Abstract
The transcription factor E-twenty-six version 5 (ETV5) has been linked with obesity in genome-wide association studies. Moreover, ETV5-deficient mice (knockout; KO) have reduced body weight, lower fat mass, and are resistant to diet-induced obesity, directly linking ETV5 to the regulation of energy balance and metabolism. ETV5 is expressed in hypothalamic brain regions that regulate both metabolism and HPA axis activity, suggesting that ETV5 may also modulate HPA axis function. In order to test this possibility, plasma corticosterone levels were measured in ETV5 KO and wildtype (WT) mice before (pre-stress) and after (post-stress) a mild stressor (intraperitoneal injection). ETV5 deficiency increased both pre- and post-stress plasma corticosterone, suggesting that loss of ETV5 elevated glucocorticoid tone. Consistent with this idea, ETV5 KO mice have reduced thymus weight, suggestive of increased glucocorticoid-induced thymic involution. ETV5 deficiency also decreased the mRNA expression of glucocorticoid receptor (GR), mineralocorticoid receptor (MR), and vasopressin receptor 1A in the hypothalamus, without altering vasopressin, corticotropin-releasing hormone, or oxytocin mRNA expression. In order to test whether reduced MR and GR expression affected glucocorticoid negative feedback, a dexamethasone suppression test was performed. Dexamethasone reduced plasma corticosterone in both ETV5 KO and WT mice, suggesting that glucocorticoid negative feedback was unaltered by ETV5 deficiency. In summary, these data suggest that the obesity-associated transcription factor ETV5 normally acts to diminish circulating glucocorticoids. This might occur directly via ETV5 actions on HPA-regulatory brain circuitry, and/or indirectly via ETV5-induced alterations in metabolic factors that then influence the HPA axis.
Keywords: ETV5, stress, hypothalamic-pituitary-adrenocortical (HPA) axis, glucocorticoids, metabolism
1.0 INTRODUCTION
ETV5 was first associated with obesity in multiple human genome-wide association studies (Willer et al. 2009; Thorleifsson et al. 2009). More recently, ETV5 has been functionally-linked with obesity. ETV5-deficient (knockout; KO) mice have reduced body weight and adiposity compared to wildtype (WT) littermates (Gutierrez-Aguilar et al. 2014; Schlesser et al. 2008). Furthermore, they are resistant to high fat diet-induced obesity, suggesting that ETV5 normally promotes body fat and body weight gain (Gutierrez-Aguilar et al. 2014). ETV5 KO mice are also glucose intolerant (despite reduced adiposity), due at least in part to impaired insulin secretion, suggesting that ETV5 also plays an important role in glucose metabolism (Gutierrez-Aguilar et al. 2014). Importantly, ETV5 mRNA is expressed in brain regions that are critical for the regulation of energy balance (e.g., arcuate (ARC) and ventromedial (VMN) hypothalamic nuclei) and this expression varies with nutritional state, suggesting that ETV5 may contribute to metabolic regulation, at least in part, via its role in the brain (Boender, van Rozen, and Adan 2012; Schmid et al. 2012; Gutierrez-Aguilar et al. 2012).
Metabolism is also influenced by the hypothalamic-pituitary-adrenocortical (HPA) axis. The HPA axis is a major neuroendocrine system that has a low basal level of activity that is markedly increased in response to stress (i.e., a real or perceived threat to homeostasis or well-being) (reviewed in (Ulrich-Lai and Herman 2009)). In this system, neurons in the paraventricular nucleus of hypothalamus (PVN) release corticotropin releasing hormone (CRH), vasopressin (AVP) and other releasing hormones from their terminals in the median eminence. These releasing hormones act on the anterior pituitary to release adrenocorticotrophic releasing hormone (ACTH) in the systemic circulation. ACTH then acts on the adrenal cortex to induce the production and release of glucocorticoids (e.g., cortisol in humans and corticosterone in rodents). Glucocorticoids exert negative feedback on the HPA axis by acting on their receptors (glucocorticoid receptors (GR) and mineralocorticoid receptors (MR)) in the brain and pituitary to decrease CRH and AVP expression, inhibit ACTH release, and limit further HPA axis activity (Kageyama and Suda 2009). Furthermore, glucocorticoids act on peripheral metabolic tissues to mobilize stored energy by: 1) stimulating gluconeogenesis in the liver (Baxter 1976); 2) enhancing lipolysis in the adipose tissue (Peckett, Wright, and Riddell 2011); and 3) modifying insulin secretion (Lambillotte, Gilon, and Henquin 1997; Dinneen et al. 1993). All of these adaptations lead to an acute hyperglycemic state, thus providing energy to fuel appropriate behavioral and cognitive responses to stress.
Importantly, HPA axis function is reciprocally influenced by metabolic state. For instance, metabolism-regulatory brain regions (e.g., ARC, VMH) and metabolic hormones (e.g., insulin, leptin) all influence HPA axis activity (reviewed in (Ulrich-Lai and Ryan 2014). Taken together with the clear role that ETV5 plays in metabolic regulation (described above), this suggests that ETV5 is also well-positioned to regulate HPA axis activity. The present work addresses this hypothesis by measuring (1) pre- and post-stress plasma corticosterone, (2) hypothalamic expression of stress-regulatory genes, and (3) glucocorticoid negative feedback, in ETV5 KO and WT mice.
2.0 MATERIALS AND METHODS
2.1 Animals
The ETV5 KO mouse line was kindly donated by Dr. K. M. Murphy (Chen et al. 2005). KO and WT male littermate mice were bred in-house using breeding pairs that were heterozygous for a loss-of-function on ETV5. Mice from 6–9 litters were used in each experiment. These animals were individually housed under a 12 h/12 h light/dark cycle (lights on at 06:00h, lights off at 18:00 h) and allowed ad libitum access to water and pelleted standard chow (LM-485; Harlan-Teklad, Indianapolis, IN, USA). The University of Cincinnati Institutional Animal Care and Use Committee approved all animal protocols.
2.2 Pre- and post-stress plasma corticosterone
In a previous study (Gutierrez-Aguilar et al. 2014), a glucose tolerance test (GTT) was performed in ETV5 KO and WT mice at 12 weeks of age. Mice were briefly fasted (06:00–10:00 h), and at 10:00 h, tail blood samples were quickly collected immediately prior to (0 min) and at 15 and 30 min after intraperitoneal (i.p.) glucose injection (1.5 mg/g of body weight) for measurement of plasma glucose and insulin. In the present work, plasma corticosterone was measured by radioimmunoassay (RIA) in these plasma samples via a RIA kit (ICN Biomedical, Costa Mesa, Calif., USA) following manufacturer’s instructions, as previously described (Ulrich-Lai et al. 2010), in order to assess the HPA response to the mild stress associated with the GTT procedure (e.g., i.p. injection). The minimum detection of the RIA Kit is 12.5 ng/ml.
2.3 Dexamethasone suppression test
ETV5 KO and WT mice were given a low dose of the synthetic glucocorticoid dexamethasone (0.1 mg/kg, s.c.) vs. saline vehicle at 20 weeks of age. At 2 hours after dexamethasone injection, a tail blood sample was quickly collected (0 min). Mice were then immediately placed into a new, clean home cage (this cage change served as a mild acute stressor) and tail blood samples were quickly collected at 30 and 60 min for determination of plasma corticosterone.
2.4 Adrenal and thymus weights
Mice (at 16 weeks of age) were sacrificed by decapitation at 10:00 h. Adrenal and thymus glands were collected, cleaned and weighed as general indices of chronic HPA axis tone. Adrenal weight is often used as an indirect index of cumulative ACTH exposure, as ACTH stimulates adrenal growth (Ulrich-Lai et al. 2006). Thymus weight is often used as an indirect index of cumulative glucocorticoid exposure, as glucocorticoids induce thymic shrinkage (Ulrich-Lai et al. 2006; Dimitrijevic et al. 2012).
2.5 Semiquantitative real-time PCR
The hypothalamus was also quickly collected at time of sacrifice, and total RNA was extracted with Trizol reagent (Invitrogen, Carlsbad, CA) and purified by RNeasy Mini Kit (Qiagen, Valencia, CA), according to manufacturer’s instructions. cDNA was produced by SuperScript First Strand Synthesis Kit (Invitrogen, Carlsbad, CA). mRNA expression was measured on an ABI 7900HT real-time PCR system with the following Taqman probes: arginine vasopressin (avp; Mm00437761_g1), arginine vasopressin receptor 1A (avpr1a; Mm00444092_m1), glucocorticoid receptor (GR) nuclear receptor subfamily 3, group C, member 1 (nr3c1; Mm00433832_m1), mineral glucocorticoid receptor (MR) nuclear receptor subfamily 3, group C, member 2 (nr3c2; Mm01241596_m1), oxytocin (oxy; Mm00726655_s1), corticotropin releasing hormone (crh; Mm01293920_s1), and the housekeeping gene the ribosomal protein L32 (L32; Mm02528467_g1). Samples were run in triplicate and the mRNA expression was expressed relative to the housekeeping L32 gene.
2.6 Statistical analysis
All results are shown as means ± SEM. Statistical analyses were performed using GraphPad Prism 6 (GraphPad Software, San Diego, CA, USA). For organ weight and gene expression data, two-tailed t-tests were used to compare the 2 genotypes. For plasma corticosterone data, a two-way ANOVA with repeated measures, followed by a Bonferroni post-hoc test, was used to compare Treatment Group and Time. Plasma corticosterone area-under-the-curve (AUC) was calculated using the trapezoidal rule and analyzed by 2-tailed t-test. Statistical significance was taken as p<0.05.
3.0 RESULTS
3.1 Pre- and post-stress plasma corticosterone
Prior to stress (0 min), plasma corticosterone was ~2.3 fold higher in the ETV5 KO mice compared to their WT littermates (Figure 1A). Exposure to mild stress (i.p. injection) increased plasma corticosterone in both ETV5 KO and WT mice. However, the difference between the genotypes persisted after stress exposure (30 and 60 min), such that plasma corticosterone remained higher (~1.6 fold) in the ETV5 KO mice after stress. These data indicate that ETV5 deficiency elevates both pre- and post-stress plasma corticosterone. Consistent with this, analysis of the plasma corticosterone area-under-the-curve shows that the total integrated plasma corticosterone response is greater in KO mice (Figure 1B).
Figure 1.
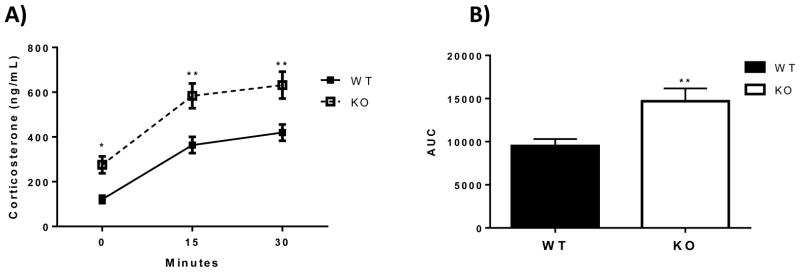
A) ETV5 deficiency increases pre- and post-stress plasma corticosterone. Plasma corticosterone was assessed in ETV5 knockout (KO) and wildtype (WT) littermate mice prior to (0 min) and after (15 and 30 min) a mild stressor (i.p. injection). Two-way ANOVA with repeated measures followed by Bonferroni post-hoc test, *p<0.05, **p<0.01. B) Analysis of area-under-the-curve (AUC) shows increased integrated plasma corticosterone in KO mice (two-tailed t-test, **p<0.01). Data are presented as mean ± SEM. n = 7 KO – 8 WT/group.
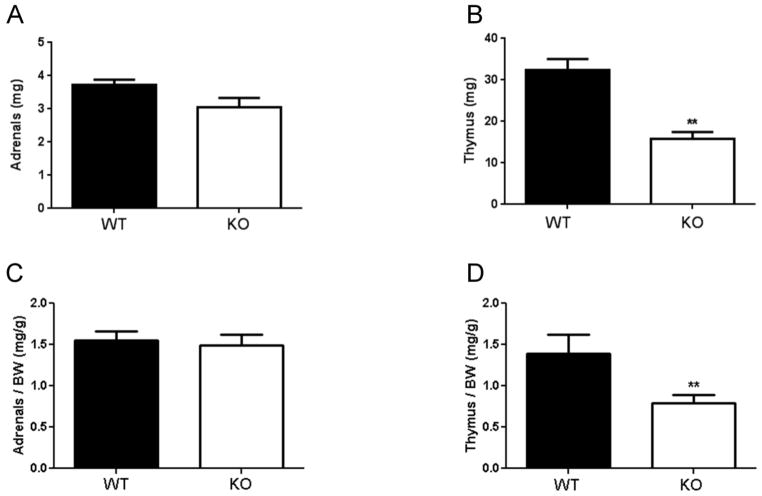
3.2 Adrenal and thymus weights
Neither total adrenal weight (Figure 2A), nor adrenal weight normalized to body weight (Figure 2C), differed by genotype. In contrast, thymus weight (Figure 2B) and thymus weight normalized to body weight (Figure 2D) were both reduced in ETV5 KO mice when compared to WT littermates. These data indicate that ETV5 deficiency reduces thymus weight even after considering differences in overall body weight, suggesting the possibility that ETV5 KO mice experienced a history of chronically elevated glucocorticoids.
Figure 2.
ETV5 deficiency reduced thymus, but not adrenal, weight. (A) Total (left + right) adrenal gland weight, (B) thymus weight, (C) adrenal weight normalized to body weight, and (D) thymus weight normalized to body weight, of ETV5 knockout (KO) and wildtype (WT) littermate mice. Data are presented as mean ± SEM. Two-tailed t-tests, **p<0.01. n = 5 KO-6 WT/group.
3.3 Hypothalamic stress-related gene expression
In order to assess whether differences in plasma corticosterone were accompanied by changes in central HPA regulation, the expression of several stress-related genes was assessed in hypothalamic tissue from ETV5 KO and WT mice (Figure 3). The expression of nr3c1 (GR), nr3c2 (MR), and avpr1a (vasopressin receptor 1a) mRNAs were significantly down-regulated in the hypothalamus of ETV5 KO mice compared to WT littermates. In contrast, avp, crh and oxy mRNAs did not differ between genotypes. These data suggest that ETV5 deficiency reduces the mRNA expression of some stress-regulatory genes in the hypothalamus. More specifically, reduced expression of MR and GR is often associated with reduced glucocorticoid negative feedback (Herman, Adams, and Prewitt 1995; Mizoguchi et al. 2003), suggesting this as a possible mechanism for elevated plasma corticosterone in ETV5 deficient mice.
Figure 3.
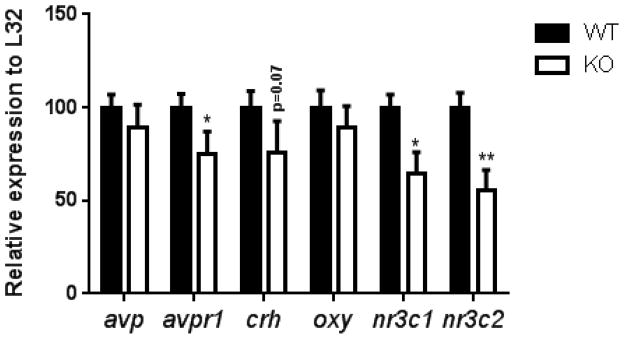
ETV5 deficiency reduced the mRNA expression of several stress-regulatory genes in the hypothalamus. Arginine vasopressin (avp), arginine vasopressin receptor (avpr1), corticotropin-releasing hormone (crh), oxytocin (oxy), glucocorticoid receptor (nr3c1) and mineralocorticoid receptor (nr3c2). Data are presented as mean ± SEM. Two-tailed t-tests, *p<0.05, **p<0.01. n = 7 KO – 8 WT/group.
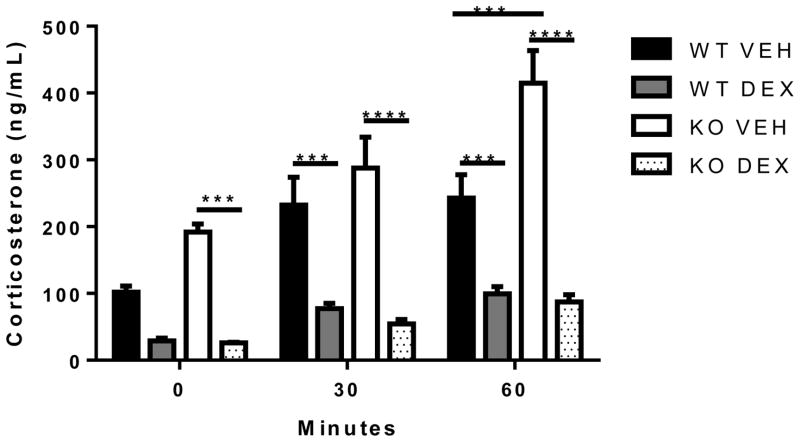
3.4 Dexamethasone suppression test
Since altered expression of corticosterone receptors (Figure 3) could influence the ability of glucocorticoids to exert negative feedback on the HPA axis, this possibility was tested in a dexamethasone suppression test. In this test, the synthetic glucocorticoid dexamethasone is administered and its ability to suppress subsequent stress-induced corticosterone secretion is used as an index of glucocorticoid negative feedback. In saline-injected mice, plasma corticosterone was generally higher in ETV5 KO mice (compared to WT littermate controls), particularly at 60 min after cage change stress (Figure. 4), consistent with earlier results (Figure 1A). Moreover, dexamethasone significantly reduced plasma corticosterone in WT mice at 30 and 60 min, and in KO mice at 0, 30 and 60 min. These data suggest that ETV5 deficiency does not prevent effective glucocorticoid negative feedback.
Figure 4.
ETV5 deficiency does not prevent glucocorticoid negative feedback in a dexamethasone suppression test. Two hours after dexamethasone (DEX) or vehicle (VEH) administration, plasma corticosterone was assessed before (0 min) and after (30 and 60 min) a mild stressor (cage change) in ETV5 knockout (KO) and wildtype (WT) littermate mice. Data are presented as mean ± SEM. Two-way ANOVA with repeated measures followed by Bonferroni post-hoc test, *p<0.001. n = 4 KO – 5 WT/group.
4.0 DISCUSSION
ETV5 was recently linked with obesity (Thorleifsson et al. 2009), and it plays an important role in regulating energy balance and glucose metabolism (Gutierrez-Aguilar et al. 2014). The present work addressed the hypothesis that ETV5 also contributes to HPA axis regulation. ETV5 KO mice had increased pre- and post-stress plasma corticosterone and decreased thymus weight, suggesting they experienced chronically elevated glucocorticoid tone. MR and GR mRNA expression was reduced in the hypothalamus of ETV5 KO mice, but a dexamethasone suppression test showed intact glucocorticoid negative feedback. Taken together, these data show that ETV5 deficiency elevates glucocorticoids, suggesting that ETV5 normally acts to decrease glucocorticoid tone.
4.1 HPA axis regulation by actions of ETV5 in brain
ETV5 is highly expressed in brain, including several hypothalamic brain regions (e.g., ARC, VMH) that are important for regulation of both metabolism and HPA activity (reviewed in (Ulrich-Lai and Ryan 2014), suggesting that ETV5 may influence HPA axis activity via its actions in brain. Consistent with this idea, the mRNA expression of several HPA-regulatory genes, including MR and GR, are down-regulated in the hypothalamus of ETV5 KO mice, implying that decreased glucocorticoid negative feedback could be responsible for the elevated HPA axis tone. However, assessment of the glucocorticoid negative feedback by dexamethasone suppression test showed intact negative feedback in ETV5 deficient mice, indicating that this is unlikely to the mechanism by which ETV5 regulates glucocorticoids levels. Instead the down-regulation of MR and GR expression may be the consequence of the increased plasma corticosterone, since chronically-elevated glucocorticoids can decrease brain MR and GR expression – though usually to a great extent in forebrain than hypothalamus (Herman, Adams, and Prewitt 1995; Mizoguchi et al. 2003).
4.2 HPA axis regulation by actions of ETV5 in pituitary and adrenal
HPA axis activity can also be regulated at the level of the pituitary (e.g., increased ACTH response to CRH/AVP) (Aguilera 1994) and adrenal gland (e.g., increased corticosterone response to ACTH) (Ulrich-Lai, Arnhold, and Engeland 2006; Spiga et al. 2011; Ulrich-Lai and Engeland 2002). ETV5 appears to be ubiquitously expressed in mice and humans (Hollenhorst, Jones, and Graves 2004; Chotteau-Lelievre et al. 2001), suggesting that ETV5 may contribute to HPA regulation via direct actions in pituitary or adrenal. Unaltered adrenal gland weight in ETV5 KO mice could indicate that ETV5 deficiency does not alter pituitary secretion of ACTH, but unfortunately this possibility could not be directly assessed since the volume of blood collected from mice is too small to permit measurement of plasma ACTH. Alternatively, ETV5 may act locally in the adrenal to regulate glucocorticoid production. For instance, ETV5 is important for the development and function of other steroidogenic tissues, such as testes and ovaries (Schlesser et al. 2008; Eo et al. 2011). However, in these tissues ETV5 deficiency results in diminished steroid hormone production, suggesting that this is unlikely to explain the elevated glucocorticoids that are seen in ETV5 KO mice. Otherwise, it is also possible that ETV5 could increase adrenal responsivity to ACTH, or else increase corticosteroid-binding globulin leading to greater total (bound plus free) circulating glucocorticoids – though these possibilities need to be tested.
4.3 Role of ETV5 in mediating interactions between the HPA axis and metabolism
Lastly, ETV5 could influence HPA axis activity indirectly via its marked effects on energy balance and metabolic state. For instance, ETV5 KO mice have reduced adiposity (Gutierrez-Aguilar et al. 2014), which can influence HPA axis tone. ETV5 KO mice also have altered levels of metabolic hormones (e.g., reduced insulin) (Gutierrez-Aguilar et al. 2014), that can themselves influence HPA axis activity.
On the other hand, elevated glucocorticoids could contribute to the metabolic abnormalities that are observed in ETV5 KO mice, including reduced insulin secretion, glucose intolerance, and hyperglycemia. For example, glucocorticoids can suppress glucose-stimulated insulin secretion in vivo (Plat et al. 1996; Dinneen et al. 1993) and in vitro (Billaudel and Sutter 1979; Barseghian and Levine 1980; Lambillotte, Gilon, and Henquin 1997; Gremlich, Roduit, and Thorens 1997). In addition, glucocorticoids can promote hyperglycemia (Mangos et al. 2000; Rizza, Mandarino, and Gerich 1982) by stimulating liver gluconeogenesis (Baxter 1976). Consistent with this idea, glucose intolerance in men is associated with elevated plasma cortisol (Reynolds et al. 2001) Taken together, this suggests that increased HPA axis tone in ETV5 KO mice may contribute, at least in part, to their metabolic dysfunction.
4.4 Overall summary and conclusions
Taken together, this work indicates that ETV5, a transcription factor that was recently associated with obesity in multiple human genome wide association studies, also regulates the HPA axis. More specifically, genetic ETV5 deficiency elevates plasma corticosterone, suggesting that ETV5 normally acts to diminish circulating glucocorticoids. This might occur directly via ETV5 actions on the HPA axis system, and/or indirectly via ETV5-induced alterations in metabolic factors that then influence the HPA axis.
HIGHLIGHTS.
Loss of ETV5 elevates glucocorticoid tone.
ETV5 deficiency decreases GR, MR and avpr1 hypothalamic transcripts.
ETV5 deficiency does not alter avp, crh, or oxy mRNA expression.
Glucocorticoid negative feedback is unaltered by ETV5 deficiency.
Acknowledgments
We thank K.M. Murphy from Washington University and the Howard Hughes Medical Institute for providing the ETV5 KO mouse line.
FUNDING
This work was supported by the National Institute of Health (grants DK093848 to RJS and R01 DK091425 to YMUL).
Footnotes
DUALITY OF INTERST
The authors declare that there is no duality of interest associated with this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–50. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- Barseghian G, Levine R. Effect of corticosterone on insulin and glucagon secretion by the isolated perfused rat pancreas. Endocrinology. 1980;106:547–52. doi: 10.1210/endo-106-2-547. [DOI] [PubMed] [Google Scholar]
- Baxter JD. Glucocorticoid hormone action. Pharmacol Ther B. 1976;2:605–69. doi: 10.1016/0306-039x(76)90010-6. [DOI] [PubMed] [Google Scholar]
- Billaudel B, Sutter BC. Direct effect of corticosterone upon insulin secretion studied by three different techniques. Horm Metab Res. 1979;11:555–60. doi: 10.1055/s-0028-1092779. [DOI] [PubMed] [Google Scholar]
- Boender AJ, van Rozen AJ, Adan RA. Nutritional state affects the expression of the obesity-associated genes Etv5, Faim2, Fto, and Negr1. Obesity (Silver Spring) 2012;20:2420–5. doi: 10.1038/oby.2012.128. [DOI] [PubMed] [Google Scholar]
- Chen C, Ouyang W, Grigura V, Zhou Q, Carnes K, Lim H, Zhao GQ, Arber S, Kurpios N, Murphy TL, Cheng AM, Hassell JA, Chandrashekar V, Hofmann MC, Hess RA, Murphy KM. ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature. 2005;436:1030–4. doi: 10.1038/nature03894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotteau-Lelievre A, Dolle P, Peronne V, Coutte L, de Launoit Y, Desbiens X. Expression patterns of the Ets transcription factors from the PEA3 group during early stages of mouse development. Mech Dev. 2001;108:191–5. doi: 10.1016/s0925-4773(01)00480-4. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic M, Stanojevic S, Kustrimovic N, Leposavic G. End-point effector stress mediators in neuroimmune interactions: their role in immune system homeostasis and autoimmune pathology. Immunol Res. 2012;52:64–80. doi: 10.1007/s12026-012-8275-9. [DOI] [PubMed] [Google Scholar]
- Dinneen S, Alzaid A, Miles J, Rizza R. Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J Clin Invest. 1993;92:2283–90. doi: 10.1172/JCI116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eo J, Shin H, Kwon S, Song H, Murphy KM, Lim JH. Complex ovarian defects lead to infertility in Etv5−/− female mice. Mol Hum Reprod. 2011;17:568–76. doi: 10.1093/molehr/gar021. [DOI] [PubMed] [Google Scholar]
- Gremlich S, Roduit R, Thorens B. Dexamethasone induces posttranslational degradation of GLUT2 and inhibition of insulin secretion in isolated pancreatic beta cells. Comparison with the effects of fatty acids. J Biol Chem. 1997;272:3216–22. doi: 10.1074/jbc.272.6.3216. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Aguilar R, Kim DH, Casimir M, Dai XQ, Pfluger PT, Park J, Haller A, Donelan E, Park J, D’Alessio D, Woods SC, MacDonald PE, Seeley RJ. The role of the transcription factor ETV5 in insulin exocytosis. Diabetologia. 2014;57:383–91. doi: 10.1007/s00125-013-3096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez-Aguilar R, Kim DH, Woods SC, Seeley RJ. Expression of new loci associated with obesity in diet-induced obese rats: from genetics to physiology. Obesity (Silver Spring) 2012;20:306–12. doi: 10.1038/oby.2011.236. [DOI] [PubMed] [Google Scholar]
- Herman JP, Adams D, Prewitt C. Regulatory changes in neuroendocrine stress-integrative circuitry produced by a variable stress paradigm. Neuroendocrinology. 1995;61:180–90. doi: 10.1159/000126839. [DOI] [PubMed] [Google Scholar]
- Hollenhorst PC, Jones DA, Graves BJ. Expression profiles frame the promoter specificity dilemma of the ETS family of transcription factors. Nucleic Acids Res. 2004;32:5693–702. doi: 10.1093/nar/gkh906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama K, Suda T. Regulatory mechanisms underlying corticotropin-releasing factor gene expression in the hypothalamus. Endocr J. 2009;56:335–44. doi: 10.1507/endocrj.k09e-075. [DOI] [PubMed] [Google Scholar]
- Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Invest. 1997;99:414–23. doi: 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangos GJ, Walker BR, Kelly JJ, Lawson JA, Webb DJ, Whitworth JA. Cortisol inhibits cholinergic vasodilation in the human forearm. Am J Hypertens. 2000;13:1155–60. doi: 10.1016/s0895-7061(00)01201-2. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Ishige A, Aburada M, Tabira T. Chronic stress attenuates glucocorticoid negative feedback: involvement of the prefrontal cortex and hippocampus. Neuroscience. 2003;119:887–97. doi: 10.1016/s0306-4522(03)00105-2. [DOI] [PubMed] [Google Scholar]
- Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60:1500–10. doi: 10.1016/j.metabol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- Plat L, Byrne MM, Sturis J, Polonsky KS, Mockel J, Fery F, Van Cauter E. Effects of morning cortisol elevation on insulin secretion and glucose regulation in humans. Am J Physiol. 1996;270:E36–42. doi: 10.1152/ajpendo.1996.270.1.E36. [DOI] [PubMed] [Google Scholar]
- Reynolds RM, Walker BR, Syddall HE, Andrew R, Wood PJ, Whorwood CB, Phillips DI. Altered control of cortisol secretion in adult men with low birth weight and cardiovascular risk factors. J Clin Endocrinol Metab. 2001;86:245–50. doi: 10.1210/jcem.86.1.7145. [DOI] [PubMed] [Google Scholar]
- Rizza RA, Mandarino LJ, Gerich JE. Cortisol-induced insulin resistance in man: impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor detect of insulin action. J Clin Endocrinol Metab. 1982;54:131–8. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- Schlesser HN, Simon L, Hofmann MC, Murphy KM, Murphy T, Hess RA, Cooke PS. Effects of ETV5 (ets variant gene 5) on testis and body growth, time course of spermatogonial stem cell loss, and fertility in mice. Biol Reprod. 2008;78:483–9. doi: 10.1095/biolreprod.107.062935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid PM, Heid I, Buechler C, Steege A, Resch M, Birner C, Endemann DH, Riegger GA, Luchner A. Expression of fourteen novel obesity-related genes in Zucker diabetic fatty rats. Cardiovasc Diabetol. 2012;11:48. doi: 10.1186/1475-2840-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiga F, Waite EJ, Liu Y, Kershaw YM, Aguilera G, Lightman SL. ACTH-dependent ultradian rhythm of corticosterone secretion. Endocrinology. 2011;152:1448–57. doi: 10.1210/en.2010-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorleifsson G, Walters GB, Gudbjartsson DF, Steinthorsdottir V, Sulem P, Helgadottir A, Styrkarsdottir U, Gretarsdottir S, Thorlacius S, Jonsdottir I, Jonsdottir T, Olafsdottir EJ, Olafsdottir GH, Jonsson T, Jonsson F, Borch-Johnsen K, Hansen T, Andersen G, Jorgensen T, Lauritzen T, Aben KK, Verbeek AL, Roeleveld N, Kampman E, Yanek LR, Becker LC, Tryggvadottir L, Rafnar T, Becker DM, Gulcher J, Kiemeney LA, Pedersen O, Kong A, Thorsteinsdottir U, Stefansson K. Genome-wide association yields new sequence variants at seven loci that associate with measures of obesity. Nat Genet. 2009;41:18–24. doi: 10.1038/ng.274. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Arnhold MM, Engeland WC. Adrenal splanchnic innervation contributes to the diurnal rhythm of plasma corticosterone in rats by modulating adrenal sensitivity to ACTH. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1128–35. doi: 10.1152/ajpregu.00042.2003. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Christiansen AM, Ostrander MM, Jones AA, Jones KR, Choi DC, Krause EG, Evanson NK, Furay AR, Davis JF, Solomon MB, de Kloet AD, Tamashiro KL, Sakai RR, Seeley RJ, Woods SC, Herman JP. Pleasurable behaviors reduce stress via brain reward pathways. Proc Natl Acad Sci U S A. 2010;107:20529–34. doi: 10.1073/pnas.1007740107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Engeland WC. Adrenal splanchnic innervation modulates adrenal cortical responses to dehydration stress in rats. Neuroendocrinology. 2002;76:79–92. doi: 10.1159/000064426. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Figueiredo HF, Ostrander MM, Choi DC, Engeland WC, Herman JP. Chronic stress induces adrenal hyperplasia and hypertrophy in a subregion-specific manner. Am J Physiol Endocrinol Metab. 2006;291:E965–73. doi: 10.1152/ajpendo.00070.2006. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Ryan KK. Neuroendocrine circuits governing energy balance and stress regulation: functional overlap and therapeutic implications. Cell Metab. 2014;19:910–25. doi: 10.1016/j.cmet.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O’Rahilly S, Purmann C, Rees MG, Ridderstrale M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, Waeber G, Wallace C, Watanabe RM, Waterworth DM, Watkins N, Witteman JC, Zeggini E, Zhai G, Zillikens MC, Altshuler D, Caulfield MJ, Chanock SJ, Farooqi IS, Ferrucci L, Guralnik JM, Hattersley AT, Hu FB, Jarvelin MR, Laakso M, Mooser V, Ong KK, Ouwehand WH, Salomaa V, Samani NJ, Spector TD, Tuomi T, Tuomilehto J, Uda M, Uitterlinden AG, Wareham NJ, Deloukas P, Frayling TM, Groop LC, Hayes RB, Hunter DJ, Mohlke KL, Peltonen L, Schlessinger D, Strachan DP, Wichmann HE, McCarthy MI, Boehnke M, Barroso I, Abecasis GR, Hirschhorn JN Consortium Wellcome Trust Case Control; and ANthropometric Traits Consortium Genetic Investigation of. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]