Abstract
LPS-induced TLR4 activation alters cellular bioenergetics and triggers proteolytic cleavage of AMPKα and HIF-1α expression in leukocytes. In human leukocytes, and more specifically neutrophils, AMPKα cleavage yields 55- and 35-kD protein fragments. Here we address the mechanism by which AMPKα is cleaved, and it’s relevance to human health. Our data indicate that AMPKα cleavage is linked to matrix metalloproteinase (MMP9) expression and that both are required for mTORC1 and S6K1 activation, and HIF-1α expression, in LPS stimulated human and mice leukocytes. Three key observations support this conclusion. First, no changes in AMPKα and TLR4 signaling intermediates (mTORC1/S6K1/ HIF-1α) were detected in LPS-stimulated MMP9-deficient mice leukocytes. Second, recombinant MMP9 cleaved human AMPKα ex vivo, producing degradation products similar in size to those detected following LPS-stimulation. Third, MMP9 inhibitors prevented AMPKα degradation and HIF-1α expression in LPS-activated human leukocytes, while AMPK activators blocked MMP9 and HIF-1α expression. Significantly, AMPKα degradation, MMP9, and TLR4 signaling intermediates were all detected in leukocytes from Type 2 diabetes mellitus (T2DM) patients and patients following cardiopulmonary bypass (CPB) surgery. Plasma from these two patient cohorts induced AMPKα cleavage and TLR4 signaling intermediates in healthy donor leukocytes and either a TLR4 inhibitor or polymyxin prevented these outcomes. Detection of AMPKα degradation, MMP9 expression, and TLR4 signaling intermediates described herein in leukocytes, the most readily available human cells for clinical investigation, may provide a powerful tool for further exploring the role of TLR4 signaling in human diseases, and lead to identification of new, context-specific therapeutic modalities for precision medicine.
Introduction
Toll-like receptors (TLRs) are a family of transmembrane pattern-recognition receptors that respond to ligands derived from pathogens and host-tissues. These receptors are central to innate immune cells activation. In humans there are 10 functional TLRs. TLR4, the most extensively studied TLR in humans (1-4), is activated by lipopolysaccharide (LPS; endotoxin), derived from Gram-negative bacteria, and a variety of endogenous ligands. These ligands include proteins derived from damaged tissues (4-8), proteins released by tissues that are stressed by excess nutrients (9), and saturated fatty acids (10). Despite significant progress in our understanding of TLR4 signaling, complete understanding of molecular mechanisms that underlie the earliest TLR4 induced responses in human leukocytes, and how chronic low-grade activation of these responses might contribute to human diseases, is lacking.
Analyses of tissue biopsies from critically ill patients revealed reduced ATP levels, suggesting that severe inflammation contributes to a decline in cellular bioenergetics (11, 12). Subsequent studies showed that in addition to well-described systemic responses (2, 13), LPS triggers within minutes after administration to human subjects profound metabolic changes in leukocytes, in part, through transcriptional regulation of proteins that contribute to mitochondrial ATP production (14). The transcriptional changes occur in conjunction with a decline in cellular ATP levels and dramatic changes in expression of two key regulators of cellular metabolism: AMP-activated protein kinase α subunit (AMPKα)2 and hypoxia-inducible transcription factor-1 α subunit (HIF-1α) (15).
AMPK is a heterotrimer α–β–γ serine/threonine kinase that monitors the cellular energy status (16). A decline in cellular ATP levels and an increase in AMP:ATP and ADP:ATP ratio promote AMPK activation (17). Activated AMPK switches on ATP-generating catabolic pathways while switching off ATP-consuming anabolic pathways, such as protein synthesis (16). AMPK suppresses protein synthesis through inactivation of mTORC1, a multi-subunit complex that includes the catalytic subunit mTOR and the regulatory-associated protein of mTOR (Raptor) (18). AMPK inactivates mTORC1 by indirect and direct mechanisms. AMPK can phosphorylate TSC2, which then acts on RHEB to inhibit mTORC1 (19). AMPK can also phosphorylate Raptor at Ser-792 (20). Phosphorylated Raptor binds 14-3-3 proteins. This interaction inhibits the binding of Raptor to mTOR, leading to mTORC1 inactivation (20). mTORC1 regulates the rate of protein synthesis through interactions with two proteins, p70 ribosomal protein S6 kinase (S6K1) and eIF4E-binding protein (4E-BP1). mTORC1 phosphorylates S6K1 at Thr-389, a site required for S6K1 activation (21, 22). By shifting the balance from energy consuming processes to energy production, AMPK improves the efficiency of cellular energy homeostasis.
AMPK activation requires phosphorylation of Thr-172 within AMPKα. LKB1 and CaMKK are two serine/threonine kinases that activate AMPK (23-27). Phosphatases PP2C and PPP2A dephosphorylate AMPKα Thr-172 triggering AMPK inactivation (28, 29). We reported recently that LPS-induced changes in cellular bioenergetics are accompanied by rapid, and yet transient, proteolytic cleavage of AMPKα (15, 30) followed by an increase in HIF-1α expression. These observations suggested that the possibility that the proteolytic cleavage of AMPKα is an alternative mechanism employed by TLR4 to regulate cellular bioenergetics and HIF-1α expression.
HIF-1 is a transcription factor that regulates cellular bioenergetics by upregulating glycolysis while reducing mitochondrial activity (31, 32). HIF-1 is a heterodimer composed of HIF-1α and HIF-1β subunits. HIF-1β is expressed constitutively. Although HIF-1α is degraded rapidly under normoxic conditions, it is stabilized under hypoxic conditions. However, LPS/TLR4 stabilize the expression of HIF-1α in leukocytes under either hypoxic or normoxic conditions (31-33). The expression of HIF-1α is below detection level in mice myeloid cells (which are predominantly neutrophils). Nonetheless, myeloid cells genetically deficient in HIF-1α exhibited dramatically reduced ATP levels, indicating that HIF-1 plays a role in these cells irrespective of their activation state (34). HIF-1α deficiency also impaired cytokines production, and myeloid cells migration, invasion, and bacterial killing capacity, establishing that HIF-1 is a key regulator of both cellular bioenergetics and inflammatory responses (34, 35).
Given these data, and the immediate response of leukocytes when faced with danger signals, it seemed plausible that TLR4 might utilize a unique regulatory mechanism to inactivate AMPKα and to induce HIF-1 activation in these cells. Indeed, our data show that AMPKα cleavage is regulated by intracellular MMP9, and that both AMPKα cleavage and MMP9 expression are required for mTORC1 activation and HIF-1α expression. Furthermore, we show that leukocytes from two patients cohorts express a signaling phenotype that is similar to that induced by LPS, and that this phenotypes is induced by a soluble TLR4 ligand present in patient’s blood. The TLR4-like signaling phenotype described in this study could provide a tool for future determination of TLR4 signaling in human diseases.
MATERIALS and METHODS
Antibodies, reagents, and inhibitors
The following antibodies were used at the indicated dilution: Actin (A2066; 1:1000) from Sigma. HIF-1α (sc-10790; 1:250), AMPKα (sc-25792; 1:1000), and MMP9 (sc-10737; 1:1000) from Santa Cruz Biotechnology, phospho-Raptor (Ser792) (#2083; 1:1000), Raptor (#2280; 1:200), phospho-p70 S6 Kinase (Thr389) (#9205; 1:1000), and phospho-AMPKα (Thr172) (#2535; 1:1000) were from Cell signaling Technology. The source of reagents and final concentrations used are as follows: LPS (lipopolysaccharide from Escherichia coli 0111:B4, Sigma). Where indicated, blood samples were treated with LPS at 10 ng/ml. Mice were challenged with LPS at 3 mg/kg body weight. CLI-095 (TAK-242; Invivogen; 3 μM), Polymyxin (Invivogen, 50 μg/ml), LY294002 (Cayman Chemical; 10μM), Rapamycin (Tocris Bioscience; 100 nM), A769662 (LC Labs; 100 nM), metformin (Sigma; 10 μM). MMP2/MMP9 inhibitor I ((inhibitor 1 (In. 1); 240 nM)), MMP2/MMP9 inhibitor IV (inhibitor 2; 27 nM), and MMP9 inhibitor I (inhibitor 3; 50 nM) were all from Millipore.
Human Subjects
The Rutgers Health Sciences Institutional Review Board approved the study. Written informed consent was obtained from all participants prior to inclusion in the study. T2DM and non-diabetic patients were recruited from Endocrinology clinics at Rutgers RWJMS. Patient demographics are presented in Table 1. Patients weighing less than 110 lbs, with an autoimmune disease, who have undergone major surgery in the past 3 months, with a current infection, with a symptomatic heart disease, and patients older than 89 years old were excluded from the study. The cardiac surgery patients were recruited from Rutgers RWJMS and Robert Wood Johnson University Hospital. Patient demographics are presented in Table 2. Premenopausal women, patients on insulin, with re-operative surgery, on pre-operative steroid therapy, on non-steroidal anti-inflammatory medication other than aspirin, and patients maintained on immunosuppressive medications or chemotherapeutic agents were excluded from the study. LPS (0.1 ng/kg) was administered in vivo as previously described (15). For in vitro studies, blood drawn into EDTA-containing tubes was separated into aliquots, and treated with LPS (10 ng/ml) or the specified inhibitors for the indicated time. Leukocytes were isolated as described (15). Lysates containing equal protein amounts were analyzed by immunoblotting. Neutrophils were isolated using Ficoll-Hypaque (Sigma-Aldrich) centrifugation followed by dextran (MW= 500,000) sedimentation. For the “mixing experiments”, patient blood samples were sedimented at unit gravity for 1.5 hours. The upper plasma fraction was recovered leaving the cellular fraction intact. The plasma fraction was next centrifuged for 10 min at 1800×g to remove residual cells. The plasma was stored at −70°C. Healthy donor’s blood was either not treated (N.T.), or treated for 1 hour with DMSO (vehicle; 0.5%), with the TLR-4 inhibitor CLI-095 (TAK-242; 3 μM), the AMPKα activator A769662 (100 nM), the MMP2/MMP9 inhibitor I (In. 1; 240 nM), or the PI-3K inhibitor LY294002 (10μM). The samples were then centrifuged for 5 min at 1800×g at 4°C. The upper plasma fraction was removed and replaced with an equal volume of patient’s plasma. The samples were rocked gently for 2 hour. The healthy donor’s leukocytes were then isolated and analyzed by immunoblotting. In another set of experiments, patient plasma was treated for 2 hours with polymyxin at 50 microgram/ml, before mixing it with healthy donor’s leukocytes.
Table 1.
Non-diabetics and Type 2 diabetics patient characteristics a
| Characteristics | Non-diabetics | T2DM |
p value between groups |
|---|---|---|---|
| Total number | 10 | 13 | |
| Age (years) | 61.4±4.1 | 61.1±2.1 | |
| Sex (Male/Female) | 5/5 | 10/3 | |
| BMI (kg/m2) | 34±3.3 | 29.5±0.9 | |
| FPG (mg/dL) | 101±5.3 | 147±14.1 | 0.024 |
| HbA1C (%) | 5.9±0.1 | 7.7±0.3 | 0.002 |
| Cholesterol (mg/dL) | 157±14 | 171±16 | |
| HDL cholesterol (mg/dL) | 49.0±4.5 | 49.7±2.3 | |
| LDL cholesterol (mg/dL) | 89.0±10.4 | 96.0±13.7 | |
| Triglyceride (mg/dL) | 94.4±20.3 | 126.7±11.7 | |
| Alanine aminotransferase (IU/L) | 28.0±8.6 | 30.3±6.4 | |
| Aspartate aminotransferase (IU/L) | 18.0±2.4 | 27.3±3.2 |
Means ± standard errors of the means
BMI, Body mass index; FPG, fasting plasma glucose; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Table 2.
Cardiac surgery with cardio pulmonary bypass patient characteristics a
| Characteristic | N=26 |
|---|---|
| Age (years) | 68±2.3 |
| Sex (Male/Female) | 15/12 |
| BMI (kg/m2) | 30±1.3 |
| FPG (mg/dL) | 120±4.6 |
| HbA1C (%) | 5.9±0.1 |
| Cholesterol (mg/dL) | 179±11 |
| HDL cholesterol (mg/dL) | 55.0±4.1 |
| LDL cholesterol (mg/dL) | 94.8±6.4 |
| Triglyceride (mg/dL) | 122.7±21.9 |
| Alanine aminotransferase (IU/L) | 32.6±6.9 |
| Aspartate aminotransferase (IU/L) | 34.7±9.3 |
| OR time (hrs) | 3.3±0.1 |
| CPB time (hrs) | 1.2±0.1 |
Means ± standard errors of the means.
In vitro MMP9 degradation assay
Recombinant Human MMP9 (purchased from R&D systems) was activated by incubation with p-aminophenylmercuric acetate ((AMPA; final concentration 1 μM; (Sigma)) for 24 hours at 37°C. For controls, whole blood was divided. One part was stimulated with LPS (10 ng/ml) for 2 hours. Leukocytes were isolated and lysed in RIPA buffer. The other part was used for unstimulated leukocytes isolation. The leukocytes were lysed in buffer containing 25 mM Tris-HCl (pH 7.5), 100 mM NaCl, and 0.1% (v/v) NP-40. The lysates were incubated for 30 min with AMPA at a final concentration of 1μM, or with 2 μg of recombinant MMP9 pre-activated with AMPA. The reactions were stopped with the addition of sample buffer.
Immunoprecipitation
Leukocytes were lysed with lysis buffer containing 25mM Tris/HCl (pH 7.5), 50mM KCl, 2mM MgCl2, 1mM EDTA, 0.5% Triton X-100, 0.5 mM PMSF and Complete protease inhibitor cocktail (Roche). The lysate was pre-cleared with 50 μl of normal rabbit serum (Santa Cruz) and protein A/G agarose beads (Santa Cruz) (20 μL). AMPKα antibody (1:150) (Santa Cruz) or MMP9 antibody (1:50) (Santa Cruz) were added to the cell lysate and incubated with gentle rocking overnight at 4°C. Afterwards, protein A/G agarose beads (20 μL) were added to the lysates, and the samples were incubated with gentle rocking for 3 hours at 4°C. Lysate was spun down and the pellet was washed 5 times with 500 μL of cell lysis buffer. The pellet was resuspended in 20 μL 4× sodium dodecyl sulfate sample buffer and heated to 97°C for 5 minutes. Subsequently, the samples were analyzed by western blotting. The PVDF membrane was blocked with 10% skim milk and incubated overnight with the specified antibodies. The specific signal was amplified by HRP-conjugated secondary antibodies, developed by ECL substrate (Pierce) and visualized by autoradiography.
Animal studies
Animal studies were approved by the Rutgers RWJMS Institutional Animal Care and Use Committee. Normal C57/BL6 mice and MMP9 null mice were purchased from Jackson laboratory. Animals were challenged with a bolus dose of LPS (i.p. injection, 3 mg/kg in 300 μl of saline). The mice were sacrificed by CO2 inhalation. Blood was obtained by heart puncture and leukocytes were isolated using the protocol used for human leukocytes (30).
Results
LPS induces AMPKα proteolytic cleavage, Raptor Ser-792 dephosphorylation, S6K1 Thr-389 phosphorylation, HIF-1α and MMP9 expression in human and mice leukocytes
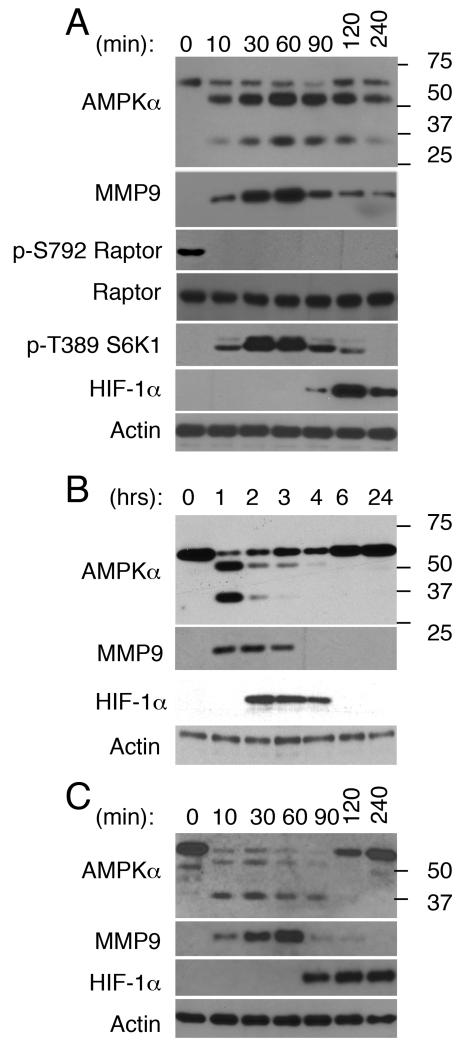
AMPK can inhibit mTORC1 by direct phosphorylation of its critical subunit Raptor at Ser-792 (20), while mTORC1 phosphorylates S6K1 at Thr-389, a site required for S6K1 activation (21, 22). Recently we reported that administration of LPS to human subjects triggers proteolytic cleavage of AMPKα (63-kD) in leukocytes, yielding two new protein bands approximately 50- and 35-kD in size (15). We surmised that AMPKα degradation contributes to AMPK inactivation. If true, AMPKα degradation should correlate with mTORC1 and S6K1 activation. Analyses of human leukocytes treated with LPS in vitro confirmed this possibility since AMPKα degradation, Raptor dephosphorylation at Ser-792, and S6K1 phosphorylation at Thr-389 were all detected as early as 10 min post-LPS stimulation (Fig 1A). Though S6K1 phosphorylation at Thr-389 was also seen by 10 min, it peaked 30-60 min post-treatment. HIF-1α expression, on the other hand, was first detected by 90 min.
FIGURE 1. LPS-induced changes in AMPKa expression correlate with Raptor dephosphorylation at Ser-792 and S6K1 phosphorylation at Thr-389, both indicative of mTORC1 activation, as well as increases in MMP9 and HIF-1a expression in human leukocytes and neutrophils.
In vitro and in vivo LPS-induced changes in human leukocytes and neutrophils were characterized by western blotting. Actin served as a loading control throughout. (A) Leukocytes were isolated from blood samples activated with LPS in vitro for the indicated time, or (B) blood samples obtained from subjects 0-24 hours after these subjects were challenged with LPS. (C) Neutrophils were isolated and then stimulated with LPS in vitro for the indicated time.
Neutrophils are the most abundant leukocyte cell type in human blood, constituting 50-65% of all circulating leukocytes at steady state. By 2 hours post-LPS infusion to humans, neutrophils constitute ~90% of all circulating blood leukocytes (15). Based on this information it seemed reasonable that the changes detected in leukocytes challenged with LPS in vivo for 2 hours (Fig 1B)(15) reflect changes that unfold in neutrophils. Indeed, purified neutrophils stimulated with LPS in vitro (Fig 1C) exhibited temporal changes in AMPKα and HIF-1α expression, which reproduced those seen in leukocytes challenged with LPS in vitro and in vivo (Fig. 1A and 1B).
Since prior studies showed that LPS triggers a rapid increase in matrix metalloproteinase 9 (MMP9) transcripts and protein expression in neutrophils (36), while others linked AMPK activation to the regulation of MMP9 expression in mice fibroblasts (37), and ii), we next asked whether the increase in intracellular MMP9 expression is sufficiently rapid to potentially account for the change in AMPKα expression. Intracellular MMP9 was detected within 10 min in both LPS-stimulated leukocytes (Fig 1A) and neutrophils (Fig 1C), and its levels continued to rise reaching a peak by 60 min. Intracellular MMP9 was also expressed in leukocytes challenged with LPS in vitro (Fig 1B). These data establish that the onset of AMPKα cleavage and the increase in intracellular MMP9 expression are both rapid and overlap.
Recently we reported that administration of LPS to mice triggers temporal changes in ATP levels, AMPKα and HIF-1α expression in leukocytes (30). However, AMPKα proteolytic fragments of 50- and 35-kD, which are seen in human leukocytes, are not detected in leukocytes from LPS-challenged mice. Here we examined and confirmed that as seen in human leukocytes, the changes in AMPKα expression correlate with Raptor Ser-792 dephosphorylation and S6K1 Thr-389 phosphorylation in mice leukocytes challenged with LPS in vivo (Fig 2A).
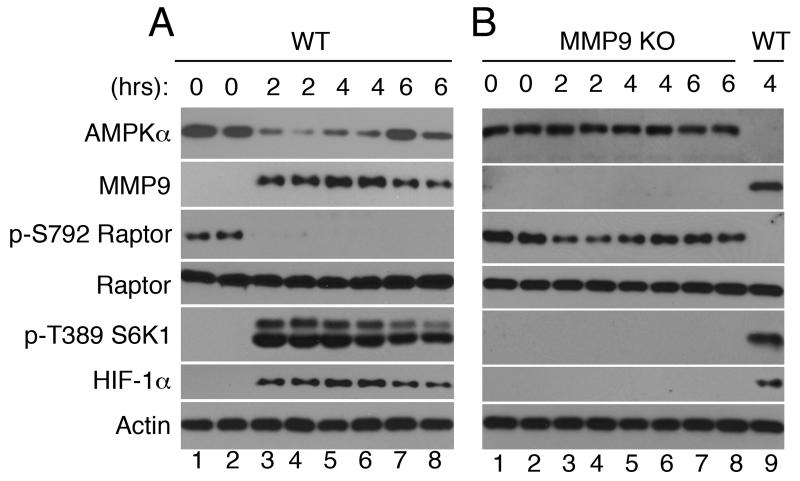
FIGURE 2. Characterization of changes in protein expression/activation in leukocytes from wild type and MMP9 knockout mice challenged with LPS in vivo.
(A) Wild type and (B) MMP9 knockout mice were challenged with LPS (3 mg/kg) for the indicated time. Each lane represents a sample obtained from a single mouse. Leukocytes obtained from a wild type mouse challenged with LPS for 4 hours served as a positive control (B, lane 9).
AMPKα degradation and the increase in intracellular MMP9 expression are related events
Since AMPKα is cleaved as soon as intracellular MMP9 expression reaches detection level (Fig 1), we wanted to know whether MMP9 is required for AMPKα degradation. To this end, we challenged genetically deficient MMP9 mice with LPS. Strikingly, AMPKα degradation, Raptor Ser-792 dephosphorylation, S6K1 Thr-389 phosphorylation, and HIF-1α expression, were all absent in LPS-challenged MMP9 deficient mice leukocytes (Fig. 2B). These data establish that intracellular MMP9 is required for AMPKα degradation, mTORC1 and S6K1 activation, as well as HIF-1α expression in mice leukocytes activated with LPS in vivo.
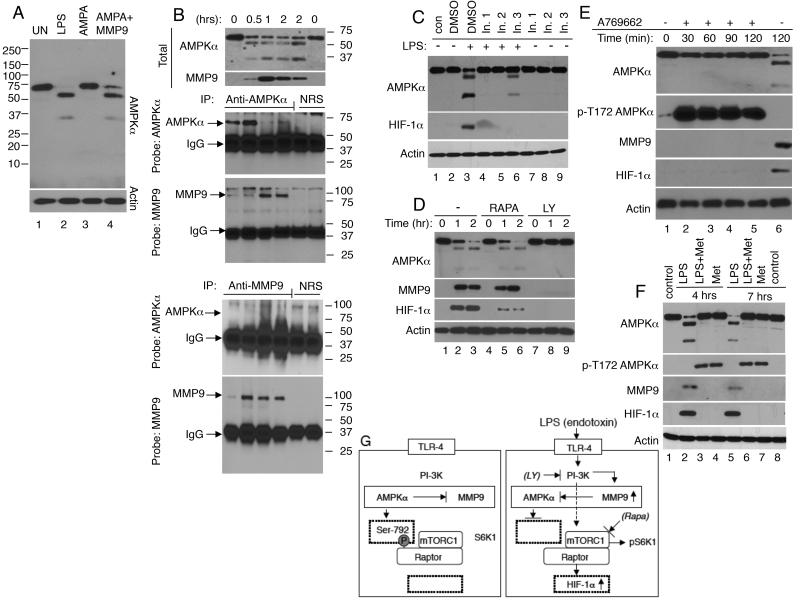
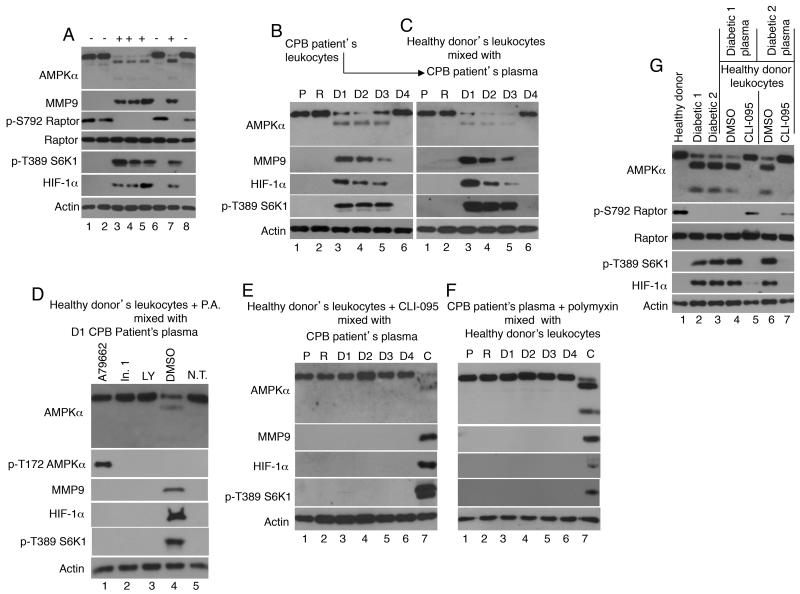
As human neutrophils are not amenable to genetic manipulations, we undertook four complementary approaches to determine whether the increase in MMP9 expression and AMPKα degradation are related events. Firstly, we tested whether activated MMP9 could cleave AMPKα in vitro. An unstimulated human leukocyte lysate sample was incubated for 30 min with recombinant MMP9, which was pre-activated by incubation with p-aminophenylmercuric acetate (AMPA). A second sample was incubated with AMPA alone. Controls included unstimulated and LPS stimulated leukocyte lysates. Activated recombinant MMP9, but not AMPA alone, triggered AMPKα degradation and appearance of AMPKα degradation products indistinguishable in size from those seen in LPS-stimulated leukocytes (Fig. 3A lanes 2 and 4). Secondly, we asked whether there was an association between AMPKα and MMP9. Lysates from leukocytes treated with LPS in vitro for the indicated time were immunoprecipitated with antisera directed against AMPKα, MMP9, or control antisera. A very low level of MMP9 was detected in AMPKα immunoprecipitates of leukocytes exposed to LPS for 0 or 0.5 hours (Fig. 3B). Though the level of full-sized AMPKα declined with time, the abundance of MMP9 in the immunoprecipitates increased at 1-2 hours post-LPS stimulation, likely reflecting an increase in AMPKα/MMP9 interaction as the levels of intracellular MMP9 increased. The MMP9 immunoprecipitates showed a significant increase in MMP9 expression 0.5-2 hours post-LPS stimulation, but no intact AMPKα was detected these samples. These data suggest that only a small fraction of total intracellular MMP9 interacts with AMPKα. Since the polyclonal AMPKα antibodies used in this study were raised against a carboxyl terminal end peptide, it appears that MMP9 remains associated with the carboxyl-terminal end of AMPKα even after AMPKα is cleavage. Thirdly, we examined the effect of three distinct MMP9 inhibitors on LPS/TLR4 signaling. Two of three MMP9 inhibitors prevented AMPKα cleavage and the increase in HIF-1α expression (Fig. 3C). Fourthly, we examined the effect of the PI3K inhibitor, LY294002, and mTORC1 inhibitor, rapamycin, on LPS-induced responses. LY294002 prevented LPS-induced AMPKα cleavage and the increases in MMP9 and HIF-1α expression, while rapamycin suppressed HIF-1α expression (38) having no effect on AMPKα or MMP9 expression (Fig. 3D). Taken together, all four approaches support the possibility that intracellular MMP9 contributes to AMPKα degradation either directly or indirectly.
FIGURE 3. Evidence that AMPKα and MMP9 function within a feedback loop in human leukocytes.
(A) To determine whether activated MMP9 could cleave MMP9 in vitro, unstimulated leukocytes were lysed (UN; lane 1), or treated for 30 min with p-aminophenylmercuric acetate (AMPA; lane 3), or with recombinant AMPA-activated MMP9 (2 μg; lane 4). As a control whole blood leukocytes were treated with LPS (10 ng/ml) for 2 hours, and then isolated and lysed (lane 2). (B) To ask whether AMPKα and MMP9 interact, leukocytes stimulated with LPS in vitro for the indicated time were lysed and analyzed by immunoblotting (upper panel) or subjected to immnoprecipitation (IP) with antisera directed against AMPKα, MMP9, or control normal sera (NRS). MMP9 was clearly visible in AMPKα immunoprecipitates from leukocytes exposed to LPS for 1-2 hours, but not in control NRS immunoprecipitates. Intact AMPKα was not detected in the MMP9 immunoprecipitates suggesting that only a small fraction of total intracellular MMP9 interacts with AMPKα. (C) To examine the effect of MMP9 inhibitors on TLR4-signaling, human leukocytes were untreated (control; con) or treated for 2 hours with DMSO (vehicle; 0.5%; lanes 2 and 3), MMP2/MMP9 inhibitor I (In. 1; 240 nM; lanes 4 and 7), MMP2/MMP9 inhibitor IV (In. 2; 27 nM; lanes 5 and 8), or MMP9 inhibitor I (In. 3; 50 nM; lanes 6 and 9), and then left unstimulated (−; lanes 1-2 and 7-9) or stimulated with LPS for 90 minutes (+; lanes 3-6). MMP2/MMP9 inhibitor I and inhibitor IV suppressed the cleavage of AMPKα and HIF-1α expression. (D) To examine the effect of mTORC1 and PI-3K inhibitors on TLR4-signaling, human leukocytes were treated for 2 hours with DMSO (0.5%; lanes 1-3), rapamycin (RAPA;100 nM; lanes 4-6), or LY294002 (LY; 10 μM; lanes 7-9), and then stimulated with LPS for 0-2 hours. The mTORC1 inhibitor, rapamycin inhibited the expression of HIF-1α, while the PI-3K inhibitor, LY prevented the changes in AMPKα, MMP9 and HIF-1α expression. (E and F) To determine whether AMPK activation contributes to MMP9 expression, human leukocytes were untreated or pre-treated with two AMPK activators, (E) A769662 (100 nM) and (F) metformin (10 μM). (E) Leukocytes were untreated (lanes 1 and 6) or treated for 2 hours with A769662, and then with LPS (10 ng/ml) for the indicated time. (F) Untreated leukocytes were isolated and lysed at time 0 (lane 1) and 7 hours later (lane 8). Leukocytes were treated for 4 hours (lanes 2-4) or 7 hours (lanes 5-7) with LPS alone, LPS plus metformin (Met) or metformin alone. A769662 and metformin induced robust AMPKα phosphorylation at Thr-172. Neither AMPKα cleavage, nor MMP9 and HIF-1α expression were detected in leukocytes treated with A769662 plus LPS or metformin plus LPS. (G) A working model of TLR4 signaling in leukocytes. We propose that upon engagement of TLR4, PI-3K is activated and contributes to an increase in MMP9 expression and AMPKα cleavage. This leads to dephosphorylation of Raptor at Ser-792 enabling mTORC1 activation. Activated mTORC1 phosphorylates S6K1 at Thr-389 and induces HIF-1α expression.
Studies showed that activated AMPKα suppresses the expression of MMP9 in fibroblasts (37). To examine whether this scenario is relevant to leukocytes, leukocytes were treated with two AMPK activators, A769662 and metformin (39). As expected, both A769662 and metformin triggered AMPKα phosphorylation at Thr-172 (Fig 3 E and F). No Thr-172 phosphorylated protein band was detected when leukocytes were treated with LPS alone (Fig 3E lane 6 and 3F lanes 2 and 5). Significantly, phosphorylated AMPKα remained intact and neither MMP9 nor HIF-1α expression was detected in leukocytes pretreated with A769662 or metformin and then with LPS (Fig 3E and 3F). Collectively, these data indicate that AMPK and intracellular MMP9 function within a negative signaling feedback loop. Our working model is presented in Fig 3G.
AMPKα proteolytic cleavage, Raptor Ser-792 dephosphorylation, S6K1 Thr-389 phosphorylation, HIF-1α and MMP9 expression are all detected in leukocytes from Type 2 diabetes mellitus patients, and patients after cardiopulmonary bypass surgery
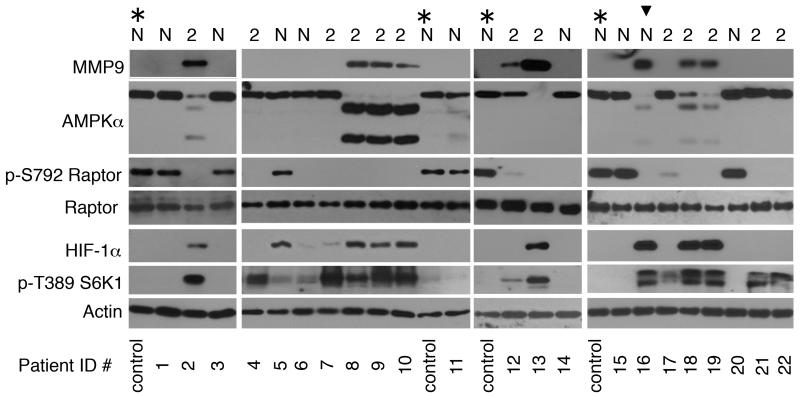
To determine whether the TLR4-signaling phenotype identified in LPS-stimulated leukocytes is relevant to human health, we studied leukocytes from two patient cohorts. The first cohort included 13 patients with Type 2 diabetes mellitus (T2DM), a disease that is associated with mild, chronic activation of the immune system, and 10 non-diabetics (Patient demographics are presented in Table 1). T2DM patients were defined by HbA1C ≥ 6.4 (40) or current treatment for diabetes. Eight of 13 (61%) patients with T2DM showed AMPKα cleavage, increases in MMP9 and HIF-1α expression, as well as dephosphorylation of Raptor at Ser-792 and S6K1 phosphorylation at Thr-389 (Fig 4). One patient initially classified as non-diabetic who exhibited leukocyte activation markers (patient ID # 16), was found subsequently to have a HbA1C of 6.0% and fasting glucose of 117 mg/dL, placing this patient within the range of pre-diabetes (40).
FIGURE 4. Leukocytes from patients with Type 2 diabetes mellitus (T2DM) exhibit a TLR4-like signaling phenotype.
Blood samples were obtained from non-diabetic (N) and Type 2 (2) diabetes mellitus patients (patient characteristics are presented in Table 1). (*; control) denotes a non-diabetic patient sample used repeatedly as a control. The researchers were blinded to the patient demographics while the samples were being analyzed. Leukocyte lysates were analyzed by immunoblotting. Arrowhead denotes a non-diabetic patient with a HbA1C of 6.0% and fasting plasma glucose of 117 mg/dL.
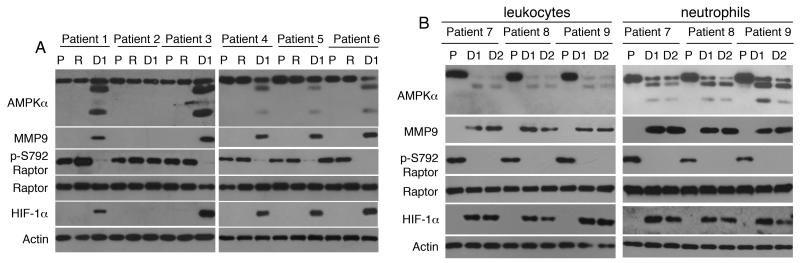
Since TLR4 is activated by a variety of endogenous ligands, we reasoned that patients scheduled for elective surgery could serve as a model for assessing TLR4 responses when endogenous “danger signals” are likely to be present. After obtaining informed consent, we enrolled and studied a cohort of 26 patients scheduled for elective cardiopulmonary bypass (CPB) surgery for coronary or valvular heart disease (patient demographics are presented in Table 2). Blood samples were obtained on the morning of the surgery, in the recovery room, and daily for up to 4 days post-surgery. Leukocytes from 20 of 26 (77%) patients showed the signaling phenotype that is characteristic to cells treated with LPS on Day 1 post-surgery (Fig 5A and 5B). Neutrophils displayed an identical signaling profile (Fig 5B).
FIGURE 5. Leukocytes from patients after cardiac surgery with cardiopulmonary bypass (CPB) exhibit a TLR4-like signaling phenotype.
Blood samples were obtained from patients (n=26) (patient characteristics are presented in Table 2) scheduled for elective cardiac surgery with CPB on the morning of the surgery (pre-surgery; P), in the recovery room (R), and on days 1 and 2 post-surgery (D1, D2). (A) Twenty of 26 patients studied exhibited AMPKα cleavage, MMP9 expression, Raptor dephosphorylation at Ser-792 and HIF-1α expression on day 1 post-surgery. Patient 2 was one of six patients who did not exhibit TLR4 activation markers in their leukocytes. Actin served as a loading control throughout. (B) Leukocytes and neutrophils obtained from three patients on the morning of the surgery (pre-surgery; P), day 1 (D1) and day 2 (D2) post-surgery exhibited an identical protein expression pattern.
A soluble and transmissible leukocyte activator is present within T2DM and CPB patient’s plasma
We then sought to determine whether the signaling pattern of CPB patients leukocytes was cell-intrinsic or reflected presence of a circulating, and potentially transmittable, TLR4-activating ligand. To this end, we prepared plasma and cellular fractions from patients blood samples and conducted a series of “mixing experiment”. In these experiments, plasma derived from patient blood was added to and mixed with cellular fractions isolated from a healthy donor’s blood. As proof-of-concept, we mixed plasma from 8 CPB patient samples obtained on day 1 post-surgery with healthy donor’s blood cells (Fig 6A). Four of these 8 CPB (marked “+”) showed a TLR4-like signaling phenotype on day 1 post-surgery, and four did not (marked “-”). Following mixing, the normal donor’s leukocytes reproduced the entire TLR4-like signaling phenotype initially detected in patient leukocytes. Significantly, only plasma from CPB patients who exhibited the characteristic TLR4-like signaling phenotype in their own leukocytes induced expression of TLR4 signaling components in healthy donor’s leukocytes.
FIGURE 6. Plasma from patients after cardiac surgery with cardiopulmonary bypass (CPB) and from T2DM patients contains a soluble factor that activates TLR4 signaling in healthy donor’s leukocytes.
We used a “mixing approach” to determine presence of TLR4 ligands in patient blood. In these experiments blood from patients and healthy donors was separated each separated into leukocyte and plasma fractions. The plasma fractions recovered from the patient samples were then mixed with healthy donor leukocytes. The signaling responses detected in healthy donor leukocytes reflected presence of a stimulating ligand in the patient blood/plasma. (A) As proof-of-principal, plasma fraction from 8 different CPB patients was mixed with leukocytes from a healthy donor. (+) and (−) indicate, respectively, which patient did or did show the TLR4-signaling phenotype in their own leukocytes. These data demonstrate that only plasma from (+) patients induced activation of healthy donor’s leukocytes. (B-E) Blood samples obtained from a CPB patient pre-surgery (P), in the recovery room (R), and on four consecutive days post-surgery (D1-D4) were separated into plasma and cellular fractions. (B) The patient leukocytes exhibited a protein expression pattern similar to that induced by LPS on days 1-3, but not on day 4 or prior to surgery. (C) Plasma obtained from the blood samples analyzed in Fig 6B were mixed with healthy donor leukocytes. Following mixing the health donor’s leukocytes exhibited the signaling phenotype first seen in the patient leukocytes. (D) Pre-incubation of healthy donor’s leukocytes with pharmacologic agents (P.A.) including an AMPKα activator (A769662; lane 1), a MMP9 inhibitor (inhibitor 1; lane 2), or a PI-3K inhibitor (LY294002), prior to mixing with patient’s plasma from the sample shown in Figure 6B lane 3 inhibited all patient plasma induced responses. Also shown are the phenotypes of healthy donor’s leukocytes treated with DMSO alone (0.5% vehicle control; lane 4) or not treated (N.T. lane 5). (E) Incubation of the healthy donor’s leukocytes with a TLR4 inhibitor, CLI-095, prior to mixing with patient plasma inhibited all patient plasma induced responses. (F) Patient plasma from the samples shown in Fig 6B were pretreated with polymyxin and then mixed with healthy donor’s leukocytes. (E and F, lane 7) Leukocytes from the sample shown in Fig 6B lane 3 served as a positive control. (G) Blood samples obtained from a healthy donor and two T2DM patients who exhibited a TLR4-like signaling phenotype in their own leukocytes were used in a “mixing experiment”. Healthy donor’s leukocytes were untreated (lanes 1-3), or were pretreated for 1 hour with DMSO (0.5%; lanes 4 and 6) or CLI-095 (TAK-242; lanes 5 and 7) prior to mixing with patient’s plasma.
In a second series of experiments we examined the signaling phenotype of leukocytes obtained from CPB patient blood prior to surgery as well as the four consecutive days following the surgery. AMPKα cleavage, increased MMP9 and HIF-1α expression, and S6K1 phosphorylation were detected in blood leukocytes obtained on days 1-3 following CPB, but not on day 4 or prior to surgery (Fig 6B), suggesting that the changes in leukocyte signaling reflect an acute response that ends by day 4. Furthermore, after mixing with plasma from the CPB patient samples shown in Fig 6B, healthy donor’s leukocytes expressed a similar signaling phenotype (Fig 6C). To ask whether the signaling pathway summarized in the model shown in Figure 3G is relevant to responses induced by patient plasma, healthy donor’s leukocytes were pretreated with pharmacologic agents described earlier (please see Fig 3 C-F), including the AMPKα activator (A769662), the MMP9 inhibitor ((Inhibitor 1; In. 1)), and the PI-3K inhibitor (LY294002) prior to exposure to patient plasma obtained on day 1 post-surgery. All three pharmacologic agents, but not the DMSO control, inhibited the patient’s plasma induced responses (Fig 6D). Then, to determine whether the component in T2DM and CPB patient plasma that regulates leukocyte activation is a TLR4 ligand, healthy donor’s cellular fractions were treated with CLI-095 (TAK-242), a specific TLR4 signaling inhibitor (41), prior to mixing and incubation with CPB patient plasma samples from the samples shown in Fig 6B. CLI-095 prevented the CPB patient plasma-induced changes in healthy donor’s leukocytes (Fig 6E). To address the possibility that endogenous LPS is present in patient plasma, CPB patient’s plasma from the samples shown in Fig 6B were treated with the antibiotic polymyxin prior to mixing with the healthy donor’s leukocytes (Fig 6F). Polymyxin is a natural polypeptide antibiotic that binds the lipid A moiety of LPS and thus preventing LPS binding to TLR4 (42). Polymyxin prevented CPB patient plasma-induced changes in the healthy donor’s leukocytes (Fig 6E). A “mixing experiment” in which plasma from two T2DM patients was mixed with healthy donor’s leukocytes showed that the patient leukocytes phenotype could be transferred to healthy donor’s leukocytes (Figure 6G lanes 4 and 5), and that the TLR-4 inhibitor, CLI-095 prevented these responses (Fig 6G lanes 5 and 7). These data link the characteristic pattern of activation of multiple indicators of TLR4 signaling to presence of a TLR4 activating factor/ligand, and possibly LPS itself, in patient plasma.
Discussion
In this study we set to identify the mechanism by which AMPKα is cleaved in response to LPS stimulation, and how this event is linked to HIF-1α expression, a key regulator of cellular bioenergetics and myeloid cell functions (34, 35). In considering which protease might cleave AMPKα, MMP9 came to mind since LPS triggers within minutes a robust increase in MMP9 transcripts and protein expression in human neutrophils (36). In addition, MMPs are known to contribute to all phases of inflammation by cleaving substrates such as cytokines and chemokines (43). The striking absence of AMPKα cleavage, Raptor Ser-792 dephosphorylation, S6K1 Thr-389 phosphorylation, and HIF-1α expression in MMP9-deficient mice leukocytes challenged with LPS in vivo provided the first concrete evidence that MMP9 is required for AMPKα cleavage and TLR4 signaling. We propose that intracellular MMP9 also regulates the expression of AMPKα in human leukocytes/neutrophils based on the following lines of evidence: i) the increase in intracellular MMP9 protein expression in leukocytes treated with LPS in vivo or in vitro is sufficiently rapid (10 min) to account for AMPKα cleavage; ii) MMP9 is detected in AMPKα immunoprecipitates indicating that the two proteins interact; iii) activated recombinant MMP9 cleaves human AMPKα in vitro producing two proteolytic fragments similar in size to those detected in LPS-treated leukocytes and neutrophils; iv) two of three MMP9 inhibitors, as well as the PI-3K inhibitor LY294002 blocked both AMPKα degradation and HIF-1α expression; v) AMPK activators which triggered AMPKα phosphorylation at Thr-172 prevented the expression of MMP9 in LPS-treated leukocytes. We conclude that AMPKα and MMP9 interact/function within a negative feedback loop. When expressed, MMP9 contributes either directly or indirectly to AMPKα degradation, whereas, as seen in fibroblasts (37), activated AMPKα suppresses the expression of MMP9.
MMPs, including MMP9, are secreted and degrade extracellular matrix proteins and other extracellular substrates. The possibility that intracellular MMP9 has an intracellular function and degrades intracellular protein(s) in leukocytes is surprising, but not without precedent. Studies from Schulz’s group (44, 45), have demonstrate that MMP2, the MMP with the closest sequence similarity to MMP9 (46, 47), degrades a number of cytoskeletal proteins in cardiac myocytes subjected to ischemia reperfusion injury (48, 49). MMPs are commonly activated when their pro-peptide is removed by cleavage. However, MMP-1, -2, and -8, and-9 are all also activated in the presence of peroxnitrite (ONOO−) (50, 51). Since activated leukocytes/neutrophils produce large amounts of peroxnitrite, it is plausible that this potent oxidizing compound is used not only to kill microorganisms but also to activate intracellular MMP9 once expressed in leukocytes. It is particularly interesting to note that peroxnitrite-activated MMPs can revert from an active to an inactive state in the presence of reducing agents (50). These data highlight the possible existence of a signaling mechanism that links presence of reactive oxygen species to mTORC1, S6K1 and HIF-1 activation.
The relationship between HIF-1α and mTORC1 is complex. Under chronic hypoxic conditions HIF-1α suppresses mTORC1 through REDD1, BNIP3, or PML, which act at distinct points upstream of mTORC1 (52-56). A reversed signaling pathway, positioning mTORC1 upstream to HIF-1α, was uncovered in PC-12 cells exposed to very short periods (15-30s) of hypoxia (referred to as intermittent hypoxia) (57). Intermittent hypoxia contributed to NADPH oxidase activation and generation of reactive oxygen species in PC-12 cells (57). When taken as a whole, the model that emerges is one in which reactive oxygen species activate MMP9 triggering AMPKα degradation, mTORC1 activation, and then HIF-1α expression. O’Neill and Hardie have recently proposed that AMPK activation limits inflammation (58) by suppressing the expression of HIF-1α. Our data support this possibility and highlight a novel mechanism by which the negative regulatory effects of AMPK relative to HIF-1α expression are switched off to enable leukocytes to fully commit to their inflammatory functions.
Leukocytes synthesize and secret a variety of inflammatory mediators, including pro- and anti-inflammatory cytokines, shortly after activation (59). Our data show that the proteolytic cleavage of AMPKα is associated with mTORC1 and S6K1 activation. These data suggest that AMPKα inactivation has two roles: i) to enable HIF-1 activation, and ii) to enable a rapid increase in protein synthesis.
This study also investigated whether the TLR4 signaling phenotype described herein is clinically relevant. Our data demonstrate that leukocytes from two disparate patient cohorts, patients with T2DM and patients after CPB, exhibit a signaling phenotype that is similar to that induced by LPS. In addition, plasma from these patients appears to contain a soluble and transferable ligand(s) that can trigger TLR4-like signaling in healthy donor leukocytes. Furthermore, pharmacologic response modifiers that inhibited LPS-induced responses in vitro, also prevented patient plasma induced responses in healthy donor’s leukocytes, suggesting involvement of common signaling mediators in both scenarios. Whether these or other pharmacological agents could reverse the signaling pathway in patient leukocytes once the pathway is already activated and in the presence of the activating ligand(s), is currently undetermined.
The data highlight the likely involvement of TLR4 in physiologic stress responses that are induced by endogenous agonists, and/or gut-derived LPS. We were unable to detect presence of LPS in patient blood using commercially available Limulus Amebosyte Lysate (LAL) based assays (detection limit 0.1 EU/ml). However, whether LPS can be detected in human serum is currently controversial (60, 61). We reported that an LPS concentration as low as 0.1 ng/kg triggers onset of TLR4 signaling in healthy subjects leukocytes in the absence of systemic responses (15). LPS used in those studies had an activity of 10 EU/ng. Estimated blood volume per kg body weight is 75 ml for men and 65 ml for women. Administration of 0.1 ng/kg would have equaled to 1 EU/75ml for men and 1 EU/65ml blood for women, or 0.013-0.015 EU/ml. Furthermore, the effect of even lower LPS concentrations might be augmented by presence of additional TLR4 ligands, such as fibronectin fragments and/or saturated fatty acids (4-10) in patients blood.
In conclusion, this study describes a novel TLR4-signaling arm that is functional in leukocytes/neutrophils, and required for regulating AMPK, mTORC1, S6K1, and HIF-1. The study also demonstrates that this signaling pathway is clinically relevant and significant since it is expressed in patient leukocytes. Analyses of leukocytes from larger and more diverse patient cohorts could provide the basis for better understanding of TLR4 signaling in chronic diseases and opportunities for appropriate therapeutic interventions.
Acknowledgments
The study was supported in part by the NIH NIEHS sponsored Rutgers Center for Environmental Exposures and Disease, Grant # NIEHS P30ES005022, and funds from NJ Health Foundation (to B.H. and L.Y.L.)
Footnotes
Abbreviations used in this study: AMPK, AMP-activated protein kinase; CPB, cardiopulmonary bypass; HIF-1, hypoxia inducible factor 1; MMP9, matrix metalloproteinase 9; mTORC1, mammalian target of rapamycin complex-1; Raptor, regulatory-associated protein of mTOR; S6K1, p70 ribosomal protein S6 kinase; T2DM, Type 2 diabetes mellitus
REFRENCES
- 1.Beutler B. TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol. 2002;270:109–120. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- 2.Lowry SF. Human endotoxemia: a model for mechanistic insight and therapeutic targeting. Shock. 2005;24(Suppl 1):94–100. doi: 10.1097/01.shk.0000191340.23907.a1. [DOI] [PubMed] [Google Scholar]
- 3.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Rakoff-Nahoum S, Medzhitov R. Toll-like receptors and cancer. Nat Rev Cancer. 2009;9:57–63. doi: 10.1038/nrc2541. [DOI] [PubMed] [Google Scholar]
- 5.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 6.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 7.Chen GY, Nunez G. Sterile inflammation: sensing and reacting to damage. Nat Rev Immunol. 2010;10:826–837. doi: 10.1038/nri2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. The Journal of biological chemistry. 2004;279:7370–7377. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 9.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fink MP. Cytopathic hypoxia. Mitochondrial dysfunction as mechanism contributing to organ dysfunction in sepsis. Crit Care Clin. 2001;17:219–237. doi: 10.1016/s0749-0704(05)70161-5. [DOI] [PubMed] [Google Scholar]
- 12.Brealey D, Brand M, Hargreaves I, Heales S, Land J, Smolenski R, Davies NA, Cooper CE, Singer M. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet. 2002;360:219–223. doi: 10.1016/S0140-6736(02)09459-X. [DOI] [PubMed] [Google Scholar]
- 13.Andreasen AS, Krabbe KS, Krogh-Madsen R, Taudorf S, Pedersen BK, Moller K. Human endotoxemia as a model of systemic inflammation. Curr Med Chem. 2008;15:1697–1705. doi: 10.2174/092986708784872393. [DOI] [PubMed] [Google Scholar]
- 14.Calvano SE, Xiao W, Richards DR, Felciano RM, Baker HV, Cho RJ, Chen RO, Brownstein BH, Cobb JP, Tschoeke SK, Miller-Graziano C, Moldawer LL, Mindrinos MN, Davis RW, Tompkins RG, Lowry SF, Large IA. Scale Collab Res Program. 2005. A network-based analysis of systemic inflammation in humans. Nature. 437:1032–1037. doi: 10.1038/nature03985. Epub 2005 Aug 1031. [DOI] [PubMed] [Google Scholar]
- 15.Haimovich B, Zhang Z, Calvano JE, Calvano SE, Kumar A, Macor MA, Corbett S, Coyle SM, Lowry SF. Cellular Metabolic Regulators: Novel Indicators of Low-Grade Inflammation in Humans. Ann Surg. 2013 doi: 10.1097/SLA.0b013e31829a4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev. 2011;25:1895–1908. doi: 10.1101/gad.17420111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, Hardie DG. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. The Journal of biological chemistry. 1996;271:27879–27887. doi: 10.1074/jbc.271.44.27879. [DOI] [PubMed] [Google Scholar]
- 18.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 20.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pullen N, Thomas G. The modular phosphorylation and activation of p70s6k. FEBS Lett. 1997;410:78–82. doi: 10.1016/s0014-5793(97)00323-2. [DOI] [PubMed] [Google Scholar]
- 22.Pearson RB, Dennis PB, Han JW, Williamson NA, Kozma SC, Wettenhall RE, Thomas G. The principal target of rapamycin-induced p70s6k inactivation is a novel phosphorylation site within a conserved hydrophobic domain. Embo J. 1995;14:5279–5287. doi: 10.1002/j.1460-2075.1995.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Makela TP, Alessi DR, Hardie DG. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol. 2003;2:28. doi: 10.1186/1475-4924-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. doi: 10.1016/j.cmet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 25.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. The Journal of biological chemistry. 2005;280:29060–29066. doi: 10.1074/jbc.M503824200. [DOI] [PubMed] [Google Scholar]
- 26.Hardie DG, Scott JW, Pan DA, Hudson ER. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003;546:113–120. doi: 10.1016/s0014-5793(03)00560-x. [DOI] [PubMed] [Google Scholar]
- 27.Neumann D, Suter M, Tuerk R, Riek U, Wallimann T. Co-expression of LKB1, MO25alpha and STRADalpha in bacteria yield the functional and active heterotrimeric complex. Mol Biotechnol. 2007;36:220–231. doi: 10.1007/s12033-007-0029-x. [DOI] [PubMed] [Google Scholar]
- 28.Park S, Scheffler TL, Rossie SS, Gerrard DE. AMPK activity is regulated by calcium-mediated protein phosphatase 2A activity. Cell Calcium. 2013;53:217–223. doi: 10.1016/j.ceca.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Wang MY, Unger RH. Role of PP2C in cardiac lipid accumulation in obese rodents and its prevention by troglitazone. Am J Physiol Endocrinol Metab. 2005;288:E216–221. doi: 10.1152/ajpendo.00004.2004. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Lowry SF, Guarente L, Haimovich B. Roles of SIRT1 in the acute and restorative phases following induction of inflammation. The Journal of biological chemistry. 2010;285:41391–41401. doi: 10.1074/jbc.M110.174482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semenza GL. HIF-1: upstream and downstream of cancer metabolism. Curr Opin Genet Dev. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papandreou I, Cairns RA, Fontana L, Lim AL, Denko NC. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer T, Yamanishi Y, Clausen BE, Forster I, Pawlinski R, Mackman N, Haase VH, Jaenisch R, Corr M, Nizet V, Firestein GS, Gerber HP, Ferrara N, Johnson RS. HIF-1alpha is essential for myeloid cell-mediated inflammation. Cell. 2003;112:645–657. doi: 10.1016/s0092-8674(03)00154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peyssonnaux C, Datta V, Cramer T, Doedens A, Theodorakis EA, Gallo RL, Hurtado-Ziola N, Nizet V, Johnson RS. HIF-1alpha expression regulates the bactericidal capacity of phagocytes. J Clin Invest. 2005;115:1806–1815. doi: 10.1172/JCI23865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pugin J, Widmer MC, Kossodo S, Liang CM, Preas H. L. n., Suffredini AF. Human neutrophils secrete gelatinase B in vitro and in vivo in response to endotoxin and proinflammatory mediators. Am J Respir Cell Mol Biol. 1999;20:458–464. doi: 10.1165/ajrcmb.20.3.3311. [DOI] [PubMed] [Google Scholar]
- 37.Morizane Y, Thanos A, Takeuchi K, Murakami Y, Kayama M, Trichonas G, Miller J, Foretz M, Viollet B, Vavvas DG. AMP-activated protein kinase suppresses matrix metalloproteinase-9 expression in mouse embryonic fibroblasts. The Journal of biological chemistry. 2011;286:16030–16038. doi: 10.1074/jbc.M110.199398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 39.Goransson O, McBride A, Hawley SA, Ross FA, Shpiro N, Foretz M, Viollet B, Hardie DG, Sakamoto K. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. The Journal of biological chemistry. 2007;282:32549–32560. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Inzucchi SE. Clinical practice. Diagnosis of diabetes. N Engl J Med. 2012;367:542–550. doi: 10.1056/NEJMcp1103643. [DOI] [PubMed] [Google Scholar]
- 41.Matsunaga N, Tsuchimori N, Matsumoto T, Ii M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol Pharmacol. 2010;79:34–41. doi: 10.1124/mol.110.068064. [DOI] [PubMed] [Google Scholar]
- 42.Rifkind D. Prevention by polymyxin B of endotoxin lethality in mice. J Bacteriol. 1967;93:1463–1464. doi: 10.1128/jb.93.4.1463-1464.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanlaere I, Libert C. Matrix metalloproteinases as drug targets in infections caused by gram-negative bacteria and in septic shock. Clin Microbiol Rev. 2009;22:224–239. doi: 10.1128/CMR.00047-08. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulz R. Intracellular targets of matrix metalloproteinase-2 in cardiac disease: rationale and therapeutic approaches. Annu Rev Pharmacol Toxicol. 2007;47:211–242. doi: 10.1146/annurev.pharmtox.47.120505.105230. [DOI] [PubMed] [Google Scholar]
- 45.Sariahmetoglu M, Crawford BD, Leon H, Sawicka J, Li L, Ballermann BJ, Holmes C, Berthiaume LG, Holt A, Sawicki G, Schulz R. Regulation of matrix metalloproteinase-2 (MMP-2) activity by phosphorylation. FASEB J. 2007;21:2486–2495. doi: 10.1096/fj.06-7938com. [DOI] [PubMed] [Google Scholar]
- 46.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Van den Steen PE, Dubois B, Nelissen I, Rudd PM, Dwek RA, Opdenakker G. Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9) Crit Rev Biochem Mol Biol. 2002;37:375–536. doi: 10.1080/10409230290771546. [DOI] [PubMed] [Google Scholar]
- 48.Ali MA, Cho WJ, Hudson B, Kassiri Z, Granzier H, Schulz R. Titin is a target of matrix metalloproteinase-2: implications in myocardial ischemia/reperfusion injury. Circulation. 2010;122:2039–2047. doi: 10.1161/CIRCULATIONAHA.109.930222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sawicki G, Leon H, Sawicka J, Sariahmetoglu M, Schulze CJ, Scott PG, Szczesna-Cordary D, Schulz R. Degradation of myosin light chain in isolated rat hearts subjected to ischemia-reperfusion injury: a new intracellular target for matrix metalloproteinase-2. Circulation. 2005;112:544–552. doi: 10.1161/CIRCULATIONAHA.104.531616. [DOI] [PubMed] [Google Scholar]
- 50.Okamoto T, Akaike T, Nagano T, Miyajima S, Suga M, Ando M, Ichimori K, Maeda H. Activation of human neutrophil procollagenase by nitrogen dioxide and peroxynitrite: a novel mechanism for procollagenase activation involving nitric oxide. Arch Biochem Biophys. 1997;342:261–274. doi: 10.1006/abbi.1997.0127. [DOI] [PubMed] [Google Scholar]
- 51.Okamoto T, Akaike T, Sawa T, Miyamoto Y, van der Vliet A, Maeda H. Activation of matrix metalloproteinases by peroxynitrite-induced protein S-glutathiolation via disulfide S-oxide formation. The Journal of biological chemistry. 2001;276:29596–29602. doi: 10.1074/jbc.M102417200. [DOI] [PubMed] [Google Scholar]
- 52.Wouters BG, Koritzinsky M. Hypoxia signalling through mTOR and the unfolded protein response in cancer. Nat Rev Cancer. 2008;8:851–864. doi: 10.1038/nrc2501. [DOI] [PubMed] [Google Scholar]
- 53.Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, Cordon-Cardo C, Simon MC, Rafii S, Pandolfi PP. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. [DOI] [PubMed] [Google Scholar]
- 54.Li Y, Wang Y, Kim E, Beemiller P, Wang CY, Swanson J, You M, Guan KL. Bnip3 mediates the hypoxia-induced inhibition on mammalian target of rapamycin by interacting with Rheb. The Journal of biological chemistry. 2007;282:35803–35813. doi: 10.1074/jbc.M705231200. [DOI] [PubMed] [Google Scholar]
- 55.Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr. Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sofer A, Lei K, Johannessen CM, Ellisen LW. Regulation of mTOR and cell growth in response to energy stress by REDD1. Mol Cell Biol. 2005;25:5834–5845. doi: 10.1128/MCB.25.14.5834-5845.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674–685. doi: 10.1002/jcp.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O’Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 59.Wright HL, Moots RJ, Bucknall RC, Edwards SW. Neutrophil function in inflammation and inflammatory diseases. Rheumatology (Oxford) 2010;49:1618–1631. doi: 10.1093/rheumatology/keq045. [DOI] [PubMed] [Google Scholar]
- 60.Pussinen PJ, Havulinna AS, Lehto M, Sundvall J, Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34:392–397. doi: 10.2337/dc10-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Romaschin AD, Walker PM. Endotoxin activity in whole blood by neutrophil chemiluminescence-A novel analytical paradigm. Clin Chem. 2000;46:1504–1506. [PubMed] [Google Scholar]