Abstract
Background
Circulating epithelial cell (CEC) isolation has provided diagnostic and prognostic information for a variety of cancers, previously supporting their identity as circulating tumor cells in the literature. However, we report CEC findings in patients with benign, pre-malignant, and malignant pancreatic lesions using a size-selective filtration device.
Study Design
Peripheral blood samples were drawn from patients found to have pancreatic lesions on preoperative imaging at a surgical clinic. Blood was filtered using ScreenCell® devices, which were evaluated microscopically by a pancreatic cytopathologist. Pathological data and clinical outcomes of these patients were obtained from medical records over a one year follow-up period.
Results
Nine healthy volunteers formed the control group and were found to be negative for CECs. There were 179 patients with pancreatic lesions that formed the study cohort. CECs were morphologically similar in patients with a variety of pancreatic lesions. Specifically, CECs were identified in 51 of 105 pancreatic ductal adenocarcinomas (PDAC) (49%), 7 of 11 neuroendocrine tumors (64%), 13 of 21 intraductal papillary mucinous neoplasms (62%), and 6 of 13 patients with chronic pancreatitis. Rates of CEC identification were similar in patients with benign, premalignant, and malignant lesions (p=0.41). In addition, CECs findings in PDAC patients were not associated with poor prognosis.
Conclusions
While CECs were not identified in healthy volunteers, they were identified in patients with benign, premalignant, and malignant pancreatic lesions. The presence of CECs in patients presenting with pancreatic lesions is not diagnostic of malignancy, nor is it prognostic for patients with PDAC.
Pancreatic lesions are being identified with increasing frequency due to improvements in imaging and widespread use of computed tomography (1). Determining the malignant potential of these lesions can be challenging and currently requires further imaging, invasive tissue sampling, or surgical resection. Peripheral blood testing for circulating epithelial cells (CECs) has been studied in several solid organ malignancies to obtain diagnostic and prognostic information (2, 3). These cells in the peripheral blood are frequently referred to as circulating tumor cells (CTCs) due to their reported association with a known malignancy.
Circulating epithelial cells (CEC) have previously been described as cells from a primary lesion that have acquired the ability to enter the circulation (4). When identified in patients with a primary malignancy, they are thought to represent cells in transit with the potential to establish distant metastases. CECs have been identified in patients with breast, lung, and colorectal cancers at various clinical stages (2, 3, 5-8). Previous studies have reported an association between CEC presence and poor prognosis using a single blood test and with serial testing over treatment regimens (6, 7, 9). Identifying CECs in pancreatic ductal adenocarcinoma (PDAC) patients and animal models has been described previously with varying success (7, 10-13).
Detection of CECs in peripheral blood samples of cancer patients has been performed using several different cell isolation techniques (14, 15). Peripheral blood CEC isolation can be performed using cytometric or immunologic techniques based on immunocytochemical staining with monoclonal antibodies against epithelial proteins. Each technique varies in level of technical complexity and cost. One technique described by Desitter and colleagues uses ScreenCell® devices to isolate CECs based on their large size compared to other cells in the circulation. Once isolated, these cells can be evaluated by a cytopathologist.
This study evaluates the CEC findings in patients with pancreatic lesions using the ScreenCell® technique. The primary aim of this study is to determine if CECs can be used to diagnose pancreatic malignancy, specifically PDAC, in preoperative patients with a pancreatic lesion. The secondary aim of this study is to determine if the presence of CECs in PDAC patients is associated with worse prognosis based on histology or clinical outcomes.
Methods
Study Design
This is a prospective cohort study of patients presenting to an academic surgical clinic with a pancreatic lesion. These patients presented for operative evaluation for procedures performed between October 2011 and October 2013, and medical records were reviewed for follow-up period of one year. Nine healthy volunteers were also included in this study as a control group. Informed consent was obtained prior to blood draws in accordance with Massachusetts General Hospital IRB. Peripheral blood samples were obtained for CEC assessment using the ScreenCell® technique prior to operative intervention or chemotherapy and processed through the filtration devices within 3 hours (16). Clinical, pathologic, and radiologic data were obtained from the medical record including vital status.
Inclusion criteria for this prospective study was attendance to the surgical clinic and a diagnosis of a pancreatic lesion. All patients were seen by a pancreatic surgeon for potential operative intervention of resection, diagnostic laparotomy, or palliative bypass. Only adult patients, over the age of 18 years, with pathologic specimens reviewed at our institution were included in this study. Patients with a pathologic diagnosis of PDAC were identified for further subgroup analysis. All patient information was collected until the patient expired, was lost to follow-up, or until the end of the study period. Controls included healthy volunteers at least 18 years of age who had no known history of malignancy or pancreatic disease.
Obtaining Clinical and Pathologic Data
Patient demographic information including age, sex, and race was obtained from the electronic medical record. Radiologic, pathologic, and clinic notes were reviewed to obtain data on tumor characteristics (tumor type, size, histologic grade, tumor invasion, and lymph node status), disease progression, and treatment plans. Date of death was determined using the electronic medical record. Time to progression is reported for resection patients from time of resection to time to diagnosis of metastatic or recurrent primary tumor growth. Time after last recorded clinic visit was censored for survival analysis.
Testing for CECs
After being filtered through the ScreenCell® (Paris, France) filtration devices according to the manufacturer’s instructions, slides were prepared with Giemsa stain (Haem 3, Fisher® USA) or toluidine blue stain. CEC identification on the stained filter slides was performed by a pancreatic cytopathologist who was blinded to the final histologic diagnosis of the pancreatic lesion. Slides were interpreted as non-diagnostic/negative, suspicious, or positive (malignant-appearing) for CECs based on established cytomorphologic criteria for pancreatic carcinoma (17). Malignant-appearing CECs were epithelioid cells with markedly enlarged (8-20x filter pore size) irregular, hyperchromatic nuclei and scant, well-defined cytoplasm (Figure 1A) or smaller epithelioid cells (2-7 × filter pore size) with round to oval nuclei, occasional nuclear groove and ill-defined but visible cytoplasm in small (Figure 1B) or large clusters (Figure 1C). Cells suspicious for CECs were enlarged, clumped cells with molded nuclei but poorly defined or absent cytoplasm (Figure 1D).
Figure 1.
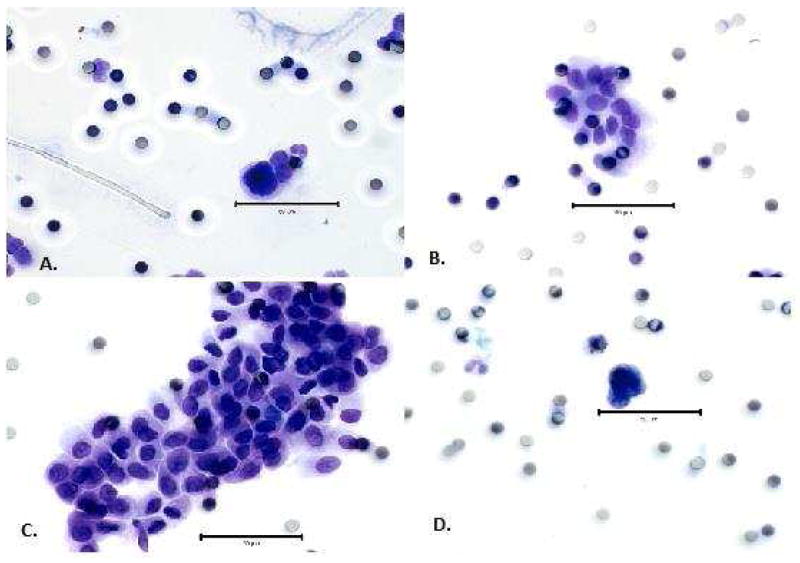
Circulating epithelial cell cytomorphology shows positive (A) single malignant-appearing epithelioid cells with large irregular nuclei; clusters of smaller malignant-appearing epithelioid cells in (B) small clusters; and (C) large clusters. (D) Cells with enlarged, clumped, molded nuclei without intact cytoplasm were suspicious for circulating epithelial cells. The bar represents 50 μm for size comparison.
Data Analysis
Data was assessed for normality and missing values. Univariate analysis was performed comparing CEC positive and non-diagnostic (negative or suspicious) patients. Fisher exact test and general trend test was used to compare categorical data. Continuous normal data was described with mean and standard deviation and compared using t-test. Survival and time to disease progression analysis was depicted using Kaplan Meier curves and compared using Wilcoxon log rank test. Time to disease progression describes time to recurrence at the surgical resection site or identification of metastatic disease from time of resection. Statistical analysis was performed using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). α <0.05 with two tail testing was used.
Results
Description of Cohort
Nine healthy volunteers and 179 patients with pancreatic lesions were evaluated for the presence of CECs. Patients included in the study had a variety of benign, pre-malignant, and malignant pancreatic lesions identified on histology, including PDAC (59%), intraductal papillary mucinous neoplasm (12%), chronic pancreatitis (7%), neuroendocrine tumor (6%), cholangiocarcinoma (5%), ampullary lesions (4%), and other lesions (7%). The other pancreatic and peri-pancreatic lesions included serous cystadenoma, mucinous cystic neoplasm, solid pseudopapillary neoplasm, splenic epidermoid cyst, duodenal polyp and cancer, and B-cell lymphoma.
Circulating Epithelial Cells
No healthy controls were found to have suspicious or malignant-appearing CECs. For all patients with pancreatic lesions, 58 (32%) were negative for CECs, 94 (53%) had malignant-appearing CECs, and 27 (15%) had cells suspicious CECs. Table 1 describes the distribution of CECs found in patients with different final histology. Patients with pancreatitis had malignant-appearing CECs in 6 (46%) and suspicious CECs in 4 (23%) of 13 cases. Thirty-eight percent of patients with IPMN did not have CECs and 62% had malignant appearing CECs. Malignant-appearing and suspicious CECs were identified in patients with PDAC in 49% and 19% of cases, respectively.
Table 1.
Circulating Epithelial Cell Findings in Patients with Different Final Histology
| Variable | n | CEC negative | CEC positive | CEC suspicious | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Healthy controls | 9 | 9 | 100 | 0 | 0 | 0 | 0 |
| All patients | 179 | 58 | 32 | 94 | 53 | 27 | 15 |
| Chronic pancreatitis | 13 | 4 | 31 | 6 | 46 | 3 | 23 |
| IPMN | 21 | 8 | 38 | 13 | 62 | 0 | 0 |
| NET | 11 | 1 | 9 | 7 | 64 | 3 | 27 |
| PDAC | 105 | 34 | 32 | 51 | 49 | 20 | 19 |
| Cholangiocarcinoma | 8 | 4 | 50 | 4 | 50 | 0 | 0 |
| Ampullary lesion | 8 | 5 | 63 | 3 | 38 | 0 | 0 |
| Other lesions | 13 | 2 | 15 | 10 | 77 | 1 | 8 |
CEC, circulating epithelial cell; IPMN, intraductal papillary mucinous neoplasm; NET, neuroendocrine tumor; PDAC, pancreatic ductal adenocarcinoma.
The pancreatic lesions were grouped into three categories for comparison 1) inflammatory lesions, including pancreatitis, 2) neoplasia/premalignant lesions, including mucinous cystic neoplasm, serous cystic neoplasm, duodenal adenoma, ampullary adenoma, non-invasive intraductal papillary mucinous neoplasm, and well-differentiated neuroendocrine tumor, and 3) malignant lesions, including PDAC, metastatic or invasive neuroendocrine tumor, solid pseudopapillary neoplasm, invasive intraductal papillary mucinous neoplasm, cholangiocarcinoma, duodenal adenocarcinoma, ampullary adenocarcinoma, and lymphoma. One patient was found to have a splenic epidermoid cyst, which was excluded from this comparison. The distribution of circulating epithelial cell findings of these groups and the control group can be seen in Figure 2. There is no difference in the distribution of CECs (non-diagnostic versus positive) among these three groups (p=0.41).
Figure 2.
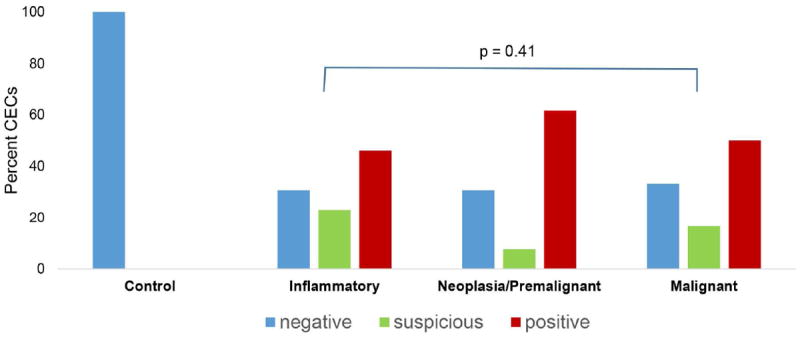
Distribution of circulating epithelial cells (CECs) in different pancreas lesions among study participants grouped as controls and patients with inflammatory (n = 13), neoplasia/premalignant (n = 39), and malignant (n = 126) lesions, and comparison of positive and non-diagnostic (negative and suspicious). Circulating epithelial cells findings among the patients with pancreatic lesions, p=0.41.
Malignant-appearing CECs had a similar appearance regardless of the pancreatic lesion histology. In addition, all of the CECs were histologically similar to fine needle aspiration biopsy samples of diagnostically malignant cells of conventional PDAC. Figure 3 illustrates representative slides of malignant-appearing CECs from patients with different final histology, including patients with PDAC, neuroendocrine tumor, and chronic pancreatitis. A fine needle aspiration biopsy cytology slide from a patient with PDAC is also shown for comparison.
Figure 3.
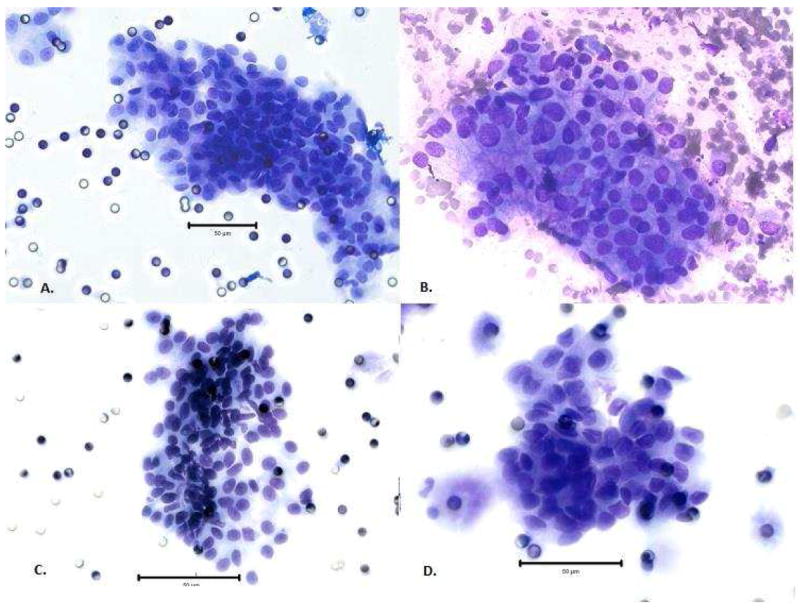
Large clusters of circulating epithelial cells (CECs) seen in the peripheral blood of patients with (A) ductal adenocarcinoma resemble (B) cells diagnostic of ductal adenocarcinoma on fine needle aspiration biopsy. Peripheral blood CECs of patients with (C) well-differentiated neuroendocrine tumor and (D) chronic pancreatitis have a similar appearance as well. The bar represents 50 μm for size comparison.
Subgroup Analysis: PDAC Patients
There were 105 patients that underwent CEC testing from the surgical clinic who were found to have PDAC on final pathology. The mean age of PDAC patients was 68 +/- 10 years with a range of 43 to 87 years old, and 54 patients were male (51%). The mean tumor size was 3.6 +/- 1.6 cm. Fifty-seven percent of PDAC tumors were found to be moderately differentiated, and rates of perineural invasion, small vessel invasion, and large vessel invasion were 86.8%, 56.8%, and 54.7%, respectively. Sixty-nine percent of PDAC patients had positive lymph nodes.
Fifty-one of 105 PDAC patients (49%) were found to have malignant-appearing CECs on cytologic analysis, and 34 PDAC patients (32%) were found to be negative for CECs. There were no statistically significant differences between the CEC positive and negative patients as seen in Table 2. The mean age of patients with malignant-appearing CECs was 68 +/-9.6 years, and 51% of these patients were male. The average size of tumor was 3.5 +/- 1.4 cm and 3.3 +/- 1.5 cm in the CEC positive and negative patients, respectively. There was no association between presence of malignant-appearing CECs and poor prognostic markers, including neurovascular invasion or tumor differentiation (Table 2). The location of the tumor had no correlation to the presence of malignant-appearing CECs, and the frequency of tumors in the head of the pancreas with CEC positive cases (76.5%) was no different than in CEC negative patients (70.6%). A similar percent of patients presented with resectable disease with 72.5% versus 82.4% undergoing pancreatic resection in the malignant-appearing and negative CEC groups, respectively. Presence of malignant-appearing CECs was not associated with advance T, N or M stage. Malignant-appearing CECs were found in PDAC patients with stages Ia-IV (Figure 4). Twenty-two percent of malignant-appearing CEC patients died during the study period compared to 24% of CEC negative patients. Censored survival analysis revealed no difference in overall survival (p=0.69) or disease progression (p=0.51) between the two groups (Figure 5a and 5b).
Table 2.
Demographic and Clinical Characteristics of Patients with Pancreatic Ductal Adenocarcinoma
| Variable | All patients, n=105 | CEC negative, n=34 | CEC positive, n=51 | CEC suspicious, n=20 | p Value |
|---|---|---|---|---|---|
| Age, y, mean±SD | 68±10.0 | 69±10.0 | 68±9.6 | 66±11.3 | 0.54 |
| Sex, male, n (%) | 54 (51.4) | 18 (52.9) | 26 (51.0) | 10 (50.0) | 0.86 |
| Race, white, n (%) | 56 (91.8) | 18 (90.0) | 26 (92.9) | 12 (92.3) | 0.54 |
| Tumor characteristics | |||||
| Size of lesion, cm, mean±SD | 3.5±1.6 | 3.3±1.5 | 3.5±1.4 | 3.8±2.1 | 0.54 |
| Location of tumor, n (%) | 0.25 | ||||
| Head | 76 (72.4) | 24 (70.6) | 39 (76.5) | 13 (65.0) | |
| Tail | 16 (15.2) | 8 (23.5) | 6 (11.8) | 2 (10.0) | |
| Other, body, neck, uncinate | 27 (25.7) | 2 (5.8) | 6 (11.8) | 5 (25.0) | |
| Grade, n (%) | 0.75 | ||||
| Well differentiated | 5 (6.5) | 1 (3.7) | 3 (8.1) | 1 (7.7) | |
| Moderately differentiated | 44 (57.1) | 16 (59.3) | 20 (54.1) | 8 (61.5) | |
| Poorly differentiated | 28 (36.4) | 10 (37.0) | 14 (37.8) | 4 (30.8) | |
| Positive LNs, n (%) | 54 (69.2) | 17 (63.0) | 29 (76.3) | 8 (61.5) | 0.24 |
| Perineural invasion, n (%) | 66 (86.8) | 21 (77.8) | 34 (91.9) | 11 (91.7) | 0.15 |
| Small vessel invasion, n (%) | 42 (56.8) | 14 (53.8) | 23 (63.9) | 5 (41.7) | 0.45 |
| Large vessel invasion, n (%) | 41 (54.7) | 15 (60.0) | 19 (50.0) | 7 (58.3) | 0.45 |
| Resectable, n (%) | 77 (73.3) | 28 (82.4) | 37 (72.5) | 12 (60) | 0.43 |
| Pancreaticoduodenectomy, n (%) | 62 (80.5) | 20 (58.8) | 31 (60.8) | 11 (55.0) | |
| Distal pancreatectomy, n (%) | 15 (19.5) | 8 (23.5) | 6 (11.8) | 1 (5.0) |
CEC, circulating epithelial cell; SD, standard deviation; LNs, lymph nodes.
Figure 4.
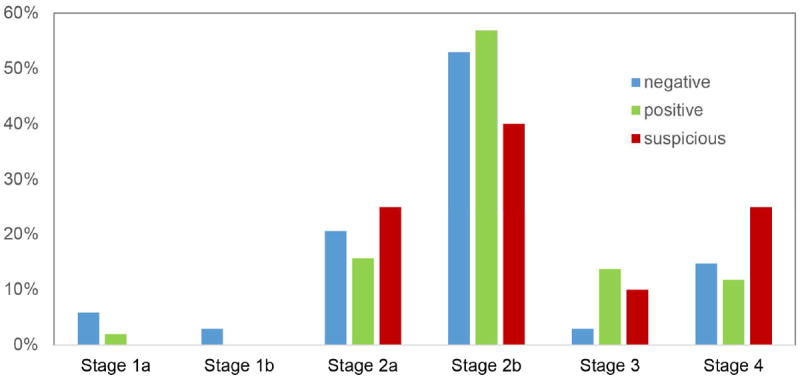
Distribution of circulating epithelial cells in patients at different stages of pancreatic ductal adenocarcinoma.
Figure 5.
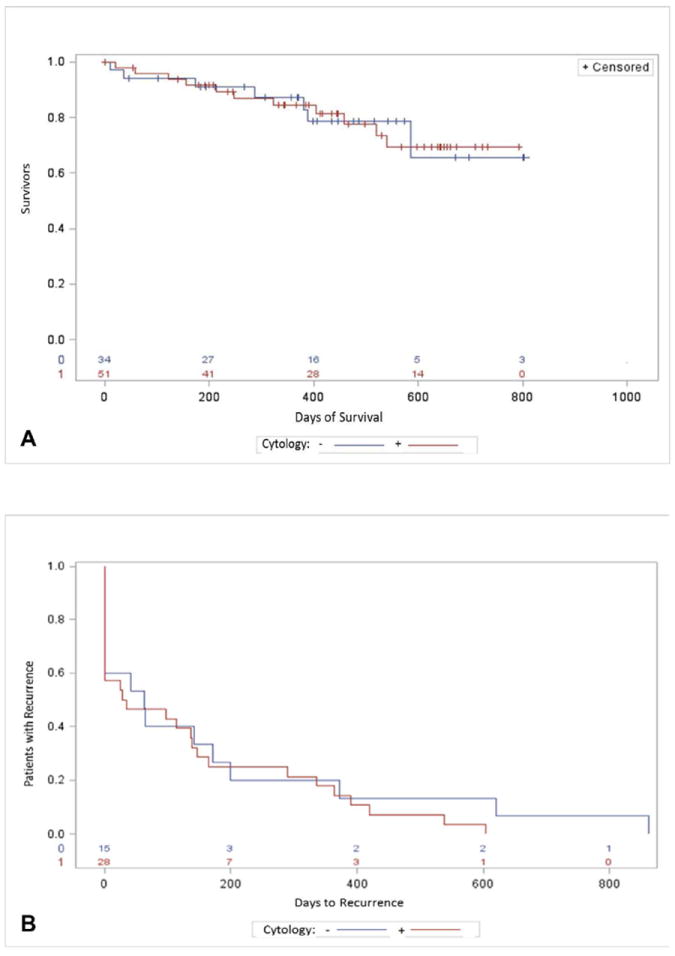
Patients with ductal adenocarcinoma. (A) Overall survival with and without circulating epithelial cells, p = 0.69; and (B) time to disease progression with and without circulating epithelial cells, p = 0.51. Red line, with circulating epithelial cells; blue line, without circulating epithelial cells.
Discussion
While CECs were not found in healthy patients without a history of pancreatic disease, this study shows that CECs may be detected in patients with a variety of benign, pre-malignant and malignant pancreatic lesions. The appearance of the malignant-appearing CECs was similar to the appearance of conventional PDAC on fine needle aspiration biopsy, regardless of the pancreatic lesion histology. Nearly half of patients with PDAC were found to have malignant-appearing CECs. However, the presence of CECs was not associated with a specific stage of disease or poor prognostic features on histology. Furthermore, PDAC patients with and without CECs had similar overall survival and time to progression of disease during a median follow-up period of one year.
Previous studies have reported that CEC isolation could be used as a liquid biopsy tool for several types of cancers including pancreatic cancer (2, 18-21). However, the low yield of antibody-dependent CEC isolation techniques made the test less useful for clinical diagnostic purposes (22-24). While this study reveals that the ScreenCell® technique has an improved CEC capture rate of 49% in PDAC patients, our study shows that the CECs defined by cytologic criteria alone may not be diagnostic of malignancy. Although healthy control patients did not have CECs, patients with a variety of benign, premalignant, and malignant pancreaticobiliary lesions were found to have CECs in the peripheral blood. Until these cells are further characterized and identified with molecular and long-term outcome analysis, the identification of CECs by cytopathologic evaluation alone cannot confirm a diagnosis of PDAC.
In addition, isolated CECs identified in a pretreatment blood sample were not able to provide prognostic information in PDAC patients. Ma et al. summarize findings from studies over a decade who report that there is potential benefit of using PDAC CECs for prognostic purposes (9). Many studies evaluating the utility of CECs for prognostic purposes evaluate multiple time point sampling, including pre and post treatment or intraoperative evaluation of CECs (12, 24-29). Our study describes the use of a single pretreatment peripheral blood sample for CEC identification. Single sample pretreatment testing for CECs did not provide information regarding PDAC patient prognosis including overall survival or time to disease progression.
The findings of this study call into question the origin and function of cells in the peripheral blood classified as CTCs and CECs. What is interesting and different from the automated immunofluorescence antibody identification is that cells were evaluated by an expert pancreatic cytopathologist and scored according to standards of cytologic and nuclear atypia used to clinically diagnosis malignancies. These cytologic criteria have a long history of accuracy in identifying malignancy (17). However, it remains unclear if these cells identified in patients with benign pancreatic pathology represent sub-clinical undiagnosed cancer from another location, or from a prior malignancy. It is also possible that these malignant appearing CECs may precede the formation of pathologically defined invasive cancer suggesting that these CECs may be a biomarker for lesions destined to become invasive cancer. Longer term follow-up is needed to explore these hypotheses.
Rhim et al using a different geometrically enhanced differential immune-capture (GEDI) microfluidic platform detected CECs from 33% of patients with Sendai negative IPMNs (Rhim 2014 Gastroenterology). These observations in addition to work in genetically engineered mouse models in which CECs were identified in advance of invasive PDAC suggest that these cells may represent early occult cancer cells (Rhim et al Cell 2012). It is possible that in benign disease CECs are released into the circulation as part of an inflammatory response; however, their strikingly similar appearance to PDAC fine needle aspiration specimens suggests these cell are more than just cytologic atypia resulting from inflammation. Further molecular or genetic evaluation of these cells may provide insight into their significance.
The findings of this study must be considered in light of some important strengths and limitations. First, all specimens in our study population were prospectively collected and confirmed by pathology, allowing us to compare patients with benign, premalignant or malignant lesions. Second, cells captured on the filtration device could be cytologically characterized by an expert cytopathologist using standard methods that clinically define malignancy. However, this study was unable to define the significance of CECs in patients with no known malignancy over the one year study period. The surgical patient population included in this study markedly differs from the patient populations of previous studies revealing potential prognostic significance of CTCs including fewer patients with metastatic disease (28, 30). Furthermore, our sub-group analysis of PDAC patients evaluated the rate of disease progression and survival, which are likely underestimates due to delay in reporting of deaths, lack of identification or testing for metastasis, or loss to follow-up. However, these findings are unlikely to be disproportionate between the CEC positive and negative groups and are accounted for with censored comparisons. Finally, this study only reports findings based on cytopathologic evaluation of size-filtered CECs. While the malignant-appearing CECs had a similar appearance to conventional PDAC fine needle aspiration specimens, the significance of their presence in benign disease remains unclear.
There are three potential clinical uses of this type of blood test: screening, diagnostic, or surveillance. Due to the low sensitivity found in this study, this current method of CEC identification is not useful as a screening tool to identify patients who might have pancreas cancer. In addition, the specificity of this test is low, making it a poor diagnostic test at this time. This is important to note because a positive CEC finding may lead some clinicians to recommend unnecessary operations if they are unaware of the high false positive rate. Future studies assessing the genomic make-up of these isolated CECs should be performed to understand the significance of their identification in the peripheral blood. Information from genomic studies will likely improve the specificity of this test, making it more clinically useful as a diagnostic test for pancreas cancer. Furthermore, this study does not address the use of this CEC identification method as a surveillance test due to the single time point of peripheral blood sampling. To assess the clinical utility of this test as a surveillance tool, future studies will require the longitudinal collection of peripheral blood samples to assess the presence of CECs over time.
Circulating cells in the peripheral blood have been described as a promising diagnostic and prognostic tool for patients with cancer. This study questions previous findings of CTC studies with the identification of malignant-appearing CECs in patients with benign, premalignant, and malignant pancreas lesions using the ScreenCell® size-based isolation technique to capture cells and standard cytologic criteria to classify these cells as malignant. While these cells were absent in healthy volunteers, it is unclear what their significance is given their presence in benign disease. This study finds that the presence of malignant-appearing CECs is not diagnostic for malignancy when evaluating patients with a variety of peripancreatic lesions. In addition, the presence of these cells in PDAC patients is not associated with a particular stage of disease or worse prognosis. Further studies evaluating long term follow up in addition to molecular and genetic characteristics of these circulating cells may shed light on their significance in patients with malignant and benign disease.
Acknowledgments
The authors thank Dr Yvon E. Cayre, Dr Nakul P. Valsangkar, Dr Sabikun Nahar, John Lindsay, David Znaty, and Dr. Fe for their technical assistance, critical discussion, and filtration devices.
Support: Dr Thayer was supported by NIH grant R01CA169086, and Dr Cauley was supported by NIH grant R25CA092203.
Footnotes
Disclosure Information: ScreenCell provided filtration devices, critical discussion, and technical assistance for this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fernández-del Castillo CM, J T, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427–434. doi: 10.1001/archsurg.138.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lianidou ES, Strati A, Markou A. Circulating tumor cells as promising novel biomarkers in solid cancers. Crit Rev Clin Lab Sci. 2014;51:160–171. doi: 10.3109/10408363.2014.896316. [DOI] [PubMed] [Google Scholar]
- 3.de Albuquerque A, Kubisch I, Ernst D, et al. Development of a molecular multimarker assay for the analysis of circulating tumor cells in adenocarcinoma patients. Clin Lab. 2012;58:373–384. [PubMed] [Google Scholar]
- 4.Cen P, Ni X, Yang J, et al. Circulating tumor cells in the diagnosis and management of pancreatic cancer. Biochimica et biophysica acta. 2012;1826:350–356. doi: 10.1016/j.bbcan.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahbari NN, Aigner M, Thorlund K, et al. Meta-analysis shows that detection of circulating tumor cells indicates poor prognosis in patients with colorectal cancer. Gastroenterol. 2010;138:1714–1726. doi: 10.1053/j.gastro.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Muller V, Riethdorf S, Rack B, et al. Prognostic impact of circulating tumor cells assessed with the CellSearch System and AdnaTest Breast in metastatic breast cancer patients: the DETECT study. Breast Cancer Res. 2012;14:R118. doi: 10.1186/bcr3243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giordano A, Egleston BL, Hajage D, et al. Establishment and validation of circulating tumor cell-based prognostic nomograms in first-line metastatic breast cancer patients. Clin Cancer Res. 2013;19:1596–1602. doi: 10.1158/1078-0432.CCR-12-3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mudan S, Giakoustidis A, Thillainayagam AV, et al. Clinical utility of circulating tumor cell measurement in the diagnosis of indeterminate lesions of the pancreas. Fut Oncol. 2010:177–179. doi: 10.2217/fon.09.156. [DOI] [PubMed] [Google Scholar]
- 9.Ma XL, Li YY, Zhang J, et al. Prognostic role of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Asian Pac J Cancer Prev. 2014;15:6015–6020. doi: 10.7314/apjcp.2014.15.15.6015. [DOI] [PubMed] [Google Scholar]
- 10.George TJ, Jr, Ogunwobi OO, Sheng W, et al. Tissue is the Issue: circulating tumor Cells in Pancreatic Cancer. J Gastrointest Cancer. 2014;45:S222–225. doi: 10.1007/s12029-014-9638-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han L, Chen W, Zhao Q. Prognostic value of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Tumour Biol. 2014;35:2473–2480. doi: 10.1007/s13277-013-1327-5. [DOI] [PubMed] [Google Scholar]
- 12.He XY, Yuan YZ. Advances in pancreatic cancer research: moving towards early detection. World J Gastroenterol. 2014;20:11241–11248. doi: 10.3748/wjg.v20.i32.11241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabbaghian MS, Rothberger G, Alongi AP, et al. Levels of elevated circulating endothelial cell decline after tumor resection in patients with pancreatic ductal adenocarcinoma. Anticancer Res. 2010;30:2911–2917. [PubMed] [Google Scholar]
- 14.Maheswaran S, Sequist LV, Nagrath S, et al. Detection of mutations in EGFR in circulating lung-cancer cells. N Engl J Med. 2008;359:366–377. doi: 10.1056/NEJMoa0800668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagrath S, Sequist LV, Maheswaran S, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235–1239. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desitter I, Guerrouahen BS, Benali-Furet N, et al. A new device for rapid isolation by size and characterization of rare circulating tumor cells. Anticancer Res. 2011;31:427–441. [PubMed] [Google Scholar]
- 17.Pitman M. In: Pancreas in Comprehensive Cytopathology. Bibbo, Wilbur, editors. London: Elsevier; 2014. [Google Scholar]
- 18.Yu M, Ting DT, Stott SL, et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature. 2012;487:510–513. doi: 10.1038/nature11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhim AD, Thege FI, Santana SM, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterol. 2014;146:647–651. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ozkumur E, Shah AM, Ciciliano JC, et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci Transl Med. 2013;5:179. doi: 10.1126/scitranslmed.3005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ilie M, Hofman V, Long-Mira E, et al. “Sentinel” circulating tumor cells allow early diagnosis of lung cancer in patients with chronic obstructive pulmonary disease. PloS One. 2014;9:e111–597. doi: 10.1371/journal.pone.0111597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gall TM, Frampton AE, Krell J, et al. Is the detection of circulating tumor cells in locally advanced pancreatic cancer a useful prognostic marker? Expert Rev Mol Diag. 2013;13:793–796. doi: 10.1586/14737159.2013.845091. [DOI] [PubMed] [Google Scholar]
- 23.Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106:508–516. doi: 10.1038/bjc.2011.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bidard FC, Huguet F, Louvet C, et al. Circulating tumor cells in locally advanced pancreatic adenocarcinoma: The ancillary CirCe 07 study to the LAP 07 trial. Ann Oncol. 2013;24:2057–2061. doi: 10.1093/annonc/mdt176. [DOI] [PubMed] [Google Scholar]
- 25.Uchikura K, Takao S, Nakajo A, et al. Intraoperative molecular detection of circulating tumor cells by reverse transcription-polymerase chain reaction in patients with biliary-pancreatic cancer is associated with hematogenous metastasis. Ann Surg Oncol. 2002;9:364–70. doi: 10.1007/BF02573871. [DOI] [PubMed] [Google Scholar]
- 26.Thege FI, Lannin TB, Saha TN, et al. Microfluidic immunocapture of circulating pancreatic cells using parallel EpCAM and MUC1 capture: characterization, optimization and downstream analysis. Lab Chip. 2014;14:1775–1784. doi: 10.1039/c4lc00041b. [DOI] [PubMed] [Google Scholar]
- 27.Torphy RJ, Tignanelli CJ, Kamande JW, et al. Circulating tumor cells as a biomarker of response to treatment in patient-derived xenograft mouse models of pancreatic adenocarcinoma. PloS One. 2014;9:e89–474. doi: 10.1371/journal.pone.0089474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurihara T, Itoi T, Sofuni A, et al. Detection of circulating tumor cells in patients with pancreatic cancer: a preliminary result. J Hepatobiliary Pancreat Surg. 2008;15:189–195. doi: 10.1007/s00534-007-1250-5. [DOI] [PubMed] [Google Scholar]
- 29.Hofman V, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;17:827–835. doi: 10.1158/1078-0432.CCR-10-0445. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Zhao Z, Liu R. Comment on Han L, et al.: Prognostic value of circulating tumor cells in patients with pancreatic cancer: a meta-analysis. Tumour Biol. 2014;35:8353–8354. doi: 10.1007/s13277-014-2507-7. [DOI] [PubMed] [Google Scholar]


