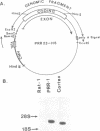
Abstract
The rat M1 muscarinic receptor gene was cloned and expressed in a rat cell line lacking endogenous muscarinic receptors. Assignment of the cloned receptors to the M1 class was pharmacologically confirmed by their high affinity for the M1-selective muscarinic antagonist pirenzepine and low affinity for the M2-selective antagonist AF-DX-116. Guanylyl imidodiphosphate [Gpp(NH)p] converted agonist binding sites on the receptor, from high-affinity to the low-affinity state, thus indicating that the cloned receptors couple to endogenous G-proteins. The cloned receptors mediated both adenylate cyclase inhibition and phosphoinositide hydrolysis, but by different mechanisms. Pertussis toxin blocked the inhibition of adenylate cyclase (indicating coupling of the receptor to inhibitory G-protein), but did not affect phosphoinositide turnover. Furthermore, the stimulation of phosphoinositide hydrolysis was less efficient than the inhibition of adenylate cyclase. These findings demonstrate that cloned M1 receptors are capable of mediating multiple responses in the cell by coupling to different effectors, possibly to different G-proteins.
Full text
PDF
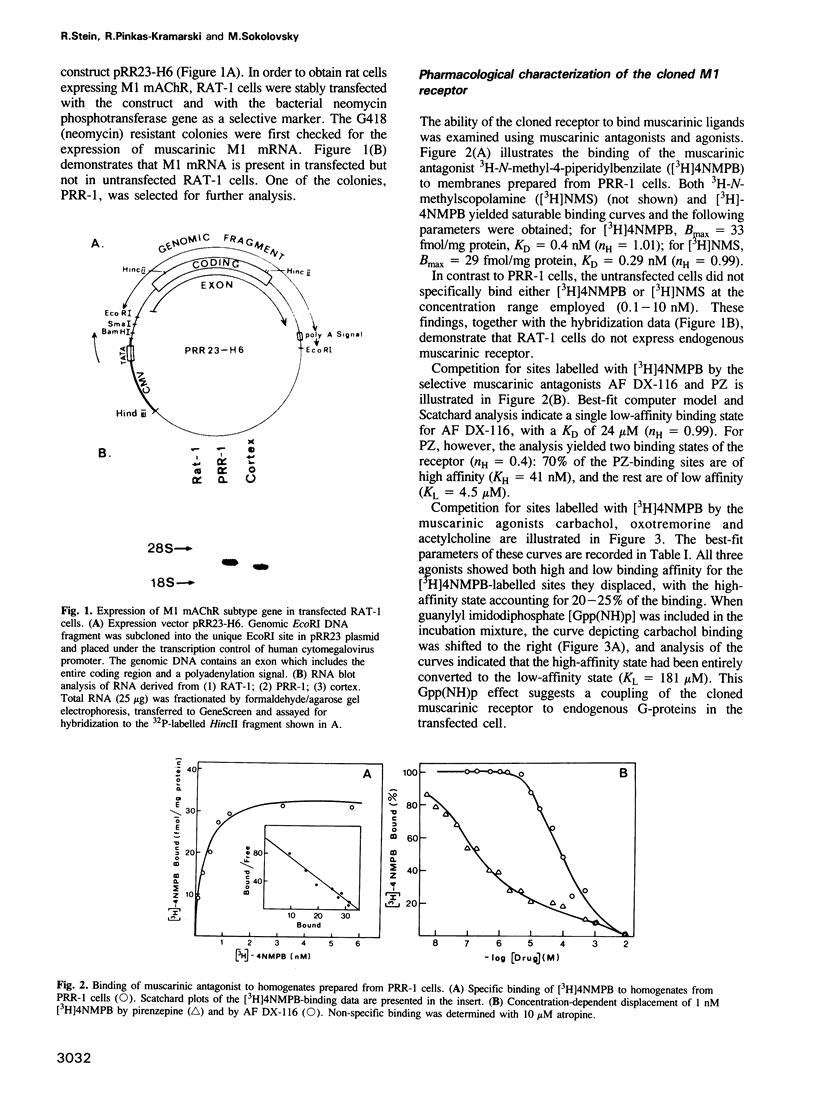
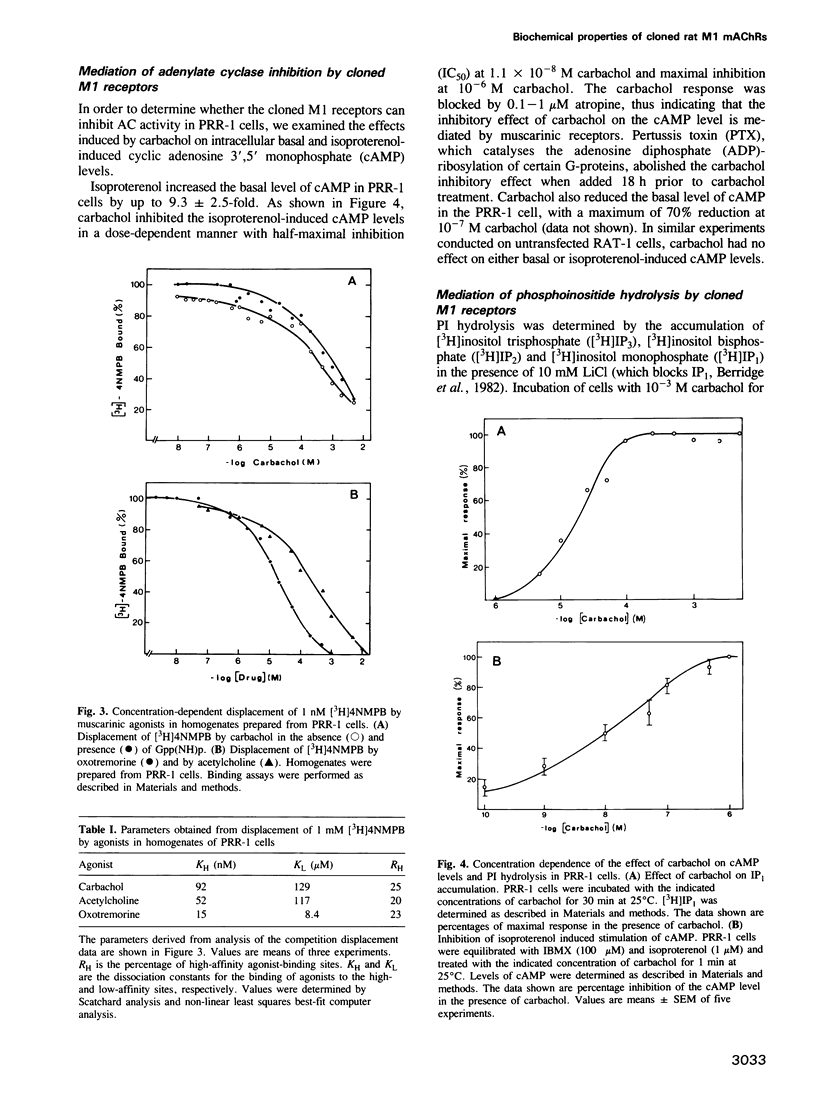


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama K., Watson M., Roeske W. R., Yamamura H. I. High-affinity [3H]pirenzepine binding to putative M1 muscarinic sites in the neuroblastoma x glioma hybrid cell line (NG 108-15). Biochem Biophys Res Commun. 1984 Feb 29;119(1):289–297. doi: 10.1016/0006-291x(84)91650-4. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Winslow J. W., Peralta E. G., Peterson G. L., Schimerlik M. I., Capon D. J., Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987 Oct 30;238(4827):672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Lithium amplifies agonist-dependent phosphatidylinositol responses in brain and salivary glands. Biochem J. 1982 Sep 15;206(3):587–595. doi: 10.1042/bj2060587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Buckley N. J., Jones S. V., Bonner T. I. Expression of a cloned muscarinic receptor in A9 L cells. Mol Pharmacol. 1987 Oct;32(4):450–455. [PubMed] [Google Scholar]
- Cohen-Armon M., Garty H., Sokolovsky M. G-protein mediates voltage regulation of agonist binding to muscarinic receptors: effects on receptor-Na+ channel interaction. Biochemistry. 1988 Jan 12;27(1):368–374. doi: 10.1021/bi00401a055. [DOI] [PubMed] [Google Scholar]
- Fukuda K., Kubo T., Akiba I., Maeda A., Mishina M., Numa S. Molecular distinction between muscarinic acetylcholine receptor subtypes. Nature. 1987 Jun 18;327(6123):623–625. doi: 10.1038/327623a0. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldo E., Hammer R., Ladinsky H. Distribution of muscarinic receptor subtypes in rat brain as determined in binding studies with AF-DX 116 and pirenzepine. Life Sci. 1987 Mar 2;40(9):833–840. doi: 10.1016/0024-3205(87)90031-2. [DOI] [PubMed] [Google Scholar]
- Gurwitz D., Kloog Y., Sokolovsky M. High affinity binding of [3H]acetylcholine to muscarinic receptors. Regional distribution and modulation by guanine nucleotides. Mol Pharmacol. 1985 Sep;28(3):297–305. [PubMed] [Google Scholar]
- Gurwitz D., Sokolovsky M. Dual pathways in muscarinic receptor stimulation of phosphoinositide hydrolysis. Biochemistry. 1987 Jan 27;26(2):633–638. doi: 10.1021/bi00376a039. [DOI] [PubMed] [Google Scholar]
- Haga K., Haga T., Ichiyama A. Reconstitution of the muscarinic acetylcholine receptor. Guanine nucleotide-sensitive high affinity binding of agonists to purified muscarinic receptors reconstituted with GTP-binding proteins (Gi and Go). J Biol Chem. 1986 Aug 5;261(22):10133–10140. [PubMed] [Google Scholar]
- Jagodzinski L. L., Sargent T. D., Yang M., Glackin C., Bonner J. Sequence homology between RNAs encoding rat alpha-fetoprotein and rat serum albumin. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3521–3525. doi: 10.1073/pnas.78.6.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumblatt J. E., Tischler A. S. Regulation of muscarinic ligand binding sites by nerve growth factor in PC12 phaeochromocytoma cells. Nature. 1982 May 13;297(5862):152–154. doi: 10.1038/297152a0. [DOI] [PubMed] [Google Scholar]
- Kloog Y., Egozi Y., Sokolovsky M. Characterization of muscarinic acetylcholine receptors from mouse brain: evidence for regional heterogeneity and isomerization. Mol Pharmacol. 1979 May;15(3):545–558. [PubMed] [Google Scholar]
- Kloog Y., Sokolovsky M. Muscarinic binding to mouse brain receptor sites. Biochem Biophys Res Commun. 1978 Apr 14;81(3):710–717. doi: 10.1016/0006-291x(78)91410-9. [DOI] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Kubo T., Maeda A., Sugimoto K., Akiba I., Mikami A., Takahashi H., Haga T., Haga K., Ichiyama A., Kangawa K. Primary structure of porcine cardiac muscarinic acetylcholine receptor deduced from the cDNA sequence. FEBS Lett. 1986 Dec 15;209(2):367–372. doi: 10.1016/0014-5793(86)81144-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Masters S. B., Martin M. W., Harden T. K., Brown J. H. Pertussis toxin does not inhibit muscarinic-receptor-mediated phosphoinositide hydrolysis or calcium mobilization. Biochem J. 1985 May 1;227(3):933–937. doi: 10.1042/bj2270933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor Z., Koch Y., Chobsieng P., Zor U. Pituitary cyclic AMP production and mechanism of luteinizing hormone release. FEBS Lett. 1975 Oct 15;58(1):318–321. doi: 10.1016/0014-5793(75)80288-2. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange P. G., Birdsall N. J., Burgen A. S. Ligand-binding propeties of the muscarinic acetylcholine receptor in mouse neuroblastoma cells. Biochem J. 1978 Jun 15;172(3):495–501. doi: 10.1042/bj1720495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatani A., Codina J., Brown A. M., Birnbaumer L. Direct activation of mammalian atrial muscarinic potassium channels by GTP regulatory protein Gk. Science. 1987 Jan 9;235(4785):207–211. doi: 10.1126/science.2432660. [DOI] [PubMed] [Google Scholar]