Abstract
Staphylococcus aureus is the primary cause of skin and skin structure infections (SSSI) in the USA. Alpha-hemolysin (Hla), a pore-forming toxin secreted by S. aureus and a major contributor to tissue necrosis, prompts recruitment of neutrophils critical for host defense against S. aureus infections. However, the failure to clear apoptotic neutrophils can result in damage to host tissues, suggesting that mechanisms of neutrophil clearance are essential to limiting Hla-mediated dermonecrosis. We hypothesized that CD36, a scavenger receptor which facilitates recognition of apoptosing cells, would play a significant role in regulating Hla-mediated inflammation and tissue injury during S. aureus SSSI. Here we show that CD36 on macrophages negatively regulates dermonecrosis caused by Hla-producing S. aureus. This regulation is independent of bacterial burden, as CD36 also limits dermonecrosis caused by intoxication with sterile bacterial supernatant or purified Hla. Dermonecrotic lesions of supernatant intoxicated CD36−/− mice are significantly larger, with increased neutrophil accumulation and IL-1β expression, compared to CD36+/+ (wild-type) mice. Neutrophil depletion of CD36−/− mice prevents this phenotype, demonstrating the contribution of neutrophils to tissue injury in this model. Furthermore, administration of CD36+/+, but not CD36−/−, macrophages near the site of intoxication reduces dermonecrosis, IL-1β production and neutrophil accumulation to levels seen in wild-type mice. This therapeutic effect is reversed by inhibiting actin polymerization in the CD36+/+ macrophages, supporting a mechanism of action whereby CD36-dependent macrophage phagocytosis of apoptotic neutrophils regulates Hla-mediated dermonecrosis. Together, these data demonstrate that CD36 is essential for controlling the host innate response to S. aureus skin infection.
Introduction
Staphylococcus aureus is the primary cause of skin and skin structure infections (SSSI) presenting to emergency departments throughout the US (1, 2). SSSIs include an intense local inflammatory response, which often precedes the formation of necrotic lesions. Although S. aureus secretes numerous virulence factors, the formation of necrotic lesions is largely due to the action of the pore-forming toxin alpha-hemolysin (Hla) (3–7). In recent years, the cellular receptor for Hla, the metalloprotease ADAM10, and its contribution to dermonecrosis, has been well-characterized (3, 5, 8–12). In contrast, little is known regarding mechanisms used by the host to control local inflammation and limit Hla-mediated tissue injury. Insight into these mechanisms of host control could inform novel approaches to limit the pathogenesis of S. aureus SSSIs.
Neutrophils are critical for host defense against S. aureus and utilize a variety of mechanisms to kill the bacteria to limit invasive infection (reviewed in (13)). However, neutrophils are short-lived and contain many noxious substances which, if released, are toxic to host tissues (14, 15). Therefore, the clearance of apoptotic neutrophils, prior to the loss of membrane integrity during secondary necrosis, is essential to limiting tissue damage. Importantly, Hla contributes to the recruitment of circulating neutrophils (16), suggesting that mechanisms of neutrophil clearance are essential to limiting Hla-mediated dermonecrosis.
The scavenger receptor CD36 is a membrane glycoprotein present on many mammalian cells, in particular monocytes and macrophages (reviewed in (17)), which is primarily known for its contribution to atherosclerosis (18, 19). However, CD36 also contributes to host innate defense against S. aureus. In conjunction with Toll-like receptor 2 (TLR2), CD36 recognizes S. aureus cell wall diacylglycerides, facilitating bacterial phagocytosis and cytokine production (20, 21). Importantly, CD36 also enables macrophage recognition and clearance of apoptotic neutrophils (22–25), suggesting an important role for CD36 in controlling the host response to skin infection; however the contribution of this receptor to the regulation of Hla-mediated dermonecrosis has not been investigated. We hypothesized that CD36 on macrophages would play a significant role in regulating local Hla-mediated inflammation and dermonecrosis, independent of its role in bacterial phagocytosis.
Using a mouse model of S. aureus dermonecrosis, here we show that CD36 negatively regulates dermonecrosis following infection with Hla-producing S. aureus. At early time points post-infection, this regulation is independent of bacterial clearance, as CD36 also limits dermonecrosis following subcutaneous intoxication with S. aureus secreted virulence factors and purified Hla. CD36−/− mice intoxicated with sterile S. aureus supernatant show significantly increased dermonecrosis, with increased neutrophil accumulation and local IL-1β expression, compared to CD36+/+ (wild-type) mice. This phenotype is prevented by neutrophil depletion, pointing to the contribution of neutrophils to tissue injury in this model. Importantly, therapeutic administration of CD36+/+, but not CD36−/−, macrophages near the site of intoxication limits dermonecrosis, IL-1β production, and neutrophil accumulation. Furthermore, whereas the administration of CD36+/+ macrophages significantly reduces the presence of necrotic neutrophils at the site of intoxication, this is reversed by pharmacological blockade of macrophage actin polymerization. This supports a mechanism of action whereby CD36-mediated macrophage phagocytosis of apoptotic neutrophils plays a significant role in host regulation of Hla-mediated dermonecrosis. Together, these data demonstrate for the first time that, independent of its role in bacterial clearance (20), CD36 expression on macrophages plays an important role in host control of inflammation and skin injury during S. aureus skin infection.
Materials and Methods
Bacterial strains and growth conditions
S. aureus USA300 LAC and its isogenic agr-deletion mutant (LACΔagr) were provided by Dr. Frank DeLeo (Rocky Mountain Laboratories, NIH/NIAID) and Dr. Michael Otto (NIH/NIAID). The isogenic hla deletion mutant (LACΔhla) was generously provided by Dr. Juliane Bubeck-Wardenburg (University of Chicago). Bacteria were grown in trypticase soy broth (TSB) at 37°C to early exponential phase as previously described (26) and CFUs determined by plating serial dilutions on blood agar (Becton Dickinson, Franklin Lakes, NJ).
Mouse model of S. aureus skin and soft tissue infection
Animal work in this study was carried out at the AAALAC accredited Animal Research Facility of the University of New Mexico Health Sciences Center in strict accordance with recommendations in the Eighth Edition of The Guide for the Care and Use of Laboratory Animals and the USA Animal Welfare Act. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of New Mexico. C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, ME) and CD36−/− mice, provided by Dr. Maria Febbraio, were bred within our facilities.
The mouse model of dermonecrosis was performed as previously described (27). Briefly, twenty-four hours before infection, male, age-matched mice (8–12 weeks, 22–28 g) were anesthetized by isoflurane and hair was removed from the right flank using Nair (Church and Dwight Co., Ewing, NJ). On the day of infection, 50µL of sterile saline containing 8 × 106 CFUs of the indicated S. aureus strain was injected subcutaneously into the right flank of anesthetized mice. Mice were weighed daily and photographed for abscess and ulcer area which was measured using ImageJ analysis (28). At the indicated time points post-infection, mice were sacrificed by CO2 asphyxiation and the abscesses (area = 2.25 cm2) and spleens were collected. For CFU determinations, abscesses and spleens were collected in bead-beating tubes containing 2.3 mm Zirconia/Silica beads (BioSpec Products Inc., Bartlesville, OK) and 1 mL of HBSS− (Gibco, Grand Island, NY) with 0.2% Human serum albumin (Sigma-Aldrich, St. Louis, MO). Tissue was disrupted for 3 min in 1 min intervals using a Mini-Bead Beater-24 (BioSpec Products Inc.). Homogenates were diluted into PBS containing 0.1% Triton X-100 and sonicated, followed by plating serial dilutions on blood agar. For cytokine and western blot analyses, abscess homogenates were clarified by centrifugation at 12,500 × g and supernatant was stored at −80°C until use as described below. For intoxication experiments, mice were subcutaneously injected with 50 µL of 0.2 micron filtered supernatant from an 18-hour culture of the indicated strain of S. aureus or with 1 µg of purified Hla in 50 µL of sterile saline. For neutrophil depletion experiments, CD36−/− mice were given 100 µg of anti-mouse Ly6G (Clone 1A8, BioXCell, West Lebanon, NH) (29) or isotype control (Rat IgG2a, BioXCell) by intraperitoneal injection on the day before and the day of infection.
Quantification of cytokines and MPO
Cytokines were measured in clarified supernatant from homogenized abscesses using custom designed multiplex assays (Millipore, Billerica, MA) according to the manufacturer’s directions. Myeloperoxidase was measured in clarified supernatant from homogenized abscesses with the ELISA Mouse Myeloperoxidase DuoSet kit (R&D systems, Minneapolis, MN) according to manufacturer’s directions.
Expression and purification of recombinant Hla and mutant H35L
The coding sequence for the mature Hla was PCR amplified from USA300 LAC genomic DNA using the following primers for insertion into the expression vector pET-28a(+) (Novagen, EMD Millipore, Billerica, MA) using the 5’ NcoI and a 3’ XhoI restriction sites: HylA F, 5'- GAGATACCATGGCAGATTCTGATATTAATATTAAAACCGG and HylA R, 5'- CATTATCTCGAGATTTGTCATTTCTTCTTTTTCCCAATCGATTTTATATC (Integrated DNA Technologies, Coralville, IA). The resulting construct includes an N-terminal methionine and a C-terminal poly-histidine tag (LEHHHHHH). This construct was transformed into the cloning strain 5-alpha F’ Iq (New England BioLabs, Ipswich, MA). The H35L variant (HlaH35L) was constructed using Q5 Site Directed Mutagenesis Kit (New England Biolabs) according to manufacturer’s instructions and using the following primers: Q5SDM F, 5’- ATGGATAGAActgAGCATCCAAACAACAAAC and Q5SDM R, 5’- AGTAATAACTGTAGCGAAG. The HlaH35L variant construct was again inserted into pET-28a(+) (Novagen). Both Hla constructs were transformed into BL21 Star (DE3) Competent E. coli (Life Technologies, Grand Island, NY) for expression. Bacteria were grown in Terrific Broth with 30 µg/mL kanamycin at 37 °C, 220 rpm and expression induced by 400 µM IPTG at an OD600 of 0.7. The biomass was harvested 3 hrs post-induction by centrifugation and the cell pellet was frozen at −80 °C. The pellet thawed in 5 volumes of lysis buffer (50 mM Tris-Cl, 150 mM NaCl, 0.5 mg/mL lysozyme, 2 mM EDTA, Pierce Universal Nuclease for Cell Lysis (Life Technologies), pH 8.0) and incubated for one hour at 37°C with rotation. Remaining intact cells were lysed by sonication and cellular debris removed by centrifugation (14000 × g for 30 min at 4 °C). Clarified lysate was loaded on a GE Healthcare C 10/200 column containing Clontech Talon Metal Affinity Resin (Mountain View, CA) pre-equilibrated in binding buffer (50 mM Tris-Cl, 150 mM NaCl, pH 8.0). After washing, bound protein was eluted with 50 mM Tris-Cl, 150 mM NaCl and 300 mM imidazole, pH 7.0. The eluted protein was then further purified and buffer exchanged using a GE Healthcare Sephacryl S-400 16/60 prepacked column (Pittsburgh, PA) equilibrated in PBS. The final protein ran as a single band on SDS/PAGE (data not shown). Recombinant protein was filtered for endotoxin removal (0.2 µm Acrodisc® Unit with Mustang™ E membrane, Pall Life Science, Ann Arbor, MI) and tested for endotoxin according to manufacturer’s directions (ToxinSensor™ Chromogenic LAL Endotoxin Assay, GenScript, Piscataway, NJ) before use. The activity of Hla and the inactivity of HlaH35L were verified using the rabbit red blood cell lysis assay as previously described (30).
Western blot analyses
Clarified abscess homogenates were rapidly thawed at 37°C and protein concentrations determined by A280nm absorbance (Nanodrop 1000 Spectrophotometer, Thermo Fisher Scientific, Wilmington, DE). Equivalent amounts of total protein were separated by SDS-PAGE on 16% Tris-Glycine gels (Novex Life Technologies, Grand Island, NY) prior to transfer to polyvinylidene fluoride membranes. Membranes were blocked with 1% BSA in Tris-buffered saline (20 mM Tris pH 7.5, 150 mM NaCl) for 30 min at 4°C, then probed with rabbit anti-mouse Caspase-1 p10 (Santa Cruz Biotechnology Inc., Dallas, TX) or rabbit anti-mouse IL-1β (Abcam, Cambridge, MA) antibodies for 1 h at 22°C. Unbound primary antibody was removed by repeated washing with TBST (TBS with 0.1% Tween20). Membranes were developed using nitro-blue tetrazolium and 5-bromo-4-chloro-39-indolyphosphate (NBT/BCIP: Pierce Biotechnology Inc., Rockford, IL) following incubation with goat anti-rabbit IgG-alkaline phosphatase (AP) conjugated secondary antibody (Sigma-Aldrich, St. Louis, MO). Imaging was performed using a Protein Simple FluorChem R imaging system (Protein Simple, Santa Clara, CA).
Quantitative RT-PCR
Abscess tissue (area = 2.25 cm2) was collected in RNALater and RNA isolated using Qiazol according to manufacturer’s instructions (Qiagen, Valencia, CA). RNA was purified using RNeasy Kits (Qiagen) and stored at −80°C until use. cDNA was generated by high capacity cDNA RT kit with RNAse inhibitor and random hexamer primers (Applied Biosystems, Foster City, CA) on a PTC-200 Peltier thermocycler (Bio-Rad, Hercules, CA). qPCR was performed using Taqman Gene Expression master mix (Applied Biosystems) and an ABI7000 Real Time PCR system. Gene expression was quantified using SDS RQ Manager Version 1.2.2 software (Applied Biosystems) relative to hprt or gapdh using Prime Time Predesigned qPCR Assays for il-1beta, tnfα, cxcl1, nlrp3, asc and caspase-1 (Integrated DNA technologies, Coralville, IA).
Flow cytometry
Abscess sections were collected in MACs storage solution (Miltenyi Biotec Inc., Auburn, CA) and enzymatically digested in gentle MACs C tubes using 350ug/mL Liberase TL (Roche, Indianapolis, IN), 2mg/mL Hyaluronidase (Sigma-Aldrich), and 0.25 kU/mL DNase (Sigma-Aldrich) in RMPI 1640 Media (ATCC, Manassas, VA) for 2 hours while shaking at 37°C. Digestion was halted with ice cold RPMI (Manassas, VA) supplemented with 10% FBS. Single cell suspensions were prepared by mechanical disruption in C tubes using the Gentle MACs Dissociator (Miltenyi Biotec) followed by filtration through a 70 µm cell strainer. Total cell numbers and live and dead counts were determined by trypan blue staining using the TC 20 Automated Cell Counter system (Bio-Rad, Hercules, CA). Cells were centrifuged 800 × g for 3 minutes and resuspended in cold 0.075% sodium azide (Sigma-Aldrich), 0.5% BSA in PBS and blocked for 30 minutes with 2% BSA, followed by a 1 hour incubation at 4°C with anti-mouse Ly6G (BioXCell, West Lebanon, NH) conjugated to Alexa Fluor 488 (Protein Labeling Kit, Molecular Probes Inc., Eugene, OR), anti-mouse CD36-Alexa Fluor 647 (Biolegend, San Diego, CA), anti-mouse F4/80-PE (Abcam), or isotype controls. Where indicated, Ly6G+ cells were isolated with an anti-Ly6G MicroBead Kit according to manufacturer’s instructions (Miltenyi Biotec Inc.). Cells were washed with PBS prior to analysis by flow cytometry (Accuri C6, BD Biosciences, San Jose, CA). Data was further analyzed using FlowJo vX software (FlowJo LCC, Ashland, OR).
Bone marrow-derived neutrophils
Mouse femurs and tibias were collected and bone marrow recovered by flushing with warm Dulbecco’s Modified Eagle’s Medium (4 mM L-glutamine, 4500 mg/L glucose, 1 mM sodium pyruvate, and 1500 mg/L sodium bicarbonate) (ATCC) using a 26-gauge needle. Recovered cells were centrifuged at 800 × g for 3 min and Ly6G+ cells (neutrophils) isolated as described above. Neutrophils were incubated in LAC supernatant for 1 h at 37°C and 5% CO2. Cell supernatant was collected for cytokine analysis or cells were lysed with RLT buffer (Qiagen) and RNA purified using an RNeasy Protect Mini Kit (Qiagen). Purified RNA was stored at −80°C until use for qPCR.
Peritoneal macrophages
Mouse peritoneal macrophages were collected by peritoneal lavage with 5 mL warm Dulbecco’s Modified Eagle’s Medium (ATCC) followed by filtration through a 70 µm cell strainer. Cells were centrifuged at 800 × g for 3 min then resuspended in Dulbecco’s Modified Eagle’s Medium with 10% FBS, 10mM Hepes, 2mM L-glutamine, 1% penicillin/streptomycin prior to plating and overnight incubation at 37°C in 5% CO2. Cells were washed twice with Dulbecco’s PBS to remove non-adherent cells. Macrophages were collected in Dulbecco’s PBS via scraping and enumerated using the TC 20 Automated Cell Counter (Bio-Rad). Macrophages (1.0 × 104 cells in 100 µL) were administered to LAC intoxicated mice by subcutaneous injection adjacent to the site of intoxication. For phagocytosis inhibition, adherent cells were incubated with 10 µM cytochalasin B or saline control for 1 hour at 37°C + 5% CO2, and washed with Dulbecco’s PBS before injection into mice.
Statistical analysis
Statistical analyses were performed using Prism 6.0 software (Graph Pad Software, Inc., La Jolla, CA). In vitro data were analyzed by Student’s t test and in vivo results by the Mann-Whitney U test for non-parametric data.
Results
CD36 limits dermonecrosis during S. aureus SSSI
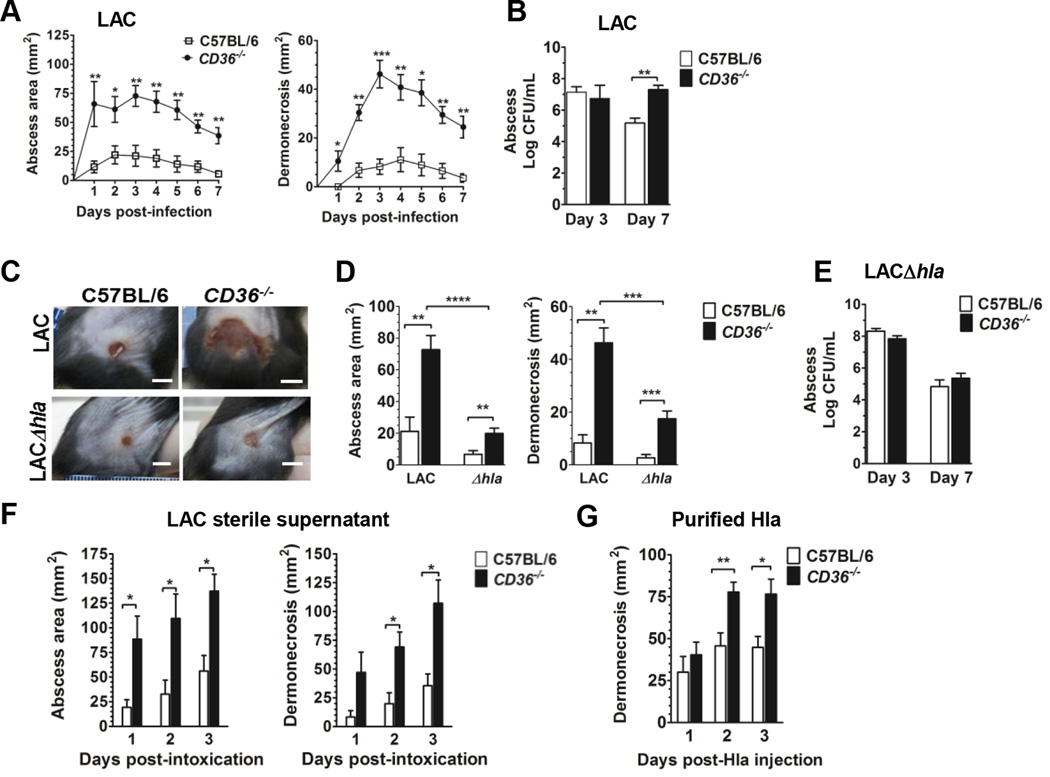
To determine whether CD36 contributes to limiting pathogenesis during S. aureus SSSI, we infected C57BL/6 (wild-type) and CD36−/− mice by subcutaneous injection with the CA-MRSA USA300 isolate LAC. Compared to wild-type mice, CD36−/− mice had significantly larger abscesses and increased dermonecrosis on days 1–7 post-infection (Fig. 1A). Importantly, at the apex of dermonecrosis on day three post-infection, there was no difference in local bacterial burden between CD36−/− and wild-type mice (Fig. 1B), suggesting that the dermonecrotic phenotype at this early time point was independent of bacterial burden. However, by day 7 post-infection, CD36−/− mice showed increased bacterial burden at the site of infection compared to wild-type mice, consistent with the previously demonstrated role of CD36 in S. aureus phagocytosis (20). Therefore, these data suggest that CD36 plays a protective role against S. aureus dermonecrosis and, at early time points post-infection, this protection is independent of its role in bacterial clearance.
FIGURE 1.
CD36−/− mice have increased dermonecrosis in response to S. aureus infection and alpha hemolysin. (a) Area of abscess (left), dermonecrosis (right) and (b) bacterial burden of mice subcutaneously infected with LAC (8 × 106 CFU). N=8 mice per group from two independent experiments. (c) Representative images of infection sites taken on day 3 post-infection (LAC, 8 × 106 CFU; LACΔhla 1.1 × 107 CFU) (scale bar = 5 mm). (D) Abscess area and dermonecrosis on day 3 post-infection with LAC or LACΔhla. N=8 (LAC) and 20 (LACΔhla) mice per group from two and four independent experiments, respectively. (E) Bacterial burden at the site of infection 3 and 7 days post-infection with LACΔhla (1.1 × 107 CFU). N=8 mice per group from two independent experiments. (F) Mice were intoxicated by subcutaneous injection with LAC sterile supernatant (18 hour culture). Area of abscess and dermonecrosis were measured daily. N=6 mice per group. (G) Area of dermonecrosis of mice injected subcutaneously with 1 µg of purified Hla. N=8 mice per group from two independent experiments. Data shown as mean + SEM. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001.
CD36 is a negative regulator of alpha-hemolysin mediated dermonecrosis
Hla is a secreted, pore-forming toxin which is a major contributor to dermonecrosis during S. aureus SSSI (3–6). To determine whether Hla is necessary for increased dermonecrosis in the absence of CD36, we infected wild-type and CD36−/− mice with a LAC isogenic hla-deletion mutant (LACΔhla) (31). At the same inoculum used for LAC (8 × 106 CFU), mice infected with LACΔhla failed to develop dermonecrosis, consistent with previous reports (data not shown) (4, 32). However, at a higher inoculum (1.1 ×107 CFU), LACΔhla infected CD36−/− mice showed small, but increased abscess formation and dermonecrosis on day three post-infection compared to wild-type mice (Fig. 1C, D). Notably, both measures were significantly reduced compared to CD36−/− mice infected with LAC, demonstrating that Hla is a major contributor to the dermonecrotic phenotype in the knockout mice. Furthermore, mice infected with a LAC isogenic agr deletion mutant (LACΔagr) (1.1 ×107 CFU) did not develop dermonecrosis (33–36) (data not shown), supporting the role of other agr-regulated factors in dermonecrosis (33, 34, 37). Importantly, in contrast to infection with LAC, there was no significant difference in day seven bacterial burden in mice infected with LACΔhla (1.1 ×107 CFU) (Fig. 1E). This demonstrates that CD36 is important for host clearance of Hla-expressing S. aureus at later time points during SSSI. Together, these data demonstrate that while CD36 largely affords protection against Hla-mediated dermonecrosis, its protection can extend to dermonecrosis caused by other agr-regulated virulence factors.
To confirm that the role of CD36 in limiting Hla-mediated dermonecrosis at early time points is independent of its contribution to bacterial clearance and, based on the results above, also independent of any potential contribution to agr regulation (38), we intoxicated mice by subcutaneous injection of sterile filtered supernatant from overnight cultures (39) of LAC or LACΔhla. Whereas LACΔhla intoxicated mice did not develop dermonecrosis (Supplemental Fig. 1A), CD36−/− mice intoxicated with LAC supernatant showed significantly increased abscess size and dermonecrosis (Fig. 1F), compared to wild-type controls. Furthermore, CD36−/− mice subcutaneously injected with purified recombinant Hla (Fig. 1G) also showed significantly increased dermonecrosis compared to wild-type mice, the timing of which was indistinguishable from abscess formation. As expected, mice injected with the purified inactive HlaH35L mutant (40) did not develop dermonecrosis (data not shown). Together, these results confirm that CD36 is a negative regulator of Hla-mediated dermonecrosis, independent of its role in phagocytosis and bacterial clearance.
CD36 limits inflammatory cytokine production associated with dermonecrosis
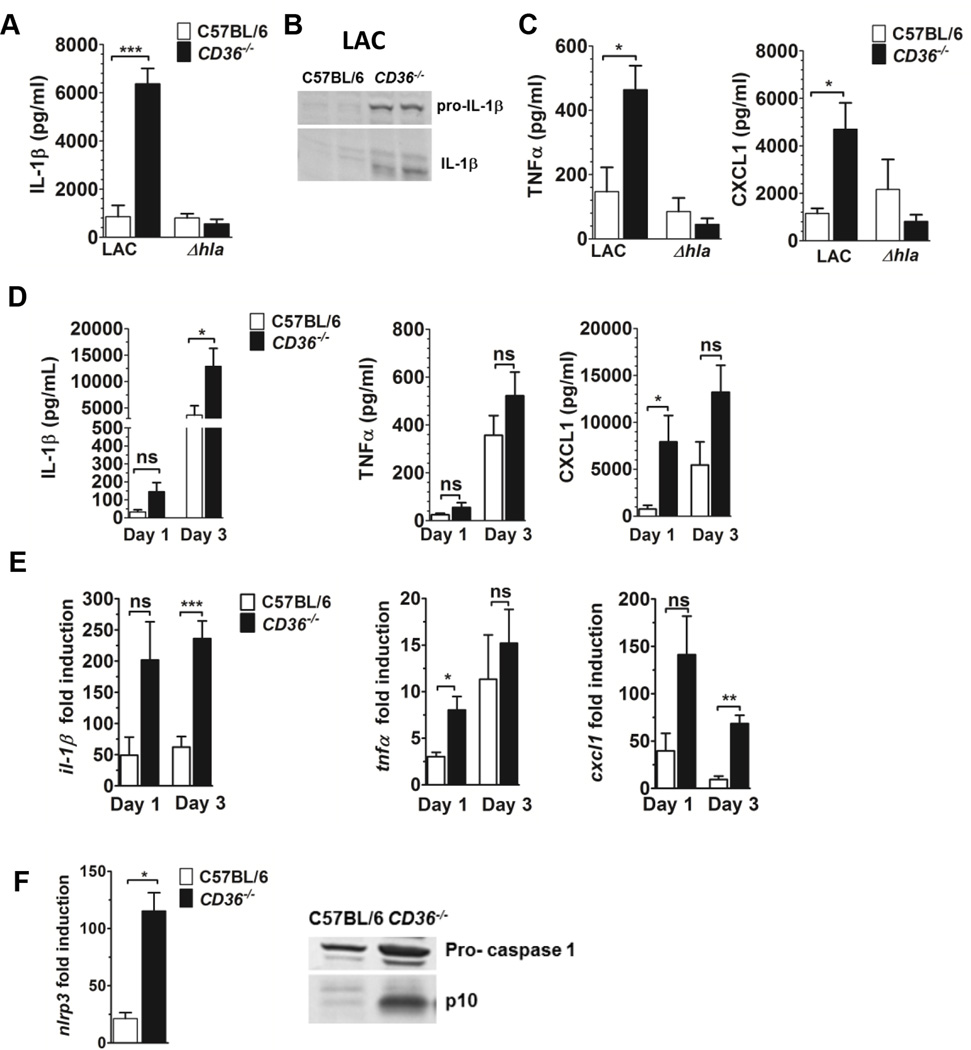
Given that Hla contributes to activation of the NLRP3 inflammasome and production of IL-1β (41–43), we predicted that the increased dermonecrosis in LAC infected CD36−/− mice would be associated with increased local levels of IL-1β compared to wild-type controls. As expected, CD36−/− mice infected with LAC, but not with LACΔhla, showed significantly increased local pro- and active IL-1β on day 3 post-infection (Fig. 2A,B) compared to wild-type mice (~ increase 7.4-fold). In addition, both TNFα and CXCL1 were increased at the site of infection in LAC, but not LACΔhla, infected CD36−/− mice compared to controls (Fig. 2C). These results suggest that CD36 regulates inflammatory cytokine expression in the skin of S. aureus infected mice in a Hla-dependent manner.
FIGURE 2.
CD36−/− mice have increased local pro-inflammatory cytokine production and NLRP3 inflammasome activation in response to S. aureus secreted virulence factors. (A–C) Mice were subcutaneously infected with LAC (8 × 106 CFU) or LACΔhla (1.1 × 107 CFU). The following were measured in clarified abscess tissue homogenate on day 3 post-infection: (A) IL-1β, (B) Western blot for pro- and active-IL-1β. (C) TNFα and CXCL1 expression. N=4–5 mice per group. (D–F) Mice were intoxicated by subcutaneous injection with LAC sterile supernatant (18 hour culture) and the following measured in injection site tissue homogenate on days 1 and 3 post-intoxication:(D) Cytokine expression and (E) il-1b, tnfα and cxcl1 transcription. (F) nlrp3 transcription and caspase-1 expression and activation (Western blot), in LAC intoxicated mice on day 3 post-intoxication. N=4–8 mice per group. Data reported as mean ± SEM. ns, not significant; *, p<0.05; **, p<0.01; ***, p<0.001.
To confirm that CD36-mediated regulation of inflammatory cytokine production is also independent of bacterial burden, we measured cytokines in the skin of mice on days 1 and 3 following intoxication with LAC sterile supernatant. As expected, local IL-1β TNFα and CXCL1 levels increased for both mouse groups between days 1 and 3 post-intoxication. LAC intoxicated CD36−/− mice showed significantly increased local IL-1β expression on day 3 post-intoxication compared to wild-type controls (~ increase 3.5-fold), while TNFα and CXCL1 levels trended higher in the knockout mice with differences in day 1 CXCL1 levels reaching statistical significance (Fig. 2D). Additionally, local transcription of these cytokines was higher in LAC intoxicated CD36−/− versus wild-type mice, with significant increases in il-1β and cxcl1 transcription on day 3, and in tnfα transcription on day 1, post-intoxication (Fig. 2E). Importantly, consistent with the lack of differential IL-1β production in LACΔhla infected mice (Fig. 2A), CD36−/− mice intoxicated with LACΔhla supernatant showed significantly less local il-1β transcription on day 1 post-intoxication compared to CD36−/− mice intoxicated with LAC supernatant (Supplemental Fig. 1B). Together, these data demonstrate that, independent of bacterial burden, CD36 regulates IL-1β expression in the skin in response to Hla within the S. aureus virulence secretome.
Since local IL-1β levels were elevated in both LAC infected and supernatant intoxicated CD36−/− mice, we postulated that these mice would also show increased local transcription of components of the NLRP3 inflammasome, including nlrp3, asc and caspase-1 (reviewed in (44)). As predicted, CD36−/− mice subcutaneously intoxicated with LAC supernatant showed significantly increased local nlrp3 transcription on day 3 post-intoxication compared to wild-type controls (Fig. 2F). Furthermore, while there was no difference in local transcript levels of asc or capase-1 (data not shown), there was increased active caspase-1 (p10) in the skin of LAC intoxicated CD36−/− mice compared to controls (Fig. 2F), indicating increased local NLRP3 inflammasome activation in the absence of CD36.
CD36 regulates neutrophil accumulation driven by S. aureus secreted virulence factors
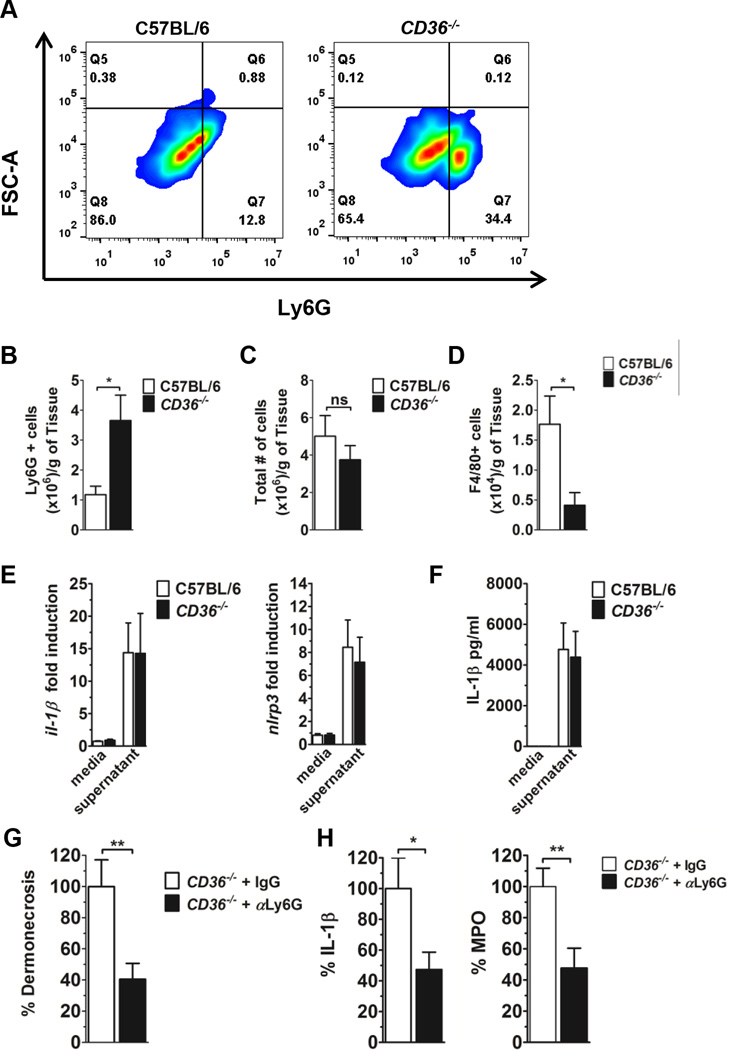
Neutrophil-derived IL-1β is sufficient for abscess formation during S. aureus SSSI, and neutrophils have been shown to produce IL-1β in response to Hla (45). Therefore, we predicted that the increased dermonecrosis and IL-1β production in LAC supernatant-intoxicated CD36−/− mice would be associated with increased local neutrophil accumulation. To address this, we subcutaneously intoxicated mice with LAC supernatant and prepared single cell suspensions of injection site tissue on day 1 post-intoxication. As predicted, CD36−/− mice had significantly increased neutrophil (Ly6G+) accumulation at the site of intoxication compared to wild-type mice (Fig. 3A,B). Furthermore, although the total number of cells present did not differ, CD36−/− mice had fewer macrophages (F4/80+) at the site of intoxication, compared to controls (Fig. 3C,D). Importantly, to our knowledge, no deficiencies in macrophage levels in CD36−/− mice have been reported. Therefore, these data suggest a role for CD36 in regulating immune cell accumulation in the skin in response to S. aureus secreted virulence factors.
FIGURE 3.
CD36−/− mice have increased local neutrophil accumulation compared to wild-type mice following LAC supernatant injection. Flow cytometry analysis of single cells suspensions from injection site tissue on day 1 post-LAC intoxication of C57BL6 and CD36−/− mice. (A,B) Presence of Ly6G+ cells, (C) total cell count and (D) F4/80+ cells at the site of intoxication. N=6 mice per group from two independent experiments. (E) il-1b and nlrp3 transcription and (F) IL-1β expression by bone marrow derived neutrophils in response to 1 h incubation with LAC sterile supernatant or media control. N=6 from two independent experiments. (G) Day 1 dermonecrosis, (H) along with MPO and local IL-1β levels, from supernatant intoxicated CD36−/− mice following treatment with anti-Ly6G or isotype control. Values are expressed as percent of isotype control. N=12–15 mice/group from four independent experiments. Data are mean ± SEM. ns, not significant; *, p<0.05; **, p<0.01.
To begin to address the mechanism of increased dermonecrosis, immune cell accumulation and IL-1β production in the skin of LAC intoxicated CD36−/− mice, we first asked whether neutrophils from CD36−/− mice produced increased IL-1β in response to Hla, compared to neutrophils from wild-type controls. Using flow cytometry, we were unable to detect CD36 on neutrophils isolated from wild-type mice (data not shown), suggesting that the presence or absence of this protein on neutrophils themselves did not contribute to the dermonecrotic phenotype in CD36−/− mice. In addition, bone marrow-derived neutrophils from wild-type and CD36−/− mice showed similar il-1β and nlrp3 transcription, and IL-1β production, upon exposure to LAC supernatant (Fig. 3E,F). Therefore, these results point to a mechanism whereby CD36 negatively regulates dermonecrosis and IL-1β production in the skin by controlling neutrophil accumulation in response to S. aureus secreted virulence factors, rather than by directly affecting the neutrophil response to Hla.
If neutrophil accumulation at the site of S. aureus intoxication is responsible for the increased dermonecrosis in CD36−/− mice, then depletion of neutrophils prior to intoxication should prevent the enhanced dermonecrotic phenotype. As expected, on day 1 post-intoxication, CD36−/− mice treated with antibody targeting Ly6G to deplete neutrophils, showed significantly reduced dermonecrosis, local IL-1β production and local myeloperoxidase (MPO) levels, a surrogate for neutrophil accumulation (45), compared to isotype treated controls (Fig. 3G). Together, these data demonstrate that CD36 protects against neutrophil-dependent skin injury induced by S. aureus secreted virulence factors.
We next asked whether increased dermonecrosis and neutrophil accumulation in CD36−/− mice resulted from increased neutrophil influx. Maximum neutrophil accumulation in the developing lesion has been reported to occur at six hours following S. aureus infection (39, 46). Therefore, we assessed local MPO levels, as an indicator of neutrophil accumulation, along with IL-1β, TNFα and CXCL1 levels at six hours following LAC infection of wild-type and CD36−/− mice. At this time point, there was no significant difference in MPO, IL-1β, TNFα or CXCL1 expression at the site intoxication between the two mouse groups (Supplement Fig. 2), suggesting that differences in neutrophil accumulation in the absence of CD36 do not result from increased neutrophil influx early post-infection.
Macrophage CD36 is sufficient to limit dermonecrosis in CD36−/− mice
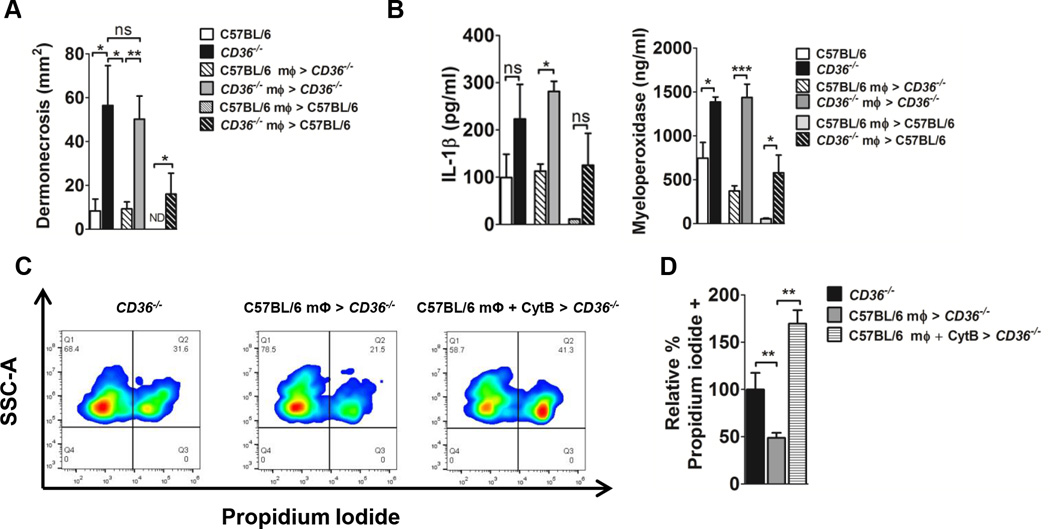
CD36 on macrophages facilitates clearance of apoptotic neutrophils, thus limiting tissue damage which follows loss of membrane integrity in dying cells and the subsequent release of noxious cell contents (14, 22–25). If CD36 on macrophages is necessary to limit tissue damage caused by S. aureus secreted virulence factors, then administration of macrophages from wild-type (CD36+/+) mice, but not from CD36−/− mice, should prevent the excessive dermonecrotic phenotype in CD36−/− mice following S. aureus intoxication. As expected, compared to untreated CD36−/− mice, local subcutaneous administration of wild-type macrophages to CD36−/− mice four hours post-intoxication was sufficient to significantly reduce day one dermonecrosis, whereas administration of macrophages from CD36−/− mice had no effect (Fig. 4A). Administration of CD36+/+ macrophages to CD36−/− mice also reduced local IL-1β and myeloperoxidase (MPO) levels (Fig. 4B). Furthermore, administration of CD36+/+, but not CD36−/−, macrophages to wild-type mice prevented dermonecrosis, with further reductions in local IL-1β and MPO levels (Fig. 4A,B). Therefore, the ability to prevent excessive dermonecrosis in the CD36−/− mice by administration of CD36+/+, but not the same number of CD36−/− macrophages, suggests that the protective mechanism of action is dependent on the presence of CD36 on the macrophages after reaching the site of inflammation.
FIGURE 4.
CD36+/+ macrophages negatively regulate the dermonecrotic phenotype of LAC intoxicated CD36−/− mice. Mice were intoxicated by subcutaneous injection with LAC sterile supernatant. 4 h later, mice were either left untreated or peritoneal macrophages were injected adjacent to the site of intoxication. (A) Area of dermonecrosis and local (B) IL-1β and MPO levels measured day 1 post-intoxication. Data reported as mean± SEM. N=4–6 mice per group from two independent experiments. (C,D) Membrane integrity of Ly6G+ cells from intoxication site skin sections measured by flow cytometry as percent propidium iodide uptake relative to CD36−/− controls. N=3–4 mice/group. ns, not significant; ND, not detected; *, p<0.05; **, p<0.01; ***, p<0.001.
To determine whether inhibition of dermonecrosis by administration of CD36+/+ macrophages is associated with a local reduction in dying neutrophils, we used propidium iodide staining (47) to assess the loss of membrane integrity in neutrophils isolated from the site of intoxication. As expected, neutrophils isolated from the site of intoxication of CD36−/− mice showed reduced propidium iodide (PI) staining following CD36+/+ macrophage treatment compared to untreated controls (Fig. 4C,D). Furthermore the reduction in PI uptake was reversed by pre-incubating macrophages with cytochalasin to inhibit phagocytosis, consistent with the previously described role of CD36 in macrophage phagocytosis and clearance of apoptotic neutrophils (22–25). Together, these data demonstrate that CD36 expression on macrophages is necessary to limit skin injury associated with inappropriate neutrophil accumulation in response S. aureus secreted virulence factors, and suggest that the mechanism of action is via CD36-mediated phagocytosis and clearance of apoptotic neutrophils.
Discussion
Host innate defense against S. aureus skin infections is a highly orchestrated process aimed at clearing the infection and minimizing tissue damage. If left unchecked, however, the innate immune response to S. aureus infection, and to virulence factors such as Hla, can itself contribute to tissue injury. Here we report that CD36, a scavenger receptor that facilitates macrophage clearance of apoptotic neutrophils (22–25), plays a major role in regulating Hla-mediated skin injury. In the absence of CD36, neutrophils recruited to the skin, in large part by Hla (16), can accumulate, become necrotic and ultimately cause tissue damage likely due to the release of their toxic cellular contents. Importantly, the administration of CD36-bearing macrophages is sufficient to limit dermonecrosis in mice lacking CD36, and to prevent dermonecrosis in wild-type mice. CD36+/+ macrophage administration significantly reduces necrotic neutrophil accumulation at the site of intoxication, a benefit which is negated by obstructing macrophage actin polymerization. Therefore, these data support a mechanism of action whereby CD36-mediated macrophage phagocytosis of apoptotic neutrophils plays a significant role in host regulation of Hla-mediated dermonecrosis. Although further research is needed, these results may suggest that therapies targeted at local upregulation of CD36 on macrophages could prove efficacious in ameliorating S. aureus-mediated skin injury.
The formation of neutrophil-rich abscesses is considered a host defense mechanism for preventing bacterial dissemination and pathogenesis in response to S. aureus skin infection. However, this innate neutrophil response must be tightly controlled to limit self-damage to host tissues. The potential damage resulting from unregulated neutrophil accumulation is suggested by the following: (i) neutrophils produce IL-1β to propagate their own recruitment (45, 48, 49), (ii) they are short-lived and subject to lysis by S. aureus-secreted virulence factors (13, 50, 51), and (iii) necrotic neutrophils release toxic substances, such as proteases and elastase (52), as well as DAMPS (danger-associated molecular patterns) such as High-mobility group box 1 protein (HMGB1) (53), which all contribute to tissue damage. Here we report that CD36 contributes to regulation of the host innate response to virulence factors secreted by S. aureus by limiting accumulation of dead or dying neutrophils during SSSI. It is important to note that, in addition to having more neutrophils at the site of inflammation compared to controls, S. aureus intoxicated CD36−/− mice also had fewer macrophages, suggesting a potential role for CD36 in macrophage accumulation in this model. However, local administration of CD36+/+, but not an equivalent number of CD36−/− macrophages, prevented the enhanced dermonecrotic phenotype of CD36−/− mice. Therefore, the primary role of CD36 in regulating the host innate response in this model is in preventing excessive neutrophil accumulation once macrophages are at the site of infection or intoxication.
CD36 is a multifunctional protein with numerous and diverse ligands, co-receptors and functions (17, 18). Whether the downstream effects of CD36 signaling are pro- or anti-inflammatory depend on the nature of the ligand and the accompanying co-receptors. CD36 was first classified as a scavenger receptor and best studied for its role in sterile inflammation and atherosclerosis. In conjunction with TLR4 and TLR6, CD36-mediated uptake of oxidized low density lipoprotein (oxLDL) results in the formation of intracellular cholesterol crystals, which drive activation of the NLRP3 inflammasome and secretion of the pro-inflammatory cytokine IL-1β (54, 55). This in turn leads to foam cell formation and atherosclerosis (reviewed in (18)). CD36 also contributes to the host inflammatory response to Gram-positive pathogens. In this respect, CD36 mediates production of reactive oxygen species in response to Propionibacterium acnes (56), phagocytosis of S. aureus (20), and, together with TLR2, recognition of S. aureus cell wall components (21). Importantly, each of these functions results in a pro-inflammatory cytokine response (20, 21, 56). In contrast, CD36 suppresses early pro-inflammatory cytokine production by both macrophages and epithelial cells in response to lipotechoic acid-phosphocholine complexes from Streptococcus pneumoniae (57). Our findings extend the known anti-inflammatory contributions of CD36 to protection against Hla-mediated S. aureus dermonecrosis, and suggest that therapeutic modulation of CD36 expression during skin infection could both enhance bacterial clearance (20) and limit tissue damage caused by the host response to S. aureus virulence factors.
Supplementary Material
Acknowledgments
We thank Dr. Hattie Gresham for helpful discussions and critical insight, and Dr. Matthew Campen, Dr. Seth Daly and Kathleen Triplett for critical review of the manuscript. We also thank Dr. Joseph Duncan for important reagents, and Dr. Bradley Elmore, Dr. Julie Hutt and Brett Manifold-Wheeler for technical support.
This work was supported by National Institutes of Health, National Institute of Allergy and Infectious Diseases grant to P.R.H. (RO1AI091917) and corresponding training supplement to M.J.C.
Footnotes
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA, Group EMINS. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 2.Talan DA, Krishnadasan A, Gorwitz RJ, Fosheim GE, Limbago B, Albrecht V, Moran GJ, Group EMINS. Comparison of Staphylococcus aureus from skin and soft-tissue infections in US emergency department patients, 2004 and 2008. Clin. Infect. Dis. 2011;53:144–149. doi: 10.1093/cid/cir308. [DOI] [PubMed] [Google Scholar]
- 3.Inoshima N, Wang Y, Bubeck Wardenburg J. Genetic requirement for ADAM10 in severe Staphylococcus aureus skin infection. J. Invest. Dermatol. 2012;132:1513–1516. doi: 10.1038/jid.2011.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy AD, Bubeck Wardenburg J, Gardner DJ, Long D, Whitney AR, Braughton KR, Schneewind O, DeLeo FR. Targeting of alpha-hemolysin by active or passive immunization decreases severity of USA300 skin infection in a mouse model. J Infect Dis. 2010;202:1050–1058. doi: 10.1086/656043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sampedro GR, DeDent AC, Becker RE, Berube BJ, Gebhardt MJ, Cao H, Bubeck Wardenburg J. Targeting Staphylococcus aureus alpha-toxin as a novel approach to reduce severity of recurrent skin and soft-tissue infections. J Infect Dis. 2014;210:1012–1018. doi: 10.1093/infdis/jiu223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kobayashi SD, Malachowa N, Whitney AR, Braughton KR, Gardner DJ, Long D, Bubeck Wardenburg J, Schneewind O, Otto M, Deleo FR. Comparative analysis of USA300 virulence determinants in a rabbit model of skin and soft tissue infection. J Infect Dis. 2011;204:937–941. doi: 10.1093/infdis/jir441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tkaczyk C, Hamilton MM, Datta V, Yang XP, Hilliard JJ, Stephens GL, Sadowska A, Hua L, O’Day T, Suzich J, Stover CK, Sellman BR. Staphylococcus aureus alpha toxin suppresses effective innate and adaptive immune responses in a murine dermonecrosis model. PLoS One. 2013;8:e75103. doi: 10.1371/journal.pone.0075103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Becker RE, Berube BJ, Sampedro GR, DeDent AC, Bubeck Wardenburg J. Tissue-specific patterning of host innate immune responses by Staphylococcus aureus alpha-toxin. J. Innate Immun. 2014;6:619–631. doi: 10.1159/000360006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berube BJ, Wardenburg JB. Staphylococcus aureus alpha-Toxin: Nearly a Century of Intrigue. Toxins (Basel) 2013;5:1140–1166. doi: 10.3390/toxins5061140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inoshima I, Inoshima N, Wilke GA, Powers ME, Frank KM, Wang Y, Wardenburg JB. A Staphylococcus aureus pore-forming toxin subverts the activity of ADAM10 to cause lethal infection in mice. Nat. Med. 2011;17:1310–1314. doi: 10.1038/nm.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powers ME, Kim HK, Wang Y, Wardenburg JB. ADAM10 mediates vascular injury induced by Staphylococcus aureus alpha-hemolysin. J Infect Dis. 2012;206:352–356. doi: 10.1093/infdis/jis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilke GA, Wardenburg JB. Role of a disintegrin and metalloprotease 10 in Staphylococcus aureus alpha-hemolysin-mediated cellular injury. Proc. Natl. Acad. Sci. U. S. A. 2010;107:13473–13478. doi: 10.1073/pnas.1001815107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigby KM, DeLeo FR. Neutrophils in innate host defense against Staphylococcus aureus infections. Semin. Immunopathol. 2012;34:237–259. doi: 10.1007/s00281-011-0295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiss SJ. Tissue destruction by neutrophils. N. Engl. J. Med. 1989;320:365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- 15.Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 16.Bartlett AH, Foster TJ, Hayashida A, Park PW. Alpha-toxin facilitates the generation of CXC chemokine gradients and stimulates neutrophil homing in Staphylococcus aureus pneumonia. J Infect Dis. 2008;198:1529–1535. doi: 10.1086/592758. [DOI] [PubMed] [Google Scholar]
- 17.Silverstein RL, Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Science signaling. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp. Mol. Med. 2014;46:e99. doi: 10.1038/emm.2014.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collot-Teixeira S, Martin J, McDermott-Roe C, Poston R, McGregor JL. CD36 and macrophages in atherosclerosis. Cardiovasc. Res. 2007;75:468–477. doi: 10.1016/j.cardiores.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 20.Stuart LM, Deng J, Silver JM, Takahashi K, Tseng AA, Hennessy EJ, Ezekowitz RA, Moore KJ. Response to Staphylococcus aureus requires CD36-mediated phagocytosis triggered by the COOH-terminal cytoplasmic domain. J. Cell Biol. 2005;170:477–485. doi: 10.1083/jcb.200501113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, Crozat K, Sovath S, Shamel L, Hartung T, Zahringer U, Beutler B. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 22.Navazo MD, Daviet L, Savill J, Ren Y, Leung LL, McGregor JL. Identification of a domain (155–183) on CD36 implicated in the phagocytosis of apoptotic neutrophils. J Biol Chem. 1996;271:15381–15385. doi: 10.1074/jbc.271.26.15381. [DOI] [PubMed] [Google Scholar]
- 23.Ren Y, Silverstein RL, Allen J, Savill J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J. Exp. Med. 1995;181:1857–1862. doi: 10.1084/jem.181.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Savill J, Hogg N, Haslett C. Macrophage vitronectin receptor, CD36, and thrombospondin cooperate in recognition of neutrophils undergoing programmed cell death. Chest. 1991;99:6S–7S. doi: 10.1378/chest.99.3_supplement.6s-a. [DOI] [PubMed] [Google Scholar]
- 25.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 1992;90:1513–1522. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rothfork JM, Timmins GS, Harris MN, Chen X, Lusis AJ, Otto M, Cheung AL, Gresham HD. Inactivation of a bacterial virulence pheromone by phagocyte-derived oxidants: new role for the NADPH oxidase in host defense. Proc. Natl. Acad. Sci. U. S. A. 2004;101:13867–13872. doi: 10.1073/pnas.0402996101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malachowa N, Kobayashi SD, Braughton KR, DeLeo FR. Mouse model of Staphylococcus aureus skin infection. Methods Mol. Biol. 2013;1031:109–116. doi: 10.1007/978-1-62703-481-4_14. [DOI] [PubMed] [Google Scholar]
- 28.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daley JM, Thomay AA, Connolly MD, Reichner JS, Albina JE. Use of Ly6G–specific monoclonal antibody to deplete neutrophils in mice. J. Leukoc. Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 30.Bernheimer AW. Assay of hemolytic toxins. Methods Enzymol. 1988;165:213–217. doi: 10.1016/s0076-6879(88)65033-6. [DOI] [PubMed] [Google Scholar]
- 31.Bubeck Wardenburg J, Bae T, Otto M, Deleo FR, Schneewind O. Poring over pores: alpha-hemolysin and Panton-Valentine leukocidin in Staphylococcus aureus pneumonia. Nat. Med. 2007;13:1405–1406. doi: 10.1038/nm1207-1405. [DOI] [PubMed] [Google Scholar]
- 32.Berube BJ, Sampedro GR, Otto M, Wardenburg JB. The psmalpha Locus Regulates Production of Staphylococcus aureus Alpha-Toxin during Infection. Infect. Immun. 2014;82:3350–3358. doi: 10.1128/IAI.00089-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montgomery CP, Boyle-Vavra S, Daum RS. Importance of the global regulators Agr and SaeRS in the pathogenesis of CA-MRSA USA300 infection. PLoS One. 2010;5:e15177. doi: 10.1371/journal.pone.0015177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheung GY, Wang R, Khan BA, Sturdevant DE, Otto M. Role of the accessory gene regulator agr in community-associated methicillin-resistant Staphylococcus aureus pathogenesis. Infect. Immun. 2011;79:1927–1935. doi: 10.1128/IAI.00046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sully EK, Malachowa N, Elmore BO, Alexander SM, Femling JK, Gray BM, DeLeo FR, Otto M, Cheung AL, Edwards BS, Sklar LA, Horswill AR, Hall PR, Gresham HD. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog. 2014;10:e1004174. doi: 10.1371/journal.ppat.1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Daly SM, Elmore BO, Kavanaugh JS, Triplett KD, Figueroa M, Raja HA, El-Elimat T, Crosby HA, Femling JK, Cech NB, Horswill AR, Oberlies NH, Hall PR. omega-Hydroxyemodin Limits Staphylococcus aureus Quorum Sensing-Mediated Pathogenesis and Inflammation. Antimicrob. Agents Chemother. 2015;59:2223–2235. doi: 10.1128/AAC.04564-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R, Braughton KR, Kretschmer D, Bach THL, Queck SY, Li M, Kennedy AD, Dorward DW, Klebanoff SJ, Peschel A, Deleo FR, Otto M. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007;13:1510–1514. doi: 10.1038/nm1656. [DOI] [PubMed] [Google Scholar]
- 38.Peterson MM, Mack JL, Hall PR, Alsup AA, Alexander SM, Sully EK, Sawires YS, Cheung AL, Otto M, Gresham HD. Apolipoprotein B Is an innate barrier against invasive Staphylococcus aureus infection. Cell Host Microbe. 2008;4:555–566. doi: 10.1016/j.chom.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright JS, 3rd, Jin R, Novick RP. Transient interference with staphylococcal quorum sensing blocks abscess formation. Proc. Natl. Acad. Sci. U. S. A. 2005;102:1691–1696. doi: 10.1073/pnas.0407661102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jursch R, Hildebrand A, Hobom G, Tranum-Jensen J, Ward R, Kehoe M, Bhakdi S. Histidine residues near the N terminus of staphylococcal alpha-toxin as reporters of regions that are critical for oligomerization and pore formation. Infect. Immun. 1994;62:2249–2256. doi: 10.1128/iai.62.6.2249-2256.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bubeck Wardenburg J, Schneewind O. Vaccine protection against Staphylococcus aureus pneumonia. J. Exp. Med. 2008;205:287–294. doi: 10.1084/jem.20072208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munoz-Planillo R, Franchi L, Miller LS, Nunez G. A critical role for hemolysins and bacterial lipoproteins in Staphylococcus aureus-induced activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3942–3948. doi: 10.4049/jimmunol.0900729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Craven RR, Gao X, Allen IC, Gris D, Bubeck Wardenburg J, McElvania-Tekippe E, Ting JP, Duncan JA. Staphylococcus aureus alpha-hemolysin activates the NLRP3-inflammasome in human and mouse monocytic cells. PLoS One. 2009;4:e7446. doi: 10.1371/journal.pone.0007446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sutterwala FS, Haasken S, Cassel SL. Mechanism of NLRP3 inflammasome activation. Ann. N. Y. Acad. Sci. 2014;1319:82–95. doi: 10.1111/nyas.12458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cho JS, Guo Y, Ramos RI, Hebroni F, Plaisier SB, Xuan C, Granick JL, Matsushima H, Takashima A, Iwakura Y, Cheung AL, Cheng G, Lee DJ, Simon SI, Miller LS. Neutrophil-derived IL-1beta is sufficient for abscess formation in immunity against Staphylococcus aureus in mice. PLoS Path. 2012;8 doi: 10.1371/journal.ppat.1003047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ford CW, Hamel JC, Stapert D, Yancey RJ. Establishment of an experimental model of a Staphylococcus aureus abscess in mice by use of dextran and gelatin microcarriers. J. Med. Microbiol. 1989;28:259–266. doi: 10.1099/00222615-28-4-259. [DOI] [PubMed] [Google Scholar]
- 47.Nygaard TK, Pallister KB, DuMont AL, DeWald M, Watkins RL, Pallister EQ, Malone C, Griffith S, Horswill AR, Torres VJ, Voyich JM. Alpha-toxin induces programmed cell death of human T cells, B cells, and monocytes during USA300 infection. PLoS One. 2012;7:e36532. doi: 10.1371/journal.pone.0036532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller LS, O’Connell RM, Gutierrez MA, Pietras EM, Shahangian A, Gross CE, Thirumala A, Cheung AL, Cheng G, Modlin RL. MyD88 mediates neutrophil recruitment initiated by IL-1R but not TLR2 activation in immunity against Staphylococcus aureus. Immunity. 2006;24:79–91. doi: 10.1016/j.immuni.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 49.Miller LS, Pietras EM, Uricchio LH, Hirano K, Rao S, Lin H, O’Connell RM, Iwakura Y, Cheung AL, Cheng G, Modlin RL. Inflammasome-mediated production of IL-1beta is required for neutrophil recruitment against Staphylococcus aureus in vivo. J Immunol. 2007;179:6933–6942. doi: 10.4049/jimmunol.179.10.6933. [DOI] [PubMed] [Google Scholar]
- 50.Alonzo F, 3rd, Torres VJ. Bacterial survival amidst an immune onslaught: the contribution of the Staphylococcus aureus leukotoxins. PLoS Pathog. 2013;9:e1003143. doi: 10.1371/journal.ppat.1003143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Surewaard BG, Haas CJd, Vervoort F, Rigby KM, DeLeo FR, Otto M, Strijp JAv, Nijland R. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell. Microbiol. 2013;15:1427–1437. doi: 10.1111/cmi.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henson PM, Johnston RB., Jr Tissue injury in inflammation. Oxidants, proteinases, and cationic proteins. J. Clin. Invest. 1987;79:669–674. doi: 10.1172/JCI112869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raucci A, Palumbo R, Bianchi ME. HMGB1: a signal of necrosis. Autoimmunity. 2007;40:285–289. doi: 10.1080/08916930701356978. [DOI] [PubMed] [Google Scholar]
- 54.Sheedy FJ, Grebe A, Rayner KJ, Kalantari P, Ramkhelawon B, Carpenter SB, Becker CE, Ediriweera HN, Mullick AE, Golenbock DT, Stuart LM, Latz E, Fitzgerald KA, Moore KJ. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013 doi: 10.1038/ni.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stewart CR, Stuart LM, Wilkinson K, Gils JMv, Deng J, Halle A, Rayner KJ, Boyer L, Zhong R, Frazier WA, Lacy-Hulbert A, Khoury JE, Golenbock DT, Moore KJ. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat. Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grange PA, Chereau C, Raingeaud J, Nicco C, Weill B, Dupin N, Batteux F. Production of superoxide anions by keratinocytes initiates P. acnes-induced inflammation of the skin. PLoS Pathog. 2009;5:e1000527. doi: 10.1371/journal.ppat.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharif O, Matt U, Saluzzo S, Lakovits K, Haslinger I, Furtner T, Doninger B, Knapp S. The scavenger receptor CD36 downmodulates the early inflammatory response while enhancing bacterial phagocytosis during pneumococcal pneumonia. J Immunol. 2013;190:5640–5648. doi: 10.4049/jimmunol.1202270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.