Abstract
Objectives
Salivary gland adenoid cystic carcinoma (ACC) is rare, aggressive, and challenging to treat. Many ACCs have a t(6;9) chromosomal translocation resulting in a MYB-NFIB fusion gene, but the clinical significance is unclear. The purposes of this study were to describe the clinicopathologic factors impacting survival and to determine the prevalence and clinical significance of MYB-NFIB fusion.
Study Design
Case series.
Methods
Medical records of patients treated for ACC of the head and neck from 1974 to 2011 were reviewed and clinicopathologic data recorded. FISH was used to detect MYB rearrangement in archival tumor tissue as a marker of MYB-NFIB fusion.
Results
158 patients were included with median follow-up 75.1 months. Median overall survival was 171.5 months (95%CI=131.9–191.6) and median disease-free survival was 112.0 months (95%CI=88.7–180.4). Advanced stage was associated with decreased overall survival (adjusted ptrend<0.001), and positive margins were associated with decreased disease-free survival (adjusted HR=8.80, 95%CI=1.25–62.12, p=0.029). 91 tumors were evaluable using FISH, and 59 (65%) had evidence of a MYB-NFIB fusion. MYB-NFIB-positive tumors were more likely than MYB-NFIB-negative tumors to originate in minor salivary glands (adjusted PR=1.51, 95%CI=1.07–2.12, p=0.019). MYB-NFIB tumor status was not significantly associated with disease-free or overall survival (HR=1.53, 95% CI=0.77–3.02, p=0.22 and HR=0.91, 95% CI=0.46–1.83, p=0.80, respectively, for MYB-NFIB-positive compared with MYB-NFIB negative tumors).
Conclusion
Stage and margin status were important prognostic factors for ACC. Tumors with evidence of MYB-NFIB fusion were more likely to originate in minor salivary glands, but MYB-NFIB tumor status was not significantly associated with prognosis.
Keywords: Adenoid cystic carcinoma, salivary gland neoplasms, MYB, MYB-NFIB fusion gene, minor salivary glands, survival, disease-free survival
Introduction
Adenoid cystic carcinoma (ACC) of the salivary glands is a rare malignancy comprising 10% of salivary gland neoplasms.1 ACC is slow-growing but aggressive, with high rates of late recurrence and distant metastasis.2–4 Treatment for ACC is extremely challenging, with current therapy limited to surgery and/or radiation, and no reliable chemotherapeutic options available for long-term disease control.1,4 Previously identified determinants of outcomes for ACC include stage, cervical lymph node metastases, margin status, perineural invasion, minor salivary gland site of origin, and histological pattern.3,5–9
MYB is a transcription factor with important roles in the regulation of cell proliferation, survival and differentiation.10 Overactivation of the MYB oncogene has recently been described as a hallmark of ACC, noted in over 80% of ACCs but not in other salivary gland neoplasms.11–13 MYB over-activation is often, but not always, the result of a chromosomal translocation, t(6;9)(q22–23;p23–24), resulting in fusion of the MYB oncogene with the transcription factor gene NFIB. This translocation is found in 29–86% of ACCs.11,13–17 The MYB-NFIB fusion protein may represent an important new therapeutic target for ACC; however, the clinical and prognostic significance of the MYB-NFIB fusion gene is unclear.
The purposes of this study were to describe our institution’s experience with ACC and determine the clinicopathologic factors that impact survival, and to assess the prevalence and clinical significance of MYB-NFIB fusion in ACCs treated at our institution.
Materials and methods
This study was approved by the Johns Hopkins School of Medicine Institutional Review Board. Patients treated for ACC of the head and neck at Johns Hopkins Medical Institutions from 1974 to 2011 were included. The medical records for these patients were retrospectively reviewed and demographic and disease-related data were recorded.
Fluorescence in situ hybridization
Fluorescence in situ hybridization (FISH) was performed on formalin-fixed paraffin-embedded (FFPE) sections in a tissue microarray (TMA) using a commercially available MYB dual-color break apart probe (ZTV-Z-2143–200, ZytoVision, Germany). The probe is designed to detect translocations involving the chromosomal region 6q23.3 harboring the MYB gene, and has two components: a probe labeled with a green fluorochrome that hybridizes proximal to and covers the 5’ end of the MYB gene, and a probe labeled with an orange fluorochrome that hybridizes ~180kb distal to the 3’ end of the MYB gene. Prior to hybridization, the TMA slide was deparaffinized using a VP 2000 processor (Abbott Molecular, Des Plains, IL). The slides and the MYB probe were co-denatured at 80 °C for 7 minutes and allowed to anneal over night at 37 °C in a humidified atmosphere. At the end of the incubation the slides were washed in 2 X SSC/0.3% NP-40 for 2 min at 72 °C and for 2 min at room temperature in 2 X SSC. The slides were counterstained with DAPI, and a cover slip was applied using Vectashield mounting medium (H-1000, Vector Laboratories, Inc.). A fluorescence microscope was used to evaluate the probe pattern. Interphase nuclei with two fusion signals of one orange and one green fluorochrome lack rearrangement at band 6q23.3 and are scored as normal (MYB-NFIB negative). A signal pattern consisting of one orange/green fusion signal and one orange and one green signal at distance from each other indicates one normal 6q23.3 locus and one locus affected by a translocation. This pattern was considered a marker of MYB-NFIB translocation (MYB-NFIB positive).
In several cases, signal patterns different from the normal (two fusion signals) and the typical rearranged (one fusion, one green, one orange signal) patterns were observed (e.g., one fusion and one green signal, one fusion and one red signal, or amplification of the green signal). These cases were re-evaluated using an in-house designed MYB-NFIB fusion probe. The probe identifies the fusion gene on the derivative chromosome 6 that results from a translocation t(6;9)(q22–23;p23–24). The probe covering the MYB gene consists of two BAC clones: RP11–104D9 and RP11–614H6. The BAC DNA is labeled by nick translation with Green-dUTP (Abbott Molecular). The probe is approximately 360 kb long, covers the MYB gene, and extends beyond the 5’ end of the gene. The NFIB probe consists also of two BAC clones: RP11–589C16 and RP11–413D24. The BAC DNA is labeled by nick translation with Orange-dUTP (Abbott Molecular). The probe is approximately 370 kb long and covers the 3’ end of the NFIB gene. The signal pattern in a normal cell consists of two green signals (chromosomes 6) and two orange signals (chromosomes 9). In the case of a rearrangement leading to a MYB-NFIB fusion the predicted signal pattern is: one green signal (chromosome 6), one orange signal (chromosome 9) and one green/orange fusion signal (derivative chromosome 6) (MYB-NFIB positive). Of the 17 cases that could not conclusively be interpreted with the MYB break apart probe and were re-evaluated with the MYB-NFIB fusion probe, only two did not show the gene fusion. These two cases were considered indeterminate.
Statistical analysis
Descriptive variables were summarized with frequencies and proportions for categorical variables, and medians with 95% confidence intervals (95%CI) or ranges for continuous variables. The association between clinical variables of interest and MYB-NFIB status was evaluated using a generalized linear model with extension to the binomial family18,19 in both univariate and bivariate models, and reported as unadjusted and adjusted prevalence ratios (PR and aPR). Survival rates were estimated using the Kaplan-Meier method20. Overall survival (OS) was defined as time from diagnosis to time of death from any cause. Disease-free survival (DFS) was defined as time from diagnosis to time of local, regional or distant recurrence. Patients with distant metastases at the time of diagnosis, or who died of primary disease without recurrence, were considered to not have received definitive treatment and were excluded from the DFS analysis. Follow-up time was calculated from diagnosis to event of interest or the last known-follow up. Survival curves were compared using the log-rank test for equality of survival functions. Unadjusted and adjusted hazard ratios (HR and aHR) were estimated using the Cox regression model. This retrospective study had 80% power to detect a 2.9-fold or greater difference in survival by MYB-NFIB tumor status. Data analysis was performed using STATA 11.2 (College Station, TX, 2012). A p-value ≤ 0.05 was considered statistically significant.
Results
Clinical and pathologic features
The study population was comprised of 158 patients with ACC. Median age at the time of diagnosis was 51.5 years (range, 17–94 years). Clinical and pathologic characteristics are displayed in Table 1. The most common tumor site was the parotid gland (N=38, 25%). A greater proportion of tumors arose from minor (N=85, 57%) than major salivary glands (N=65, 43%). Histologically, most tumors were observed to have a cribriform pattern (N=78, 70%) and perineural invasion (N=68, 83%). Tumors with positive margins (N=73, 66%) were significantly more likely to have perineural invasion than those with negative or close margins (78% compared with 22%, p=0.026), and were also more likely to have local recurrence (29% compared with 8%, p=0.017).
Table 1.
Clinicopathologic characteristics of patients with adenoid cystic carcinoma at Johns Hopkins Medical Institutions from 1974 to 2011.
| Patient Characteristics (N=158) | N (%) |
|---|---|
| Age | |
| <55 years | 79 (53) |
| ≥55 years | 71 (47) |
| Gender | |
| Male | 62 (39) |
| Female | 96 (61) |
| Smoking history | |
| No | 62 (52) |
| Yes | 58 (48) |
| Salivary gland type | |
| Major | 65 (43) |
| Minor | 85 (57) |
| Primary site | |
| Parotid | 38 (25) |
| Submandibular gland | 23 (15) |
| Oral cavity | 35 (23) |
| Nose & paranasal sinuses | 31 (21) |
| Other | 23 (15) |
| Predominant pattern | |
| Tubular | 16 (14) |
| Cribriform | 78 (70) |
| Solid | 17 (15) |
| Perineural invasion | |
| No | 14 (17) |
| Yes | 68 (83) |
| Overall Stage | |
| I | 21 (19) |
| II | 21 (19) |
| III | 18 (17) |
| IV | 48 (44) |
| Treatment | |
| Surgery and radiation | 107 (84) |
| Surgery | 15 (12) |
| RadiationA | 6 (5) |
| Neck dissection | |
| No | 57 (50) |
| Yes | 56 (50) |
| Nodal metastases found on neck dissection | |
| No | 41 (76) |
| Yes | 13 (24) |
| Margin status | |
| Negative | 21 (19) |
| Close | 16 (15) |
| Positive | 73 (66) |
| Recurrence | |
| Local recurrence | |
| No | 97 (69) |
| Yes | 44 (31) |
| Regional recurrence | |
| No | 132 (95) |
| Yes | 7 (5) |
| New distant metastases | |
| No | 99 (71) |
| Yes | 41 (29) |
| Any recurrence after initial treatmentB | |
| No | 70 (52) |
| Yes | 64 (48) |
| Last known vital status | |
| Alive | 78 (53) |
| Expired | 70 (47) |
Two patients receiving radiation also received chemotherapy and were excluded from further analysis.
Any recurrence includes local or regional recurrence and new distant metastases.
Survival
Median follow-up time was 75.1 months (range, 0.5–360.0 months). Five-year OS was 80% (95%CI=71–86%) and median OS was 171.5 months (95%CI=131.9–190.6). At the time of last follow-up, 78 (53%) of patients were alive. In univariate analysis, factors associated with decreased OS included older age (HR=1.04, 95%CI=1.02–1.06, p<0.001 for each year of age), nodal metastases at the time of neck dissection (HR=3.09, 95%CI=1.20–7.98, p=0.020), and more advanced overall stage (ptrend<0.001) (Table 2). OS was also significantly decreased among patients treated with single modality therapy (surgery or radiation) compared to those treated with surgery and adjuvant radiation (HR=3.01, 95%CI=1.35–6.72, p=0.007). In multivariate analysis, more advanced overall stage (adjusted ptrend<0.001) and older age at diagnosis (aHR=1.03, 95%CI=1.01–1.05, p=0.001) were independently associated with OS (Table 2).
Table 2.
Clinicopathologic characteristics associated with overall and disease-free survival.
| Overall Survival | Disease-free Survival | |||
|---|---|---|---|---|
| Groups compared | Univariate HR; 95% CI (p-value) |
MultivariateA aHR; 95% CI (p-value) |
Univariate HR; 95% CI (p-value) |
MultivariateA aHR; 95% CI (p-value) |
| Age (years, continuous) | ||||
| 1.04; 1.02–1.06 (<0.001) | 1.03; 1.01–1.05 (0.001) | 0.99; 0.98–1.01 (0.52) | 1.01; 0.99–1.04 (0.39) | |
| Gender | ||||
| Male | REF | REF | ||
| Female | 0.86; 0.53–1.41 (0.55) | 0.84; 0.50–1.40 (0.50) | ||
| Smoking history | ||||
| No | REF | REF | ||
| Yes | 0.73; 0.41–1.28; 0.27 | 0.92; 0.52–1.63 (0.78) | ||
| Salivary gland type | ||||
| Major | REF | REF | ||
| Minor | 0.81; 0.50–1.33 (0.41) | 1.04; 0.63–1.70 (0.89) | ||
| Predominant pattern | ||||
| Tubular | REF | REF | ||
| Cribriform | 0.60; 0.25–1.47 (0.27) | 0.88; 0.33–2.30 (0.79) | ||
| Solid | 1.01; 0.36–2.86 (0.98) | 1.46; 0.48–4.50 (0.51) | ||
| Perineural invasion | ||||
| No | REF | REF | ||
| Yes | 1.91; 0.67–5.42 (0.23) | 1.94; 0.58–6.49 (0.28) | ||
| Overall Stage | ||||
| I | REFB | REFB | REFC | REFC |
| II | 2.45; 0.83–7.24 (0.11) | 1.92; 0.66–5.64 (0.23) | 2.17; 0.73–6.43 (0.16) | 3.40; 0.94–12.32 (0.062) |
| III | 3.82; 1.35–10.85 (0.012) | 3.94; 1.28–12.07 (0.017) | 2.58; 0.76–8.80 (0.13) | 3.20; 0.96–10.69 (0.059) |
| IV | 5.07; 2.05–12.57 (<0.001) | 5.15; 2.12–12.54 (<0.001) | 3.20; 1.24–8.30 (0.017) | 2.46; 0.88–6.85 (0.085) |
| Treatment | ||||
| Surgery and radiation | REF | REF | REF | REF |
| Surgery or radiation | 3.01; 1.35–6.72 (0.007) | 2.21; 0.92–5.32 (0.076) | 3.13; 1.47–6.63 (0.003) | 1.51; 0.41–5.59 (0.54) |
| Nodal metastases on neck dissectionD | ||||
| No | REF | REF | ||
| Yes | 3.09; 1.20–7.98 (0.020) | 1.28; 0.35–4.63 (0.70) | ||
| Margin status | ||||
| Negative | REF | REFE | REFE | |
| Close | 1.16; 0.44–3.04 (0.76) | 5.98; 1.30–29.86 (0.029) | 7.04; 0.89–55.94 (0.065) | |
| Positive | 1.58; 0.75–3.30 (0.23) | 6.93; 1.64–29.24 (0.008) | 8.80; 1.25–62.12 (0.029) | |
Adjusted models include all variables with adjusted hazard ratios reported
ptrend<0.001 for univariate analysis and multivariate analysis.
ptrend=0.014 for univariate analysis and ptrend=0.23 for multivariate analysis.
Nodal metastases on neck dissection was not included in multivariate analysis because of the small number of patients undergoing neck dissection (N=56).
ptrend=0.0034 for univariate analysis and ptrend=0.039 for multivariate analysis.
Abbreviations: HR, hazard ratio; aHR, adjusted hazard ratio
Five-year DFS was 65% (95%CI=55–73%) and median DFS was 112.0 months (95%CI=88.7–180.4). More advanced overall stage was associated with decreased DFS (ptrend=0.014), as were receipt of single modality therapy compared with surgery and adjuvant radiation (HR=3.31, 95%CI=1.47–6.63, p=0.003) and close or positive surgical margins (HR=5.98, 95%CI=1.30–29.86, p=0.029 for close and HR=6.93, 95%CI=1.64–29.24, p=0.008 for positive compared with negative margins). In multivariate analysis, margin status was the only independent predictor of DFS; positive margins were independently associated with an eight-fold decrease in DFS (aHR=8.80, 95%CI=1.25–62.12, p=0.029) (Table 2).
MYB-NFIB rearrangement
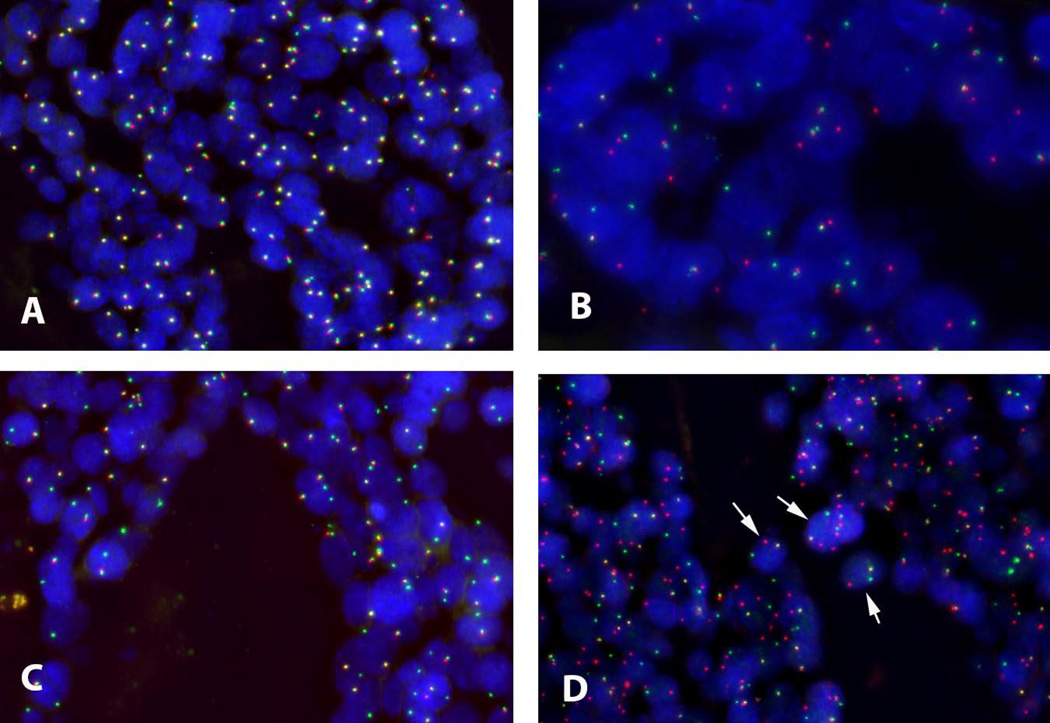
FISH was used to determine MYB rearrangement as a marker of MYB-NFIB fusion tumor status for 93 ACC specimens, of which 91 tumors were considered evaluable for analysis. Of these, 59 (65%) tumors were MYB-NFIB positive and 32 (35%) were MYB-NFIB negative (Figure 1).
Figure 1. Representative FISH assay results.
A: MYB break apart assay using commercial MYB dual-color break apart probe. Normal pattern (MYB-rearrangement negative) with two fusion signals in each cell (5’ MYB green fluorophore, 3’ MYB orange fluorophore). B: MYB break-apart assay abnormal pattern (MYB-rearrangement positive) with one fusion signal, one 3’ MYB orange signal, and one 5’ MYB green signal. C: MYB break-apart assay atypical pattern with one fusion signal and one green signal but no orange, requiring clarification using the MYB-NFIB fusion probe shown in D. D: MYB-NFIB fusion assay using laboratory designed probe. The pattern of one fusion (5’ MYB green fluorophore /3’ NFIB orange fluorophore), one orange (3’ NFIB) and one green (5’ MYB) signal confirms MYB-NFIB positive tumor status for the tumor shown in C. Arrows indicate cells representative of MYB-NFIB fusion pattern.
There were no significant differences in the clinical and pathologic characteristics of cases with MYB-NFIB tumor status available compared with cases that did not have MYB-NFIB tumor status available (Supplementary Table 1).
Clinicopathologic factors associated with MYB-NFIB positive tumor status were considered (Table 3). Tumors arising from minor salivary glands were more likely to be MYB-NFIB positive (PR=1.52, 95%CI=1.06–2.18, p=0.023), and female gender was significantly associated with MYB-NFIB positive tumor status (PR=1.44, 95%CI=1.01–2.05, p=0.041). Tumors that recurred or had nodal metastases at the time of neck dissection were more likely to be MYB-NFIB positive, although these differences were not statistically significant (p=0.15 and p=0.14). Age, gender, smoking history, stage and perineural invasion were not associated with MYB-NFIB status (Table 3). In bivariate analysis, both minor salivary gland origin (aPR=1.51, 95%CI=1.07–2.12, p=0.019) and female gender (aPR=1.49, 95%CI=1.05–2.11, p=0.026) remained significantly associated with MYB-NFIB positive tumor status.
Table 3.
Clinicopathologic characteristics compared with MYB-NFIB tumor status.
| Characteristics | MYB-NFIB(−) N (%) |
MYB-NFIB(+) N (%) |
Prevalence ratio MYB-NFIB(+) vs. MYB-NFIB(−) PR (95% CI) |
p-value |
|---|---|---|---|---|
| Total (N=91) | ||||
| 59 (63) | 32 (34) | |||
| Age | ||||
| <55 years | 14 (34) | 27 (66) | REF | |
| ≥55 years | 16 (36) | 29 (64) | 0.98 (0.72–1.33) | 0.89 |
| Gender | ||||
| Male | 18 (48) | 19 (52) | REF | |
| Female | 14 (26) | 40 (74) | 1.44 (1.01–2.05) | 0.041 |
| Smoking history | ||||
| No | 13 (38) | 21 (62) | REF | |
| Yes | 12 (31) | 27 (69) | 1.12 (0.80–1.57) | 0.51 |
| Salivary gland type | ||||
| Major | 18 (50) | 18 (50) | REF | |
| Minor | 12 (24) | 38 (76) | 1.52 (1.06–2.18) | 0.023 |
| Predominant pattern | ||||
| Tubular | 5 (45) | 6 (54) | REF | |
| Cribriform | 18 (30) | 42 (70) | 1.28 (0.73–2.26) | 0.39 |
| Solid | 5 (45) | 6 (54) | 1.00 (0.46–2.15) | 1.00 |
| Margin status | ||||
| Negative | 4 (36) | 7 (64) | REF | |
| Close | 4 (40) | 6 (60) | 0.94 (0.48–1.86) | 0.86 |
| Positive | 16 (38) | 26 (62) | 0.97 (0.58–1.62) | 0.92 |
| Perineural invasion | ||||
| No | 3 (33) | 6 (67) | REF | |
| Yes | 17 (40) | 25 (60) | 0.89 (0.53–1.52) | 0.68 |
| Overall Stage | ||||
| I | 5 (63) | 3 (38) | REF | |
| II | 5 (31) | 11 (69) | 1.82 (0.70–4.79) | 0.22 |
| III | 4 (29) | 10 (71) | 1.90 (0.73–4.98) | 0.19 |
| IV | 9 (31) | 20 (69) | 1.84 (0.72–4.68) | 0.20 |
| Nodal metastases on neck dissection | ||||
| No | 9 (45) | 11 (55) | REF | |
| Yes | 2 (20) | 8 (80) | 1.45 (0.88–2.41) | 0.14 |
| Any recurrenceA | ||||
| No | 16 (42) | 22 (58) | REF | |
| Yes | 10 (26) | 28 (74) | 1.27 (0.91–1.77) | 0.15 |
Abbreviations: PR, prevalence ratio
Any recurrence includes local or regional recurrence and new distant metastases.
MYB-NFIB and survival
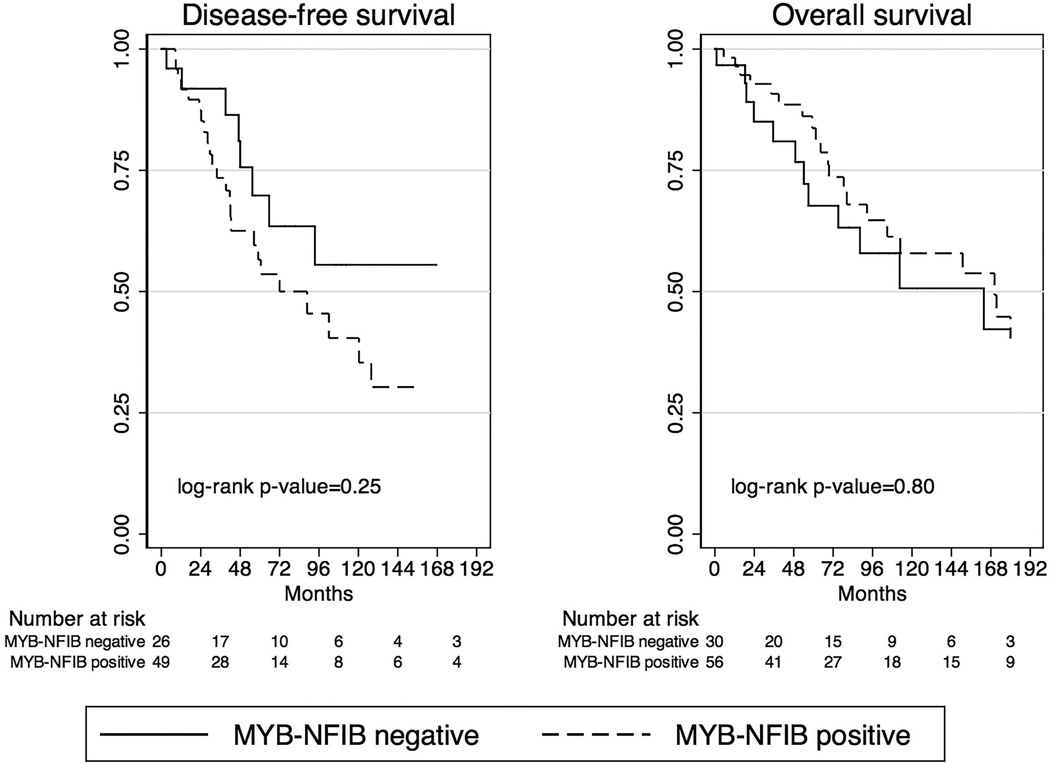
Median follow-up time for patients evaluated for MYB-NFIB tumor status was 62.6 months (range, 1.0–357.4 months). Five-year OS was 84% (95%CI=70–92%) for MYB-NFIB positive compared with 68% (95%CI=45–82%) for MYB-NFIB negative tumors, and median OS was 170.3 months (95%CI=92.6–237.3) for MYB-NFIB positive compared with 163.7 months (95%CI=57.0–270.5) for MYB-NFIB negative tumors. There was no difference in OS by MYB-NFIB tumor status (HR=0.91, 95% CI=0.46–1.83, p=0.80).
In contrast, MYB-NFIB positive tumors exhibited a trend toward decreased DFS. Five-year DFS was 54% (95%CI=37–68%) for MYB-NFIB positive compared with 70% (95%CI=44–85%) for MYB-NFIB negative tumors, and median DFS was 88.7 months (95%CI=42.0–127.8) for MYB-NFIB positive compared with 187.2 months (95%CI=55.4–318.9) for MYB-NFIB negative tumors (Figure 2). The association of MYB-NFIB tumor status with decreased DFS was not, however, statistically significant (HR=1.53, 95% CI 0.77–3.02, p=0.22), and was further attenuated after adjustment for minor versus major salivary gland site of origin (adjusted HR=1.18, 95%CI 0.51–2.71, p=0.70).
Figure 2. Disease-free and overall survival by MYB-NFIB tumor status.
Discussion
ACC of the head and neck is uncommon and difficult to treat due to its high propensity for late recurrence and distant metastasis, sometimes many years after initial diagnosis and treatment.1,3,4 In this study, we have elucidated important prognostic factors for both overall and disease-free survival, and explored clinicopathologic correlates of the MYB-NFIB fusion gene.
Clinical outcomes
Similar to previous studies,3,5 we found that ACCs were frequently of minor salivary gland origin (57%) and had a high rate of perineural invasion (83%). Recurrences were largely local (31%) or distant (29%), with regional recurrence in only 5% of cases, consistent with the known patterns of ACC recurrence.3 Our five-year rates of OS (80%) and DFS (65%) are also consistent with previous reports.3,5,21
Advanced overall stage was the strongest independent predictor of poor OS in our study, and was also significantly associated with decreased DFS. Stage was therefore a more important prognostic factor than histologic pattern, perineural invasion or site of origin. Indeed, overall stage is known to correlate with outcomes for ACC.3,6–8,21
Margin status was the only factor independently associated with DFS, with a significant trend toward decreasing DFS with closer proximity of disease to the surgical margin. Positive margins were also associated with greater risk of local failure, consistent with previous reports.22,23 These associations are not surprising, especially given that in this and other studies,3 tumors with positive margins were also significantly more likely to demonstrate perineural invasion. Tumors that are challenging to surgically extirpate appear to have a more aggressive biological phenotype and higher likelihood of recurrence. The current treatment paradigm for ACC with positive margins is surgical resection followed by adjuvant radiation therapy,4,24,25 and our results support the indication for adjuvant therapy in these cases.
MYB-NFIB tumor status
The MYB oncogene is overexpressed in 89–97% ACCs of the head and neck.11,13 A subset of these (29–86%) are characterized by an activating MYB-NFIB fusion resulting from a variable balanced t(6;9)(q22–23;p23–24) translocation event that is not found in other salivary gland cancers.11,13,15–17 Overexpression of MYB RNA and the Myb transcription factor protein are associated with the MYB-NFIB fusion, but also occur independent of the translocation, suggesting additional mechanisms of MYB dysregulation.11,13,15,16
The combination of an initial MYB break-apart probe with a confirmatory MYB-NFIB fusion probe that was used for the FISH assay in this study has not previously been used in ACC of the head and neck. The MYB break-apart probe alone detects a break in the 6q23.2–q23.3 locus containing the MYB gene that most likely results in a MYB-NFIB fusion gene, as has been well described,13,14,17 and our use of a confirmatory MYB-NFIB fusion probe ensured that indeterminate cases were properly categorized. The prevalence of MYB-NFIB positive tumors in this study was 65%, which is similar to previous FISH studies in formalin-fixed tissue.11,15,17
The clinical significance, if any, of the MYB-NFIB fusion gene has not been well established. We demonstrated a higher prevalence of the MYB-NFIB fusion in tumors from minor salivary glands and among females, and a trend toward higher likelihood of recurrence and decreased DFS with MYB-NFIB positive tumor status, although there was no association with OS. The significance of these results is unclear. Importantly, our sample size was underpowered to detect with certainty the observed difference in DFS by MYB-NFIB status (HR=1.53, 95% CI 0.77–3.02, p=0.22), which was further attenuated after adjusting for the fact that MYB-NFIB positive tumors were predominantly from minor salivary glands. However, several previous studies have also reported borderline associations of the MYB-NFIB fusion in ACC with poor prognostic parameters, including perineural invasion15, local recurrence15, and decreased overall survival.16 Taken together with our study, this suggests a possible trend towards more aggressive disease with MYB-NFIB positive tumor status. Larger studies are necessary to determine with certainty whether the MYB-NFIB fusion is of prognostic significance, or is simply a biomarker of ACC carcinogenesis and potential therapeutic target.
It may be the case that MYB overexpression, independent of the mechanism by which it occurs, is a key event in ACC pathogenesis. Indeed, current evidence indicates that MYB is dysregulated by diverse and complex mechanisms in ACC,13,17 and overexpression of the MYB transcript 5’ end was found in one study to be independently associated with decreased overall survival.16 In this scenario, the weak associations of MYB-NFIB tumor status with prognosis found in ours and other studies may simply reflect a greater importance of MYB overexpression than the MYB-NFIB fusion per se in influencing outcomes. Sequencing studies have suggested a possible separate role for NFIB as well.17,26 Further research is required to describe the role of MYB or NFIB dysregulation individually, and in the context of the MYB-NFIB fusion.
Strengths and limitations
The strengths of this study are the report of clinicopathologic variables that impact outcomes in ACC, contributing to our understanding of prognostic determinants for this rare disease. In addition, the MYB break-apart and MYB-NFIB fusion FISH assay is a new tool that may be used in studying the role of the MYB-NFIB fusion and MYB alterations in ACC. However, this assay is limited in that it is possible that a subset of tumors with a MYB translocation pattern on FISH actually harbored an atypical translocation. In addition, detection of the MYB-NFIB translocation by RT-PCR has been shown to be lower in formalin-fixed compared with frozen tissue (44–57% compared with 86%)11,13,15,17 so that the use of formalin-fixed tissue in the present study may lower the sensitivity of our assay for MYB-NFIB positive tumor status and limit the interpretation of clinicopathologic correlates. Other limitations of this study include the retrospective nature of the medical chart review and the relatively small sample size with FISH assay results available, resulting in inadequate power to detect significant survival differences by MYB-NFIB tumor status.
Conclusion
In summary, stage and margin status are important prognostic indicators for ACC. The MYB-NFIB fusion may be more prevalent in tumors with aggressive features, but was not significantly associated with prognosis in our study. Further research is indicated to determine both the clinical significance and therapeutic targeting potential of the MYB-NFIB fusion gene.
Supplementary Material
Acknowledgments
Location of research: This work was performed in the Johns Hopkins University School of Medicine Department of Otolaryngology-Head and Neck Surgery (EMR, MT, SL, CF, PKH) and Johns Hopkins University School of Medicine Department of Pathology (RY, JAB).
Financial support: Research reported in this manuscript was supported by the National Institute of Dental and Craniofacial Research (NIDCR) and NIH grant R01- DE023227 (PKH), The University of Texas M. D. Anderson Cancer Center Career Development Award (JAB) and NIDCR grant 2T32DC000027-26 (EMR). The content is solely the responsibility of the authors and does not necessarily represent the official view of the National Institutes of Health.
Footnotes
Conflict of interest: The authors report no conflict of interest.
The research in this manuscript was presented at the Combined Sections Meeting of the Triological Society at Coronado Island, CA, USA, January 22–24, 2015.
Supplementary Items
Supplementary Table 1: Clinical and pathologic characteristics of cases evaluated for MYB-NFIB status compared with those not evaluated.
References
- 1.Bradley PJ. Adenoid cystic carcinoma of the head and neck: a review. Current opinion in otolaryngology & head and neck surgery. 2004;12:127–132. doi: 10.1097/00020840-200404000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Jones AS, Hamilton JW, Rowley H, Husband D, Helliwell TR. Adenoid cystic carcinoma of the head and neck. Clinical otolaryngology and allied sciences. 1997;22:434–443. doi: 10.1046/j.1365-2273.1997.00041.x. [DOI] [PubMed] [Google Scholar]
- 3.Khan AJ, DiGiovanna MP, Ross DA, et al. Adenoid cystic carcinoma: a retrospective clinical review. Int J Cancer. 2001;96:149–158. doi: 10.1002/ijc.1013. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Shao C, Tan ML, Mu D, Ferris RL, Ha PK. Molecular biology of adenoid cystic carcinoma. Head & neck. 2012;34:1665–1677. doi: 10.1002/hed.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marcinow A, Ozer E, Teknos T, et al. Clinicopathologic predictors of recurrence and overall survival in adenoid cystic carcinoma of the head and neck: A single institutional experience at a tertiary care center. Head & neck. 2013 doi: 10.1002/hed.23523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oplatek A, Ozer E, Agrawal A, Bapna S, Schuller DE. Patterns of recurrence and survival of head and neck adenoid cystic carcinoma after definitive resection. Laryngoscope. 2010;120:65–70. doi: 10.1002/lary.20684. [DOI] [PubMed] [Google Scholar]
- 7.Huang M, Ma D, Sun K, Yu G, Guo C, Gao F. Factors influencing survival rate in adenoid cystic carcinoma of the salivary glands. International journal of oral and maxillofacial surgery. 1997;26:435–439. doi: 10.1016/s0901-5027(97)80008-2. [DOI] [PubMed] [Google Scholar]
- 8.Norberg-Spaak L, Dardick I, Ledin T. Adenoid cystic carcinoma: use of cell proliferation, BCL-2 expression, histologic grade, and clinical stage as predictors of clinical outcome. Head & neck. 2000;22:489–497. doi: 10.1002/1097-0347(200008)22:5<489::aid-hed8>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 9.Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Archives of otolaryngology--head & neck surgery. 1999;125:149–152. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 10.Ramsay RG, Gonda TJ. MYB function in normal and cancer cells. Nat Rev Cancer. 2008;8:523–534. doi: 10.1038/nrc2439. [DOI] [PubMed] [Google Scholar]
- 11.Brill LB, 2nd, Kanner WA, Fehr A, et al. Analysis of MYB expression and MYB-NFIB gene fusions in adenoid cystic carcinoma and other salivary neoplasms. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2011;24:1169–1176. doi: 10.1038/modpathol.2011.86. [DOI] [PubMed] [Google Scholar]
- 12.Stephens PJ, Davies HR, Mitani Y, et al. Whole exome sequencing of adenoid cystic carcinoma. The Journal of clinical investigation. 2013;123:2965–2968. doi: 10.1172/JCI67201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Persson M, Andren Y, Moskaluk CA, et al. Clinically significant copy number alterations and complex rearrangements of MYB and NFIB in head and neck adenoid cystic carcinoma. Genes, chromosomes & cancer. 2012;51:805–817. doi: 10.1002/gcc.21965. [DOI] [PubMed] [Google Scholar]
- 14.Persson M, Andren Y, Mark J, Horlings HM, Persson F, Stenman G. Recurrent fusion of MYB and NFIB transcription factor genes in carcinomas of the breast and head and neck. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:18740–18744. doi: 10.1073/pnas.0909114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.West RB, Kong C, Clarke N, et al. MYB expression and translocation in adenoid cystic carcinomas and other salivary gland tumors with clinicopathologic correlation. The American journal of surgical pathology. 2011;35:92–99. doi: 10.1097/PAS.0b013e3182002777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitani Y, Li J, Rao PH, et al. Comprehensive analysis of the MYB-NFIB gene fusion in salivary adenoid cystic carcinoma: Incidence, variability, and clinicopathologic significance. Clin Cancer Res. 2010;16:4722–4731. doi: 10.1158/1078-0432.CCR-10-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho AS, Kannan K, Roy DM, et al. The mutational landscape of adenoid cystic carcinoma. Nature genetics. 2013;45:791–798. doi: 10.1038/ng.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zocchetti C, Consonni D, Bertazzi PA. Estimation of prevalence rate ratios from cross-sectional data. International journal of epidemiology. 1995;24:1064–1067. doi: 10.1093/ije/24.5.1064. [DOI] [PubMed] [Google Scholar]
- 19.McCullagh P, Nelder JA. Generalized linear models. London ; New York: Chapman and Hall; 1989. [Google Scholar]
- 20.Kaplan EPM. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958:457–481. [Google Scholar]
- 21.Spiro RH, Huvos AG. Stage means more than grade in adenoid cystic carcinoma. American journal of surgery. 1992;164:623–628. doi: 10.1016/s0002-9610(05)80721-4. [DOI] [PubMed] [Google Scholar]
- 22.Garden AS, Weber RS, Morrison WH, Ang KK, Peters LJ. The influence of positive margins and nerve invasion in adenoid cystic carcinoma of the head and neck treated with surgery and radiation. Int J Radiat Oncol Biol Phys. 1995;32:619–626. doi: 10.1016/0360-3016(95)00122-F. [DOI] [PubMed] [Google Scholar]
- 23.Prokopakis EP, Snyderman CH, Hanna EY, Carrau RL, Johnson JT, D'Amico F. Risk factors for local recurrence of adenoid cystic carcinoma: the role of postoperative radiation therapy. American journal of otolaryngology. 1999;20:281–286. doi: 10.1016/s0196-0709(99)90028-5. [DOI] [PubMed] [Google Scholar]
- 24.Chen AM, Granchi PJ, Garcia J, Bucci MK, Fu KK, Eisele DW. Local-regional recurrence after surgery without postoperative irradiation for carcinomas of the major salivary glands: implications for adjuvant therapy. Int J Radiat Oncol Biol Phys. 2007;67:982–987. doi: 10.1016/j.ijrobp.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 25.Dillon PM, Chakraborty S, Moskaluk CA, Joshi PJ, Thomas CY. Adenoid Cystic Carcinoma: A Review of Recent Advances, Molecular Targets and Clinical Trials. Head & neck. 2014 doi: 10.1002/hed.23925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitani Y, Rao PH, Futreal PA, et al. Novel chromosomal rearrangements and break points at the t(6;9) in salivary adenoid cystic carcinoma: association with MYB-NFIB chimeric fusion, MYB expression, and clinical outcome. Clin Cancer Res. 2011;17:7003–7014. doi: 10.1158/1078-0432.CCR-11-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.