Abstract
The prevalence of child and adolescent obesity in the United States increased dramatically between 1970 and 2000, and there are few indications that the rates of childhood obesity are decreasing. Obesity is associated with myriad medical, psychological, and neurocognitive abnormalities that impact children’s health and quality of life. Genotypic variation is important in determining the susceptibility of individual children to undue gains in adiposity; however, the rapid increase in pediatric obesity prevalence suggests that changes to children’s environments and/or to their learned behaviors may dramatically affect body weight regulation. This paper presents an overview of the epidemiology, consequences, and etiopathogenesis of pediatric obesity, serving as a general introduction to the subsequent papers in this Special Issue that address aspects of childhood obesity and cognition in detail.
Keywords: Children, Adolescents, Hyperphagia, Overeating, Etiology
Introduction
Among children in the US, the percentage of children classified as obese, according to the U.S. Centers for Disease Control growth standards, has more than tripled since the 1970s (Ogden, Carroll, Curtin, Lamb, & Flegal, 2010; Ogden et al., 2006; Ogden, Carroll, Kit, & Flegal, 2014; Ogden, Flegal, Carroll, & Johnson, 2002). Obesity-related diseases rarely seen in children previously, including obesity-associated sleep apnea (Muzumdar & Rao, 2006), non-alcoholic fatty liver disease with resultant cirrhosis (Molleston, White, Teckman, & Fitzgerald, 2002), and type 2 diabetes (Dabelea et al., 2014; Pettitt et al., 2014), are increasingly diagnosed in children and adolescents. Because childhood onset obesity frequently persists into adulthood, it is also associated with increased long-term morbidity and mortality (Must, Jacques, Dallal, Bajema, & Dietz, 1992). For all these reasons, it is crucial to understand the causes and consequences of childhood obesity. This paper serves as a general introduction to the subsequent papers in this Special Issue that more specifically address childhood obesity and cognition by presenting an overview of the epidemiology, consequences, and etiopathogenesis of pediatric obesity.
Body mass index-based definitions and the epidemiology of pediatric obesity
“Obesity” is defined as the accumulation and storage of excess body fat, while “overweight” is weight in excess of a weight reference standard (Ogden & Flegal, 2010). Because there are no consensus criteria defining childhood obesity on the basis of excessive body adipose tissue, weight-based classification based on body mass index (BMI, kg/m2) has been routinely used for both epidemiological and clinical purposes.
The BMI typically decreases immediately after birth, increases through the first 6–8 months (WHO Multicentre Growth Reference Study Group, 2006), decreases until age 5–7 years, with corresponding decreases in percentage body fat (Garn & Clark, 1976) and then increases for the reminder of childhood up to adult levels. Because the distribution of BMI changes dramatically with age and differs by sex in children and adolescents, age- and sex-specific BMI percentiles rather than raw BMI values are used for BMI-based classification (Rolland-Cachera et al., 1982). The reference standards most commonly used in the United States for evaluating children’s BMI are the 2000 Centers for Disease Control and Prevention (CDC, 2000) growth charts that provide age- and sex-specific standards for ages 2 to 18 (Kuczmarski et al., 2000, 2002). These charts supply smoothed percentiles for BMI that were constructed using a modified LMS (lambda, mu, and sigma) estimation procedure (Kuczmarski et al., 2002) from data obtained in nationally representative U.S. surveys conducted between 1963 and 1980 (Kuczmarski et al., 2000). More recent data were not included because of the marked increases in BMI that were seen in subsequent U.S. surveys (Flegal, Ogden, Wei, Kuczmarski, & Johnson, 2001; Troiano & Flegal, 1998). Because of the paucity of data for children at the greatest BMIs in the data sets used, the top percentile defined by the CDC 2000 growth charts is the 97th percentile.
Before 2010, CDC 2000 growth charts for ages 2 to 18 years demarcated the 85th to 94.99th percentiles for BMI as “at risk for overweight” and ≥95th BMI percentile as “overweight” (Himes & Dietz, 1994). These cut points were subsequently renamed “overweight” for the 85th to 94.99th BMI percentiles and “obese” for ≥95th BMI percentile, to be consistent with recommendations by other groups (Barlow, 2007; Barlow & Dietz, 1998; Himes & Dietz, 1994; Koplan, Liverman, & Kraak, 2005; Krebs et al., 2007; Obesity: preventing and managing the global epidemic, report of a WHO consultation, 2000; Ogden & Flegal, 2010; Physical status: The use and interpretation of anthropometry, 1995; Koplan, Liverman, & Kraak, 2004).
There are limitations to the use of BMI-based standards to define obesity (Barlow & Dietz, 1998; Barlow, 2007; Flegal, Ogden et al., 2010; Himes & Dietz, 1994; Krebs et al., 2007) because BMI cannot discriminate between lean and fat mass (Wellens et al., 1996) and thus excess body fatness cannot be measured directly from weight and height (Himes & Dietz, 1994). However, there is a high correlation between fat mass and BMI among children (Field et al., 2003; Mei et al., 2002), and the majority of children with BMI ≥95th percentile have high adiposity (Freedman, Mei, Srinivasan, Berenson, & Dietz, 2007). Nevertheless, about 25% of U.S. children with BMI ≥95th percentile do not appear to have particularly high amounts of body fat (Flegal, Ogden et al., 2010); thus BMI is a first screening tool to identify children who may be overfat. BMI percentiles may be particularly inaccurate for children belonging to some racial and ethnic minorities (Flegal, Ogden et al., 2010).
An expert committee convened by the American Medical Association proposed recognition of the 99th BMI percentile as a cut point to classify children with severe obesity who are likely to be at increased risk for cardiovascular risk factors (Barlow, 2007). However, estimates of the cut points for the 99th percentile for age and sex are considered unstable. Extreme percentiles extrapolated from the CDC-supplied LMS parameters do not match well to the empirical data for the 99th percentile. A statistically defensible cut point for severe (sometimes called “extreme”) obesity based on available U.S. data is 120% of the smoothed 95th percentile (Flegal et al., 2009). Many investigators also include all adolescents with BMI ≥35 kg/m2 in the severe/extreme obesity group (Kelly et al., 2013; Koebnick et al., 2010). Children with extreme/severe obesity are at even higher risk for the complications of obesity detailed below (Kelly et al., 2013).
Over the past 50 years, global trends suggest that the prevalence of obesity among children (using BMI-based criteria) has increased significantly (Lobstein, 2010; Wang & Lobstein, 2006). Since the 1960s, prevalence rates have quadrupled in many countries (Lobstein, Baur, & Uauy, 2004). Based on the CDC 2000 BMI standards, among those ages 2–19 years, in 2012, 31.8% had BMI ≥85th percentile, 16.9% (approximately 12.7 million children) had BMI ≥95th percentile (Ogden et al., 2014) and in 2010, 12.3% had BMI ≥97th percentile (Ogden, Carroll, Kit, & Flegal, 2012). Certain racial and ethnic minority populations, especially African Americans, Hispanics, and American Indians, are at particular risk for obesity, while Asian children appear to have lower BMI-based risk of obesity (Flegal, Carroll, Ogden, & Curtin, 2010; Ogden et al., 2014; Spiegel & Alving, 2005). Although some recent data suggest obesity rates have stabilized in children and may even have decreased in those ages 2–5 years (Flegal, Carroll, Kit, & Ogden, 2012; Flegal, Carroll et al., 2010; Ogden et al., 2010, 2012, 2014; Yanovski & Yanovski, 2011), the obesity prevalence among children and adolescents remains alarmingly high.
Consequences of pediatric obesity
Pediatric overweight and obesity are of concern because of both immediate and later onset health consequences (Daniels, 2009). Children at the highest levels of BMI are usually at the greatest risk of obesity-associated adverse health outcomes (Koplan et al., 2004). Obesity in childhood is more likely to lead to adult obesity (Freedman et al., 2007) and to the tracking of poor health throughout adulthood; thus obesity appears to be a major contributor to many preventable causes of morbidity. The risk of adult obesity appears higher for older obese children, for those with more severe obesity, and for those with obese parents. (Whitaker, Wright, Pepe, Seidel, & Dietz, 1997). Some data suggest that those with extremely high BMI percentiles (significantly above the 97th percentile) are even more likely to have tracking of obesity into adulthood (Freedman et al., 2007). Although there is variation in the estimates among studies examining the question of persistence, it appears that approximately 40% of obese children become obese adults (Freedman et al., 2004; Must & Strauss, 1999; Power, Lake, & Cole, 1997). The appearance of obesity-associated conditions in childhood has been shown to lead to an earlier onset of related medical complications (Pavkov et al., 2006). Some (Jeffreys, McCarron, Gunnell, McEwen, & Smith, 2003; Must et al., 1992) but not all (Gray, Lee, Sesso, & Batty, 2011; Juonala et al., 2011) studies suggest that pediatric obesity itself has a unique impact on later health independent of adult weight; regardless, there is unanimity that pediatric obesity is a strong risk factor for adult obesity and its complications. The current U.S. childhood obesity epidemic thus has the potential to reverse the improvements in life-expectancy that occurred during the 20th century in the U.S. (Olshansky et al., 2005) and to cause more functional disability in those who survive to old age (Alley & Chang, 2007).
Cardiovascular disease
Obese and overweight youth are more likely to have cardiovascular risk factors resulting in cardiac structural and hemodynamic alterations (Freedman et al., 2007) including hypertension (Speiser et al., 2005), increases in ventricular mass (Daniels, 2009) endothelial dysfunction, with carotid artery intimal medial thickening, and early coronary and aortic fatty streaks and fibrous plaque (Freedman et al., 2004; Tounian et al., 2001), as well as atherosclerosis (Berenson et al., 1998; Daniels, 2009; McGill et al., 2002). Analyses have suggested, however, that there is little evidence that childhood BMI is an independent risk factor for adult cardiovascular risk once adult BMI is taken into consideration (Lloyd, Langley-Evans, & McMullen, 2010).
Dyslipidemia
Childhood obesity is associated with dyslipidemia, with the most common abnormality being elevated triglycerides and decreased high-density lipoprotein (HDL) cholesterol (Daniels, 2009). Elevated low-density lipoprotein (LDL)-cholesterol is also seen in obese children; however, the association between adiposity and LDL-cholesterol is weaker than that of adiposity with triglycerides and HDL-cholesterol (Daniels, 2011). BMI is also positively associated with likelihood for LDL particle size <25.5 nm (Shimabukuro, Sunagawa, & Ohta, 2004). Childhood dyslipidemia has been shown to persist and to be a predictor of adult dyslipidemia, adult carotid intimal media thickness, and other cardiovascular disease risks (Nadeau, Maahs, Daniels, & Eckel, 2011) (Lauer, Lee, & Clarke, 1988).
Impaired glucose homeostasis
Obesity is commonly accompanied by insulin resistance and hyperinsulinemia, which precede and play a major role in the development of type 2 diabetes mellitus (T2DM) (Shulman, 2000). In children, total body fat and visceral fat are positively associated with fasting insulin (Caprio et al., 1995; Freedman et al., 1987; Gutin et al., 1994), and impaired insulin sensitivity may worsen with duration of obesity (Le Stunff & Bougneres, 1994). Some data suggest as many as 21% of obese adolescents and 25% of obese children may have impaired glucose tolerance (Sinha et al., 2002), although most studies report much lower prevalence (Uwaifo, Elberg, & Yanovski, 2002). The increasing incidence of pediatric T2DM has paralleled the increasing prevalence of obesity. It has been estimated that more than 20% of all new cases of pediatric-onset diabetes among adolescents are now T2DM (Dabelea et al., 2014) and the overall prevalence of T2DM in children ages ≥10 years is 0.46 per 1000 children, a 30% increase since 2001 (Dabelea et al., 2014; Liese et al., 2006). Development of T2DM adds on higher risk for cardiovascular disease than obesity alone. Among Pima Indians, the onset of T2DM during childhood or adolescence has been associated with a markedly earlier age for development of end-stage renal disease and a significant increase in mortality rate before age 55 years (Pavkov et al., 2006).
Metabolic syndrome
The metabolic syndrome refers to the clustering of insulin resistance, hypertension, dyslipidemia, and obesity, and this condition has been associated with increased risk of cardiovascular disease and T2DM in adults. There is no consensus definition for the metabolic syndrome in pediatrics, but there are sets of criteria derived from the adult criteria that use percentile-based cut points for children (Daniels, 2009). Increasing BMI and insulin resistance during childhood are strong predictors of the metabolic syndrome (Shaibi & Goran, 2008; Srinivasan, Myers, & Berenson, 2002). In the NHANES 1999–2002 survey data, the overall prevalence of metabolic syndrome among U.S. adolescents ages 12 to 19 years ranged from 2.0% to 9.4% depending on the definition used, whereas among obese adolescents, the prevalence ranged from 12.4% to 44.2% (Cook, Auinger, Li, & Ford, 2008). However, the clinical utility of the metabolic syndrome in pediatrics remains unclear (Daniels, 2009). There is also quite limited stability for the diagnosis of metabolic syndrome among children and adolescents (Goodman, Daniels, Meigs, & Dolan, 2007; Goodman et al., 2009; Gustafson et al., 2009; Stanley, Chen, & Goodman, 2014). A detailed scientific statement appraising the evidence for utility of the metabolic syndrome in pediatrics is available from the American Heart Association (Steinberger et al., 2009).
Pulmonary comorbidities
It is estimated that up to 33% of obese children have obstructive sleep apnea (OSA) (Marcus et al., 1996; Tauman & Gozal, 2006; Wing et al., 2003). Among severely obese adolescents, 55% have polysomnographic findings consistent with OSA (Tauman & Gozal, 2006). Central hypoventilation syndrome also has been described in obese children (Tauman & Gozal, 2006). Pediatric studies have also documented an association between obesity and asthma (Jensen, Collins, Gibson, & Wood, 2011).
Gastrointestinal comorbidities
Gastroesophageal reflux, nonalcoholic fatty liver disease (NAFLD), cholelithiasis, and gallstones are increased among obese pediatric patients. A 13% prevalence of gastroesophageal reflux has been observed in obese children (Pashankar, Corbin, Shah, & Caprio, 2009). NAFLD has been shown to occur in 2.6–25% of obese children and adolescents from small epidemiological studies using indirect diagnosis tests such as liver enzymes or ultrasound (Socha et al., 2009), while an autopsy study reported a 9.6% prevalence in children ages 2–19 years and a higher prevalence among obese children (38%) (Schwimmer, Deutsch et al., 2003). NAFLD can potentially progress to nonalcoholic steatohepatitis (NASH) or to hepatic fibrosis and cirrhosis (Socha et al., 2009). NAFLD histology shows more fibrosis in children than adults (Schwimmer et al., 2006). Gallstones also have been shown to be more common in obese adolescents, with a higher prevalence observed in obese girls (Koebnick et al., 2012).
Orthopedic complications
A higher frequency of musculoskeletal discomfort and/or impairment of mobility (Taylor et al., 2006) and eased risk of fractures (Goulding, Grant, & Williams, 2005) has been documented in obese children and adolescents. Tibia vara (Blount’s disease) and slipped capital femoral epiphysis (SCFE) are the most common orthopedic problems in obese children (Daniels, 2009), resulting from mechanical stress on the developing skeletal system. Blount’s disease usually occurs in severely obese boys age 9 years or older and presents with bowing of the tibia and abnormal gait. SCFE presents with a waddling gait, limitation of hip movement, and/or pain in the hip or knee joints. SCFE occurs more commonly among obese African-American than non-African American males (Loder, Aronson, & Greenfield, 1993).
Psychosocial and neurocognitive issues
Obese children are more likely to have psychological distress including low self-esteem, higher rates of anxiety disorders, body image disturbance, and depressive symptoms (Hesketh, Wake, & Waters, 2004; Reeves, Postolache, & Snitker, 2008). There is also evidence suggesting increased risk for obesity among depressed youth (Richardson et al., 2003). Obese children and adolescents reported significantly lower health-related quality of life compared to their normal-weight peers, and they rated their health-related quality of life as low (Fallon et al., 2005), in some studies as low as that of children being treated for cancer (Schwimmer, Burwinkle, & Varni, 2003). Experiences of teasing and bullying have been shown to be higher among obese children and adolescents (Griffiths, Wolke, Page, & Horwood, 2006; Neumark-Sztainer et al., 2002; Young-Hyman et al., 2006). Weight-related teasing may result in unhealthy weight-control behaviors such as binge- or loss of control eating, which could cause further weight gain among overweight and obese youth (Tanofsky-Kraff et al., 2006; Tanofsky-Kraff, Yanovski et al., 2009).
With regard to neurocognitive function, many manuscripts have documented cross-sectional associations between body weight and neurocognitive dysfunction among children (Cserjesi, Molnar, Luminet, & Lenard, 2007; Kamijo et al., 2012, 2014; Li, Dai, Jackson, & Zhang, 2008; Verdejo-Garcia et al., 2010). A recent critical review of the literature found support for an inverse relationship between body weight/adiposity and executive functioning, attention, visuospatial performance, and motor skill (Liang, Matheson, Kaye, & Boutelle, 2014). Several of the genetic syndromes associated with obesity (reviewed below) are also associated with significant neurocognitive defects. It is possible that neurocognitive defects are etiologic factors causing or worsening obesity; it is also likely that at least some of the observed issues in obese children are the result of obesity and its medical complications. These concepts, as well as the possibility that specific macro- and micro-nutrients in the diet may change neurocognitive function in a fashion that worsens the predisposition toward obesity, are explored in some of the subsequent papers in this Special Issue.
Puberty
Obesity is associated with early onset of the larche (Crocker et al., 2014; Juul et al., 2006; Kaplowitz, Slora, Wasserman, Pedlow, & Herman-Giddens, 2001) and slightly earlier menarche among girls (Anderson, Dallal, & Must, 2003; de Ridder et al., 1992; Freedman et al., 2002; Garn & Haskell, 1959; Jaruratanasirikul, Mo-suwan, & Lebel, 1997; St George, Williams, & Silva, 1994; Wattigney, Srinivasan, Chen, Greenlund, & Berenson, 1999). In contrast, obese boys are more likely to have delayed onset of gonadarche (Crocker et al., 2014; Lee et al., 2010; Wang, 2002). Excess adipose tissue results in elevated estrogenic effects because of several factors, including greater aromatase expression in adipose tissue, low levels of sex hormone-binding globulin, and possibly other contributors such as high-fat diets (Jasik & Lustig, 2008).
Hyperandrogenism and polycystic ovary syndrome
Among girls, peripubertal obesity is associated with significant hyperandrogenism, which is particularly marked in the pre- and early pubertal period (McCartney et al., 2007). Elevated insulin levels are thought to be the mechanism leading to hyperandrogenism (McCartney et al., 2007). Excess adiposity may thus cause polycystic ovary syndrome and may be associated with anovulation resulting in irregular menses (oligomenorrhea or amenorrhea), elevated androgens with or without clinical hyperandrogenism (hirsutism, acne, and male-pattern hair loss), and cystic ovaries. In adults, 30% to 75% of women with polycystic ovary syndrome have obesity (Ehrmann, 2005).
Mortality
Some (Jeffreys et al., 2003; Must et al., 1992) but not all (Gray et al., 2011) studies have suggested that pediatric obesity has a unique impact on subsequent mortality. For example, in 1992, investigators (Must et al., 1992) reported that adolescent obesity was independently associated with an increased risk of mortality among 508 men in the Harvard Growth Study, but a subsequent analysis (Gray et al., 2011) found no evidence for an independent effect of high BMI in early adulthood on cardiovascular mortality risk in a study of 18,995 participants in the Harvard Alumni Study. Nevertheless, projection analysis by Olshansky and colleagues (2005) predicted a shorter life span for the current generation of U.S. children, largely because of obesity and all its related comorbidities.
Etiopathogenesis of pediatric obesity
Obesity is clearly an environmentally-induced disorder; our genetic endowments have changed minimally during the last 40 years, yet the prevalence of abnormally high BMI in US children has tripled – an observation that can only be explained by changes in external factors affecting children’s energy economy (Fig. 1). Obesity is also just as clearly a genetic disease, because all available data suggest that 60–80% of the observed variance in human body weight can be accounted for by inherited factors (Wardle, Carnell, Haworth, & Plomin, 2008). Finally, there is good evidence for gene × environment interactions for obesity risk alleles (Ahmad et al., 2011; Andreasen et al., 2008; Demerath et al., 2013; Garver et al., 2013; Qi et al., 2012; Rampersaud et al., 2008; Rosenquist et al., 2015). Thus, for obesity, “genetic background loads the gun, but the environment pulls the trigger” (Bray, 2004).
Fig. 1.
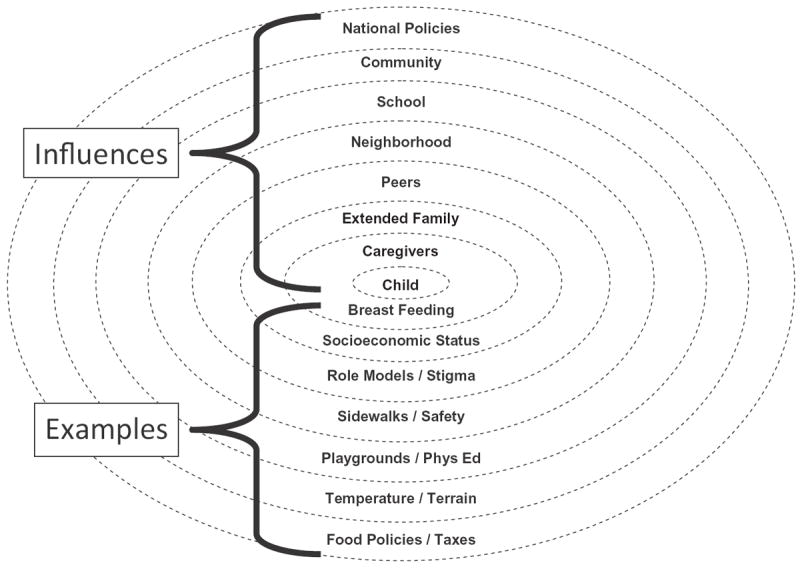
A Social–Ecological Model of Influences on pediatric obesity. Levels of environmental influence begin with the family environment and extend to larger spheres of influence and include peers, neighborhoods, schools, community, and national factors. Examples of influences within each of these spheres are also given. For example, neighborhood environment may influence children’s activity if there are no sidewalks or if safe areas for play are not available. (Figure courtesy of Denise E Wilfley, PhD, St. Louis, MO; adapted with permission.)
More than 300 genetic loci that are potentially involved in human body weight regulation have been identified through analyses in humans, rodents, and Caenorhabditis elegans (Ashrafi et al., 2003; Rankinen et al., 2006), and at least 32 have genome-wide significance (Speliotes et al., 2010). Some exceedingly rare gene variants affect gene function and behavior to such an extent that obesity results even without a particularly “obesogenic” environment, but the vast majority of genetic differences are presumed to affect body weight enough to cause obesity only in a permissive environment. Conditions known to influence body weight include:
Classical endocrine disorders associated with weight gain
Children with identifiable endocrinopathies are believed to comprise only a small minority of children referred for evaluation of overweight, on the order of 2–3% (Crino et al., 2003). Hypothyroidism is associated with a BMI increase in children of 1–2 BMI units (i.e., only a few kg) (Ning & Yanovski, 2006). Because children with hypothyroidism usually have diminished linear growth, BMI may be high even though weight does not exceed the 95th percentile (Abbassi, Rigterink, & Cancellieri, 1980). Growth hormone (GH) deficiency is also associated with diminished linear growth that is accompanied by continued increase in body weight. GH deficiency leads to increased fat mass, especially in a central distribution, along with decreased lean mass. Cushing’s Syndrome usually causes central obesity, although weight gain may be more global in children, and it is also associated with markedly diminished height velocity (Greening et al., 2006; Magiakou et al., 1994). Insulinomas are quite rare in children, with an incidence rate of ~4 per 50,000,000 per year. Elevated insulin production leads to increased food intake to counter lower blood sugars and therefore leads to obesity (Bonfig, Kann, Rothmund, & Schwarz, 2007; Dizon, Kowalyk, & Hoogwerf, 1999).
Structural disorders of the hypothalamus associated with weight gain
Hypothalamic obesity may arise after injury to, or congenital malformation of, the hypothalamus. For example, loss of function of the hypothalamic developmental factor Sim1 leads to obesity in humans (Holder, Butte, & Zinn, 2000; Hung et al., 2007). Obesity occurs in approximately 50% of children treated surgically for craniopharyngioma (Hoffman et al., 1992; Muller et al., 2001; Srinivasan et al., 2004).
Leptin signaling pathway genes
Rare inactivating mutations affecting genes in the leptin signaling pathway may account for as much as 3 or 4% of severe, early-onset obesity. Inactivating mutations affecting both alleles of the genes coding for leptin (Montague et al., 1997; Strobel, Issad, Camoin, Ozata, & Strosberg, 1998), the leptin receptor (Clement et al., 1998; Farooqi, Wangensteen et al., 2007), pro-opiomelanocortin (POMC) (Challis et al., 2002; Creemers et al., 2008; Farooqi et al., 2006; Krude et al., 1998, 2003; Lee et al., 2006), and enzymes that process POMC, such as prohormone convertase 1 (Farooqi, Volders et al., 2007; Jackson et al., 1997, 2003) have also been found in pediatric patients. Abnormalities causing inactivation in genes affecting leptin receptor signal transduction, such as SH2B1 are also associated with obesity and neurocognitive defects (Bochukova et al., 2010; Doche et al., 2012). Some data suggest that ciliopathies such as those causing Bardet Biedl Syndromes may disrupt leptin signaling (Feuillan et al., 2011; Seo et al., 2009). Homozygous and heterozygous inactivating mutations in the melanocortin 4 receptor (MC4R) cause obesity and hyperphagia during childhood (Farooqi et al., 2003) and are the most common known cause of severe, early onset obesity accounting for, in some series, as many as 3% of such children (Farooqi et al., 2000). Rare mutations in MRAP2, a protein essential for MC4R function, are also associated with pediatric obesity (Asai et al., 2013). Some data also support a role for polymorphisms in the MC3R for regulation of body weight, particularly in African American children (Feng et al., 2005; Savastano et al., 2009). Brain derived neurotrophic factor (BDNF) is believed to function downstream from MC4R in the leptin signaling pathway. Haploinsufficiency for BDNF has been suggested to be the cause of pediatric-onset obesity and contribute to neurocognitive problems in patients with WAGR syndrome (Han et al., 2008, 2013). A heterozygous inactivating mutation in the gene coding for the BDNF receptor has also been found to be associated with obesity, seizures, and developmental delay (Yeo et al., 2004).
Syndromic obesity
Many genetic syndromes (Table 1) involve obesity as part of their presentation but only a small fraction of obese children have one of these etiologies. Importantly, developmental delay or neurocognitive defects are reported in the majority of these syndromes. It is possible that some individuals with non-syndromic obesity have less-damaging mutations in one or more of these genes that may in part account for the greater incidence of developmental delay among obese individuals.
Table 1.
Genetic syndromes commonly associated with obesity or overgrowth.
| Genetic syndrome | Gene(s)/chromosomal location(s) | Associated with neurocognitive deficits? |
|---|---|---|
| Achondroplasia | FGFR3/4p16.3 | No |
| Alström Syndrome | ALMS1/2p13.1 | Yes |
| Bardet Biedl Syndromes | Multiple/Multiple | Yes |
| Beckwith–Wiedemann Syndrome | CDKN1C, KCNQ1OT1/H19,/11p15, 11p14 | Rarely |
| Borjeson–Forssman–Lehmann Syndrome | PHF6/Xq26.2 | Yes |
| Carpenter syndrome | RAB23/6p11.2 | Yes |
| Congenital disorder of glycosylation 1a | PMM2/16p13.2 | Yes |
| Cohen Syndrome | COH1 (VPS13B)/8q22.2 | Yes |
| Cowden syndrome | PTEN/10q23.31 | Yes |
| Fragile X | FMR1/Xq27.3 | Yes |
| MEHMO Syndrome | Xp22.13-p21.1 | Yes |
| Meningomyelocele | Multiple/Multiple | Variable |
| MORM Syndrome | INPP5E/9q34.3 | Yes |
| Prader Willi Syndrome | SNRPN, NDN/15q11.2 | Yes |
| Pseudohypoparathyroidism 1a | GNAS/20q13.32 | Yes |
| Simpson–Golabi–Behmel Syndrome | GPC3/Xq26.2 | Variable |
| Smith–Magenis Syndrome | RAI1/17p11.2 | Yes |
| Sotos Syndrome 1 | NSD1/5q35.2-q35.3 | Yes |
| Sotos Syndrome 2 | NFIX/19p13.2 | Yes |
| Turner Syndrome | Multiple/X | Variable |
| Ulnar–mammary Schinzel Syndrome | TBX3/12q24.21 | Yes |
| WAGR Syndrome with obesity | BDNF/11p14.1 | Yes |
| Weaver syndrome | EZH2/7q36 | Yes |
| Wilson–Turner Syndrome | HDAC8/Xq13.1 | Yes |
Common allelic variation in genes that may affect energy balance
Single Nucleotide Polymorphisms (SNPs) and copy number variation in many genes and chromosomal regions have been found to be associated with body weight or body composition (Meyre et al., 2009; Scherag et al., 2010; Speliotes et al., 2010; Thorleifsson et al., 2009; Wheeler et al., 2013; Willer et al., 2009). The mechanisms explaining how identified gene regions might change energy balance are often not fully understood. The most widely replicated finding is linkage of the FTO gene locus with body weight (Dina et al., 2007; Frayling et al., 2007; Hinney et al., 2007; Hunt et al., 2008; Scuteri et al., 2007). FTO mRNA is highly expressed in brain areas important for regulation of energy- and reward-driven consumption (Fredriksson et al., 2008). Some limited data also suggest that children with less common alleles in FTO may have greater food intake (Cecil, Tavendale, Watt, Hetherington, & Palmer, 2008; Wardle, Llewellyn, Sanderson, & Plomin, 2008), reduced satiety (Wardle, Carnell, Haworth, Farooqi et al., 2008) and greater prevalence of loss of control over their eating (Tanofsky-Kraff, Han et al., 2009). It remains unclear whether the protein coded by FTO itself (Church et al., 2010) or other genes regulated by the FTO locus (Smemo et al., 2014; Stratigopoulos et al., 2008) account for these associations.
Pre-conception, maternal, and intrauterine factors
Beyond straightforward genetic links between parent and child body weight, there is evidence that the maternal environment can have great consequences for subsequent risks for both obesity and its metabolic complications (Ludwig & Currie, 2010; Whitaker & Dietz, 1998). For example, high pre-pregnancy maternal weight and pregnancy weight gain are risk factors for infants to be born large for their gestational age (Whitaker & Dietz, 1998; Yu et al., 2013) and for subsequent development of metabolic abnormalities including impaired glucose homeostasis. Maternal diabetes also has an unquestioned role in predisposing infants to large size and subsequent type 2 diabetes (Dabelea et al., 2000, 2008; Gillman, Rifas-Shiman, Berkey, Field, & Colditz, 2003). Inadequate intrauterine growth also appears associated with childhood obesity, type 2 diabetes, and coronary heart disease (Barker, 2007; Hales & Barker, 1992). The notion that the intrauterine environment may be of paramount importance for subsequent child health is sometimes referred to as the “Developmental Origins of Health and Disease” model, but is also known as the Barker Hypothesis, after David Barker, who reinvigorated this area of research and carried out many studies documenting that fetal re-programming by the intrauterine environment can have lasting impact on children’s health (Bateson et al., 2004).
Acquired obesity
Many medications may lead to weight gain, including insulin secretagogues, glucocorticoids, antipsychotics, mood stabilizers, antidepressants, anticonvulsants, antihypertensives, antihistamines, and chemotherapeutic agents (Maayan & Correll, 2011; Malone, 2005). An avian form of adenovirus, AD36, has been found to cause increased adiposity in infected chickens (Dhurandhar, Kulkarni, Ajinkya, & Sherikar, 1992) and some studies suggest that humans with antibodies to AD36 (indicating past infection) also tend to have higher rates of obesity (Atkinson et al., 2005; Dhurandhar, Kulkarni, Ajinkya, Sherikar, & Atkinson, 1997).
As outlined in Fig. 1, and as suggested by the recent increases in the prevalence of obesity, the sociocultural and ecological environment plays a major role in determining who becomes obese (Hawkins, Cole, & Law, 2009; Keith et al., 2006; Lang & Rayner, 2007). Arizona Pima Indians who live on a reservation have much higher rates of obesity and diabetes than their genetically-related counterparts in an isolated Mexican village (Ravussin, Valencia, Esparza, Bennett, & Schulz, 1994). Asian and Hispanic adolescents born in the United States have a higher prevalence of obesity than immigrant members of the same community (Popkin & Udry, 1998). Finally, the differential response of some people to environmental conditions that lead to obesity may be the result of epigenetic changes – alterations in gene expression related to disease risk that are modified by the environment during development (Almen et al., 2014; van Dijk, Molloy, Varinli, Morrison, & Muhlhausler, 2015). Some of the papers included in this Special Issue will address mechanisms through which the environment may predispose susceptible individuals to develop obesity.
Conclusion
Pediatric obesity is a complex disorder, with myriad contributing environmental, biopsychosocial, genetic, and epigenetic factors. It causes significant medical, psychosocial, and neurocognitive abnormalities during childhood. The persistence of obesity into adulthood makes understanding the causes of excessive adiposity during childhood of paramount importance to prevent its impact on long-term health and quality of life.
The subsequent papers in this Special Issue discuss one of the most interesting proposed mechanisms for the establishment and maintenance of pediatric obesity; these manuscripts examine the potential effects of obesity and diet composition on brain substrates for cognition, elucidate the cognitive processes influenced by childhood weight and nutritional status, and provide data from intervention studies aimed at improving cognition to affect body weight in children. It is hoped that this Special Issue will promote future research in this area that will ultimately aid efforts to prevent and treat pediatric obesity.
Footnotes
Funding: NIH Intramural Research Program Grant 1ZIAHD000641 (J. Yanovski) from the National Institute of Child Health and Human Development with supplemental funding from the Office of Behavioral and Social Sciences Research. Disclaimers: The opinions and assertions expressed herein are those of the author and are not to be construed as reflecting the views of the DHHS or the U.S. Public Health Service.
References
- Abbassi V, Rigterink E, Cancellieri RP. Clinical recognition of juvenile hypothyroidism in the early stage. Clinical Pediatrics. 1980;19:782–786. doi: 10.1177/000992288001901201. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Lee IM, Pare G, Chasman DI, Rose L, Ridker PM, et al. Lifestyle interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes Care. 2011;34:675–680. doi: 10.2337/dc10-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alley DE, Chang VW. The changing relationship of obesity and disability, 1988–2004. JAMA: The Journal of the American Medical Association. 2007;298:2020–2027. doi: 10.1001/jama.298.17.2020. [DOI] [PubMed] [Google Scholar]
- Almen MS, Nilsson EK, Jacobsson JA, Kalnina I, Klovins J, Fredriksson R, et al. Genome-wide analysis reveals DNA methylation markers that vary with both age and obesity. Gene. 2014;548:61–67. doi: 10.1016/j.gene.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Anderson SE, Dallal GE, Must A. Relative weight and race influence average age at menarche. Results from two nationally representative surveys of US girls studied 25 years apart. Pediatrics. 2003;111:844–850. doi: 10.1542/peds.111.4.844. [DOI] [PubMed] [Google Scholar]
- Andreasen CH, Stender-Petersen KL, Mogensen MS, Torekov SS, Wegner L, Andersen G, et al. Low physical activity accentuates the effect of the FTO rs9939609 polymorphism on body fat accumulation. Diabetes. 2008;57:95–101. doi: 10.2337/db07-0910. [DOI] [PubMed] [Google Scholar]
- Asai M, Ramachandrappa S, Joachim M, Shen Y, Zhang R, Nuthalapati N, et al. Loss of function of the melanocortin 2 receptor accessory protein 2 is associated with mammalian obesity. Science. 2013;341:275–278. doi: 10.1126/science.1233000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi K, Chang FY, Watts JL, Fraser AG, Kamath RS, Ahringer J, et al. Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421:268–272. doi: 10.1038/nature01279. [DOI] [PubMed] [Google Scholar]
- Atkinson RL, Dhurandhar NV, Allison DB, Bowen RL, Israel BA, Albu JB, et al. Human adenovirus-36 is associated with increased body weight and paradoxical reduction of serum lipids. International Journal of Obesity (2005) 2005;29:281–286. doi: 10.1038/sj.ijo.0802830. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The origins of the developmental origins theory. Journal of Internal Medicine. 2007;261:412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- Barlow SE. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity. Summary report. Pediatrics. 2007;120(Suppl. 4):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- Barlow SE, Dietz WH. Obesity evaluation and treatment. Expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics. 1998;102:E29. doi: 10.1542/peds.102.3.e29. [DOI] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D’Udine B, Foley RA, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Berenson GS, Srinivasan SR, Bao W, Newman WP, 3rd, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. The New England Journal of Medicine. 1998;338:1650–1656. doi: 10.1056/NEJM199806043382302. [DOI] [PubMed] [Google Scholar]
- Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, et al. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–670. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfig W, Kann P, Rothmund M, Schwarz HP. Recurrent hypoglycemic seizures and obesity. Delayed diagnosis of an insulinoma in a 15 year-old boy. Final diagnostic localization with endosonography. Journal of Pediatric Endocrinology and Metabolism. 2007;20:1035–1038. doi: 10.1515/jpem.2007.20.9.1035. [DOI] [PubMed] [Google Scholar]
- Bray GA. The epidemic of obesity and changes in food intake. The Fluoride Hypothesis. Physiology and Behavior. 2004;82:115–121. doi: 10.1016/j.physbeh.2004.04.033. [DOI] [PubMed] [Google Scholar]
- Caprio S, Hyman L, Limb C, McCarthy S, Lange R, Sherwin R, et al. Central adiposity and its metabolic correlates in obese adolescent girls. American Journal of Physiology Endocrinology and Metabolism. 1995;269:E118–E126. doi: 10.1152/ajpendo.1995.269.1.E118. [DOI] [PubMed] [Google Scholar]
- Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. The New England Journal of Medicine. 2008;359:2558–2566. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- Challis BG, Pritchard LE, Creemers JW, Delplanque J, Keogh JM, Luan J, et al. A missense mutation disrupting a dibasic prohormone processing site in pro-opiomelanocortin (POMC) increases susceptibility to early-onset obesity through a novel molecular mechanism. Human Molecular Genetics. 2002;11:1997–2004. doi: 10.1093/hmg/11.17.1997. [DOI] [PubMed] [Google Scholar]
- Church C, Moir L, McMurray F, Girard C, Banks GT, Teboul L, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nature Genetics. 2010;42:1086–1092. doi: 10.1038/ng.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clement K, Vaisse C, Lahlou N, Cabrol S, Pelloux V, Cassuto D, et al. A mutation in the human leptin receptor gene causes obesity and pituitary dysfunction. Nature. 1998;392:398–401. doi: 10.1038/32911. [DOI] [PubMed] [Google Scholar]
- Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. The Journal of Pediatrics. 2008;152:165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Creemers JW, Lee YS, Oliver RL, Bahceci M, Tuzcu A, Gokalp D, et al. Mutations in the amino-terminal region of proopiomelanocortin (POMC) in patients with early-onset obesity impair POMC sorting to the regulated secretory pathway. The Journal of Clinical Endocrinology and Metabolism. 2008;93:4494–4499. doi: 10.1210/jc.2008-0954. [DOI] [PubMed] [Google Scholar]
- Crino A, Greggio NA, Beccaria L, Schiaffini R, Pietrobelli A, Maffeis C. Diagnosis and differential diagnosis of obesity in childhood. Minerva Pediatrica. 2003;55:461–470. [PubMed] [Google Scholar]
- Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, et al. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. The Journal of Clinical Endocrinology and Metabolism. 2014;99:E1519–E1529. doi: 10.1210/jc.2014-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserjesi R, Molnar D, Luminet O, Lenard L. Is there any relationship between obesity and mental flexibility in children? Appetite. 2007;49:675–678. doi: 10.1016/j.appet.2007.04.001. [DOI] [PubMed] [Google Scholar]
- de Ridder CM, Thijssen JH, Bruning PF, Van den Brande JL, Zonderland ML, Erich WB. Body fat mass, body fat distribution, and pubertal development. A longitudinal study of physical and hormonal sexual maturation of girls. The Journal of Clinical Endocrinology and Metabolism. 1992;75:442–446. doi: 10.1210/jcem.75.2.1639945. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Hanson RL, Lindsay RS, Pettitt DJ, Imperatore G, Gabir MM, et al. Intrauterine exposure to diabetes conveys risks for type 2 diabetes and obesity. A study of discordant sibships. Diabetes. 2000;49:2208–2211. doi: 10.2337/diabetes.49.12.2208. [DOI] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis EJ, Lamichhane AP, D’Agostino RB, Jr, Liese AD, Vehik KS, et al. Association of intrauterine exposure to maternal diabetes and obesity with type 2 diabetes in youth. The SEARCH Case-Control Study. Diabetes Care. 2008;31:1422–1426. doi: 10.2337/dc07-2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, et al. Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA: The Journal of the American Medical Association. 2014;311:1778–1786. doi: 10.1001/jama.2014.3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels SR. Complications of obesity in children and adolescents. International Journal of Obesity (2005) 2009;33(Suppl. 1):S60–S65. doi: 10.1038/ijo.2009.20. [DOI] [PubMed] [Google Scholar]
- Daniels SR. Lipid concentrations in children and adolescents. It is not all about obesity. The American Journal of Clinical Nutrition. 2011;94:699–700. doi: 10.3945/ajcn.111.022483. [DOI] [PubMed] [Google Scholar]
- Demerath EW, Choh AC, Johnson W, Curran JE, Lee M, Bellis C, et al. The positive association of obesity variants with adulthood adiposity strengthens over an 80-year period. A gene-by-birth year interaction. Human Heredity. 2013;75:175–185. doi: 10.1159/000351742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhurandhar NV, Kulkarni P, Ajinkya SM, Sherikar A. Effect of adenovirus infection on adiposity in chicken. Veterinary Microbiology. 1992;31:101–107. doi: 10.1016/0378-1135(92)90068-5. [DOI] [PubMed] [Google Scholar]
- Dhurandhar NV, Kulkarni PR, Ajinkya SM, Sherikar AA, Atkinson RL. Association of adenovirus infection with human obesity. Obesity Research. 1997;5:464–469. doi: 10.1002/j.1550-8528.1997.tb00672.x. [DOI] [PubMed] [Google Scholar]
- Dina C, Meyre D, Gallina S, Durand E, Korner A, Jacobson P, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nature Genetics. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- Dizon AM, Kowalyk S, Hoogwerf BJ. Neuroglycopenic and other symptoms in patients with insulinomas. The American Journal of Medicine. 1999;106:307–310. doi: 10.1016/s0002-9343(99)00021-2. [DOI] [PubMed] [Google Scholar]
- Doche ME, Bochukova EG, Su HW, Pearce LR, Keogh JM, Henning E, et al. Human SH2B1 mutations are associated with maladaptive behaviors and obesity. The Journal of Clinical Investigation. 2012;122:4732–4736. doi: 10.1172/JCI62696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrmann DA. Polycystic ovary syndrome. The New England Journal of Medicine. 2005;352:1223–1236. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- Fallon EM, Tanofsky-Kraff M, Norman AC, McDuffie JR, Taylor ED, Cohen ML, et al. Health-related quality of life in overweight and nonoverweight black and white adolescents. The Journal of Pediatrics. 2005;147:443–450. doi: 10.1016/j.jpeds.2005.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Drop S, Clements A, Keogh JM, Biernacka J, Lowenbein S, et al. Heterozygosity for a POMC-null mutation and increased obesity risk in humans. Diabetes. 2006;55:2549–2553. doi: 10.2337/db06-0214. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O’Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. The New England Journal of Medicine. 2003;348:1085–1095. doi: 10.1056/NEJMoa022050. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Volders K, Stanhope R, Heuschkel R, White A, Lank E, et al. Hyperphagia and early-onset obesity due to a novel homozygous missense mutation in prohormone convertase 1/3. The Journal of Clinical Endocrinology and Metabolism. 2007;92:3369–3373. doi: 10.1210/jc.2007-0687. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Wangensteen T, Collins S, Kimber W, Matarese G, Keogh JM, et al. Clinical and molecular genetic spectrum of congenital deficiency of the leptin receptor. The New England Journal of Medicine. 2007;356:237–247. doi: 10.1056/NEJMoa063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqi IS, Yeo GS, Keogh JM, Aminian S, Jebb SA, Butler G, et al. Dominant and recessive inheritance of morbid obesity associated with melanocortin 4 receptor deficiency. The Journal of Clinical Investigation. 2000;106:271–279. doi: 10.1172/JCI9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng N, Young SF, Aguilera G, Puricelli E, Adler-Wailes DC, Sebring NG, et al. Co-occurrence of two partially inactivating polymorphisms of MC3R is associated with pediatric-onset obesity. Diabetes. 2005;54:2663–2667. doi: 10.2337/diabetes.54.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillan PP, Ng D, Han JC, Sapp JC, Wetsch K, Spaulding E, et al. Patients with Bardet-Biedl syndrome have hyperleptinemia suggestive of leptin resistance. The Journal of Clinical Endocrinology and Metabolism. 2011;96:E528–E535. doi: 10.1210/jc.2010-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field AE, Laird N, Steinberg E, Fallon E, Semega-Janneh M, Yanovski JA. Which metric of relative weight best captures body fatness in children? Obesity Research. 2003;11:1345–1352. doi: 10.1038/oby.2003.182. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US Adults, 1999–2010. JAMA: The Journal of the American Medical Association. 2012;307:491–497. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA: The Journal of the American Medical Association. 2010;303:235–241. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Ogden CL, Wei R, Kuczmarski RL, Johnson CL. Prevalence of overweight in US children. Comparison of US growth charts from the Centers for Disease Control and Prevention with other reference values for body mass index. The American Journal of Clinical Nutrition. 2001;73:1086–1093. doi: 10.1093/ajcn/73.6.1086. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Ogden CL, Yanovski JA, Freedman DS, Shepherd JA, Graubard BI, et al. High adiposity and high body mass index-for-age in US children and adolescents overall and by race-ethnic group. The American Journal of Clinical Nutrition. 2010;91:1020–1026. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegal KM, Wei R, Ogden CL, Freedman DS, Johnson CL, Curtin LR. Characterizing extreme values of body mass index-for-age by using the 2000 Centers for Disease Control and Prevention growth charts. The American Journal of Clinical Nutrition. 2009;90:1314–1320. doi: 10.3945/ajcn.2009.28335. [DOI] [PubMed] [Google Scholar]
- Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredriksson R, Hagglund M, Olszewski PK, Stephansson O, Jacobsson JA, Olszewska AM, et al. The obesity gene, FTO, is of ancient origin, up-regulated during food deprivation and expressed in neurons of feeding-related nuclei of the brain. Endocrinology. 2008;149:2062–2071. doi: 10.1210/en.2007-1457. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Dietz WH, Tang R, Mensah GA, Bond MG, Urbina EM, et al. The relation of obesity throughout life to carotid intima-media thickness in adulthood. The Bogalusa Heart Study. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2004;28:159–166. doi: 10.1038/sj.ijo.0802515. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Relation of age at menarche to race, time period, and anthropometric dimensions. The Bogalusa Heart Study. Pediatrics. 2002;110:e43. doi: 10.1542/peds.110.4.e43. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Inter-relationships among childhood BMI, childhood height, and adult obesity. The Bogalusa Heart Study. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2004;28:10–16. doi: 10.1038/sj.ijo.0802544. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents. The Bogalusa Heart Study. The Journal of Pediatrics. 2007;150:12–17.e12. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Freedman DS, Srinivasan SR, Burke GL, Shear CL, Smoak CG, Harsha DW, et al. Relation of body fat distribution to hyperinsulinemia in children and adolescents. The Bogalusa Heart Study. American Journal of Clinical Nutrition. 1987;46:403–410. doi: 10.1093/ajcn/46.3.403. [DOI] [PubMed] [Google Scholar]
- Garn SM, Clark DC. Trends in fatness and the origins of obesity Ad Hoc Committee to Review the Ten-State Nutrition Survey. Pediatrics. 1976;57:443–456. [PubMed] [Google Scholar]
- Garn SM, Haskell JA. Fat and growth during childhood. Science. 1959;130:1711–1712. doi: 10.1126/science.130.3390.1711. [DOI] [PubMed] [Google Scholar]
- Garver WS, Newman SB, Gonzales-Pacheco DM, Castillo JJ, Jelinek D, Heidenreich RA, et al. The genetics of childhood obesity and interaction with dietary macronutrients. Genes & Nutrition. 2013;8:271–287. doi: 10.1007/s12263-013-0339-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman MW, Rifas-Shiman S, Berkey CS, Field AE, Colditz GA. Maternal gestational diabetes, birth weight, and adolescent obesity. Pediatrics. 2003;111:e221–e226. doi: 10.1542/peds.111.3.e221. [DOI] [PubMed] [Google Scholar]
- Goodman E, Daniels SR, Meigs JB, Dolan LM. Instability in the diagnosis of metabolic syndrome in adolescents. Circulation. 2007;115:2316–2322. doi: 10.1161/CIRCULATIONAHA.106.669994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman E, Li C, Tu YK, Ford E, Sun SS, Huang TT. Stability of the factor structure of the metabolic syndrome across pubertal development. Confirmatory factor analyses of three alternative models. The Journal of Pediatrics. 2009;155(S5):e1–e8. doi: 10.1016/j.jpeds.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. Journal of Bone and Mineral Research. 2005;20:2090–2096. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- Gray L, Lee IM, Sesso HD, Batty GD. Body weight in early and mid-adulthood in relation to subsequent coronary heart disease mortality: 80-year follow-up in the Harvard Alumni Study. Archives of Internal Medicine. 2011;171:1768–1770. doi: 10.1001/archinternmed.2011.486. discussion 1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening JE, Storr HL, McKenzie SA, Davies KM, Martin L, Grossman AB, et al. Linear growth and body mass index in pediatric patients with Cushing’s disease or simple obesity. Journal of Endocrinological Investigation. 2006;29:885–887. doi: 10.1007/BF03349191. [DOI] [PubMed] [Google Scholar]
- Griffiths LJ, Wolke D, Page AS, Horwood JP. Obesity and bullying. Different effects for boys and girls. Archives of Disease in Childhood. 2006;91:121–125. doi: 10.1136/adc.2005.072314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson JK, Yanoff LB, Easter BD, Brady SM, Keil MF, Roberts MD, et al. The stability of metabolic syndrome in children and adolescents. The Journal of Clinical Endocrinology and Metabolism. 2009;94:4828–4834. doi: 10.1210/jc.2008-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutin B, Islam S, Manos T, Cucuzzo N, Smith C, Stachura ME. Relation of percentage of body fat and maximal aerobic capacity to risk factors for atherosclerosis and diabetes in black and white seven-to-eleven-year-old children. The Journal of Pediatrics. 1994;125:847–852. doi: 10.1016/s0022-3476(05)81997-3. [DOI] [PubMed] [Google Scholar]
- Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus. The thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- Han JC, Liu QR, Jones M, Levinn RL, Menzie CM, Jefferson-George KS, et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. The New England Journal of Medicine. 2008;359:918–927. doi: 10.1056/NEJMoa0801119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JC, Thurm A, Golden Williams C, Joseph LA, Zein WM, Brooks BP, et al. Association of brain-derived neurotrophic factor (BDNF) haploinsufficiency with lower adaptive behaviour and reduced cognitive functioning in WAGR/11p13 deletion syndrome. Cortex; A Journal Devoted to the Study of the Nervous System and Behavior. 2013;49:2700–2710. doi: 10.1016/j.cortex.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins SS, Cole TJ, Law C. An ecological systems approach to examining risk factors for early childhood overweight. Findings from the UK Millennium Cohort Study. Journal of Epidemiology and Community Health. 2009;63:147–155. doi: 10.1136/jech.2008.077917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh K, Wake M, Waters E. Body mass index and parent-reported self-esteem in elementary school children. Evidence for a causal relationship. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2004;28:1233–1237. doi: 10.1038/sj.ijo.0802624. [DOI] [PubMed] [Google Scholar]
- Himes JH, Dietz WH. Guidelines for overweight in adolescent preventive services. Recommendations from an expert committee. The Expert Committee on Clinical Guidelines for Overweight in Adolescent Preventive Services. The American Journal of Clinical Nutrition. 1994;59:307–316. doi: 10.1093/ajcn/59.2.307. [DOI] [PubMed] [Google Scholar]
- Hinney A, Nguyen TT, Scherag A, Friedel S, Bronner G, Muller TD, et al. Genome Wide Association (GWA) study for early onset extreme obesity supports the role of fat mass and obesity associated gene (FTO) variants. PLoS ONE. 2007;2:e1361. doi: 10.1371/journal.pone.0001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HJ, De Silva M, Humphreys RP, Drake JM, Smith ML, Blaser SI. Aggressive surgical management of craniopharyngiomas in children. Journal of Neurosurgery. 1992;76:47–52. doi: 10.3171/jns.1992.76.1.0047. [DOI] [PubMed] [Google Scholar]
- Holder JL, Jr, Butte NF, Zinn AR. Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Human Molecular Genetics. 2000;9:101–108. doi: 10.1093/hmg/9.1.101. [DOI] [PubMed] [Google Scholar]
- Hung CC, Luan J, Sims M, Keogh JM, Hall C, Wareham NJ, et al. Studies of the SIM1 gene in relation to human obesity and obesity-related traits. International Journal of Obesity (2005) 2007;31:429–434. doi: 10.1038/sj.ijo.0803443. [DOI] [PubMed] [Google Scholar]
- Hunt SC, Stone S, Xin Y, Scherer CA, Magness CL, Iadonato SP, et al. Association of the FTO gene with BMI. Obesity. 2008;16:902–904. doi: 10.1038/oby.2007.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RS, Creemers JW, Farooqi IS, Raffin-Sanson ML, Varro A, Dockray GJ, et al. Small-intestinal dysfunction accompanies the complex endocrinopathy of human proprotein convertase 1 deficiency. The Journal of Clinical Investigation. 2003;112:1550–1560. doi: 10.1172/JCI18784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RS, Creemers JW, Ohagi S, Raffin-Sanson ML, Sanders L, Montague CT, et al. Obesity and impaired prohormone processing associated with mutations in the human prohormone convertase 1 gene. Nature Genetics. 1997;16:303–306. doi: 10.1038/ng0797-303. [DOI] [PubMed] [Google Scholar]
- Jaruratanasirikul S, Mo-suwan L, Lebel L. Growth pattern and age at menarche of obese girls in a transitional society. Journal of Pediatric Endocrinology and Metabolism. 1997;10:487–490. doi: 10.1515/JPEM.1997.10.5.487. [DOI] [PubMed] [Google Scholar]
- Jasik CB, Lustig RH. Adolescent obesity and puberty. The “perfect storm”. Annals of the New York Academy of Sciences. 2008;1135:265–279. doi: 10.1196/annals.1429.009. [DOI] [PubMed] [Google Scholar]
- Jeffreys M, McCarron P, Gunnell D, McEwen J, Smith GD. Body mass index in early and mid-adulthood, and subsequent mortality. A historical cohort study. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2003;27:1391–1397. doi: 10.1038/sj.ijo.0802414. [DOI] [PubMed] [Google Scholar]
- Jensen ME, Collins CE, Gibson PG, Wood LG. The obesity phenotype in children with asthma. Paediatric Respiratory Reviews. 2011;12:152–159. doi: 10.1016/j.prrv.2011.01.009. [DOI] [PubMed] [Google Scholar]
- Juonala M, Magnussen CG, Berenson GS, Venn A, Burns TL, Sabin MA, et al. Childhood adiposity, adult adiposity, and cardiovascular risk factors. The New England Journal of Medicine. 2011;365:1876–1885. doi: 10.1056/NEJMoa1010112. [DOI] [PubMed] [Google Scholar]
- Juul A, Teilmann G, Scheike T, Hertel NT, Holm K, Laursen EM, et al. Pubertal development in Danish children. Comparison of recent European and US data. International Journal of Andrology. 2006;29:247–255. doi: 10.1111/j.1365-2605.2005.00556.x. discussion 286–290. [DOI] [PubMed] [Google Scholar]
- Kamijo K, Khan NA, Pontifex MB, Scudder MR, Drollette ES, Raine LB, et al. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity. 2012;20:2406–2411. doi: 10.1038/oby.2012.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamijo K, Pontifex MB, Khan NA, Raine LB, Scudder MR, Drollette ES, et al. The negative association of childhood obesity to cognitive control of action monitoring. Cerebral Cortex (New York, N Y : 1991) 2014;24:654–662. doi: 10.1093/cercor/bhs349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls. Relation to increased body mass index and race. Pediatrics. 2001;108:347–353. doi: 10.1542/peds.108.2.347. [DOI] [PubMed] [Google Scholar]
- Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, et al. Putative contributors to the secular increase in obesity. Exploring the roads less traveled. International Journal of Obesity (2005) 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, et al. Severe obesity in children and adolescents. Identification, associated health risks, and treatment approaches. A scientific statement from the American Heart Association. Circulation. 2013;128:1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- Koebnick C, Smith N, Black MH, Porter AH, Richie BA, Hudson S, et al. Pediatric obesity and gallstone disease. Journal of Pediatric Gastroenterology and Nutrition. 2012;55:328–333. doi: 10.1097/MPG.0b013e31824d256f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koebnick C, Smith N, Coleman KJ, Getahun D, Reynolds K, Quinn VP, et al. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. The Journal of Pediatrics. 2010;157:26–31, e22. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koplan JP, Liverman CT, Kraak VA, editors. Preventing childhood obesity Health in balance. Washington, DC: Institute of Medicine, National Academies Press; 2004. [PubMed] [Google Scholar]
- Koplan JP, Liverman CT, Kraak VI. Preventing childhood obesity. Health in the balance. Executive summary. Journal of the American Dietetic Association. 2005;105:131–138. doi: 10.1016/j.jada.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Krebs NF, Himes JH, Jacobson D, Nicklas TA, Guilday P, Styne D. Assessment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl. 4):S193–S228. doi: 10.1542/peds.2007-2329D. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Luck W, Horn R, Brabant G, Gruters A. Severe early-onset obesity, adrenal insufficiency and red hair pigmentation caused by POMC mutations in humans. Nature Genetics. 1998;19:155–157. doi: 10.1038/509. [DOI] [PubMed] [Google Scholar]
- Krude H, Biebermann H, Schnabel D, Tansek MZ, Theunissen P, Mullis PE, et al. Obesity due to proopiomelanocortin deficiency. Three new cases and treatment trials with thyroid hormone and ACTH4-10. The Journal of Clinical Endocrinology and Metabolism. 2003;88:4633–4640. doi: 10.1210/jc.2003-030502. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts. United States. Advance Data. 2000:1–27. [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, et al. 2000 CDC Growth Charts for the United States. Methods and development. Vital and Health Statistics Series 11, Data From the National Health Survey. 2002:1–190. [PubMed] [Google Scholar]
- Lang T, Rayner G. Overcoming policy cacophony on obesity. An ecological public health framework for policymakers. Obesity Reviews. 2007;8(Suppl. 1):165–181. doi: 10.1111/j.1467-789X.2007.00338.x. [DOI] [PubMed] [Google Scholar]
- Lauer R, Lee J, Clarke W. Factors affecting the relationship between childhood and adult cholesterol levels. The Muscatine Study. Pediatrics. 1988;82:309–318. [PubMed] [Google Scholar]
- Le Stunff C, Bougneres P. Early changes in postprandial insulin secretion, not in insulin sensitivity, characterize juvenile obesity. Diabetes. 1994;43:696–702. doi: 10.2337/diab.43.5.696. [DOI] [PubMed] [Google Scholar]
- Lee JM, Kaciroti N, Appugliese D, Corwyn RF, Bradley RH, Lumeng JC. Body mass index and timing of pubertal initiation in boys. Archives of Pediatrics and Adolescent Medicine. 2010;164:139–144. doi: 10.1001/archpediatrics.2009.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Challis BG, Thompson DA, Yeo GS, Keogh JM, Madonna ME, et al. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metabolism. 2006;3:135–140. doi: 10.1016/j.cmet.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Li Y, Dai Q, Jackson JC, Zhang J. Overweight is associated with decreased cognitive functioning among school-age children and adolescents. Obesity. 2008;16:1809–1815. doi: 10.1038/oby.2008.296. [DOI] [PubMed] [Google Scholar]
- Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. International Journal of Obesity. 2014;38:494–506. doi: 10.1038/ijo.2013.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese AD, D’Agostino RB, Jr, Hamman RF, Kilgo PD, Lawrence JM, Liu LL, et al. The burden of diabetes mellitus among US youth. Prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118:1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- Lloyd LJ, Langley-Evans SC, McMullen S. Childhood obesity and adult cardiovascular disease risk. A systematic review. International Journal of Obesity. 2010;34:18–28. doi: 10.1038/ijo.2009.61. [DOI] [PubMed] [Google Scholar]
- Lobstein T. Obesity and the economics of prevention Fit not fat. Paris: Organization for Economic Cooperation and Development; 2010. Special focus II. The size and risks of the international epidemic of child obesity. [Google Scholar]
- Lobstein T, Baur L, Uauy R. Obesity in children and young people. A crisis in public health. Obesity Reviews. 2004;5(Suppl. 1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- Loder RT, Aronson DD, Greenfield ML. The epidemiology of bilateral slipped capital femoral epiphysis. A study of children in Michigan. The Journal of Bone and Joint Surgery American Volume. 1993;75:1141–1147. doi: 10.2106/00004623-199308000-00003. [DOI] [PubMed] [Google Scholar]
- Ludwig DS, Currie J. The association between pregnancy weight gain and birthweight. A within-family comparison. Lancet. 2010;376:984–990. doi: 10.1016/S0140-6736(10)60751-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L, Correll CU. Weight gain and metabolic risks associated with antipsychotic medications in children and adolescents. Journal of Child and Adolescent Psychopharmacology. 2011;21:517–535. doi: 10.1089/cap.2011.0015. [DOI] [PubMed] [Google Scholar]
- Magiakou MA, Mastorakos G, Oldfield EH, Gomez MT, Doppman JL, Cutler GB, Jr, et al. Cushing’s syndrome in children and adolescents. Presentation, diagnosis, and therapy. The New England Journal of Medicine. 1994;331:629–636. doi: 10.1056/NEJM199409083311002. [DOI] [PubMed] [Google Scholar]
- Malone M. Medications associated with weight gain. The Annals of Pharmacotherapy. 2005;39:2046–2055. doi: 10.1345/aph.1G333. [DOI] [PubMed] [Google Scholar]
- Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM. Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatric Pulmonology. 1996;21:176–183. doi: 10.1002/(SICI)1099-0496(199603)21:3<176::AID-PPUL5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- McCartney CR, Blank SK, Prendergast KA, Chhabra S, Eagleson CA, Helm KD, et al. Obesity and sex steroid changes across puberty. Evidence for marked hyperandrogenemia in pre- and early pubertal obese girls. The Journal of Clinical Endocrinology and Metabolism. 2007;92:430–436. doi: 10.1210/jc.2006-2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill HC, Jr, McMahan CA, Herderick EE, Zieske AW, Malcom GT, Tracy RE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation. 2002;105:2712–2718. doi: 10.1161/01.cir.0000018121.67607.ce. [DOI] [PubMed] [Google Scholar]
- Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. The American Journal of Clinical Nutrition. 2002;75:978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- Meyre D, Delplanque J, Chevre JC, Lecoeur C, Lobbens S, Gallina S, et al. Genome-wide association study for early-onset and morbid adult obesity identifies three new risk loci in European populations. Nature Genetics. 2009;41:157–159. doi: 10.1038/ng.301. [DOI] [PubMed] [Google Scholar]
- Molleston JP, White F, Teckman J, Fitzgerald JF. Obese children with steatohepatitis can develop cirrhosis in childhood. The American Journal of Gastroenterology. 2002;97:2460–2462. doi: 10.1111/j.1572-0241.2002.06003.x. [DOI] [PubMed] [Google Scholar]
- Montague CT, Farooqi IS, Whitehead JP, Soos MA, Rau H, Wareham NJ, et al. Congenital leptin deficiency is associated with severe early-onset obesity in humans. Nature. 1997;387:903–908. doi: 10.1038/43185. [DOI] [PubMed] [Google Scholar]
- Muller HL, Bueb K, Bartels U, Roth C, Harz K, Graf N, et al. Obesity after childhood craniopharyngioma. German multicenter study on pre-operative risk factors and quality of life. Klinische Padiatrie. 2001;213:244–249. doi: 10.1055/s-2001-16855. [DOI] [PubMed] [Google Scholar]
- Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. The New England Journal of Medicine. 1992;327:1350–1355. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1999;23(Suppl. 2):S2–S11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- Muzumdar H, Rao M. Pulmonary dysfunction and sleep apnea in morbid obesity. Pediatric Endocrinology Reviews. 2006;3(Suppl. 4):579–583. [PubMed] [Google Scholar]
- Nadeau KJ, Maahs DM, Daniels SR, Eckel RH. Childhood obesity and cardiovascular disease. Links and prevention strategies. Nature Reviews Cardiology. 2011;8:513–525. doi: 10.1038/nrcardio.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumark-Sztainer D, Falkner N, Story M, Perry C, Hannan PJ, Mulert S. Weight-teasing among adolescents. Correlations with weight status and disordered eating behaviors. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 2002;26:123–131. doi: 10.1038/sj.ijo.0801853. [DOI] [PubMed] [Google Scholar]
- Ning C, Yanovski JA. Endocrine disorders associated with pediatric obesity. In: Goran M, Sothern M, editors. Handbook of pediatric obesity. Boca Raton, FL: CRC Press; 2006. p. 135. [Google Scholar]
- Obesity. Preventing and managing the global epidemic, report of a WHO consultation. World Health Organization Technical Report Series. 2000;894:i–xii. 1–253. [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA: The Journal of the American Medical Association. 2010;303:242–249. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA: The Journal of the American Medical Association. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA: The Journal of the American Medical Association. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA: The Journal of the American Medical Association. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. National Health Statistics Reports. 2010:1–5. [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA: The Journal of the American Medical Association. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, et al. A potential decline in life expectancy in the United States in the 21st century. The New England Journal of Medicine. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- Pashankar DS, Corbin Z, Shah SK, Caprio S. Increased prevalence of gastroesophageal reflux symptoms in obese children evaluated in an academic medical center. Journal of Clinical Gastroenterology. 2009;43:410–413. doi: 10.1097/MCG.0b013e3181705ce9. [DOI] [PubMed] [Google Scholar]
- Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA: The Journal of the American Medical Association. 2006;296:421–426. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- Pettitt DJ, Talton J, Dabelea D, Divers J, Imperatore G, Lawrence JM, et al. Prevalence of diabetes in U.S. youth in 2009. The SEARCH for diabetes in youth study. Diabetes Care. 2014;37:402–408. doi: 10.2337/dc13-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical status. The use and interpretation of anthropometry. Vol. 854. Geneva, Switzerland: World Health Organization; 1995. [PubMed] [Google Scholar]
- Popkin BM, Udry JR. Adolescent obesity increases significantly in second and third generation U.S. immigrants. The National Longitudinal Study of Adolescent Health. The Journal of Nutrition. 1998;128:701–706. doi: 10.1093/jn/128.4.701. [DOI] [PubMed] [Google Scholar]
- Power C, Lake JK, Cole TJ. Measurement and long-term health risks of child and adolescent fatness. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity. 1997;21:507–526. doi: 10.1038/sj.ijo.0800454. [DOI] [PubMed] [Google Scholar]
- Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, et al. Sugar-sweetened beverages and genetic risk of obesity. The New England Journal of Medicine. 2012;367:1387–1396. doi: 10.1056/NEJMoa1203039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampersaud E, Mitchell BD, Pollin TI, Fu M, Shen H, O’Connell JR, et al. Physical activity and the association of common FTO gene variants with body mass index and obesity. Archives of Internal Medicine. 2008;168:1791–1797. doi: 10.1001/archinte.168.16.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankinen T, Zuberi A, Chagnon YC, Weisnagel SJ, Argyropoulos G, Walts B, et al. The human obesity gene map. The 2005 update. Obesity. 2006;14:529–644. doi: 10.1038/oby.2006.71. [DOI] [PubMed] [Google Scholar]
- Ravussin E, Valencia ME, Esparza J, Bennett PH, Schulz LO. Effects of a traditional lifestyle on obesity in Pima Indians. Diabetes Care. 1994;17:1067–1074. doi: 10.2337/diacare.17.9.1067. [DOI] [PubMed] [Google Scholar]
- Reeves GM, Postolache TT, Snitker S. Childhood obesity and depression. Connection between these growing problems in growing children. International Journal of Child Health and Human Development. 2008;1:103–114. [PMC free article] [PubMed] [Google Scholar]
- Richardson LP, Davis R, Poulton R, McCauley E, Moffitt TE, Caspi A, et al. A longitudinal evaluation of adolescent depression and adult obesity. Archives of Pediatrics and Adolescent Medicine. 2003;157:739–745. doi: 10.1001/archpedi.157.8.739. [DOI] [PubMed] [Google Scholar]
- Rolland-Cachera MF, Sempe M, Guilloud-Bataille M, Patois E, Pequignot-Guggenbuhl F, Fautrad V. Adiposity indices in children. The American Journal of Clinical Nutrition. 1982;36:178–184. doi: 10.1093/ajcn/36.1.178. [DOI] [PubMed] [Google Scholar]
- Rosenquist JN, Lehrer SF, O’Malley AJ, Zaslavsky AM, Smoller JW, Christakis NA. Cohort of birth modifies the association between FTO genotype and BMI. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:354–359. doi: 10.1073/pnas.1411893111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savastano DM, Tanofsky-Kraff M, Han JC, Ning C, Sorg RA, Roza CA, et al. Energy intake and energy expenditure among children with polymorphisms of the melanocortin-3 receptor. The American Journal of Clinical Nutrition. 2009;90:912–920. doi: 10.3945/ajcn.2009.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherag A, Dina C, Hinney A, Vatin V, Scherag S, Vogel CI, et al. Two new loci for body-weight regulation identified in a joint analysis of genome-wide association studies for early-onset extreme obesity in French and German study groups. PLoS Genetics. 2010;6:e1000916. doi: 10.1371/journal.pgen.1000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA: The Journal of the American Medical Association. 2003;289:1813–1819. doi: 10.1001/jama.289.14.1813. [DOI] [PubMed] [Google Scholar]
- Schwimmer JB, Deutsch R, Kahen T, Lavine JE, Stanley C, Behling C. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118:1388–1393. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- Schwimmer JB, Deutsch R, Rauch JB, Behling C, Newbury R, Lavine JE. Obesity, insulin resistance, and other clinicopathological correlates of pediatric nonalcoholic fatty liver disease. The Journal of Pediatrics. 2003;143:500–505. doi: 10.1067/S0022-3476(03)00325-1. [DOI] [PubMed] [Google Scholar]
- Scuteri A, Sanna S, Chen WM, Uda M, Albai G, Strait J, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genetics. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Guo DF, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Human Molecular Genetics. 2009;18:1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaibi GQ, Goran MI. Examining metabolic syndrome definitions in overweight Hispanic youth. A focus on insulin resistance. The Journal of Pediatrics. 2008;152:171–176. doi: 10.1016/j.jpeds.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimabukuro T, Sunagawa M, Ohta T. Low-density lipoprotein particle size and its regulatory factors in school children. The Journal of Clinical Endocrinology and Metabolism. 2004;89:2923–2927. doi: 10.1210/jc.2003-031818. [DOI] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. The Journal of Clinical Investigation. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. The New England Journal of Medicine. 2002;346:802–810. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- Smemo S, Tena JJ, Kim KH, Gamazon ER, Sakabe NJ, Gomez-Marin C, et al. Obesity-associated variants within FTO form long-range functional connections with IRX3. Nature. 2014;507:371–375. doi: 10.1038/nature13138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socha P, Horvath A, Vajro P, Dziechciarz P, Dhawan A, Szajewska H. Pharmacological interventions for nonalcoholic fatty liver disease in adults and in children. A systematic review. Journal of Pediatric Gastroenterology and Nutrition. 2009;48:587–596. doi: 10.1097/MPG.0b013e31818e04d1. [DOI] [PubMed] [Google Scholar]
- Speiser PW, Rudolf MC, Anhalt H, Camacho-Hubner C, Chiarelli F, Eliakim A, et al. Childhood obesity. The Journal of Clinical Endocrinology and Metabolism. 2005;90:1871–1887. doi: 10.1210/jc.2004-1389. [DOI] [PubMed] [Google Scholar]
- Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nature Genetics. 2010;42:937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel AM, Alving BM. Executive summary of the Strategic Plan for National Institutes of Health Obesity Research. The American Journal of Clinical Nutrition. 2005;82:211S–214S. doi: 10.1093/ajcn/82.1.221S. [DOI] [PubMed] [Google Scholar]
- Srinivasan S, Ogle GD, Garnett SP, Briody JN, Lee JW, Cowell CT. Features of the metabolic syndrome after childhood craniopharyngioma. The Journal of Clinical Endocrinology and Metabolism. 2004;89:81–86. doi: 10.1210/jc.2003-030442. [DOI] [PubMed] [Google Scholar]
- Srinivasan SR, Myers L, Berenson GS. Predictability of childhood adiposity and insulin for developing insulin resistance syndrome (syndrome X) in young adulthood. The Bogalusa Heart Study. Diabetes. 2002;51:204–209. doi: 10.2337/diabetes.51.1.204. [DOI] [PubMed] [Google Scholar]
- St George IM, Williams S, Silva PA. Body size and the menarche. The Dunedin Study. The Journal of Adolescent Health. 1994;15:573–576. doi: 10.1016/1054-139x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- Stanley TL, Chen ML, Goodman E. The typology of metabolic syndrome in the transition to adulthood. The Journal of Clinical Endocrinology and Metabolism. 2014;99:1044–1052. doi: 10.1210/jc.2013-3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. Progress and challenges in metabolic syndrome in children and adolescents. A scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009;119:628–647. doi: 10.1161/CIRCULATIONAHA.108.191394. [DOI] [PubMed] [Google Scholar]
- Stratigopoulos G, Padilla SL, LeDuc CA, Watson E, Hattersley AT, McCarthy MI, et al. Regulation of Fto/Ftm gene expression in mice and humans. American Journal of Physiology – Regulatory Integrative and Comparative Physiology. 2008;294:R1185–R1196. doi: 10.1152/ajpregu.00839.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel A, Issad T, Camoin L, Ozata M, Strosberg AD. A leptin missense mutation associated with hypogonadism and morbid obesity. Nature Genetics. 1998;18:213–215. doi: 10.1038/ng0398-213. [DOI] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Cohen ML, Yanovski SZ, Cox C, Theim KR, Keil M, et al. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics. 2006;117:1203–1209. doi: 10.1542/peds.2005-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Han JC, Anandalingam K, Shomaker LB, Columbo KM, Wolkoff LE, et al. The FTO gene rs9939609 obesity-risk allele and loss of control over eating. The American Journal of Clinical Nutrition. 2009;90:1483–1488. doi: 10.3945/ajcn.2009.28439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanofsky-Kraff M, Yanovski SZ, Schvey NA, Olsen CH, Gustafson J, Yanovski JA. A prospective study of loss of control eating for body weight gain in children at high risk for adult obesity. The International Journal of Eating Disorders. 2009;42:26–30. doi: 10.1002/eat.20580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauman R, Gozal D. Obesity and obstructive sleep apnea in children. Paediatric Respiratory Reviews. 2006;7:247–259. doi: 10.1016/j.prrv.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-Kraff M, Adler-Wailes DC, et al. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117:2167–2174. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, et al. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nature Genetics. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children. A prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- Troiano RP, Flegal KM. Overweight children and adolescents. Description, epidemiology, and demographics. Pediatrics. 1998;101:497–504. [PubMed] [Google Scholar]
- Uwaifo GI, Elberg J, Yanovski JA. Impaired glucose tolerance in obese children and adolescents. The New England Journal of Medicine. 2002;347:290–292. [PubMed] [Google Scholar]
- van Dijk SJ, Molloy PL, Varinli H, Morrison JL, Muhlhausler BS. Epigenetics and human obesity. International Journal of Obesity. 2015;39:85–97. doi: 10.1038/ijo.2014.34. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Exposito M, Schmidt-Rio-Valle J, Fernandez-Serrano MJ, Cruz F, Perez-Garcia M, et al. Selective alterations within executive functions in adolescents with excess weight. Obesity. 2010;18:1572–1578. doi: 10.1038/oby.2009.475. [DOI] [PubMed] [Google Scholar]
- Wang Y. Is obesity associated with early sexual maturation? A comparison of the association in American boys versus girls. Pediatrics. 2002;110:903–910. doi: 10.1542/peds.110.5.903. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. International Journal of Pediatric Obesity. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- Wardle J, Carnell S, Haworth CMA, Plomin R. Evidence for a strong genetic influence on childhood adiposity despite the force of the obesogenic environment. The American Journal of Clinical Nutrition. 2008;87:398–404. doi: 10.1093/ajcn/87.2.398. [DOI] [PubMed] [Google Scholar]
- Wardle J, Carnell S, Haworth CM, Farooqi IS, O’Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. The Journal of Clinical Endocrinology and Metabolism. 2008;93:3640–3643. doi: 10.1210/jc.2008-0472. [DOI] [PubMed] [Google Scholar]
- Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. International Journal of Obesity (2005) 2008;33:42–45. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- Wattigney WA, Srinivasan SR, Chen W, Greenlund KJ, Berenson GS. Secular trend of earlier onset of menarche with increasing obesity in black and white girls. The Bogalusa Heart Study. Ethnicity and Disease. 1999;9:181–189. [PubMed] [Google Scholar]
- Wellens RI, Roche AF, Khamis HJ, Jackson AS, Pollock ML, Siervogel RM. Relationships between the Body Mass Index and body composition. Obesity Research. 1996;4:35–44. doi: 10.1002/j.1550-8528.1996.tb00510.x. [DOI] [PubMed] [Google Scholar]
- Wheeler E, Huang N, Bochukova EG, Keogh JM, Lindsay S, Garg S, et al. Genome-wide SNP and CNV analysis identifies common and low-frequency variants associated with severe early-onset obesity. Nature Genetics. 2013;45:513–517. doi: 10.1038/ng.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker RC, Dietz WH. Role of the prenatal environment in the development of obesity. The Journal of Pediatrics. 1998;132:768–776. doi: 10.1016/s0022-3476(98)70302-6. [DOI] [PubMed] [Google Scholar]
- Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. The New England Journal of Medicine. 1997;337:869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatrica Supplementum. 2006;450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x. [DOI] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, et al. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nature Genetics. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing YK, Hui SH, Pak WM, Ho CK, Cheung A, Li AM, et al. A controlled study of sleep related disordered breathing in obese children. Archives of Disease in Childhood. 2003;88:1043–1047. doi: 10.1136/adc.88.12.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanovski SZ, Yanovski JA. Obesity prevalence in the United States. Up, down, or sideways? The New England Journal of Medicine. 2011;364:987–989. doi: 10.1056/NEJMp1009229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo GS, Connie Hung CC, Rochford J, Keogh J, Gray J, Sivaramakrishnan S, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nature Neuroscience. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- Young-Hyman D, Tanofsky-Kraff M, Yanovski SZ, Keil M, Cohen ML, Peyrot M, et al. Psychological status and weight-related distress in overweight or at-risk-for-overweight children. Obesity. 2006;14:2249–2258. doi: 10.1038/oby.2006.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity. A systematic review and meta-analysis. PLoS ONE. 2013;8:e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]


