Abstract
Antiretroviral therapy (ART) induces rapid suppression of viral replication and a progressive replenishment of CD4+ T cells in HIV-infected individuals. However, the effect of ART on restoring pre-existing memory CD4+ T cells specific for common co-pathogens is still unclear. To better understand the dynamics of antigen-specific CD4+ T cells during ART, we assessed the frequency, functional capacity and memory profile of CD4+ T cells specific for Mycobacterium tuberculosis (Mtb) and cytomegalovirus (CMV) in 15 HIV-infected individuals before and one year after ART initiation.
After ART initiation, the frequency of Mtb-specific CD4+ T cells showed little change, while CMV-specific CD4+ T cells were significantly lower (p=0.003). There was no difference in the polyfunctional or memory profile of antigen-specific CD4+ T cells before and after ART. The replenishment of antigen-specific CD4+ T cells correlated with the memory differentiation profile of these cells prior to ART. Pathogen-specific CD4+ T cells exhibiting a late differentiated profile (CD45RO+CD27−) had a lower capacity to replenish (p=0.019, r=−0.5) compared to cells with an early differentiated profile (CD45RO+CD27+; p=0.04, r=0.45).
In conclusion, restoration of co-pathogen-specific memory CD4+ T cells during treated HIV infection is related to their memory phenotype, where early differentiated cells (such as most Mtb-specific cells) have a higher replenishment capacity compared to late differentiated cells (such as most CMV-specific cells). These data identify an important, hitherto unrecognized, factor that may limit restoration of co-pathogen immunity in HIV-infected individuals on ART.
INTRODUCTION
The hallmarks of untreated HIV infection are a progressive loss of CD4+ T cells, sustained cellular activation and chronic inflammation (1-3). In addition to the numerical depletion of CD4+ T cells, HIV can also alter the functional capacity of these cells, impairing their proliferative potential, altering their cytokine secretion profiles and changing their phenotypic characteristics in response to HIV antigens as well as various co-pathogens (4-7). Both of these quantitative and qualitative alterations can lead to increased susceptibility to opportunistic infections, including tuberculosis (TB), Candidiasis and Human Papilloma Virus (HPV) infection (8). Indeed, HIV is the best-recognized risk factor for TB disease even before profound CD4+ T cell deficiency (9, 10). The introduction of antiretroviral therapy (ART) has drastically decreased morbidity and mortality in HIV-infected individuals (11), inducing a rapid reduction of plasma viral load and a progressive repletion of CD4+ T cells (12). Although the clinical benefit of ART is undeniable, the extent to which ART can fully “normalize” functional immunity remains unclear (13). HIV-infected individuals on ART exhibit a differential degree of recovery of co-pathogen-specific CD4+ T cell responses, depending on the pathogen they target (14-20). For example, it has been shown that the restoration of CMV-specific CD4+ T cells occurs early after ART (19), but appears to be short-lived (15). Conversely, Candida-specific CD4 responses recover slowly (16). Contrasting data exist on the degree of recovery of Mycobacterium tuberculosis (Mtb)-specific CD4+ T cell responses upon ART. Jambo et al. showed in a cross-sectional study that the frequency and polyfunctional profile of Mtb-specific CD4+ T cell responses were similar in ART-naïve or treated individuals (20), while Sutherland et al. reported that ART increases the polyfunctional capacity of these cells (18). Other studies described only a partial reconstitution of Mtb-specific CD4+ T cell responses after ART (14, 17).
It is of particular importance to define the factors that associate with successful pathogen-specific CD4+ T cell recovery upon ART, as limited “normalization” of functional CD4+ T cell responses could account for sustained incidence of opportunistic infections. Several parameters influence the degree and dynamics of recovery of the overall CD4+ T cell compartment in response to ART, such as age, CD4 count at the time of treatment initiation, and timing of ART initiation after HIV infection (21-23). However, it is still unclear why CD4+ T cells of different pathogen specificities have different profiles of restoration, and the mechanisms mediating this variable recovery of memory CD4+ T cells to co-pathogens are still incompletely understood. Thus, to better understand the effect of successful ART on the dynamics of recovery of co-pathogen-specific CD4+ T cells, we compared the magnitude, functional capacity and memory differentiation profiles of Mtb- and CMV-specific CD4+ T cells before and one year after ART initiation in a cohort of HIV-infected individuals, and HIV-uninfected controls.
MATERIALS AND METHODS
Study participants
Blood samples were collected from 15 women participating in the CAPRISA 002 study, a cohort study following HIV-infected women from HIV seroconversion until five years on treatment. The cohort is situated in KwaZulu-Natal, South Africa, and has been previously described (24, 25). Participants were selected based on sample availability. Blood samples from 9 HIV-uninfected participants were provided from the CAPRISA 004 vaginal microbicide (1% tenofovir) gel trial (26). An additional 14 HIV-uninfected participants from CAPRISA 004 were studied for immune activation. HIV-uninfected participants were from the same community as the HIV-infected individuals and age-matched; they were either in the pre-intervention phase or in the placebo arm of the trial. For HIV-infected individuals, the time of infection was estimated either as the same date as a prospective RNA positive/antibody negative measurement or taken as the midpoint between the last antibody negative test and first antibody positive enzyme-linked immunosorbent assay test. Participants in the cohort were offered ART according to South African national HIV treatment guidelines (at a CD4 count of <200 cells/mm3 prior to October 2012; <350 cells/mm3 until the present). Eight of the 15 participants were taking standard first-line therapy (TDF/3TC/EFV), 3 were on ddI-EC/3TC/EFV, and 1 each on d4T/3TC/EFV, TDF/3TC/NVP, d4T/3TC/NVP and AZT/3TC/LPV/r. Two participants switched drug regimens while on study, namely CAP200 (EFV/3TC/ddI-EC to EFV/3TC/TDF at month 11), and CAP255 (EFV/3TC/d4T to EFV/3TC/AZT at month 10). No participants had active TB during the study period, or exhibited any immune reconstitution disease upon HIV treatment. Ethical approval for the study was obtained from the University of KwaZulu-Natal and University of Cape Town Research Ethics Committees. All participants provided written informed consent to participate in the study.
Determination of plasma viral load and CD4 counts
Plasma HIV viral loads and CD4 counts were quantified at each study visit. Over the course of the study, the viral load PCR assay switched from Roche AMPLICOR HIV-1 monitor test version 1.5 [lower detection limit (LDL) of 400 RNA copies/ml], to Roche Taqman version 1.0 on June 1, 2010 (LDL 40 RNA copies/ml), and then to Roche Taqman version 2.0 on January 9, 2012 (LDL 20 RNA copies/ml). Absolute blood CD4 and CD8 T cell counts were measured using the FACSCalibur TruCOUNT method (BD Biosciences) and expressed as cells/mm3. Plasma samples matching the visits where PBMC were studied were tested for antibodies to CMV and CMV DNA. Seropositivity was determined using the Cobas CMV IgG Assay (Roche), and CMV viral load was detected and quantified using a CMV R-gene PCR kit (Argene), with a detection limit of 30 copies/ml.
Cell preparation
PBMC were isolated by standard Ficoll-Hypaque density gradient centrifugation (Amersham Pharmacia) and cryopreserved in 90% heat-activated fetal calf serum (FCS, Invitrogen) plus 10% DMSO and stored in liquid nitrogen until needed. Cryopreserved PBMC were thawed and rested in R10 [RPMI 1640 containing 10% heat-inactivated FCS (Sigma) and 50 U/ml of penicillin-streptomycin] at 37°C and 5% CO2 for 8 hours prior to antigen stimulation.
Antigens and cell stimulation
Following resting, PBMC were stimulated using Mtb Purified Protein Derivative (PPD, 10 μg/ml; Statens Serum Institute), a pool of CMV peptides consisting of 138 peptides (15mers overlapping by 11 amino acids) covering the entire HCMV pp65 protein (2 μg/ml; NIH AIDS Reagent Program) and Staphylococcal enterotoxin B (SEB, 2.5 μg/ml; Sigma), used as a positive control. Of note, we found no major differences in the memory profile, cytokine secretion potential and polyfunctional capacity between peptide and protein-stimulated PBMC (Bunjun et al., manuscript in preparation). Stimulations were performed in the presence of co-stimulatory antibodies, anti-CD28 and anti-CD49d (both at 1 μg/ml; BD Biosciences) at 37°C for 16 hours. Brefeldin A (BFA; Sigma) was added after 1 hour at a concentration of 10 μg/ml. Surface and intracellular cytokine staining was performed at the end of the incubation period. Sufficient cells were available for assessing CMV and SEB responses in only 12/15 HIV-infected participants.
Surface phenotypic and intracellular cytokine staining and flow cytometry
The following antibodies were used for surface and intracellular staining: CD3-APC-H7 (SK7; BD Biosciences), CD4-PE-Cy5.5 (S3.5; Invitrogen), CD8-Qdot 705 (3B5; Invitrogen), CD27-PE-Cy5 (1A4CD27; R&D Systems), CD45RO-ECD (UCHL1; R&D Systems), IFN-γ-Alexafluor 700 (B27; BD Biosciences), IL-2-APC (MQ1-17H12; BD Biosciences), TNF-α-PE-Cy7 (MAb11; eBiosciences), CD14-PacBlue (Tük4; Invitrogen), CD19-PacBlue (SJ25-CI; Invitrogen) and a violet amine viability dye (“Vivid”; Molecular Probes). All antibodies were titrated to obtain optimal concentrations prior to use. After stimulation, PBMC were first stained with Vivid for 20 min at room temperature. Cells were then surface stained with CD4, CD8, CD45RO, CD27, CD14 and CD19 antibodies. Cells were fixed and permeabilized using Cytofix/Cytoperm buffer (BD Biosciences) and stained intracellularly with CD3, IL-2, TNF-α and IFN-γ. Cells were then re-suspended in 1× CellFix (BD Biosciences). Samples were acquired on a BD Fortessa using FACSDiva software and analyzed using FlowJo (version 9.8.2; TreeStar). The gating strategy is presented in Supplementary Figure S1. A positive cytokine response was defined as at least twice the background (in the presence of co-stimulatory antibodies and no antigen). For phenotyping the memory profile of cells producing cytokines, a cut-off of 40 events was used. Polyfunctionality of antigen-specific cells was analyzed using Pestle (version 1.7) and Spice (version 5.35) software (27). Immune activation was measured by surface staining of unstimulated cells using the CD3, CD4, CD8, CD14 and CD19 antibodies listed above, as well as CD38-APC (HIT2) and HLA-DR-APC-Cy7 (L243) (both BD Biosciences), along with the inclusion of Vivid.
Statistical Analysis
Statistical analyses were performed using GraphPad Prism (version 5.0; San Diego). Non-parametric statistical tests were used for all comparisons. The Mann-Whitney U test and the Wilcoxon Signed Rank test were used for unmatched and paired samples, respectively, and the Kruskal-Wallis ANOVA using Dunn’s test for multiple comparisons. Correlations were performed using the Spearman Rank test. A p-value of <0.05 was considered statistically significant.
RESULTS
Effect of ART on the restoration of co-pathogen-specific CD4+ T cells
In order to better understand the replenishment dynamics of CD4+ T cells specific for co-pathogens in HIV-infected individuals on treatment, we studied 15 HIV-infected individuals before and after ART initiation. The cohort consisted of women infected with HIV-1 for a median of 4.9 years (IQR: 3.8-5.8 years) at the time of treatment initiation (Table 1). Pre-ART samples were obtained at a median of 1.7 months (IQR: 0.8-2.5) prior to starting ART. The median plasma viral load was 21,955 HIV RNA copies/ml (IQR: 4,743-52,800), and the median CD4 count was 289 cells/mm3 (IQR: 193-322). After a median of 12 months of treatment (IQR: 11-12.3 months), all individuals exhibited some degree of viral suppression (p<0.0001, Supplementary Figure S2A), which was below the limit of detection of the assay in 12/15 individuals and <300 RNA copies/ml in the remainder. An increase in absolute CD4 count occurred in 13/15 individuals over the period measured (p<0.0001, Supplementary Figure S2B). The median fold change in absolute CD4 count was 1.9 (IQR: 1.6-2.3), and when adjusted for length of treatment, the replenishment rate of CD4+ T cells was 20 cells/mm3/month (IQR: 14-26, data not shown); this was accompanied by a concomitant doubling of the CD4/CD8 ratio (0.27 vs. 0.66; p=0.0007, Supplementary Figure S2C). As previously reported (21-23), the degree of CD4 reconstitution was inversely related to absolute CD4 count at ART initiation (p<0.0001, r=−0.85) and the duration of HIV infection (p=0.016, r=−0.61, data not shown). We also measured immune activation before and after ART using the markers CD38 and HLA-DR. There was a significant reduction in CD4+ and CD8+ T cells co-expressing CD38+ and HLA-DR+ (p=0.0002 and p=0.0001, Supplementary Figure S2D), as well as HLA-DR+ alone (p=0.0001 for both subsets). Residual levels of immune activation after one year of ART remained significantly greater than healthy, HIV-uninfected individuals for both markers (Supplementary Figure S2D). We found no association between the degree of CD4 reconstitution and CD4+ T cell activation before or after ART (data not shown).
Table 1. Characteristics of study participants.
|
|
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
Pre-ART |
Post-ART |
|||||||
| PID | Pre-ART duration of infection (y) | Viral load (RNA copies/ml) | CD4 count (cells/mm3) | CD4:CD8 ratio | Months before ART initiation | Viral load (RNA copies/ml) | CD4 count (cells/mm3) | CD4:CD8 ratio | Months after ART initiation |
|
|
|
|
|||||||
| CAP200 | 3.8 | 423,000 | 135 | 0.24 | 2.5 | <20 | 496 | 0.68 | 13.5 |
| CAP206 | 5.2 | 198,277 | 289 | 0.15 | 0.2 | <40 | 512 | 0.50 | 11.0 |
| CAP222 | 6.1 | 3,049 | 319 | 0.61 | 3.2 | <20 | 619 | 1.24 | 11.3 |
| CAP237 | 5.2 | 4,743 | 308 | 0.57 | 0.0 | <20 | 528 | 1.05 | 12.0 |
| CAP244 | 7.3 | 21,955 | 318 | 0.32 | 0.0 | 273 | 647 | 0.53 | 12.3 |
| CAP248 | 6.6 | 29,258 | 363 | 0.26 | 2.8 | 121 | 305 | 0.27 | 6.7 |
| CAP255 | 3.7 | 8,830 | 226 | 0.18 | 3.8 | <20 | 484 | 0.53 | 18.1 |
| CAP257 | 4.8 | 52,800 | 170 | 0.19 | 2.0 | <20 | 494 | 0.47 | 12.0 |
| CAP262 | 5.8 | 1,130 | 338 | 1.69 | 1.7 | <20 | 329 | 3.13 | 12.1 |
| CAP267 | 5.4 | 41,338 | 269 | 0.27 | 1.3 | <20 | 347 | 0.35 | 8.7 |
| CAP268 | 4.2 | 4,537 | 186 | 0.18 | 0.9 | <20 | 426 | 0.63 | 12.0 |
| CAP276 | 1.4 | 22,000 | 232 | 0.37 | 2.3 | <400 | 627 | 1.03 | 20.2 |
| CAP277 | 4.9 | 7,997 | 322 | 0.22 | 1.0 | 29 | 535 | 0.66 | 10.0 |
| CAP279 | 2.7 | 58,100 | 193 | 0.40 | 0.8 | <20 | 400 | 1.48 | 12.0 |
| CAP316 | 4.1 | 5,612 | 436 | 0.33 | 2.4 | <20 | 708 | 0.67 | 11.8 |
|
|
|
|
|||||||
| Median | 4.9 | 21,955 | 289 | 0.27 | 1.7 | <20 | 496 | 0.66 | 12 |
| IQR | 3.8 - 5.8 | 4,743 - 52,800 | 193 - 322 | 0.95 - 0.40 | 0.8 – 2.5 | <20 - 40 | 400 - 619 | 0.5 - 1.05 | 11 - 12.3 |
|
|
|
|
|||||||
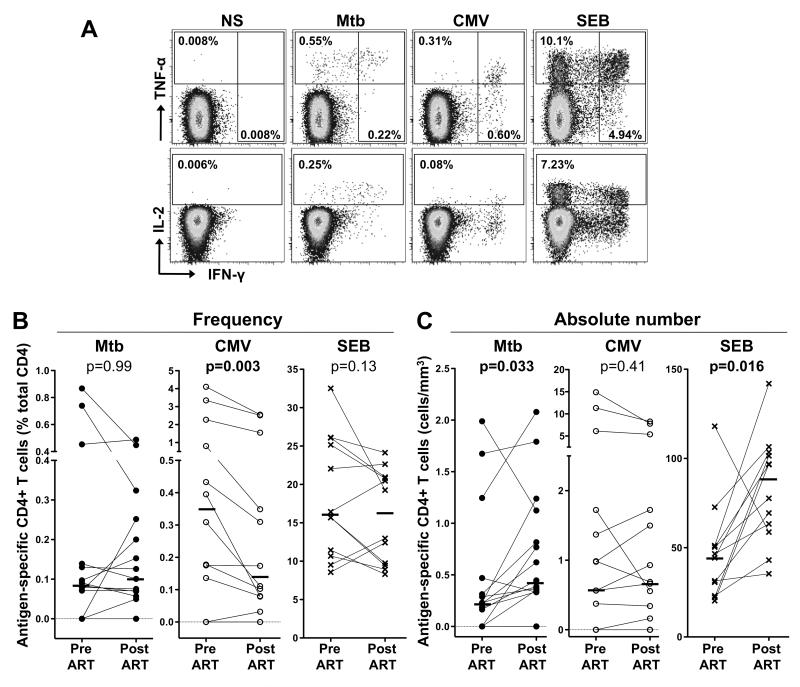
To determine whether the dynamics of co-pathogen-specific CD4+ T cells mirror the reconstitution of the total CD4+ T cell population, we measured the magnitude of Mtb- and CMV-specific CD4+ T cell responses, as well as SEB-reactive CD4+ cells, before and after ART initiation. Figure 1A shows representative flow cytometry plots of cytokine production (IFN-γ, IL-2 and TNF-α) by CD4+ T cells in response to Mtb, CMV and SEB stimulation in one HIV-infected patient (CAP267). At baseline (before ART initiation), 11/15 individuals tested had a detectable Mtb response, with a median frequency of 0.08% of total CD4+ T cells (range, 0-0.9%; Figure 1B). CMV responses were detected in 10/12 individuals tested and the median frequency was 0.35% (range: 0-4.1%). SEB responses were detectable in 12/12 participants with with a median frequency of 16% (range: 8.6-32.5%). The frequency of Mtb-specific and SEB-responding CD4+ T cells were comparable pre- and post-ART, whilst the frequency of CMV-specific CD4+ T cells was significantly lower after treatment (median 0.35% vs 0.14%, p=0.003, Figure 1C). To take into account variation in absolute CD4 counts across the cohort, the absolute number of antigen-specific CD4+ T cells was calculated. The number of Mtb-specific and SEB-responding CD4+ T cells increased significantly after ART (p=0.033 and p=0.016, respectively), whilst the number of CMV-specific CD4+ T cells remained unchanged upon treatment (Figure 1C), consistent with the decreased frequency in the context of increases in absolute CD4 numbers. Overall, these data reveal that the dynamics of reconstitution of antigen-specific CD4+ T cells vary according to their pathogen specificity.
FIGURE 1. Effect of ART on Mtb-, CMV- and SEB-specific CD4+ T cell responses.
(A) Representative flow cytometry plots of the expression of TNF-α, IFN-γ and IL-2 from CD4+ T cells after stimulation with Mtb and CMV antigens and SEB, in one study participant. NS corresponds to unstimulated PBMC. The frequency of cytokine-producing cells expressed as a percentage of the total CD4+ T cell population are indicated. Frequency (B) and absolute number (C) of Mtb-, CMV- and SEB-specific CD4+ T cell responses before and after ART. Horizontal bars represent the median. Statistical comparisons were performed using a Wilcoxon matched pairs test.
Relationship between the dynamics of restoration of total and co-pathogen-specific CD4+ T cells
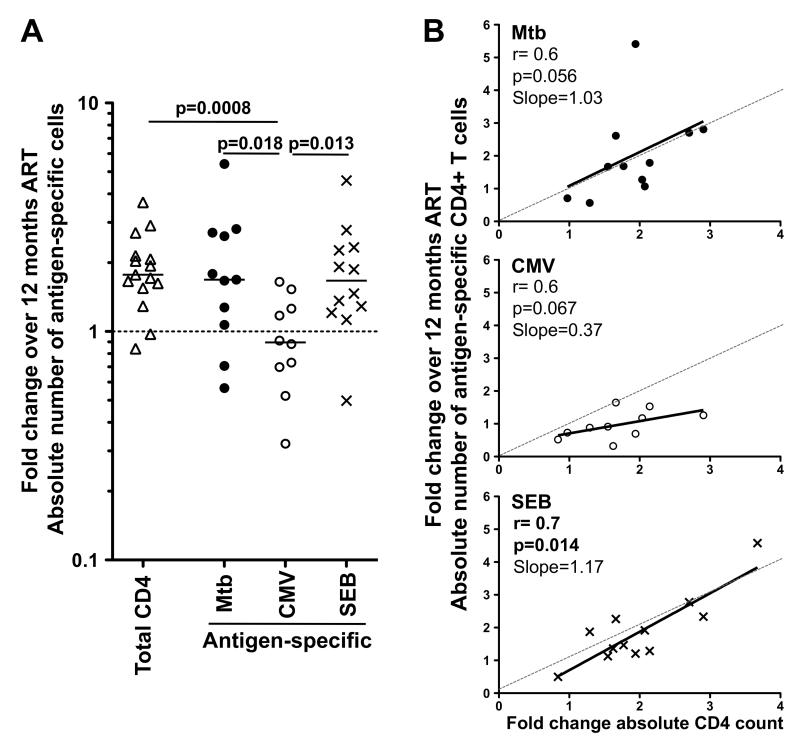
To further investigate the restoration dynamics of co-pathogen-specific CD4+ T cells, we examined the relationship between the fold change in the absolute number of antigen-specific CD4+ T cells pre- and post-ART (in individuals with detectable antigen responses at both time points, n=11 for Mtb, n=10 for CMV and n=12 for SEB), and the absolute total CD4 count variation. Figure 2A shows that whilst the magnitude of cell replenishment of Mtb-specific and SEB-responding CD4+ T cells was comparable to that of total CD4+ T cells (approximately a two-fold increase over the 12 months of ART), the extent of restoration of CMV-specific CD4+ T cells was significantly lower (p=0.018, p=0.013 and p=0.0008, to Mtb, SEB and total CD4+ cells, respectively). Consistent with these observations, the degree of restoration of Mtb- and SEB-responding CD4+ T cells corresponded closely with the overall CD4 recovery, as measured in fold-change of the absolute CD4 count, with a slope close to 1 (Figure 2B). In contrast, for CMV-specific CD4+ T cell responses the slope was 0.37, indicating a reduced replenishment rate of these cells compared to the total CD4 compartment (Figure 2B). Of note, whilst all study participants were CMV seropositive, none had detectable CMV viral replication at either visit, pre- or post-ART, indicating that a difference in CMV antigen load at the two visits was not a contributing factor to the reduced replenishment capacity of CMV-specific T cells.
FIGURE 2. Restoration dynamics of Mtb-, CMV- and SEB-specific CD4+ T cells after ART.
(A) Fold change in the total, Mtb-, CMV- and SEB-specific absolute CD4+ T cell count over 12 months of ART. The horizontal dotted line indicates no change from the baseline pre-ART time point. Statistical comparisons were performed using a non-parametric Mann-Whitney test. (B) Association between the fold change of the absolute number of antigen-specific CD4+ T cells and the fold change in the absolute CD4 count pre- and post-ART. Statistical associations were performed by a two-tailed non-parametric Spearman rank correlation. The solid line represents a linear regression fit, and the dashed line depicts the ideal slope of 1.
Effect of ART on the functional capacity of co-pathogen-specific CD4+ T cells
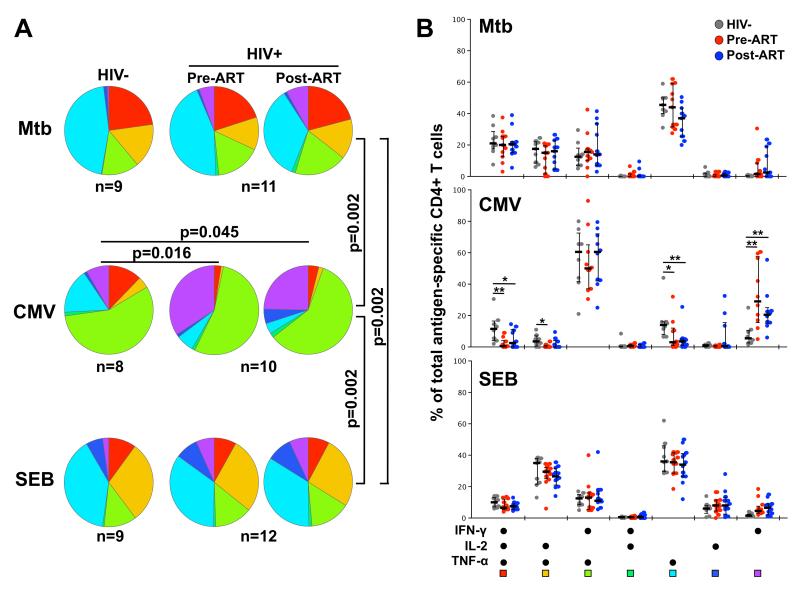
ART has been shown to gradually restore the polyfunctional capacity of HIV-specific T cells (28, 29), but the effect of ART on the functionality of CD4+ T cells specific for other pathogens is unclear. Thus, we evaluated the cytokine secretion profiles of Mtb-, CMV- and SEB-reactive CD4+ T cells before and after ART initiation in detail (Figure 3). We included 9 HIV-uninfected individuals in this analysis for comparison. Our first finding was that the cytokine production profile of CD4+ T cells varied significantly according to their specificity (p=0.002, Figure 3A), with Mtb-specific responses consisting of a greater proportion of polyfunctional cells producing all three cytokines, in addition to a predominant TNF-α monofunctional subset (~40% of the response), whilst CMV-specific CD4+ responses exhibited predominantly a IFN-γ+TNF-α+ phenotype (~60%; Figure 3B). ART did not significantly alter the cytokine secretion profile of CD4+ T cells from baseline, regardless of their specificity for Mtb, CMV or SEB (Figure 3A&B). Moreover, the polyfunctional characteristics of Mtb-specific and SEB-responsive CD4+ T cell responses were comparable to those observed in HIV-uninfected individuals. In contrast, CMV responses were significantly skewed in HIV-infected individuals compared to HIV-uninfected subjects (p=0.016 and p=0.045 for pre- and post-ART, Figure 3A), with a lower proportion of cells co-producing IFN-γ, IL-2 and TNF-α, and a higher proportion of IFN-γ monofunctional cells (Figure 3B). These data demonstrate that, unlike for Mtb-specific CD4 responses, HIV infection impaired the functional potential of CMV-specific CD4 responses towards a less polyfunctional profile, and one year of ART did not restore the CMV functional profile.
FIGURE 3. Polyfunctional capacity of Mtb-, CMV- and SEB-specific CD4+ T cells in HIV-infected and uninfected individuals pre- and post-ART.
Pie charts (A) and graphs (B) representing the cytokine secretion ability of Mtb-, CMV- and SEB-specific CD4+ T cell responses in HIV-uninfected individuals (n=9) and HIV-infected individuals pre-and post-ART initiation. Each section of the pie chart represents a specific combination of cytokines, as indicated by the color at the bottom of the graph. Horizontal bars depict the median with interquartile range indicated. Statistical comparisons were performed using a Wilcoxon rank-sum test. *p < 0.05, **p < 0.01, ***p < 0.001.
Impact of the memory phenotype of pathogen-specific CD4+ T cells on their replenishment potential upon ART
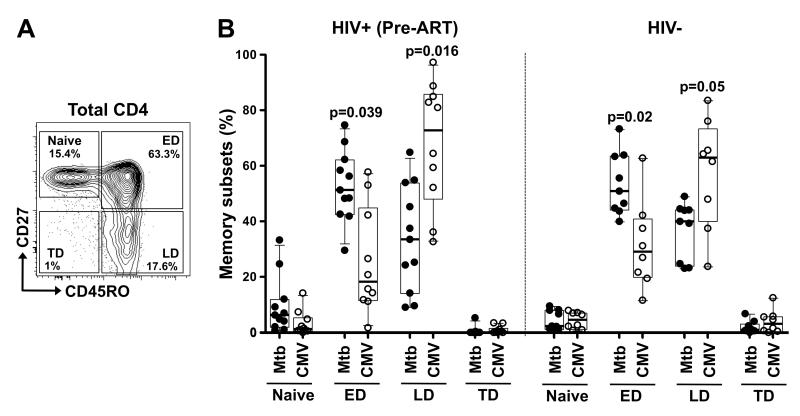
Depending on their memory differentiation profile, CD4+ T cells are endowed with distinct survival and proliferation capacities (30, 31). Thus, to investigate whether the cell maturation phenotype could impact the replenishment capacity of co-pathogen-specific CD4+ T cells, we first compared the differentiation profiles of Mtb- and CMV-specific CD4+ T cells at baseline, prior to ART initiation. Figure 4A shows a representative contour plot of distinct memory subsets in the CD4 compartment pre-ART in one HIV-infected individual. Based on the expression of CD45RO and CD27, we were able to discriminate four different memory subpopulations (32), namely: Naïve (CD45RO-CD27+), Early Differentiated (ED: CD45RO+CD27+, encompassing central and transitional memory subsets), Late Differentiated (LD: CD45RO+CD27−, encompassing effector memory cells) and Terminally Differentiated (TD: CD45RO-CD27−, or effector cells). In HIV-infected subjects, although Mtb- and CMV-specific CD4+ T cells were represented in both ED and LD subsets, they comprised significantly distinct profiles (Figure 4B, left panel). Mtb-specific cells exhibited primarily an early differentiated profile [median 51%, interquartile range (IQR) 42-62], whereas CMV-specific cells demonstrated a substantial enrichment of the late differentiated phenotype (median 73%, IQR 48-86), as previously described (33). Of note, ART did not markedly alter the overall memory differentiation profile of Mtb-specific or CMV-specific CD4+ T cells (Supplementary Figure S3). In addition, HIV-uninfected individuals exhibited similarly distinctive memory CD4 differentiation profiles for Mtb and CMV (Figure 4B, right panel).
FIGURE 4. Memory profiles of Mtb- and CMV-specific CD4+ T cells in HIV-infected and uninfected individuals.
(A) Representative example of total CD4 memory subset distribution in one HIV-infected individual. Naïve: CD45RO−CD27+, Early Differentiated (ED: CD45RO+CD27+), Late Differentiated (LD: CD45RO+CD27−) and Terminally Differentiated (TD: CD45RO-CD27−). The frequencies of each subset are indicated. (B) Memory profile of Mtb- and CMV-specific CD4+ T cells in HIV-infected individuals pre-ART (left panel) and HIV-uninfected individuals (right panel). Results are shown as box and whisker (10-90 percentile) plots. Each dot depicts an individual and the horizontal bar is the median. Statistical comparisons were performed using a one-way ANOVA non-parametric Kruskal-Wallis test. *p < 0.05, **p < 0.01, ***p < 0.001.
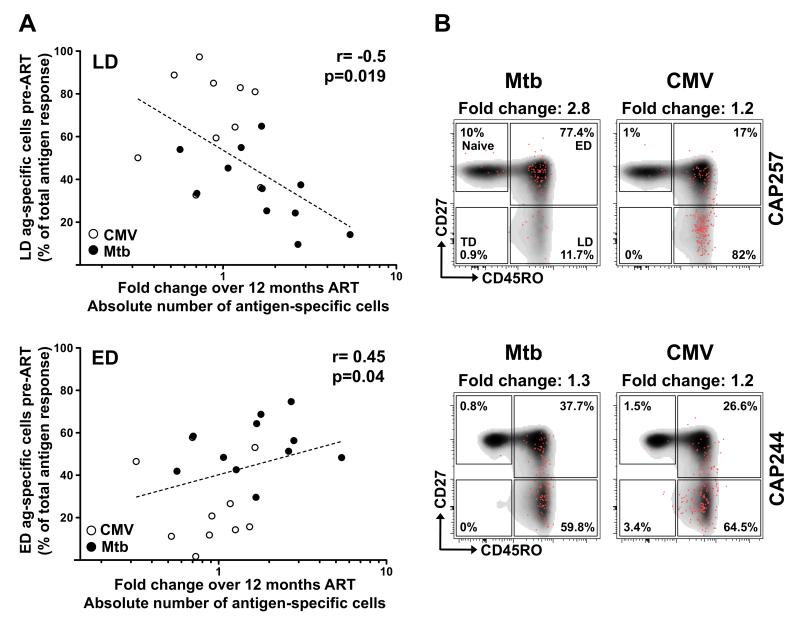
We next investigated the relationship between the memory phenotype of pathogen-specific CD4+ T cells at the time of ART initiation and the capacity of these cells to be maintained and/or replenished upon treatment. Figure 5A shows that the fold change in the absolute number of pathogen-specific CD4+ T cells over 12 months on ART inversely correlated with the proportion of cells exhibiting a late differentiated phenotype (p=0.019, r=−0.5). Consistent with this, a positive correlation was observed between the fold change in the absolute number of pathogen-specific CD4+ T cells and the proportion of these cells exhibiting an early differentiated phenotype (p=0.04, r=0.45). Of note, the proportion of late-differentiated Mtb-specific CD4+ T cell responses (in the absence of CMV responses) also correlated inversely with the fold change in the absolute number of Mtb-specific cells (r=−0.62, p=0.038, data not shown), and so too when ‘naïve-like’ Mtb-specific cells were grouped together with ED cells, we observed a trend towards a positive association with the fold change in the absolute number of Mtb-specific cells (r=0.54, p=0.05, data not shown). This relationship between the memory profile and the ART-induced repletion potential of pathogen-specific CD4+ T cells is further illustrated in Figure 5B, showing specific examples from two participants. In CAP257, where the pre-ART CMV-specific CD4 response showed an enrichment in cells with a late differentiated phenotype (82%), their ability to expand over 12 months on ART was considerably lower than Mtb-specific CD4 responses, characterized by an early differentiated phenotype (77.4%) (Fold change 1.2 for the CMV response vs. 2.8 for the Mtb response). In CAP244, where the memory profile of both Mtb and CMV-specific responses were similar (~60% of late differentiated cells), the fold change in the absolute number of CD4+ T cells to both pathogens was comparable.
FIGURE 5. Relationship between the memory profile of Mtb- and CMV-specific CD4+ T cells and the dynamics of restoration of these cells.
(A) Association between the proportion of antigen-specific CD4+ T cells exhibiting a late differentiated (LD; top panel) or early differentiated (ED; bottom panel) profile with the fold change in antigen-specific CD4+ T cell absolute count after ART. Statistical associations were performed by a two-tailed non-parametric Spearman rank correlation. (B) Representative examples of the memory profile of Mtb- or CMV-specific CD4+ T cells pre-ART in two individuals. The frequencies of each subset are indicated. For each example, the fold change in antigen-specific absolute CD4+ T cell count pre-and post-ART is indicated at the top of each plot.
Overall, these data reveal that the ability of co-pathogen-specific memory CD4+ T cells to expand upon ART appears to be related to their memory phenotype, where early differentiated cells have a higher replenishment capacity compared to late differentiated cells.
DISCUSSION
The introduction of ART has changed the clinical pattern of HIV infection significantly, with considerable reductions in morbidity and mortality. Whilst it is clearly established that ART leads to a progressive replenishment of the CD4+ T cell compartment in the majority of cases, the extent to which pre-existing co-pathogen-specific CD4+ T cells are restored, and the causes of variable restoration, are poorly defined. In this study, we investigated the dynamics of restoration of memory CD4+ T cells specific for a bacterial co-pathogen (Mtb) and a viral pathogen (CMV) in 15 HIV-infected individuals in response to ART. Our main finding was that the extent of reconstitution of pathogen-specific cells was related to their memory differentiation profile at the time of ART initiation, where cells exhibiting an early differentiation memory profile (including central memory and transitional memory cells) had a higher replenishment potential compared to late differentiated (effector memory) T cells.
Multiple mechanisms contribute to the increase in CD4+ T cells in blood in response to antiretroviral treatment for HIV (34). Over the first few months of ART, there is a redistribution of CD4+ T cells from the lymph nodes to the blood (35, 36), leading to a rapid initial rise in CD4+ counts. Moreover, homeostatic cell proliferation (37), decreased cell death (38) and increased thymic output (39, 40) also play a role in the replenishment of CD4+ T cells. While the CD4 absolute cell count at the time of treatment initiation is one of the main factors dictating the level to which CD4+ T cells are restored (21, 23), other parameters such as the activation level of T cells at the time of treatment (41), age (42, 43) or active co-infections (44, 45) also influence the degree of reconstitution of the CD4 compartment. Thus, CD4+ T cell recovery upon ART appears to be dependent on both the extent of immune damage at the time of treatment initiation and the regenerative capacities of these cells.
We demonstrate here that despite successful viral suppression and robust CD4 gains in the majority of participants, the dynamics of Mtb- and CMV-specific CD4+ T cell recovery were distinct. This shows that not all T cell subpopulations have the same potential to be replenished. Several factors and/or cellular attributes could account for these differences. Firstly, the regenerative capacities of pathogen-specific CD4+ T cells could be determined by their intrinsic ability to survive or proliferate, and it has been clearly established that CD4+ T cells exhibiting an early differentiated memory profile have an enhanced survival and proliferative potential compared to cells with a late differentiated phenotype (30, 31). Our data are in accordance with these observations, showing that the extent of replenishment of pathogen-specific CD4+ T cells correlates with their memory status, with superior recovery observed for antigen-specific CD4+ T cells exhibiting an early memory phenotype. Mechanistically, cell responsiveness to common γ-chain cytokines may account for the distinct renewal potential of these different cell subsets (46-48). In our study, the replenishment potential of Mtb- or CMV-specific CD4+ T cells did not associate with their ability to produce IL-2 (data not shown), however we have observed that responsiveness to IL-7 distinguishes ED and LD CD4+ subsets, with ED memory T cells being more responsive to exogenous IL-7 and IL-2 (as measured by Stat5 phosphorylation), compared to LD memory CD4+ T cells (Riou et al., unpublished data). Further analysis comparing the level of expression of cytokine receptors and cytokine-induced proliferation potential between Mtb- and CMV-specific CD4+ T cells would be needed to define the role of these signaling pathways in the restoration of immune memory cells upon ART.
Although Mtb responses displayed primarily an early differentiated phenotype, there were substantial inter-individual differences, ranging from 30% to 75% of the response. The CD4+ memory profile is dictated by numerous factors, including continual antigen exposure in high burden settings (49), previous TB, TB treatment and cure (50-52). A recent study carried out in a similar geographic setting as the present study noted dynamic changes in the T cell response to Mtb in the context of latent TB infection and HIV co-infection (53). It is worth noting that we also detected antigen-specific cells with a ‘naïve-like’ phenotype, previously described for Mtb and other infections (51, 54, 55). Although making up only a minor proportion of the total Mtb- and CMV-specific response, these cells expanded post ART in the majority of individuals compared to other memory subsets, that were characterized by more variable expansion and contraction. For CMV, this expansion led to a significantly greater frequency of this subset after ART. These cells may fall within the recently described memory stem cell subset, comprising memory cells endowed with a greater ability to proliferate and persist long-term compared to central memory (56, 57). Thus, the composition of the memory pool at the time of ART initiation may influence the restoration of antigen-specific CD4+ T cells.
To determine whether short-term ART affected not just quantitative but also qualitative aspects of co-pathogen immunity, we assessed the ability of cells to simultaneously secrete multiple cytokines, which for some infections, such as HIV and leishmaniasis, are a correlate of immune control or protection (58, 59). Although the significance of polyfunctional cells in TB is unclear (48), we demonstrated that over 50% of Mtb-specific CD4+ cells produced three or two cytokines simultaneously, a profile that did not significantly change after ART; moreover, this was similar to the profile from HIV-uninfected individuals. Our data are in apparent contrast to previous findings (18) showing that ART leads to an improved polyfunctional profile in Mtb-specific CD4+ T cell after short-term treatment, but this may be due to cohort differences, as the participants in that study were characterized by more advanced immunosuppression and higher viral loads than in the present study. Since it has been reported that the impairment of the functional capacity of Mtb-specific CD4+ T cells is related to HIV disease status (60), it is likely that differences in the severity of HIV pathology at the time of treatment initiation between the two studied groups could account for these discrepancies. The polyfunctional profile we observed for CMV-specific CD4+ cells is consistent with that previously reported (33), with simultaneous IFN-γ and TNF-α, and IFN-γ alone dominating the response. The CMV profile also did not change after ART, and in this case was less polyfunctional compared to HIV-infected individuals. Our study only examined paired samples from 12 months after ART, and it is possible that prolonged ART may restore CMV functional profiles (29).
It is important to note that although we demonstrate an expansion of Mtb-specific CD4+ T cells after ART, restoration of the magnitude and measures of functionality of the Mtb-specific CD4+ Th1 response determined by the production of three cytokines is a coarse measure of functional immunity to TB, and may not reflect protective immunity. Several additional CD4+ T cell subsets and cytokines contribute to TB immunity (61-64), and we have found an even greater diversity of CD4+ T cell subsets specific for Mtb (Riou et al., submitted for publication). An additional issue is that we do not know the extent of pathogen-specific CD4+ recovery in tissues upon ART, which appears defective for TB in the lungs even after prolonged viral suppression (20). These considerations may account for the persistently greater susceptibility of successfully treated HIV-infected individuals to TB (65).
Our study had several limitations. We were constrained by the availability of paired samples prior to and after ART, limiting us to studying 15 individuals in detail. Testing the generalizability of our findings in larger cohorts is warranted. Also, we focused our study on short-term restoration of pathogen-specific immunity (after 1 year of ART), and although study participants exhibited suppressed HIV replication, decreased immune activation and improved CD4 counts, studying responses after longer-term therapy may provide further important insights and more definitive conclusions on the restoration of pathogen-specific immunity. Furthermore, the participants we studied had nadir CD4 counts of >135 cells/mm3 (median 289 cells/mm3), and we did not have access to participants commencing treatment at lower CD4 counts (e.g. <50 cells/mm3). These highly immunocompromised patients would be the most relevant group to study with respect to susceptibility to CMV disease. In this context, although CMV-specific cells replenished more poorly than Mtb-specific cells after ART, they were present at a high frequency prior to ART initiation, that may well have been sufficient to provide protective immunity to CMV. Moreover, the extent of CD4 depletion at the time of ART initiation could impact the degree and dynamics of antigen-specific CD4+ T cell recovery. Lastly, we delineated memory subsets based on two markers, CD27 and CD45RO. These markers cannot differentiate between central memory and transitional memory cells within the CD27+CD45RO+ ED subset, and inclusion of a marker such as CCR7 would afford an additional degree of distinction that may reveal further differences in the ability of these memory subsets to expand after ART, and should be included in future studies of reconstitution of pathogen-specific immunity. Further research addressing these important limitations is needed to confirm and extend our findings.
Understanding the host and cellular factors that contribute to successful immune restoration on effective antiretroviral treatment for HIV, including both the overall CD4+ T cell compartment and co-pathogen-specific CD4 immunity, is of crucial importance, as only partial recovery of these cells could result in a persistently heightened risk of particular opportunistic infections. In this report, we showed that the renewal potential of pre-existing pathogen-specific CD4+ T cells was related to their memory differentiation profile. It will be important to determine whether this principle holds true in a wider context for other co-pathogens. Incomplete restoration of functional immunity and the persistence of elevated susceptibility to particular co-infections have implications for targeted interventions in the treated HIV-infected population. Overall, our findings underscore the complexity of immune reconstitution on ART, and the importance of preserving functional immunity with early ART (66).
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to all CAPRISA 002 study participants who are continuing to make an important personal contribution to HIV research. The scientific and supportive role of the whole CAPRISA 002 and CAPRISA 004 study and protocol team is gratefully acknowledged. We thank Mrs Kathryn Norman for administrative assistance, and Tracey Müller for managing the sample repository. We thank the two anonymous reviewers for their helpful comments to strengthen and improve the clarity of the manuscript. The following reagent was obtained through the AIDS Reagent Program, Division of AIDS, NIAID, NIH: HCMV pp65 peptide pool.
This work was funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH), the Office of the Director (OD) and the Department of Health and Human Services Grant R01 AI084387 (to W.A.B.), U19 A151794 (to S.A.K.), R21 AI115977 (to C.R.) and the South African Medical Research Council. The clinical trial from which some of the participants were drawn (CAPRISA 004) was supported by the United States Agency for International Development (USAID), FHI360 [USAID co-operative agreement #GPO-A-00-05-00022-00, contract #132119]. W.A.B. is supported by a Wellcome Trust Intermediate Fellowship in Public Health and Tropical Medicine (089832/Z/09/Z).
Footnotes
CONTRIBUTION:
Conceived and designed the experiments: C.R., N.J.G., L.W., Q.A.K., S.A.K. and W.A.B. Performed the experiments: R.F.T., A.P.S. and L.M. Analyzed the data: C.R. and R.F.T. Contributed reagents/materials/analysis tools: N.J.G., N.S., Q.A.K. and S.A.K. Wrote the paper: C.R., R.F.T. and W.A.B. All authors approved the final manuscript.
Disclosure: The authors have no conflict of interest.
REFERENCES
- 1.Moir S, Chun TW, Fauci AS. Pathogenic mechanisms of HIV disease. Annual review of pathology. 2011;6:223–248. doi: 10.1146/annurev-pathol-011110-130254. [DOI] [PubMed] [Google Scholar]
- 2.Okoye AA, Picker LJ. CD4(+) T-cell depletion in HIV infection: mechanisms of immunological failure. Immunological reviews. 2013;254:54–64. doi: 10.1111/imr.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doitsh G, Galloway NL, Geng X, Yang Z, Monroe KM, Zepeda O, Hunt PW, Hatano H, Sowinski S, Munoz-Arias I, Greene WC. Cell death by pyroptosis drives CD4 T-cell depletion in HIV-1 infection. Nature. 2014;505:509–514. doi: 10.1038/nature12940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shearer GM, Clerici M. Early T-helper cell defects in HIV infection. AIDS. 1991;5:245–253. doi: 10.1097/00002030-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Luciano AA, Lederman MM, Valentin-Torres A, Bazdar DA, Sieg SF. Impaired Induction of CD27 and CD28 Predicts Naive CD4 T Cell Proliferation Defects in HIV Disease. The Journal of Immunology. 2007;179:3543–3549. doi: 10.4049/jimmunol.179.6.3543. [DOI] [PubMed] [Google Scholar]
- 6.Morou A, Palmer BE, Kaufmann DE. Distinctive features of CD4+ T cell dysfunction in chronic viral infections. Current opinion in HIV and AIDS. 2014;9:446–451. doi: 10.1097/COH.0000000000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boswell KL, Paris R, Boritz E, Ambrozak D, Yamamoto T, Darko S, Wloka K, Wheatley A, Narpala S, McDermott A, Roederer M, Haubrich R, Connors M, Ake J, Douek DC, Kim J, Petrovas C, Koup RA. Loss of circulating CD4 T cells with B cell helper function during chronic HIV infection. PLoS Pathogens. 2014;10:e1003853. doi: 10.1371/journal.ppat.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saharia KK, Koup RA. T cell susceptibility to HIV influences outcome of opportunistic infections. Cell. 2013;155:505–514. doi: 10.1016/j.cell.2013.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sonnenberg P, Glynn JR, Fielding K, Murray J, Godfrey-Faussett P, Shearer S. How soon after infection with HIV does the risk of tuberculosis start to increase? A retrospective cohort study in South African gold miners. The Journal of Infectious Diseases. 2005;191:150–158. doi: 10.1086/426827. [DOI] [PubMed] [Google Scholar]
- 10.Geldmacher C, Zumla A, Hoelscher M. Interaction between HIV and Mycobacterium tuberculosis: HIV-1-induced CD4 T-cell depletion and the development of active tuberculosis. Current opinion in HIV and AIDS. 2012;7:268–275. doi: 10.1097/COH.0b013e3283524e32. [DOI] [PubMed] [Google Scholar]
- 11.Williams BG, Lima V, Gouws E. Modelling the impact of antiretroviral therapy on the epidemic of HIV. Current HIV research. 2011;9:367–382. doi: 10.2174/157016211798038533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guihot A, Bourgarit A, Carcelain G, Autran B. Immune reconstitution after a decade of combined antiretroviral therapies for human immunodeficiency virus. Trends in Immunology. 2011;32:131–137. doi: 10.1016/j.it.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV-1 infection. Blood. 2011;117:5582–5590. doi: 10.1182/blood-2010-12-322453. [DOI] [PubMed] [Google Scholar]
- 14.Schluger NW, Perez D, Liu YM. Reconstitution of immune responses to tuberculosis in patients with HIV infection who receive antiretroviral therapy. Chest. 2002;122:597–602. doi: 10.1378/chest.122.2.597. [DOI] [PubMed] [Google Scholar]
- 15.Keane NM, Price P, Lee S, Almeida CA, Stone SF, James I, French MA. Restoration of CD4 T-cell responses to cytomegalovirus is short-lived in severely immunodeficient HIV-infected patients responding to highly active antiretroviral therapy. HIV medicine. 2004;5:407–414. doi: 10.1111/j.1468-1293.2004.00245.x. [DOI] [PubMed] [Google Scholar]
- 16.Burgess K, Price P, James IR, Stone SF, Keane NM, Lim AY, Warmington JR, French MA. Interferon-gamma responses to Candida recover slowly or remain low in immunodeficient HIV patients responding to ART. Journal of Clinical Immunology. 2006;26:160–167. doi: 10.1007/s10875-006-9008-4. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson KA, Seldon R, Meintjes G, Rangaka MX, Hanekom WA, Maartens G, Wilkinson RJ. Dissection of regenerating T-Cell responses against tuberculosis in HIV-infected adults sensitized by Mycobacterium tuberculosis. American Journal of Respiratory and Critical Care Medicine. 2009;180:674–683. doi: 10.1164/rccm.200904-0568OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland JS, Young JM, Peterson KL, Sanneh B, Whittle HC, Rowland-Jones SL, Adegbola RA, Jaye A, Ota MO. Polyfunctional CD4(+) and CD8(+) T cell responses to tuberculosis antigens in HIV-1-infected patients before and after anti-retroviral treatment. Journal of Immunology. 2010;184:6537–6544. doi: 10.4049/jimmunol.1000399. [DOI] [PubMed] [Google Scholar]
- 19.Hsu DC, Kerr SJ, Iampornsin T, Pett SL, Avihingsanon A, Thongpaeng P, Zaunders JJ, Ubolyam S, Ananworanich J, Kelleher AD, Cooper DA. Restoration of CMV-specific-CD4 T cells with ART occurs early and is greater in those with more advanced immunodeficiency. PloS One. 2013;8:e77479. doi: 10.1371/journal.pone.0077479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jambo KC, Banda DH, Afran L, Kankwatira AM, Malamba RD, Allain TJ, Gordon SB, Heyderman RS, Russell DG, Mwandumba HC. Asymptomatic HIV-infected individuals on antiretroviral therapy exhibit impaired lung CD4(+) T-cell responses to mycobacteria. American Journal of Respiratory and Critical Care Medicine. 2014;190:938–947. doi: 10.1164/rccm.201405-0864OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, Cavassini M, Bernasconi E, Rickenbach M, Hirschel B, Battegay M, Swiss HIVCS. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clinical Infectious Diseases. 2005;41:361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 22.Aiuti F, Mezzaroma I. Failure to reconstitute CD4+ T-cells despite suppression of HIV replication under HAART. AIDS reviews. 2006;8:88–97. [PubMed] [Google Scholar]
- 23.Le T, Wright EJ, Smith DM, He W, Catano G, Okulicz JF, Young JA, Clark RA, Richman DD, Little SJ, Ahuja SK. Enhanced CD4+ T-cell recovery with earlier HIV-1 antiretroviral therapy. The New England Journal of Medicine. 2013;368:218–230. doi: 10.1056/NEJMoa1110187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mlisana K, Werner L, Garrett NJ, McKinnon LR, van Loggerenberg F, Passmore JA, Gray CM, Morris L, Williamson C, Abdool Karim SS, A. P. o. R. i. S. A. S. T. Centre for the Rapid disease progression in HIV-1 subtype C-infected South African women. Clinical Infectious Diseases. 2014;59:1322–1331. doi: 10.1093/cid/ciu573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Loggerenberg F, Mlisana K, Williamson C, Auld SC, Morris L, Gray CM, Abdool Karim Q, Grobler A, Barnabas N, Iriogbe I, Abdool Karim SS, C. A. I. S. Team Establishing a cohort at high risk of HIV infection in South Africa: challenges and experiences of the CAPRISA 002 acute infection study. PloS One. 2008;3:e1954. doi: 10.1371/journal.pone.0001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D, C. T. Group Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry. Part A: the journal of the International Society for Analytical Cytology. 2011;79:167–174. doi: 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mahnke YD, Greenwald JH, DerSimonian R, Roby G, Antonelli LR, Sher A, Roederer M, Sereti I. Selective expansion of polyfunctional pathogen-specific CD4(+) T cells in HIV-1-infected patients with immune reconstitution inflammatory syndrome. Blood. 2012;119:3105–3112. doi: 10.1182/blood-2011-09-380840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehr M, Cahenzli J, Haas A, Price DA, Gostick E, Huber M, Karrer U, Oxenius A. Emergence of polyfunctional CD8+ T cells after prolonged suppression of human immunodeficiency virus replication by antiretroviral therapy. Journal of Virology. 2008;82:3391–3404. doi: 10.1128/JVI.02383-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual Review of Immunology. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 31.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 32.Riou C, Treurnicht F, Abrahams MR, Mlisana K, Liu MK, Goonetilleke N, Koup R, Roederer M, Abdool Karim S, de Bruyn G, Williamson C, Gray CM, Burgers WA, C. S. Team Increased memory differentiation is associated with decreased polyfunctionality for HIV but not for cytomegalovirus-specific CD8+ T cells. Journal of Immunology. 2012;189:3838–3847. doi: 10.4049/jimmunol.1201488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Casazza JP, Betts MR, Price DA, Precopio ML, Ruff LE, Brenchley JM, Hill BJ, Roederer M, Douek DC, Koup RA. Acquisition of direct antiviral effector functions by CMV-specific CD4+ T lymphocytes with cellular maturation. The Journal of Experimental Medicine. 2006;203:2865–2877. doi: 10.1084/jem.20052246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson EM, Sereti I. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunological reviews. 2013;254:343–354. doi: 10.1111/imr.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bucy RP, Hockett RD, Derdeyn CA, Saag MS, Squires K, Sillers M, Mitsuyasu RT, Kilby JM. Initial increase in blood CD4(+) lymphocytes after HIV antiretroviral therapy reflects redistribution from lymphoid tissues. The Journal of Clinical Investigation. 1999;103:1391–1398. doi: 10.1172/JCI5863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pakker NG, Notermans DW, de Boer RJ, Roos MT, de Wolf F, Hill A, Leonard JM, Danner SA, Miedema F, Schellekens PT. Biphasic kinetics of peripheral blood T cells after triple combination therapy in HIV-1 infection: a composite of redistribution and proliferation. Nature Medicine. 1998;4:208–214. doi: 10.1038/nm0298-208. [DOI] [PubMed] [Google Scholar]
- 37.Marchetti G, Gori A, Casabianca A, Magnani M, Franzetti F, Clerici M, Perno CF, Monforte A, Galli M, Meroni L. Comparative analysis of T-cell turnover and homeostatic parameters in HIV-infected patients with discordant immune-virological responses to HAART. AIDS. 2006;20:1727–1736. doi: 10.1097/01.aids.0000242819.72839.db. [DOI] [PubMed] [Google Scholar]
- 38.Piconi S, Trabattoni D, Gori A, Parisotto S, Magni C, Meraviglia P, Bandera A, Capetti A, Rizzardini G, Clerici M. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS. 2010;24:1991–2000. doi: 10.1097/QAD.0b013e32833c93ce. [DOI] [PubMed] [Google Scholar]
- 39.Delgado J, Leal M, Ruiz-Mateos E, Martinez-Moya M, Rubio A, Merchante E, de la Rosa R, Sanchez-Quijano A, Lissen E. Evidence of thymic function in heavily antiretroviral-treated human immunodeficiency virus type 1-infected adults with long-term virologic treatment failure. The Journal of Infectious Diseases. 2002;186:410–414. doi: 10.1086/341561. [DOI] [PubMed] [Google Scholar]
- 40.Dion ML, Bordi R, Zeidan J, Asaad R, Boulassel MR, Routy JP, Lederman MM, Sekaly RP, Cheynier R. Slow disease progression and robust therapy-mediated CD4+ T-cell recovery are associated with efficient thymopoiesis during HIV-1 infection. Blood. 2007;109:2912–2920. doi: 10.1182/blood-2006-09-047308. [DOI] [PubMed] [Google Scholar]
- 41.Goicoechea M, Smith DM, Liu L, May S, Tenorio AR, Ignacio CC, Landay A, Haubrich R. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV-1 DNA. The Journal of Infectious Diseases. 2006;194:29–37. doi: 10.1086/504718. [DOI] [PubMed] [Google Scholar]
- 42.Kaufmann GR, Bloch M, Finlayson R, Zaunders J, Smith D, Cooper DA. The extent of HIV-1-related immunodeficiency and age predict the long-term CD4 T lymphocyte response to potent antiretroviral therapy. AIDS. 2002;16:359–367. doi: 10.1097/00002030-200202150-00007. [DOI] [PubMed] [Google Scholar]
- 43.Allers K, Bosel D, Epple HJ, Karcher H, Schmidt W, Kunkel D, Geelhaar-Karsch A, Schinnerling K, Moos V, Schneider T. Effect of age on the CD4(+) T-cell impairment in HIV-infected persons without and with cART. Journal of Acquired Immune Deficiency Syndromes. 2014;66:7–15. doi: 10.1097/QAI.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 44.Almeida M, Cordero M, Almeida J, Orfao A. Abnormal cytokine production by circulating monocytes and dendritic cells of myeloid origin in ART-treated HIV-1+ patients relates to CD4+ T-cell recovery and HCV co-infection. Current HIV research. 2007;5:325–336. doi: 10.2174/157016207780636524. [DOI] [PubMed] [Google Scholar]
- 45.Kaufmann GR, Elzi L, Weber R, Furrer H, Giulieri S, Vernazza P, Bernasconi E, Hirschel B, Battegay M, H. I. V. C. S. Swiss Interruptions of cART limits CD4 T-cell recovery and increases the risk for opportunistic complications and death. AIDS. 2011;25:441–451. doi: 10.1097/QAD.0b013e3283430013. [DOI] [PubMed] [Google Scholar]
- 46.Ahlers JD, Belyakov IM. Molecular pathways regulating CD4(+) T cell differentiation, anergy and memory with implications for vaccines. Trends in Molecular Medicine. 2010;16:478–491. doi: 10.1016/j.molmed.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Hodge JN, Srinivasula S, Hu Z, Read SW, Porter BO, Kim I, Mican JM, Paik C, Degrange P, Di Mascio M, Sereti I. Decreases in IL-7 levels during antiretroviral treatment of HIV infection suggest a primary mechanism of receptor-mediated clearance. Blood. 2011;118:3244–3253. doi: 10.1182/blood-2010-12-323600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Prezzemolo T,G, Guggino M, La Manna P, Di Liberto D, Dieli F, Caccamo N. Functional Signatures of Human CD4 and CD8 T Cell Responses to Mycobacterium tuberculosis. Frontiers in Immunology. 2014;5:180. doi: 10.3389/fimmu.2014.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schuetz A, Dirks J, Sester U, Haule A, Elias N, Geldmacher C, Sanga E, Maboko L, Reither K, Hoelscher M, Meyerhans A, Sester M. Pathogen prevalence may determine maintenance of antigen-specific T-cell responses in HIV-infected individuals. AIDS. 2012;26:695–700. doi: 10.1097/QAD.0b013e3283519a89. [DOI] [PubMed] [Google Scholar]
- 50.Adekambi T, Ibegbu CC, Kalokhe AS, Yu T, Ray SM, Rengarajan J. Distinct effector memory CD4+ T cell signatures in latent Mycobacterium tuberculosis infection, BCG vaccination and clinically resolved tuberculosis. PloS One. 2012;7:e36046. doi: 10.1371/journal.pone.0036046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marin ND, Paris SC, Rojas M, Garcia LF. Reduced frequency of memory T cells and increased Th17 responses in patients with active tuberculosis. Clinical and Vaccine Immunology. 2012;9:1667–1676. doi: 10.1128/CVI.00390-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tapaninen P, Korhonen A, Pusa L, Seppala I, Tuuminen T. Effector memory T-cells dominate immune responses in tuberculosis treatment: antigen or bacteria persistence? The International Journal of Tuberculosis and Lung Disease. 2010;14:347–355. [PubMed] [Google Scholar]
- 53.Mitchell JE, Chetty S, Govender P, Pillay M, Jaggernath M, Kasmar A, Ndung’u T, Klenerman P, Walker BD, Kasprowicz VO. Prospective monitoring reveals dynamic levels of T cell immunity to Mycobacterium tuberculosis in HIV infected individuals. PloS One. 2012;7:e37920. doi: 10.1371/journal.pone.0037920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Caccamo N, Meraviglia S, La Mendola C, Guggino G, Dieli F, Salerno A. Phenotypical and functional analysis of memory and effector human CD8 T cells specific for mycobacterial antigens. Journal of Immunology. 2006;177:1780–1785. doi: 10.4049/jimmunol.177.3.1780. [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee S, Clark CE, Lugli E, Roederer M, Nutman TB. Filarial Infection Modulates the Immune Response to Mycobacterium tuberculosis through Expansion of CD4+ IL-4 Memory T Cells. Journal of Immunology. 2015;194:2706–2714. doi: 10.4049/jimmunol.1402718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gattinoni L, Lugli E, Ji Y, Pos Z, Paulos CM, Quigley MF, Almeida JR, Gostick E, Yu Z, Carpenito C, Wang E, Douek DC, Price DA, June CH, Marincola FM, Roederer M, Restifo NP. A human memory T cell subset with stem cell-like properties. Nature Medicine. 2011;17:1290–1297. doi: 10.1038/nm.2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lugli E, Dominguez MH, Gattinoni L, Chattopadhyay PK, Bolton DL, Song K, Klatt NR, Brenchley JM, Vaccari M, Gostick E, Price DA, Waldmann TA, Restifo NP, Franchini G, Roederer M. Superior T memory stem cell persistence supports long-lived T cell memory. The Journal of Clinical Investigation. 2013;123:594–599. doi: 10.1172/JCI66327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, Lederman MM, Benito JM, Goepfert PA, Connors M, Roederer M, Koup RA. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood. 2006;107:4781–4789. doi: 10.1182/blood-2005-12-4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nature Medicine. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 60.Day CL, Mkhwanazi N, Reddy S, Mncube Z, van der Stok M, Klenerman P, Walker BD. Detection of polyfunctional Mycobacterium tuberculosis-specific T cells and association with viral load in HIV-1-infected persons. The Journal of Infectious Diseases. 2008;197:990–999. doi: 10.1086/529048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McBride A, Konowich J, Salgame P. Host defense and recruitment of Foxp3(+) T regulatory cells to the lungs in chronic Mycobacterium tuberculosis infection requires toll-like receptor 2. PLoS Pathogens. 2013;9:e1003397. doi: 10.1371/journal.ppat.1003397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Scriba TJ, Kalsdorf B, Abrahams DA, Isaacs F, Hofmeister J, Black G, Hassan HY, Wilkinson RJ, Walzl G, Gelderbloem SJ, Mahomed H, Hussey GD, Hanekom WA. Distinct, specific IL-17- and IL-22-producing CD4+ T cell subsets contribute to the human anti-mycobacterial immune response. Journal of Immunology. 2008;180:1962–1970. doi: 10.4049/jimmunol.180.3.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, Kaushal D, Reinhart TA, Randall TD, Khader SA. CXCR5+ T helper cells mediate protective immunity against tuberculosis. Journal of Clinical Investigation. 2013;123:712–726. doi: 10.1172/JCI65728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wozniak TM, Saunders BM, Ryan AA, Britton WJ. Mycobacterium bovis BCG-specific Th17 cells confer partial protection against Mycobacterium tuberculosis infection in the absence of gamma interferon. Infection and Immunity. 2010;78:4187–4194. doi: 10.1128/IAI.01392-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Girardi E, Sabin CA, d’Arminio Monforte A, Hogg B, Phillips AN, Gill MJ, Dabis F, Reiss P, Kirk O, Bernasconi E, Grabar S, Justice A, Staszewski S, Fatkenheuer G, Sterne JA, C. Antiretroviral Therapy Cohort Incidence of Tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clinical Infectious Diseases. 2005;41:1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 66.Danel C, Gabillard D, Le Carrou J, Anglaret X, Moh R, Eholie S, Ménan H, Badje A, Kouame G, Ntakpe JB. Early ART and IPT in HV-infected African adults with high CD4 count (Temprano trial); Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, USA. 2015; Abstract 115LB. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.