Abstract
Androgens act widely in the body in both central and peripheral sites. Prior studies indicate that in the mouse, suprachiasmatic nucleus (SCN) cells bear androgen receptors (ARs). The SCN of the hypothalamus in mammals is the locus of a brain clock that regulates circadian rhythms in physiology and behavior. Gonadectomy results in reduced AR expression in the SCN and in marked lengthening of the period of free-running activity rhythms. Both responses are restored by systemic administration of androgens, but the site of action remains unknown. Our goal was to determine whether intracranial androgen implants targeted to the SCN are sufficient to restore the characteristic free-running period in gonadectomized male mice. The results indicate that hypothalamic implants of testosterone propionate in or very near the SCN produce both anatomical and behavioral effects, namely increased AR expression in the SCN and restored period of free-running locomotor activity. The effect of the implant on the period of the free-running locomotor rhythm is positively correlated with the amount of AR expression in the SCN. There is no such correlation of period change with amount of AR expression in other brain regions examined, namely the preoptic area, bed nucleus of the stria terminalis and premammillary nucleus. We conclude that the SCN is the site of action of androgen effects on the period of circadian activity rhythmicity.
Keywords: hypothalamus, brain clock, testosterone, locomotor activity, sex difference, androgen receptor, sex steroid, light, free-running period
Introduction
The suprachiasmatic nucleus (SCN) of the hypothalamus is the locus of a master circadian clock controlling behavioral and physiological rhythms (Klein et al., 1991). Among other rhythms, the SCN regulates circadian rhythms in gonadal hormone secretion, and in turn hormones feedback to influence SCN functions (Fernandez-Guasti et al., 2000; Karatsoreos et al., 2007a; Kashon et al., 1996). A role for gonadal hormones in the maturation of circadian rhythmicity has been suggested in rodents and in non-human primates (Hagenauer et al., 2011a; Hagenauer et al., 2011b; Hagenauer and Lee, 2011; Hummer et al., 2012; Melo et al., 2010; Sellix et al., 2013). In humans, there is a correlation between chronotype (morningness or eveningness) and circulating hormone concentrations during aging (Harman et al., 2001; Roenneberg et al., 2004).
In male mice, lack of testosterone dramatically affects locomotor activity rhythms. Gonadectomy results in a marked alteration of circadian behaviors, including a longer free-running circadian period, reduced precision of the activity onset, less early night activity, reduced overall activity levels, and a large decrease in androgen receptor (AR) expression in brain regions typically expressing AR. Systemic replacement of androgens by slow release silastic capsules restores these responses to those of the intact animal (Butler et al., 2012; Daan et al., 1975; Iwahana et al., 2008; Karatsoreos et al., 2007a). Circadian period and precision are both rescued in gonadectomized (GDX) mice by testosterone propionate (TP), as well as by the non-aromatizable androgen, dihydrotestosterone (Karatsoreos et al., 2007a), indicating mediation by the androgen receptor. In contrast, total daily activity is restored fully by TP but only partially by dihydrotestosterone, suggesting that both ARs and estrogen receptors mediate this aspect of behavior.
AR-containing cells are localized to numerous brain regions and to peripheral tissues (Dart et al., 2013), but the precise locus at which androgens affect circadian behavior is not known. ARs have been observed in the SCN of several species, including mouse, ferret, and human (Hagenauer and Lee, 2011; Karatsoreos et al., 2007a), though there are species differences in their concentration and distribution (Jahan et al., 2015). In the mouse, these ARs are specifically localized in retinorecipient cells of the SCN core subregion (Iwahana et al., 2008; Karatsoreos et al., 2007a). Hormone removal/replacement studies show specific effects on circadian behavior (Karatsoreos et al., 2007a), light responsiveness of the circadian clock (Butler et al., 2012), and structure of the SCN (Karatsoreos et al., 2011). Therefore, the aim of the present study was to determine whether androgen treatment directed specifically to the SCN would suffice to alter the period of free-running circadian locomotor rhythms.
Materials and Methods
Animals and housing
Male C57BL/6J mice (Charles River Laboratories, Kingston, NY, n=24) aged 7 weeks were housed individually on pine shavings in clear polycarbonate cages (32× 14 × 13 cm) equipped with running wheels (13 cm diameter). Cages were placed in light-tight chambers with independent lighting control and ventilation (Phenome Technologies, Inc., Skokie, IL). Animals were provided with ad libitum access to food and water, and maintained in constant dim red light (peak wavelength 639 nm, half-maximal width 18 nm, Avago Techologies, San Jose, CA; (Butler and Silver, 2011). Illuminance was 0.3 lux at the cage floor (ILT1700, International Light Technologies, Peabody, MA, USA). All animal maintenance and experimental protocols were approved by Columbia University's Institutional Animal Care and Use Committee.
Experimental groups
After two weeks of baseline behavioral monitoring, mice (n=24) were assigned randomly to one of the following groups, termed: Intact (no manipulations, n=4), Gonadectomized (GDX, n=3), and GDX-TP Implanted (GDX-TP; n=17). The Intact and GDX groups served as immunohistochemical controls to confirm previous work on AR expression in the SCN (Butler et al., 2012; Iwahana et al., 2008; Karatsoreos et al., 2007a). All mice were first tested in the running wheels when they were intact. At that point, the GDX and GDX-TP groups continued in the testing apparatus for two more weeks. Two weeks after gonadectomy, mice in the GDX-TP group received an intracranial implant containing androgen (details below), and monitored for an additional 12 days.
Wheel-running behavior
Free-running behavior was recorded continuously. Wheel revolutions per 10 min bin were stored on a computerized data acquisition system (VitalView, Respironics, Inc, Murraysville, PA: currently Starr Life Sciences, Oakmont, PA). Period and daily onset of activity bouts was performed using Clocklab (Actimetrics, Wilmette, IL, USA). To calculate the precision of onset of activity, the daily difference between the actual and the projected free-running onset time was tracked, using Clocklab. The standard deviation of these daily differences is reported as the precision. Thus, the smaller the standard deviation, the more precise is the animal’s onset from day to day.
Gonadectomy
Mice were deeply anesthetized with ketamine (70 mg/kg, i.p.) and xylazine (5 mg/kg, i.p.), and buprenorphine (0.5 mg/kg, s.c.) was used as an analgesic. GDX was performed by abdominal incision and removal of both testes. Muscle and fascia were closed using surgical silk, and the overlying skin was sutured.
Steroid implants
Implants were made by mixing TP (Steraloids, Inc., Newport, RI) in beeswax at a ratio of 1:5 TP:wax (Veney and Rissman, 2000). The mixture was spread in a thin layer, and a 26-gauge, blunt-ended stainless steel guide cannula (C315GA/SPC; Plastics One, Roanoke, VA) was tamped to create a pellet 400µm in length and 260 µm in diameter. Intracranial implantation was performed using a stereotaxic instrument. The cannula containing the TP pellet was directed at a point above the SCN, using the Franklin and Paxinos Atlas of the Mouse (1997) with the following coordinates in relation to bregma: AP= −0.5mm ML=−0.5mm DV=−5.3mm from skull surface. The guide cannula was then raised 0.4 mm and the pellet was expelled using a fitted dummy cannula through the guide. The spread of steroid from the source was assessed by expression of AR in the hypothalamus of the castrated animals.
Perfusion and immunochemistry
To explore the effects of androgens on AR expression at the end of the behavioral study, the brains from 4 intact, 3 castrated, and 17 GDX-TP-implanted animals were collected (only 4 Intact and 3 GDX animals were used here as both their behavior and their SCN AR expression has been published previously) (Butler et al., 2012; Daan et al., 1975; Iwahana et al., 2008; Karatsoreos et al., 2007a). Animals were deeply anesthetized (pentobarbital: 200 mg/kg i.p.) and perfused intracardially with 50 ml saline followed by 100 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.3. Brains were post-fixed for 4 h at 4°C, cryoprotected in 20% sucrose in 0.1 M PB overnight, and sliced at 50µm on a cryostat. Tissue was processed for single-label immunochemistry using antibodies against AR. Free-floating sections were blocked in normal donkey serum for 1 h in PB with 0.1% Triton X (PBT), and then incubated for 48 h in AR primary antibody made in rabbit (Santa Cruz, CA; 1:1000) in PB with 0.3% Triton-X. Following the primary incubation, sections were washed 3 × 10 min with PBT, and then placed into a donkey anti-rabbit secondary conjugated to CY3 (1:200, Jackson ImmunoResearch, West Grove, PA) for 2 h. Sections were washed in PB, mounted onto gel-coated slides, and dehydrated in a graded series of alcohols (50–100%). Coverslips were applied with Krystalon (EM Science, Gibbstown, NJ).
Quantification of AR
Images of the brain nuclei that showed high AR expression in intact animals [SCN, preoptic area (POA), bed nucleus of the stria terminalis (BNST) and premammillary nucleus (PMN)] were captured with a Nikon Eclipse E800 epifluorescent microscope (Nikon, Tokyo, Japan) equipped with a cooled CCD camera (Retiga Exi; Q-Imaging, Surrey, Canada), using Q-capture Pro software (Q-Imaging). Sections were excited and emission filtered using filter cubes for Cy3. Images were transferred to Image J (NIH, Bethesda, MD). The freehand drawing tool was used to measure the optical density (OD) of these nuclei and of non-stained areas (background) in the same images. Relative optical density (ROD) was calculated by subtracting background OD from nuclei OD. Distance between implant and SCN was measured using the straight line tool in Image J. If the implant was not on the same section as the SCN, the location of the implant was projected onto the slide bearing the SCN, and a line was drawn from SCN border to the projected implant border (distance A). The number of sections separating SCN and implant was counted and multiplied by 50 micrometer (distance B). Distance was the length of the hypotenuse of the right triangle formed by sides A and B. Distances between implant and the other three regions were calculated similarly.
Statistical analysis
For comparison of animals when intact, GDX, and GDX-TP implanted, differences in AR-immunoreactivity (AR-ir) in various brain regions and period of locomotor activity period were analyzed using a two-way ANOVA, followed by Tukey HSD tests, and were considered statistically significant at the p < 0.05 level. Period comparison between animals when intact, GDX, and GDX-TP implanted was done using a repeated measures ANOVA. The effect size is indicated (η2, Eta Squared). Linear regression analysis was used to establish the correlation between AR expression and distance of implants from specific nuclei, and the change in period among hormone conditions.
The period of activity (hours ± S.E.M) within individual animals (N=15/17) while intact, GDX, and GDX-TP implanted, was analyzed using a Chi square periodogram in Clocklab (Actimetrics Inc., Wilmette, IL). For one animal, activity was low and irregular after castration, so its period could not be calculated. For the other animal, the brain was not processed due to poor perfusion. Effects of the distance between the site of the implant to various nuclei on period of free-running rhythm, and on SCN AR-ir were analyzed by Pearson Product Moment Correlation.
T-tests were used to compare GDX and TP-GDX states of the animals for the following measures: activity levels, precision of activity onset, change in period, and also to compare the effects on period of effective and ineffective implants. The Cohen’s d effect size is indicated. Linear regression was used to calculate the correlation between change in activity level and precision of activity onset.
Results
GDX and hormone replacement affect androgen receptors expression
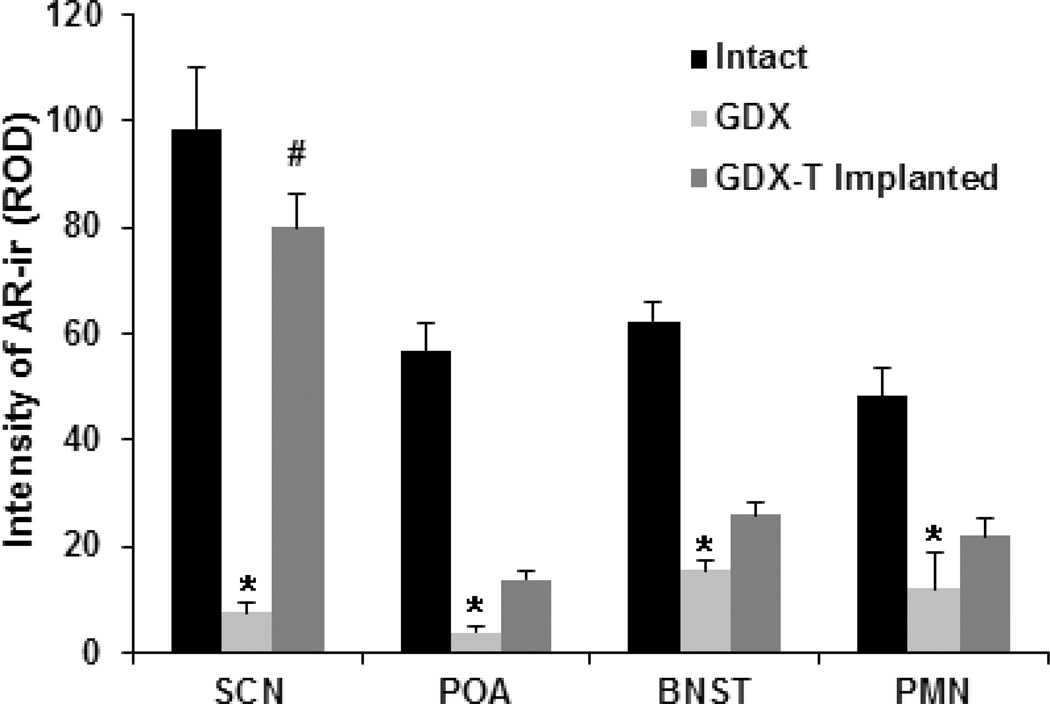
The intensity of AR-ir differs markedly among experimental groups. This is demonstrated in photomicrographs of the SCN, POA, BNST and PMN (Fig. 1) and in quantitative analyses of these regions (Fig. 2). AR expression is high in the SCN, POA, BNST and PMN of intact animals and much reduced in each of these brain regions in GDX animals. GDX-TP implanted mice have increased AR expression in the SCN compared to GDX animals. On the side of the implant, AR expression varies by brain region [F(3,80)=21.2, p<0.001, η2=0.44], and hormonal state [F(2,80)=58.7, p<0.001, η2=0.59] and there is an interaction between brain region and hormonal state [F(6,80)=7.2, p<0.001]. SCN expression of AR decreases after GDX (p<0.001) and increases after TP implant (p<0.001) to a level not significantly different for that seen when animals were intact (p=0.07, Tukey test). In the POA, BNST and PMN, AR-ir is reduced in GDX and TP-implanted animals compared to their intact state (p=0.003, Tukey test), but there are no significant differences in AR-ir expression between the TP-implanted and the GDX conditions (p>0.5, Tukey test).
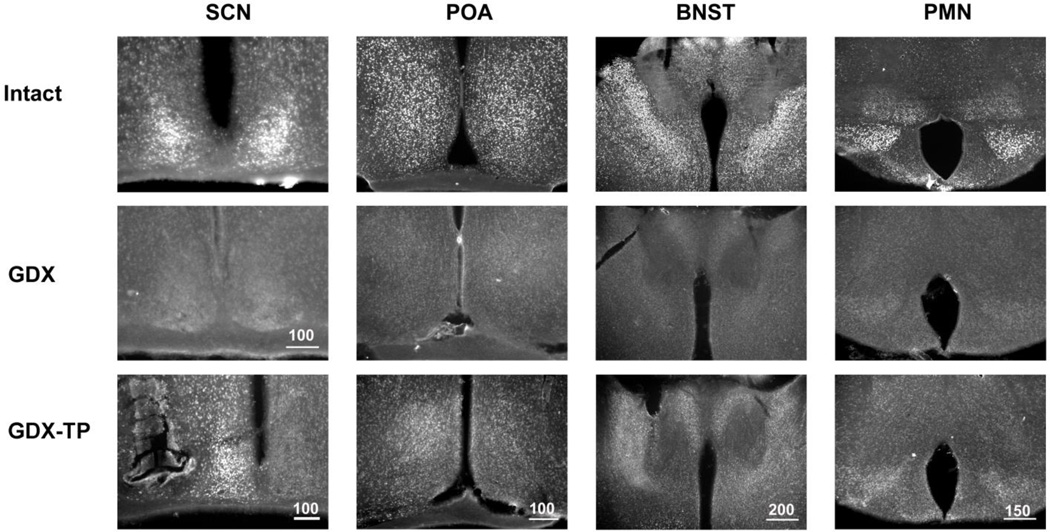
Figure 1.
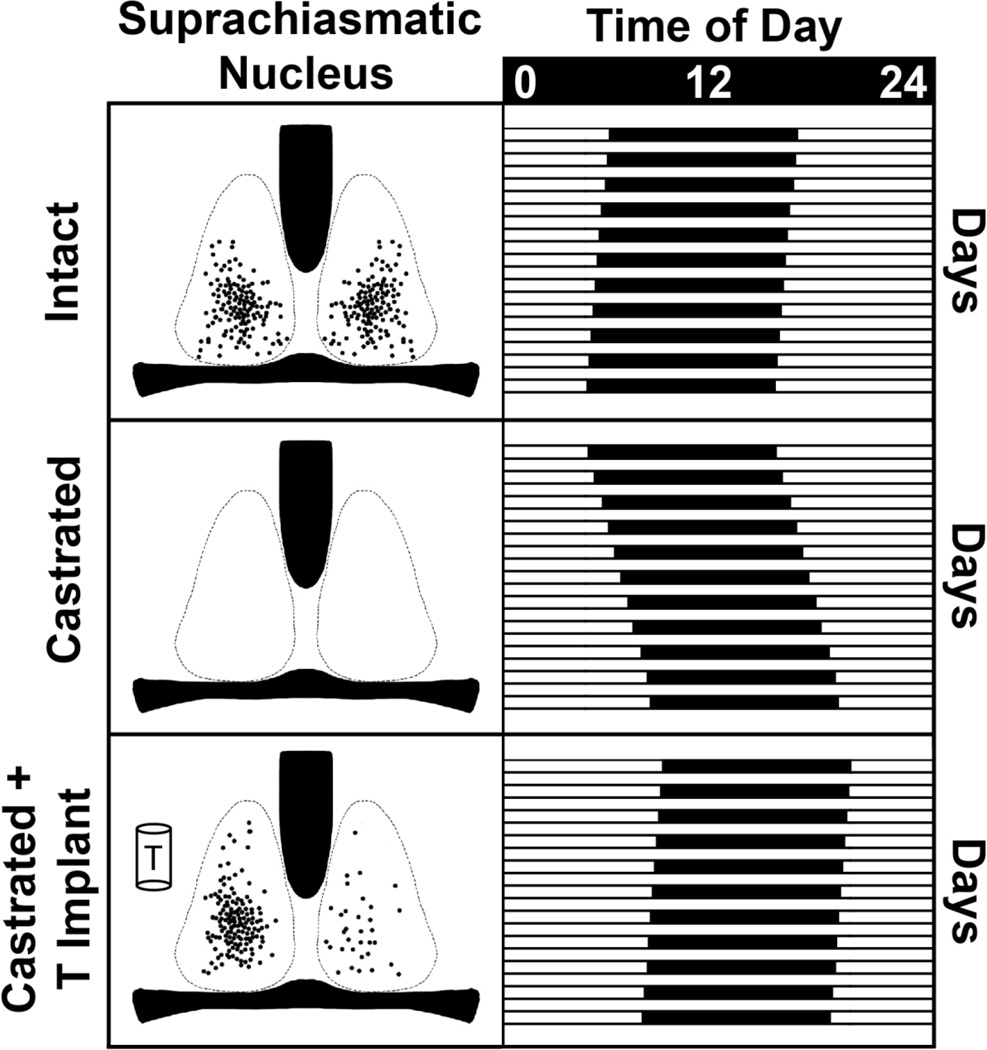
Photomicrographs show AR-ir in the SCN, POA, BNST and PMN of one representative animal from each experimental group, namely Intact, GDX and Implanted with TP. The Intact animal has high AR expression in each nucleus; the GDX, very low or no expression. The GDX-TP implanted animal with the pellet close to the SCN (bottom left panel) has high expression of AR in the SCN on the side of the implant and very little expression in the contralateral SCN. Other regions express some AR-ir, mainly on the side of the implant. Scale bar length is shown in microns.
Figure 2.
Bar graphs show average AR expression (mean ± SEM relative optical density, ROD) in SCN, POA, BNST and PMN on the side of the implant in the Intact, GDX, and GDX-TP experimental groups. AR expression is reduced by GDX compared to intact in all brain regions, and this is rescued by the T implant only in the SCN.* p<0.01 GDX compared to Intact; # p<0.001 T-Implanted compared to GDX.
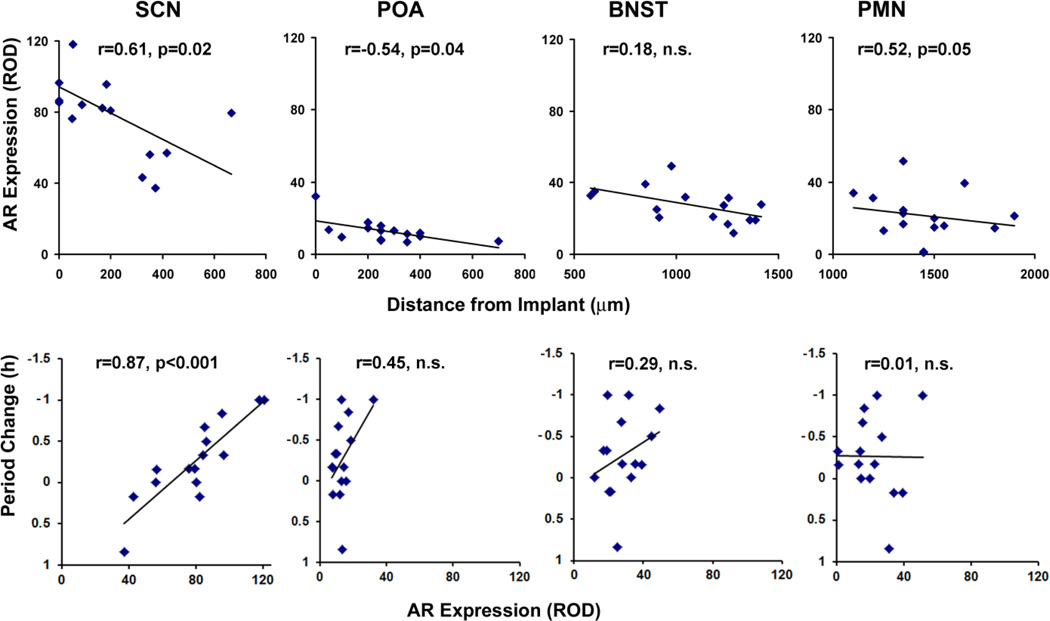
AR expression and circadian period
Overall the implant proximity to the SCN determined AR expression levels such that the closer the implant site was to the SCN, the greater AR expression in this nucleus (r=0.61, p=0.02, n=15). This was also the case for the POA and PMN, but not for the BNST (Fig. 3, upper panels). The hormonal state had significant effects on period (repeated measures ANOVA: F(2, 50)= 7.65, p=0.002, η2=0.32). Following GDX, the period of locomotor activity increased from 24.03 ± 0.06 to 24.48 ± 0.06 (Tukey test: p=0.002) and following implantation of TP pellets, the period decreased to 24.17 ± 0.13 (Tukey test: p=0.03). Importantly, the period shortening effect varied by implant site; the decrease in period was positively correlated with the amount of AR expression in the SCN (Fig 3, bottom panels; see actogram on Fig.4; Table 1). This change in period was not correlated, however, with AR expression in any of the other brain regions studied. Both activity levels and precision of activity onset were increased following GDX-TP implantation compared to the GDX condition (140% increase in activity, t(14)=3.9, p=0.002, d=2.01 and 30.3% increase in precision, t(14)=7.0, d=3.61, p<0.001).
Figure 3.
Top panels: Correlation between site of TP implant and amount of AR expression ipsilateral to side of implant, measured for SCN, POA, BNST and PMN for GDX-TP animals. Bottom panels: Correlation between intensity of ipsilateral AR expression SCN, POA, BNST and change in the period of the free-running rhythm. Each point represents one animal.
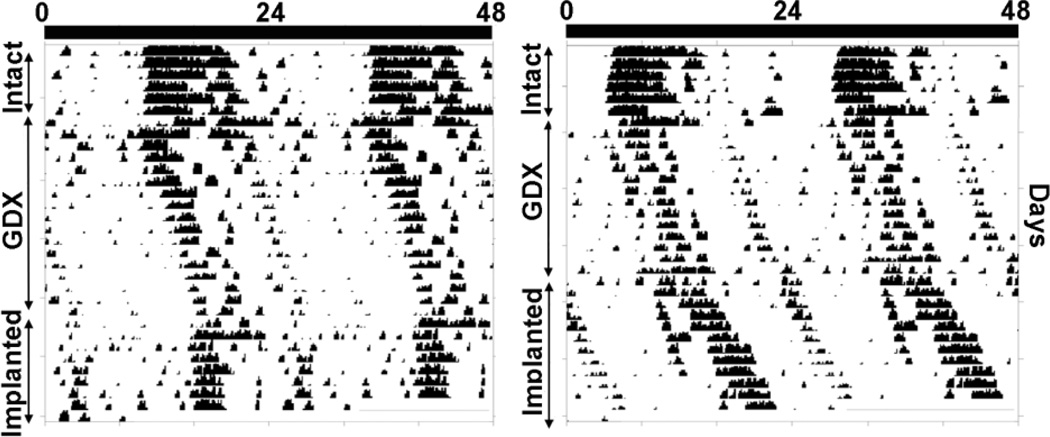
Figure 4.
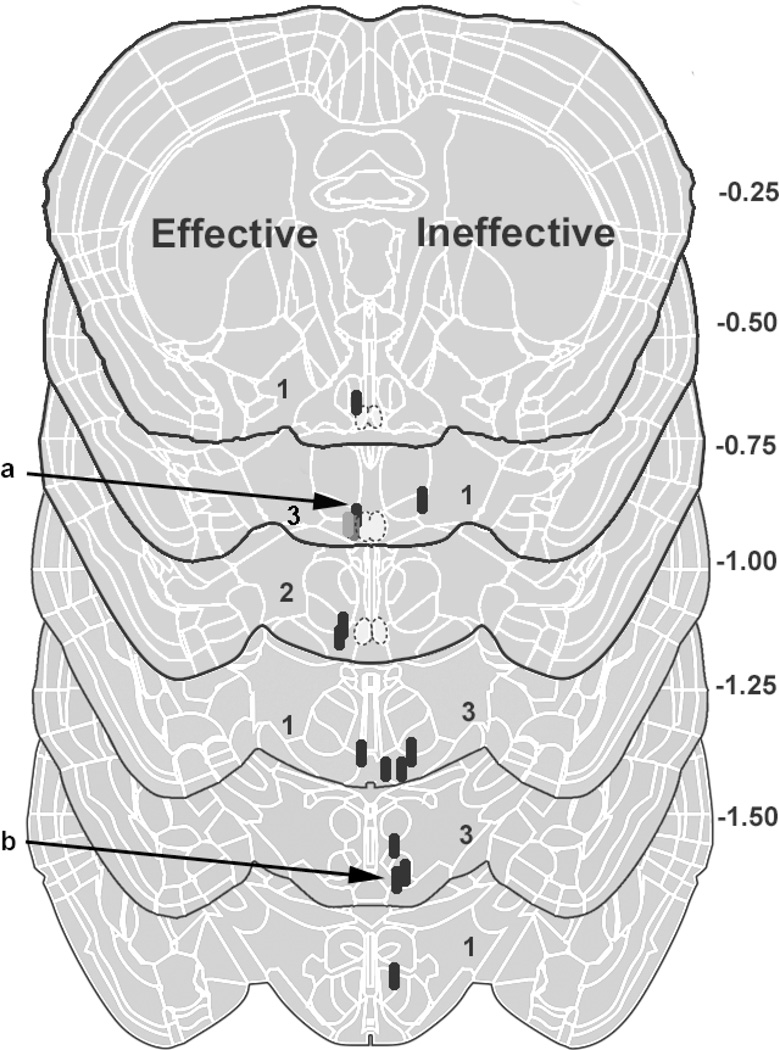
Double-plotted actograms showing the change in free-running period of wheel-running activity of two representative animals while intact, following GDX, and after implantation of TP. The intact animals have a free-running period of ~24 hours. Following GDX the period of activity onset lengthens in both animals. The animal shown in the left panel has an implant close to the SCN (labeled “a” in Fig. 5). The animal shown in the right panel bears an implant far from the SCN (labeled “b” in Fig. 5).
Table 1.
The correlation between the change in period following implantation of the GDX animals, and AR expression is measured in two ways: ipsilateral (ipsi-) to the implant and bilaterally (Bi-) (N=15).
| Ipsi- SCN |
Bi-SCN | Ipsi- POA |
Bi-POA | Ipsi- BNST |
Bi- BNST |
Ipsi- PMN |
Bi-PMN | |
|---|---|---|---|---|---|---|---|---|
| r | .87 | .57 | .45 | .25 | .29 | .24 | .01 | .02 |
| p | 0.00003 | 0.02 | ns | ns | ns | ns | ns | ns |
A number of other variables were explored, including the relationships among AR expression levels, amount of activity, and precision of activity onset. In each case the statistical analyses did not reach significance, as follows: There was no significant correlation between change in amount of activity (before and after TP implantation) and amount of AR expression in any brain region tested (SCN: r=0.009, p=0.97; POA: r=0.08, p=0.8; BNST: r=0.48, p=0.07; PMN: r=0.18, p=0.52). Similarly, there was no significant correlation between increased onset precision (before to after TP implantation) and AR expression in any brain region (SCN: r=0.11, p=0.69; POA: r=0.01, p=0.97; BNST: r=0.62, p=0.83; PMN: r=0.33, p=0.24). Additionally, the change in period seen after TP implantation was not correlated to changes in amount of activity (R=0.06, p=0.84) or changes in onset precision (R=0.23, p=0.41).
For a final analysis, animals were separated into those with implants that were effective in reducing period and those with ineffective implants that did not affect period (Fig. 5). Even though period only increased in one group, the amount of activity increased in both groups [t(6)=2.6, p=0.04, d=1.97, and t(7)=2.7, p=0.03, d=1.91, respectively]. Similarly, onset precision improved significantly, regardless of whether the TP implant was effective [t(6)=4.3, p=0.005, d=3.25] or ineffective [t(7)=6.4, p=0.001, d=4.53] in decreasing circadian period.
Figure 5.
Schematics of brain sections show TP implant placement. For orientation the numbers to the right of the schematics indicate distance from bregma (millimeters). The SCN is located at bregma −0.25to −0.75. Each implant site is shown by a black oval, and as some sites overlap, the number of implants at each level is also given. Implants that resulted in a period decrease of >15 min are shown on the left side of the schematic, while those that resulted in a period decrease of <15 min on the right. The locations of implants of animals whose actograms are shown in Fig. 4 are indicated by the letters “a” for the left actogram and “b” for the right actogram.
Discussion
Androgens act widely in the body at both central and peripheral sites, though our understanding of unique effects at these various sites of action is limited. Because the specialized role of the SCN as a brain clock is well established, this nucleus presents an opportunity to examine direct effects of androgens at this site on circadian rhythms, and the consequences for behavior of these androgen actions. Previous research has shown that circulating androgens influence the free-running period of locomotor rhythms and that the SCN bears androgen receptors. In the present study, we demonstrate that TP implants in the SCN in the castrated male are sufficient to restore the free-running period of circadian locomotor activity. The data strongly support the sufficiency of SCN androgenic signaling in control of circadian period.
Numerous lines of evidence indicate that circadian clock parameters are affected by AR-dependent mechanisms. First, circadian period can be completely restored in GDX animals with dihydrotesterone, which is not aromatizable (Karatsoreos et al., 2007a). Second, ER knockout does not alter circadian period in male mice (Blattner and Mahoney, 2012). It is noteworthy that estrogen receptors, specifically ERβ, are also expressed in the SCN, though in the shell rather than in the core (Vida et al., 2008). Third, in other species, estrogen does not shorten period in males even though it does in females (Zucker et al., 1980). The role of aromatase in the SCN has not been studied directly, but aromatase knockout mice do not experience the same period lengthening as do wild type mice (Brockman et al., 2011). The authors suggest an important organizational role for estrogens in the maturation of the circadian system though an activational role remains possible. Finally, we note that experiments with dihydrotesterone do not necessarily implicate AR alone, because of its potential conversion to 3-beta-Diol (Handa et al., 2008). Flutamide antagonism of dihydrotesterone effects would support an AR dependent mechanism.
Effects of the implanted pellets
Gonadectomy reduces AR expression in the brain, and systemic treatment restores it (Lu et al., 1998). Prior reports have shown that intracranial androgen implants effectively modulate behavior and neural activity (Lund et al., 2006; Sharma and Rissman, 1994; Veney and Rissman, 2000; Williamson et al., 2010). In implanted mice, we found that shortening of circadian period was correlated with AR expression in the SCN but not with AR expression in the POA, PMN, or BNST. The high AR expression in the SCN was seen on the side ipsilateral to the implanted pellet, and very low or no expression in the contralateral side attests to the localized release of androgen from the implant. Nevertheless, the unilateral AR in the SCN was sufficient to modulate the circadian period of locomotor activity. Given the correlation between change in period and unilateral SCN AR expression, it is possible that bilateral SCN implants would result in an even greater change in period.
In the present study, activity levels increased when GDX animals received a TP implant, but there was no correlation between the change in period and the increase in activity. Thus, the TP mediated period decrease seen here is not due to activity increase. There is mixed evidence of a relationship between amount of activity and free-running period. In hamsters exposed to a novel running wheel for 3 hours, free-running period lengthens, but only in those with very high activity (Weisgerber et al., 1997). In control Hsd:ICR mice, there was no correlation between total activity and period, though there was a strong relationship between period and time spent running and a weak one with running speed (Koteja et al., 2003). These data suggest a nuanced relationship between activity behavior and circadian period; the relationship in humans is not yet clear (Monk, 2005).
Effect of androgens on SCN network function
Although neurons of the SCN can act as autonomous oscillators, circadian timing in the SCN tissue is an emergent property of the SCN network (Welsh et al., 2010; Yan et al., 2007). Communication among neurons is an important aspect in determining circadian function. Thus, factors that modulate central nervous system functional connectivity may contribute importantly to normal SCN function. Anatomically, the SCN is composed of a ventrolateral core and a dorsomedial shell. AR’s are highly localized to the ventrolateral core area of the nucleus (Karatsoreos and Silver, 2007b; Mong et al., 2011). The projections of AR-containing cells form a dense plexus in the core, with their fibers exiting the SCN dorsally, indicating that their efferents contribute to output signals of the brain clock that modulate circadian activity in target brain regions (Karatsoreos et al., 2007a). We previously reported that GDX increases the density of astrocytic processes in the SCN and decreases the apparent number of synapses as revealed by reduced pre- and post-synaptic markers (Karatsoreos et al., 2011). Therefore, androgens may affect circadian behavior by the plastic reorganization of the SCN.
Effect of androgens on photic input to the SCN
Endocrine factors can modulate sensitivity to external cues. In the retinorecipient core of the SCN, light and androgen signals converge on the same neurons (Karatsoreos et al., 2004; Morin and Allen, 2006). We have found that GDX alters the SCN’s sensitivity to photic cues, but the direction of effect depends on the duration and phase of light exposure as well as on the outcome measure used. First, GDX blunts the immediate photic response as indicated by less light-induced FOS in the SCN core (Karatsoreos et al., 2007a). Despite this reduction in the first order response, clock genes respond differently. GDX reduces the early-night Per2 response without affecting Per1 and enhances the late-night Per1 response without affecting Per2 (Karatsoreos et al., 2011). Most behavioral responses on the other hand point towards enhanced sensitivity to light. Phase delays in response to light pulses are larger in GDX mice (advances unchanged) (Karatsoreos et al., 2011). Also, GDX increases the sensitivity of the circadian system to tonic light exposure. SCN photic sensitivity can be tested in conditions of constant light by the “Aschoff effect”, the phenomenon of period lengthening as a function of light intensity (Aschoff, 1960). We found that intact and GDX mice have similar free-running periods in constant darkness, but in constant light, GDX mice always have longer free-running periods than intact mice, over a range of light intensities (Butler and Silver, 2011).
The juxtaposition of reduced immediate early gene response to an acute light pulse with greater behavioral responsiveness may point to cellular versus network differences. As indicated above, GDX has strong effects on SCN network morphology. Such hormone-dependent structural plasticity could affect the propagation of photic information from first-order retinorecipient neurons to the rest of the SCN network (Karatsoreos et al., 2011). Importantly, the data presented here show that androgens act locally to alter circadian period and that androgen treatment of the SCN is necessary for these behavioral changes.
Sex differences in circadian organization
Though sex differences in circadian organization have been described, a satisfactory explanation for function remains elusive. Period is shorter in females in humans by 6 min, (Duffy et al., 2011), rats by 12 min, (Schull et al., 1989), and hamsters by 4 min (n.s.) (Davis et al., 1983). Even small period differences can alter the phase of rhythms. For example, the 6 min faster rhythm in women is associated with their core body temperature minimum occurring ~90 min earlier than in men (Cain et al., 2010). Experimental lengthening of circadian period by only 30 min with heavy water delays activity onset by 4 hours (Eskes and Zucker, 1978). In comparison, the ~60 min changes observed with GDX and TP replacement dwarf these sex differences.
The SCN may be an example of endocrine compensation (De Vries, 2004): rather than cause an overt sex difference, the AR in males functions to minimize sex differences in circadian organization. This may ensure appropriate entrainment to the light-dark cycle. It is interesting to speculate that the behavioral implication is that androgen actions on circadian rhythms in the SCN enable male and female mice to inhabit overlapping temporal niches in their daily activity. In the absence of androgens, males have very different temporal patterns of activity, with much reduced activity early in the night (Karatsoreos et al., 2007a). Others have suggested that earlier burrow emergence may increase the chances of encountering mates (Morin et al., 1977). Such suggestions will remain conjectural until evaluated in natural contexts, where daily patterns of activity can deviate from that observed in the laboratory and where other environmental pressures may drive the timing of activity (Gattermann et al., 2008; Hut et al., 2012; Kavanau, 1969; Levy et al., 2007; Smale et al., 2003).
Relevance to human health
Understanding how gonadal hormones affect rhythmicity is important to both health and disease in responses thought to have a circadian underpinning in humans. Gonadal steroids modulate both circadian daily rhythms and sleep patterns (Empson and Purdie, 1999; Manber and Armitage, 1999; Manber and Bootzin, 1997; Purdie et al., 1995). Rhythms and sleep change during development and with aging, and there is a strong correspondence between circadian changes and changes in circulating hormone concentration (Roenneberg et al., 2004; Harman et al., 2001). This is supported by a limited number of reports regarding the role of gonadal hormones in organizing circadian rhythms during puberty (Hagenauer et al., 2011a; Hagenauer et al., 2011b). Plasticity of this nature allows organisms to display varied responses to environmental changes, such as those represented in seasonal cycles in temperate zones. Androgens play a key role in the plasticity of neural circuits, as evidenced by their impact on brain circuitry and animal behavior.
Figure 6.
Highlights.
We explore site of androgens effects on the period of activity rhythms in mice
Gonadectomy results in lengthening of the free-running period of activity rhythms
Following gonadectomy, AR expression is reduced in the suprachiasmatic nucleus
Testosterone propionate (TP) implants in the SCN reinstate SCN AR expression
TP implants in the SCN restore the normal circadian period in the castrated animal
Acknowledgments
Supported by grants NS37919 and NSF1256105 to RS. We thank Malini Riddle for help with the figures and Domingo Rodriguez for excellent animal husbandry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Zina Model, Email: model.zina@gmail.com.
Matthew P. Butler, Email: butlema@ohsu.edu.
Joseph LeSauter, Email: jlesauter@barnard.edu.
Rae Silver, Email: Rae.Silver@columbia.edu.
References
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harbor symposia on quantitative biology. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Blattner MS, Mahoney MM. Circadian parameters are altered in two strains of mice with transgenic modifications of estrogen receptor subtype 1. Genes, brain, and behavior. 2012;11:828–836. doi: 10.1111/j.1601-183X.2012.00831.x. [DOI] [PubMed] [Google Scholar]
- Brockman R, Bunick D, Mahoney MM. Estradiol deficiency during development modulates the expression of circadian and daily rhythms in male and female aromatase knockout mice. Hormones and behavior. 2011;60:439–447. doi: 10.1016/j.yhbeh.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Butler MP, Karatsoreos IN, LeSauter J, Silver R. Dose-dependent effects of androgens on the circadian timing system and its response to light. Endocrinology. 2012;153:2344–2352. doi: 10.1210/en.2011-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, Silver R. Divergent photic thresholds in the non-image-forming visual system: entrainment, masking and pupillary light reflex. Proceedings. Biological sciences / The Royal Society. 2011;278:745–750. doi: 10.1098/rspb.2010.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain SW, Dennison CF, Zeitzer JM, Guzik AM, Khalsa SB, Santhi N, Schoen MW, Czeisler CA, Duffy JF. Sex differences in phase angle of entrainment and melatonin amplitude in humans. J Biol Rhythms. 2010;25:288–296. doi: 10.1177/0748730410374943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S, Damassa D, Pittendrigh CS, Smith ER. An effect of castration and testosterone replacement on a circadian pacemaker in mice (Mus musculus) Proc Natl Acad Sci U S A. 1975;72:3744–3747. doi: 10.1073/pnas.72.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dart DA, Waxman J, Aboagye EO, Bevan CL. Visualising androgen receptor activity in male and female mice. PLoS One. 2013;8:e71694. doi: 10.1371/journal.pone.0071694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis FC, Darrow JM, Menaker M. Sex differences in the circadian control of hamster wheel-running activity. The American journal of physiology. 1983;244:R93–R105. doi: 10.1152/ajpregu.1983.244.1.R93. [DOI] [PubMed] [Google Scholar]
- De Vries GJ. Minireview: Sex differences in adult and developing brains: compensation, compensation, compensation. Endocrinology. 2004;145:1063–1068. doi: 10.1210/en.2003-1504. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang AM, Phillips AJ, Munch MY, Gronfier C, Wyatt JK, Dijk DJ, Wright KP, Jr, Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. Proc Natl Acad Sci U S A 108 Suppl. 2011;3:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empson JA, Purdie DW. Effects of sex steroids on sleep. Ann Med. 1999;31:141–145. doi: 10.3109/07853899708998790. [DOI] [PubMed] [Google Scholar]
- Eskes GA, Zucker I. Photoperiodic regulation of the hamster testis: dependence on circadian rhythms. Proc Natl Acad Sci U S A. 1978;75:1034–1038. doi: 10.1073/pnas.75.2.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Guasti A, Kruijver FP, Fodor M, Swaab DF. Sex differences in the distribution of androgen receptors in the human hypothalamus. J Comp Neurol. 2000;425:422–435. doi: 10.1002/1096-9861(20000925)425:3<422::aid-cne7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Gattermann R, Johnston RE, Yigit N, Fritzsche P, Larimer S, Ozkurt S, Neumann K, Song Z, Colak E, Johnston J, McPhee ME. Golden hamsters are nocturnal in captivity but diurnal in nature. Biology letters. 2008;4:253–255. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, King AF, Possidente B, McGinnis MY, Lumia AR, Peckham EM, Lee TM. Changes in circadian rhythms during puberty in Rattus norvegicus: developmental time course and gonadal dependency. Hormones and behavior. 2011a;60:46–57. doi: 10.1016/j.yhbeh.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Ku JH, Lee TM. Chronotype changes during puberty depend on gonadal hormones in the slow-developing rodent, Octodon degus. Hormones and behavior. 2011b;60:37–45. doi: 10.1016/j.yhbeh.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenauer MH, Lee TM. Time for testosterone: the suprachiasmatic nucleus gets sexy. Endocrinology. 2011;152:1727–1730. doi: 10.1210/en.2011-0198. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Pak TR, Kudwa AE, Lund TD, Hinds L. An alternate pathway for androgen regulation of brain function: activation of estrogen receptor beta by the metabolite of dihydrotestosterone, 5alpha-androstane-3beta,17beta-diol. Hormones and behavior. 2008;53:741–752. doi: 10.1016/j.yhbeh.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR Baltimore Longitudinal Study of, A. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. The Journal of clinical endocrinology and metabolism. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- Hummer DL, Peckham EM, Lee TM. Estradiol acts during a post-pubertal sensitive period to shorten free-running circadian period in male Octodon degus. Eur J Neurosci. 2012;36:3051–3058. doi: 10.1111/j.1460-9568.2012.08228.x. [DOI] [PubMed] [Google Scholar]
- Hut RA, Kronfeld-Schor N, van der Vinne V, De la Iglesia H. In search of a temporal niche: environmental factors. Prog Brain Res. 2012;199:281–304. doi: 10.1016/B978-0-444-59427-3.00017-4. [DOI] [PubMed] [Google Scholar]
- Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Hormones and behavior. 2008;53:422–430. doi: 10.1016/j.yhbeh.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan MR, Kokubu K, Islam MN, Matsuo C, Yanai A, Wroblewski G, Fujinaga R, Shinoda K. Species differences in androgen receptor expression in the medial preoptic and anterior hypothalamic areas of adult male and female rodents. Neuroscience. 2015;284:943–961. doi: 10.1016/j.neuroscience.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Karatsoreos IN, Butler MP, Lesauter J, Silver R. Androgens modulate structure and function of the suprachiasmatic nucleus brain clock. Endocrinology. 2011;152:1970–1978. doi: 10.1210/en.2010-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007a;148:5487–5495. doi: 10.1210/en.2007-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Silver R. Minireview: The neuroendocrinology of the suprachiasmatic nucleus as a conductor of body time in mammals. Endocrinology. 2007b;148:5640–5647. doi: 10.1210/en.2007-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Yan L, LeSauter J, Silver R. Phenotype matters: identification of light-responsive cells in the mouse suprachiasmatic nucleus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:68–75. doi: 10.1523/JNEUROSCI.1666-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashon ML, Arbogast JA, Sisk CL. Distribution and hormonal regulation of androgen receptor immunoreactivity in the forebrain of the male European ferret. J Comp Neurol. 1996;376:567–586. doi: 10.1002/(SICI)1096-9861(19961223)376:4<567::AID-CNE6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kavanau JL. Influences of light on activity of the small mammals, Peromyscus spp., Tamias striatus, and Mustela rixosa. Experientia. 1969;25:208–209. doi: 10.1007/BF01899128. [DOI] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus. The Mind's Clock. New York: Oxford University Press; 1991. p. 467. [Google Scholar]
- Koteja P, Swallow JG, Carter PA, Garland T., Jr Different effects of intensity and duration of locomotor activity on circadian period. J Biol Rhythms. 2003;18:491–501. doi: 10.1177/0748730403256998. [DOI] [PubMed] [Google Scholar]
- Levy O, Dayan T, Kronfeld-Schor N. The relationship between the golden spiny mouse circadian system and its diurnal activity: an experimental field enclosures and laboratory study. Chronobiol Int. 2007;24:599–613. doi: 10.1080/07420520701534640. [DOI] [PubMed] [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139:1594–1601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- Lund TD, Hinds LR, Handa RJ. The androgen 5alpha-dihydrotestosterone and its metabolite 5alpha-androstan-3beta, 17beta-diol inhibit the hypothalamo-pituitary-adrenal response to stress by acting through estrogen receptor beta-expressing neurons in the hypothalamus. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:1448–1456. doi: 10.1523/JNEUROSCI.3777-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manber R, Armitage R. Sex, steroids, and sleep: a review. Sleep. 1999;22:540–555. [PubMed] [Google Scholar]
- Manber R, Bootzin RR. Sleep and the menstrual cycle. Health Psychol. 1997;16:209–214. doi: 10.1037//0278-6133.16.3.209. [DOI] [PubMed] [Google Scholar]
- Melo PR, Belisio AS, Menezes AA, Azevedo CV. Influence of seasonality on circadian motor activity rhythm in common marmosets during puberty. Chronobiol Int. 2010;27:1420–1437. doi: 10.3109/07420528.2010.501416. [DOI] [PubMed] [Google Scholar]
- Mong JA, Baker FC, Mahoney MM, Paul KN, Schwartz MD, Semba K, Silver R. Sleep, rhythms, and the endocrine brain: influence of sex and gonadal hormones. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:16107–16116. doi: 10.1523/JNEUROSCI.4175-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH. Aging human circadian rhythms: conventional wisdom may not always be right. J Biol Rhythms. 2005;20:366–374. doi: 10.1177/0748730405277378. [DOI] [PubMed] [Google Scholar]
- Morin LP, Allen CN. The circadian visual system, 2005. Brain research reviews. 2006;51:1–60. doi: 10.1016/j.brainresrev.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Morin LP, Fitzgerald KM, Rusak B, Zucker I. Circadian organization and neural mediation of hamster reproductive rhythms. Psychoneuroendocrinology. 1977;2:73–98. doi: 10.1016/0306-4530(77)90035-x. [DOI] [PubMed] [Google Scholar]
- Purdie DW, Empson JA, Crichton C, Macdonald L. Hormone replacement therapy, sleep quality and psychological wellbeing. Br J Obstet Gynaecol. 1995;102:735–739. doi: 10.1111/j.1471-0528.1995.tb11433.x. [DOI] [PubMed] [Google Scholar]
- Roenneberg T, Kuehnle T, Pramstaller PP, Ricken J, Havel M, Guth A, Merrow M. A marker for the end of adolescence. Curr Biol. 2004;14:R1038–R1039. doi: 10.1016/j.cub.2004.11.039. [DOI] [PubMed] [Google Scholar]
- Schull J, Walker J, Fitzgerald K, Hiilivirta L, Ruckdeschel J, Schumacher D, Stanger D, McEachron DL. Effects of sex, thyro-parathyroidectomy, and light regime on levels and circadian rhythms of wheel-running in rats. Physiology & behavior. 1989;46:341–346. doi: 10.1016/0031-9384(89)90001-2. [DOI] [PubMed] [Google Scholar]
- Sellix MT, Murphy ZC, Menaker M. Excess androgen during puberty disrupts circadian organization in female rats. Endocrinology. 2013;154:1636–1647. doi: 10.1210/en.2012-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma UR, Rissman EF. Testosterone implants in specific neural sites activate female sexual behaviour. Journal of neuroendocrinology. 1994;6:423–432. doi: 10.1111/j.1365-2826.1994.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Smale L, Lee T, Nunez AA. Mammalian diurnality: some facts and gaps. J Biol Rhythms. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- Veney SL, Rissman EF. Steroid implants in the medial preoptic area or ventromedial nucleus of the hypothalamus activate female sexual behaviour in the musk shrew. Journal of neuroendocrinology. 2000;12:1124–1132. doi: 10.1046/j.1365-2826.2000.00567.x. [DOI] [PubMed] [Google Scholar]
- Vida B, Hrabovszky E, Kalamatianos T, Coen CW, Liposits Z, Kallo I. Oestrogen receptor alpha and beta immunoreactive cells in the suprachiasmatic nucleus of mice: distribution, sex differences and regulation by gonadal hormones. Journal of neuroendocrinology. 2008;20:1270–1277. doi: 10.1111/j.1365-2826.2008.01787.x. [DOI] [PubMed] [Google Scholar]
- Weisgerber D, Redlin U, Mrosovsky N. Lengthening of circadian period in hamsters by novelty-induced wheel running. Physiology & behavior. 1997;62:759–765. doi: 10.1016/s0031-9384(97)00192-3. [DOI] [PubMed] [Google Scholar]
- Welsh DK, Takahashi JS, Kay SA. Suprachiasmatic nucleus: cell autonomy and network properties. Annu Rev Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M, Bingham B, Gray M, Innala L, Viau V. The medial preoptic nucleus integrates the central influences of testosterone on the paraventricular nucleus of the hypothalamus and its extended circuitries. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:11762–11770. doi: 10.1523/JNEUROSCI.2852-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Karatsoreos I, LeSauter J, Welsh DK, Kay SA, Foley DK, Silver R. Exploring spatiotemporal organizatio of the SCN circuits, Clocks and Rhythms. Cold Spring Harbor Laboratory Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker I, Fitzgerald KM, Morin LP. Sex differentiation of t-e circadian system in the golden hamster. The American journal of physiology. 1980;238:R97–R101. doi: 10.1152/ajpregu.1980.238.1.R97. [DOI] [PubMed] [Google Scholar]