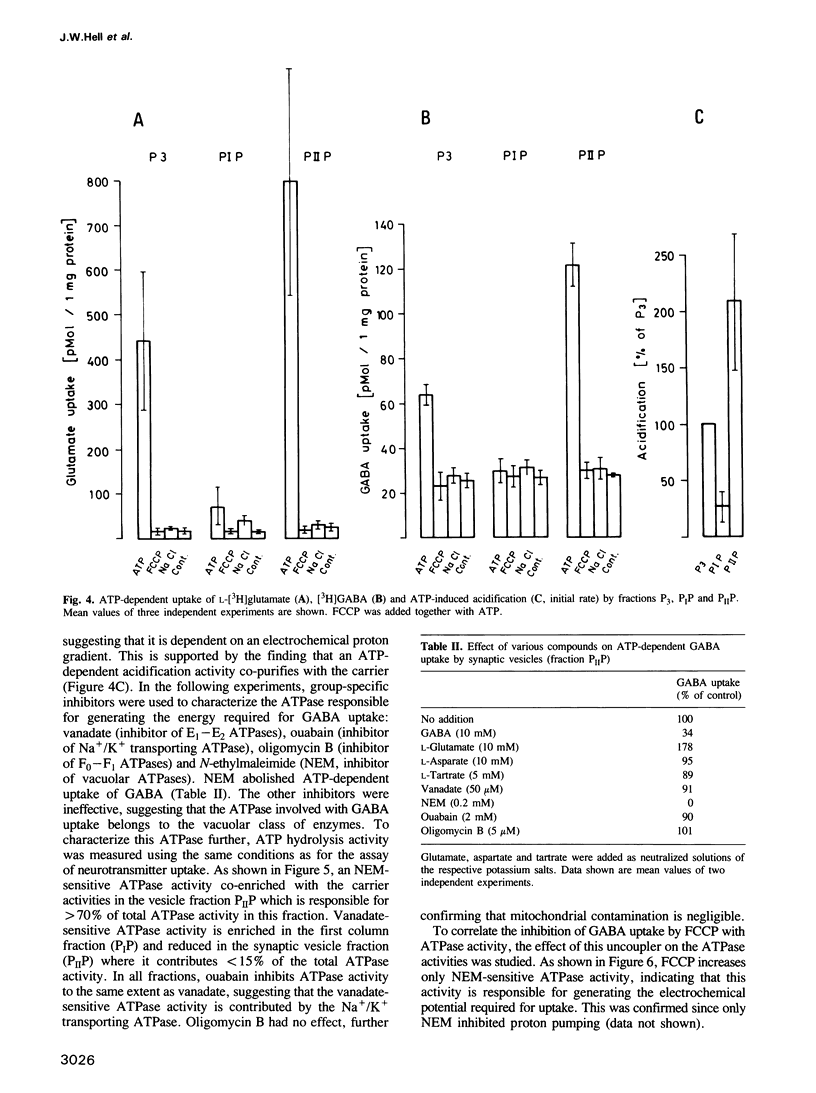
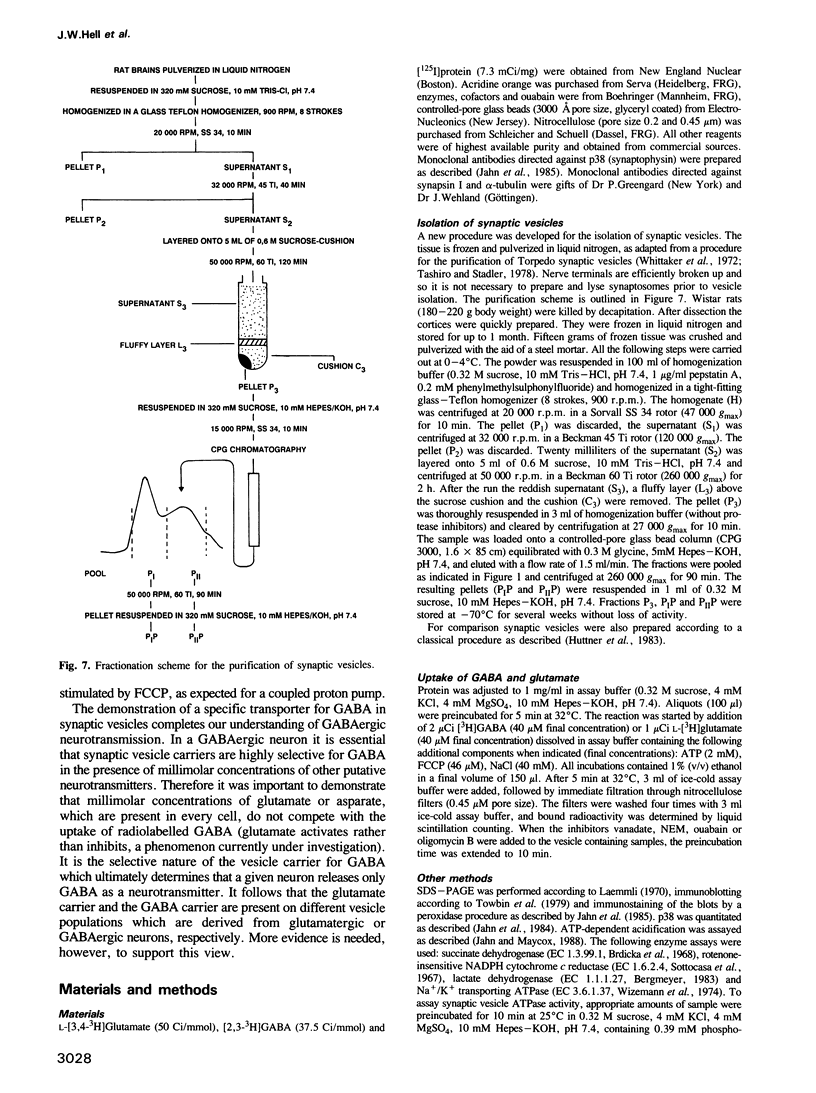
Uptake of GABA was demonstrated in rat brain synaptic vesicles which were prepared by a new and efficient procedure. The uptake activity co-purified with the synaptic vesicles during the isolation procedure. The purity of the vesicle fraction was rigorously examined by analysis of marker enzymes and marker proteins and also by immunogold electron microscopy using antibodies against p38 (synaptophysin). Contamination by other cellular components was negligible, indicating that GABA uptake by the synaptic vesicle fraction is specific for synaptic vesicles and not due to the presence of other structure possessing GABA uptake or binding activities. GABA uptake was ATP dependent and similar to the uptake of glutamate, which was assayed for a comparison. Both uptake activities were independent of sodium. They were inhibited by the uncoupler carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone, indicating that the energy for the uptake is provided by an electrochemical proton gradient. This gradient is generated by a proton ATPase of the vacuolar type as suggested by the effects of various ATPase inhibitors on neurotransmitter uptake and proton pumping. Competition experiments revealed that the transporters for GABA and glutamate are selective for the respective neurotransmitters.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelides K. J. Transport of catecholamines by native and reconstituted rat heart synaptic vesicles. J Neurochem. 1980 Oct;35(4):949–962. doi: 10.1111/j.1471-4159.1980.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brdiczka D., Pette D., Brunner G., Miller F. Kompartimentierte Verteilung von Enzymen in Rattenlebermitochondrien. Eur J Biochem. 1968 Jul;5(2):294–304. doi: 10.1111/j.1432-1033.1968.tb00370.x. [DOI] [PubMed] [Google Scholar]
- Ceccarelli B., Hurlbut W. P. Vesicle hypothesis of the release of quanta of acetylcholine. Physiol Rev. 1980 Apr;60(2):396–441. doi: 10.1152/physrev.1980.60.2.396. [DOI] [PubMed] [Google Scholar]
- De Camilli P., Greengard P. Synapsin I: a synaptic vesicle-associated neuronal phosphoprotein. Biochem Pharmacol. 1986 Dec 15;35(24):4349–4357. doi: 10.1016/0006-2952(86)90747-1. [DOI] [PubMed] [Google Scholar]
- DeLorenzo R. J., Freedman S. D. Calcium dependent neurotransmitter release and protein phosphorylation in synaptic vesicles. Biochem Biophys Res Commun. 1978 Jan 13;80(1):183–192. doi: 10.1016/0006-291x(78)91121-x. [DOI] [PubMed] [Google Scholar]
- Disbrow J. K., Gershten M. J., Ruth J. A. Uptake of L-[3H] glutamic acid by crude and purified synaptic vesicles from rat brain. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1221–1227. doi: 10.1016/0006-291x(82)92130-1. [DOI] [PubMed] [Google Scholar]
- Fykse E. M., Fonnum F. Uptake of gamma-aminobutyric acid by a synaptic vesicle fraction isolated from rat brain. J Neurochem. 1988 Apr;50(4):1237–1242. doi: 10.1111/j.1471-4159.1988.tb10599.x. [DOI] [PubMed] [Google Scholar]
- Harlos P., Lee D. A., Stadler H. Characterization of a Mg2+-ATPase and a proton pump in cholinergic synaptic vesicles from the electric organ of Torpedo marmorata. Eur J Biochem. 1984 Nov 2;144(3):441–446. doi: 10.1111/j.1432-1033.1984.tb08485.x. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Reese T. S. Evidence for recycling of synaptic vesicle membrane during transmitter release at the frog neuromuscular junction. J Cell Biol. 1973 May;57(2):315–344. doi: 10.1083/jcb.57.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B., Schiebler W., Greengard P., De Camilli P. Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol. 1983 May;96(5):1374–1388. doi: 10.1083/jcb.96.5.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Greengard P. A quantitative dot-immunobinding assay for proteins using nitrocellulose membrane filters. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1684–1687. doi: 10.1073/pnas.81.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R., Schiebler W., Ouimet C., Greengard P. A 38,000-dalton membrane protein (p38) present in synaptic vesicles. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4137–4141. doi: 10.1073/pnas.82.12.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner B. I., Schuldiner S. Mechanism of transport and storage of neurotransmitters. CRC Crit Rev Biochem. 1987;22(1):1–38. doi: 10.3109/10409238709082546. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maron R., Kanner B. I., Schuldiner S. The role of a transmembrane pH gradient in 5-hydroxy tryptamine uptake by synaptic vesicles from rat brain. FEBS Lett. 1979 Feb 15;98(2):237–240. doi: 10.1016/0014-5793(79)80190-8. [DOI] [PubMed] [Google Scholar]
- Matthew W. D., Tsavaler L., Reichardt L. F. Identification of a synaptic vesicle-specific membrane protein with a wide distribution in neuronal and neurosecretory tissue. J Cell Biol. 1981 Oct;91(1):257–269. doi: 10.1083/jcb.91.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A., Baker R. R., Morris S. J., Whittaker V. P. The preparation and characterization of synaptic vesicles of high purity. Brain Res. 1976 Jun 11;109(2):285–309. doi: 10.1016/0006-8993(76)90531-x. [DOI] [PubMed] [Google Scholar]
- Naito S., Ueda T. Adenosine triphosphate-dependent uptake of glutamate into protein I-associated synaptic vesicles. J Biol Chem. 1983 Jan 25;258(2):696–699. [PubMed] [Google Scholar]
- Naito S., Ueda T. Characterization of glutamate uptake into synaptic vesicles. J Neurochem. 1985 Jan;44(1):99–109. doi: 10.1111/j.1471-4159.1985.tb07118.x. [DOI] [PubMed] [Google Scholar]
- Nelson N. The vacuolar proton-ATPase of eukaryotic cells. Bioessays. 1987 Dec;7(6):251–254. doi: 10.1002/bies.950070605. [DOI] [PubMed] [Google Scholar]
- Parsons S. M., Bahr B. A., Gracz L. M., Kaufman R., Kornreich W. D., Nilsson L., Rogers G. A. Acetylcholine transport: fundamental properties and effects of pharmacologic agents. Ann N Y Acad Sci. 1987;493:220–233. doi: 10.1111/j.1749-6632.1987.tb27203.x. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reichardt L. F., Kelly R. B. A molecular description of nerve terminal function. Annu Rev Biochem. 1983;52:871–926. doi: 10.1146/annurev.bi.52.070183.004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudnick G. ATP-driven H+ pumping into intracellular organelles. Annu Rev Physiol. 1986;48:403–413. doi: 10.1146/annurev.ph.48.030186.002155. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro T., Stadler H. Chemical composition of cholinergic synaptic vesicles from Torpedo marmorata based on improved purification. Eur J Biochem. 1978 Oct 16;90(3):479–487. doi: 10.1111/j.1432-1033.1978.tb12627.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P., Essman W. B., Dowe G. H. The isolation of pure cholinergic synaptic vesicles from the electric organs of elasmobranch fish of the family Torpedinidae. Biochem J. 1972 Jul;128(4):833–845. doi: 10.1042/bj1280833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B., Franke W. W. Identification and localization of synaptophysin, an integral membrane glycoprotein of Mr 38,000 characteristic of presynaptic vesicles. Cell. 1985 Jul;41(3):1017–1028. doi: 10.1016/s0092-8674(85)80082-9. [DOI] [PubMed] [Google Scholar]
- Wizemann V., Christian A. L., Wiechmann J., Schulz I. The distribution of membrane bound enzymes in the acini and ducts of the cat pancreas. Pflugers Arch. 1974 Feb 18;347(1):39–47. doi: 10.1007/BF00587053. [DOI] [PubMed] [Google Scholar]




