Abstract
Background
Cue-induced methamphetamine craving increases after prolonged forced (experimenter-imposed) abstinence from the drug (incubation of methamphetamine craving). Here, we determined whether this incubation phenomenon would occur under conditions that promote voluntary (self-imposed) abstinence. We also determined the effect of the novel mGluR2 positive allosteric modulator, AZD8529, on incubation of methamphetamine craving after forced or voluntary abstinence.
Methods
We trained rats to self-administer palatable food (6 sessions) and then to self-administer methamphetamine under two conditions: 12 sessions (9-hr/day) or 50 sessions (3-hr/day). We then assessed cue-induced methamphetamine seeking in extinctions test after 1 or 21 abstinence days. Between tests, the rats underwent either forced abstinence (no access to the food- or drug-paired levers) or voluntary abstinence for 19 days (achieved via a discrete choice procedure between methamphetamine and palatable food; 20 trials per day). We also determined the effect of subcutaneous injections of AZD8529 (20 and 40 mg/kg) on cue-induced methamphetamine seeking 1 or 21 days after forced or voluntary abstinence.
Results
Under both training and abstinence conditions, cue-induced methamphetamine seeking in the extinction tests was higher after 21 abstinence days than after 1 day (incubation of methamphetamine craving). AZD8529 decreased cue-induced methamphetamine seeking on day 21 but not day 1 of forced or voluntary abstinence.
Conclusions
We introduce a novel animal model to study incubation of drug craving and cue-induced drug seeking after prolonged voluntary abstinence, mimicking the human condition of relapse after successful contingency management treatment. Our data suggest that PAMs of mGluR2 should be considered for relapse prevention.
Keywords: psychostimulants, palatable food, discrete choice, self-administration, extended access, incubation of drug craving, abstinence, relapse, addiction models, mGluR2/3, positive allosteric modulator, glutamate
Introduction
Methamphetamine addiction is characterized by high relapse rates (1–3). Human drug relapse is often precipitated by exposure to drug-associated cues that provoke drug craving (4), now a formal criterion in the DSM-V (5). On the basis of clinical observations, Gawin and Kleber (6) proposed that cue-induced cocaine craving progressively increases during early abstinence and remains elevated for extended time periods. An analogous phenomenon, termed ‘incubation of drug craving’ has been observed in rats trained to self-administer different drugs of abuse (7–11), including methamphetamine (12). In rodent models, incubation of drug craving refers to the time-dependent increases in cue-induced drug seeking during abstinence (13). Subsequent studies showed that incubation of drug craving also occurs in humans addicted to nicotine, alcohol, and methamphetamine (14–16). These findings provide support for the translational potential of using rodent incubation models to identify pharmacological targets for relapse prevention.
The abstinence period preceding relapse testing in studies using animal models is typically experimenter-imposed or forced; this is achieved either by removal of the laboratory animal from the drug self-administration environment or through extinction training (17, 18). However, in humans, abstinence is often voluntary (self-imposed) with drug available in the addict’s environment but forgone in favor of other non-addictive alternative rewards (19, 20). Current animal models of relapse after voluntary abstinence rely on punishment-induced suppression of drug intake (21–26). These models, as well as other relapse/reinstatement models (27, 28), do not capture the choice-based suppression of drug intake due to the presence of alternative non-drug rewards more typical of addicts (29, 30). Indeed, evidence from contingency management studies in humans, show that the availability of non-drug rewards (e.g., monetary vouchers), given in exchange for ‘clean’ urine samples, can maintain abstinence for many months (31, 32). Other drug addiction treatment strategies are also derived from operant learning principles, including community reinforcement approach (33) and behavioral self-control training (34). Importantly, when contingency management discontinues, most addicts relapse to drug use (3, 35).
Based on these considerations and results from recent studies showing that when given a mutually exclusive choice between cocaine or methamphetamine and palatable foods, most rats prefer the non-drug rewards over these drugs (36–39), we introduce here a rat model of incubation of drug craving after prolonged voluntary abstinence. Voluntary abstinence is achieved using a mutually exclusive discrete-choice procedure in which the alternative reward is palatable food (37–39).
In the present study, we trained rats to self-administer palatable food pellets and then trained them to self-administer methamphetamine using two established procedures to model addiction: extended daily access drug self-administration procedure (40, 41) and a variation of a long-term training procedure used to identify addicted rats based on DSM-IV criteria (42, 43), the latter, a procedure that has not been previously used in incubation of drug craving studies. Subsequently, we exposed the rats to 3 weeks of voluntary abstinence using our recently established mutually exclusive choice procedure in which most rats strongly prefer palatable food over methamphetamine (39). We then assessed cue-induced methamphetamine seeking in extinction tests performed on abstinence days 1 and 21. For comparison purposes, we assessed incubation of methamphetamine craving after forced (experimenter-imposed) abstinence, the established incubation of craving model (44).
A second goal of our study was to pharmacologically characterize incubation of methamphetamine craving after voluntary (and forced) abstinence. For this purpose, we used AZD8529, a new and highly selective positive allosteric modulator (PAM) of metabotropic glutamate receptors 2 (mGluR2) (45) that was recently shown to inhibit cue- and drug-induced reinstatement of nicotine seeking in squirrel monkeys (46). mGluR2 PAMs bind to an allosteric site of the receptor and selectively activate these receptors in the presence of glutamate (47, 48). mGluR2s are expressed primarily on presynaptic glutamate neurons and their activation by PAMs or agonists like LY379268 decreases evoked glutamate release (49). However, from a medication development perspective, there are limitations with LY379268 and related mGluR2/3 orthosteric agonists (see Discussion) that have led to the development of selective mGluR2 PAMs like AZD8529 (50, 51).
Materials and Methods
See supplement 1 for details on subjects, drugs, intravenous surgery, apparatus, procedures, abstinence phase, extinction tests, and statistical analyses.
Specific experiments
Exp. 1: Incubation of methamphetamine craving after short-term extended daily access (9 hr/session; 12 sessions): comparison between forced and voluntary abstinence (Fig. 1)
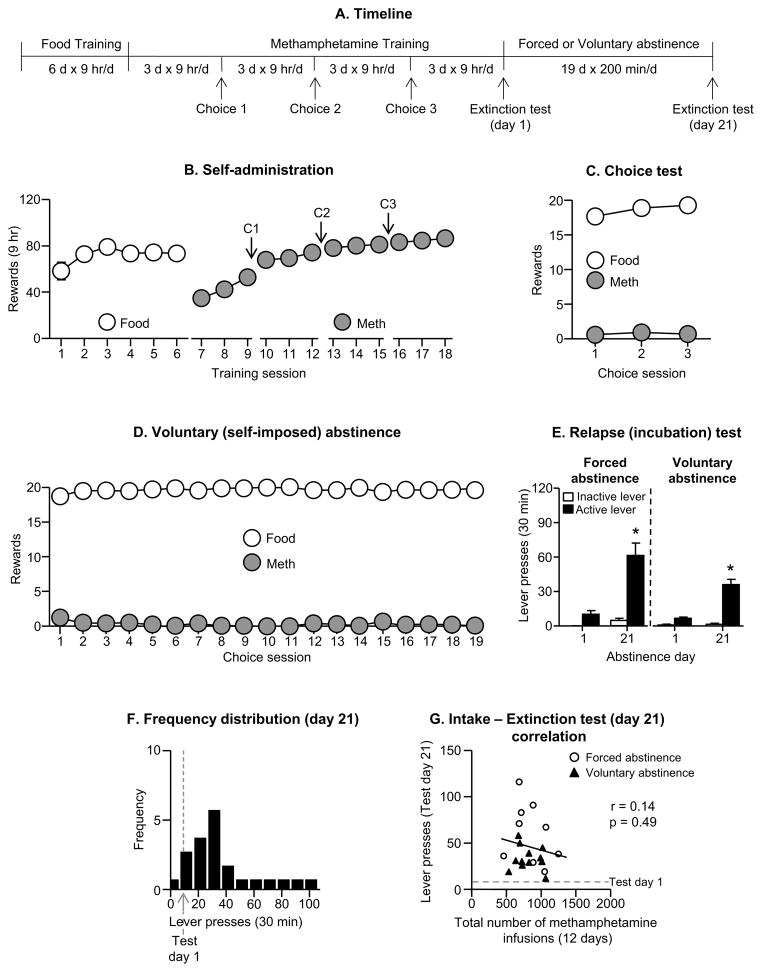
Figure 1. Incubation of methamphetamine craving after short-term extended daily access (9 hr/session, 12 sessions).
(A) Timeline of the experiment. (B) Self-administration: Mean±SEM number of food rewards (5 palatable food pellets/reward delivery) or methamphetamine infusions (0.1 mg/kg/infusion) during the 9-hr sessions. (C) Discrete choice tests: Mean±SEM of food reward and methamphetamine infusions earned during the 3 discrete choice sessions that were performed during training (20 trials every 10 min). (D) Voluntary abstinence: Mean±SEM of food reward and methamphetamine infusions earned during the 19 discrete choice sessions (20 trials every 10 min). (E) Relapse (extinction) tests: Mean±SEM of lever presses on the previously active lever and on the inactive lever during the 30 min extinction sessions. * Different from day 1 within each abstinence condition, p<0.01 (n=9–12 per group). (F) Frequency distribution: Frequency distribution of active lever presses over 30 min extinction test. The abscissa scale was divided into 10 bins, each spanning 10 units. (G) Extinction test correlation: A dimensional analysis based on the sample tested (n=21) shows lack of correlation between the total number of methamphetamine infusions earned during the training sessions and the total number of lever presses during the extinction test on abstinence day 21. The dashed line in grey refers to the number of active lever presses on abstinence day 1.
We compared incubation of methamphetamine craving after forced (the established model (7, 52)) and voluntary abstinence under extended daily access training conditions (22, 53). We used 2 groups of rats (n=9–12 per group) in a mixed experimental design that included the between-subjects factor of Abstinence Condition (forced, voluntary) and the within-subjects factor of Abstinence Day (1, 21). The experiment consisted of 3 phases: training phase, discrete choice tests, and the relapse test.
Training
We first trained the rats (n=21) to self-administer palatable food pellets (6 sessions, 9 hr/session; 5 pellets per reward delivery) and then trained them to self-administer methamphetamine (9 hr/session; 12 sessions; 0.1 mg/kg/infusion per reward delivery).
Discrete choice tests
We determined food versus methamphetamine choice after every three consecutive drug self-administration sessions in both groups (3 choice tests) and for 19 days in the voluntary abstinence group.
Relapse (incubation) test
We tested the forced and voluntary abstinence rats for cue-induced methamphetamine seeking under extinction conditions on abstinence days 1 and 21. The extinction test sessions were 30 min in order to minimize carry-over effect and extinction learning/experience during day 1 testing, which may decrease incubated cue-induced drug seeking during day 21 testing.
Exp. 2: Incubation of methamphetamine craving after a long-term limited daily access (3 hr/session; 50 sessions): comparison between forced and voluntary abstinence (Fig. 2)
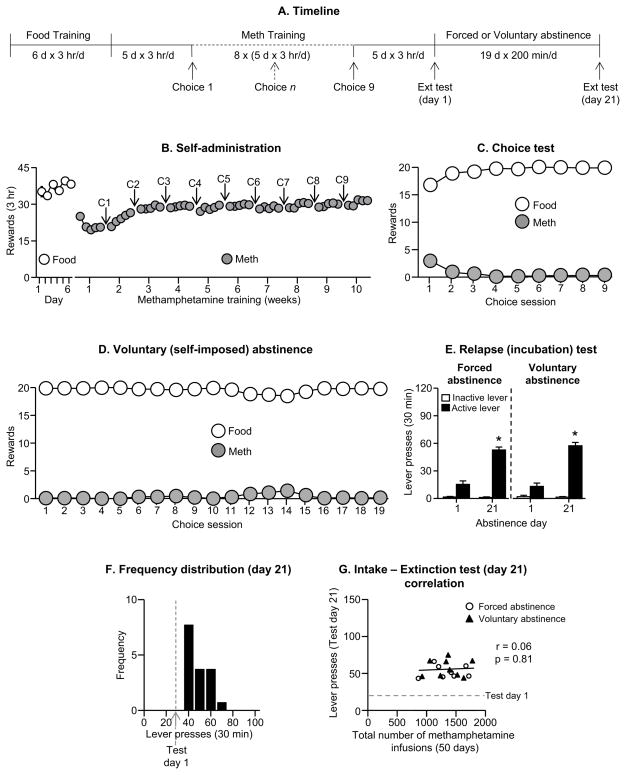
Figure 2. Incubation of methamphetamine craving after long-term limited daily access (3 hr/day, 50 sessions).
(A) Timeline of the experiment. (B) Self-administration: Mean±SEM number of food rewards or methamphetamine infusions during the 3-hr sessions. (C) Discrete choice tests: Mean±SEM of food reward and methamphetamine infusions earned during the 9 discrete choice sessions that were performed during training. (D) Voluntary abstinence: Mean±SEM of food reward and methamphetamine infusions earned during the 19 discrete choice sessions. (E) Relapse (extinction) tests: Mean±SEM of lever presses on the previously active lever and on the inactive lever during the 30 min extinction sessions. * Different from day 1 within each abstinence condition, p<0.01 for (n=8–9 per group). (F) Frequency distribution: Frequency distribution of active lever presses over 30 min extinction test. (G) Extinction test correlation: A dimensional analysis based on the sample tested (n=17) shows lack of correlation between the total number of methamphetamine infusions earned during the training sessions and the total number of lever presses during the extinction test on abstinence day 21. The dashed line in grey refers to the number of active lever presses on abstinence day 1.
We compared incubation of methamphetamine craving after forced or voluntary abstinence following a drug training procedure (50 sessions for 3 hr/day) that is based on the procedure used in the addiction model of Deroche-Gamonet, Belin, and Piazza (42, 43, 54). We used 2 groups of rats (n=8–9 per group) in a mixed experimental design that included the between-subjects factor of Abstinence Condition (forced, voluntary) and the within-subjects factor of Abstinence Day (1, 21). The experiment consisted of 3 phases: training phase, discrete choice tests, and the relapse test.
Training
We first trained the rats (n=17) to self-administer palatable food pellets (6 days, 3 hr/day; 5 pellets per reward delivery) and then trained them to self-administer methamphetamine (0.1 mg/kg/infusion) for 3 hr/day for 50 days (5 training days per week).
Discrete choice tests
We determined food versus methamphetamine choice in both groups after every five consecutive methamphetamine self-administration sessions (9 choice tests) and during 19 days for the voluntary abstinence group. The day after each choice day during training, we gave the rats a rest day before the next weekly cycle of 5 methamphetamine self-administration training days, 1 choice day, and 1 rest day.
Relapse test
We tested the forced and voluntary abstinence groups for cue-induced methamphetamine seeking under extinction conditions (30 min test session) on abstinence days 1 and 21.
Exp. 3: Effect of AZD8529 on incubation of methamphetamine craving after forced or voluntary abstinence (Fig. 3)
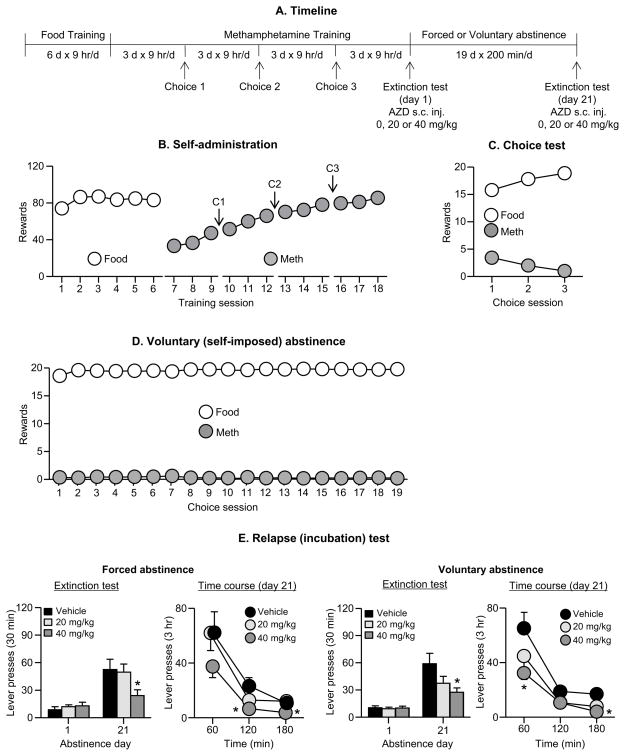
Figure 3. Effect of AZD8529 on incubation of methamphetamine craving after forced or voluntary abstinence.
(A) Timeline of the experiment. (B) Self-administration: Mean±SEM number of food rewards or methamphetamine infusions during the 9-hr sessions (12 sessions). (C) Discrete choice tests: Mean±SEM of food rewards and methamphetamine infusions earned during the 3 discrete choice sessions that were performed during training. (D) Voluntary abstinence: Mean±SEM of food reward and methamphetamine infusions earned during the 19 discrete choice sessions. (E) Relapse (extinction) tests: Left side shows the data from the forced abstinence group and the right side from the voluntary abstinence group. For each condition, the left column shows active lever-presses during the 30 min extinction test on abstinence day 1 and the first 30 min of the 3-hr extinction test on day 21. The right column shows the time course of the 3-hr extinction test on abstinence day 21. Data are mean±SEM of lever presses on the previously active lever during the extinction sessions. We injected vehicle or AZD8529 (20 or 40 mg/kg, s.c) 3 hr before the test sessions. * Different from day 1 within each abstinence condition, p<0.01; forced abstinence, n=8–9 per dose; voluntary abstinence, n=14–16 per dose.
We determined the effect of systemic injections of vehicle or AZD8529 (20 or 40 mg/kg, s.c) on incubation of methamphetamine craving. We used 6 groups of rats (forced Abstinence: n=8–9; voluntary Abstinence: n=14–16) in a mixed experimental design that included the between-subjects factor of Abstinence Condition (forced, voluntary), AZD8529 Dose (0,20, 40 mg/kg), and the within-subjects factor of Abstinence Day (1, 21). The experiment consisted of three phases: training phase, discrete choice tests, and the relapse test.
Training
We first trained the rats to self-administer palatable food pellets (6 days, 9 hr/day; 5 pellets per reward delivery) and then trained them to self-administer methamphetamine (9 hr/session; 12 sessions; 0.1 mg/kg/infusion per reward delivery).
Discrete choice tests
We determined food versus methamphetamine choice in both groups after every 3 consecutive drug self-administration sessions (3 choice tests) and during 19 days for the voluntary abstinence group. We habituated the rats to the injection procedure for 2 days during this phase.
Relapse test
We tested the forced and voluntary abstinence rats for cue-induced methamphetamine seeking under extinction conditions on abstinence days 1 and 21. The duration of the test session on day 1 was 30 min and on day 21 was 3 hr. We used a longer test session on day 21 in order to obtain more information on the behavioral effect of AZD8529 (3-hr pretreatment time) on ‘incubated’ cue-induced methamphetamine seeking. As mentioned above, the duration of the test session on day 1 was 30 min to minimize carry-over effect and extinction learning/experience during day 1 testing, which may decrease incubated cue-induced drug seeking during day 21 testing.
Exp. 4. Effect of AZD8529 on high rate food-reinforced responding
The goal of Exp. 4 was to rule out that the effect of AZD8529 on cue-induced drug seeking on abstinence day 21 was due to motor deficits. For this purpose, we retrained rats (n=23) previously used in Exp. 3 to self-administer food pellets for 1-hr per day for 2 days. During the subsequent test session, we injected different groups of rats with vehicle, 20, or 40 mg/kg of AZD8529 (n=7–8 per group) 3 hr prior to the session and measured pellet intake and lever presses during the test session.
Results
Exp. 1: Incubation of methamphetamine craving after short-term extended daily access (9 hr/session; 12 sessions): comparison between forced and voluntary abstinence (Fig. 1A)
Food and methamphetamine training
The rats increased their food and methamphetamine intake over sessions (Fig. 1B), and as in our previous study, extended access (9 hr/d) of methamphetamine led to strong escalation of drug intake (26, 53, 55). The repeated measures ANOVA showed a significant effect of Session for both food [F(5,100)=3.5, p<0.01] and methamphetamine [F(11,220)=37.5, p<0.001]. During the 3 discrete choice sessions, the rats showed a strong preference for the food (p<0.01, Fig. 1C).
Abstinence phase
During the 3-week abstinence period, the rats in the voluntary abstinence group showed a strong preference for food (p<0.01), resulting in either no or minimal (1–2 infusions per day) methamphetamine intake (Fig. 1D).
Relapse (extinction) tests
Cue-induced methamphetamine seeking in the extinction tests was higher after 21 abstinence days than after 1 day, demonstrating ‘incubation of methamphetamine craving’ after both forced and voluntary abstinence (Fig. 1E). The statistical analysis of active lever-presses included the between-subjects factor of Abstinence Condition (forced, voluntary), the within-subjects factor of Abstinence Day (1, 21), and inactive lever-presses as a covariate. This analysis showed a significant main effect of Abstinence Day (F(1,17)=17.5, p<0.01) but not Abstinence Condition or an interaction between the two factors (p values>0.1).
Correlations and frequency distribution
We examined the correlation (Pearson r) between the total number of methamphetamine infusions earned during the training sessions and the total number of lever presses during the extinction tests on abstinence day 21. We found no correlations between these two measures (r=0.14, p>0.1) (Fig. 1G). In Fig. 1F we show the frequency distribution of extinction responding during testing on abstinence day 21 in reference to the mean of day 1 extinction responding.
Exp. 2: Incubation of methamphetamine craving after a long-term limited daily access (3 hr/session; 50 sessions): comparison between forced and voluntary abstinence (Fig. 2A)
Food and methamphetamine training
The number of food rewards earned remained stable during the 6 food training sessions (p>0.05 for Session effect). The rats increased the number of methamphetamine rewards earned over during the first 10 training days (Fig. 2B), as indicated by a significant effect of Session [F(49,784)=18.1, p<0.01]. During the 9 discrete choice sessions, the rats showed a strong preference for the food (p<0.01, Fig. 2C).
Abstinence phase
During the 3-week abstinence period, the rats in the voluntary abstinence condition, showed a strong preference for food, resulting in either no or minimal methamphetamine intake (p<0.01, Fig. 2D).
Relapse (extinction) tests
Cue-induced methamphetamine seeking in the extinction tests was higher after 21 abstinence days than after 1 day after both forced and voluntary abstinence (Fig. 2E). The statistical analysis of active lever presses included the between-subjects factor of Abstinence Condition, the within-subjects factor of Abstinence Day, and inactive lever-presses as a covariate. This analysis showed a significant main effect of Abstinence Day (F(1,13)=73.9, p<0.01) but not Abstinence Condition or an interaction between the two factors (p values>0.1).
Correlations and frequency distribution
We examined the correlation (Pearson r) between the total number of methamphetamine infusions earned during the training sessions and the total number of lever presses during the extinction tests on abstinence day 21. We found no correlations between these two measures (r=0.06, p>0.1) (Fig. 2G). In Fig. 2F we show the frequency distribution of extinction responding during testing on abstinence day 21 in reference to the mean of day 1 extinction responding.
Exp. 3: Effect of AZD8529 on incubation of methamphetamine craving after forced or voluntary abstinence (Fig. 3A)
Food and methamphetamine training
The rats modestly increased their food intake and as in Exp. 1, strongly escalated their methamphetamine intake over days (Fig. 3B). The repeated measures ANOVA showed a significant effect of Session for both food [F(5,355)=7.3, p<0.01] and methamphetamine [F(11,781)=215.6, p<0.01]. During the 3 discrete choice tests, the rats showed a strong preference for the food (p<0.01, Fig. 3C).
Abstinence phase
During the 3-week abstinence period, the rats in the voluntary abstinence condition showed a strong preference for food (p<0.01), resulting in either no or minimal methamphetamine intake (Fig. 3D).
Relapse (extinction) tests
Systemic injections of AZD8528 dose-dependently decreased cue-induced methamphetamine seeking in the extinction tests on abstinence day 21 but not day 1; this effect occurred after either forced or voluntary abstinence (Fig. 3E). The statistical analysis of the 30 min test data (the test duration on day 1) included the between-subjects factors of Abstinence Condition and AZD8529 Dose, the within-subjects factor of Abstinence Day, and inactive lever-presses as a covariate. This analysis showed a significant interaction between Abstinence Day and AZD8529 Dose [F(2,64)=4.7, p<0.01], reflecting the selective effect of AZD8529 cue-induced methamphetamine seeking during late but not early abstinence. We also analyzed the data from the entire 3-hr day 21 test-session using the factors of Abstinence Condition, AZD8529 Dose, and Session Hour. This analysis showed significant effects of AZD8529 Dose [F(2,66)=6.5, p<0.01] and Session Hour [F(2,132)=90.4, p<0.01] but no significant effects of Abstinence Condition or interactions between the 3 factors (p>0.1). These statistical results indicate that AZD8529 decreased the response to the methamphetamine cues during the extinction tests, but had no effect on within-session extinction learning (Fig. 3E). Additional post-hoc analyses showed that the effect of AZD8529 on lever responding on day 21 extinction test was significant at the 40 mg/kg but not the 20 mg/kg dose (see figures).
Exp. 4. Effect of AZD8529 on high rate food-reinforced responding
The goal of Exp. 4 was to rule out that the effect of AZD8529 on the cue-induced drug seeking on abstinence day 21 is due to motor deficits or some other non-specific performance impairments. We retrained rats (n=23) previously used in Exp. 3 to self-administer the palatable food pellets and determined the effect AZD8529 (0, 20 or 40 mg/kg, n=7–8 per dose) on ongoing food-reinforced responding. We found that systemic AZD8529 injections had no effect on food self-administration (Fig. 4).
Figure 4. Effect of AZD8529 on food self-administration.
Mean±SEM number of food rewards, and active and inactive lever-presses during the 1-hr test sessions. We injected vehicle or AZD8529 (20 or 40 mg/kg, s.c) 3 hr before the test sessions.
Discussion
There are two main findings in our study. First, time-dependent increases in cue-induced methamphetamine seeking (incubation of methamphetamine craving) were reliably observed after prolonged periods of choice-based voluntary abstinence. This effect was observed under two different self-administration procedures that are widely used to model drug addiction: extended daily access drug self-administration procedure (40, 41) and a long-term training procedure used to identify addicted rats based on DSM-IV criteria (42, 43, 54). Second, the mGluR2 PAM AZD8529 decreased ‘incubated’ cue-induced methamphetamine seeking after prolonged voluntary or forced abstinence but had no effect on either ‘non-incubated’ drug seeking during early abstinence or food-reinforced responding. The latter finding indicates that AZD8529 effects on cue-induced methamphetamine seeking are not due to motor deficits or some other non-specific side effects. Finally, our results indicate that incubation of methamphetamine craving after voluntary abstinence is as robust as incubation after forced abstinence.
Implications for animal models of drug addiction and relapse
The goal of our study was to develop a rat model to study incubation of drug craving that occurs after long-term voluntary abstinence. Our model was inspired in part by the recent findings of ‘resurgence’ (reinstatement) of cocaine or alcohol seeking after extinction when an alternative food reward is removed (56, 57). In the operant conditioning paradigm, ‘resurgence’ refers to the recovery of an extinguished operant response after discontinuation of reinforcement of an alternative response (58–60).
We have developed our animal model, because in current animal models of relapse abstinence is experimenter-imposed (or forced) either via extinction training or by removing the rat from the self-administration chambers (17, 18, 27, 28). Additionally, recent animal models of relapse that do rely on voluntary abstinence are based on punishment-induced suppression of drug intake (21–26), an aspect of drug addiction that is not captured in our choice-based relapse model.
These existing relapse/reinstatement models deviate from the human condition where abstinence is often voluntary due to the availability of alternative non-drug rewards (19, 20). This is exemplified in the contingency management treatment procedure where the availability of non-drug rewards, given in exchange for ‘clean’ urine samples, can maintain abstinence for many months (31, 32, 61). However, when contingency management discontinues, most addicts relapse to drug use (3, 35). We propose that incubation of cue-induced drug seeking after an alternative reward choice-based voluntary abstinence in our rat model is analogous to the human condition of relapse to drug use after termination of long-term contingency management treatment. Our model also mimics to some degree relapse that occurs in more natural settings when former addicts lose important alternative non-drug rewards that maintains abstinence (a steady job, social relationships, etc).
A question that derives from our study is whether similar or different neurobiological mechanisms control incubation of methamphetamine craving after voluntary versus forced abstinence. Potential evidence for mechanistic differences is the lower magnitude of ‘incubated’ cue-induced drug seeking in the voluntary abstinence condition than in the forced abstinence condition in Exp. 1 (Fig. 1E). However, this effect was not observed in Exp. 2 under different training conditions (Fig. 2E) or in Exp. 3 under the same training conditions of Exp. 1 (Fig. 3E, vehicle groups in the voluntary and forced abstinence conditions). Additionally, AZD8529 had a similar effect on ‘incubated’ cue-induced methamphetamine seeking after voluntary and forced abstinence, suggesting mechanistic similarities. Regardless, the absence of mechanistic differences in our studies using neuropharmacological manipulations does not rule out the possibility of unique brain areas and circuits mediating incubation of drug craving after voluntary versus forced abstinence.
Finally, the results of our study may also have implications for animal models of addiction. Specifically, drug addiction is often characterized by reduced behavioral responding for non-drug rewards or diminished control over behavior by non-drug rewards (5, 62, 63). This aspect of human drug addiction was not observed in our study in which we used two established procedures to model addiction: extended daily access drug self-administration procedure (40, 41) and long-term training procedure used to identify addicted rats based on DSM-IV criteria (42, 43).
In agreement with our recent study (39) and previous observations of Ahmed and colleagues with cocaine (38, 64), the rats in our study strongly preferred the palatable food over methamphetamine after extended access (9 hr/d) methamphetamine self-administration. We also found no evidence for a shift in food-methamphetamine choice during the extended drug self-administration training (50 sessions) in which some cocaine-trained rats develop ‘addiction-like’ behavior, as assessed by progressive ratio responding, resistance to shock-induced suppression of drug-reinforced responding, and high non-reinforced responding (43, 54). However, in our study we did not explicitly monitor the emergence of these behavioral measures and our sample size was relatively small (n=17). A question for future research, which will require a significantly larger sample, is whether a switch in food-drug preference would emerge in 15–20% of the rats who develop the addiction phenotype in the long-term training addiction model (43, 54).
Effect of AZD8529 on incubation of methamphetamine craving
In our initial pharmacological characterization of incubation of methamphetamine craving after voluntary abstinence we found that the novel mGuR2 PAM AZD8529 decreased this incubation, as well as incubation of methamphetamine craving after forced abstinence. AZD8529’s effect on cue-induced methamphetamine seeking was only observed on abstinence day 21 but not day 1, suggesting a selective effect on ‘incubated’ cue-induced drug seeking. However, this selective time-dependent effect should be interpreted with caution because of a potential floor effect due to low responding on day 1.
Our data extend the recent finding that AZD8529 decreases nicotine self-administration and cue- and drug-induced reinstatement of nicotine seeking in squirrel monkeys (46). There is also evidence that another mGluR2 PAM (BINA) decreases cocaine self-administration and cue-induced reinstatement in rats (65).
These results are in agreement with the literature on the effect of Group II mGluR agonists on the behavioral effects of drugs in different animal models of addiction (66, 67). Group II mGluRs comprises of two Gi-coupled receptor subtypes: mGluR2 and mGluR3 (49). As mentioned in the Introduction, mGluR2s are expressed primarily on presynaptic glutamate neurons and their activation leads to decreased evoked glutamate release (49, 68). mGluR3s are expressed primarily postsynaptic neurons and on glia and their physiological function is unknown (49, 69). The prototype drug used to study the function of Group II mGluRs is LY379268, which binds to both mGluR2 and mGluR3 (49, 70). LY379268 and related compounds decrease reinstatement induced by re-exposure to discrete, discriminative, or contextual cues that were previously associated with alcohol (71), heroin (72, 73), cocaine (74, 75), and methamphetamine (76) self-administration. LY379268 also decreases cocaine priming-induced reinstatement in squirrel monkeys (77) and nicotine and methamphetamine self-administration in rats (78, 79). We also found that systemic and central amygdala LY379268 injections decrease incubation of cocaine craving after forced abstinence (80). Based on our recent findings on the role of central amygdala in incubation of methamphetamine craving after forced abstinence (53), we speculate that mGluR2 in this brain area also play a role in AZD8529’s effect on incubation of methamphetamine craving. Additionally, our data are in agreement with recent results demonstrating that inhibition of glutamate transmission in corticostriatal circuits by mGluR1 PAMs (81) or optogenetic manipulations (81–83) decreases incubation of cocaine craving.
Finally, as discussed by Justinova et al. (46), from a medication development perspective, AZD8529 appears to have a more favorable profile than LY379268. This is because LY379268 exhibits low bioavailability (84), tolerance can develop to its behavioral effects (78), and it potently activates the mGluR3 subtype whose physiological function is unknown (49). In this regard, in squirrel monkeys, Justinova et al., (46) did not observe any tolerance to the effects of AZD8529 on nicotine self-administration or reinstatement after repeated injections of drug doses that are well tolerated in human studies (45), making the compound a potential suitable candidate for relapse prevention.
Concluding remarks
We introduce a novel and reliable animal model to study incubation of drug craving and cue-induced drug seeking after voluntary abstinence, potentially mimicking the human condition of relapse after successful contingency management or other conditions that promote alternative reward choice-based abstinence. Our data further suggest that PAMs of mGluR2 should be considered for relapse prevention in psychostimulant users. Finally, our results raise two important questions for future research. First, as previous studies demonstrate that the mechanisms of opiate- and psychostimulant reward and relapse/reinstatement are not the same (27, 85–89), one question is whether incubation of drug craving after voluntary abstinence would also occur in rats with a history of opiate self-administration. The second question is whether similar or different mechanisms control incubation of drug craving after forced versus volitional abstinence. Our initial results with AZD8529 suggest mechanistic similarities but additional research will be required to resolve this question.
Supplementary Material
Acknowledgments
Research was supported by the National Institute on Drug Abuse, Intramural Research Program funds (YS and GS). N.J.M. received support from Early Career Fellowship 1053308 by the National Health and Medical Research Council. We thank Alan Cross (AstraZeneca) for providing AZD8529 and David Epstein and Kenzie Preston for helpful comments.
Footnotes
Author’s contributions: DC, MV, TZ, XL, SA, RM, NM, FL, JMB carried out the experiments, DC, MV, and YS performed data analysis. DC, GS, FL, and YS designed the study and wrote the manuscript with MV. All authors critically reviewed the content and approved the final version before submission.
All authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elkashef A, Vocci F, Hanson G, White J, Wickes W, Tiihonen J. Pharmacotherapy of methamphetamine addiction: an update. Subst Abus. 2008;29:31–49. doi: 10.1080/08897070802218554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rawson RA, Marinelli-Casey P, Anglin MD, Dickow A, Frazier Y, Gallagher C, et al. A multi-site comparison of psychosocial approaches for the treatment of methamphetamine dependence. Addiction. 2004;99:708–717. doi: 10.1111/j.1360-0443.2004.00707.x. [DOI] [PubMed] [Google Scholar]
- 3.Roll JM. Contingency management: an evidence-based component of methamphetamine use disorder treatments. Addiction. 2007;102(Suppl 1):114–120. doi: 10.1111/j.1360-0443.2006.01774.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans. Ann N Y Acad Sci. 1992;654:400–415. doi: 10.1111/j.1749-6632.1992.tb25984.x. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Dsm-5. American Psychiatric Pub Incorporated; 2013. [Google Scholar]
- 6.Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- 7.Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neisewander JL, Baker DA, Fuchs RA, Tran-Nguyen LT, Palmer A, Marshall JF. Fos protein expression and cocaine-seeking behavior in rats after exposure to a cocaine self-administration environment. J Neurosci. 2000;20:798–805. doi: 10.1523/JNEUROSCI.20-02-00798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shalev U, Morales M, Hope B, Yap J, Shaham Y. Time-dependent changes in extinction behavior and stress-induced reinstatement of drug seeking following withdrawal from heroin in rats. Psychopharmacology. 2001;156:98–107. doi: 10.1007/s002130100748. [DOI] [PubMed] [Google Scholar]
- 10.Bienkowski P, Rogowski A, Korkosz A, Mierzejewski P, Radwanska K, Kaczmarek L, et al. Time-dependent changes in alcohol-seeking behaviour during abstinence. Eur Neuropsychopharmacol. 2004;14:355–360. doi: 10.1016/j.euroneuro.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 11.Abdolahi A, Acosta G, Breslin FJ, Hemby SE, Lynch WJ. Incubation of nicotine seeking is associated with enhanced protein kinase A-regulated signaling of dopamine- and cAMP-regulated phosphoprotein of 32 kDa in the insular cortex. Eur J Neurosci. 2010;31:733–741. doi: 10.1111/j.1460-9568.2010.07114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shepard JD, Bossert JM, Liu SY, Shaham Y. The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry. 2004;55:1082–1089. doi: 10.1016/j.biopsych.2004.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- 14.Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, et al. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang G, Shi J, Chen N, Xu L, Li J, Li P, et al. Effects of length of abstinence on decision-making and craving in methamphetamine abusers. PLoS One. 2013;8:e68791. doi: 10.1371/journal.pone.0068791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li P, Wu P, Xin X, Fan YL, Wang GB, Wang F, et al. Incubation of alcohol craving during abstinence in patients with alcohol dependence. Addict Biol. 2014 doi: 10.1111/adb.12140. [DOI] [PubMed] [Google Scholar]
- 17.Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology (Berl) 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- 19.Marlatt AG. Models of relapse and relapse prevention: a commentary. Exp Clin Psychopharmacol. 1996;4:55–60. [Google Scholar]
- 20.Epstein DH, Preston KL. The reinstatement model and relapse prevention: a clinical perspective. Psychopharmacology. 2003;168:31–41. doi: 10.1007/s00213-003-1470-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Panlilio L, Thorndike E, Schindler C. Reinstatement of punishment-suppressed opioid self-administration in rats: an alternative model of relapse to drug abuse. Psychopharmacology. 2003;168:229–235. doi: 10.1007/s00213-002-1193-0. [DOI] [PubMed] [Google Scholar]
- 22.Cooper A, Barnea-Ygael N, Levy D, Shaham Y, Zangen A. A conflict rat model of cue-induced relapse to cocaine seeking. Psychopharmacology. 2007;194:117–125. doi: 10.1007/s00213-007-0827-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 24.Pelloux Y, Murray JE, Everitt BJ. Differential vulnerability to the punishment of cocaine related behaviours: effects of locus of punishment, cocaine taking history and alternative reinforcer availability. Psychopharmacology (Berl) 2014 doi: 10.1007/s00213-014-3648-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marchant NJ, Khuc TN, Pickens CL, Bonci A, Shaham Y. Context-induced relapse to alcohol seeking after punishment in a rat model. Biol Psychiatry. 2013;73:256–262. doi: 10.1016/j.biopsych.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krasnova IN, Marchant NJ, Ladenheim B, McCoy MT, Panlilio LV, Bossert JM, et al. Incubation of methamphetamine and palatable food craving after punishment-induced abstinence. Neuropsychopharmacology. 2014;39:2008–2016. doi: 10.1038/npp.2014.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bossert JM, Marchant NJ, Calu DJ, Shaham Y. The reinstatement model of drug relapse: recent neurobiological findings, emerging research topics, and translational research. Psychopharmacology (Berl) 2013;229:453–476. doi: 10.1007/s00213-013-3120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchant NJ, Li X, Shaham Y. Recent developments in animal models of drug relapse. Curr Opin Neurobiol. 2013;23:675–683. doi: 10.1016/j.conb.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ahmed SH. Validation crisis in animal models of drug addiction: beyond non-disordered drug use toward drug addiction. Neurosci Biobehav Rev. 2010;35:172–184. doi: 10.1016/j.neubiorev.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Banks ML, Negus SS. Preclinical Determinants of Drug Choice under Concurrent Schedules of Drug Self-Administration. Adv Pharmacol Sci. 2012;2012:281768. doi: 10.1155/2012/281768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Preston KL, Umbricht A, Epstein DH. Abstinence reinforcement maintenance contingency and one-year follow-up. Drug Alcohol Depend. 2002;67:125–137. doi: 10.1016/s0376-8716(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 32.Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu Rev Psychol. 2004;55:431–461. doi: 10.1146/annurev.psych.55.090902.142033. [DOI] [PubMed] [Google Scholar]
- 33.Azrin NH. Improvements in the community-reinforcement approach to alcoholism. Behav Res Ther. 1976;14:339–348. doi: 10.1016/0005-7967(76)90021-8. [DOI] [PubMed] [Google Scholar]
- 34.Hester RK, Delaney HD. Behavioral Self-Control Program for Windows: results of a controlled clinical trial. J Consult Clin Psychol. 1997;65:686–693. doi: 10.1037//0022-006x.65.4.686. [DOI] [PubMed] [Google Scholar]
- 35.Silverman K, DeFulio A, Sigurdsson SO. Maintenance of reinforcement to address the chronic nature of drug addiction. Prev Med. 2012;55(Suppl):S46–53. doi: 10.1016/j.ypmed.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, et al. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013;23:581–587. doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 39.Caprioli D, Zeric T, Thorndike E, Venniro M. Persistent palatable food preference in rats with a history of limited and extended access to methamphetamine self-administration. Addiction Biology. 2015 doi: 10.1111/adb.12220. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- 41.Ahmed SH. Escalation of drug use. Neuromethods. 2011;53:267–292. [Google Scholar]
- 42.Deroche-Gamonet V, Belin D, Piazza PV. Evidence for addiction-like behavior in the rat. Science. 2004;305:1014–1017. doi: 10.1126/science.1099020. [DOI] [PubMed] [Google Scholar]
- 43.Piazza PV, Deroche-Gamonet V. A multistep general theory of transition to addiction. Psychopharmacology (Berl) 2013;229:387–413. doi: 10.1007/s00213-013-3224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cross AJ. AZD8529 – an mGluR2 positive allosteric modulator for the treatment of schizophrenia. American College of Neuropsychopharmacology Annual Meeting; Hollywood, Florida. 2013. [Google Scholar]
- 46.Justionva Z, Panlilio LV, Secci ME, Redhi GH, Schindler CW, Cross AJ, et al. The novel mGluR2 positive allosteric modulator, AZD8529, decreases nicotine self-administration and relapse in squirrel monkeys. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2015.01.014. (accepted pending revisions) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaffhauser H, Rowe BA, Morales S, Chavez-Noriega LE, Yin R, Jachec C, et al. Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2. Mol Pharmacol. 2003;64:798–810. doi: 10.1124/mol.64.4.798. [DOI] [PubMed] [Google Scholar]
- 48.Galici R, Jones CK, Hemstapat K, Nong Y, Echemendia NG, Williams LC, et al. Biphenyl-indanone A, a positive allosteric modulator of the metabotropic glutamate receptor subtype 2, has antipsychotic- and anxiolytic-like effects in mice. J Pharmacol Exp Ther. 2006;318:173–185. doi: 10.1124/jpet.106.102046. [DOI] [PubMed] [Google Scholar]
- 49.Schoepp DD. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther. 2001;299:12–20. [PubMed] [Google Scholar]
- 50.Conn PJ, Lindsley CW, Jones CK. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol Sci. 2009;30:25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 52.Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- 53.Li X, Zeric T, Kambhampati S, Bossert JM, Shaham Y. The central amygdala nucleus is critical for incubation of methamphetamine craving. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2014.320. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deroche-Gamonet V, Piazza PV. Psychobiology of cocaine addiction: Contribution of a multi-symptomatic animal model of loss of control. Neuropharmacology. 2014;76(Pt B):437–449. doi: 10.1016/j.neuropharm.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 55.Theberge FR, Li X, Kambhampati S, Pickens CL, St Laurent R, Bossert JM, et al. Effect of chronic delivery of the Toll-like receptor 4 antagonist (+)-naltrexone on incubation of heroin craving. Biol Psychiatry. 2013;73:729–737. doi: 10.1016/j.biopsych.2012.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quick SL, Pyszczynski AD, Colston KA, Shahan TA. Loss of alternative non-drug reinforcement induces relapse of cocaine-seeking in rats: role of dopamine D(1) receptors. Neuropsychopharmacology. 2011;36:1015–1020. doi: 10.1038/npp.2010.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Podlesnik CA, Jimenez-Gomez C, Shahan TA. Resurgence of alcohol seeking produced by discontinuing non-drug reinforcement as an animal model of drug relapse. Behav Pharmacol. 2006;17:369–374. doi: 10.1097/01.fbp.0000224385.09486.ba. [DOI] [PubMed] [Google Scholar]
- 58.Bouton ME. Why behavior change is difficult to sustain. Prev Med. 2014;68C:29–36. doi: 10.1016/j.ypmed.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leitenberg H, Rawson RA, Bath K. Reinforcement of competing behavior during extinction. Science. 1970;169:301–303. doi: 10.1126/science.169.3942.301. [DOI] [PubMed] [Google Scholar]
- 60.Winterbauer NE, Bouton ME. Mechanisms of resurgence II: Response-contingent reinforcers can reinstate a second extinguished behavior. Learn Motiv. 2011;42:154–164. doi: 10.1016/j.lmot.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stitzer M, Petry N. Contingency management for treatment of substance abuse. Annu Rev Clin Psychol. 2006;2:411–434. doi: 10.1146/annurev.clinpsy.2.022305.095219. [DOI] [PubMed] [Google Scholar]
- 62.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 64.Ahmed SH. The science of making drug-addicted animals. Neuroscience. 2012;211:107–125. doi: 10.1016/j.neuroscience.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 65.Jin X, Semenova S, Yang L, Ardecky R, Sheffler DJ, Dahl R, et al. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35:2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kenny PJ, Markou A. The ups and downs of addiction: role of metabotropic glutamate receptors. Trends Pharmacol Sci. 2004;25:265–272. doi: 10.1016/j.tips.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 67.Liechti ME, Markou A. Role of the glutamatergic system in nicotine dependence: implications for the discovery and development of new pharmacological smoking cessation therapies. CNS Drugs. 2008;22:705–724. doi: 10.2165/00023210-200822090-00001. [DOI] [PubMed] [Google Scholar]
- 68.Xi ZX, Ramamoorthy S, Baker DA, Shen H, Samuvel DJ, Kalivas PW. Modulation of group II metabotropic glutamate receptor signaling by chronic cocaine. J Pharmacol Exp Ther. 2002;303:608–615. doi: 10.1124/jpet.102.039735. [DOI] [PubMed] [Google Scholar]
- 69.Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 70.Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, et al. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J Med Chem. 1999;42:1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- 71.Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, et al. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- 72.Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bossert JM, Poles GC, Sheffler-Collins SI, Ghitza UE. The mGluR2/3 agonist LY379268 attenuates context- and discrete cue-induced reinstatement of sucrose seeking but not sucrose self-administration in rats. Behav Brain Res. 2006;173:148–152. doi: 10.1016/j.bbr.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 74.Baptista MA, Martin-Fardon R, Weiss F. Preferential effects of the metabotropic glutamate 2/3 receptor agonist LY379268 on conditioned reinstatement versus primary reinforcement: comparison between cocaine and a potent conventional reinforcer. J Neurosci. 2004;24:4723–4727. doi: 10.1523/JNEUROSCI.0176-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Peters J, Kalivas PW. The group II metabotropic glutamate receptor agonist, LY379268, inhibits both cocaine- and food-seeking behavior in rats. Psychopharmacology. 2006;186:143–149. doi: 10.1007/s00213-006-0372-9. [DOI] [PubMed] [Google Scholar]
- 76.Kufahl PR, Watterson LR, Nemirovsky NE, Hood LE, Villa A, Halstengard C, et al. Attenuation of methamphetamine seeking by the mGluR2/3 agonist LY379268 in rats with histories of restricted and escalated self-administration. Neuropharmacology. 2013;66:290–301. doi: 10.1016/j.neuropharm.2012.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Adewale AS, Platt DM, Spealman RD. Pharmacological stimulation of group ii metabotropic glutamate receptors reduces cocaine self-administration and cocaine-induced reinstatement of drug seeking in squirrel monkeys. J Pharmacol Exp Ther. 2006;318:922–931. doi: 10.1124/jpet.106.105387. [DOI] [PubMed] [Google Scholar]
- 78.Liechti ME, Lhuillier L, Kaupmann K, Markou A. Metabotropic glutamate 2/3 receptors in the ventral tegmental area and the nucleus accumbens shell are involved in behaviors relating to nicotine dependence. J Neurosci. 2007;27:9077–9085. doi: 10.1523/JNEUROSCI.1766-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crawford JT, Roberts DC, Beveridge TJ. The group II metabotropic glutamate receptor agonist, LY379268, decreases methamphetamine self-administration in rats. Drug Alcohol Depend. 2013;132:414–419. doi: 10.1016/j.drugalcdep.2013.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- 81.Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, et al. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci. 2014;17:73–80. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ma YY, Lee BR, Wang X, Guo C, Liu L, Cui R, et al. Bidirectional modulation of incubation of cocaine craving by silent synapse-based remodeling of prefrontal cortex to accumbens projections. Neuron. 2014;83:1453–1467. doi: 10.1016/j.neuron.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee BR, Ma YY, Huang YH, Wang X, Otaka M, Ishikawa M, et al. Maturation of silent synapses in amygdala-accumbens projection contributes to incubation of cocaine craving. Nat Neurosci. 2013;16:1644–1651. doi: 10.1038/nn.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dhanya RP, Sidique S, Sheffler DJ, Nickols HH, Herath A, Yang L, et al. Design and synthesis of an orally active metabotropic glutamate receptor subtype-2 (mGluR2) positive allosteric modulator (PAM) that decreases cocaine self-administration in rats. J Med Chem. 2011;54:342–353. doi: 10.1021/jm1012165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ettenberg A. Opponent process properties of self-administered cocaine. Neurosci Biobehav Rev. 2004;27:721–728. doi: 10.1016/j.neubiorev.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 86.Ettenberg A. The runway model of drug self-administration. Pharmacol Biochem Behav. 2009;91:271–277. doi: 10.1016/j.pbb.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Badiani A. Substance-specific environmental influences on drug use and drug preference in animals and humans. Curr Opin Neurobiol. 2013;23:588–596. doi: 10.1016/j.conb.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 88.Badiani A, Belin D, Epstein D, Calu D, Shaham Y. Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci. 2011;12:685–700. doi: 10.1038/nrn3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peters J, Pattij T, De Vries TJ. Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends Pharmacol Sci. 2013;34:689–695. doi: 10.1016/j.tips.2013.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.