Abstract
Background and Aims
To balance competing cardiovascular benefits and metabolic risks of statins, markers of type 2 diabetes (T2D) susceptibility are needed. We sought to define a competing risk/benefit of statin therapy on T2D and cardiovascular disease (CVD) events using liver attenuation and coronary artery calcification (CAC).
Methods and Results
3,153 individuals from the Multi-Ethnic Study of Atherosclerosis (MESA) without CVD, T2D/impaired fasting glucose, or baseline statin therapy had CT imaging for CAC and hepatic attenuation (hepatic steatosis). Cox models and rates of CVD and T2D were calculated to assess the role of liver attenuation in T2D and the relative risks/benefits of statins on CVD and T2D. 216 T2D cases were diagnosed at median 9.1 years follow-up. High liver fat and statin therapy were associated with diabetes (HR 2.06 [95%CI 1.52–2.79, P<0.0001] and 2.01 [95%CI 1.46–2.77, P<0.0001], respectively), after multivariable adjustment. With low liver fat and CAC=0, the number needed to treat (NNT) for statin to prevent one CVD event (NNT 218) was higher than the number needed to harm (NNH) with an incident case of T2D (NNH 68). Conversely, those with CAC >100 and low liver fat were more likely to benefit from statins for CVD reduction (NNT 29) relative to T2D risk (NNH 67). Among those with CAC >100 and fatty liver, incremental reduction in CVD with statins (NNT 40) was less than incremental risk increase for T2D (NNH 24).
Conclusions
Liver fat is associated with incident T2D and stratifies competing metabolic/CVD risks with statin therapy. Hepatic fat may inform T2D surveillance and lipid therapeutic strategies.
INTRODUCTION
Recent society guideline recommendations have lowered the threshold for initiation of statin therapy for the primary prevention of cardiovascular events, resulting in as many as 12.8 million new prescriptions for statin therapy. Most of these individuals do not have established CVD and the majority will never experience a cardiovascular event.(1) These recommendations occur in the context of recent data suggesting an association between statin therapy and type 2 diabetes (T2D).(2) Although multiple large randomized trials demonstrated an average CVD benefit from statin therapy exceeding average harm from increased rate of T2D,(3) the U.S. Food and Drug Administration has required a warning regarding risk of T2D with statin therapy since February 2012. Just as risk stratification modalities such as the Pooled Cohort Equations and coronary artery calcium (CAC) scores have enabled targeting of statin therapy to those who are most likely to benefit, there is need for improved stratification of T2D risk among patients treated with statins.
In this regard, hepatic lipid accumulation (“steatosis”) has emerged as a weight-independent risk factor of poorer insulin sensitivity, subclinical atherosclerosis and a pro-inflammatory phenotype—factors relevant to both T2D and CVD risk. Hepatic x-ray attenuation—a well-validated computed tomographic (CT) marker of steatosis(4, 5)—is easily measured simultaneous with CAC score assessment. We hypothesized that the simultaneous measurement of both indices would provide an effective tool for identification of subgroups with greatest potential benefit and greatest risk of harm from statin therapy, thereby allowing for greater personalization of treatment.
We present results of a study that included 3,153 individuals in the Multi-Ethnic Study of Atherosclerosis without T2D and not on statin therapy at study entry who underwent chest CT imaging at baseline study visit and subsequent follow-up for incident T2D and CVD events. We investigated the impact of liver fat on T2D and CVD risk independent of established clinical risk markers. Furthermore, we determined whether an approach guided by coronary artery calcium scoring and hepatic attenuation simultaneously might identify T2D risk in individuals on statin therapy for improved surveillance.
METHODS
Study Population
The MESA study design has been previously described in detail.(6) In brief, the MESA study enrolled 6,814 individuals of diverse ethnicities (white, African American, Chinese American and Hispanic) from six American sites. MESA participants were free of cardiovascular disease (history of myocardial infarction, angina pectoris, prior revascularization, heart failure, atrial fibrillation, stroke, or peripheral arterial disease) at study onset. Derivation of our study cohort is shown in Supplemental Figure 1. The final cohort included 3,153 individuals who underwent CT scanning with quantification of coronary artery calcium score and hepatic attenuation at Exam 1. Informed consent was obtained from all subjects and institutional review board approval was obtained.
Baseline demographics, history, medications, fasting blood glucose, and clinical examination were assessed at 5 clinic visits (Exams 1–5; between 2000–2011). Metabolic syndrome was adjudicated at each MESA visit by National Cholesterol Education Panel Adult Treatment Panel III guidelines.(7) Hypertension at Exam 1 was defined as systolic blood pressure ≥ 140 mmHg or diastolic blood pressure ≥ 90 mmHg or either self-reported history of hypertension or use of anti-hypertensive medication. Diabetes was defined by American Diabetes Association 2003 guidelines (defined by fasting glucose and/or treatment for diabetes).(8) Individuals were followed for incident T2D (as defined above) as well as composite hard CVD events including myocardial infarction, resuscitated cardiac arrest, stroke (not including transient ischemic attacks) and death from coronary heart disease or stroke. Statin use was determined directly, as participants brought in their medication bottles to MESA clinic visits, and a technician recorded the medication name and strength. Intensity of statin therapy was graded based on a modified version of the classification in the 2013 ACC/AHA guidelines(9) (Supplemental Table 1).
Measurement of Liver Fat and Coronary Calcification
Measurement of liver attenuation has been previously described.(10) Briefly, liver attenuation was measured as the average intensity of two regions approximately 1 cm2 each within the parenchyma of the right hepatic lobe, avoiding vascular structures and hepatic cysts. To assess the presence of a dose-response effect, we also categorized liver attenuation into quartiles corresponding to: ≤57, 57.5–62.5, 62.6–67.5 and ≥67.6 HU, respectively. We also used the previously validated threshold of ≤40 Hounsfield units (HU) to be a marker of significant hepatic steatosis.(11) Coronary artery calcium score was determined as described in prior MESA reports and categorized as 0, 1–100 and >100.(12)
Statistical Analysis
All variables were examined for normality and parametric or non-parametric tests were selected as appropriate. We compared baseline characteristics between those without statin prescriptions and those with incident statin use with Kruskal-Wallis, Fisher exact and chi-square tests for continuous, dichotomous and categorical variables, respectively. Two-sided P-values <0.05 were considered significant. All analyses were performed in SAS 9.4 (SAS Institute, Cary, NC) or R 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
Survival Analysis for Diabetes
We estimated rates of incident diabetes per 10 person-years using Poisson regression, after confirming the absence of significant over-dispersion. We used a generalized additive model with Poisson regression to explore the non-linear relationship between liver attenuation and rate of incident diabetes. Using generalized linear models, we computed unadjusted rates across quartiles of liver attenuation, as well as rates adjusted for race, gender, family history of diabetes, total weekly intentional exercise, fasting glucose and C-reactive protein levels at exam 1. Age, BMI, waist circumference, systolic blood pressure, and serum HDL and triglycerides were entered as time-varying covariates at each MESA examination. We analyzed the impact of hepatic steatosis and statin treatment using a discrete-time Cox survival model, adjusting for time-varying and time-invariant covariates as in Poisson regression. If T2D and statin therapy occurred concurrently in the same MESA examination, T2D was not considered statin-associated. We assessed improvement in model fit with the global χ2 and Akaike information criterion (AIC), discrimination with the c-index and risk assessment with the net reclassification improvement (NRI).(13) Confidence intervals for NRI were computed using bootstrapping. In order to further assess the impact of residual confounding, we constructed a logistic regression model for the initiation of statin therapy (c-index 0.77, 95% CI 0.75–0.79). We used the predicted probability of statin initiation as a propensity score. The Cox regression was then repeated (1) stratifying by deciles of the propensity score and (2) adjusting for the propensity score. Finally, we examined whether appropriateness of statin prescription was related to incident diabetes using a contemporary definition of appropriateness (>5% 10-year risk threshold for appropriateness by the pooled cohort equations or a calcium score ≥100).
Survival Analysis for CVD Endpoints
We constructed Kaplan-Meier survival curves for incident hard cardiovascular events for the entire cohort based on calcium score strata (0, 1–100 and >100) and liver attenuation (quartile 1 vs 2–4). We evaluated the significance of these survival differences with the log-rank test.
Evaluation of Competing Risks from Statin Therapy
We divided the study population based on liver attenuation (quartile 1 vs quartiles 2–4). These two groups were subdivided based on calcium score (0, 1–100, >100). We used a Poisson regression of the entire study cohort to estimate the adjusted rate of incident diabetes per 10 person-years in each of the resulting six groups under two conditions: non-use and use of statins. We used unadjusted Poisson regression to estimate rates of incident hard CVD events per 10 person-years in each of the six groups. We estimated the potential reduction in CVD events by multiplying the observed hard CVD event rate by the 22% risk reduction observed in a recent meta-analysis (14). This approach has been taken in prior work within MESA(12, 15). These estimates of risk differences for diabetes and hard CVD events with and without statin treatment were then used to compute numbers needed to harm (NNH) and treat (NNT), respectively.
RESULTS
Baseline Characteristics, Stratified by Statin Prescription
Baseline characteristics of the MESA study population are shown in Table 1. Compared to participants who were not on statin therapy at baseline or in follow-up (“no statin” group; N=2,237), individuals started on statin therapy after MESA Exam 1 were more likely to be older with greater baseline metabolic risk at baseline study visit (by body mass index, waist circumference, blood pressure, fasting glucose, C-reactive protein and metabolic syndrome diagnosis; all P<0.01). The median coronary calcium score was slightly higher among those initiated on statin therapy (0 vs 1.6, P<0.0001). Liver attenuation by CT was slightly lower in patients referred for statin therapy (63 vs. 62 HU; P=0.003). The racial and gender distribution was similar in both groups.
Table 1.
Baseline Characteristics of the study population, stratified by no statin at followup or use of statin at follow-up.
| All Subjects (N=3153) |
No Statin (N=2237) |
Incident Statin Use (N=916) |
P-Value | |
|---|---|---|---|---|
| Age (y) | 59.0 [51.0–68.0] | 58.0 [50.0–68.0] | 61.0 [54.0–69.0] | <0.0001 |
| Male Gender | 1384 (43.9) | 992 (44.3) | 392 (42.8) | 0.43 |
| Race | 0.004 | |||
| White | 1235 (39.2) | 834 (37.3) | 401 (43.8) | |
| Asian | 394 (12.5) | 297 (13.3) | 97 (10.6) | |
| Black | 855 (27.1) | 627 (28.0) | 228 (24.9) | |
| Hispanic | 669 (21.2) | 479 (21.4) | 190 (20.7) | |
| Weight (lb) | 165.7 [143.0–192.0] | 165.0 [142.3–190.0] | 168.0 [144.1–194.9] | 0.02 |
| BMI (kg/m2) | 27.0 [24.1–30.3] | 26.8 [23.9–30.2] | 27.5 [24.6–30.9] | <0.0001 |
| Waist Circumference (cm) | 95.0 [86.0–103.5] | 94.2 [85.2–102.9] | 96.5 [87.6–105.0] | <0.0001 |
| Systolic BP (mmHg) | 120.3 [108.0–136.0] | 118.5 [107.0–134.5] | 124.5 [112.5–140.0] | <0.0001 |
| Diastolic BP (mmHg) | 71.5 [64.5–78.0] | 71.0 [64.5–77.5] | 72.5 [65.5–79.0] | <0.0001 |
| Hypertension | 1116 (35.4) | 693 (31.0) | 423 (46.2) | <0.0001 |
| Antihypertensive Rx | 838 (26.6) | 514 (23.0) | 324 (35.4) | <0.0001 |
| Smoking Status | 0.19 | |||
| Never | 1678 (53.2) | 1181 (52.8) | 497 (54.3) | |
| Former | 1099 (34.9) | 774 (34.6) | 325 (35.5) | |
| Current | 375 (11.9) | 281 (12.6) | 94 (10.3) | |
| Family history of diabetes | 1062 (33.7) | 726 (32.5) | 336 (36.7) | 0.02 |
| Metabolic Syndrome | 644 (20.4) | 390 (17.4) | 254 (27.7) | <0.0001 |
| Glucose (mg/dl) | 86.0 [81.0–91.0] | 86.0 [80.0–91.0] | 87.0 [81.0–91.0] | 0.002 |
| CRP (mg/l) | 1.7 [0.8–4.0] | 1.6 [0.7–3.8] | 2.0 [1.0–4.6] | <0.0001 |
| Exercise (MET•min/week) | 885.0 [210.0–2100.0] | 870.0 [180.0–2100.0] | 907.5 [210.0–2100.0] | 0.8 |
| Coronary Calcium | ||||
| Agatston Score | 0.0 [0.0–36.7] | 0.0 [0.0–23.0] | 1.6 [0.0–105.8] | <0.0001 |
| CAC=0 | 1863 (59.1) | 1410 (63.0) | 453 (49.5) | <0.0001 |
| CAC 1–100 | 745 (23.6) | 517 (23.1) | 228 (24.9) | |
| CAC>100 | 545 (17.3) | 310 (13.9) | 235 (25.7) | |
| Liver Attenuation (HU) | 62.5 [57.5–67.5] | 63.0 [58.0–67.5] | 62.0 [56.0–67.5] | 0.003 |
| Fatty Liver (≤40 HU) | 146 (4.6) | 86 (3.8) | 60 (6.6) | 0.001 |
| Maximum Stain Intensity | <0.0001 | |||
| No Statin | 2237 (70.9) | 2237 (100.0) | 0 (0.0) | |
| Statin of Unknown Intensity | 6 (0.2) | 0 (0.0) | 6 (0.7) | |
| Low-Intensity Statin | 145 (4.6) | 0 (0.0) | 145 (15.8) | |
| Moderate-Intensity Statin | 652 (20.7) | 0 (0.0) | 652 (71.2) | |
| High-Intensity Statin | 113 (3.6) | 0 (0.0) | 113 (12.3) |
Abbreviations: Hx=history, HU=Hounsfield units. Covariates are reported in the population available.
Liver Fat is Associated with Incident T2D and Reclassifies Risk of T2D Independent of Clinical Risk Factors and Statin Use
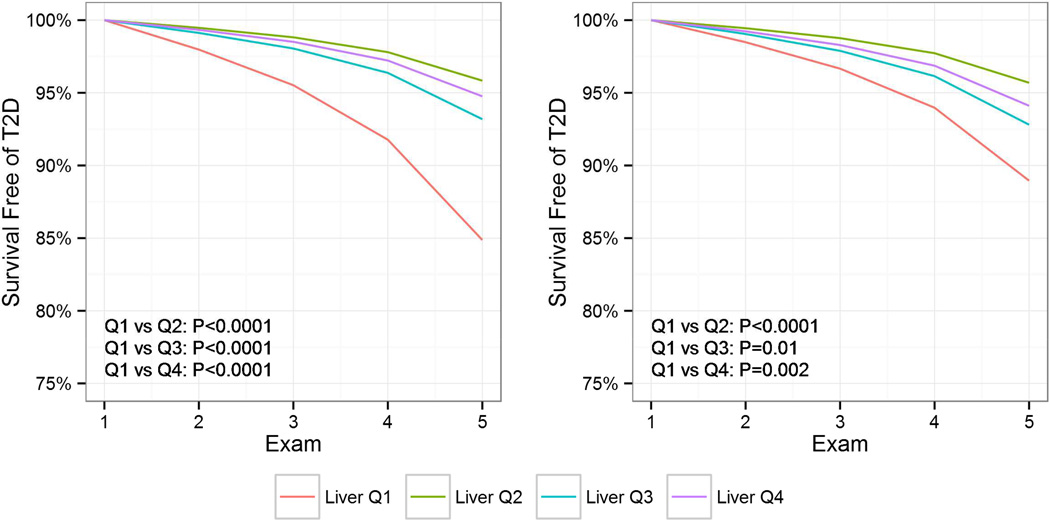
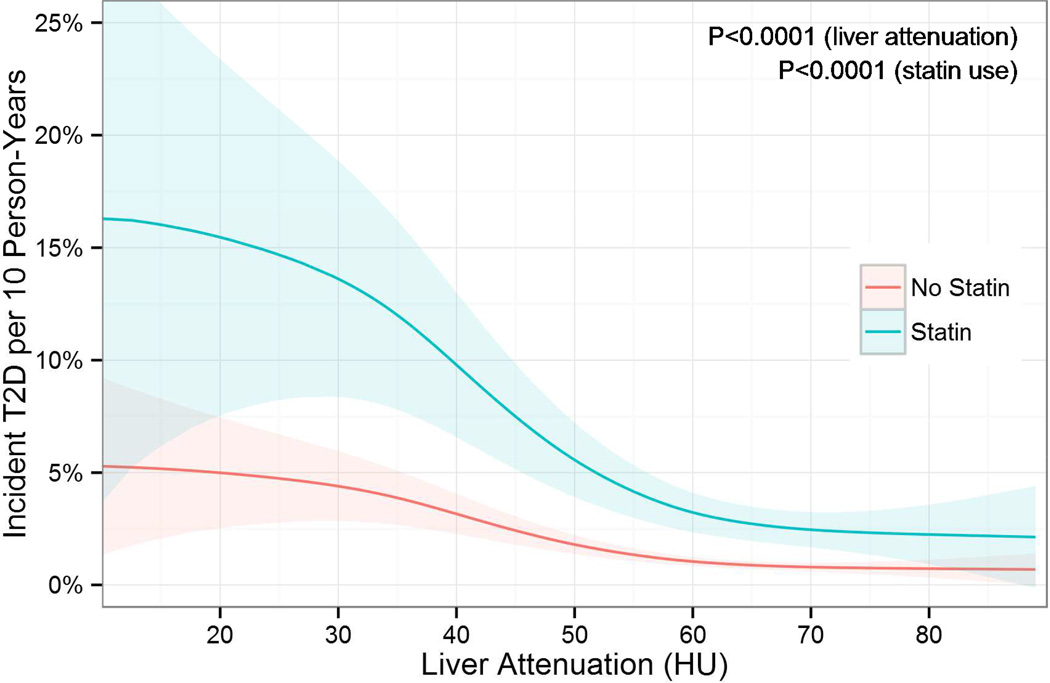
A total 216 cases of incident T2D were diagnosed in 3,153 patients (6.9%) at a median 9.1 years of follow-up (IQR 4.8–9.5 years) (Supplemental Table 2). The lowest quartile of hepatic attenuation (indicating the greatest degree of hepatic steatosis) was associated with a markedly increased risk of incident T2D (Figure 1). Notably, risk began to rise even above previously suggested thresholds for fatty liver of ≤40 HU (Figure 2). The greatest risk of incident T2D was among individuals who both received statin therapy at follow-up and were in lowest quartile of hepatic attenuation (Supplemental Figure 2).
Figure 1.
Liver Steatosis is Associated with Incident Diabetes. Survival free of type 2 diabetes mellitus (T2D) using discrete-time Cox regression. Q1–4 indicate quartiles 1–4. HU=Hounsfield units. Both unadjusted data (left) and data adjusted for age*, gender, race, family history of diabetes, weight*, waist circumference*, systolic blood pressure*, exercise, metabolic syndrome*, glucose*, high-sensitivity C-reactive protein and statin use* (right) are presented. *indicates time-varying covariates.
Figure 2.
Risk of Incident Diabetes across Liver Fat, Stratified by Statin Use. The incidence of diabetes increases non-linearly with decreasing liver attenuation in Hounsfield units (HU) and is much greater with statin use (cyan) than in non-statin users (pink). Importantly, risk of diabetes is increased even above previously described thresholds of ≤40 HU for fatty liver. Unadjusted incidence rate curves and 95% confidence intervals were computed with Poisson regression using a generalized additive model with a log link function.
In multivariable Cox survival analysis, the presence of a fatty liver (quartile 1 of hepatic attenuation; HR=2.06, 95% CI 1.52–2.79; P<0.0001) was associated with incident T2D risk, independent of statin initiation, established clinical and metabolic risk factors (Table 2). The addition of fatty liver significantly improved net reclassification (NRI=0.32, 95% CI 0.05–0.58) beyond statin use and clinical/metabolic risk factors. The favorable NRI was largely driven by the NRI among individuals who did not develop diabetes (NRInon-events=0.41, 95% CI 0.37–0.44), indicating powerful negative predictive value. There was no effect modification of the association between hepatic attenuation and T2D by age, gender, race, weight, waist circumference, BMI or statin use. We found similar results when a threshold of ≤40 HU was used to define a fatty liver. After multivariable adjustment, the relationship between statin therapy and incident diabetes was not altered by the intensity of statin therapy. When this analysis was repeated with propensity-stratification or propensity-adjustment for statin initiation, nearly identical results were obtained. Finally, appropriateness of statin therapy was not associated with incident diabetes.
Table 2.
Multivariable Survival Analysis for Incident Diabetes
| Model 1 | Model 2 | Model 3 | ||||
| Fit Statistic | P | Fit Statistic | P | Fit Statistic | P | |
| Global χ2 | 1846.8 | ref | 1828.7 | <0.0001 | 1807.4 | <0.0001 |
| AIC | 1874.8 | ref | 1858.7 | <0.0001 | 1839.4 | <0.0001 |
| C-Index | 0.73 [0.66–0.80] | ref | 0.74 [0.66–0.81] | 0.38 | 0.74 [0.67–0.82] | 0.7 |
| NRI | 0.335 [0.093–0.562] | <0.05 | 0.318 [0.049–0.579] | <0.05 | ||
| NRI (Events) | −0.346 [−0.592--0.12] | <0.05 | −0.091 [−0.357-0.167] | >0.05 | ||
| NRI (Non-Events) | 0.651 [0.705-0.105] | <0.05 | 0.409 [0.371–0.443] | <0.05 | ||
| Hazard Ratio | P | Hazard Ratio | P | Hazard Ratio | P | |
| Age (per 10 y)* | 0.84 [0.71–0.99] | 0.04 | 0.81 [0.68–0.96] | 0.01 | 0.81 [0.69–0.96] | 0.02 |
| Race | ||||||
| White | 1.00 | ref | 1.00 | ref | 1.00 | Ref |
| Chinese | 1.76 [1.06–2.92] | 0.03 | 1.88 [1.13–3.13] | 0.02 | 1.78 [1.07–2.98] | 0.03 |
| Black | 1.77 [1.22–2.58] | 0.003 | 1.91 [1.31–2.79] | 0.0008 | 2.1 [1.44–3.08] | 0.0001 |
| Hispanic | 1.45 [0.98–2.14] | 0.06 | 1.56 [1.06–2.31] | 0.03 | 1.47 [0.99–2.18] | 0.06 |
| Female Gender | 1.47 [1.06–2.03] | 0.02 | 1.49 [1.07–2.06] | 0.02 | 1.49 [1.07–2.07] | 0.02 |
| Family history of diabetes | 1.51 [1.14–2] | 0.004 | 1.47 [1.11–1.95] | 0.007 | 1.43 [1.08–1.9] | 0.01 |
| BMI* | 1 [0.95–1.05] | 0.92 | 1 [0.95–1.05] | 0.9 | 0.99 [0.95–1.04] | 0.78 |
| Waist Circumference (per 10 cm)* | 1.33 [1.1–1.6] | 0.002 | 1.33 [1.1–1.6] | 0.003 | 1.28 [1.06–1.54] | 0.009 |
| Systolic BP (per 10 mmHg)* | 0.97 [0.9–1.04] | 0.43 | 0.97 [0.9–1.05] | 0.5 | 0.97 [0.9–1.05] | 0.51 |
| HDL (per 10 mg/dl) | 0.9 [0.8–1.02] | 0.12 | 0.9 [0.8–1.02] | 0.12 | 0.93 [0.83–1.05] | 0.23 |
| Triglycerides (per 10 mg/dl) | 1.01 [0.99–1.03] | 0.22 | 1.01 [0.99–1.03] | 0.22 | 1.01 [0.99–1.03] | 0.52 |
| Exercise (per 1000 MET•min/wk) | 1.09 [1.07–1.12] | 0.76 | 1.01 [0.95–1.07] | 0.72 | 1.01 [0.95–1.08] | 0.66 |
| Glucose (per 10 mg/dl) | 0.98 [0.96–1] | 0.2 | 1.02 [0.99–1.04] | 0.19 | 1.01 [0.98–1.04] | 0.44 |
| CRP (mg/l) | 1.09 [1.07–1.12] | 0.76 | 1.01 [0.95–1.07] | 0.72 | 1.01 [0.95–1.08] | 0.66 |
| Statin Initiation* | 2.05 [1.49–2.82] | <0.0001 | 2.01 [1.46–2.77] | <0.0001 | ||
| Fatty Liver (Q1 Attenuation) | 2.06 [1.52–2.79] | <0.0001 | ||||
indicates time-varying covariates.
Abbreviations: Akaike information criterion (AIC), NRI=continuous net reclassification improvement, HU=Hounsfield units, HDL= high density lipoprotein, Q1 indicates lowest quartile. Hazard ratios in fully adjusted models for fatty liver were similar when interleukin-6 was replaced for CRP and homeostatic model of insulin resistance was replaced for fasting glucose.
Coronary Calcification and Liver Fat Identify Competing Cardiovascular Benefit and Risks of Statin Treatment
At a median follow-up of 10.4 years (IQR 9.8–10.8 years), a total of 152 hard cardiovascular events (4.8%) were observed in the study population. While coronary artery calcium score was associated with CVD events at long-term follow-up similar to prior MESA results, hepatic attenuation was not associated with CVD, except in the intermediate calcium score stratum (CAC 1–100; Supplemental Figure 3). The distribution of calcium score was not different based on liver attenuation (Supplemental Figure 4).
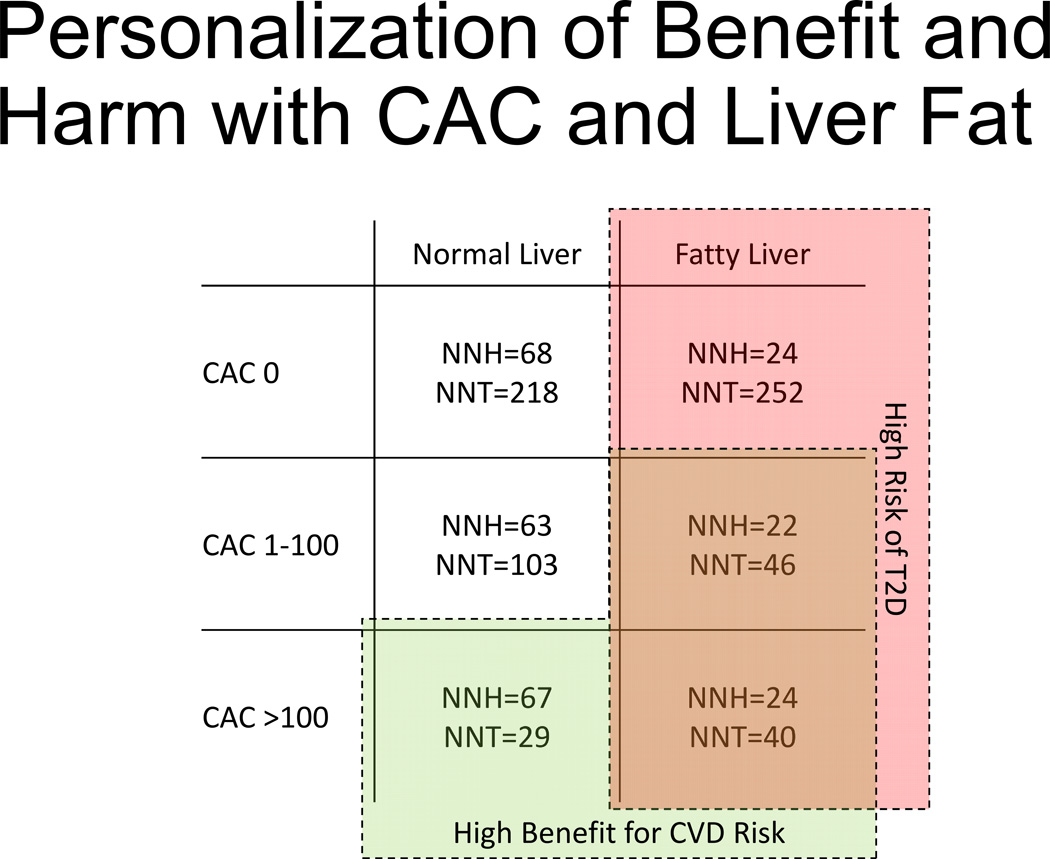
We investigated whether hepatic attenuation and coronary artery calcification could be used jointly to balance T2D risk and CVD risk (Figure 3). In individuals with less liver fat (quartiles 2–4) and either zero or intermediate calcium score, the expected absolute CVD risk reduction from statin therapy is 0.5% and 1.0%, respectively, corresponding to number needed to treat with a statin (NNT) 218 and 103, respectively, to prevent one CVD event. Conversely, the expected absolute T2D risk increase from statin therapy is 1.5% and 1.6% for zero and intermediate calcium score, respectively, corresponding to number needed to harm for an incident case of T2D (NNH) 68 and 63, respectively. The expected absolute T2D risk increase was consistently higher (4.2–4.5%) and NNH (22–24) was consistently lower among individuals with a fatty liver (quartile 1), regardless of calcium score.
Figure 3.
Personalization of Benefit and Harm with Coronary Artery Calcium Score and Liver Fat. Numbers of persons needed to treat (NNT) with statins to avoid one hard cardiovascular disease (CVD) event and numbers persons needed to harm (NNH) with statins to cause one additional incident type 2 diabetes (T2D) case in categories of coronary artery calcium score (CAC) and fatty liver (defined as quartile 1 of liver attenuation) or normal liver (quartiles 2–4 of liver attenuation). Subgroups with CVD risk reduction high benefit (low NNT) are outlined in green (both bottom and right middle cells). Subgroups with high risk of T2D are shaded in red (right column). Overlapping regions appear brown.
Among MESA participants with higher calcium score (CAC >100) and less liver fat (“normal” liver), the expected absolute CVD risk reduction was 3.4%, corresponding to NNT to prevent one CVD event 29, which was lower than an absolute T2D risk increase of 1.5% (NNH for one additional T2D case 67). Conversely, among those with higher calcium score and a fatty liver, the expected absolute CVD risk reduction is 2.5% (NNT 40 for CVD prevention), compared to a 4.2% expected absolute T2D risk increase with statins (NNH 24 for an additional T2D case).
DISCUSSION
Recent guideline updates have resulted in over 12 million American adults newly qualifying for statin therapy(1). This expansion has brought to the forefront a debate over whether further expansion of statin therapy appropriately balances reduction in CVD risk with the potential for increased T2D associated with statin therapy. In this study, we have demonstrated that hepatic fat—a metabolic marker reflecting systemic inflammation and insulin resistance—can reclassify risk of T2D beyond traditional clinical risk factors and statin prescription. Importantly, hepatic fat improves risk assessment for T2D beyond variables included in validated diabetes risks scores(16). Rates of incident T2D with statin therapy were greater among MESA participants with low hepatic attenuation (higher liver fat). Using coronary artery calcium score, a well-established marker of CVD risk(12), we found that hepatic attenuation and CAC score stratify incident T2D and CVD risk in a largely separable manner. Among individuals with zero-to-intermediate CAC score (≤100), CVD risk reduction with statin therapy was accompanied by a high risk of incident T2D, especially among those with a fatty liver. While MESA participants with higher CAC scores (>100) and lower liver fat had substantial CVD risk reduction with a modest risk of incident T2D, those with a fatty liver had CVD risk reduction (relative to incident T2D risks) with statin therapy. The primary clinical implication of these findings is that hepatic fat and coronary artery calcium score—both simultaneously attained in one non-contrast, low radiation dose CT scan—can identify individuals requiring greater surveillance for incident T2D while on clinically indicated statin therapy for primary CVD prevention. While it is clear that T2D and CVD are not clinically equivalent outcomes, this approach may serve to further refine the risk/benefit calculus for CVD prevention beyond calcium score alone in an ever-growing, statin-eligible population.
The liver is a critical pathophysiologic mediator of glycemic control and insulin sensitivity, linked to inflammation, obesity, subclinical cardiovascular disease and T2D.(17–24) Our results extend prior work(10, 25) by establishing the ability of CT markers of hepatic steatosis to effectively reclassify risk of incident T2D in a multi-ethnic, normoglycemic cohort over long-term follow-up beyond longitudinal changes in weight, waist circumference (an anthropometric marker of visceral adiposity), fasting glucose and incident statin prescription. Similarly, our results are in concert with a recent report from the MESA study suggesting poor reclassification metrics for CVD events with addition of liver attenuation to the Framingham risk score and coronary artery calcium score.(26) We additionally found that the distribution of hepatic attenuation does not vary based on coronary artery calcium score. In this context, our results define hepatic attenuation and coronary artery calcification as largely orthogonal, complementary biomarkers of metabolic and CVD risk, respectively, accessible in a single, non-contrast, low radiation dose CT examination currently accepted for risk assessment in CVD.(27)
These findings reach therapeutic relevance in their implications on surveillance and risk-benefit assessments for the increasing population of individuals eligible for statin therapy. As reported in a recent study from Pencina and colleagues from the National Health and Nutrition Examination Survey, the newly published ACC/AHA guidelines will extend statin prescriptions among individuals aged 60–75 years without established CVD to 77.3% of the population (for a 7.5% 10-year risk threshold) and up to 90% (for a 5% risk threshold; relative to 47.8% with previous guidelines).(1) Given the goal to reduce incident CVD events through aggressive primary prevention therapy, the ACC/AHA has also advocated the use of coronary artery calcium scoring (a class IIa indication) to further refine risk in asymptomatic individuals at intermediate CVD risk (defined as 10–20% 10-year risk in prior guidelines).(27) Indeed, recent studies from MESA suggest that individuals with a zero calcium score are at very low risk of CVD,(15) fueling suggestions that calcium score may be a method to focus therapy to those at highest risk. Regardless of which additional index is used, these considerations call for some additional, targeted risk stratification in primary prevention statin prescription.
There has been much controversy as to whether statin prescription per se increases diabetes risk or may be a marker of individuals at higher risk. Accordingly, effect size estimates that suggest that statin therapy imposes a higher risk for diabetes are highly variable, depending on study design. In a meta-analysis of published randomized statin trials (over 90,000 individuals), the hazard ratio for incident diabetes was 1.09, with treatment of 255 patients with statins for 4 years yielding 1 additional diabetes case(28). On the other hand, in an observational study of over 25,000 individuals with propensity score matching (similar techniques as used in our study), statin use was associated with an odds ratio of 1.87 (95% CI 1.67–2.01) for incident diabetes(29), similar as observed in MESA. Indeed, despite attempts at propensity matching to account for variation, inherent variability in populations between randomized controlled studies and observational cohorts may account for some of the heterogeneity in effect size: as observed here, individuals receiving statin therapy in MESA were at substantially higher cardiometabolic risk (Table 1). Nevertheless, recent population-level Mendelian randomization studies suggest a potential mechanistic basis for statin-mediated HMG-CoA reductase inhibition on increased weight and incident type 2 diabetes (odds ratio 1.12)(30). In the wake of the available evidence, our results do not endorse using imaging to withhold statin therapy: on the contrary, our results suggest that statin therapy is critical to disease prevention, but increased surveillance utilizing easily-accessed hepatic fat content obtained by CT at the time of coronary calcium scoring could be used to further risk stratify individuals for incident diabetes risk.
The results of our study should be viewed in the context of its design. As MESA is a prospective cohort study, we observed a significant imbalance in metabolic risk factors between those individuals who did and did not receive statin therapy in follow-up (Table 1). Despite consistent results after propensity stratification or adjustment, the absolute measure of the association between statin therapy and incident T2D is therefore susceptible to residual confounding, and should be approached with caution (especially given lower effect sizes reported in previous randomized studies). The primary goal of our work was to examine the role of hepatic steatosis in balancing risk prediction (not estimating magnitude of statin-associated T2D). While we do not maintain that incident T2D and CVD are clinically equivalent outcomes, the imperative to improve risk stratification and early treatment by identifying those individuals at highest metabolic and CVD risk remains an important goal in disease prevention. Although we did not use data from MESA to derive statin-associated risk reduction for this cohort, we utilized estimates reported in a comprehensive meta-analysis that demonstrated consistent results across many high-quality randomized studies. Finally, while we did not specifically examine interactions between fatty liver and genetic variants in this study, we recognize this is an important area of future study.
In conclusion, we found that CT markers of hepatic steatosis are independently associated with and reclassify risk of T2D regardless of age, gender, race, change in weight or statin prescription. Hepatic attenuation by CT identified individuals at highest risk of T2D across strata of coronary artery calcification, introducing a novel method to balance the risk-benefit calculus of metabolic and CVD outcomes with statin therapy in the context of a growing statineligible population.
Supplementary Material
Highlights.
We investigated individuals from the Multi-Ethnic Study of Atherosclerosis with CT imaging for liver attenuation
Risk of diabetes was increased with greater liver fat by CT
We found that liver fat and coronary artery calcification can be used to identify those individuals at highest and lowest risks of diabetes and cardiovascular disease
Further research into the use of multi-modality imaging to stratify cardiometabolic risk is warranted
ACKNOWLEDGEMENTS
MESA was supported by contracts NO1-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute. All other authors have no financial disclosures relevant to the content of this manuscript. Dr. Shah is supported by a Fellow-to-Faculty Award from the American Heart Association. Dr. Allison was supported by funding for the MESA Abdominal Body Composition Ancillary study from the National Heart, Lung, and Blood Institute (R01-HL088451). MESA is supported by contracts NO1-HC-95159 through N01-HC-95169 from the National Heart, Lung, and Blood Institute (NHLBI). Jingzhong Ding and Matthew J. Budoff are supported by R01-HL-085323 and R01-HL-071739, respectively, from NHLBI. Dr. Murthy had full access to all of the data in the study and takes responsibility for the integrity of the data, the accuracy of the data analysis, and acknowledgment of all persons vital to the production of this manuscript. Dr. Murthy has minor stock in General Electric. There are no other disclosures for any other authors relevant to the content of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Pencina MJ, Navar-Boggan AM, D'Agostino RB, Sr, Williams K, Neely B, Sniderman AD, et al. Application of New Cholesterol Guidelines to a Population-Based Sample. N Engl J Med. 2014 doi: 10.1056/NEJMoa1315665. [DOI] [PubMed] [Google Scholar]
- 2.Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA. 2011;305(24):2556–2564. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 3.Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380(9841):565–571. doi: 10.1016/S0140-6736(12)61190-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ricci C, Longo R, Gioulis E, Bosco M, Pollesello P, Masutti F, et al. Noninvasive in vivo quantitative assessment of fat content in human liver. J Hepatol. 1997;27(1):108–113. doi: 10.1016/s0168-8278(97)80288-7. [DOI] [PubMed] [Google Scholar]
- 5.Limanond P, Raman SS, Lassman C, Sayre J, Ghobrial RM, Busuttil RW, et al. Macrovesicular hepatic steatosis in living related liver donors: correlation between CT and histologic findings. Radiology. 2004;230(1):276–280. doi: 10.1148/radiol.2301021176. [DOI] [PubMed] [Google Scholar]
- 6.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. American journal of epidemiology. 2002;156(9):871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 7.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 8.Expert Committee on the D, Classification of Diabetes M. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 2003;26(Suppl 1):S5–S20. doi: 10.2337/diacare.26.2007.s5. [DOI] [PubMed] [Google Scholar]
- 9.Stone NJ, Robinson J, Lichtenstein AH, Bairey Merz CN, Lloyd-Jones DM, Blum CB, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013 doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.McAuley PA, Hsu FC, Loman KK, Carr JJ, Budoff MJ, Szklo M, et al. Liver attenuation, pericardial adipose tissue, obesity, and insulin resistance: the Multi-Ethnic Study of Atherosclerosis (MESA) Obesity (Silver Spring) 2011;19(9):1855–1860. doi: 10.1038/oby.2011.191. [DOI] [PubMed] [Google Scholar]
- 11.Zeb I, Li D, Nasir K, Katz R, Larijani VN, Budoff MJ. Computed tomography scans in the evaluation of fatty liver disease in a population based study: the multi-ethnic study of atherosclerosis. Acad Radiol. 2012;19(7):811–818. doi: 10.1016/j.acra.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, et al. Associations between C-reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population-based cohort study. Lancet. 2011;378(9792):684–692. doi: 10.1016/S0140-6736(11)60784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27(2):157–172. doi: 10.1002/sim.2929. discussion 207-12. [DOI] [PubMed] [Google Scholar]
- 14.Cholesterol Treatment Trialists C. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bittencourt MS, Blaha MJ, Blankstein R, Budoff M, Vargas JD, Blumenthal RS, et al. Polypill therapy, subclinical atherosclerosis, and cardiovascular events-implications for the use of preventive pharmacotherapy: MESA (Multi-Ethnic Study of Atherosclerosis) J Am Coll Cardiol. 2014;63(5):434–443. doi: 10.1016/j.jacc.2013.08.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson PW, Meigs JB, Sullivan L, Fox CS, Nathan DM, D'Agostino RB., Sr Prediction of incident diabetes mellitus in middle-aged adults: the Framingham Offspring Study. Arch Intern Med. 2007;167(10):1068–1074. doi: 10.1001/archinte.167.10.1068. [DOI] [PubMed] [Google Scholar]
- 17.Stefan N, Kantartzis K, Haring HU. Causes and metabolic consequences of Fatty liver. Endocr Rev. 2008;29(7):939–960. doi: 10.1210/er.2008-0009. [DOI] [PubMed] [Google Scholar]
- 18.Targher G. Non-alcoholic fatty liver disease, the metabolic syndrome and the risk of cardiovascular disease: the plot thickens. Diabet Med. 2007;24(1):1–6. doi: 10.1111/j.1464-5491.2007.02025.x. [DOI] [PubMed] [Google Scholar]
- 19.Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168(15):1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- 20.Kotronen A, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Tissue specificity of insulin resistance in humans: fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia. 2008;51(1):130–138. doi: 10.1007/s00125-007-0867-x. [DOI] [PubMed] [Google Scholar]
- 21.Kotronen A, Westerbacka J, Bergholm R, Pietilainen KH, Yki-Jarvinen H. Liver fat in the metabolic syndrome. J Clin Endocrinol Metab. 2007;92(9):3490–3497. doi: 10.1210/jc.2007-0482. [DOI] [PubMed] [Google Scholar]
- 22.Roden M. Mechanisms of Disease: hepatic steatosis in type 2 diabetes--pathogenesis and clinical relevance. Nat Clin Pract Endocrinol Metab. 2006;2(6):335–348. doi: 10.1038/ncpendmet0190. [DOI] [PubMed] [Google Scholar]
- 23.Sookoian S, Pirola CJ. Non-alcoholic fatty liver disease is strongly associated with carotid atherosclerosis: a systematic review. J Hepatol. 2008;49(4):600–607. doi: 10.1016/j.jhep.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 24.Zelber-Sagi S, Nitzan-Kaluski D, Halpern Z, Oren R. Prevalence of primary nonalcoholic fatty liver disease in a population-based study and its association with biochemical and anthropometric measures. Liver Int. 2006;26(7):856–863. doi: 10.1111/j.1478-3231.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 25.Zeb I, Katz R, Nasir K, Ding J, Rezaeian P, Budoff MJ. Relation of nonalcoholic fatty liver disease to the metabolic syndrome: the Multi-Ethnic Study of Atherosclerosis. J Cardiovasc Comput Tomogr. 2013;7(5):311–318. doi: 10.1016/j.jcct.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 26.Yeboah J, Carr JJ, Terry JG, Ding J, Zeb I, Liu S, et al. Computed tomography-derived cardiovascular risk markers, incident cardiovascular events, and all-cause mortality in nondiabetics: the Multi-Ethnic Study of Atherosclerosis. Eur J Prev Cardiol. 2013 doi: 10.1177/2047487313492065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56(25):e50–e103. doi: 10.1016/j.jacc.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 28.Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, de Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375(9716):735–742. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 29.Mansi I, Frei CR, Wang CP, Mortensen EM. Statins and New-Onset Diabetes Mellitus and Diabetic Complications: A Retrospective Cohort Study of US Healthy Adults. J Gen Intern Med. 2015 doi: 10.1007/s11606-015-3335-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swerdlow DI, Preiss D, Kuchenbaecker KB, Holmes MV, Engmann JE, Shah T, et al. HMG-coenzyme A reductase inhibition, type 2 diabetes, and bodyweight: evidence from genetic analysis and randomised trials. Lancet. 2015;385(9965):351–361. doi: 10.1016/S0140-6736(14)61183-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.