Abstract
Physical activity has beneficial effects on not only improving some disease conditions but also by preventing the development of multiple disorders. Experiments in this study examined the effects of wheel running on intakes of chow and palatable diet e.g. high fat (HF) or high sucrose (HS) diet in male and female Sprague Dawley rats. Experiment 1 demonstrated that acute wheel running results in robust HF or HS diet avoidance in male rats. Although female rats with running wheel access initially showed complete avoidance of the two palatable diets, the avoidance of the HS diet was transient. Experiment 2 demonstrated that male rats developed decreased HF diet preferences regardless of the order of diet and wheel running access presentation. Running associated changes in HF diet preference in females, on the other hand, depended on the testing schedule. In female rats, simultaneous presentation of the HF diet and running access resulted in transient complete HF diet avoidance whereas running experience prior to HF diet access did not affect the high preference for the HF diet. Ovariectomy in females resulted in HF diet preference patterns that were similar to those in male rats during simultaneous exposure of HF and wheel running access but similar to intact females when running occurred before HF exposure. Overall, the results demonstrated wheel running associated changes in palatable diet preferences that were in part sex dependent. Furthermore, ovarian hormones play a role in some of the sex differences. These data reveal complexity in the mechanisms underlying exercise associated changes in palatable diet preference.
Keywords: Wheel running, Diet preference, Sex difference, Ovariectomy, Ovarian hormones
1. Introduction
An appropriate amount of exercise has multiple beneficial effects on both mental and physical health. Exercise ameliorates anxiety or depression [1], enhances insulin sensitivity and thus glucose metabolism [2-4], and maintains or improves cognitive function [5, 6] in humans and animals. Physical activity is also highly recommended as a method to prevent multiple health issues such as obesity and cardiovascular disease [7, 8]. Furthermore, exercise facilitates healthy weight maintenance and weight reduction in overweight or obese subjects [9, 10]. In rodents, acute wheel running decreases food intake and body weight for a number of days [11]. When the running wheel access is provided over a longer duration, food intake is increased but the body weight remains lower than that of sedentary controls [12]. Furthermore, wheel running can prevent obesity in diet [13] or genetically induced rodent obesity models [14]. Despite these known effects, aspects of the mechanisms though which exercise impacts energy balance, however, remain unclear.
One potential mechanism that could contribute to body weight control with exercise is through altering dietary preferences. Rats introduced to a sucrose solution and running wheels simultaneously run in the wheels but avoid the sweet solution [15]. When solid palatable food is provided e.g. high fat (HF) diet pellet, wheel running can significantly reduce intakes and thus decrease preferences of a previously preferred HF diet in a two-diet (standard chow vs. HF) choice feeding schedule [16, 17]. HF diet preferences are reduced further in male F344 and Brown Norway hybrid rats when both wheel running access and the HF diet are novel and introduced simultaneously [18]. The effects of simultaneous introduction of wheel running access and a novel HF diet were severe. The running rats not only avoided the HF diet but also greatly reduced chow intakes to ~1/4 of their baseline intakes. A 10% weight loss was observed within 3 days as a consequence of running with severe intake reduction [18].
The current study aimed to further explore the effects of wheel running access on diet preference. Only HF diet was tested in the previous study. Whether wheel running specifically changes preferences to HF diets is unknown. Furthermore, a previous study has demonstrated sex differences in wheel running associated response to palatable sweetened milk [19]. Accordingly, we hypothesized that wheel running may differently decrease palatable diet preference, regardless of the diet composition e.g. HF or high sugar, in male and female rats and ovarian hormones contribute to these sex differences. These hypotheses were tested in Sprague-Dawley rats. In Experiment 1, wheel running effects on diet preferences to HF and high sucrose (HS) palatable diets in both male and female rats were determined. Experiment 2 further examined the effects of long term and repeated exposure to wheel running and HF diets and determined the roles of ovarian hormones in the sex differences observed in both experiments.
2. Materials and methods
2.1. Experiment 1
Subjects
Twenty five male (250-275 g) and 24 female (150-175 g) Sprague-Dawley rats (SD, Harlan, Frederick MD) were the subjects of experiment 1. The ages of the male and female rats were similar (7-8 weeks). Due to the limited number of running wheel cages (wheel diameter 36 cm and width 11 cm, Wahmann, Timonium, MD), the experiments were conducted in two cohorts. On arrival, rats were divided into sedentary (Sed) and wheel running (WR) groups. The Sed rats were individually housed in metal wire-mesh hanging cages while the WR rats were placed into a black plexiglass box with a locked running wheel attached. Temperature (65 – 76 °C) and humidity (20 – 70 %) of the housing room was monitored daily. The light/dark cycle was 12 h on//off with light on at 0100 and light off at 1300. There were 10-days of habituation before the experimental procedures began. During this period, all rats had ad libitum access to water and a standard high carbohydrate chow diet (Harlan 2018, 3.1 kcal/g). Water, food intake with spillage and body weight were measured daily between 0800 and 1000. All procedures were approved by the Institutional Animal Care and Use Committee of the Johns Hopkins University.
Procedures
After habituation, rats in Sed and WR groups were further divided into two subgroups that were introduced to either a 60% high fat (HF, Research Diets D12492, 5.24 kcal/g) or a 50% high sucrose (HS, Research Diets D10001, 3.9 kcal/g) diet. This design resulted in four groups: SedHF, SedHS, WRHF and WRHS. On the first day of the novel palatable diet introduction, rats were weighed and food was removed at 0800. Two hours before the dark onset (1100), the chow and the novel diet (HS or HF) were placed in the cage. At the time of novel palatable diet presentation, the wheels were unlocked for the WR rats. That is the palatable diet and access to voluntary running were both novel for the WR rats. Wheel running activity was recorded in real time, and analyzed later through a computer with MedPC IV software (Med Associates Inc, Georgia, VT). This access to palatable diets and wheel running regimen continued for 6 days. After 6 days, the palatable diets were removed and wheels for the WR rats were locked.
After 1 wk without wheel running access and access to chow diet only, a 30 min intake test was conducted. The chow diet was removed 3 hrs before light off. At dark onset, the rats were provided with 2.9-3.3 g of either HS or HF diet in a hopper consistent with their previous assignment. After 30 min, the hopper was removed and the residual food was recorded. A summary of the experimental design and procedures is demonstrated in Table 1.
Table 1.
Novel palatable diet avoidance
| Procedures Groups | baseline | Diet Choice (6 days) | 1 wk off | 30 min Intake Test |
|---|---|---|---|---|
| SedHF | sedentary | chow + HF | sedentary | HF |
| WRHF | chow only | chow + WR+HF | chow only | HF |
| SedHS | chow + HS | HS | ||
| WRHS | chow + WR+HS | HS |
2.2 Experiment 2
Subjects
The source, age and weight of the SD rats (Harlan, Frederick MD) were the same as described in Experiment 1. There were 55 rats (18 males and 37 females) included in this experiment. To understand the role of female sex hormones on the different responses to palatable diet and running wheel access in males and female rats, 18 of the female rats were ovariectomized. Ovariectomy was performed by the supplier. Again, due to the limited number of wheels, the experiments were conducted in three cohorts. The housing condition and light/dark cycle were the same as in Experiment 1.
Procedures
The goal of this experiment was to determine how long term and intermittent exercise affect palatable diet preference in both sexes. Based on the results from Experiment 1, while the exercise induced avoidance was similar for the HF and HS diet, it appeared that the effects on the HF diet preference were more robust. Thus, only the HF vs. chow choice was used in this experiment.
In this experiment, HF and voluntary running access occurred at two periods of time that were separated by 2 wks without running wheel access and with access to chow only. After habituation, the rats in each of the three cohorts were divided into a Sed and two WR groups. Similar to Experiment 1, the Sed rats were introduced to the HF diet and had simultaneous access to both the chow and HF diets (SedHF). The first WR group had the same treatment as in Experiment 1 (WRHF). They were given access to the HF diet and wheel running simultaneously. Previous studies have examined the effects of wheel running on HF intake when the HF diet had been available prior to running wheel access but not the other way around [16, 17]. Thus, the second WR group received access to running wheel with chow only for four days first. Four days of wheel running before HF diet introduction was chosen based on our previous data showing that acute wheel running resulted in significant reduction of food intake and intakes normally began to increase toward baseline levels after four days. Beginning on the fifth day, the HF diet was introduced and from that point on this group also had access to both wheel running and the HF diet (WR_HF). This 1st exposure to HF and voluntary running lasted for 15 days (D 1-15). The second exposure occurred 2 wks after all rats had been sedentary with access to the chow diet alone. During the 2nd exposure, the SedHF rats received the same two-diet access as the 1st exposure and both of the WR groups gained simultaneous access to the HF diet and wheel running on the same day. The 2nd exposure lasted for 9 days (D 30-38). A summary of the experimental design and procedures is demonstrated in Table 2.
Table 2.
Long term wheel running and repeated exposure
| Procedures Groups | baseline | HF choice (day 1-15) | 2 wks off (day 16-29) | 2nd exposure (day 30-38) |
|---|---|---|---|---|
| SedHF | sedentary chow only | chow + HF | sedentary chow only | chow + HF |
| WRHF | chow + WR+HF | chow + WR+HF | ||
| WR_HF | chow + WR (4
days) ↓ chow + WR+HF (11 days) |
chow + WR+HF |
2.3. Statistical Analysis
Raw data included body weight (BW), wheel running activity and daily absolute intakes of chow, HS and HF diets. In order to compare daily energy intakes between males (M), females (F) and ovariectomized females (OVX), the data were normalized to energy intake per 100 g of BW. Daily palatable diet preference ratio was calculated as kcal intake of the palatable diet divided by the total kcal intake on that day. Preference ratio > 0.5 means that the rats prefer the palatable to the chow diet. In Experiment 1 results from 25 males and 24 females were included in the data analysis. In Experiment 2, results from 1 rat in each group of male WRHF and WR_HF and female WRHF were excluded due to inappropriate functioning of the running wheels. Thus, data included in Experiment 2 was from 16 males, 18 females and 18 ovariectomized female rats. The data were analyzed by repeated-measures [time (BW, intakes and activity across days), within or between groups] and factorial ANOVA with diet (chow vs. HF or HS), wheel running access (Sed and WR), and sex (M, F and OVX) as factors, and post-hoc Newman-Keuls tests as appropriate using Statistica 7.1 (Tulsa, OK). Data are presented as mean ± standard error of the mean (SEM).
3. Results
3.1. Experiment 1
3.1.1. Diet preference and energy intake
Chow vs. HF diet
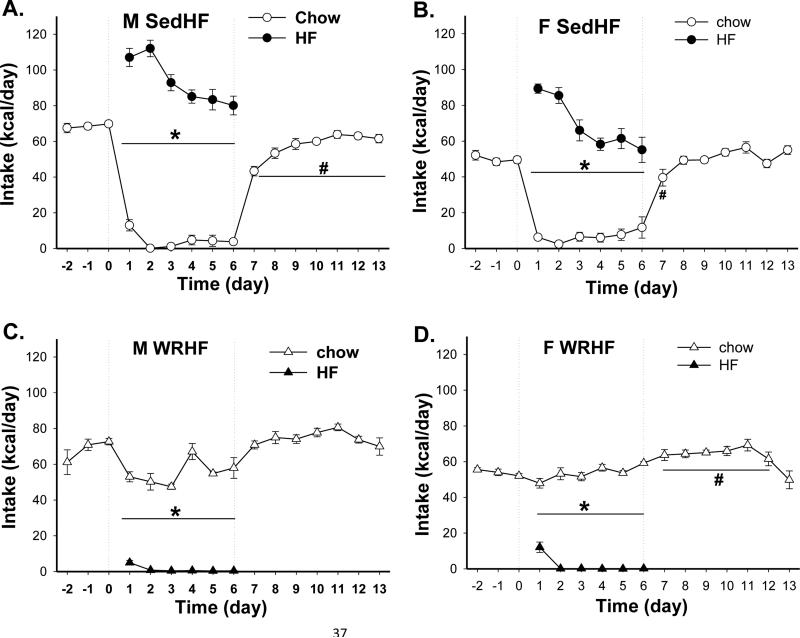
The diet intake patterns of WR rats were completely the reverse of those in Sed rats. When the HF diet was introduced, both male and female Sed rats (n=7 and 6, respectively) showed hyperphagic responses. The caloric intake of the HF diet was almost twice their baseline chow intake for two days (Fig.1A & B). Throughout the six days period, the Sed rats consumed significantly more HF than chow diet (M vs. F: F(1, 12)=692.3, p<0.0001 vs. F(1, 10)=193.1, p<0.0001). That is, both males and females preferred the HF diet and no overall significant effect of sex in HF diet preference was observed [F(1, 21)=1.0, p=0.3]. However, male Sed rats had higher preference to the HF diet than did female rats (preference ratios: 0.95 ± 0.02 vs. 0.82 ± 0.09, p<0.05) on the 6th day of the two-diet choice feeding protocol. The chow and HF diet intakes differed significantly in both male and female WR rats (Fig. 1 C & D, n=6/group). Rats with running wheel access consumed the chow diet and completely avoided the HF diet [comparisons of chow and HF intakes; M vs. F: F(1, 10)=557.8, p<0.0001 vs. F(1, 10)=5006.4, p<0.0001].
Fig. 1.
Chow and HF intake patterns in male (M) and female (F) groups. Diet choice and wheel running occurred during between day 0 and 6. (A) & (B) Male and female SedHF rats (n= 7 and 6, respectively) preferred the HF diet throughout the 6 days diet choice period. (C) & (D) Male and female WRHF rats (n=6/group) sampled the HF diet initially and then completely avoided the HF diet for the rest of the diet choice period. *: p< 0.05 between chow and HF intakes
Differences between Sed and WR rats were also revealed in their daily energy intake [exercise effect, F(1, 21)=147.5, p<0.0001]. In Sed rats, total intakes during the diet choice period were significantly higher than their baseline intakes (post-hoc p<0.03). In WR rats, males reduced total intake whereas females showed no change in total intake during the diet choice period [sex effect, F(1, 21)=33.9, p<0.0001].
Chow vs. HS diet
Sed and WR rats (n=6/group) demonstrated similar differences in diet intake patterns to those in the chow vs. HF diet choice condition. Sed rats consumed the HS diet whereas the WR rats avoided it. During the six day diet choice period, both Sed male and female rats consumed more HS diet than the chow diet [Fig. 2 A & B; M vs. F: F(1, 10)=56.9, p<0.0001 vs. F(1, 10)=8.7, p<0.02]. Male SedHS rats consumed more HS than the chow diet throughout the diet-choice period. Female SedHS rats, on the other hand, decreased their HS diet intake over time. Thus, by the end of 6 days, the HS preference ratio was higher in males (0.75) than in females (0.58). Similar to the chow vs. HF choice condition, both male and female WR rats sampled the HS diet on the first day of exposure and then completely avoided the HS diet for the next two days [Fig. 2 C & D; M vs. F: F(1, 10)=1684, p<0.0001 vs. F(1, 10)=34.8, p<0.0002]. A sex difference occurred starting on day 4 of the diet-choice schedule. Male rats continued to avoid the HS diet until the end of the diet-choice schedule. In contrast, female rats began to consume the HS diet and by the 6th day, their HS and chow diet intakes were the same. Thus, overall there were sex differences in HS preference ratios [effects of exercise and sex × exercise, F(1, 20)=111.1 and 7.1 respectively, both p<0.02; sex × exercise × time, F(5, 100)=2.5, p<0.04].
Fig. 2.
Chow and HS intake patterns in male (M) and female (F) groups (n=6/group). Diet choice and wheel running occurred during between day 0 and 6. (A) Male SedHS rats preferred the HS diet throughout the 6 days diet choice period. (B) Female SedHS rats preferred the HS diet on days 1-4 but not on days 5-6. (C) Male WRHS rats sampled the HS diet initially and then completely avoided the HS diet for the rest of the diet preference period. (D) Female WRHS rats sampled the HS diet, completely avoided it and then gradually increased intake to equal amount with the chow diet by the 6th day of diet choice. *: chow vs. HF, p< 0.05; #: vs. day 0 intake, p<0.05
Similar to the chow vs. HF condition, total energy intake differed in SedHS and WRHS rats. Compared to chow baseline, male and female SedHS rats consumed significantly more energy for 2 and 3 days into the two-diet choice period, respectively. During wheel running access, only male but not female rats reduced energy intake [effects of sex, exercise and sex × exercise interaction, F(1,20)= 21.3, 30.2 and 4.7 respectively, all p<0.05]. After all rats were returned to the sedentary condition with access to only the chow diet, only male SedHS rats showed a reduction in intake on the first day (post-hoc p<0.0001) of chow access only again.
3.1.2. Wheel running activity
Both male and female WR rats increased running activity over the 6 days period of running wheel access [time effect, F(5, 100)=17.4, P<0.0001; M: from 439.3 ± 82 to 938.7 ± 119.8, F: from 1898.3 ± 304.1 to 4458.3 ± 440.4]. Females ran significantly more than did male rats [sex effect, F(1, 20)=62.0, p< 0.0001]. Finally, individual palatable diets did not affect running activity because no significant difference was found between WRHF and WRHS [diet effect, F(1, 20)=2.3, p=0.14; average running revolution across the 6 days: WRHF vs. WRHS, M: 800.3 ± 122.1 vs. 611.4 ± 84.0; F: 2480.9 ± 502.6 vs. 3570.7 ± 269.3].
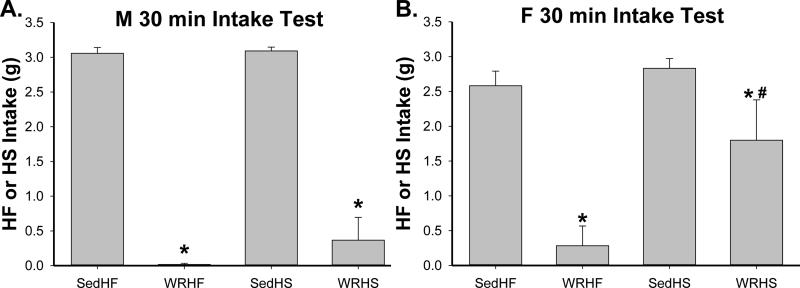
3.1.3. 30-min intake test
The results of 30-min intake test revealed effects of exercise and diet [Fig. 3, effects of diet, exercise, sex × exercise and diet × exercise, F(1, 41)= 8.0, 144.0, 10.3 and 4.4 respectively, all p<0.05]. In SedHF rats, males consumed all and females consumed most of the HF diet provided within 30 min. Although no longer running, male WR rats continued to completely avoid whereas females sampled the HF diet. In HS rats, both male and female Sed rats consumed all of the HS diet provided. Unlike the HF condition, WRHS males sampled whereas females consumed most of the HS diet (post-hoc p<0.0006) provided.
Fig. 3.
HF or HS intakes during the 30-min intake test. (A) In males, Sed rats consumed all 3 g HF or HS diet provided within the 30 min period whereas previously WR ones continued to avoid the palatable diet. (B) In females, Sed rats consumed most of the 3 g HF or HS diet provided within the 30 min period. Previously running WRHF rats continued to avoid the HF diet whereas previously running WRHS rats consumed most HS diet provided. *: SedHF vs. WRHF or SedHS vs. WRHS, p< 0.05; #: M WRHS vs. F WRHS, p<0.05
3.1.4. Body weight
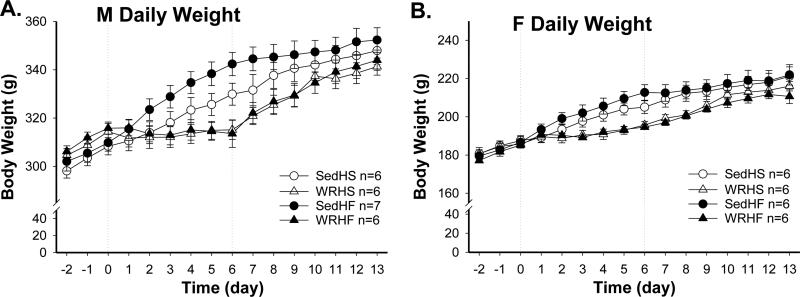
Body weight results are consistent with the energy intake and the expected wheel running suppression of weight gain [effects of sex and exercise, F(1, 41)=2533.3 and 12.6 respectively, both p<0.001]. In both males and females, SedHF rats weighed significantly more than WRHF rats during the 6 days of diet-choice period (Fig. 4 A & B). Percent BW gain between the day before diet-choice and the day of the 30-min intake test revealed sex difference [effects of sex, exercise and sex × exercise, F= (1, 41)= 96.7, 19.6, 4.9 respectively, all p<0.04]. In males, Sed groups gained significantly more weight than did WR groups [Sed vs. WR, HF: 13.7 ± 0.6 vs. 8.9 ± 0.7 %, post-hoc p<0.004; HS: 12.8 ± 0.8 vs. 8.6 ± 0.4 %, post-hoc p<0.01]. In females, however, no significant differences in percent weight gain were found in Sed and WR groups (Sed vs. WR, HF: 18.8 ± 1.5 vs. 16.8 ± 1.2 % and HS: 18.1 ± 1.1 vs. 17.1 ± 1.1 %, both post-hoc p>0.4).
Fig. 4.
Daily body weight in male (A) and female (B) rats throughout Experiment 1. Diet choice and wheel running access occurred from day 1 to day 6.
3.2. Experiment 2
3.2.1. Diet intake and preference
3.2.1.1. 1st exposure
SedHF condition
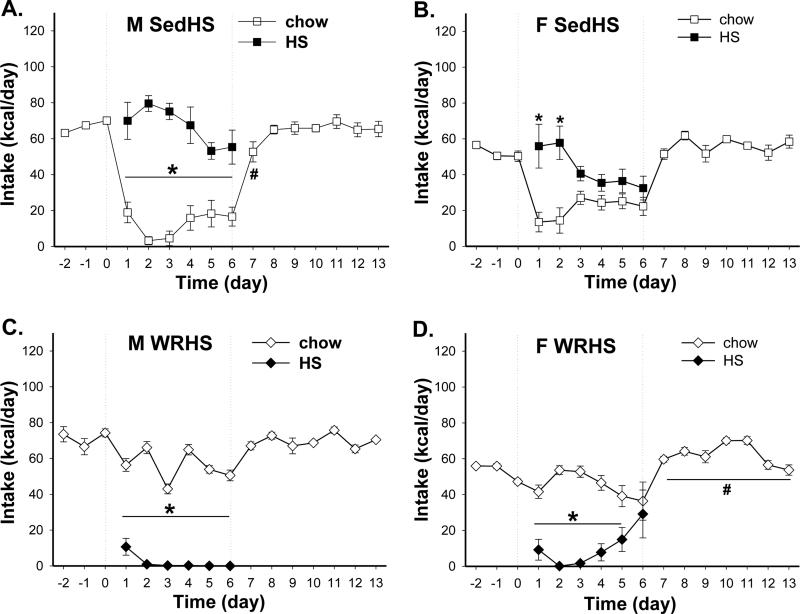
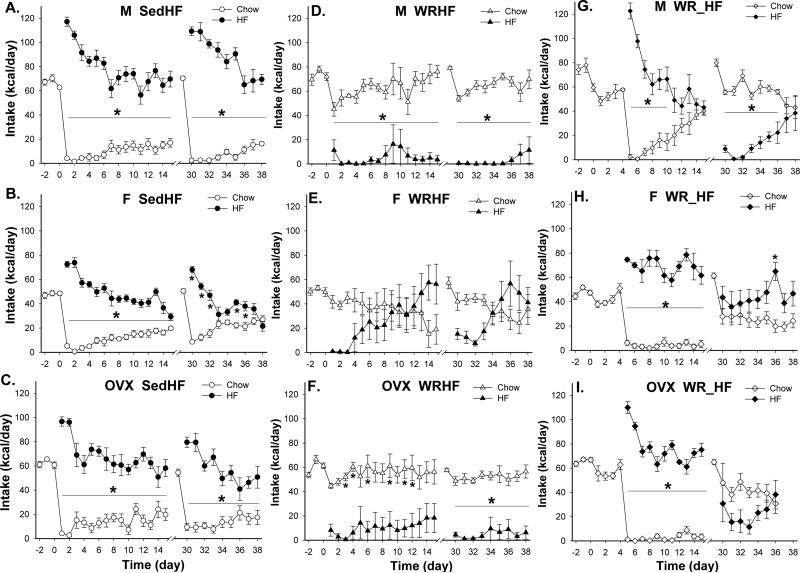
Consistent with results of Experiment 1, all Sed rats showed hyperphagic responses when they were introduced to the HF diet. Male (n=6), intact (n=7) and ovariectomied female (n=6) SedHF rats all consumed more HF diet than chow diet throughout the 15 days choice period [Fig. 5 A-C, day 1 – 15; M vs. F vs. OVX: F(1, 10)=203 vs. F(1, 12)=199.3 vs. F(1, 10)=84.4, all p<0.0001]. Nonetheless, males and ovariectomized female rats stabilized their HF diet intake whereas the intact female rats reduced their intake over time. Thus, comparisons of HF diet preference ratios reveal significant effects of time and time × sex [F(14, 224)=16.2 and (28, 224)=1.9, both p<0.006]. By the 15th day, male and ovariectomized SedHF preferred the HF diet more than did the intact female SedHF rats (preference ratios M vs. F vs. OVX: 0.80 ± 0.05 vs. 0.60 ± 0.04 vs. 0.74 ± 0.06).
Fig. 5.
Chow and HF intake patterns in male (M), intact (F) and ovariectomized (OVX) female groups. (A - C) Both M and OVX SedHF rats (n=6/group) maintained high HF diet intake during 1st (day 0 - 15) and 2nd (day 30 - 38) exposure period. In F SedHF rats (n=7), HF intakes decreased and chow intakes increased over time during the two diet choice period. (D – F) Both M and OVX WRHF rats (n=5 and 6, respectively) avoided the HF diet during each exposure period. In contrast, F WRHF rats (n=5) gradually increased HF diet intake across days during each diet choice period. (G – I) During the 1st exposure period, F and OVX WR_HF rats (n=6/group) maintained high HF diet intake throughout the 11-day diet choice period. In contrast, M WR_HF rats (n=5) went from large consumption of the HF diet to equal intakes of the chow and the HF diet by the end of the diet choice period. During the 2nd exposure, the M and OVX WR_HF groups consumed more chow than HF diet. In contrast, F WR_HF rats consumed more HF than chow diet. Circle symbols represent SedHF groups, triangle symbols represent WRHF groups and diamond symbols represent WR_HF groups. *: p< 0.05 between chow and HF intakes; Note: Data from the last 2 days of the OVX WR_HF group was not included due to experimental error.
WRHF condition
Simultaneous access to wheel running and HF diet reduced HF diet intake (Fig. 5 D-F, day 1 - 15). Chow diet intakes were significantly greater than HF diet intakes in both male and ovariectomized female WRHF rats (n=5 and 6, respectively) throughout the 15 day period [M vs. OVX: F(1, 8)=68.7 vs. F(1, 10)=20.9, both p<0.002]. In contrast, intact females (n=5) sampled and avoided the HF diet for the first 3 days and then gradually increased the intakes of the HF diet to more than that of the chow diet from day 13 to day 15 [effects of diet, time, and diet × time: F(1, 8)= 0.4, p=0.53, F(14, 112)=1.1, p=0.37 and F(14, 112)=4.9, p<0.0001]. In the intact female WRHF rats, however, post hoc tests did not reveal any significant difference in chow and HF diet intake at any time point. If comparisons include intakes of the first 6 days of the two-diet choice, the chow intakes were significantly greater than HF diet intakes from day 1 to day 3 (post hoc p<0.05). Overall, HF diet preference ratios of male and ovariectomized female WRHF rats were < 0.23 [HF preference ratio effects of time and time × sex: F(14, 182)=4.3 and F(28, 182)=2.2, both p<0.001]. In contrast, the HF diet preference ratios of intact female WRHF rats during day 13 and 15 were >0.6. These data indicated that simultaneous access to exercise and a novel HF diet results in avoidance to the HF diet. The effect persisted over time in males but not females. These sex differences can be eliminated by ovariectomy in females.
WR_HF condition
When wheel running access was allowed 4 days before the introduction of chow vs. HF diet choice, male (n=5) but not female rats reduced HF diet intake over time (Fig 5 G-I, day 1 – 15). All rats initially showed hyperphagic responses to the HF diet when it was introduced. Intact and ovariectomized females (n=6/group) consumed significantly more HF than chow diet throughout the diet choice period [F vs. OVX: F(1, 10)=209.9 vs. F(1,10)=660.8, both p<0.0001] whereas male rats gradually decreased HF diet intake and the intakes of the two diets did not differ by the end of the 15 days period [F(1, 8)=1, 8)=45.2, p<0.0001]. Overall, HF preference ratios of intact and ovariectomized female WR_HF maintained > 0.86 and those of male WR_HF rats decreased from 0.99 on the first day to 0.51 on the last day of the two-diet choice exposure [effects of sex, time and sex × time in HF preference ratios: F(2, 14)=10.6, (10, 140)=10.9 and (20, 140)=6.6, all p<0.002]. Thus, unlike the in the WRHF condition, ovariectomy did not eliminate the sex difference in wheel running associated changes of HF diet preference in the WR_HF condition.
Finally, analysis of HF diet preference between SedHF and WRHF groups revealed an effect of exercise [F(1, 29)=128.4, p<0.0001] and significant interactions of sex × exercise [F(2, 29)=3.9, P<0.04], time × sex [F(28, 406)=1.6, P<0.03], time × exercise [F(14, 406)=12.7, P<0.0001] and sex × exercise × time [F(28, 406)=3.5, P<0.0001]. During this 1st exposure period, the WR groups included rats with simultaneous running and diet choice (WRHF) or with delayed diet choice (WR_HF) schedules. The ad lib access to the chow and HF diet only overlapped for 11 days (day 5-15) between the two treatment groups. Analysis of the 11 days HF diet preferences in WRHF and WR_HF condition revealed significant effects of sex [F(2, 27)=5.1, p<0.02], diet choice schedule [simultaneous vs. delayed, F(1, 27)=77.8, p<0.0001], time × sex [F(20, 270)=4.0, p<0.0001] and time × diet choice schedule [F(10, 270)=8.0, p<0.0001]. These data indicated that simultaneous access to wheel running and a novel HF diet resulted in persistent and transient avoidance to the HF diet in running male and female rats, respectively. Moreover, delayed access to the HF diet in males with wheel running access resulted in a gradual decrease in HF diet preference but in females, the high preference to the HF diet persisted as long as the rats were running. Ovariectomy eliminated the sex differences observed in the simultaneous but not in the delayed HF diet choice schedule.
3.2.1.2. 2nd exposure
After 2 wks without wheel running access and with ad lib access to chow, all rats were exposed again to the HF vs. chow choice schedule (Fig. 5, day 30 - 38). Diet intake patterns were similar to their 1st exposure in the SedHF and WRHF conditions in males, females and ovariectomized females. Male and ovariectomized female SedHF rats consistently consumed significantly more HF than chow diet throughout the 9 days period [Fig. 5 A & C, day 30 – 38; M vs. OVX: F(1, 8)=68.6 vs. F(1, 10)=55.1, both p< 0.0001]. Intact female SedHF rats decreased their HF diet intake over time and the chow and HF intakes were no longer different by day 4 of diet choice [Fig. 5B, day 30 – 38, F(1, 12)=43.6, p<0.0001]. For the WRHF condition, males and ovariectomized females consumed mostly the chow diet and avoided the HF diet during the entire 9 days period [Fig. 5 D & F, day 30 – 38; M vs. OVX: F(1, 8)=230.3 vs. F(1, 10)=71.3, both p<0.0001]. Intact WRHF females consumed more chow than the HF diet for 4 days and then intakes of the two diets were similar [Fig. 5E, day 30 – 38; effects of diet and diet × time: F(1, 8)=0.26, p=0.62 and F(8, 64)=7.93, p<0.0001].
The WR_HF groups demonstrated different diet intake patterns from their 1st exposure (Fig. 5 G – H). Unlike the previous exposure where running began a few days before the HF diet was introduced, access to running and the HF diet were introduced simultaneously during this exposure. Under such schedule, male WR_HF rats consumed small amounts of the HF diet initially, completely avoided it and then gradually increased their HF diet intake to the level of chow intakes by the 9th day of the schedule [Fig. 5G, day 30 - 38, F(1, 8)= 68.6, p<0.0001)]. In intact female WR_HF rats, intake of the HF diet was greater than intake of the chow diet throughout the 9 days period [Fig. 5H, day 30 - 38, effects of diet and diet × time: F(1, 10)= 4.58, p=0.06 and F(8, 80)=2.56, p<0.02]. Ovariectomized WR_HF females consumed less HF than chow diet during this exposure period but the differences were not significant [Fig. 5I, day 30 - 38, effects of diet and diet × time: F(1, 10)= 2.94, p=0.12 and F(6, 60)=2.63, p<0.03].
Due to experimental error, data from the last 2 days of ovariectomized WR_HF females were missing. Thus, repeated measure ANOVA comparing HF diet preference ratios among all three experimental conditions included only the first 7 days of the two-diet choice. The analysis revealed significant exercise effects and sex differences in all three experimental conditions (SedHF, WRHF and WR_HF) among the males, females and ovariectomized females [effects of sex, exercise, sex × exercise and sex × exercise × time: F(2, 43)=7.3, (2, 43)=87.4, (4, 43)=9.6, and (24, 258)=2.6, all p<0.002]. Overall, HF diet preference patterns were similar between males and ovariectomized females and they were different from those of the intact females during this 2nd exposure period.
3.2.2. Energy intake per 100 g BW
Energy intake normalized to body weight during Experiment 2 is summarized in Table 3. Data were analyzed to compare intakes of baselines (D 0 and D 29 ) and the first 4 days (D 1-4 and D 30-33) and the last 11 days during the 1st exposure (D 5-15) and the last 5 days during the 2nd exposure (D 34-38). As mentioned in Method 2.2, the first 4 days after running was the representative of acute wheel running induced anorexia if the effect occurs.
Table 3.
Normalized energy intake in Experiment 2
| average intake (kcal/100 g BW/day) | 1st Exposure | 2nd Exposure | |||||
|---|---|---|---|---|---|---|---|
| Day | D0 | D 1-4 | D 5-15 | D29 | D 30-33 | D 34-38 | |
| Group / Diet | chow | chow + HF (chow only in WR_HF) | chow + HF | chow | chow + HF | chow + HF | |
| M | SedHF | 20.8 ± 0.2 (a) | 32.5 ± 0.8 (b) | 23.6 ± 0.6 (a) | 17.9 ± 0.4 (a) | 26.1 ± 0.6 (b) | 20.5 ± 0.6 (a) |
| WRHF | 23.2 ± 0.9 (a) | 18.0 ± 0.8 (b) | 22.0 ± 0.9 (a) | 20.6 ± 0.4 (a) | 16.0 ± 0.6 (bc) | 18.9 ± 0.7 (ab) | |
| WR_HF | 19.6 ± 1.0 (a) | 17.7 ± 0.5 (a) | 26.2 ± 0.7 (b) | 21.6 ± 1.0 (a) | 17.4 ± 0.8 (bc) | 21.1 ± 1.7 (ab) | |
| F | SedHF | 26.8 ± 0.9 (a) | 36.2 ± 0.6 (b) | 27.1 ± 0.3 (a) | 21.8 ± 0.6 (a) | 27.1 ± 0.5 (b) | 23.5 ± 0.3 (a) |
| WRHF | 26.7 ± 1.7 (a) | 25.0 ± 1.7 (a) | 34.3 ± 1.2 (b) | 24.4 ± 2.0 (a) | 24.2 ± 1.0 (a) | 31.5 ± 1.9 (b) | |
| WR_HF | 26.8 ± 1.3 (a) | 24.3 ± 1.3 (a) | 38.0 ± 1.5 (b) | 27.0 ± 0.6 (a) | 29.4 ± 2.1 (ab) | 30.4 ± 1.8 (bc) | |
| OVX | SedHF | 31.4 ± 1.3 (a) | 42.9 ± 1.1 (b) | 31.6 ± 0.3 (a) | 19.0 ± 0.9 (a) | 27.5 ± 0.6 (b) | 21.4 ± 0.6 (a) |
| WRHF | 32.1 ± 1.4 (a) | 28.5 ± 1.1 (b) | 30.6 ± 0.9 (a) | 20.8 ± 0.6 (a) | 19.3 ± 0.7 (a) | 21.4 ± 0.8 (a) | |
| WR_HF | 32.8 ± 0.6 (a) | 27.7 ± 1.0 (b) | 33.0 ± 0.8 (a) | 21.8 ± 0.6 (a) | 21.1 ± 1.5 (a) | 22.2 ± 1.0 (a) | |
Different letters indicate significant difference in the same group within the Exposure period according to post hoc tests. Note that due to experimental error, average intake for D 34-38 in the WR_HF group of OVX was actually average intake of D 34-36. (M: male, F: female, OVX: ovariectomized female)
1st exposure
Comparisons were made using repeated measures ANOVA including sex (male, female, and ovariectomized female) and exercise (SedHF, WRHF and WR_HF) as factors. At baseline, energy intake in females was significantly greater than in males and ovariectomy did not eliminate this difference [effects of sex and sex × time: F(2, 43)=157.5 and F(4, 86)=13.2, both p<0.0001; M vs. F vs. OVX: 21.2 vs. 26.8 vs. 32.1 kcal/100 g, post hoc p<0.0001]. Significant increases in energy intake during the first 4 days of HF diet exposure were seen in the SedHF condition of male, intact and ovariectomized female rats [effect of sex × exercise × tiem: F(8, 86)=4.3, p<0.0003]. Wheel running induced anorexia during the first 4 days of wheel running was significant in male and ovariectomized female rats but not intact female rats [effect of exercise and sex × exercise: F (2, 43)=25.4 and F(4, 43)=3.5, both p<0.02].
2nd exposure
Before the onset of this exposure, baseline chow intakes in females were significantly greater than those in male and ovariectomized female rats [effects of sex and sex × time: F(2, 43)=58.1 and F(4, 86)=5.8, both p<0.0004; M vs. F vs. OVX: 19.9 vs. 24.2 vs. 20.5 kcal/100 g, post hoc p<0.0001]. Significant increases in energy intake during the first 4 days of HF diet exposure were seen in the SedHF condition of male, intact and ovariectomized female rats [effect of sex × exercise × tiem: F(8, 86)=4.1, p<0.0004]. During this exposure, however, only male rats showed significant wheel running induced decreases in intake. Neither intact nor ovariectomized female wheel running rats showed changes in intake during this 2nd running period [effect of exercise and sex × exercise: F (2, 43)=3.2 and F(4, 43)=6.4, both p<0.05].
3.2.3. Wheel running activity
1st exposure
Consistent with results from Experiment 1, sex differences in running activity were observed [sex effect, F(2, 27)=53.4, p<0.0001]. Both males and intact females increased daily running activity over the 15 days period [time effect, F(14, 378)=35.2, p<0.0001; M: from 884.0 ± 203.4 to 3581.8 ± 1143.7, F: from 2854.0 ± 549.1 to 12335.2 ± 1405.4] and average daily running activity was significantly higher in intact females (WRHF vs. WR_HF = 6736.8 ± 1036.2 vs. 7654.6 ± 868.1) than in males (WRHF vs. WR_HF = 1918.2 ± 422.1 vs. 2386.6 ± 632.4; sex effect, F(2, 27)=53.3, p<0.0001; M vs. F, post-hoc p<0.0002). The differences in running activity appeared to be mediated by ovarian hormones because running activity in ovariectomized females was significantly reduced to the level of males (WRHF vs. WR_HF = 1506.7 ± 98.6 vs. 1230.1 ± 73.7; M vs. OVX, post-hoc p>0.5). Daily running activity in ovariectomized females maintained similar each day and did not increase over time.
2nd exposure
The levels and patterns of running activity during the 2nd exposure were quite similar to those during the 1st exposure. Both males and intact females increased daily running across the 9 days period (M: from 1385.0 ± 292.2 to 3805.6 ± 1397.4, F: from 3508.0 ± 496.2 to 12319.8 ± 1692.8) and ovariectomized females had similar amount of running activity each day [effects of time and sex, F(8, 216)=12.5 and (2, 27)=51.6, both p<0.0001]. Average daily running activity were similar in males (WRHF vs. WR_HF = 1943 ± 477.6 vs. 2951.5 ± 945.2) and ovariectomized females (WRHF vs. WR_HF = 1375.0 ± 74.9 vs. 1158.0 ± 46.6; M vs. OVX, post-hoc p>0.2), which were significantly less than those in intact females [WRHF vs. WR_HF = 6672.0 ± 716.5 vs. 7673.5 ± 1082.5; sex effect, F(2, 27)=46.8, p<0.0001; F vs. M or OVX, post-hoc p<0.0006].
3.2.4. Body weight
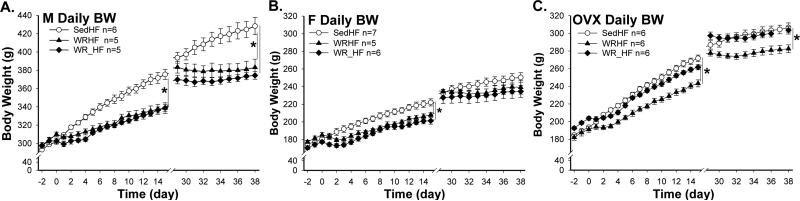
1st exposure
Compared to SedHF rats, wheel running suppressed weight gain across all groups [effects of exercise and sex: F(2, 43)= 81.0 and 131.5, each p<0.0001; Fig. 6 A – C, day 0 - 15 ]. Across males, females and ovariectomized females, SedHF rats (% weight gain: M, F and OVX = 24.5 ± 1.5, 22.8 ± 1.0, and 40.4 ± 1.7 %) gained more weight than WR ones during the experimental period. The effects of running on BW gain in WRHF and WR_HF of males (% weight gain, 9.3 ± 1.6 vs. 12.0 ± 1.0 %), intact females (12.4 ± 1.7 vs. 13.5 ± 1.2 %), and ovariectomized females (27.4 ± 1.1 vs. 28.5 ± 1.5 %) were similar i.e. no significant differences in weight gain between WRHF and WR_HF conditions.
Fig. 6.
Daily body weight in male (A., M), intact female (B., F) and ovariectomized female (C., OVX) groups throughout Experiment 2. *: group BW, p<0.05
2nd exposure
Patterns of weight gain during the 2nd exposure were similar to those in the 1st exposure (Fig. 6 A – C, day 29 - 38) in that wheel running again suppressed weight gain [exercise effect, F(2,43)=23.1, p<0.0001]. The sex effect on weight gain, however, disappeared during this wheel running and diet choice exposure [F(2, 43)=0.4, p=0.7]. Across males, females and ovariectomized females, SedHF rats (% weight gain: M, F & OVX = 8.7 ± 1.0, 7.7 ± 1.0, & 6.9 ± 0.9 %) gained more weight than WR rats during the 2nd exposure period. The effects of running on BW gain in WRHF and WR_HF of males (% weight gain, −0.1 ± 0.9 vs. 1.2 ± 0.4 %), intact females (1.8 ± 2.4 vs. 3.2 ± 2.2 %), and ovariectomized females (1.8 ± 0.9 vs. 2.1 ± 0.9 %) were similar i.e. no significant differences in weight gain.
4. Discussion
This study revealed sex differences in wheel running induced changes in palatable diet preference in SD rats. When wheel running access and a novel palatable HF or HS diet were provided simultaneously, male rats showed persistent avoidance to the palatable diets for not only during but also after the running had stopped. In contrast, female rats avoided the palatable diets for a few days but then gradually increased intakes of the palatable diets to prefer them before the running access stopped. In another schedule when wheel running occurred a few days before the access to a novel HF diet, both male and female rats initially showed hyperphagic response to the HF diet. However, HF diet intakes of the male rats gradually declined to no preference between the HF and the standard chow diets while female rats maintained high intake and preference for the diet throughout the running period. Finally, both male and females with prior experience with simultaneous wheel running and HF diet consumption decreased HF diet preference when the two stimuli were provided again after a period without the access to them. The sex differences in wheel running induced changes of palatable diet preference are partially mediated by ovarian hormones.
Consistent with a previous study in F344 and Brown Norway hybrid male rats [18], wheel running produces complete HF diet avoidance. The palatable diet avoidance is not specific to the HF diet as the same avoidance occurred to a HS diet. Unlike the F344 and Brown Norway hybrid rats, a hybrid of strains with hyperactive/hyper-reactive (F344) and hypoactive (Brown Norway) hypothalamic-pituitary-adrenal (HPA) axis to stress [20], simultaneous presentation of the palatable diet and wheel running in SD rats, a strain with a different HPA reactivity level, did not result in severe anorexia and significant weight loss. Moreover, the results revealed sex differences in sedentary and wheel running associated palatable diet preference that have not been previously reported. When sedentary, males maintained preference to HF or HS throughout the diet choice periods. Female rats, on the other hand, gradually decreased their preference to the HF or HS diet and eventually consumed equal amount of palatable and chow diets i.e. no preference between the two diets. The sex differences in sedentary palatable diet choice are mediated by ovarian hormones because ovariectomized females maintained their HF diet preference throughout the testing period as did the male rats. When wheel running and HF diet were provided simultaneously (WRHF), males persistently and females transiently avoided the palatable diet. Again, this difference was eliminated by ovariectomy. When wheel running occurred before the introduction of the HF diet choice e.g. WR_HF, males gradually decreased their HF diet intake from high preference to no preference. In contrast, female WR_HF rats maintained high preference throughout the exposure period and ovariectomy had no effect on such a preference pattern. Accordingly, the results of this study demonstrated that some but not all of the sex differences in exercise induced changes in palatable diet preference are mediated by ovarian hormones.
The exercise induced complete avoidance to HF and HS diets persisted longer in males than in female rats. The persistent palatable diet avoidance was consistent between diets (HF or HS) and between experiments (short term exposure in Experiment 1 and long term, repeated exposure in Experiment 2) in males. That is, exercise universally resulted in a prolonged palatable diet avoidance in males. The avoidance lasted when the rats were no longer running as demonstrated by the results of the 30 min intake test in Experiment 1. In contrast, the phenomena in females were more complex. Female WRHF rats in Experiment 1 completely avoided the HF diet throughout the period of running. On the other hand, female WRHS rats avoided the HS diet completely for only 2 days. Since the palatable diet avoidance effect was more robust and persisted longer in the chow vs. HF diet choice condition in both sexes, this diet choice condition was applied in Experiment 2 to study the effects of long term and repeated exercise exposure. Female WRHF rats in this experiment demonstrated similar palatable diet preference pattern to the female WRHS rats in Experiment 1. During the 1st exposure, female running rats avoided the HF diet for only 3 days and actually preferred the HF diet by the end of 15 days running (Fig. 6E). At first glance, it may appear that the persistence of HF diet avoidance as a result of the simultaneous onset of HF diet and wheel running access in female rats in Experiment 1 and 2 were inconsistent. The 30 min intake test in Experiment 1, however, showed that the WRHF experienced female rats consumed some while the WRHF experienced male rats still completely avoided the HF diet (Fig. 3). Such result indicates that female WRHF rats could begin to consume the HF diet earlier than the male WRHF rats if the running and two-diet choice continued longer than 6 days in Experiment 1.
Nevertheless, the different durations of palatable diet avoidance in WRHF females between experiments raises the question of whether the ovarian hormonal status during running and diet choice schedule affects the length of the exercise induced palatable diet avoidance. Experiments in this study involved long term daily measurement of weight and intakes and to avoid interference with those measurements, ovarian cycle was not monitored. Variable hormonal status among female rats could contribute to the inconsistent duration of exercise induced palatable diet avoidance observed between rats within a group as well as between experiments in WRHF intact females. When ovarian hormones were excluded by ovariectomy, exercise induced HF diet avoidance in females became similar to that in males (Fig. 6 D - E). These results suggest that the fast recovery of the HF diet preference in running females is primarily due to ovarian hormones (progesterone and estrogen). The inclusion of ovariectomized females, however, did not distinguish between potential roles of progesterone and estrogen in exercise induced palatable diet avoidance. Further studies are necessary to elucidate the roles of specific ovarian hormones on the initiation and maintenance of palatable diet avoidance induced by exercise.
The persistence of exercise induced palatable diet avoidance may also depend on the form of the palatable diet e.g. liquids or solids. Previous studies have demonstrated that exercise e.g. wheel running and forced swimming suppressed the intake of palatable sweet solutions in male rats [21, 22]. The degree of intake suppression depends on the volume/duration of exercise, the longer the exercise, the more suppression of sweet solution intakes. Thus, a complete suppression of 24% sucrose intake was demonstrated in a paradigm similar to the current study i.e. daily 24 hr simultaneous access to chow vs. sucrose and wheel running. When the choice was between a solid chow and a sucrose solution, complete avoidance to the sucrose was transient and sucrose preference patterns were similar to the preference pattern seen in female WRHS in Experiment 1 and WRHF in Experiment 2 [15]. Thus, a transient complete avoidance of a palatable diet by wheel running can be observed in male rats with the diet in liquid form. Since previous studies with chow vs. sucrose solution choice included only male rats, it would be of interest to determine whether female rats with running wheel access would show an even less persistent avoidance or smaller decrease in preferences to the sucrose solution.
In our studies, the sex differences in HF diet preference after days of wheel running experience (the WR_HF condition) do not appear to be mediated by ovarian hormones. Previous studies have demonstrated that exercise can decrease an established HF diet preference in males [16, 17]. The inclusion of the WR_HF group in Experiment 2, which received wheel running access for four days before the introduction of the HF diet, was used to determine whether exercise changes HF diet preference with a different exercising and palatable diet exposure schedule. Under this condition, male rats initially overate and preferred the HF diet but decreased the preference as running continued. In contrast, female rats maintained overeating and high preference throughout the running and diet choice period. The results of WR_HF and WRHF schedules in combination with those from previous studies indicate that wheel running develops decreased HF diet preference in males regardless of the order of the HF diet and exercise presentation e.g. HF before exercise, simultaneous exposure or exercise before HF. In contrast, the effects of exercise on palatable diet preferences in females depend on the testing schedule. Simultaneous presentation resulted in transient avoidance of the palatable diet whereas exercise before the HF diet presentation resulted in persistent preference to the HF diet to a greater degree than the preferences in sedentary females. The greater HF diet preference of females in the WR_HF condition was not affected by ovariectomy. Thus, the data suggests that the ovarian hormones does not account for all sex differences of HF diet preference in different diet and wheel running access schedules.
The effects of exercise on HF diet choice and the roles of ovarian hormones become more complicated with intermittent exercise and HF diet choice schedule. Because all WR_HF rats had been exposed to the running wheels and the HF diet, the two stimuli were presented simultaneously in all groups during the 2nd exposure in Experiment 2. In response to this exposure, WR_HF males reduced their HF diet intakes more than did intact WR_HF females. Furthermore, ovariectomized WR_HF females exhibited similar HF diet preference patterns to the WR_HF male rats i.e. they never preferred the HF diet during this 2nd exposure indicating potential roles for ovarian hormones in HF diet preference during intermittent exercise experience (Fig. 5 G – I, days 30 - 36). Overall, these results suggest additional mechanisms other than ovarian hormones may play roles in sex differences in exercise induced alterations of HF diet preferences.
Although we did not directly assess a role for neophobia or conditioning in the exercise induced palatable diet avoidance, such an explanation seems unlikely. Previous research with sweet solutions has suggested that the reduction of palatable fluid intake after exercise e.g. wheel running [23, 24] and forced swimming [21, 22, 25] is a result of conditioned taste aversion (CTA) learning. In this case, exercise serves as an unconditioned stimulus (US) and the sweet solution is a conditioned stimulus (CS). To serve as an US in CTA, a stimulus must produce negative physiological effects e.g. malaise. It is suggested that the reduced familiar food intake (anorexia) resulted from acute exercise can be viewed as an unconditioned negative effects of exercise. Furthermore, the novelty of the CS is important in producing a robust CTA. When these rules are applied, the results that wheel running produced anorexia to the chow and complete avoidance to novel HF and HS diets (i.e. not specific to one stimulus only) in male rats support that wheel running could serve as an US to support CTA learning. The conditioning mechanism is also supported by the fact that HF or HS diet avoidance persisted when rats were no longer running (Fig. 3). On the other hand, comparisons between the 1st and 2nd exposures in Experiment 2 allowed indirect assessment of the roles of neophobia in wheel running induced palatable diet avoidance. Repeated wheel running substantially suppressed intakes to a familiar (Fig. 5G) or highly preferred HF diet (Fig. 5I) during the 2nd exposure of Experiment 2. These results are consistent with our recent report that wheel running decreases preferences for previously formed preference to a familiar HF diet [26]. Taken together, these data indicate that the decreases in HF diet preference with exercise are not simply due to neophobia or a conditioning learning. Moreover, the fact that acute running in female can produce HF diet avoidance without anorexia to the familiar chow diet (Fig. 1D) further supports the idea at other mechanisms are involved in exercise related reduction of HF diet preferences.
Results of the current experiments are consistent with previous reports on sex differences in wheel running and food intake. In general, wheel running activity is higher in females than in males [19, 27-29]. While the same difference was observed, the current study also revealed the importance of ovarian hormones on female running activity. When introduced to running wheels, both males and females gradually increase daily running and then stabilized their activity levels. Although ovariectomy reduced female running activity to the same levels of male rats, it also eliminated a pattern of increase running across days such that levels of running activity remained constant. This phenomenon suggests that ovarian hormones mediate not only the amount of running but also the motivation to increase running over time. These data are consistent with a previous report demonstrating that ovarian hormones are not required for the initiation of running activity but are related to the gradual increase in daily running activity [30].
Moreover, many studies have demonstrated food intake reduction within the first few days of wheel running in adult male rats [31, 32, 14, 11, 33]. The results of wheel running in male rats in this study were consistent with such an effect. In contrast, the phenomenon of wheel running induced anorexia in adult females is not as consistent and pronounce. Increased [31, 34, 29], no change [35] or decreased [36, 32, 33] food intake accompanying wheel running have all been reported in adult female rats. The current study further illustrates this complicated issue of exercise induced anorexia in female rats. Wheel running in female rats produced no change in energy intake in Experiment 1. During the 1st exposure of Experiment 2, energy intake in running females appeared to be reduced but the reduction did not reach statistical significance. When running access was provided again during the 2nd exposure, energy intake in those females did not decrease and even increased in one group (Table 3). Furthermore, data from ovariectomized females in this study did not provide conclusive results. If ovarian hormones are important for sex differences in exercise induced anorexia, one would expect the results in ovariectomized females to be similar to those of males. This assumption was satisfied as ovariectomized running females significantly reduced intake during the 1st exposure. The same ovariectomized females, however, did not change intake in response to running during the 2nd exposure (Table 3). In other words, the changes of energy intake in response to wheel running in ovariectomized female rats did not mirror those in male rats. Given the inconsistent results in ovariectomizd female rats, whether and how ovarian hormones contribute to sex differences in exercise induced anorexia remains unclear.
In summary, the results of this study support the hypothesis that wheel running decreases preferences to not only a HF but also a HS diet in rats. Consistent with the hypothesis, the patterns of wheel running associated decrease in palatable diet preference differ between male and female rats. The results also demonstrate that ovarian hormones play a significant role in these sex differences while revealing the complexity of mechanisms underlying exercise associated changes in palatable diet preference. The findings in this study may provide new insights to investigate mechanisms underlying disordered eating behaviors. Subjects with anorexia nervosa are characterized by avoidance to highly palatable high fat/energy diets and a large portion of them shows hyperactivity [37]. On the other hand, it has been demonstrated that obese subjects have lower activity level and often overconsume the palatable energy dense diets [38]. Physical activity or exercise appears to be a key factor influencing extreme avoidance or preference of palatable energy dense diets. Further understanding of the mechanisms underlying exercise associated changes in palatable energy dense diet preference may pique new prevention or treatment methods for disordered eating and weight management.
Highlights.
Acute running produce complete palatable diet avoidance in male and female rats.
Exercise induced palatable diet avoidance is more robust and persistent in males.
Ovariectomy eliminates some of the sex differences produced by wheel running.
Acknowledgements
This research was funded by the Klarman Family Foundation and NIH DK-19302. The authors thank Mr. Alexander Moghadam for assistant with animal care.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zschucke E, Gaudlitz K, Strohle A. Exercise and physical activity in mental disorders: clinical and experimental evidence. J Prev Med Public Health. 2013;46(Suppl 1):S12–21. doi: 10.3961/jpmph.2013.46.S.S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boersma GJ, Barf RP, Benthem L, van Dijk G, Scheurink AJ. Forced and voluntary exercise counteract insulin resistance in rats: the role of coping style. Horm Behav. 2012;62:93–8. doi: 10.1016/j.yhbeh.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 3.De Feo P, Schwarz P. Is physical exercise a core therapeutical element for most patients with type 2 diabetes? Diabetes Care. 2013;36(Suppl 2):S149–54. doi: 10.2337/dcS13-2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fedewa MV, Gist NH, Evans EM, Dishman RK. Exercise and insulin resistance in youth: a meta-analysis. Pediatrics. 2014;133:e163–74. doi: 10.1542/peds.2013-2718. [DOI] [PubMed] [Google Scholar]
- 5.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–15. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kelly ME, Loughrey D, Lawlor BA, Robertson IH, Walsh C, et al. The impact of exercise on the cognitive functioning of healthy older adults: A systematic review and meta-analysis. Ageing Res Rev. 2014 doi: 10.1016/j.arr.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, et al. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380:247–57. doi: 10.1016/S0140-6736(12)60646-1. [DOI] [PubMed] [Google Scholar]
- 8.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380:219–29. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blundell JE, King NA. Exercise, appetite control, and energy balance. Nutrition. 2000;16:519–22. doi: 10.1016/s0899-9007(00)00250-1. [DOI] [PubMed] [Google Scholar]
- 10.King NA, Hopkins M, Caudwell P, Stubbs RJ, Blundell JE. Beneficial effects of exercise: shifting the focus from body weight to other markers of health. Br J Sports Med. 2009;43:924–7. doi: 10.1136/bjsm.2009.065557. [DOI] [PubMed] [Google Scholar]
- 11.Looy H, Eikelboom R. Wheel running, food intake, and body weight in male rats. Physiol Behav. 1989;45:403–5. doi: 10.1016/0031-9384(89)90147-9. [DOI] [PubMed] [Google Scholar]
- 12.Moran TH, Bi S. Hyperphagia and obesity of OLETF rats lacking CCK1 receptors: developmental aspects. Dev Psychobiol. 2006;48:360–7. doi: 10.1002/dev.20149. [DOI] [PubMed] [Google Scholar]
- 13.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol. 2008;294:R290–301. doi: 10.1152/ajpregu.00661.2007. [DOI] [PubMed] [Google Scholar]
- 14.Kawaguchi M, Scott KA, Moran TH, Bi S. Dorsomedial hypothalamic corticotropin-releasing factor mediation of exercise-induced anorexia. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1800–5. doi: 10.1152/ajpregu.00805.2004. [DOI] [PubMed] [Google Scholar]
- 15.Satvat E, Eikelboom R. Dissociation of conditioned and unconditioned factors in the running-induced feeding suppression. Physiol Behav. 2006;89:428–37. doi: 10.1016/j.physbeh.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Liang N-C, Moran TH. Running wheel activity reduces high fat diet preference without altering expression of reward genes. Appetite. 2012;59S:e35. [Google Scholar]
- 17.Scarpace PJ, Matheny M, Zhang Y. Wheel running eliminates high-fat preference and enhances leptin signaling in the ventral tegmental area. Physiol Behav. 2010;100:173–9. doi: 10.1016/j.physbeh.2010.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scarpace ET, Matheny M, Strehler KY, Shapiro A, Cheng KY, et al. Simultaneous introduction of a novel high fat diet and wheel running induces anorexia. Physiol Behav. 2012;105:909–14. doi: 10.1016/j.physbeh.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eckel LA, Moore SR. Diet-induced hyperphagia in the rat is influenced by sex and exercise. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1080–5. doi: 10.1152/ajpregu.00424.2004. [DOI] [PubMed] [Google Scholar]
- 20.Gomez F, Lahmame A, de Kloet ER, Armario A. Hypothalamic-pituitary-adrenal response to chronic stress in five inbred rat strains: differential responses are mainly located at the adrenocortical level. Neuroendocrinology. 1996;63:327–37. doi: 10.1159/000126973. [DOI] [PubMed] [Google Scholar]
- 21.Masaki T, Nakajima S. Further evidence for conditioned taste aversion induced by forced swimming. Physiol Behav. 2005;84:9–15. doi: 10.1016/j.physbeh.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Masaki T, Nakajima S. Taste aversion in rats induced by forced swimming, voluntary running, forced running, and lithium chloride injection treatments. Physiol Behav. 2006;88:411–6. doi: 10.1016/j.physbeh.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 23.Forristall JR, Hookey BL, Grant VL. Conditioned taste avoidance induced by forced and voluntary wheel running in rats. Behav Processes. 2007;74:326–33. doi: 10.1016/j.beproc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Salvy SJ, Heth DC, Pierce WD, Russell JC. Conditioned taste aversion induced by wheel running: further evidence on wheel running duration. Behav Processes. 2004;66:101–6. doi: 10.1016/j.beproc.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima S, Masaki T. Taste aversion learning induced by forced swimming in rats. Physiol Behav. 2004;80:623–8. doi: 10.1016/j.physbeh.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Liang NC, Bello NT, Moran TH. Wheel running reduces high-fat diet intake, preference and mu-opioid agonist stimulated intake. Behav Brain Res. 2015 doi: 10.1016/j.bbr.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferreira JA, Foley AM, Brown M. Sex hormones differentially influence voluntary running activity, food intake and body weight in aging female and male rats. Eur J Appl Physiol. 2012;112:3007–18. doi: 10.1007/s00421-011-2271-y. [DOI] [PubMed] [Google Scholar]
- 28.Gentry RT, Wade GN. Sex differences in sensitivity of food intake, body weight, and running-wheel activity to ovarian steroids in rats. J Comp Physiol Psychol. 1976;90:747–54. doi: 10.1037/h0077246. [DOI] [PubMed] [Google Scholar]
- 29.Schroeder M, Shbiro L, Gelber V, Weller A. Post-weaning voluntary exercise exerts long-term moderation of adiposity in males but not in females in an animal model of early-onset obesity. Horm Behav. 2010;57:496–505. doi: 10.1016/j.yhbeh.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Ramaley JA. Development of running activity in maturing rats: dependence upon prior androgen exposure and ovarian function. Physiol Behav. 1975;15:25–9. doi: 10.1016/0031-9384(75)90274-7. [DOI] [PubMed] [Google Scholar]
- 31.Carrera O, Cerrato M, Vazquez R, Sineiro C, Gutierrez E. Gender dimorphic effects of voluntary running in laboratory rats depends on maturational status. Q J Exp Psychol (Hove) 2011;64:823–32. doi: 10.1080/17470218.2010.523473. [DOI] [PubMed] [Google Scholar]
- 32.Jones LC, Bellingham WP, Ward LC. Sex differences in voluntary locomotor activity of food-restricted and ad libitum-fed rats. Implications for the maintenance of a body weight set-point. Comp Biochem Physiol A Comp Physiol. 1990;96:287–90. doi: 10.1016/0300-9629(90)90694-n. [DOI] [PubMed] [Google Scholar]
- 33.Tokuyama K, Saito M, Okuda H. Effects of wheel running on food intake and weight gain of male and female rats. Physiol Behav. 1982;28:899–903. doi: 10.1016/0031-9384(82)90211-6. [DOI] [PubMed] [Google Scholar]
- 34.Pitts GC. Body composition in the rat: interactions of exercise, age, sex, and diet. Am J Physiol. 1984;246:R495–501. doi: 10.1152/ajpregu.1984.246.4.R495. [DOI] [PubMed] [Google Scholar]
- 35.Cortright RN, Chandler MP, Lemon PW, DiCarlo SE. Daily exercise reduces fat, protein and body mass in male but not female rats. Physiol Behav. 1997;62:105–11. doi: 10.1016/s0031-9384(97)00148-0. [DOI] [PubMed] [Google Scholar]
- 36.Eckel LA, Houpt TA, Geary N. Spontaneous meal patterns in female rats with and without access to running wheels. Physiol Behav. 2000;70:397–405. doi: 10.1016/s0031-9384(00)00278-x. [DOI] [PubMed] [Google Scholar]
- 37.Davis C. Eating disorders and hyperactivity: a psychobiological perspective. Can J Psychiatry. 1997;42:168–75. doi: 10.1177/070674379704200207. [DOI] [PubMed] [Google Scholar]
- 38.Levine JA, Lanningham-Foster LM, McCrady SK, Krizan AC, Olson LR, et al. Interindividual variation in posture allocation: possible role in human obesity. Science. 2005;307:584–6. doi: 10.1126/science.1106561. [DOI] [PubMed] [Google Scholar]