Abstract
Purpose
The purpose of this study was to utilize a visuomotor tracking task, with both the jaw and hand, to add to the literature regarding non-speech motor practice and sensorimotor integration (outside of auditory-motor integration domain) in adults who do (PWS) and do not (PWNS) stutter.
Method
Participants were 15 PWS (14 males, mean age = 27.0) and 15 PWNS (14 males, mean age = 27.2). Participants tracked both predictable and unpredictable moving targets separately with their jaw and their dominant hand, and accuracy was assessed by calculating phase and amplitude difference between the participant and the target. Motor practice effect was examined by comparing group performance over consecutive tracking trials of predictable conditions as well as within the first trial of same conditions.
Results
Results showed that compared to PWNS, PWS were not significantly different in matching either the phase (timing) or the amplitude of the target in both jaw and hand tracking of predictable and unpredictable targets. Further, there were no significant between-group differences in motor practice effects for either jaw or hand tracking. Both groups showed improved tracking accuracy within and between the trials.
Conclusion
Our findings revealed no statistically significant differences in non-speech motor practice effects and integration of sensorimotor feedback between PWS and PWNS, at least in the context of the visuomotor tracking tasks employed in the study. In general, both talker groups exhibited practice effects (i.e., increased accuracy over time) within and between tracking trials during both jaw and hand tracking. Implications for these results are discussed.
Keywords: stuttering, motor control, motor practice, sensorimotor integration, visuomotor tracking
1. Introduction
Developmental stuttering is a speech disorder characterized by disruptions in the flow of speech taking the form of repetitions, prolongations, and silent blocks. While a complex interaction of environmental, motor, emotional and cognitive-linguistic variables are believed to underlie the onset and development of stuttering, (for review see Bloodstein & Bernstein Ratner, 2008) the overt behaviors of stuttering may be the result of disruptions in the respiratory, phonatory and articulatory movements leading to the inability to smoothly transition between speech sounds. For some time, it has been argued that a better understanding of the aberrant motor control processes involved in stuttering would lay a foundation for study of potential contribution of other factors, such as environmental, emotional and/or linguistic, on the development of this disorder (Max, Guenther, Gracco, Ghosh, & Wallace, 2004).
Numerous attempts have been made to describe the status of both speech and non-speech motor systems in people who stutter (PWS). One observation that has been replicated across many studies is that the speech and non-speech (i.e., orofacial, finger and hand) movements of PWS are slower and longer in duration than those of people who do stutter (PWNS), as well as more variable or less stable (for review see Bloodstein & Bernstein Ratner, 2008; Kleinow & Smith, 2000; Max, 2004; Max et al., 2004; McClean, Kroll, & Loftus, 1990; Namasivayam & van Lieshout, 2008; Olander, Smith & Zelaznik, 2010; Smith, Sadagopan, Smits-Bandstra, De Nil, & Rochon, 2006; Walsh and Weber-Fox, 2010; Zelaznik et al., 1997; Zimmermann, 1980). These between-group and across-domain differences in movement duration, amplitude and stability, especially in non-speech (orofacial, finger, and hand) movements, suggest that stuttering may result from a more general deficit in motor control that is not speech-specific. This conclusion is supported by evidence that timing control of both speech and non-speech gestures shares a common neural substrate (Bengtsson, Ehrsson, Forssberg, & Ullén, 2005; Binkofski & Buccino, 2004).
There are several theories that attempt to explain the motor deficit believed to underlie stuttering. In the Speech Motor Skill (SMS) theory proposed by van Lieshout and colleagues (van Lieshout, Hulstijn, & Peters, 2004) in which speech production is viewed in the same realm as other fine motor skills, with individual abilities falling along a continuum from least to most skilled. It is hypothesized that PWS's abilities may be located toward the lower end of the presumed normal speech motor skill continuum. Following the SMS perspective, disfluencies are viewed as disruptions in the preparation and performance of complex motor actions in the face of cognitive-linguistic, environmental or emotional influences (Peters, Hulstijn, & van Lieshout, 2000; Namasivayam & van Lieshout, 2011). Disruptions in speech motor control are thought to be subtle and only become evident when high demands for movement accuracy and speed or increased task complexity are placed on the system. For example, Smith and her colleagues have repeatedly demonstrated that PWS's speech motor variability (as measured by a “spatiotemporal index” or degree to which the pattern of movement is consistent on repeated productions of the same utterance) is strongly affected by the length and phonological complexity of the produced utterance (Kleinow & Smith, 2000; Smith, Sadagopan, Walsh & Weber-Fox, 2010).
Despite evidence that PWS may exhibit less proficient motor performance (see evidence provided above), it is not clear if there are distinct processes within the motor system that are deficient. Both feedback and feedforward modes of control are required for skilled motor control, and it has been speculated that inefficiencies in the speech motor control of PWS could be attributed to a lack of ability to utilize and/or learn feedforward models, the overreliance of feedback or the lack of ability to integrate feedback with ongoing feedforward commands (Cai et al., 2012; Cai et al., 2014; Civier, Tasko, & Guenther, 2010; Loucks, Chon, & Han, 2012; Max et al., 2004; Tourville, Reilly, & Guenther, 2008). In the following paragraphs we will discuss research regarding the motor practice and learning abilities and utilization of sensory feedback in PWS. We will then present a case for why visuomotor tracking tasks are a good method of investigating motor practice effects and sensorimotor integration.
1.1. Motor practice effects, motor learning and stuttering
Motor learning is process that results in a long-term memory for the execution of motor skills. It is essential for the efficient and effortless execution of complex sequential movements (e.g., speaking, walking, typing, and playing musical instruments) as well as for calibrating the smoothness and accuracy of simple movements (Abbruzzese, Pelosin, & Marchese, 2008). Acquisition of motor skills is typically manifested by increased accuracy and speed of performance. It is thought that such increases in speed and accuracy of performance result from repeated exposure to a specific skill, often without conscious recollection of the prior learning episode or the rules underlying the task (Cohen & Squire, 1980).
Research has shown that motor movements, including those for speech production, require a certain degree of practice to become adult-like (Green, Moore, Higashikawa, & Steeve, 2000; Green & Nip, 2010). Proficiency in performance of those movements will likely depend on motor learning that is the result of motor practice. Within one prominent theory of motor control, the Schema Theory (Schmidt & Lee, 2005), the process of movement acquisition is viewed as an interaction between an individual's innate capacities and the type of movement to be learned. According to the SMS model previously described (van Lieshout, Hulstijn, & Peters, 2004; Namasivayam & van Lieshout, 2011) PWS may have a limited ability to benefit from motor practice and achieve lower levels of movement proficiency than PWNS after the same amount of practice.
Empirical evidence indicating less robust motor learning abilities in PWS comes from studies of sequence learning in various domains: finger tapping, syllable sequencing, and nonsense word learning. In an early study, Webster (1986) examined PWS and PWNS's abilities to learn four-element finger tapping sequences and found that, compared to PWNS, PWS made more errors and had slower response initiation times with practice. Smits-Bandstra and De Nil (2007) also used a finger-tapping sequence learning task to show that PWS, when compared to controls, showed slower response reaction time with practice. In the same study, the authors demonstrated that PWS benefitted less from practice during a speech sequencing task (also indexed by slower response initiation time) compared to PWNS.
Finally, Namasivayam and van Lieshout (2008) compared the effects of motor practice on learning nonsense words. They assessed amplitude and duration for individual lip movements as well as coordination between lip and tongue movements as measured by mean coherence (an index for frequency coupling between the two interacting gestures) and the variability of relative phase (an index of the stability of the coupling between the two interacting gestures). PWS showed similar motor practice and learning changes to those of PWNS on a nonsense word learning task, performed at fast and normal speech rates across three test sessions (two on the same day and one at least one week after the first two sessions). However, the results also indicated that at normal speaking rates after one week retention period PWNS exhibited a greater increase in mean coherence values relative to PWS, which suggested that PWS did not learn to the same extent as PWNS.
Motor learning deficits in PWS have been attributed to the use of a control strategy that relies too heavily on sensory feedback and thus yields considerable time lags in production (Max et al., 2004; Tourville, Reilly, & Guenther, 2008). This feedback-driven delay is thought to be the source of instability in the motor system that manifests as stuttering-like disfluencies (Civier, Tasko, & Guenther, 2010). Converging evidence for this view comes from work showing that PWS exhibit slow and asynchronous vocal adjustments to auditory perturbations (Cai et al., 2012; Loucks, Chon & Han, 2012). Further, recent modeling studies showing that stuttering-like disfluencies can be simulated when the model is biased away from feedforward and toward feedback as a control mechanism (Civier, Tasko, & Guenther, 2010). Additionally, there is evidence from functional imaging research supporting the notion that PWS exhibit feedforward deficits, which would perhaps lead to an over-reliance on feedback modes of control. In their activation likelihood estimation (ALE) meta-analysis of imaging studies of chronic developmental stuttering in adults, Brown et al. (2005) provide evidence of over-activation in the motor areas of PWS during speech, and a lack of activation in the auditory areas. These observations have been interpreted to indicate less competent motor performance associated with aberrant feedforward (“efference copy”) planning. As such, it has been speculated that PWS are unable to shift from feedback modes and thus do not take advantage of the feedforward control system.
In summary, findings from studies of both the speech and non-speech motor control and sequencing abilities of people who stutter lend support to the hypothesis that PWS exhibit difficulties in learning automatized speech and non-speech sequences (i.e., fingers and hands), particularly under with high task demands. Specifically, when compared to PWNS, PWS exhibit slower, longer, and more variable movements and less robust motor learning as indexed by reduced benefit from motor practice. In recent years, researchers have proposed that deficits in the efficient use of feedforward motor commands and an over-reliance on feedback mechanisms is the root of the reduced motor stability of PWS.
1.2. Purpose of the present study
In the present study, we used a novel visuomotor tracking task with different effectors (jaw and a dominant hand) to assess both motor execution ability and motor practice effects in PWS and PWNS. Moreover, we included predictable and unpredictable tracking conditions in our paradigm, which allowed us to examine tracking accuracy when the nature of the target movement called for the bias towards feedforward versus feedback strategy. Accurate tracking of predictable targets is hypothesized to rely on feedforward control of movement, whereas for unpredictable targets one has to rely on feedback movement control strategy.
Visuomotor tracking involves continuous tracking of a visually presented moving target (usually sinusoidal). The effector can be any moveable structure, such as lip, jaw, finger or hand. Visuomotor tracking has been a method of choice for the study of speech motor control in people with motor speech disorders (Ballard & Robin, 2007; Ballard, Robin, & Folkins, 2003; Ballard, Solomon, Robin, Moon, & Folkins, 2008; Folkins et al., 1995; McClean, Beukelman, & Yorkston, 1987; Willingham, Koroshetz, & Peterson, 1996; cf. Weismer, 2006; Ziegler, 2003) as well as healthy children and adults (e.g., Ballard, Robin, Woodworth, & Zimba, 2001; Clark et al., 2001). Further, it allows for the parallel analysis of motor execution and motor learning in different effector systems (e.g., jaw and hand) without the confounding factor of linguistic/phonological processing. These factors make visuomotor tracking methods desirable for investigating the generalized nature of a motor deficit across domains.
Important to the current study, tracking a moving target is an appropriate task to test motor practice effects. It allows for examination of aspects of motor control related to the planning of movement patterns by requiring participants to track either predictable or unpredictable signals. Accurate tracking of a predictable signal requires the construction of an internal model for the target motion, and is either phase synchronous or phase leading (Flowers, 1978; Moon, Zebrowski, Robin, & Folkins, 1993) indicating that target movement is anticipated by the participant. Moreover, it has been shown that participants continue to accurately produce predictable movements after the target has been removed, suggesting that predictable tracking is model-driven and relies on feedforward control (Ballard & Robin, 2007) and, thus, may rely on motor learning. By contrast, tracking of unpredictable signals involves a phase lag, since target movement cannot be anticipated, and thus the individual is forced to rely on feedback of the signal to be tracked and continually integrate the new target into the ongoing motor plan. Thus, visuomotor tracking of comparable predictable and unpredictable signals allows for assessment of feedback and feedforward modes of control in people who do and do not stutter.
Although, on the surface, visuomotor tracking is different from speech production in that it does not involve multiple overlapping and sequential articulatory gestures, there are some important similarities between the two (Ballard et al., 2001; Moon et al., 1993). Moon et al., (1993) summarized the similarities of this system with speech and advantages of using it in assessment of articulator motor control. Specifically, they point out that visuomotor tracking requires similar alternation of opening and closing gestures with peak velocity in the middle of the gesture without imposing linguistic units.
Another important advantage of a tracking task is that it allows for assessment of motor practice effects with reference to the speed of movement to be learned, which as Namasivayam and van Lieshout (2008) point out has been a limitation for most existing motor learning studies in stuttering research. Lastly, analysis of sinusoidal movement allows for simultaneous assessment of tracking accuracy in phase (timing) and amplitude domains independently, which, to our knowledge, has not been assessed previously in people who stutter, but could provide some evidence for the hypothesis of stuttering as a disorder of timing control of movements (Alm, 2004; Zimmermann, Smith, & Hanley, 1981). As pointed out by Max and Yudman (2003) previously studies have examined between-group differences in the temporal domain without simultaneously examining associated spatial measures, making it impossible to rule out the possibility that difficulties in stuttering are specific to timing as opposed to other aspects of speech movements.
We designed this study to answer three research questions. First, do PWS differ from age-matched PWNS in tracking accuracy during both manual and oral tasks, when accuracy is measured by amplitude difference (gain) and phase difference (delay) between the target and the effector? This will help to address gaps in the research with regard to whether PWS have issues with phase and amplitude tracking (Max & Yudman, 2003; Namasivayam and van Lieshout, 2008). Second, do PWS differ from PWNS in motor learning ability where learning ability is measured by the decrease in error magnitude (to be defined below) over time? This would help to illuminate whether PWS benefit less from motor practice than PWNS. Third, do PWS differ from PWNS with regard to tracking an unpredictable signal? Given that an unpredictable signal requires constant feedback integration, the visuomotor tracking of an unpredictable signal would assess the sensorimotor integration of visual feedback and will shed light on whether PWS have generalized sensorimotor integration deficit.
2. Method
2.1 Participants
Participants were fifteen adults who stutter (1 female), ranging in age from 18 to 39 years (mean=27; SD=5.8) of age and fifteen age- and gender-matched adults who do not stutter (mean = 27.2; SD=6.3). Demographic data for participants in this study is presented in table 1. All PWS were self-described to be stuttering, started stuttering in childhood and had no known neurological impairments. All but one PWS reported having received treatment for stuttering at one time in their life. Participants classified as PWS exhibited 3 or more stuttering-like disfluencies (SLDs) per 100 words (mean SLDs in 400 words = 12.02; SD=5.72) and scored 13 and higher on the Stuttering Severity Instrument -3 (SSI-3; Riley and Riley, 1994) (mean SSI-3 = 22.87; SD=5.76). Table 2 presents PWS speech characteristics and SSI-3 scores. This study's protocol was approved by the Institutional Review Board of the University of Iowa, Iowa City, Iowa. Each of the 30 participants read and then signed informed consent to participate in the present study.
Table 1.
Demographic and clinical characteristics of participants.
| Variable | Group | Minimum | Maximum | Mean | Std. Deviation |
|---|---|---|---|---|---|
| Age (years) | PWS | 18 | 39 | 27 | 5.84 |
| PWNS | 18 | 41 | 27.2 | 6.35 | |
| Education (years) | PWS | 11 | 23 | 14.73 | 3.51 |
| PWNS | 10 | 21 | 14.93 | 3.45 | |
| Years in therapy | PWS | 0 | 11 | 5 | 3.40 |
Gender: 1 female, 14 males in each group
Table 2.
Speech Characteristics of PWS (SLD=stuttering like disfluencies)
| Participant ID | % total words disfluent | % SLD | %SLDs out of total disfluencies | % SLD in reading (out of 122 words) | SSI-3 score & severity |
|---|---|---|---|---|---|
|
conversation sample 400 words | |||||
| S1 | 14 | 8 | 58 | 5 | (20) mild |
| S2 | 17 | 13 | 76 | 12 | (23) mild |
| S3 | 9 | 4 | 49 | 5 | (19) mild |
| S4 | 13.5 | 9 | 67 | 1.6 | (19) mild |
| S5 | 12 | 8.3 | 70 | 12 | (18) mild |
| S6 | 23 | 18.5 | 81 | 5 | (30) moderate |
| S7 | 11 | 6 | 56 | 9 | (20) mild |
| S8 | 28.8 | 22 | 76.5 | 6.6 | (29) moderate |
| S9 | 14 | 12 | 89 | 6 | (20) mild |
| S10 | 15 | 10 | 68 | 19.7 | (26) moderate |
| S11 | 29 | 19 | 67 | 34 | (34) severe |
| S12 | 19 | 10 | 51 | 1.6 | (18) mild |
| S13 | 9 | 6 | 74 | 3 | (13) very mild |
| S14 | 17.5 | 13.5 | 77 | 20.5 | (25) moderate |
| S15 | 27 | 21 | 79 | 11.5 | (29) moderate |
From an initial pool of 34 possible participants, one PWNS was excluded because of presence of an exclusionary health condition and 3 PWS were excluded because no measurable stuttering-like disfluencies were observed during a 10-minute conversational sample.
All thirty participants were paid volunteers, with some recruited from the University of Iowa's Wendell Johnson Speech and Hearing Clinic where they were receiving treatment for stuttering and others recruited from the University of Iowa community via e-mail advertisements. At the initial contact with potential participants they were interviewed to ensure that they had normal or corrected-to-normal vision and no exclusionary health conditions (e.g.: structural brain disease; active epilepsy; acute illness or active, confounding medical, neurological, or musculoskeletal conditions; alcoholism or other forms of drug addiction).
2.2 Data collection: Visuomotor tracking procedure
All thirty participants were tested individually during one approximately 2-3 hour visit, with the same procedure being carried out for each participant. Before testing started, the first author described the details of the study to the participants, who read and signed the informed consent form. Subsequently, each participant filled out two forms asking them for their educational history and prescription medication use. A 10-minute conversational speech sample was recorded from participants who stuttered (PWS).
In general, the testing session consisted of a series of tracking tasks performed with the jaw and dominant hand. Jaw and hand movements during tracking were recorded using Optotrak (Northern Digital, Waterloo, Ontario, Canada), an optoelectronic position measurement system that tracks the three-dimensional motion of infrared-emitting diodes (IREDs).
2.3 Tracking conditions
The order in which the two (i.e., jaw and hand) tracking conditions was presented to participants was counterbalanced. Within each condition, the order of presentation of the three levels of difficulty (to be explained below) and target frequencies (explanation follows) was randomized for each participant.
Jaw and hand tracking were each studied during 9 different tracking conditions. The effective tracking range was set at 12mm (1.2 cm) for jaw tracking and 105mm (10.5 cm) for hand tracking (see below for details).
Targets-to-track occurred at three levels of difficulty, with Level 1 being the easiest and Level 3 being the hardest. Level 1 and 2 included predictable target movements, whereas Level 3 targets were unpredictable. Level 1 consisted of sinusoids of constant amplitude and constant frequency. Level 1 targets were designed to assess participants’ ability to learn a relatively easily discernible pattern of movement. Level 2 consisted of sinusoids of constant frequency and variable amplitude, where the amplitude varied in a predictable pattern (amplitude ratios of 1, 0.67, 0.34 repeated). These targets were designed to evaluate participants’ ability to extract and learn a more complex motor pattern, not as visually obvious as the one in Level 1. Level 3 consisted of sinusoids of constant frequency and variable amplitude, where the amplitude varied in an unpredictable pattern (same amplitude ratios as Level 2). Level 3 targets required participants to continually match the jaw position with the moving target without being able to anticipate the target position.
Target sinusoids in Levels 1, 2 and 3 occurred at three frequencies, 0.3 Hz (a), 0.6 Hz (b) and 0.9 Hz (c), for a total of 9 tracking conditions. These frequencies were chosen based on previous research (Ballard & Robin, 2007; Flowers, 1978; Moon, Zebrowski, Robin, & Folkins, 1993; Robin, Jacks, Hageman, Clark, & Woodworth, 2008). The difficulty of tracking the target was expected to increase with frequency. Thus, Level 1a denotes a sinusoidal target of 0.3 Hz at a constant amplitude. Likewise, Level 2b denotes a sinusoidal target of 0.6 Hz with variable amplitude but a predictable pattern. Figure 1 presents graphical depiction of the tracking conditions used in this study.
Figure 1. Tracking Conditions.
The targets in the left, middle, and right columns have frequencies of 0.3, 0.6, and 0.9 Hz, respectively. The top row (Level 1) targets were sinusoids of constant amplitude. The middle row (Level 2) targets were sinusoids of variable amplitude, where the amplitude varied in a predictable pattern. The bottom row (Level 3) targets were sinusoids of variable amplitude, where the amplitude varied in an unpredictable pattern (same amplitude ratios as Level 2).
In the experiment, participants repeated Level 1 and Level 2 conditions for two consecutive trials so that practice effects could be examined. Level 3 conditions were not repeated and not included in the analysis of motor practice since there was no learning expected to occur due to the unpredictable nature of Level 3 target movement. Thus, level 3 conditions were only analyzed for accuracy of tracking.
2.4 Trial duration
For both hand and jaw tracking, each tracking condition (e.g. Level 1a) lasted for 60 seconds after which participants were given a 10-15 second break. If a participant felt the need, a longer break could be requested. During jaw tracking conditions participants were asked to refrain from swallowing and wait for the break to swallow if they could. A glass of water was made available to minimize participants’ dry mouth and make swallowing on purpose during the breaks easier. Participants were given a 10-15 minute break before they proceeded to the second part of the experiment (i.e., depending on counter-balanced order, either jaw or manual tracking tasks).
2.5 Jaw tracking
The participants were seated in front of a computer screen 2 meters away from the screen to avoid saccadic eye movement (Cassel, 1973). Two IREDs were attached to the participant's face with the use of two-sided adhesive tape – one marker was placed on the forehead and another marker was placed under the chin. During test trials, a sinusoidal target signal, corresponding to one of the 9 conditions (see above) appeared on the computer screen as a vertically moving black square which was 1.5 cm wide. Movement signals from the jaw were transduced via the Optotrak system using the Optotrak Application Programmer's Interface (OAPI) – commercially available software, that allows for display of kinematic data in real-time. Movement signals from the jaw were represented on the computer screen as a white square 1cm wide. Participants were instructed to keep “the white square” inside “the black square” as best as they could. They were also instructed to keep their head as still as possible and only move their jaw during tracking.
2.5.1 Jaw tracking: Calibration
The maximum extent of jaw movement was calibrated by asking participants to close their mouth comfortably with their lips together without clenching their teeth. A position of the IREDs in this configuration was acquired, which corresponded to the maximum closed jaw position. Then, the participants were asked to hold a 15 mm bite block between their incisors, and a second sample of IRED positions was acquired, which corresponded to the maximum open jaw position for calibration. The effective tracking range was set to 70% of the calibration distance (maximum open jaw position – maximum closed jaw position), so that the maximum excursion for the jaw was set at 12mm, a distance shown to correspond to jaw opening amplitude during speech (Edwards & Harris, 1990). These procedures permitted participants to maintain what was thought to be a comfortable tracking range and avoid requiring participants to close their mouth completely or open it too wide during tracking. The calibration of the Optotrak system was done separately for each participant and was performed before a pre-test practice session.
2.5.2 Jaw tracking: Pre-test practice trial
The pre-test practice trial lasted for 30 seconds. The pre-test permitted participants to get acquainted with the way the movement of their jaw translated to the movement of the white square on the computer screen. During the practice trial only the white square controlled by the participant's jaw movement was displayed on the computer screen (no target was presented). Participants were encouraged to open and close their jaw and observe the way this movement is reflected by movement of the while square on the computer screen. After the pre-test practice trial the first author inquired if the participant understood the task, and felt ready to start. After confirming that the participant was ready to start, the first author started the testing conditions in the order unique to each participant. For both jaw and hand tracking, every testing condition started with a warning tone and after a one-second-delay a moving target appeared on the computer screen for participants to track.
2.6 Hand tracking
For all hand tracking conditions, a small portable table was placed next to the participant's chair. Participants were asked to rest their dominant hand on the table top during hand tracking. The height of the table was adjusted for each participant to make their arm feel comfortable during tracking. The table also had marks used for calibration of the Optotrak and two stationary bars attached to each side of the table for infrared-emitting diode (IRED) placement.
An IRED marker was attached to the participant's middle finger on the dominant hand with the use of adhesive tape. To track the target, participants were instructed to slide their hand horizontally while keeping it rested on the tabletop. They were instructed to move their hand together with the lower arm as if it was a hand of a clock or a pendulum. To minimize movement of the wrist during tracking, participants wore a commercially available wrist stabilizer during tracking. Movements of the hand were transduced via the Optotrak system to appear on the computer screen. Movements of the hand were referenced to a marker that was stationary and was placed on one of the two stationary bars on the tabletop.
2.6.1 Hand tracking: Calibration
The maximum extent of hand movement was calibrated by asking participants to align their middle finger (with the marker placed on it) with two lines marked on the tabletop: one corresponding to the maximally closed position, and the other corresponding to the maximally open position. The effective hand tracking range was 10.5cm and used 70% of the maximal range (15cm) to avoid touching the ends of the tracking range with the hand during tracking. Before the start of each tracking trial, participants were asked to align their middle finger with the line marking the center of the tracking range on the table top. The same pre-test practice trial procedures as with the jaw tracking were implemented for hand tracking.
As mentioned above, jaw as well as hand tracking involved the same 9 conditions, with the exception that for the hand tracking conditions the target was moving in a horizontal plane, as opposed to a vertical plane for the jaw tracking.
2.7. Pre-analysis signal processing (Quantifying tracking accuracy)
Movements of the effector during tracking were measured from the Optotrak outputs, sampled at a rate of 50 Hz. During each 60 second trial, the positions of the participant's jaw or hand during tracking were recorded as x, y coordinates, together with the coordinates of the target and the corresponding time stamp.
The goal of the study was to assess tracking accuracy and its improvement over time (defined as motor learning). We examined improvements in accuracy within the first trial as well as between the first and the second trials of predictable conditions. Due to the sinusoidal nature of the target and the participant's tracking movements, magnitude and phase were chosen to quantify the accuracy of tracking. These were in turn used to assess the extent of participants’ overshoot or undershoot of the target and time lag or lead relative to the target.
We used a fitting procedure described immediately below to estimate the magnitude and phase differences between the target and the participant's tracking. Prior to implementing the fitting procedure, the raw target signals were shifted on the y-axis so that the amplitudes were symmetric around zero and then scaled so that the sinusoidal amplitude of each target had peak values of ±1 (as shown in Fig. 1). The raw participant tracking signals were shifted and scaled by identical amounts so that the relative differences were preserved.
The total time for each recording was nominally approximately 60 seconds. For analysis purposes, recordings were truncated so that they contained an integral number of cycles of the target. For the 0.3 Hz condition, each recording was truncated to contain 17 full cycles, resulting in a length of which was approximately 56.67 s. In a similar manner, the 0.6 and 0.9 Hz conditions were truncated to contain 35 and 53 full cycles, with resulting lengths of 58.33 s and 58.89 s, respectively.
The magnitude and phase of the tracking as a function of time, relative to the target, were computed using a sliding window and an ordinary least-squares fitting procedure. The window length was one-half cycle of the target sinusoid. Each subsequent time window was shifted by a quarter cycle of the target, resulting in 50% overlap with the previous time window and 3 fits per cycle. This procedure resulted in a total of 51, 105, and 159 fits for target frequencies of 0.3, 0.6, and 0.9 Hz, respectively.
Basis functions for the fitting were formed by creating sine and cosine waves one-half cycle in duration. A design matrix was formed for the model
| (1) |
where ŷ was the fit, m controlled the frequency and was set equal to 0.5 in order to create one-half cycle, n was a vector of integers from 0 to N-1, and N was the total number of samples in the window. The variable k, which represents the mean amplitude offset from zero, was expected to be zero in every case. Fits were made to both the target and the tracking waveforms. For each fit, the coefficients a, b, and k were chosen to minimize the weighted sum of squared errors between ŷ and the target or the tracking. Once the coefficients were obtained, the magnitude, M, and phase, φ, of the each fit were calculated as
| (2) |
and
| (3) |
2.7.1. Dependent Variables
The accuracy of tracking was determined through the use of three measures: (1) gain, (2) delay, and (3) total error magnitude. Gain was defined as the ratio of tracking magnitude (Mtrack) relative to target magnitude (Mtarget),
| (4) |
where magnitude was calculated by Eq. 2. Delay (expressed in cycles) was the target phase (in radians, as calculated by Eq. 3) minus the tracking phase, divided by 2π,
| (5) |
Using the sliding window described above, multiple fits were computed for each recording, resulting in multiple estimates of gain and delay (51, 105, and 159 fits for target frequencies of 0.3, 0.6, and 0.9 Hz, respectively). In order to reduce high-frequency noise in these data and make the underlying trends across time more clear, the fits were low-pass filtered with a cubic smoothing spline (implemented by the Matlab function csaps, using a smoothing parameter of 0.005).
Accurate tracking was expected to result in gain values close to 1. Gain values < 1 indicate amplitude undershoot in the tracking, while values of gain >1 indicate overshoot. Accurate tracking was also expected to result in delay values close to 0. Negative (−) delay values indicate a phase or temporal lead in the tracking, suggesting the participant anticipated the target. Positive (+) delay values indicate a phase or temporal lag in the tracking. A split between positive and negative delays suggests that the participant neither consistently anticipated nor followed the target.
The error magnitude variable was calculated based on gain and delay variables, resulting in a combined measure of overall tracking accuracy. Because gain and delay are theoretically independent, they are useful measures to examine with regard to the tracking strategies applied by the two groups. As an overall estimate of motor learning, however, it was easier to interpret the results in terms of the magnitude of tracking error. Expressed in terms of the gain and delay calculations given above (Eq. 4 and 5), error magnitude, |ε|, was defined as
| (6) |
where g is gain and d is delay. Perfectly accurate tracking would yield an error magnitude of zero; the greater the error magnitude, |ε|, the greater the total error.
The fitting procedure and calculations of gain, delay, and error magnitude were completed separately for each of the 30 participants. Figure 2 shows a representative example of tracking data along with calculated gain, delay, and error magnitude values.
Figure 2. Tracking of the target.
Example of tracking accuracy measures obtained from the fitting procedure. Top panel shows data from one participant for the first 30 second trial of Level 2a. The target is shown in black line, and the tracking in dotted line. The bottom three panels show smoothed gain, delay, and error magnitudes. The gain data shows a trend of undershoot in the first 20 seconds of tracking and overshoot towards the end of the 30 second interval. The delay data shows a trend of initial lag and subsequent tracking with a minimal deviation from the target. Error magnitude shows the overall decrease in tracking error as the trial progresses.
2.8. Motor control analysis
We compared tracking accuracy (as measured by gain and absolute values of delay) for predictable conditions (level 1 and level 2) and unpredictable conditions (level 3). Multiple estimates of each dependent variable (gain, delay) were averaged within each trial for each participant. Thus, each participant contributed one gain and one delay value per tracking trial. Statistical analysis of data was performed in IBM SPSS Statistics software using a mixed model design with group, condition, target frequency and trial as fixed factors (with repeated measures on condition, target frequency and trial) and delay or gain as the dependent variable. The model tested for the main effect of each fixed factor as well as the group by each fixed factor interactions. For the level 3 (unpredictable) condition analysis, group and target frequency were the fixed factors (with repeated measures on target frequency). The model tested for the main effect of group and target frequency as well as the group by target frequency interaction. Where applicable, the follow up tests were carried out using the Bonferroni correction for multiple comparisons.
2.9. Analysis of motor practice effect
Motor practice effect in the current investigation was defined as improvement in tracking accuracy over time. Motor practice was examined at both the between-trial and within-trial level for predictable conditions (level 1 and 2). Between-trial learning was examined by assessing the main effect of trial for predictable conditions in the mixed model design, as described above.
Within-trial motor practice was assessed by carrying out several repeated measures ANOVAs with group (PWS and PWNS) as the between-group factor and time as the within-group factor with repeated measures; 6 non-overlapping consecutive 10-second periods of tracking performance within the first 60 second tracking trial were used in the analyses.1 Error magnitude was the dependent variable. Multiple estimates of error magnitude were averaged over each time interval within the first 60 second tracking trial, thus each participant contributed six error magnitude values for the within-trial motor practice analyses. Hereafter, we refer to each consecutive time interval as time1, time2, time3, time4, time5 and time6. Where applicable, the extent of within trial increases in accuracy (i.e. motor practice effects) between groups was analyzed using the Bonferroni corrected multiple comparisons at each time interval.
3. Results
3.1. Motor control
3.1.1. Jaw tracking
3.1.1.1. Predictable signals (level 1 and 2)
Spatial tracking of target (Gain)
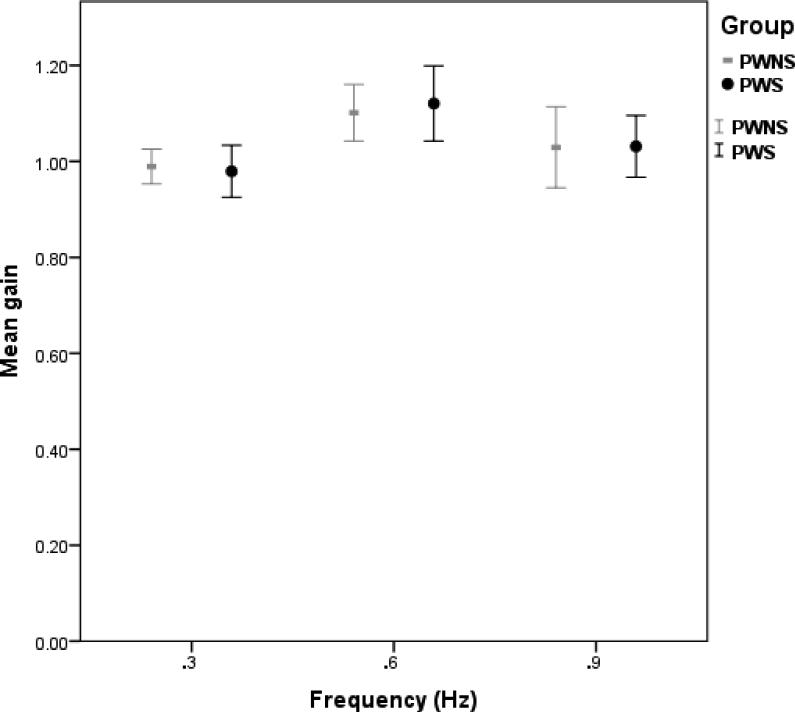
The gain variable represented accuracy with which participants were able to match the spatial location of the target. Mixed model analysis of jaw tracking of predictable signals indicated that there was a significant effect of condition (F (1, 322) =169.34; p<.001; d2=1.16) with level 1 conditions associated with greater accuracy than level 2 conditions and a significant effect of frequency (F (1, 322) =12.82; p<.001). Multiple comparisons analysis showed that .3Hz frequency was associated with more accurate tracking of the target than .6Hz (d=.34) and .9Hz (d=.49) frequencies; .6 Hz frequency was also associated with more accurate tracking than .9Hz frequency (d=.18). There was neither a significant main effect of group (F (1, 28) =.026; p=.874; d=.03) nor trial (F (1, 322) =.445; p=.505; d=.05) or group by fixed factor interactions. Figure 3 shows the mean gain values for each group.
Figure 3.
Mean gain for predictable tracking conditions (error bars: +/− 2 SE). Data from jaw tracking.
Temporal tracking of target (Delay)
Mixed model analysis of jaw tracking of predictable signals (using absolute values of delay) indicated that there was a significant effect of trial (F (1, 322) =8.11; p=.005; d=.19) with participants showing a significantly smaller target-tracker time difference (i.e. more accurate) on the second tracking trial compared to the first one, indicating a learning effect, and a significant effect of condition (F (1, 322) =202.73; p<.001; d=1.11) with level 1 conditions associated with greater accuracy than level 2 conditions. There was also a significant effect of frequency (F (2, 322) =30.25; p<.001). Multiple comparisons analysis showed that .3Hz frequency was associated with larger target-tracker time difference than .6 Hz (d= .60) and .9Hz (d=.56) frequencies. There was neither a significant main effect of group (F (1, 28) =1.31; p=.262; d=.23) nor group by fixed factor interactions. Figure 4 shows the mean absolute delay values for each group.
Figure 4.
Mean absolute delay (in seconds) for predictable tracking conditions (error bars: +/− 2 SE). Data from jaw tracking.
3.1.1.2. Unpredictable signals (level 3)
Spatial tracking of target (Gain)
Mixed model analysis of jaw tracking of unpredictable signals revealed a significant effect of frequency (F (2, 56) =12.18; p<.001). Multiple comparisons analysis indicated that .3Hz (d=1.12) and .9Hz (d=.59) frequencies were associated with better accuracy (less overshoot) than .6Hz. There was neither a significant main effect of group (F (1, 28) =.012; p=.914; d=.03) nor group by frequency interaction. Figure 5 shows the mean gain values for each group.
Figure 5.
Mean gain for unpredictable tracking conditions (error bars: +/− 2 SE). Data from jaw tracking.
Temporal tracking of target (Delay)
Mixed model analysis of jaw tracking of unpredictable signals revealed a significant effect of frequency (F (2, 56) =7.32; p=.001). Multiple comparisons analysis indicated that .3Hz frequency was associated with larger target-tracker time difference than .6 Hz (d=.80) and .9Hz (d=.41) frequencies. There was neither a significant main effect of group (F (1, 28) =2.23; p=.147; d=.43) nor group by frequency interaction. Figure 6 shows the mean absolute delay values for each group.
Figure 6.
Mean absolute delay (in seconds) for unpredictable tracking conditions (error bars: +/− 2 SE). Data from jaw tracking.
3.1.2. Hand tracking
3.1.2.1. Predictable signals
Spatial tracking of target (Gain)
Mixed model analysis of hand tracking of predictable signals showed that there was a significant effect of condition (F (1, 322) =28.63; p<.001; d=.41) with level 1 conditions associated with greater accuracy than level 2 conditions. There was also a significant effect of frequency (F (2, 322) =8.86; p<.001) with .3Hz associated with a better accuracy (less overshoot) than .6Hz (d=.46) and .9Hz (d=.24) frequencies. Analysis also revealed a significant group by frequency interaction (F (2, 322) =3.80; p=.023) in the absence of significant simple effects. Group effect was not significant (F (1, 28) =.014; p=.908; d=.03). Figure 7 shows the mean gain values for each group.
Figure 7.
Mean gain for predictable tracking conditions (error bars: +/− 2 SE). Data from hand tracking.
Temporal tracking of target (Delay)
Mixed model analysis of hand tracking of predictable signals (using absolute values of delay) indicated that there was a significant effect of trial (F (1, 322) =13.75; p<.001; d=.25) with participants showing a significantly smaller target-tracker time difference (i.e. more accurate) on the second tracking trial compared to the first one, indicating a learning effect. There was also a significant effect of condition (F (1, 322) =278.84; p<.001; d=1.34) with level 1 conditions associated with greater accuracy than level 2 conditions, and a significant group by condition interaction (F (1, 322) =4.81; p=.029). No other interactions were significant. Test of simple effects showed that there was no between-group difference for level 1 conditions, however, for level 2 conditions, there was a significant between-group difference with PWNS tracking with less delay than PWS (F (1) =4.27; p=.04; d=.31).
There was also a significant effect of frequency (F (2, 322) =6.47; p=.002). Multiple comparisons analysis showed that .3Hz frequency was associated with more accurate tracking of the target (smaller target-tracker time difference) than .6Hz (d=.28) and .9Hz (d=.25) frequencies. There was no significant effect of group (F (1, 28) =.70; p=.410; d=.17). Figure 8 shows the mean absolute delay values for each group.
Figure 8.
Mean absolute delay (in seconds) for predictable tracking conditions (error bars: +/− 2 SE). Data from hand tracking.
3.1.2.2. Unpredictable Signals
Spatial tracking of target (Gain)
Mixed model analysis of hand tracking of unpredictable signals revealed a significant effect of frequency (F (2, 56) =4.75; p=.012). Multiple comparisons analysis indicated that .3Hz (d=.66) and .6Hz (d=.47) frequencies were associated with more accurate tracking (less undershoot) of the target than .9Hz. There was neither a significant effect of group (F (1, 28) =.378; p=.544; d=.16) nor group by frequency interaction. Figure 9 shows the mean gain values for each group.
Figure 9.
Mean gain for unpredictable tracking conditions (error bars: +/− 2 SE). Data from hand tracking.
Temporal tracking of target (Delay)
Mixed model analysis of hand tracking of unpredictable signals revealed a significant effect of frequency (F (2, 56) =3.83; p=.028). Multiple comparisons analysis indicated that .6Hz frequency was associated with more accurate tracking (smaller target-tracker time difference) of the target than .9Hz (d=.55). There was also a marginally significant main effect of group (F (1, 28) =4.03; p=.055; d=.53) with PWS exhibiting larger target-tracker time difference than PWNS. Figure 10 shows the mean absolute delay values for each group.
Figure 10.
Mean absolute delay (in seconds) for unpredictable tracking conditions (error bars: +/− 2 SE). Data from hand tracking.
3.1.3. Summary of motor control analysis and between-trial motor learning analysis
PWS were not significantly different from PWNS in matching the timing and spatial position of the target during either jaw or hand tracking for either predictable (level 1 and 2) or unpredictable conditions apart from one exception. PWS exhibited a significantly larger delay than PWNS during hand tracking of level 2 conditions. For predictable conditions, level 2 conditions were associated with larger tracking errors than level 1. Both groups improved their temporal tracking accuracy (as measured by delay) from the first to the second tracking trial during predictable conditions of both jaw and hand tracking, indicating a practice effect. No practice (trial effect) was observed for spatial accuracy (as measured by gain) for either jaw or hand.
3.2. Within-trial motor practice
3.2.1. Jaw tracking: Simple sine waves/Level 1
Repeated measures ANOVA assessment (with group as the between-group factor and time as the within-group factor with repeated measures) revealed a significant main effect of time for 0.3Hz (F (1, 2.89)1 =4.92; p=.004), for 0.6Hz (F(1, 3.57)=23.46; p<.001), and 0.9Hz (F (1, 4.31)=19.17; p<.001) for all participants, with no interaction between group and time. There was no significant main effect of group. Test of within-subject contrasts showed that the significant decrease in error occurred between time1 (first 10 sec.) and time2 (second 10 sec.) with no significant decrease thereafter for 0.3Hz (F(1)=8.85; p=.006; d=.66), and for 0.6Hz (F(1)=56.71; p<.001; d=1.68). For 0.9Hz there was a significant decrease in error between time1 and time2 (F(1)=69.93; p<.001; d=1.85) and between time4 and time5 (F(1)=4.34; p=.046; d=.39).
3.2.2. Jaw tracking: Complex sine patterns/Level 2
Repeated measures ANOVA revealed a significant main effect of time for 0.3Hz (F(1,4.02)=4.75; p=.001), for 0.6Hz (F(1,4.64)=11.89; p<.001), and 0.9Hz (F(1,3.45)=12.23; p<.001) with no interaction between group and time. There was no significant main effect of group. A test of within-subject contrasts showed that the significant decrease in error occurred between time1 and time2 with no significant decrease thereafter for 0.3Hz (F(1)=16.39; p<.001; d=.65), for 0.6Hz (F(1)=25.06; p<.001; d=1.06), and for 0.9Hz (F(1)=57.70; p<.001; d=1.34) with no significant decrease thereafter.
3.2.3. Hand tracking: Simple sine waves/ Level 1
A repeated measures ANOVA revealed a significant main effect of time for 0.3Hz (F(1,2.19)=30.93; p<.001), for 0.6Hz (F(1,2.60)=33.97; p<.001), and 0.9Hz (F(1,3.41)=42.37; p<.001) for all participants, with no interaction between group and time. There was no significant main effect of group. A test of within-subject contrasts showed that the significant decrease in error occurred between time1 and time2 (F(1)=41.44; p<.001; d=1.08) and time2 and time3 (F(1)=4.06; p=.05; d=.21) with no significant decrease thereafter for 0.3Hz. There was a significant decrease in error between time1 and time2 (F(1)=52.31; p<.001; d=1.67) and time 3 and time4 (F(1)=4.01; p=.05; d=.25) for 0.6Hz, and between time1 and time2 for 0.9Hz (F(1)=115.67; p<.001; d=1.8).
3.2.4. Hand tracking: Complex sine patterns/ Level 2
A repeated measures ANOVA revealed a significant main effect of time for 0.3Hz (F(1,2.38)=9.66; p<.001), for 0.6Hz (F(1,2.53)=25.85; p<.001), and for 0.9Hz (F(1,3.25)=18.44; p<.001) for all participants, with no interaction between group and time. There was no significant main effect of group. A test of within-subject contrasts showed that the significant decrease in error occurred between time1 and time2 for the 0.3Hz (F(1)=18.43; p<.001; d=.54) and 0.9 Hz frequency (F(1)=50.23; p<.001; d=1.28) with no significant decrease thereafter. For the 0.6Hz frequency, there was a significant decrease in error between time1 and time2 (F(1)=30.22; p<.001; d=.90) and time3 and time4 (F(1)=15.36; p=.001; d=.30) with no significant decrease thereafter.
3.2.5. Summary of within-trial motor practice findings
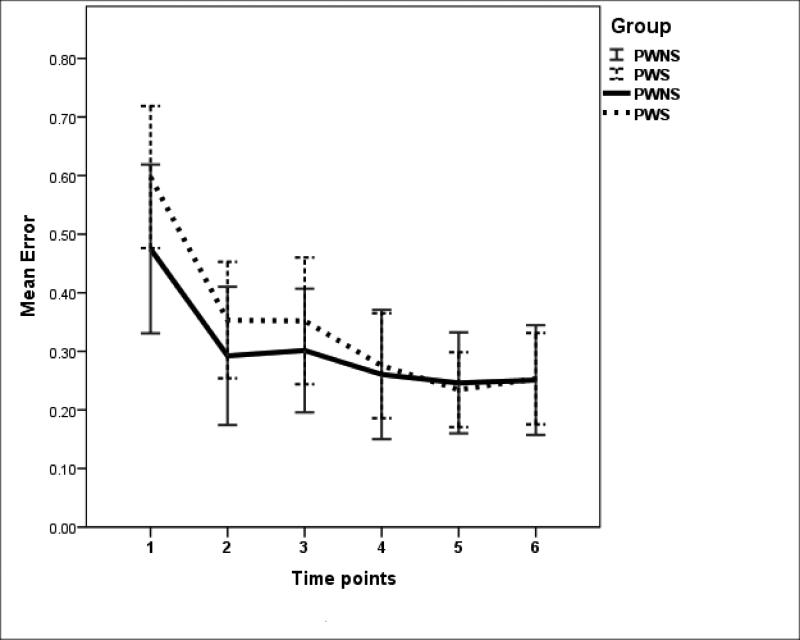
There were no significant between-group differences for within-trial motor practice for either jaw or hand, with both groups exhibiting most dramatic increase in accuracy from the first to second 10-second interval (with such increase taken as an index of practice effects). Graphic depiction of the error decrease trend is presented in Figure 11. The trend shown in Figure 11 is representative of both jaw and hand tracking within-trial error changes.
Figure 11.
Changes in error magnitude over time (error bars: +/− 2 SE). Hand tracking of level 2 0.6Hz condition.
4. Discussion
The present study resulted in two main findings. First, PWS did not differ significantly from PWNS in either temporal or amplitude matching of the target for either jaw or hand tracking in both predictable and unpredictable tracking conditions, apart from one condition – level 2 predictable hand tracking where PWS had a significantly larger delay than PWNS. Second, both groups exhibited motor practice effects (i.e., increased accuracy over time) within and between tracking trials during both jaw and hand tracking and there were no significant group differences in the rate or extent of learning. These findings will be discussed below.
4.1. Motor tracking accuracy
In the present study we used a task that allowed us to simultaneously assess the temporal and spatial aspects of motor tracking accuracy in different effector systems (i.e. jaw and hand). In this way, we addressed a limitation in prior work that examined the temporal domain alone, making it impossible to assess the contributions that the spatial aspects of movement make to stuttering. Between-group comparisons of the temporal and spatial accuracy results for both jaw and had movements were not significant, at least in the context of the task employed. While these results do not lend support to the hypothesis that PWS have a more generalized motor control deficit, it is important to consider that the PWS in our study showed a trend toward less accurate temporal tracking in both jaw and hand tracking and a significantly larger absolute delay in hand tracking of level 2 predictable conditions. Considering these results, two points are clear. First, we cannot rule out the possibility that both the predictable and unpredictable tracking tasks we used in this study, for both jaw and hand tracking, did not challenge the timing mechanisms that are critical for speech production (for review see Weismer, 2006). This becomes especially relevant in light of research assessing the interactions between motoric, linguistic and emotional variables, which suggests that PWS may experience increased susceptibility to fluency breakdowns when the system is stressed with linguistic (Smith, Sadagopan, Walsh, & Weber-Fox, 2010) and/or emotional (Conture & Walden, 2012; Conture et al., 2006) requirements. Second, our participants were adults who stutter and it is possible that they may have employed learned compensatory processes that obscured any potential underlying differences in the timing control of movements.
One factor that may have influenced the results of the present investigation is the presence of visual feedback during tracking. According to the Speech Motor Skill (SMS) approach proposed by van Lieshout and colleagues (van Lieshout, Hulstijn & Peters, 1996a, 1996b, 2004) PWS are inclined to use a less automated motor strategy which renders them more dependent on sensory information during movement execution. In the present study visual feedback was always available to participants during both jaw and hand tracking tasks, which may have influenced performance of PWS and PWNS to a different degree. Prior work has shown that children (Howell et al., 1995) and adults who stutter (Archibald & De Nil, 1999; Loucks & De Nil, 2006) performed as well as people who do not stutter in non-speech jaw movement task when visual feedback was available, and significantly less accurate in the absence of visual feedback. Researchers have theorized that the relative inaccuracy of jaw movements shown by children who stutter and adults who stutter in the absence of visual feedback may be explained by aberrant proprioceptive integration, suggesting an oral kinesthetic deficit (Archibald & De Nil, 1999; Loucks & De Nil, 2006; Loucks, De Nil, & Sasisekaran, 2007; cf. Namasivayam, van Lieshout, McIlroy, & De Nil, 2009).
In the unpredictable tracking task, PWS did not significantly differ from PWNS in their ability to match the timing and amplitude of the target. This task required constant sensorimotor integration because the ongoing motor plan had to be continually updated to match the unpredictable movement of the target. Our results suggest that PWS do not exhibit difficulties with visual-motor integration required to accurately track a moving target. It is important to note the recent evidence of difficulties in auditory-motor integration in PWS (Cai et al., 2012, 2014; Loucks et al., 2012). It is possible that if sensorimotor integration deficits do exist in PWS, they may not be generalized across all modalities and may be restricted to the auditory and/or proprioceptive domains which are salient for speech production.
4.2. Motor practice effects
In the context of the tracking tasks we used in this study, present findings do not support the hypothesis that PWS benefit less from motor practice relative to PWNS. In general, both talker groups exhibited practice effects (i.e., increased accuracy over time) within and between tracking trials during both jaw and hand tracking. Findings suggest that within-trial learning largely occurred in the initial 10 seconds of tracking exposure. However, our data also show a significant accuracy improvement between consecutive trials of predictable conditions for both groups.
Previous studies that examined learning of a motor pattern during a single testing session report conflicting results. Some report that adults who stutter have limited ability to acquire a new motor pattern as observed in the same day performance (Nielson & Nielson 1991; Smits-Bandstra et al., 2006; Webster, 1986), while others do not find significant differences between PWS and PWNS (Namasivayam & van Lieshout, 2008; Bauerly & De Nil, 2011). Our results support the latter studies, showing that PWS were able to improve their accuracy over time as much as PWNS when the testing occurred in one practice session.
Though participants in our study exhibited immediate improvement in accuracy in the first seconds of exposure to a tracking task, it is possible that this immediate learning may not be retained after a period of rest. This leads to an important point. Some have suggested (Bauerly & De Nil, 2011) that short-term learning paradigms, like the one used in the present study, do not adequately assess “learning” and should be attributed to practice effects. Learning rather must be inferred from measurement of the skill transfer to a novel task or performance improvement following a period of rest. Given that the present paradigm was not designed to assess long-term accuracy improvement and did not allow for prolonged or varied practice to take effect, our findings should be interpreted with caution. Instead, our findings are probably most germane to practice effects or “early” learning (Krebs et al., 2001). Our study does, however, provide arguably valuable information about immediate learning of a motor skill in PWS.
As pointed out by Namasivayam and van Lieshout (2008), one of the limitations in existing motor learning studies in stuttering research is that both motor practice and learning has been measured without reference to the speed of movement to be learned, or articulation rate in the case of speech motor learning. The present study attempted to address this issue by measuring changes in tracking accuracy over time under slow (0.3 Hz) medium (0.6 Hz) and fast (0.9 Hz) movement conditions. Our data suggest that PWS and PWNS improve accuracy of both jaw and hand tracking equally across varying speeds of movement, and do not support the hypothesis that PWS's motor execution or learning would be selectively compromised at fast rates of movement.
4.3. Caveats
Empirical evidence indicates that PWS may benefit from and rely on sensory feedback to a greater degree than PWNS (Archibald & De Nil, 1999; Loucks & De Nil, 2006; Max et al., 2004; Namasivayam & van Lieshout, 2008). Thus, the quality of external stimulus in the present study may have impacted the accuracy of jaw and hand tracking and the learning process. Perhaps the external “feedback” used in the present study minimized differences between PWS and PWNS, speculation that must await future study.
The current study did not examine whether there are within-group dissociations in tracking accuracy and its variability, a finding reported for linguistic skills of children who stutter (Anderson et al., 2005; Coulter et al., 2009). This becomes increasingly important given the findings that PWS may have similar performance to people who are normally fluent in linguistic and non-linguistic domains, but distribution of their performance may be a bimodal in nature (Olander et al., 2010), supporting the existence of subgroups among PWS (e.g. Conture, & Schwartz, 1988).
One salient caveat pertaining to the current study is that our paradigm did not allow for differentiation of the influence on tracking performance of motor execution abilities versus visual and attention mechanisms. Related to that is the issue of using visual feedback for movement control. It has been shown that PWS did not differ from their controls on motor tasks when visual feedback was provided. By contrast, they did not perform as well as controls in tasks without feedback. Thus, future research should incorporate motor control and learning tasks performed with and without feedback, to elucidate the role it plays in motor control of people who stutter.
Another limitation of the present study is that its methods only allowed for assessment of practice effects or immediate learning. Having participants come back several times over days or weeks to perform the same task would enable the assessment of long-term changes in accuracy and/or skill acquisition and retention, both important stages of motor learning.
Supplementary Material
Highlights.
Participants tracked a moving target with their jaw and their dominant hand separately, and accuracy for tracking both (a) predictable and (b) unpredictable target movements was assessed.
Compared to PWNS, PWS were not significantly different in matching the amplitude and timing of the target in both jaw and hand tracking of both predictable and unpredictable targets.
There were no significant between-group differences in motor practice effects for either jaw or hand tracking.
Acknowledgements
This research was supported in part by NIH research grants to Purdue (DC000559) and Vanderbilt (DC000523-14A1) Universities, as well as the University of Iowa Executive Council of Graduate and Professional Students research grant.
The authors would like to thank Edward G. Conture for reviewing earlier drafts of this paper and E. Warren Lambert for providing statistical advice. Lastly, the authors would like to thank participants in this study who made this work possible.
Biography
Victoria Tumanova
Victoria Tumanova earned her PhD in Speech and Hearing Science from the University of Iowa. She completed a postdoctoral fellowship at Vanderbilt University. She is currently an assistant professor in the Department of Communication Sciences and Disorders at Syracuse University.
Patricia Zebrowski
Tricia Zebrowski is an ASHA Fellow and Board Recognized Fluency Specialist. She has authored numerous research and clinical papers, book chapters, videos, and a manual for stuttering intervention. Her research focuses on potential risk factors in the development of different subtypes of stuttering in children and the physiological correlates of stuttering.
Shawn Goodman
Shawn Goodman earned BS and MS degrees in Audiology from Brigham Young University, and his PhD from Indiana University. He completed a postdoctoral fellowship at Boys Town National Research Hospital. He is currently an Assistant Professor in the Department of Communication Sciences and Disorders at the University of Iowa.
Rick Arenas
Rick Arenas is an Assistant Professor in the Department of Speech and Hearing Sciences at the University of New Mexico. His research interests include mechanisms of online speech monitoring as well as biological mechanisms of learning and how individual variability in these mechanisms relates to stuttering development and treatment.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
As described in the Pre-analysis signal processing section of the method, the 60 second recordings were truncated to contain an integral number of sinusoidal cycles, thus the duration of the 6th period of tracking performance was 6.67 seconds for .3 Hz condition and 8.33 seconds and 8.89 seconds for .6 and .9 Hz conditions.
Throughout the results section “d” refers to the Cohen's d, a measure of effect size.
Since both our jaw and hand tracking data in motor learning analysis for the within-trial comparisons did not satisfy the sphericity condition, the Huynh-Feldt correction was used to adjust the degrees of freedom for the F-tests.
Contributor Information
Victoria Tumanova, Current affiliation: Department of Communication Sciences and Disorders, Syracuse University, 621 Skytop Rd, Syracuse, NY 13244.
Patricia M. Zebrowski, Department of Communication Sciences and Disorders, The University of Iowa, Wendell Johnson Speech and Hearing Center, Iowa City, IA 52242, phone: +1 319-335-8735 tricia-zebrowski@uiowa.edu
Shawn S. Goodman, Department of Communication Sciences and Disorders, The University of Iowa, Wendell Johnson Speech and Hearing Center, Iowa City, IA 52242, phone: +1 319-335-8700 shawn-goodman@uiowa.edu
Richard M. Arenas, Current Affiliation: Department of Speech and Hearing Sciences, The University of New Mexico, 1700 Lomas Blvd. NE Suite 1300, Albuquerque, NM 87131 rickarenas@unm.edu phone: +1 505-277-4453
References
- Adams SG, Weismer G, Kent RD. Speaking rate and speech movement velocity profiles. Journal of Speech and Hearing Research. 1993;36:41–54. doi: 10.1044/jshr.3601.41. [DOI] [PubMed] [Google Scholar]
- Alm PA. Stuttering and the basal ganglia circuits: a critical review of possible relations. Journal of Communication Disorders. 2004;37(4):325–369. doi: 10.1016/j.jcomdis.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Anderson JD, Pellowski MW, Conture EG. Childhood stuttering and dissociations across linguistic domains. Journal of fluency disorders. 2005;30(3):219–253. doi: 10.1016/j.jfludis.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Archibald L, De Nil LF. The relationship between stuttering severity and kinesthetic acuity for jaw movements in adults who stutter. Journal of Fluency Disorders. 1999;24(1):25–42. [Google Scholar]
- Ballard KJ, Robin DA. Influence of continual biofeedback on jaw pursuit-tracking in healthy adults and in adults with apraxia plus aphasia. Journal of Motor Behavior. 2007;39(1):19–28. doi: 10.3200/JMBR.39.1.19-28. [DOI] [PubMed] [Google Scholar]
- Ballard KJ, Robin DA, Folkins JW. An integrative model of speech motor control: A response to Ziegler. Aphasiology. 2003;17(1):37–48. [Google Scholar]
- Ballard KJ, Robin DA, Woodworth G, Zimba L. Age-related changes in motor control during articulator visuomotor tracking. Journal of Speech, Language and Hearing Research. 2001;44:763–777. doi: 10.1044/1092-4388(2001/060). [DOI] [PubMed] [Google Scholar]
- Ballard KJ, Solomon NP, Robin DA, Moon JB, Folkins JW. Nonspeech Assessment of the Speech Production Mechanism. In: McNeil MR, editor. Clinical Management of Sensorimotor Speech Disorders. Thieme Medical Publishers; New York: 2008. [Google Scholar]
- Barasch CT, Guitar B, McCauley RJ, Absher RG. Disfluency and Time Perception. Journal of Speech Language and Hearing Research. 2000;43(6):1429–1439. doi: 10.1044/jslhr.4306.1429. [DOI] [PubMed] [Google Scholar]
- Bauerly KR, De Nil LF. Speech sequence skill learning in adults who stutter. Journal of Fluency Disorders. 2011;36(4):349–360. doi: 10.1016/j.jfludis.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bengtsson SL, Ehrsson HH, Forssberg H, Ullén F. Effector-independent voluntary timing: behavioural and neuroimaging evidence. European Journal of Neuroscience. 2005;22(12):3255–3265. doi: 10.1111/j.1460-9568.2005.04517.x. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G. Motor functions of the Broca's region. Brain and Language. 2004;89(2):362–369. doi: 10.1016/S0093-934X(03)00358-4. [DOI] [PubMed] [Google Scholar]
- Bloodstein O, Bernstein Ratner N. A Handbook on Stuttering. 6 ed. Delmar; Clifton Park, NY: 2008. [Google Scholar]
- Boutsen FR, Brutten GJ, Watts CR. Timing and Intensity Variability in the Metronomic Speech of Stuttering and Nonstuttering Speakers. Journal of Speech Language and Hearing Research. 2000;43(2):513–520. doi: 10.1044/jslhr.4302.513. [DOI] [PubMed] [Google Scholar]
- Brown S, Ingham RJ, Ingham JC, Laird AR, Fox PT. Stuttered and fluent speech production: an ALE meta-analysis of functional neuroimaging studies. Human Brain Mapping. 2005;25(1):105–117. doi: 10.1002/hbm.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Beal DS, Ghosh SS, Tiede MK, Guenther FH, Perkell JS. Weak responses to auditory feedback perturbation during articulation in persons who stutter: evidence for abnormal auditory-motor transformation. PloS one. 2012;7:e41830. doi: 10.1371/journal.pone.0041830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai S, Beal DS, Ghosh SS, Guenther FH, Perkell JS. Impaired timing adjustments in response to time-varying auditory perturbation during connected speech production in persons who stutter. Brain and Language. 2014;129:24–29. doi: 10.1016/j.bandl.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassel KJ. The usefulness of a temporal correlation technique in the assessment of human motor performance on a tracking device. Medical and Biological Engineering and Computing. 1973;11:755–761. doi: 10.1007/BF02478664. [DOI] [PubMed] [Google Scholar]
- Civier O, Tasko SM, Guenther FH. Overreliance on auditory feedback may lead to sound/syllable repetitions: Simulations of stuttering and fluency-inducing conditions with a neural model of speech production. Journal of Fluency Disorders. 2010;35(3):246–279. doi: 10.1016/j.jfludis.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark HM, Robin DA, McCullagh G, Schmidt RA. Motor control in children and adults during a non-speech oral task. Journal of Speech, Language, and Hearing Research. 2001;44:1015–1025. doi: 10.1044/1092-4388(2001/080). [DOI] [PubMed] [Google Scholar]
- Cohen N, Squire L. Preserved learning and retention of pattern-analyzing skill in amnesia: dissociation of knowing how and knowing that. Science. 1980;210(4466):207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- Conture EG, Walden TA. A dual diathesis-stressor model of stuttering. In: Bellakova L, Filatova Y, editors. Theoretical Issues of Fluency Disorders. Vlados; Moscow: 2012. pp. 94–127. [Google Scholar]
- Conture EG, Walden TA, Arnold HS, Graham CG, Hartfield KN, Karrass J. Communication-emotional model of stuttering. In: Ratner NB, Tetnowski J, editors. Current issues in stuttering research and practice. Vol. 2. Lawrence Erlbaum Associates; Mahwah, NJ: 2006. pp. 17–47. [Google Scholar]
- Cooper MH, Allen GD. Timing control accuracy in stutterers and non-stutterers. Journal of Speech and Hearing Research. 1977;20:55–71. doi: 10.1044/jshr.2001.55. [DOI] [PubMed] [Google Scholar]
- Coulter CE, Anderson JD, Conture EG. Childhood stuttering and dissociations across linguistic domains: a replication and extension. Journal of Fluency Disorders. 2009;34(4):257–278. doi: 10.1016/j.jfludis.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Diego-Balaguer R, Couette M, Dolbeau G, Dürr A, Youssov K, Bachoud-Lévi A-C. Striatal degeneration impairs language learning: evidence from Huntington's disease. Brain. 2008;131(11):2870–2881. doi: 10.1093/brain/awn242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Nil LF, Bosshardt HG. Studying stuttering from a neurological and cognitive information processing perspective. In: Bosshardt HG, Yaruss JS, Peters HFM, editors. Fluency disorders: Theory, research, treatment, and self-help: Proceedings of the third world conference of fluency disorders. Nijmegen University Press; Nijmegen, The Netherlands: 2001. pp. 53–58. [Google Scholar]
- De Nil LF, Kroll RM, Houle S. Functional neuroimaging of cerebellar activation during single word reading and verb generation in stuttering and nonstuttering adults. Neuroscience Letters. 2001;302(2-3):77–80. doi: 10.1016/s0304-3940(01)01671-8. [DOI] [PubMed] [Google Scholar]
- Edwards J, Harris KS. Rotation and translation of the jaw during speech. Journal of Speech and Hearing Research. 1990;33:550–562. doi: 10.1044/jshr.3303.550. [DOI] [PubMed] [Google Scholar]
- Flowers KA. Some frequency response characteristics of Parkinson's disease. Brain. 1978;101:19–34. doi: 10.1093/brain/101.1.19. [DOI] [PubMed] [Google Scholar]
- Folkins JW, Moon JB, Luschei ES, Robin DA, Tye-Murray N, Moll KL. What can nonspeech tasks tell us about speech motor disabilities? Journal of Phonetics. 1995;23(1):139–147. [Google Scholar]
- Green JR, Moore CA, Higashikawa M, Steeve RW. The physiologic development of speech motor control: Lip and jaw coordination. Journal of Speech, Language and Hearing Research. 2000;43(1):239. doi: 10.1044/jslhr.4301.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JR, Nip IS. Maassen B, van Lieshout P, editors. Some organization principles in early speech development. Speech motor control: New developments in basic and applied research. 2010:171–188. [Google Scholar]
- Howell P, Sackin S, Rustin L. Comparison of speech motor development in stutterers and fluent speakers between 7 and 12 years old. Journal of Fluency Disorders. 1995;20:243–255. [Google Scholar]
- Kleinow J, Smith A. Influences of length and syntactic complexity on the speech motor stability of the fluent speech of adults who stutter. Journal of Speech, Language and Hearing Research. 2000;43(2):548–559. doi: 10.1044/jslhr.4302.548. [DOI] [PubMed] [Google Scholar]
- Krebs H, Hogan N, Hening W, Adamovich S, Poizner H. Procedural motor learning in Parkinson's disease. Experimental Brain Research. 2001;141(4):425–437. doi: 10.1007/s002210100871. [DOI] [PubMed] [Google Scholar]
- Loucks T, Chon H, Han W. Audiovocal integration in adults who stutter. International Journal of Language and Communication Disorders. 2012;47:451–456. doi: 10.1111/j.1460-6984.2011.00111.x. [DOI] [PubMed] [Google Scholar]
- Loucks TMJ, De Nil LF. Oral kinesthetic deficit in adults who stutter: A target- accuracy study. Journal of Motor Behavior. 2006;38(3):238–247. doi: 10.3200/JMBR.38.3.238-247. [DOI] [PubMed] [Google Scholar]
- Loucks TMJ, De Nil LF, Sasisekaran J. Jaw-phonatory coordination in chronic developmental stuttering. Journal of Communication Disorders. 2007;40(3):257–272. doi: 10.1016/j.jcomdis.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Max L. Stuttering and internal models for sensorimotor control: A theoretical perspective to generate testable hypotheses. In: Maassen B, Kent R, Qeters HFM, van Lieshout PHHM, Hulstijn W, editors. Speech motor control in normal and disordered speech. Oxford University Press; Oxford, UK: 2004. pp. 357–388. [Google Scholar]
- Max L, Baldwin CJ. The role of motor learning in stuttering adaptation: Repeated versus novel utterances in a practice–retention paradigm. Journal of Fluency Disorders. 2010;35(1):33–43. doi: 10.1016/j.jfludis.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max L, Caruso AJ, Gracco VL. Kinematic analyses of speech, orofacial nonspeech, and finger movements in stuttering and nonstuttering adults. Journal of Speech, Language and Hearing Research. 2003;46(1):215–232. doi: 10.1044/1092-4388(2003/017). [DOI] [PubMed] [Google Scholar]
- Max L, Guenther FH, Gracco VL, Ghosh SS, Wallace ME. Unstable or insufficiently activated internal models and feedback-biased motor control as sources of dysfluency: A theoretical model of stuttering. Contemporary Issues in Communication Science and Disorders. 2004;31:105–122. [Google Scholar]
- Max L, Yudman E. Accuracy and variability of isochronous rhythmic timing across motor systems in stuttering versus nonstuttering individuals. Journal of Speech, Language, and Hearing Research. 2003;46:146–163. doi: 10.1044/1092-4388(2003/012). [DOI] [PubMed] [Google Scholar]
- McClean MD, Beukelman DR, Yorkston KM. Speech-muscle visuomotor tracking in dysarthric and nonimpaired speakers. Journal of Speech, Language and Hearing Research. 1987;30(2):276. doi: 10.1044/jshr.3002.276. [DOI] [PubMed] [Google Scholar]
- McClean MD, Kroll RM, Loftus NS. Kinematic analysis of lip closure in stutterers' fluent speech. Journal of Speech, Language and Hearing Research. 1990;33(4):755. doi: 10.1044/jshr.3304.755. [DOI] [PubMed] [Google Scholar]
- Moon JB, Zebrowski P, Robin DA, Folkins JW. Visuomotor tracking ability of young adult speakers. Journal of Speech and Hearing Research. 1993;36(4):672–682. doi: 10.1044/jshr.3604.672. [DOI] [PubMed] [Google Scholar]
- Namasivayam AK, van Lieshout P. Investigating speech motor practice and learning in people who stutter. Journal of Fluency Disorders. 2008;33(1):32–51. doi: 10.1016/j.jfludis.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Namasivayam AK, van Lieshout P. Speech motor skill and stuttering. Journal of Motor Behavior. 2011;43(6):477–489. doi: 10.1080/00222895.2011.628347. [DOI] [PubMed] [Google Scholar]
- Namasivayam AK, van Lieshout P, McIlroy WE, De Nil L. Sensory feedback dependence hypothesis in persons who stutter. Human Movement Science. 2009;28(6):688–707. doi: 10.1016/j.humov.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Neilson MD, Neilson PD. Adaptive model theory of speech motor control and stuttering. In: Peters HFM, Hulstijn W, Starkweather CW, editors. Speech motor control and stuttering: Proceedings of the 2nd International Conference on Speech Motor Control and Stuttering. Excerpta Medica; Amsterdam: 1991. pp. 149–156. [Google Scholar]
- Olander L, Smith A, Zelaznik HN. Evidence That a Motor Timing Deficit Is a Factor in the Development of Stuttering. J Speech Lang Hear Res. 2010;53(4):876–886. doi: 10.1044/1092-4388(2009/09-0007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters HM, Hulstijn W, van Lieshout PH. Recent developments in speech motor research into stuttering. Folia Phoniatrica et Logopaedica. 2000;52(1-3):103–119. doi: 10.1159/000021518. [DOI] [PubMed] [Google Scholar]
- Plante E, Gómez RL, Gerken L. Sensitivity to word order cues by normal and langauge/learning disabled adults. Journal of Communication Disorders. 2002;35:453–462. doi: 10.1016/s0021-9924(02)00094-1. [DOI] [PubMed] [Google Scholar]
- Robin DA, Jacks A, Hageman C, Clark HM, Woodworth G. Visuomotor tracking abilities of speakers with apraxia of speech or conduction aphasia. Brain and Language. 2008;106(2):98–106. doi: 10.1016/j.bandl.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R, Lee T. Motor control and learning: A behavioral emphasis. 4th ed. Human Kinetics; Champaign, IL: 2005. [Google Scholar]
- Schwartz HD, Conture EG. Subgrouping young stutterers: Preliminary behavioral observations. Journal of Speech, Language and Hearing Research. 1988;31(1):62. doi: 10.1044/jshr.3101.62. [DOI] [PubMed] [Google Scholar]
- Smith A, Sadagopan N, Walsh B, Weber-Fox C. Increasing phonological complexity reveals heightened instability in inter-articulatory coordination in adults who stutter. Journal of Fluency Disorders. 2010;35(1):1–18. doi: 10.1016/j.jfludis.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits-Bandstra S, De Nil LF. Sequence skill learning in persons who stutter: Implications for cortico-striato-thalamo-cortical dysfunction. Journal of Fluency Disorders. 2007;32(4):251–278. doi: 10.1016/j.jfludis.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Smits-Bandstra S, De Nil L, Rochon E. The transition to increased automaticity during finger sequence learning in adult males who stutter. Journal of Fluency Disorders. 2006;31(1):22–42. doi: 10.1016/j.jfludis.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Tourville JA, Reilly KJ, Guenther FH. Neural mechanisms underlying auditory feedback control of speech. Neuroimage. 2008;39(3):1429–1443. doi: 10.1016/j.neuroimage.2007.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lieshout PHHM, Hulstijn W, Peters HFM. From planning to articulation in speech production: What differentiates a person who stutters from a person who does not stutter. Journal of Speech and Hearing Research. 1996a;39:546–564. doi: 10.1044/jshr.3903.546. [DOI] [PubMed] [Google Scholar]
- van Lieshout PHHM, Hulstijn W, Peters HFM. Speech production in people who stutter: Testing the motor plan assembly hypothesis. Journal of Speech and Hearing Research. 1996b;39:76–92. doi: 10.1044/jshr.3901.76. [DOI] [PubMed] [Google Scholar]
- van Lieshout PHHM, Hulstijn W, Peters HFM. Searching for the weak link in the speech production chain of people who stutter: A motor skill approach. In: Maassen B, Kent RD, Peters HFM, van Lieshout PHHM, Hulstijn W, editors. Speech motor control in normal and disordered speech. Oxford University Press; Oxford: 2004. pp. 313–357. [Google Scholar]
- Webster WG. Response sequence organization and reproduction by stutterers. Neuropsychologia. 1986;24(6):813–821. doi: 10.1016/0028-3932(86)90080-1. [DOI] [PubMed] [Google Scholar]
- Weismer G. Philosophy of research in motor speech disorders. Clinical Linguistics & Phonetics. 2006;20(5):315–349. doi: 10.1080/02699200400024806. [DOI] [PubMed] [Google Scholar]
- Wieneke G, Janssen P, Brutten GJ. Variance of central timing of voiced and voiceless periods among stutterers and nonstutterers. Journal of Fluency Disorders. 1995;20(2):171–189. [Google Scholar]
- Willingham DB, Koroshetz WJ, Peterson EW. Motor skills have diverse neural bases: Spared and impaired skill acquisition in Huntington's disease. Neuropsychology. 1996;10:315–321. [Google Scholar]
- Zelaznik HN, Smith A, Franz EA, Ho M. Differences in bimanual coordination associated with stuttering. Acta Psychologica. 1997;96(3):229–243. doi: 10.1016/s0001-6918(97)00014-0. [DOI] [PubMed] [Google Scholar]
- Ziegler W. Speech motor control is task-specific: Evidence from dysarthria and apraxia of speech. Aphasiology. 17(1):3–36. [Google Scholar]
- Zimmermann G. Articulatory dynamics of fluent utterances of stutterers and nonstutterers. Journal of Speech, Language and Hearing Research. 1980;23(1):95. doi: 10.1044/jshr.2301.95. [DOI] [PubMed] [Google Scholar]
- Zimmermann GN, Smith A, Hanley JM. Stuttering: In Need of a Unifying Conceptual Framework. Journal of Speech and Hearing Research. 1981;24(1):25–31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.