Abstract
Background
As we move towards planning for clinical trials in Facioscapulohumeral Muscular Dystrophy (FSHD), a better understanding of the clinical relationship with morphological changes in FSHD muscle biopsies will be important for stratifying patients and understanding post-therapeutic changes in muscle.
Methods
We performed a prospective cross-sectional study of quadriceps muscle biopsies in 74 genetically confirmed FSHD participants (64 FSHD1, 10 FSHD2). We compared a 12-point muscle pathology grade to genetic mutation, disease severity score, and quantitative myometry.
Results
Pathology grade had moderate correlations with genetic mutation (rho=−0.45, P<0.001), clinical severity score (rho=0.53, P<0.001), disease duration (rho=0.31, P=0.03), and quantitative myometry (rho=−0.47, P<0.001). We found no difference in the frequency of inflammation between FSHD types 1 and 2.
Conclusions
The pathology grade of quadriceps muscle may be a useful marker of disease activity in FSHD, and it may have a role in stratification for future clinical trials.
Keywords: Facioscapulohumeral muscular dystrophy, DUX4, muscle pathology, pathology grade, clinical trials
Introduction
Facioscapulohumeral muscular dystrophy (FSHD) is a common muscular dystrophy (prevalence of 1:15,000) that is slowly progressive, most often characterized by (asymmetric) weakness starting in the face, shoulder girdle and arms, and later followed by weakness in distal followed by proximal lower extremities.1-3 Age of first wheelchair use is related to genetic mutation and patient age, but overall approximately 20% of patients >age 50 years may require a wheelchair.2,4
Muscle biopsies from FSHD patients show non-specific myopathic changes, including rounding of muscle fibers, degenerating and regenerating fibers, increased internal nuclei, and later in the disease course, increased fibrosis. Up to one-third of muscle biopsies have been reported to show a lymphocyte-predominant inflammatory infiltrate.5,6 The relationship of these morphological changes to traditional measures of disease severity is not known.
Recent studies have suggested that both FSHD types 1 and 2 operate through a common downstream genetic mechanism of de-repression of a retrogene, DUX4, which is not normally expressed in somatic muscle tissue.7,8 Several lines of evidence show that low levels of DUX4 expression interfere with myogenic differentiation, lead to apoptotic cell death, and make cells more susceptible to oxidative stress.9-13 The relationship of these molecular changes to morphological changes in patient muscle biopsies is not known. A pathology grading system provides an important frame of reference for interpreting molecular changes in preclinical studies. Even in the absence of direct molecular evidence of disease activity in FSHD muscle biopsies, a better understanding of the muscle pathology in the disease can be important in 2 ways as we move toward clinical trial planning: 1) as a scheme for stratifying patients, and 2) to provide potential morphological evidence of change for proof-of-concept or early phase clinical studies.
Here we performed a prospective cross-sectional morphological study comparing FSHD muscle biopsies to other measures of disease severity.
Methods
We performed a cross-sectional observational study of genetically confirmed FSHD participants at the University of Rochester Medical Center from 2002 to 2013. The study was approved by the institutional review board, and written and informed consent was obtained from all participants.
Participants
FSHD participants were between age 18 and 75 years and had genetic confirmation per previously published protocols.14,15 For FSHD type 2 CpG methylation measurements were taken after cleavage with the methylation-sensitive endonuclease FseI, using a methylation threshold of <25%7. All FSHD2 participants had SMCHD1 gene analysis.7 Participants were ineligible if they had muscle wasting that made a needle biopsy impractical.
Assessments
Participants were evaluated during a single day visit in the General Clinical Research Center. In addition to collection of muscle biopsy samples, participants also filled out a clinical history and symptom questionnaire, had bedside manual muscle testing, quantitative myometry of the biopsied muscle prior to the biopsy, and were assigned a clinical severity score. Passive range of motion of the shoulders on abduction was estimated by the evaluator at the bedside. The average of left and right sides was used for analysis (SROM). Disease duration was defined as age at time of the study minus age at diagnosis with FSHD.
Quantitative myometry was performed using the dynamic-fatigue option of the Quantitative Muscle Assessment software version 4.2 (QMA Systems, Inc., Gainesville, GA). Average peak force after 3 maximal voluntary isometric contractions was measured per previously published guidelines.16 Raw data were transformed and normalized against an extensive database of strength measurements in normal volunteers and expressed as the number of standard deviations of the strength that would be predicted for a healthy person of the same age, gender, and height.17
The clinical severity score (CSS) is a 10-grade clinical severity scale developed by Ricci et al.18 This score takes into account the extent of weakness in various body regions and considers the descending spread of symptoms from face and shoulders to pelvic and leg muscles typical of FSHD (0=unaffected to 10=severely affected).
The histopathologic samples were graded for the severity of their pathologic changes based on 10μm sections stained with Hematoxylin & Eosin and Trichrome. Between 50 and 125 mg of tissue was obtained for each evaluation. The score is a summary of a single trained neuromuscular pathologist's impression of the pathology in the sample (typically 4 slides with 2 cross-sectional samples per slide). The pathology grade uses an ordinal scale to rank each morphological characteristic of FSHD muscle biopsies between 0 and 3 (0 = normal; 1= mild; 2=moderate; and 3=severe). The scoring sheet is available from the Fields center for FSHD Research website (https://www.urmc.rochester.edu/fields-center/protocols/documents/MBxHistopathReportrevAUG2011.pdf) The following categories were scored:
Variability in fiber size
Extent of central nucleation
Necrosis/regeneration
Interstitial fibrosis
The pathology grade is a sum of the 4 categories, which yields a score between 0 and 12 (0 = normal to 12 = severe dystrophic changes). In addition samples were ranked from 0 to 3 on the extent of inflammatory infiltrates.
Muscle Biopsy
Needle muscle biopsies were obtained from the vastus lateralis. This muscle was chosen to reduce the variability due to sampling from different muscles. We chose a quadriceps muscle because we felt a less clinically affected muscle would be more likely to show FSHD-specific changes at the pathological and molecular level and would be more likely to track with progression of disease than a muscle that was more end-stage clinically or pathologically. The skin and subcutaneous tissues were anesthetized with 1% lidocaine. A 3 mm incision was made in the skin and fascia, and muscle tissue was obtained using a side-cut Bergstrom needle (4 mm internal diameter). Details of the procedure are available at: http://www.urmc.rochester.edu/fields-center/protocols/needle-muscle-biopsy.cfm.
Statistical considerations
Standard statistical methods were used to describe all groups (FSHD1, FSHD2), including calculation of the median and first and third quartiles (i.e., interquartile range, IQR). The test for differences in distribution between FSHD1 and FSHD2 employed the Kruskal-Wallis test for factors that were either continuous data, or ordered data with more than 7 levels (e.g., 0-12 pathology grade). The Pearson chi-square test was used for testing difference in frequencies among FSHD types 1 and 2. Associations between pathology grade and other measures of disease (clinical severity score, quantitative myometry, average passive shoulder range of motion, disease duration, and D4Z4 fragment size) used Spearman correlation coefficient with Fisher z-transformation and 95% confidence limits. Differences in pathology grade by group based on residual D4Z4 fragment size used a Bonferroni correction for multiple testing. Effects of age and gender on pathology grade in biopsies evaluated for downstream DUX4 markers (n=15) were evaluated using the GLM procedure. Associations between use of assistive devices (wheelchair use, cane, or walker) and pathology grade were determined using logistic regression.19 The yearly increase in odds for use of assistive devices by pathology grade was derived from the logistic regression model and presented with associated Wald 95% confidence interval.19 All P-values are 2-tailed. The box and whisker plots reflect the standard calculations of the median and first and third quartiles (box). The whisker, measured from the median, is either 1 1/2 times the box width, or the most extreme raw value, whichever is less. Individual raw values beyond the whisker are indicated with a dot. Descriptive analysis and statistical tests were conducted using SAS version 9.3 (SAS Institute Inc., Cary, NC), and STATA version 11.2 (StataCorp, College Station, TX).
Results
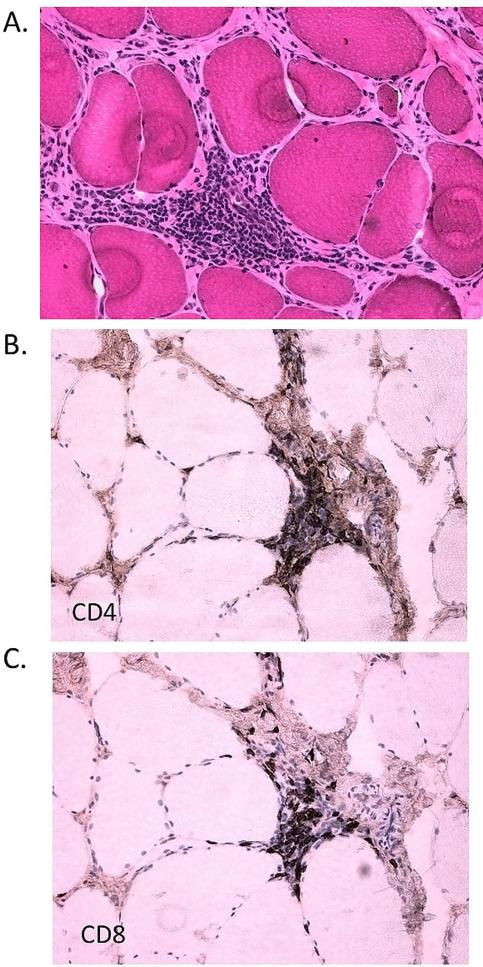
The pathology grade and clinical severity scores (CSS) were available for 74 participants (64 FSHD1 and 10 FSHD2, Table 1). Of the 10 FSHD2 participants, 6 had mutations in SMCHD1 predicted to be pathogenic. Baseline clinical characteristics of this cohort have been described previously;20 for the clinical characteristics used for correlations here no differences were seen in clinical measures between participants with FSHD types 1 or 2. Both groups were mostly men, predominately middle aged, and moderately affected. Morphological features of quadriceps biopsies were similar for types 1 and 2 and showed non-specific myopathic changes. There was no difference in pathology grade (FSHD1 median 3.0, IQR 2.0-4.0 versus FSHD2 median 3.0, IQR 2.0-4.0, P=0.78). The combined mean pathology grade for FSHD types 1 and 2 was 3.38, with a between-participant standard deviation of 1.94. The vastus lateralis is generally considered a muscle affected late in the disease course; however the vast majority of participants had pathology grade > 0 (n=72 or 97.3%), despite only moderate median clinical severity scores (median total population 5.0, IQR 3.0-6.0). We found no difference in the frequency of inflammation in FSHD types 1 and 2 (6.3% for FSHD1 versus 10% for FSHD2, P=0.66, Figure 1).
Table 1.
Pathology grade clinical characteristics
| Item | FSHD1 | FSHD2¶ | Total | P Value+ |
|---|---|---|---|---|
| n | 64 | 10 | 74 | - |
| Gender (%M) | 45 (70.3) | 7 (70.0) | 52 (70.3) | 0.98* |
| Median Age (Q1, Q3) | 50.0 (39.5, 57.0) | 51.5 (34.0, 62.0) | 50.0 (38.0, 59.0) | 0.67 |
| Median Age Dx (Q1, Q3)@ | 38.0 (24.0, 50.0) | 29.0 (18.0, 59.0) | 37.5 (24.0, 50.0) | 0.99 |
| Median D4Z4 (Q1, Q3) | 24.0 (18.5, 29.0) | 45.0 (42.0, 55.0) | 24.5 (19.0, 31.0) | <0.0001 |
| Median Meth (955 CI)% | 33.0 (28.0, 38.3) | 15.5 (12.0, 18.0) | 31.5 (23.0, 37.9) | <0.0001 |
| Median CSS (Q1, Q3) | 5.00 (3.00, 6.00) | 5.50 (5.00, 6.00) | 5.00 (3.00, 6.00) | 0.89 |
| Median Quad Path Grade (Q1, Q3) | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) | 3.00 (2.00, 4.00) | 0.78 |
| Median Quad QMT std (Q1, Q3)# | −1.29 (−2.82, −0.51) | −0.775 (−1.46, 0.032) | −1.24 (−2.51, −0.44) | 0.26 |
| Median SROM (Q1, Q3)$ | 80.0 (60.0, 135) | 80.0 (80.0, 90.0) | 80.0 (60.0, 135) | 0.96 |
6 had mutations in SMCHD1
Significance taken from the Kruskal-Wallis test
chi square test
n=50 (43 FSHD1, 7 FSHD2)
n=68 (58 FSHD1, 10 FSHD2)
n=50 (42 FSHD1, 8 FSHD2)
n=58 (49 FSHD1, 9 FSHD2), SROM = average of the passive range of motion to shoulder abduction for the left and right sides.
n=number; dx=diagnosis; Q1=first quartile; Q3=third quartile; Meth=methylation; CSS = clinical severity score; Quad=quadriceps; path=pathology; QMT=quantitative myometry; std = standard score; SROM = shoulder range of motion.
Figure 1.
Inflammation in an FSHD2 biceps muscle biopsy. CD4 lymphocyte predominant primarily perivascular inflammatory infiltrates can be seen, which are similar to FSHD1. A) Hematoxylin and eosin stain showing perivascular inflammatory infiltrate which is comprised of both B) CD4, and C) CD8 positive cells.
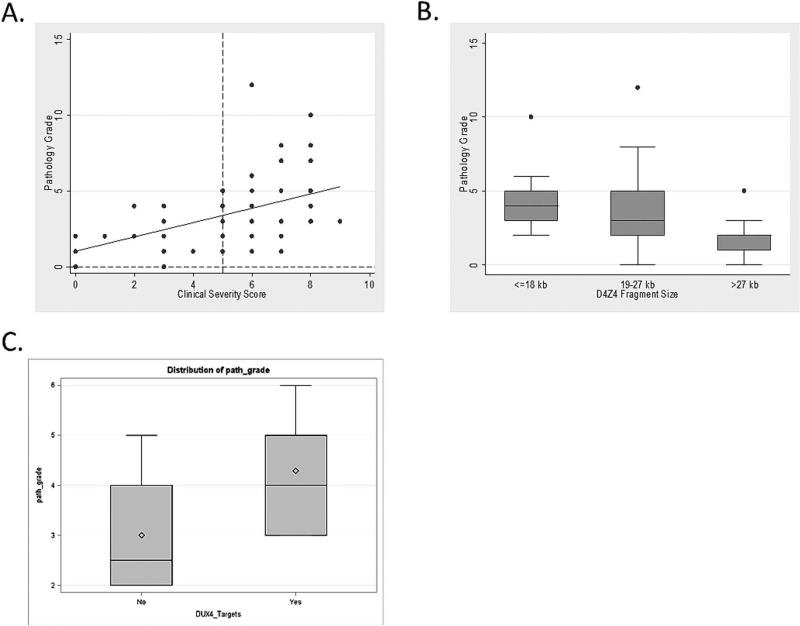
Because there were no differences in pathology grade or clinical characteristics, we grouped FSHD types 1 and 2 for correlations (with the exception of the relationship of pathology grade to the size of the residual D4Z4 fragment, which was FSHD1 only). We found moderate to strong correlations between muscle pathology grade and CSS (rho=0.53, P<0.0001), QMT standard score (rho=-0.47, P=0.0004), and average SROM (rho=-0.45, P=0.0003, Table 2). When evaluating the relationship between pathology grade and CSS a few details are notable: all but 2 participants with CSS scores less than 5 (no lower extremity involvement) showed pathological changes on muscle biopsy (Figure 2A). The pathology grade showed a moderate correlation with the size of the D4Z4 fragment for FSHD1 participants (rho=−0.45, P=0.0001). This relationship was driven largely by participants with the largest residual D4Z4 fragments who had lower pathology grades overall (>27 kb compared to ≤18 kb, difference −2.13, 95% confidence limits [CI] −3.86, -0.39, P=0.0003; and >27 kb compared to 19-27kb, difference −1.64, 95% Cl −3.16, −0.12, P=0.01, Figure 2B). For FSHD2 participants there was no significant relationship between D4Z4 methylation percentages and muscle pathology grade (n=10, r=−0.36, P=0.33).
Table 2.
Correlations to pathology grade
| Pathology Grade Versus | n | Spearman correlation coefficient (95% Confidence limits)# | P Value |
|---|---|---|---|
| CSS | 74 | 0.53 (0.34, 0.67) | <0.0001 |
| D4Z4 Fragment Size* | 64 | −0.45 (−0.63, −0.23) | 0.0001 |
| Disease duration | 50 | 0.31 (0.027, 0.54) | 0.03 |
| QMT standard score | 50 | −0.47 (−0.66, −0.22) | 0.0004 |
| SROM | 58 | −0.45 (−0.63, −0.21) | 0.0003 |
Confidence limits from Fisher Z-transformation
FSHD1
CSS = clinical severity score; QMT = quantitative myometry; SROM = shoulder range of motion.
Figure 2.
Relationships of histopathology to disease activity. A) The relationship of muscle pathology grade to clinical severity score; B) The pathology grade is lower for participants with genetic mutations >27 kb (7-10 repeats); C) The pathology grade is higher in participants expressing DUX4 targets.
The pathology grade showed a moderate correlation with disease duration, and the odds of using a wheelchair, walker, or cane increased by 37.1% for each increase of 1 in the pathology grade (95% confidence interval 7.2, 75.3, P=0.01).
A subgroup of participant muscle biopsies (n=15) were analyzed as part of a separate study to identify DUX4 downstream targets utilizing RNA-seq techniques.21 They selected 4 highly sensitive downstream DUX4 targets based on increased gene expression and DUX4 binding sites (PRAMEF2, LEUTX, KHDC1L, and TRIM43). Eight biopsies (53.3%) showed increased expression of all 4 genes. Biopsies expressing these DUX4 target genes had muscle pathology scores higher than those not expressing DUX4 targets after adjusting for gender and age (4.49, 95% CI 3.47, 5.51; versus 2.95, 95% CI 1.83, 4.07; P=0.05, Figure 2C).
Discussion
In this study the muscle pathology grade showed good cross-sectional associations with other measures of disease severity in FSHD, including genetic mutation, clinical severity grade, and quantitative myometry.
FSHD has a characteristic descending progression of weakness, starting in the face, scapular girdle, and arms, followed later by the distal, then the proximal lower extremity muscles. Involvement of the lower extremity is associated with later disease complications, such as restrictive lung disease and use of wheelchairs.2,20 Despite these observations, a recent study of functional impairment in FSHD suggested lower extremity involvement may be more common than previously appreciated, with up to one-third of participants demonstrating difficulty getting out of a chair.4 It is interesting that the pattern of progression of pathologic changes in the vastus lateralis showed a linear relationship to clinical severity scores, even for participants who, per bedside exam, did not have proximal lower extremity weakness. Moderate correlations were also seen between the pathology grade and disease duration, quantitative strength testing, and the size of the residual D4Z4 fragment for FSHD1. MRI studies support this notion, showing relationships between quantitative fat measurements of muscles in the thigh and clinical parameters like strength.22 This suggests the pathology grade may be an indicator of disease even in mildly to moderately affected muscles. Indeed over half of a sample of biopsies tested for downstream DUX4 target genes showed increased expression of all 4 targets, which corresponded to samples with higher muscle pathology grades.21 We suspect that the actual relationship of the muscle pathology grade to DUX4 downstream targets will be more complicated, e.g. not strictly linear. It is possible that expression of downstream DUX4 targets may be an early marker of muscle involvement. In this situation there would be a point beyond which you would expect the relationship of the muscle pathology grade and DUX4 downstream markers to no longer be linear, e.g. for pathologically advanced muscles. MRI and molecular studies have provided some support for this. They show inflammation by STIR sequences in otherwise structurally normal appearing muscles, which appear to also express downstream DUX4 targets23,21. Ultimately the relationship between specific pathological grades and downstream DUX4 markers will require a larger study.
Inflammation has been reported in up to one-third of skeletal muscle biopsies from patients with FSHD, and unlike other dystrophies, inflammation in FSHD tends to be perivascular.5,6 The frequency of inflammation reported here was lower than that reported previously, an observation that could be the result of standardized sampling of the quadriceps and smaller sample volumes obtained by needle biopsy. The presence of perivascular inflammation is 1 of the few characteristic pathologic changes in FSHD; its presence in both FSHD1 and 2 biopsies provides further support for a common pathophysiologic process for both forms of FSHD.
When considering stratification of participants in future FSHD clinical trials, prior studies show differences in disease severity based on genetic mutation. For FSHD type 1, participants with low residual D4Z4 repeat numbers (1-3 repeats) typically have more severe disease and an earlier age at disease onset and first wheelchair use24,4. Here we show that participants with the largest residual D4Z4 fragments (7-10 repeats) have lower overall muscle pathology scores. This corresponds with other papers which have suggested a later age of onset for patients with larger residual fragments1,4.
Limitations to this study include the small sample size. The conclusions from any such analysis are limited by the degree of variability from muscle to muscle inherent in the disease and the degree to which a small sample from a single muscle can be said to represent the overall progression of disease in a given participant.
Despite this limitation, we feel the reality is that muscle biopsies are and will remain important for muscular dystrophy clinical trials in the foreseeable future, to evaluate changes in pathology and/or molecular markers. In addition such a systematic approach to categorizing muscle pathology will be vital during pre-clinical studies. Ultimately showing an improvement in muscle pathology would be of benefit for FSHD. A tool to help quantify muscle pathological findings will be useful. A simple ordinal grading scale has the advantage of being relatively simple to implement, requiring only standard muscle histochemical stains. The relationships seen in this study suggest there may be utility in using this approach for stratification, as there are broad cross-sectional relationships of the pathology grade to clinical and genetic measures in FSHD.
In summary, the pathology grade of the quadriceps is a useful cross-sectional marker of disease severity in FSHD which may serve as a useful measure of disease progression or strategy for stratifying patients for future clinical trials. The pathology grade is related to underlying genetic mutation in FSHD type 1 and down-stream DUX4 target gene expression. A similar approach could be used to evaluate any muscle of interest in any progressive muscular dystrophy.
Acknowledgements
We would like to thank all the FSHD participants and their family members without whose support this study would not have been possible.
Study Funding
The Cellular and Molecular Pathophysiology Study in FSHD has been funded in whole or in part by the National Institutes of Health (grant # 1PO1NS069539-01) and the Fields Center for FSHD and Neuromuscular Research. The project described in this publication was supported by the University of Rochester CTSA award number UL1 RR024160 from the National Center for Research Resources and the National Center for Advancing Translational Sciences of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Statland's work on this project was supported by the Muscular Dystrophy Association's Clinical Research Training Grant, and a CTSA grant from NCATS awarded to the University of Kansas Medical Center for Frontiers: The Heartland Institute for Clinical and Translational Research # KL2TR000119.
Abbreviations
- CSS
clinical severity score
- FSHD
Facioscapulohumeral muscular dystrophy
- IQR
interquartile range
- SROM
shoulder range of motion
Footnotes
Disclosure
Dr. Statland is a consultant for Cytokinetics. Dr. Tawil is a consultant for Cytokinetics and Novartis.
References
- 1.Mostacciuolo ML, Pastorello E, Vazza G, Miorin M, Angelini C, Tomelleri G, et al. Facioscapulohumeral muscular dystrophy: epidemiological and molecular study in a north-east Italian population sample. Clin Genet. 2009;75(6):550–555. doi: 10.1111/j.1399-0004.2009.01158.x. [DOI] [PubMed] [Google Scholar]
- 2.Padberg GW, Frants RR, Brouwer OF, Wijmenga C, Bakker E, Sandkuijl LA. Facioscapulohumeral muscular dystrophy in the Dutch population. Muscle Nerve. 1995;2:S81–84. [PubMed] [Google Scholar]
- 3.Tawil R, Van Der Maarel SM. Facioscapulohumeral muscular dystrophy. Muscle Nerve. 2006;34(1):1–15. doi: 10.1002/mus.20522. [DOI] [PubMed] [Google Scholar]
- 4.Statland JM, Tawil R. Risk of functional impairment in facioscapulohumeral muscular dystrophy. Muscle Nerve. 2013 doi: 10.1002/mus.23949. [DOI] [PubMed] [Google Scholar]
- 5.Arahata K, Ishihara T, Fukunaga H, Orimo S, Lee JH, Goto K, et al. Inflammatory response in facioscapulohumeral muscular dystrophy (FSHD): immunocytochemical and genetic analyses. Muscle Nerve. 1995;2:S56–66. [PubMed] [Google Scholar]
- 6.Carpenter SKG. Pathology of Skeletal Muscle. Oxford University Press; New York: 2001. [Google Scholar]
- 7.Lemmers RJ, Tawil R, Petek LM, Balog J, Block GJ, Santen GW, et al. Digenic inheritance of an SMCHD1 mutation and an FSHD-permissive D4Z4 allele causes facioscapulohumeral muscular dystrophy type 2. Nat Genet. 2012;44(12):1370–1374. doi: 10.1038/ng.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemmers RJ, van der Vliet PJ, Klooster R, Sacconi S, Camano P, Dauwerse JG, et al. A unifying genetic model for facioscapulohumeral muscular dystrophy. Science. 2010;329(5999):1650–1653. doi: 10.1126/science.1189044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosnakovski D, Daughters RS, Xu Z, Slack JM, Kyba M. Biphasic myopathic phenotype of mouse DUX, an ORF within conserved FSHD-related repeats. PLoS One. 2009;4(9):e7003. doi: 10.1371/journal.pone.0007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kowaljow V, Marcowycz A, Ansseau E, Conde CB, Sauvage S, Matteotti C, et al. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul Disord. 2007;17(8):611–623. doi: 10.1016/j.nmd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Snider L, Asawachaicharn A, Tyler AE, Geng LN, Petek LM, Maves L, et al. RNA transcripts, miRNA-sized fragments and proteins produced from D4Z4 units: new candidates for the pathophysiology of facioscapulohumeral dystrophy. Hum Mol Genet. 2009;18(13):2414–2430. doi: 10.1093/hmg/ddp180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderplanck C, Ansseau E, Charron S, Stricwant N, Tassin A, Laoudj-Chenivesse D, et al. The FSHD atrophic myotube phenotype is caused by DUX4 expression. PLoS One. 2011;6(10):e26820. doi: 10.1371/journal.pone.0026820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wuebbles RD, Long SW, Hanel ML, Jones PL. Testing the effects of FSHD candidate gene expression in vertebrate muscle development. Int J Clin Exp Pathol. 2010;3(4):386–400. [PMC free article] [PubMed] [Google Scholar]
- 14.de Greef JC, Lemmers RJ, Camano P, Day JW, Sacconi S, Dunand M, et al. Clinical features of facioscapulohumeral muscular dystrophy 2. Neurology. 2010;75(17):1548–1554. doi: 10.1212/WNL.0b013e3181f96175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wijmenga C, Hewitt JE, Sandkuijl LA, Clark LN, Wright TJ, Dauwerse HG, et al. Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat Genet. 1992;2(1):26–30. doi: 10.1038/ng0992-26. [DOI] [PubMed] [Google Scholar]
- 16.Personius KE, Pandya S, King WM, Tawil R, McDermott MP. Facioscapulohumeral dystrophy natural history study: standardization of testing procedures and reliability of measurements. The FSH DY Group. Phys Ther. 1994;74(3):253–263. doi: 10.1093/ptj/74.3.253. [DOI] [PubMed] [Google Scholar]
- 17.Tawil R, McDermott MP, Mendell JR, Kissel J, Griggs RC. Facioscapulohumeral muscular dystrophy (FSHD): design of natural history study and results of baseline testing. FSH-DY Group. Neurology. 1994;44(3 Pt 1):442–446. doi: 10.1212/wnl.44.3_part_1.442. [DOI] [PubMed] [Google Scholar]
- 18.Ricci E, Galluzzi G, Deidda G, Cacurri S, Colantoni L, Merico B, et al. Progress in the molecular diagnosis of facioscapulohumeral muscular dystrophy and correlation between the number of KpnI repeats at the 4q35 locus and clinical phenotype. Ann Neurol. 1999;45(6):751–757. doi: 10.1002/1531-8249(199906)45:6<751::aid-ana9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Walker GA, Shostak J. Common Statistical Methods for Clinical Research. SAS Institute Inc.; Cary, NC: 2010. pp. 361–397. [Google Scholar]
- 20.Scully MA, Eichinger KJ, Donlin-Smith CM, Tawil R, Statland JM. Restrictive Lung Involvement in Facioscapulohumeral Muscular Dystrophy. Muscle Nerve. 2014 doi: 10.1002/mus.24218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao Z, Snider L, Balog J, Lemmers RJ, van der Maarel SM, Tawil R, et al. DUX4-induced gene expression is the major molecular signature in FSHD skeletal muscle. Hum Mol Genet. 2014 doi: 10.1093/hmg/ddu251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen BH, Voet NB, Nabuurs CI, Kan HE, de Rooy JW, Geurts AC, et al. Distinct Disease Phases in Muscles of Facioscapulohumeral Dystrophy Patients Identified by MR Detected Fat Infiltration. PLoS One. 2014;9(1):e85416. doi: 10.1371/journal.pone.0085416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tasca G, Pescatori M, Monforte M, Mirabella M, Iannaccone E, Frusciante R, et al. Different molecular signatures in magnetic resonance imaging-staged facioscapulohumeral muscular dystrophy muscles. PLoS One. 2012;7(6):e38779. doi: 10.1371/journal.pone.0038779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ricci G, Scionti I, Sera F, Govi M, D'Amico R, Frambolli I, et al. Large scale genotype-phenotype analyses indicate that novel prognostic tools are required for families with facioscapulohumeral muscular dystrophy. Brain. 2013;136(Pt 11):3408–3417. doi: 10.1093/brain/awt226. [DOI] [PMC free article] [PubMed] [Google Scholar]