Abstract
Recruitment of innate immune effector cells into sites of infection is a critical component of resistance to pathogen infection. Using a model of intradermal footpad injection of Candida albicans, we observed that inflammation as measured by footpad thickness and neutrophil recruitment occurred independent of adoptive immunity but was significantly reduced in MyD88−/− and IL-6−/− mice. Unexpectedly, mice that lack Langerhans cells (ΔLC) developed increased skin inflammation and expressed higher amounts of IL-6 suggesting a suppressive role for Langerhans cells. Increased inflammation also occurred in Rag1−/− ΔLC mice but was reversed by antibody-mediated ablation of natural killer (NK) cells. CXCR6+CD49a+ NK cells are a liver-resident subset that can mediate inflammatory skin responses. We found that exaggerated skin inflammation was absent in ΔLC × CXCR6−/− mice. Moreover, the exaggerated response in ΔLC mice could be adoptively transferred with liver CD49a+ NK cells. Finally, CD49a+ NK cells in ΔLC but not control mice were recruited to the skin and inhibition of their recruitment prevented the exaggerated response. Thus, in the absence of LC, CD49a+ liver NK cells display an inappropriately pro-inflammatory phenotype that results in increased local skin inflammation. These data reveal a novel function for LC in the regulation of this recently described subset of skin tropic NK cells.
Introduction
The skin is an immunologically active barrier tissue that is colonized by a diverse community of commensal microorganisms and is also a frequent site of pathogen invasion (1). The early response to infection with extracellular bacteria and fungus drives elaboration of numerous inflammatory cytokines from keratinocytes as well as from skin-resident T cells and innate lymphoid cells (ILC)(2). These cell types have the capacity to elicit a direct antimicrobial response through the elaboration of antimicrobial peptides (e.g. cathelicidin) and cytokines that enhance keratinocyte proliferation (e.g. IL-22). In addition, the recruitment of neutrophils to the site of infection through a cascade of factors including local production of IL-17 is a central component of the innate response. Patients with defective production of IL-17 are highly susceptible to skin infections with the yeast C. albicans and gram positive bacteria such as S. aureus(3).
Langerhans cells are a subset of epidermal resident dendritic cells that acquire antigen from the outermost portions of the skin before migrating to regional lymph nodes where they have the capacity to efficiently activate naïve and memory T cells(4). Using huLangerin-DTA mice that have a selective absence in the skin of Langerhans cells (henceforth referred to as ΔLC), we found that LC are necessary and sufficient for the differentiation of Th17 cells in the setting of a superficial C. albicans infection. Th17 cells migrate back into the skin and provide protection from subsequent challenge(5–7). In addition to Th17 effectors that are recruited into the skin several days after the onset of infection, populations of αβ and γδ T cells are prepositioned in the skin. These resident T cells elaborate IL-17 and drive inflammation through a mechanism that depends on commensal microorganisms, MyD88 and IL-1β (8). Mice lacking LC do not have an altered skin microbiome and have normal resistance to epicutaneous C. albicans infection(5, 9).
To explore whether innate responses to an extracellular pathogen are altered by in absence of LC, we challenged mice with intradermal Candida albicans. Unexpectedly, inflammation was exaggerated in the absence of LC and was independent of adaptive immunity. Rather, in the absence of LC, the recently described CXCR6+ CD49a+ subset of NK cells were inappropriately recruited into the skin due to a cell intrinsic defect. This unexpected finding provides a novel function for this NK subset and suggests a novel function for LC in the regulation of this skin tropic NK subset.
Material and Methods
Mice
All animals were on the C57BL/6 genetic background unless otherwise specified. HuLangerin-Cre I-Aβ (10), huLangerin-Cre MyD88(6), huLangerin-DTA (11), mice have all been previously described. Rag1−/−, IL-6−/−, IL-1β−/−, MyD88/TRIF−/− and B6.129P2-Cxcr6tm1Litt were purchased from Jackson Labs (Bar Harbor, ME). IL-23−/− mice were a kind gift from Nico Ghilardi (Genentech, San Francisco CA). NLRP3−/− and ASC−/− mice were acquired from Millenium Pharmacerticals(12). All experiments were performed with 8–10 week old age- and sex-matched littermates. Mice were housed in specific pathogen free microisolator cages and fed irradiated food and acidified water. The University of Minnesota institutional animal care and use committee approved all animal protocols.
Footpad Challenge
C. albicans was grown in YPAD medium at 30°C medium, respectively, until the OD600 reached 1.5. Cells were washed in sterile PBS and heat killed at 56°C for 60 minutes. Heat killed cells were injected at a final concentration of 5×106 cells in 20ul PBS per footpad or as indicated. Footpad thickness was measured using an engineer’s micrometer (Mitutoyo, Japan) at various time points after injection. Footpad thickness from control PBS injected contralateral footpads was subtracted to obtain the change in footpad thickness.
Flow Cytometry
Single-cell lymph node and spleen suspensions were obtained and stained as previously described(11). For Single cell liver leukocyte suspension the livers were weighted and minced in RPMI 10% FCS DNAse solution and passed through a 40 μm filter. Cells were washed and separated with a Ficoll (Sigma) gradient and washed with either PBS or HBSS. Samples were analyzed on LSR-II flow cytometers (BD Biosciences). Data were analyzed with FlowJo software (TreeStar, Ashland, OR). Isolation of NK cell subsets by flow cytometric cell sorting was performed with FACS-Aria II (BD Bioscience). Antibodies used: CD3 PE, CD45.1 APC, CD3 PE, GR1-APC, NK1.1 PECy7, PerCP eflour 710 and viability dye eFlour®780 (eBioscience); CXCR6 PE and APC (BD Bioscience); CD49a (BD Pharmingen); CD49b, CD11b PeCy7, CD45.2 PB (BioLegend).
NK Cell Adoptive transfer
Liver leukocyte single cell suspension was obtained as described above. Cells were ficoll purified, washed and resuspended in sterile PBS. 105 cells were injected i.v. into recipient mice. In some experiments, cells were first purified by FACsorting based on expression of CD45, NK1.1, DX5, and CD49a.
Pertussis toxin treatment
Pertussis toxin (Sigma) was injected at a dose of 500ng/mouse i.v. 1 hour prior to C. albicans challenge. For ex vivo treatment, ficoll purified liver leukocytes were incubated 90 minutes in complete medium supplemented with vehicle of pertussis toxin at a final concentration of 20 ng/ml prior to adoptive transfer.
Antibody depletion
Depleting antibodies 40 μl anti-asialo GM1 (Wako, Wako, TX) or 500 μg anti-NK1.1 (PK136 BioXCell) or Isotype control (C1.18.4 BioXCell) were injected i.p. one day prior to challenge.
Quantitative Real time PCR
Footpads were harvested and stored in RNAlater (Quiagen) at indicated time points. mRNA was extracted using RNAeasy kit (Qiagen, Valencia, CA) and analyzed via quantitative PCR (qPCR) with TaqMan Gene Expression Assays for IL-6 and MPO (Applied Biosystems, Carlsbad, CA), as previously described(5).
Histology
Skin samples were fixed overnight in 10% formalin, dehydrated, and embedded in paraffin. The 10 μm microtome sections were stained with HE according to the manufacturer’s instructions (Sigma-Aldrich).
Statistics
Significant differences were calculated between control group and individual experimental groups with the Student’s unpaired, two-tailed t test.
Results
Footpad swelling to C. albicans is exaggerated in the absence of Langerhans cells
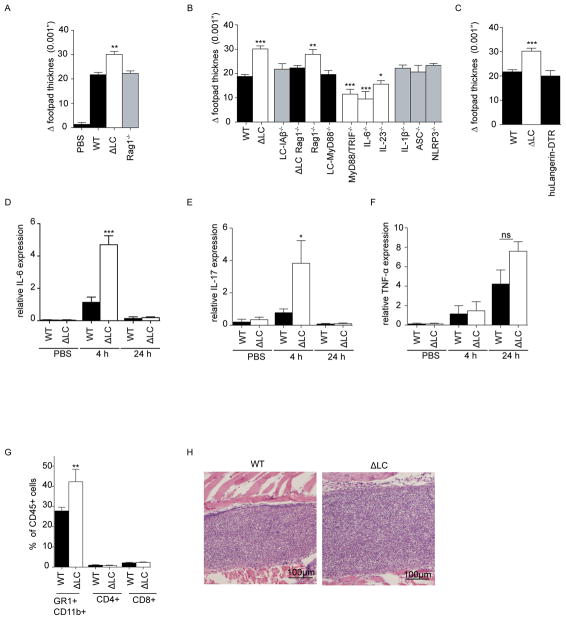
To determine whether LC are required for optimal innate responses to extracellular pathogens, we injected 5×105 heat killed C. albicans (HKCA) into the footpads of naïve mice (Figure 1A). One day later, we observed increase footpad thickness indicative of an inflammatory response. We observed a similar phenomena with a gram positive bacterium R. nasimurium (HKRN), that is commensal in our colony (Figure 1A and S1). Unexpectedly, we observed a modestly exaggerated yet persistent response to both HKCA and HKRN in huLangerin-DTA mice (ΔLC) that have a genetic ablation of Langerhans cells (Figure 1A–B)(11).
Figure 1. Skin inflammation is exaggerated in the absence of Langerhans cells.
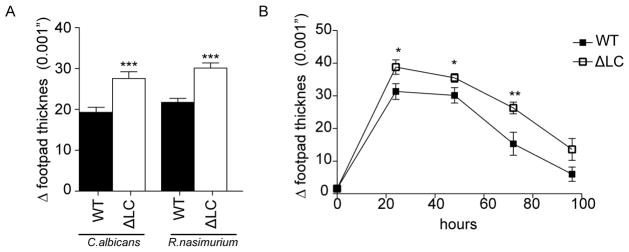
(A) Footpad swelling response of naïve WT and ΔLC after intradermal injection with 5×105 heat killed commensal bacteria Rothia nasimurium and S. epidermidis. The degree of inflammation is represented as increased footpad thickness 24 hours after challenge. R. nasimurium induces a stronger footpad swelling in WT mice and an exaggerated response in ΔLC. (B) The observed exaggerated swelling response after HKRN injection was observed over time. Each experiment included cohorts of at least 4 mice per group and were repeated at least 3 times. *p<0.05; ***p<0.001
Absence of LC results in increased IL-6 and neutrophil recruitment independent of adaptive immunity
To explore the mechanism underlying the exaggerated response seen in ΔLC mice, we first compared the HKRN response in knockout mice with selective immune defects. Unexpectedly, the footpad swelling in Rag1−/− mice was indistinguishable from controls indicating that B and T cells including dermal and epidermal γδ T cells are not required (Figure 2A). Challenge of WT and Rag1−/− mice with HKCA gave the same result (Figure 2B). Since immune responses against C. albicans have been better studied than those against R. nasimurium, the remainder of our experiments were performed using C. albicans. To confirm that the increased response in ΔLC mice was also independent of adaptive immunity, we generated ΔLC × Rag1−/−. Challenge with HKCA resulted in the same increased response seen in ΔLC mice (Figure 2B). In addition, footpad thickness was not increased in mice a LC-specific ablation of MHC-II (LC-I-Aβ) (10). Notably, we did not observe increased footpad thickness in huLangerin-DTR mice in which LC are acutely depleted prior to administration of HKCA (Figure 2C) (13). Thus, the exaggerated inflammation seen in the absence of LC requires a constitutive absence of LC and occurs independently B cells and T cells.
Figure 2. Absence of LC results in increased IL-6 and neutrophil recruitment independent of adaptive immunity.
(A and B) Footpads of naïve mice of the indicated genotype were challenged by footpad injection with 5×105 heat killed C. albicans (HKCA). The degree of inflammation is represented as the increased footpad thickness 24 hours after challenge. (C) Naïve huLangerin-DTR mice were injected with DT and 24-post ablation the footpads were intradermal challenged with 5×105 HKCA. (D–F) Expression of mRNA for IL-6, Il-17 and TNF-α was measured from total footpads in WT and ΔLC mice at 4 and 24 hours after challenge with HKCA. (G) Flow cytometric analysis of CD4+, CD8+ and GR1+CD11b+ cells isolated from footpads at 4 and 24 hours post challenge is shown. (H) Representative histology stained with H&E is shown from footpads of WT and ΔLC mice 24 hours after challenge with HCKA. Each experiment included cohorts of at least 4 mice per group and were repeated at least 3 times except for (B) that were repeated twice. Statistical comparison were made between individual experimental and control groups; *p<0.05; ***p<0.001
Recognition of C. albicans depends on inflammasome activation and recognition via TLR, especially TLR2 (3). Footpad challenge of NLRP3−/−, ASC−/− and IL-1β−/− mice with HKCA resulted in comparable inflammation as wild type mice indicating that the response occurs independent of inflammasome activation. In contrast, responses were significantly reduced in IL-6−/−, Myd88/TRIF−/− and to a lesser extent in IL-23−/− mice (Figure 2B). LC-MyD88−/− mice that have a LC-specific ablation of MyD88 develop normal responses indicating that sensing of C. albicans by LC is not required for innate responses to HKCA(6). Thus, inflammation to dermal HKCA depends on a MyD88/TRIF/IL-6 pathway and occurs independently of B cells, T cells or inflammasome activation.
To characterize the inflammatory response generated by dermal HKCA, we compared expression of cytokine mRNA at 4 and 24 hours after injection in WT and ΔLC mice. We observed greatly increased levels of IL-6 mRNA in ΔLC mice early after injection (Figure 2D). Expression of IL-17 (Figure 2E) but not TNF (Figure 2F) or IL-1β (unpublished observations) was also increased. Analysis of footpads 24 hours after HKCA by flow cytometry revealed an influx of dominated by GR1+ CD11b+ neutrophils that was consistent with histological examination of tissue (Figures 2G and H). Taken together our results demonstrate that dermal C. albicans induces an early innate immune response that is associated with expression of IL-6, IL-17 and recruitment of neutrophils that are all exaggerated in mice lacking LC through a T cell-independent mechanism.
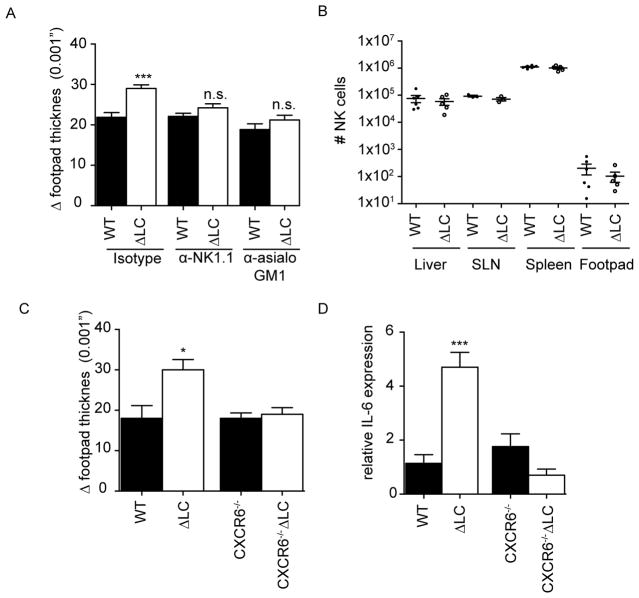
Exaggerated innate responses in LC-deficient mice requires CXCR6+ NK cells
NK cells have recently been shown to participate in response to fungal infections(14–16). To determine whether the exaggerated HKCA response in ΔLC mice required NK cells, we efficiently depleted NK cells in WT and ΔLC mice using either α-NK1.1 or α-Asialo-GM1 antibodies (Figure S2). Ablation of NK cells did not affect the response to HKCA in WT mice but reversed the exaggerated phenotype observed in ΔLC mice (Figure 3A). Under steady-state conditions, numbers of NK cell in WT and ΔLC mice were similar in LN, spleen, liver and footpad (Figure 3B). Furthermore we found that in vivo LPS stimulated WT and ΔLC mice lead to a modestly higher percentage of IFN-γ expression within liver ΔLC NK cells but not within ΔLC NK cells in the skin draining lymph nodes (Figure S3 A and B), indicating that NK cells within the liver of ΔLC mice display an altered phenotype.
Figure 3. Exaggerated innate responses in LC-deficient mice requires CXCR6+ NK cells.
(A) Cohorts of ΔLC (white bars) or littermate controls (black bars) were treated with αNK1.1, αasialo-GM1, or isotype 24 hours prior to footpad challenge with HKCA. The degree of inflammation is represented as the increased footpad thickness 24 hours after challenge. (B) Numbers of CD45+, NK1.1+ CD3− NK cells in spleen, liver, skin draining lymph node (SLN) and footpads of naive WT and ΔLC mice is shown. (C) As in (A), naïve ΔLC, WT littermate controls, ΔLC CXCR6−/− and littermate CXCR6−/− mice were challenged with HKCA and increase footpad thickness at 24 hours as well as total IL-6 mRNA at 4 hours (D) was measured. Each experiment included cohorts of at least 5 mice per group and were repeated at least 3 times except for (B) in which each symbol represents an individual mouse. *p<0.05; ***p<0.001
Recently a novel subset of CXCR6+, CD49a+, CD49b−, DX5−, EOMES− liver resident NK cells that mediate effector function in skin after hapten immunization independently of adaptive immunity has been described(17–19). Although the numbers of cells in this subset was not altered in ΔLC mice (unpublished observations), we considered whether this subset could be dysregulated in the absence of LC. CXCR6gfp/gfp mice were bred with ΔLC mice resulting in LC-deficient mice in which the NK cells are deprived of CXCR6 signaling. We observed that the absence of CXCR6 did not affect the response to HKCA in LC-sufficient mice but reversed the exaggerated footpad swelling as well as the exaggerated IL-6 seen in HKCA treated ΔLC mice (Figures 3C and D). We thus conclude that the exaggerated response observed in ΔLC mice requires CXCR6+ NK cells.
CXCR6+ CD49a+ NK cells have an intrinsic defect in LC-deficient mice
NK1.1+ CXCR6+ NK cells also express CD49a+ but lacked expression of DX5 and EOMES (Figure 4A) (17, 20, 21). To determine whether the observed enhanced skin inflammation seen in ΔLC mice results from an intrinsic defect of CD49a+ liver NK cells, we adoptively transferred 105 ficoll purified CD45+ liver leukocytes from naïve WT or ΔLC hosts into naïve WT or ΔLC recipients (Figure 4B). WT or ΔLC mice that received cells isolated from WT livers (black bars) had responses similar to WT or ΔLC controls. Importantly, WT recipients of cells isolated from the livers of ΔLC mice developed an exaggerated response similar to control ΔLC mice. Responses in ΔLC recipient mice were not additionally increased by transfer of cells from ΔLC mice.
Figure 4. CXCR6+ CD49a+ NK cells have an intrinsic defect in LC-deficient mice.
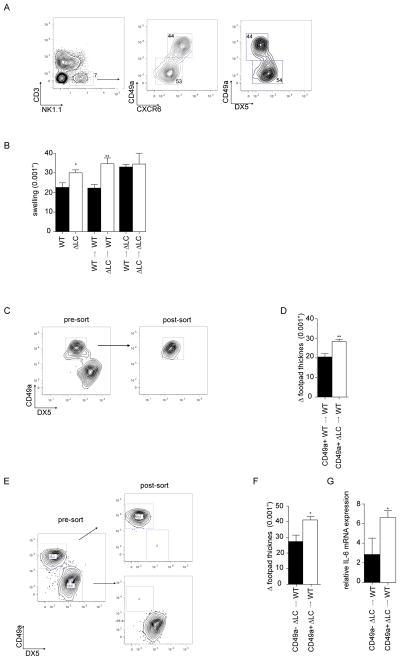
(A) NK cells isolated from livers of WT mice were identified based on expression of NK1.1 and absence of CD3. Expression of CD49a, CXCR6 and DX5 on these cells is shown. (B) Liver leukocytes were obtained from WT and ΔLC mice. 105 cells were adoptively transferred by i.v. injection into WT or ΔLC mice as indicated. Mice were challenged with HKCA 1 day after transfer and footpad thickness was measured 24 hours after challenge. C) CD3−, NK1.1+, CD49a+ DX5− NK cells obtained from livers of ΔLC or WT mice were purified by FACSorting as shown prior to adoptive transfer of 5 × 104 cells into naïve WT recipients. (D) Mice were challenged with HKCA 1 day after transfer and footpad thickness was measured 24 hours after challenge. E) As in (C) except that CD49a+DX5− and CD49a−DX5+ liver NK cells isolated from ΔLC mice were adoptively transferred into WT recipients. (F) Mice were challenged with HKCA 1 day after transfer and footpad thickness was measured 24 hours and (G) Relative IL-6 expression 4 hours after challenge. Each experiment included cohorts of at least 5 mice per group and was repeated at least 2 times.
To confirm that this effect was mediated by CD49a+ NK cells, we purified liver CD49a+ DX5− NK cells by FACS from WT and ΔLC mice prior to adoptive transfer into WT mice. Adoptive transfer of 5×104 CD49a+ DX5− liver NK cells from ΔLC mice was sufficient to transfer the response (Figure 4c and d). Importantly, CD49a+ DX5− liver NK cells from WT mice did not transfer the phenotype. Finally, we adoptively transferred FACS purified CD49a+ DX5− or CD49a−DX5+ liver NK cells isolated from ΔLC mice into naïve WT mice (Figure 4E). WT recipients of CD49a+DX5− but not CD49a−DX5+ liver NK cells were sufficient to drive exaggerated footpad swelling and increased expression of IL-6 after challenge with HKCA (Figure 4Fand G). Thus, we conclude that the observed exaggerated response in ΔLC mice results from an intrinsic effect of liver resident CD49a+ NK cells in LC-deficient mice.
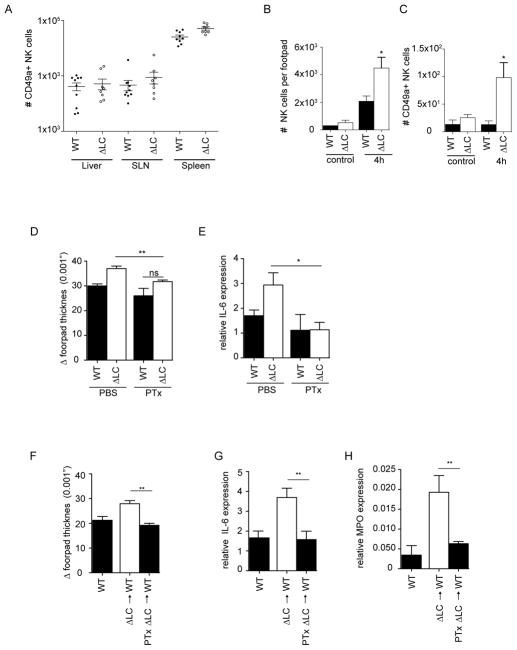
CD49a+ NK liver cells are recruited to the site of inflammation
In addition to the liver, CD49a+ NK cells are found within multiple tissues (21). We found comparable numbers of these cells in liver, skin draining LN (SLN), and spleen of WT and ΔLC mice (Figure 5A). Under steady-state conditions, these cells were also observed in equal numbers in the footpads of WT and ΔLC mice (Figure 5B). In contrast, 4 hours after challenge with HKCA the number of NK cells in the footpad increased dramatically and was further increased in ΔLC mice (Figure 5B). Notably, the number of CD49a+ NK cells in the footpad was not increased in WT mice but was increased by over 10 fold in ΔLC mice (Figure 5C).
Figure 5. CD49a+ NK liver cells are recruited to the site of inflammation.
(A) Total numbers of CD3−, NK1.1+ CD49a+ NK cells in the indicated tissue and mouse strain is shown. Each symbol represents an individual animal. (B) Total numbers of CD3− NK1.1+ NK cells, (C) CD3− NK1.1+ CD49a+ NK cells isolated from the footpads of WT and ΔLC mice 4 hours after PBS or HKCA challenge. (D) WT and ΔLC mice were treated with 500 ng of pertussis toxin or PBS i.v. 1 hour prior to challenge with HKCA. Footpad thickness was measured 24 hours and (E) Relative IL-6 expression 4 hours after challenge. (F) Total liver leukocytes isolated from ΔLC mice were incubated in vitro for 90 minutes in media supplemented with 20mg/ml pertussis toxin or vehicle prior to adoptive transfer of 105 cells into WT recipients. Mice were challenged with HKCA 1 day after transfer and footpad thickness was measured 24 hours and (G,H) Relative IL-6 and MPO expression 4 hours after challenge. Each experiment included cohorts of at least 5 mice per group and was repeated at least 2 times except for (A) in which each symbol represents an individual mouse.
To determine whether recruitment of CD49a+ NK cells into the skin is required for the phenotype, we treated WT and ΔLC mice with pertussis toxin 1 hour prior to challenge with HKCA to inhibit chemokine receptor signaling. Pretreatment with pertussis toxin inhibited the exaggerated inflammation and IL-6 normally seen at 4 hours in ΔLC mice (Figures 5D–E). To control for the possibility that treatment with pertussis toxin affects cells in addition to CD49a+ NK cells, we incubated liver leukocytes from ΔLC mice ex vivo with pertussis toxin for 90 minutes prior to adoptive transfer into WT mice. As expected liver leukocytes incubated ex vivo without pertussis toxin induced an exaggerated inflammatory response and elevated IL-6 (Figure 5F–G). In contrast, liver leukocytes pretreated with pertussis toxin failed to induce the exaggerated response. Finally, we also observed that myeloid peroxidase (MPO), a marker of neutrophil recruitment, was increased in the footpads of mice receiving mock treated liver cells and was suppressed by pertussis treatment (Figure 5H). Taken together these data demonstrate that in the absence of LC, CD49a+ liver NK cells are inappropriately recruited into the skin resulting in increased IL-6 and neutrophil recruitment.
Discussion
Herein we describe that immune responses to dermal challenge with C. albicans in naïve mice resulted in considerable inflammation as measured by increased footpad size, expression of IL-6/IL-17, and recruitment of neutrophils. The response required MyD88/TRIFF but was inflammasome independent. Notably, mice lacking LC developed exaggerated responses to C. albicans that required the presence of CXCR6+ liver-resident NK cells and was independent of T or B cells. Finally, the exaggerated response to C. albicans seen in LC-deficient mice could be adoptively transferred by CD49a+ liver-resident NK cells. These data reveal an unexpected interplay between LC and liver-resident CD49a+ NK cells resulting in suppression of the innate response to a pathogen in the skin.
CXCR6+ liver-resident NK cells were first reported as a source of T cell and B cell-independent immunity to epicutaneous application of sensitizing haptens which was later found to also occur in response to viral infection(19, 22). In depth phenotypic analysis of liver NK cells has recently revealed that CXCR6+ NK cell can be defined as CD49a+DX5−Tbet+Eomes− (17, 20). They have been suggested to be a true subset of NK cells with an expression profile and a transcription factor dependence that is distinct from other NK cells in the liver, thymus, spleen and uterus(21). Recent findings show that CD49a+ CD49b− liver cells are negative for EOMES and display a distinct gene expression pattern suggesting they may be more closely related to ILC1 cells (23, 24). Notably, CD49a+ CD49b− NK1.1+ NK cells in the liver and skin appear to be similar. These cells are thought to become activated in the regional lymph node in response to antigen prior to their CXCR6-mediated localization to liver sinusoids(25). In response to stimulus, they are recruited back into the skin where they induce a local inflammatory response. In support of this model, we find that CD49a+ NK cells are present in the skin, skin-draining lymph nodes and liver. They are also recruited into the skin. Notably, in the absence of LC, unprimed CD49a+ liver NK cells acquire a cell intrinsic phenotype that results in their recruitment into inflamed skin. We speculate that LC may function to limit CD49a+ NK cell priming as a mechanism to suppress inappropriate responses to benign stimuli.
We observed the response to heat killed C. albicans, a relatively well defined response that has been shown to rely on pathogen recognition via TLR2/Dectin-1/NLRP3 and IL-17 mediated recruitment of neutrophils(3). Our observation of a response dominated by neutrophil recruitment that depended on MyD88 and IL-6 is consistent with prior reports(26–29). NK cells are important for resistance to C. albicans infection both by augmenting PMN activity via GM-CSF and by direct killing of C. albicans(14–16). CD49a+ NK appear to be unique among NK cells in their ability to generate GM-CSF that may be responsible for their ability to enhance local inflammation in response to C. albicans(21).
The finding that LC suppress CD49a+ NK cell function is an unexpected and novel function for Langerhans cells. Interestingly, short-term ablation of LC is not sufficient to phenocopy the responses observed in long-term ablation. Thus, it is unlikely that LC modulate NK cell function at the time of challenge. Rather, we hypothesize that during ontogeny or under steady-state conditions LC either directly or indirectly modulate the function of long-lived CD49a+ liver NK cells(19). We cannot exclude the possibility that deletion of an unobserved population other than LC in our transgenic mice is responsible for the altered NK cell phenotype. We have previously reported that CD103+CD11b+ lpDC in the intestinal lamina propria are absent in ΔLC mice(30). The absence of this DC subset does not alter NK function, however, as we did not observed exaggerated C. albicans responses in CD11c+ Notch2-fl mice that lack CD103+CD11b+ lpDC but have intact numbers of LC(unpublished observations).
In summary, we report novel function for LC in the regulation of skin tropic NK cells. Several aspects of the LC-NK cell interaction such as the factors that suppress CD49a+ NK cells and nature of LC and NK cell interaction remain to be elucidated in future studies.
Supplementary Material
Footnotes
There are no conflicts of interest for any of the authors.
References
- 1.Grice EA, Segre JA. The skin microbiome. Nat Rev Microbiol. 2011;9:244–253. doi: 10.1038/nrmicro2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014 doi: 10.1038/nri3646. [DOI] [PubMed] [Google Scholar]
- 3.Hernández-Santos N, Gaffen SL. Th17 cells in immunity to Candida albicans. Cell Host Microbe. 2012;11:425–435. doi: 10.1016/j.chom.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merad M, Sathe P, Helft J, Miller J, Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Igyarto BZ, Haley K, Ortner D, Bobr A, Gerami-Nejad M, Edelson BT, Zurawski SM, Malissen B, Zurawski G, Berman J, Kaplan DH. Skin-resident murine dendritic cell subsets promote distinct and opposing antigen-specific T helper cell responses. Immunity. 2011;35:260–272. doi: 10.1016/j.immuni.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haley K, Igyarto BZ, Ortner D, Bobr A, Kashem S, Schenten D, Kaplan DH. Langerhans cells require MyD88-dependent signals for Candida albicans response but not for contact hypersensitivity or migration. J Immunol. 2012;188:4334–4339. doi: 10.4049/jimmunol.1102759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kashem SW, Igyarto BZ, Gerami-Nejad M, Kumamoto Y, Mohammed J, Jarrett E, Drummond RA, Zurawski SM, Zurawski G, Berman J, Iwasaki A, Brown GD, Kaplan DH. Candida albicans Morphology and Dendritic Cell Subsets Determine T Helper Cell Differentiation. Immunity. 2015;42:356–366. doi: 10.1016/j.immuni.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, Deming C, Quinones M, Koo L, Conlan S, Spencer S, Hall JA, Dzutsev A, Kong H, Campbell DJ, Trinchieri G, Segre JA, Belkaid Y. Compartmentalized Control of Skin Immunity by Resident Commensals. Science. 2012 doi: 10.1126/science.1225152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scholz F, Badgley BD, Sadowsky MJ, Kaplan DH. Immune mediated shaping of microflora community composition depends on barrier site. PLoS ONE. 2014;9:e84019. doi: 10.1371/journal.pone.0084019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Igyarto BZ, Jenison MC, Dudda JC, Roers A, Müller W, Koni PA, Campbell DJ, Shlomchik MJ, Kaplan DH. Langerhans cells suppress contact hypersensitivity responses via cognate CD4 interaction and langerhans cell-derived IL-10. J Immunol. 2009;183:5085–5093. doi: 10.4049/jimmunol.0901884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaplan DH, Jenison MC, Saeland S, Shlomchik WD, Shlomchik MJ. Epidermal langerhans cell-deficient mice develop enhanced contact hypersensitivity. Immunity. 2005;23:611–620. doi: 10.1016/j.immuni.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Sutterwala FS, Ogura Y, Szczepanik M, Lara-Tejero M, Lichtenberger GS, Grant EP, Bertin J, Coyle AJ, Galán JE, Askenase PW, Flavell RA. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Bobr A, Olvera-Gomez I, Igyarto BZ, Haley KM, Hogquist KA, Kaplan DH. Acute ablation of Langerhans cells enhances skin immune responses. J Immunol. 2010;185:4724–4728. doi: 10.4049/jimmunol.1001802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bär E, Whitney PG, Moor K, Sousa CRE, LeibundGut-Landmann S. IL-17 Regulates Systemic Fungal Immunity by Controlling the Functional Competence of NK Cells. Immunity. 2014;40:117–127. doi: 10.1016/j.immuni.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Li SS, Kyei SK, Timm-McCann M, Ogbomo H, Jones GJ, Shi M, Xiang RF, Oykhman P, Huston SM, Islam A, Gill MJ, Robbins SM, Mody CH. The NK Receptor NKp30 Mediates Direct Fungal Recognition and Killing and Is Diminished in NK Cells from HIV-Infected Patients. Cell Host Microbe. 2013;14:387–397. doi: 10.1016/j.chom.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Voigt J, Hünniger K, Bouzani M, Jacobsen ID, Barz D, Hube B, Löffler J, Kurzai O. Human Natural Killer Cells Acting as Phagocytes Against Candida albicans and Mounting an Inflammatory Response That Modulates Neutrophil Antifungal Activity. J Infect Dis. 2013 doi: 10.1093/infdis/jit574. [DOI] [PubMed] [Google Scholar]
- 17.Peng H, Jiang X, Chen Y, Sojka DK, Wei H, Gao X, Sun R, Yokoyama WM, Tian Z. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J Clin Invest. 2013;123:1444–1456. doi: 10.1172/JCI66381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paust S, Senman B, von Andrian UH. Adaptive immune responses mediated by natural killer cells. Immunol Rev. 2010;235:286–296. doi: 10.1111/j.0105-2896.2010.00906.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, Bienvenu J, Henry T, Debien E, Hasan UA, Marvel J, Yoh K, Takahashi S, Prinz I, de Bernard S, Buffat L, Walzer T. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. Journal of Experimental Medicine. 2014;211:563–577. doi: 10.1084/jem.20131560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, Riley JK, Zhu J, Tian Z, Yokoyama WM. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife. 2014;3:e01659. doi: 10.7554/eLife.01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 23.Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014:1–14. doi: 10.1038/nature13047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinette ML, Fuchs A, Cortez VS, Lee JS, Wang Y, Durum SK, Gilfillan S, Colonna M Immunological Genome Consortium. Transcriptional programs define molecular characteristics of innate lymphoid cell classes and subsets. Nat Immunol. 2015;16:306–317. doi: 10.1038/ni.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;12:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 26.Basu S, Quilici C, Zhang HH, Grail D, Dunn AR. Mice lacking both G-CSF and IL-6 are more susceptible to Candida albicans infection: critical role of neutrophils in defense against Candida albicans. Growth factors (Chur, Switzerland) 2008;26:23–34. doi: 10.1080/08977190801987513. [DOI] [PubMed] [Google Scholar]
- 27.Romani L, Mencacci A, Cenci E, Spaccapelo R, Toniatti C, Puccetti P, Bistoni F, Poli V. Impaired neutrophil response and CD4+ T helper cell 1 development in interleukin 6-deficient mice infected with Candida albicans. J Exp Med. 1996;183:1345–1355. doi: 10.1084/jem.183.4.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kagami S, Rizzo HL, Kurtz SE, Miller LS, Blauvelt A. IL-23 and IL-17A, but not IL-12 and IL-22, are required for optimal skin host defense against Candida albicans. J Immunol. 2010;185:5453–5462. doi: 10.4049/jimmunol.1001153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. The Journal of Immunology. 2013;190:521–525. doi: 10.4049/jimmunol.1202924. [DOI] [PubMed] [Google Scholar]
- 30.Welty NE, Staley C, Ghilardi N, Sadowsky MJ, Igyarto BZ, Kaplan DH. Intestinal lamina propria dendritic cells maintain T cell homeostasis but do not affect commensalism. Journal of Experimental Medicine. 2013 doi: 10.1084/jem.20130728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.