Abstract
Endocrine disrupting chemical (EDC) exposures during critical periods of development may influence neuronal development and the manifestation of sexually dimorphic sociability and social novelty behaviors in adulthood. In this study, we assessed the effects of gestational exposure to PCBs on the social behavior of males and females later in adulthood. A weakly estrogenic PCB mixture, Aroclor 1221 (A1221, 0.5 or 1 mg/kg) was administered to pregnant Sprague-Dawley rat dams. Both a positive control (estradiol benzoate; EB, 50 μg/kg) and negative control (dimethylsulfoxide; DMSO in sesame oil vehicle) were similarly administered to separate sets of dams. The sexes responded differently in two tasks essential to sociality. Using a three-chamber apparatus that contained a caged, same-sex, gonadectomized stimulus animal and an empty stimulus cage, we found that both sexes showed a strong preference for affiliating with a stimulus animal (vs. an empty cage), an effect that was much more pronounced in the males. In the second task, a novel and a familiar stimulus animal were caged at opposite ends of the same apparatus. Females displayed a higher degree of novelty preference than the males. During both tests, females had significantly higher social approach behaviors while male engaged in significantly more interactive behaviors with the conspecific. Of particular interest, males born of dams that received prenatal A1221 (0.5 mg/kg) exhibited an overall decrease in nose-to-nose investigations. These behavioral data suggest that the males are more sensitive to A1221 treatment than are females. In addition to behavioral analysis, serum corticosterone was measured. Females born of dams treated with A1221 (0.5 mg/kg) had significantly higher concentrations of corticosterone than the DMSO female group; males were unaffected. Females also had significantly higher corticosterone concentrations than did males. Overall, our results suggest that the effects of gestational exposure to PCBs on adult social behavior are relatively limited within this particular paradigm.
Keywords: Endocrine Disrupting Chemicals, Polychlorinated biphenyls, A1221, Nose Touching, Sociability, Social Novelty, Social Recognition, Social Memory, Sexual Dimorphism, Three-chamber test
Introduction
Prenatal exposure to endocrine disrupting chemicals (EDCs) can disrupt the neuroendocrine system, leading to alterations in adult social and sociosexual behaviors in a sexually-dimorphic manner. Most research has been conducted for bisphenol A (BPA), exposure to which causes a decrease in the territorial marking of male mice (Williams et al., 2013), as well as female-specific alteration of one-on-one social interactions in juvenile mice (Wolstenholme, 2011) and prairie voles (Sullivan et al., 2014). BPA also perturbs social recognition in mice (Wolstenholme et al., 2013). Exposure to other EDCs such as atrazine (mice: Belloni et al., 2011), PCBs (rats: Jolous-Jamshidi et al., 2010), and chlorpyrifos (mice: Venerosi et al., 2012) are associated with perturbations of normal social interactions. Polychlorinated biphenyls (PCBs) - including the Aroclor 1221 mixture (A1221) used in the current study – also disrupt sexual behavior in female rats (Chung and Clemens, 1999; Steinberg et al., 2007). However, beyond this work, studies of EDC effects on social affiliation (individual preference to associate with a conspecific) and social novelty (individual choice to affiliate with a strange versus a familiar conspecific) are limited. Research has shown sex differences in these behaviors, as male rats tend to spend more time interacting with an unfamiliar, same-sex conspecific than do females (Carrier and Kabbaj, 2012; Slamberová et al., 2011; Stack et al., 2010). However, to our knowledge there are no studies investigating the effects of gestational exposure to PCBs on this paradigm.
The purpose of this study was to provide a thorough characterization of the social behavioral phenotype caused by gestational EDC exposure. We assessed how treatment of a pregnant rat dam with A1221 during the third trimester of gestation affected the social behavior of male and female offspring later in adulthood. Two dosages of A1221 (0.5 and 1 mg/kg) were administered during the last trimester of gestation, during a critical period of sexual differentiation of the hypothalamus (Davis et al., 1996; Jacobson et al., 1980). Both positive control (estradiol benzoate; EB) and negative control (DMSO vehicle) groups were used for comparison. Using this model, we were able to address hypotheses about sex differences in performance in two types of socially relevant tests, and to test the hypothesis that prenatal exposure to EDCs in these responses would have sex-specific effects. Because it is known that male and female rats differ in their basal concentrations of corticosterone (Kitay, 1961; Gillette et al., 2014) and, further, that circulating levels of corticosterone influences social behaviors in rats (Veenit et al., 2013), we also measured concentrations of this hormone in our experimental rats.
Materials and Methods
Experimental Design
Sprague-Dawley rats were purchased from Harlan Sprague-Dawley (Houston, TX), and all animal procedures were conducted in compliance with protocols approved by IACUC at the University of Texas at Austin. They were housed in a colony room with controlled temperature (22 C) and light cycle (12:12 dark:light, lights on at 2400). Virgin females were mated with sexually experienced males. The day following successful mating, as indicated by a sperm-positive vaginal smear, was termed embryonic day 1 (E1). Male and female stimulus rats were purchased as young adults from Harlan, and gonadectomized under isoflurane anesthesia. Stimulus animals were not treated with EDCs or vehicle.
Pregnant rats were exposed to one of four treatments, administered via intraperitoneal injections, on E16 and E18, the beginning of the period of brain sexual differentiation(Davis et al., 1996; Jacobson et al., 1980). The dosages used were based on prior work conducted in the Gore lab that showed physiological, behavioral, and neuroendocrine effects (Steinberg et al., 2007, 2008; Dickerson et al., 2011a; Walker et al., 2013, 2014): (1) Vehicle (3% DMSO/sesame oil mix), (2) Estradiol benzoate (EB; 50 μg/kg), (3) Aroclor 1221 (A1221, 0.5 mg/kg), or (4) A1221 (1 mg/kg). The number of litters per treatment was 11, 11, 10, and 10, respectively. Although we did not measure body burden or tissue content in the exposed offspring, the literature suggests that maternal-fetal transfer results in an exposure to approximately 1–2 μg/kg A1221, and 100 ng/kg EB, in the fetuses (Takagi et al., 1986).
The day of parturition was called postnatal day 0 (P0). At P1, the newborn pups were weighed and their anogenital distance measured; litters were culled to 4 males and 4 females. The pups were monitored daily for eye opening, while body weights and anogenital distance were taken weekly following birth. The pups were weaned at P21 and rehoused in same-sex groups where they were monitored daily for signs of pubertal development: vaginal opening in females and preputial separation in males (Steinberg et al., 2007; Walker et al., 2012). Following vaginal opening, daily vaginal smears were taken and cell cytology was examined as a measure of estrous cyclicity in the females. Beginning at P60 animals were subjected to a battery of the following tests in random order: sociability and social novelty, mate preference, open field and elevated plus maze; fear conditioning always was the last test. The total number of behaviorally characterized animals was 82 females and 80 males. Order of testing had no effect on behavioral outcomes. Experimental rats were weighed and euthanized 30 days after testing was completed, and bloods centrifuged and frozen for hormone assay, and adrenals and gonads removed and weighed.
Hormone Radioimmunoassay
Around P90, animals were euthanized by rapid decapitation and trunk blood was collected; females were euthanized in proestrus. In addition, animals from the same litters that were not behaviorally tested were used to increase sample size; this resulted in a total number of 158 females and 153 males. 10 μl of sample from each individual was used to measure serum corticosterone concentration in a single non-human radioimmunoassay (MP Biomedicals; Corticosterone 3H RIA - 07120002). Assay sensitivity was 7.7 ng/ml, and intra-assay variability was 4.1%.
Behavioral Paradigm
A three-chamber social apparatus (100 cm × 100 cm; Stoelting, Figure 1) was used as the testing arena (Crews et al., 2012; Moy et al., 2004). Testing was conducted under dim red light during the dark period of their light-dark cycle, approximately two hours following lights out. The experimental animal was placed in the middle chamber of the apparatus, with doors to the two side chambers closed. For females, estrous cycle status on the day of testing was recorded to identify any potential differences relating to the behaviors examined. Same-sex gonadectomized stimulus animals were placed in a 7 cm × 15 cm cylindrical stimulus cage located in a corner of the lateral chambers; bars allowed for nose-to-nose investigation but did not permit further contact.
Figure 1.
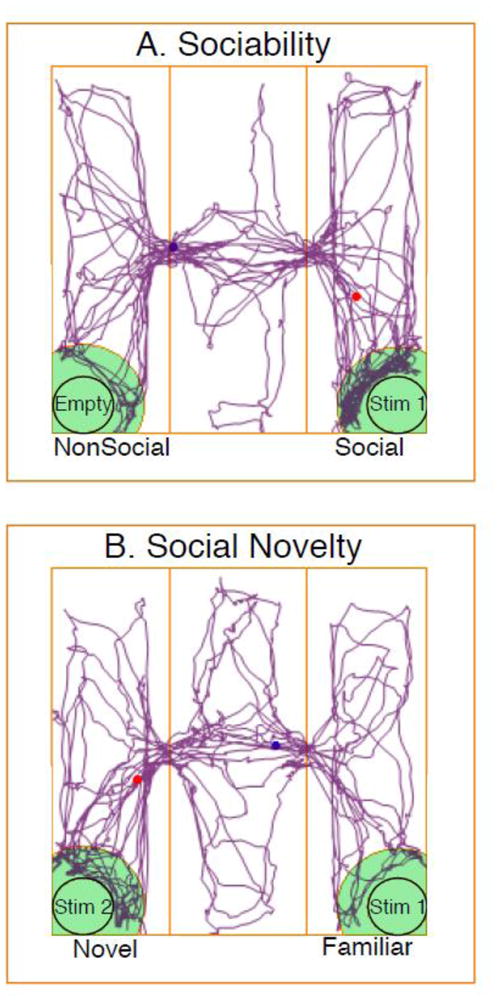
A diagram of the 3-chamber apparatus, with a representative tracking profile from Any-Maze for an individual rat, is shown for the Sociability (A) and Social Novelty (B) tests. In Sociability (A), the stimulus rat (Stim 1) was a same-sex, gonadectomized rat, and the other cage was empty. In Social Novelty (B), the same animal (Stim 1) was used again as the familiar rat, together with an unfamiliar same-sex, gonadectomized rat in the other cage (Stim 2).
Sociability and Social Novelty
A five minute habituation period was used to allow the experimental rats access to the center chamber only. The doors were then opened and the experimental animal allowed to freely move around the entire apparatus for the two ten minute periods. All behaviors were video recorded throughout the testing. The entire apparatus was dismantled and all surfaces wiped clean with a 70% ethanol solution between each test.
During the first Sociability test, one of the stimulus cages, randomly selected, held a novel same-sex (untreated by EDCs, and gonadectomized in adulthood) rat while the other stimulus cage remained empty (Figure 1A). At the test’s conclusion, the experimental animal was removed from the apparatus and temporarily placed in a holding cage. The original stimulus rat, and a novel same-sex, gonadectomized stimulus animal, were each placed into stimulus cages and were randomly placed into opposite sides of the testing arena. The experimental animal was then reintroduced to the center chamber, marking the beginning of Social Novelty. The experimental animal was then allowed to interact with the now-familiar and novel stimulus animals for ten minutes (Figure 1B).
AnyMaze (Stoelting Co.) was used to track behaviors. Automated computer-scored measures were: total distance travelled, and average speed throughout the entire apparatus. The time in proximity (defined as one body length) to the stimulus cage was also determined by the program. The video recordings of the tests were manually scored for the following behaviors: nose touching (the time each experimental animal spent in direct nose-to-nose contact with the stimulus animals), stimulus rat investigation (the time spent investigating the stimulus animal, but not necessarily nose-touching), grooming (time spent self-grooming), and rearing (time spent on hind legs without support from any walls).
Statistical Analyses
Because of non-homogeneity of behavioral datasets, the Kruskal-Wallis test was used to compare effects of treatment within sexes. A generalized extreme studentized deviate (ESD) test was used to detect outliers, limited to a maximum of two per group. Any animals that were outstanding outliers across multiple endpoints were removed from the analyses. Posthoc analyses included t-test for sex effects, Tukey HSD for treatment effects within sexes, or Steel-Dwass for treatment effects within sexes when the data did not satisfy the assumptions for parametric analyses. Cohen’s d analysis was used to determine the effect size, within each group, for the Social Novelty data. An effect size of 0.8 or higher is equivalent to Cohen’s standard LARGE, and indicates that the mean of the control group (Familiar) is at the 79th percentile and sharing 69% overlap with the comparison group (Novel). The hormone data were homogeneous and a two-way ANOVA identified main effects of treatment and sex; subsequent one-way ANOVA was performed to determine the effects of treatment within sexes. Initial statistical analyses were used to identify any potential cohort or litter effects within groups; none were identified, and therefore, analysis was conducted using individuals within a litter as separate datapoints (no more than 2 per sex per litter). This resulted in 10–11 litters per treatment, with 20–22 males and 20–22 females per endpoint for behaviors.
Results
There were no significant effects of female estrous cycle status, corticosterone concentration, litter, or cohort on any of the behavioral measures examined for Sociability or Social Novelty.
Corticosterone
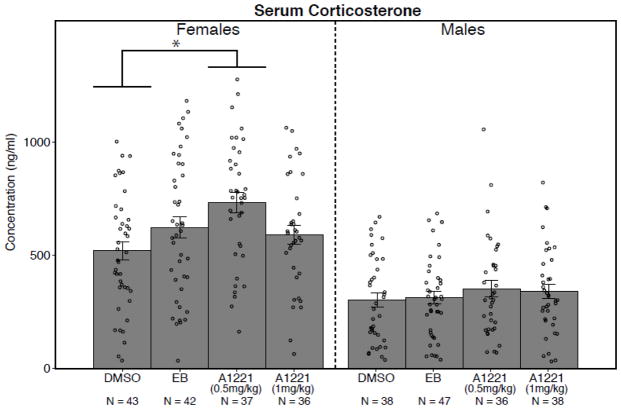
A two-way ANOVA indicated a sex difference (F1, 312 = 114.01, p < 0.0001), with females having a significantly higher serum concentration than males (Figure 2). Subsequent analyses of effects of treatment within each sex showed significant differences in females (F3,154 = 4.13, p < 0.009); a Tukey HSD post hoc test found the A1221 (0.5 mg/kg) group to have significantly higher corticosterone concentrations than the DMSO group. There were no significant differences among the male treatment groups.
Figure 2.
Circulating concentrations of corticosterone are shown for adult males and females receiving prenatal exposure to the vehicle (DMSO), estradiol benzoate (EB) or Aroclor 1221 (A1221, 0.5 or 1 mg/kg). Females had significantly higher corticosterone concentrations than males (p < 0.0001). Within females, A1221 (0.5 mg/kg) rats had significantly higher concentrations of serum corticosterone than the DMSO females. Data shown are mean ± standard error, with individual values shown as circles. *, p < 0.05. N’s are indicated below each bar.
Body weight
A Student’s t-test indicated that males (x̄ = 415 g) weighed significantly more than females (x̄ = 264 g), regardless of treatment (p < 0.0001). A one-way ANOVA within each sex revealed that rats that were prenatally treated with either dosage of A1221 had significantly greater body weight (p < 0.001) at the age at euthanasia (~P90; Table 1).
Table 1.
Somatic measures in prenatally exposed rats
| Measure | Sex | Treatment | Mean | SEM | Sex Diff. | ||
|---|---|---|---|---|---|---|---|
| Body weight (g) | Females | DMSO | 257 | ± | 4 | F < M p < 0.0001 |
|
| EB | 263 | ± | 3 | ||||
| A1221 (0.5) | 270 | ± | 2 | ||||
| A1221 (1) | 265 | ± | 3 | ||||
|
| |||||||
| Males | DMSO | 400 | ± | 9 | |||
| EB | 413 | ± | 6 | ||||
| A1221 (0.5) | 427 | ± | 7 | ||||
| A1221 (1) | 419 | ± | 4 | ||||
|
| |||||||
| Adrenal gland weight (g) | Females | DMSO | 0.057 | ± | 1.7 × 10−3 | F > M p < 0.0001 |
|
| EB | 0.059 | ± | 1.8 × 10−3 | ||||
| A1221 (0.5) | 0.065 | ± | 2.5 × 10−3 | ||||
| A1221 (1) | 0.062 | ± | 1.5 × 10−3 | ||||
|
| |||||||
| Males | DMSO | 0.048 | ± | 1.4 × 10−3 | |||
| EB | 0.05 | ± | 1.1 × 10−3 | ||||
| A1221 (0.5) | 0.049 | ± | 1.2 × 10−3 | ||||
| A1221 (1) | 0.052 | ± | 2.1 × 10−3 | ||||
|
| |||||||
| Gonad weight (g) | Females | DMSO | 0.13 | ± | 4.7 × 10−3 | N/A | |
| EB | 0.13 | ± | 4.0 × 10−3 | ||||
| A1221 (0.5) | 0.13 | ± | 3.7 × 10−3 | ||||
| A1221 (1) | 0.13 | ± | 5.0 × 10−3 | ||||
|
| |||||||
| Males | DMSO | 4.0 | ± | 5.0 × 10−2 | N/A | ||
| EB | 4.2 | ± | 6.5 × 10−2 | ||||
| A1221 (0.5) | 4.0 | ± | 5.7 × 10−2 | ||||
| A1221 (1) | 4.1 | ± | 5.0 × 10−2 | ||||
Body weight, adrenal weight, and gonad weight were sexually dimorphic. Prenatal treatment with vehicle (DMSO), estradiol benzoate (EB), and Aroclor 1221 at 0.5 or 1 mg/kg did not affect these endpoints. Data shown are mean ± SEM.
Adrenal gland weight
A two-way ANOVA (Sex x Treatment) indicated a main effect of sex, with females (x̄ = 0.06 g) having heavier adrenals than males (x̄ = 0.05 g), regardless of treatment (p < 0.0001; Table 1). However, there were no significant effects of treatment on adrenal weight in either sex.
Gonad weight
One-way ANOVA within each sex indicated no effect of treatment on ovarian weight in females, or testicular weight in males (Table 1).
Sociability Test
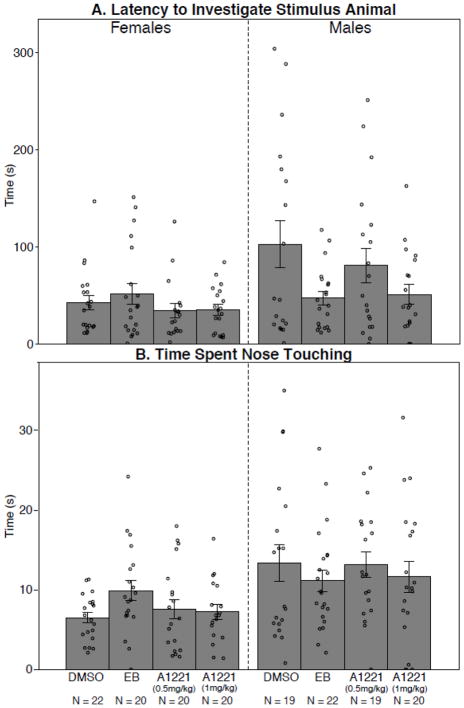
Sociability tests were completed on 82 females and 80 males. Three animals (all male) that never entered the chamber containing the stimulus animal during the first stage (Sociability) had to be excluded, as they had never interacted with the animal and could therefore not distinguish a novel from a familiar conspecific. The computer-generated data for the diagnostic behaviors of the Sociability tests are shown in Table 2A, and investigator-scored data related to behaviors that took place in proximity to a stimulus animal are shown in Table 3A. Main effects of sex were determined by grouping the treatment groups within each sex and running a Student’s t-test, with results shown in Tables 2A and 3A. Females traveled a greater distance, were faster, and engaged in more grooming and rearing than males. Males had longer latencies to investigate the stimulus animal and to engage in the first nose touch, than did females. Males also spent more time investigating the stimulus animal and nose touching than females. The sexes were equivalent in time spent in proximity to the stimulus rat. Although rats spent more time in the chamber containing a stimulus animal than the empty chamber, regardless of sex or treatment (H = 197.40; p < 0.0001), within each sex there were no treatment effects. Two representative behaviors are shown in Figure 3 for latency to investigate the stimulus animal, and time spent nose touching, illustrating the sex difference but no significant treatment effects.
Table 2.
Sociability and Social Novelty diagnostic behaviors
| A. Sociability
| |||||
|---|---|---|---|---|---|
| Measure | Sex | Treatment | Mean | SEM | Sex Diff. |
| Distance (m) | Females | DMSO | 53.0 | ± 2.6 | F > M p < 0.0001 |
| EB | 53.0 | ± 2.2 | |||
| A0.5 | 52.0 | ± 2.6 | |||
| A1.0 | 57.4 | ± 4.7 | |||
|
| |||||
| Males | DMSO | 37.7 | ± 2.8 | ||
| EB | 48.4 | ± 4.9 | |||
| A0.5 | 46.0 | ± 2.4 | |||
| A1.0 | 39.0 | ± 2.4 | |||
|
| |||||
| Speed (cm/s) | Females | DMSO | 8.8 | ± 0.4 | F > M p < 0.0001 |
| EB | 8.9 | ± 0.3 | |||
| A0.5 | 8.7 | ± 0.4 | |||
| A1.0 | 9.6 | ± 0.8 | |||
|
| |||||
| Males | DMSO | 6.3 | ± 0.4 | ||
| EB | 8.1 | ± 0.8 | |||
| A0.5 | 7.7 | ± 0.4 | |||
| A1.0 | 6.3 | ± 0.4 | |||
|
| |||||
| Grooming (s) | Females | DMSO | 6.6 | ± 1.2 | F > M p < 0.05 |
| EB | 8.1 | ± 1.9 | |||
| A0.5 | 6.3 | ± 1.3 | |||
| A1.0 | 4.1 | ± 0.7 | |||
|
| |||||
| Males | DMSO | 3.6 | ± 1.1 | ||
| EB | 4.7 | ± 1.0 | |||
| A0.5 | 3.3 | ± 0.9 | |||
| A1.0 | 5.7 | ± 1.5 | |||
|
| |||||
| Rearing (s) | Females | DMSO | 6.2 | ± 1.3 | F > M p < 0.03 |
| EB | 4.2 | ± 1.2 | |||
| A0.5 | 5.5 | ± 1.5 | |||
| A1.0 | 4.2 | ± 1.0 | |||
|
| |||||
| Males | DMSO | 2.8 | ± 0.9 | ||
| EB | 3.3 | ± 0.9 | |||
| A0.5 | 1.9 | ± 0.4 | |||
| A1.0 | 2.3 | ± 1.6 | |||
| B. Social Novelty
| |||||
|---|---|---|---|---|---|
| Measure | Sex | Treatment | Mean | SEM | Sex Diff. |
| Distance (m) | Females | DMSO | 46.6 | ± 2.3 | F > M p < 0.0001 |
| EB | 48.4 | ± 3.1 | |||
| A0.5 | 45.5 | ± 2.4 | |||
| A1.0 | 48.6 | ± 2.6 | |||
|
| |||||
| Males | DMSO | 33.9 | ± 2.1 | ||
| EB | 33.7 | ± 2.2 | |||
| A0.5 | 41.0 | ± 3.0 | |||
| A1.0 | 36.6 | ± 2.2 | |||
|
| |||||
| Speed (cm/s) | Females | DMSO | 7.9 | ± 0.4 | F > M p < 0.0001 |
| EB | 8.1 | ± 0.5 | |||
| A0.5 | 7.6 | ± 0.4 | |||
| A1.0 | 8.1 | ± 0.4 | |||
|
| |||||
| Males | DMSO | 5.6 | ± 0.3 | ||
| EB | 5.6 | ± 0.4 | |||
| A0.5 | 6.9 | ± 0.4 | |||
| A1.0 | 6.1 | ± 0.4 | |||
|
| |||||
| Grooming (s) | Females | DMSO | 13.8 | ± 2.1 | F > M p < 0.003 |
| EB | 15.4 | ± 2.7 | |||
| A0.5 | 16.5 | ± 2.8 | |||
| A1.0 | 11.3 | ± 2.2 | |||
|
| |||||
| Males | DMSO | 11.3 | ± 2.9 | ||
| EB | 11.3 | ± 1.5 | |||
| A0.5 | 10.8 | ± 1.7 | |||
| A1.0 | 8.6 | ± 1.9 | |||
|
| |||||
| Rearing (s) | Females | DMSO | 20.5 | ± 5.2 | F > M p < 0.0001 |
| EB | 9.4 | ± 2.3 | |||
| A0.5 | 11.4 | ± 3.2 | |||
| A1.0 | 10.5 | ± 2.7 | |||
|
| |||||
| Males | DMSO | 2.4 | ± 0.8 | ||
| EB | 3.9 | ± 1.1 | |||
| A0.5 | 4.1 | ± 1.3 | |||
| A1.0 | 4.2 | ± 1.6 | |||
Behaviors are shown that were computer-scored and used as diagnostic measures in the two social behavioral tests. Sex differences and direction of change are indicated for each behavior. P-values are provided when sexes were significantly different from one another. Data shown are mean ± SEM.
Table 3.
Sociability and Social Novelty behaviors in proximity to the stimulus animal
| A. Sociability
| |||||||
|---|---|---|---|---|---|---|---|
| Measure | Sex | Treatment | Chamber
|
Sex Diff. | |||
| Social | NonSocial | ||||||
|
| |||||||
| Mean | SEM | Mean | SEM | ||||
| Time in proximity to stimulus animal (s) | Females | Vehicle | 221 | ± 17.7 | 82 | ± 7.6 | F = M |
| EB | 199 | ± 9.4 | 83 | ± 9.6 | |||
| A1221 (0.5) | 201 | ± 18.5 | 68 | ± 6.8 | |||
| A1221 (1) | 220 | ± 17.7 | 68 | ± 9.0 | |||
|
| |||||||
| Males | Vehicle | 218 | ± 19.7 | 45 | ± 7.0 | ||
| EB | 216 | ± 16.4 | 52 | ± 5.5 | |||
| A1221 (0.5) | 226 | ± 13.7 | 59 | ± 6.8 | |||
| A1221 (1) | 262 | ± 25.6 | 34 | ± 4.7 | |||
|
| |||||||
| Time spent investigating stimulus animal (s) | Females | Vehicle | 133 | ± 12.2 | 44 | ± 4.8 | F < M p < 0.004 |
| EB | 127 | ± 9.2 | 46 | ± 6.5 | |||
| A1221 (0.5) | 113 | ± 10.2 | 35 | ± 4.6 | |||
| A1221 (1) | 131 | ± 12.8 | 37 | ± 5.7 | |||
|
| |||||||
| Males | Vehicle | 144 | ± 16.1 | 23 | ± 3.8 | ||
| EB | 148 | ± 12.5 | 28 | ± 3.5 | |||
| A1221 (0.5) | 159 | ± 12.1 | 33 | ± 4.4 | |||
| A1221 (1) | 167 | ± 21.5 | 16 | ± 3.0 | |||
|
| |||||||
| Latency to investigate stimulus animal (s) | Females | Vehicle | 55 | ± 11.2 | N/A | N/A | F < M p < 0.005 |
| EB | 51 | ± 10.7 | N/A | N/A | |||
| A1221 (0.5) | 48 | ± 48.5 | N/A | N/A | |||
| A1221 (1) | 40 | ± 7.0 | N/A | N/A | |||
|
| |||||||
| Males | Vehicle | 128 | ± 34.1 | N/A | N/A | ||
| EB | 78 | ± 26.2 | N/A | N/A | |||
| A1221 (0.5) | 81 | ± 17.5 | N/A | N/A | |||
| A1221 (1) | 76 | ± 26.5 | N/A | N/A | |||
|
| |||||||
| Time spent nose touching (s) | Females | Vehicle | 7.6 | ± 1.0 | N/A | N/A | F < M p < 0.0001 |
| EB | 9.9 | ± 1.3 | N/A | N/A | |||
| A1221 (0.5) | 7.6 | ± 1.2 | N/A | N/A | |||
| A1221 (1) | 8.8 | ± 1.4 | N/A | N/A | |||
|
| |||||||
| Males | Vehicle | 13.4 | ± 2.3 | N/A | N/A | ||
| EB | 11.1 | ± 1.4 | N/A | N/A | |||
| A1221 (0.5) | 13.9 | ± 1.5 | N/A | N/A | |||
| A1221 (1) | 12.9 | ± 2.0 | N/A | N/A | |||
|
| |||||||
| Latency to first nose touch (s) | Females | Vehicle | 58 | ± 11.5 | N/A | N/A | F < M p < 0.001 |
| EB | 48 | ± 8.9 | N/A | N/A | |||
| A1221 (0.5) | 65 | ± 13.8 | N/A | N/A | |||
| A1221 (1) | 72 | ± 23.0 | N/A | N/A | |||
|
| |||||||
| Males | Vehicle | 133 | ± 34.0 | N/A | N/A | ||
| EB | 79 | ± 25.6 | N/A | N/A | |||
| A1221 (0.5) | 91 | ± 17.5 | N/A | N/A | |||
| A1221 (1) | 90 | ± 30.2 | N/A | N/A | |||
| B. Social Novelty
| |||||||
|---|---|---|---|---|---|---|---|
| Measure | Sex | Treatment | Chamber
|
Sex Diff. | |||
| Familiar | Novel | ||||||
|
| |||||||
| Mean | SEM | Mean | SEM | ||||
| Time in proximity to stimulus animal (s) | Females | Vehicle | 96 | ± 8.7 | 147 | ± 11.9 | F < M p < 0.004 |
| EB | 97 | ± 12.6 | 146 | ± 14.1 | |||
| A1221 (0.5) | 92 | ± 12.7 | 154 | ± 21.5 | |||
| A1221 (1) | 110 | ± 12.3 | 129 | ± 12.6 | |||
|
| |||||||
| Males | Vehicle | 121 | ± 17.0 | 169 | ± 15.8 | ||
| EB | 98 | ± 12.1 | 194 | ± 19.3 | |||
| A1221 (0.5) | 107 | ± 12.1 | 138 | ± 13.0 | |||
| A1221 (1) | 122 | ± 14.4 | 175 | ± 11.4 | |||
|
| |||||||
| Time spent investigating stimulus animals (s) | Females | Vehicle | 54 | ± 6.5 | 74 | ± 9.2 | F < M p < 0.001 |
| EB | 50 | ± 5.5 | 76 | ± 8.2 | |||
| A1221 (0.5) | 54 | ± 9.1 | 70 | ± 7.6 | |||
| A1221 (1) | 57 | ± 7.2 | 68 | ± 8.7 | |||
|
| |||||||
| Males | Vehicle | 78 | ± 11.7 | 95 | ± 10.4 | ||
| EB | 63 | ± 9.0 | 118 | ± 11.3 | |||
| A1221 (0.5) | 66 | ± 8.4 | 87 | ± 9.2 | |||
| A1221 (1) | 78 | ± 11.8 | 101 | ± 11.2 | |||
|
| |||||||
| Time spent nose touching (s) | Females | Vehicle | 2.8 | ± 0.5 | 5.2 | ± 0.9 | F < M p < 0.001 |
| EB | 3.3 | ± 0.6 | 5.2 | ± 0.7 | |||
| A1221 (0.5) | 2.9 | ± 0.5 | 4.4 | ± 0.8 | |||
| A1221 (1) | 3.4 | ± 0.8 | 3.8 | ± 0.5 | |||
|
| |||||||
| Males | Vehicle | 4.4 | ± 1.0 | 10.1 | ± 1.7 | ||
| EB | 5.7 | ± 1.3 | 10.4 | ± 1.1 | |||
| A1221 (0.5) | 3.6 | ± 0.8 | 6.1 | ± 1.1 | |||
| A1221 (1) | 3.7 | ± 0.8 | 7.8 | ± 1.1 | |||
|
| |||||||
| Latency to first nose touch (s) | Females | Vehicle | 100 | ± 21.0 | 45 | ± 12.1 | F = M |
| EB | 138 | ± 25.9 | 41 | ± 18.3 | |||
| A1221 (0.5) | 136 | ± 31.6 | 44 | ± 14.4 | |||
| A1221 (1) | 109 | ± 25.0 | 38 | ± 19.4 | |||
|
| |||||||
| Males | Vehicle | 68 | ± 29.2 | 52 | ± 8.5 | ||
| EB | 121 | ± 26.7 | 49 | ± 11.9 | |||
| A1221 (0.5) | 170 | ± 33.1 | 52 | ± 14.0 | |||
| A1221 (1) | 138 | ± 22.9 | 39 | ± 15.7 | |||
Behaviors are shown that were investigator-scored that took place in relationship to the stimulus animal, within at least one body length. Sex differences and direction of change are indicated for each behavior. P values are provided when sexes were significantly different from one another. Data shown are mean ± SEM.
Figure 3.
Sociability test results are shown for the latency to investigate the stimulus animal (A), and the time spent nose touching (B). These measures were both sexually dimorphic, and higher in males than females (p < 0.005, 0.0001, respectively). However, no significant treatment effects within each sex were found. Data shown are mean ± standard error, with individual values shown as circles. N’s are indicated below each bar.
Social Novelty
The computer-generated data for the diagnostic behaviors of the Social Novelty tests are summarized in Table 2B and investigator-scored data related to behaviors that took place in proximity to a stimulus animal are shown in Table 3B. Similar to the Sociability test, in the Social Novelty test there were many significant sex differences: females traveled a greater distance, were faster, and engaged in more grooming and rearing than males (Table 2B). Males spent more time investigating the stimulus animal and nose touching than females, as well as time in proximity to the stimulus rat. The sexes were equivalent in the latency to the first nose touch (Table 3B).
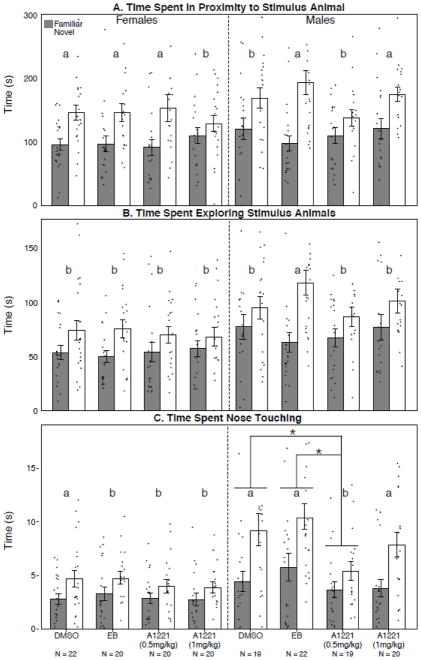
For the behaviors that took place in proximity to the stimulus animals, the dataset violated the assumptions for parametric analyses, despite any attempts at transformation. Student’s t-test identified significant effects of sex for these behaviors (Table 3B), and overall the expected preference to engage in behaviors with a novel over a familiar animal was observed. Further analysis by Cohen’s d effect size test revealed that this was altered in a sex- and dose-specific manner (Table 4). For the time spent in proximity to the stimulus animal (Figure 4A; Table 4A), three groups’ effect sizes [females (A1221, 1 mg/kg) and males (DMSO; A1221, 0.5 mg/kg)] did not meet Cohen’s d LARGE effect cut-off. For total time investigating the stimulus animal (Figure 4B; Table 4B), a LARGE effect size was only observed in EB males. Lastly, for time spent in direct nose-to-nose contact (Figure 4C; Table 4C), all non-vehicle female groups had a disrupted novelty preference; a LARGE effect size in the DMSO group was lost in the EB, and both A1221 groups. Among males, only the A1221 (0.5 mg/kg) group had an altered preference from DMSO. There was also an effect of treatment in the males, for which the A1221 (0.5 mg/kg) group spent significantly less time in direct nose-to-nose contact than both male control groups (p < 0.05; Figure 4C).
Table 4.
Cohen’s d effect size for behaviors
| A. Time in Proximity to Stimulus Animal
| |||
|---|---|---|---|
| Cohen’s d effect size | Percentile standing | Overlap (%) | |
|
|
|||
| Females | |||
| DMSO | 1.0 | 84 | 62 |
| EB | 1.6 | 95 | 42 |
| A1221 (0.5) | 0.8 | 79 | 69 |
| A1221 (1) | 0.3 | 62 | 88 |
| Males | |||
| DMSO | 0.7 | 76 | 73 |
| EB | 1.3 | 90 | 52 |
| A1221 (0.5) | 0.5 | 69 | 80 |
| A1221 (1) | 0.9 | 82 | 65 |
| B. Time Investigating Stimulus Animal
| |||
|---|---|---|---|
| Cohen’s d effect size | Percentile standing | Overlap (%) | |
|
|
|||
| Females | |||
| DMSO | 0.7 | 76 | 73 |
| EB | 0.7 | 76 | 73 |
| A1221 (0.5) | 0.5 | 69 | 80 |
| A1221 (1) | 0.2 | 58 | 92 |
| Males | |||
| DMSO | 0.2 | 58 | 92 |
| EB | 1.2 | 88 | 55 |
| A1221 (0.5) | 0.6 | 73 | 76 |
| A1221 (1) | 0.5 | 69 | 80 |
| C. Time Spent Nose Touching
| |||
|---|---|---|---|
| Cohen’s d effect size | Percentile standing | Overlap (%) | |
|
|
|||
| Females | |||
| DMSO | 1.4 | 92 | 48 |
| EB | 0.5 | 69 | 80 |
| A1221 (0.5) | 0.4 | 66 | 84 |
| A1221 (1) | 0.5 | 69 | 80 |
| Males | |||
| DMSO | 0.9 | 82 | 65 |
| EB | 0.8 | 79 | 69 |
| A1221 (0.5) | 0.5 | 69 | 80 |
| A1221 (1) | 1.0 | 84 | 62 |
Cohen’s d effect size was calculated for time spent in proximity to, investigating, and nose touching with, the familiar vs. the novel stimulus animal, in the Social Novelty test. An effect size of 0.8 or higher (in bold) is equivalent to Cohen’s standard LARGE, which indicates that the mean of the control group (Familiar) is at the 79th percentile and sharing 69% overlap with the comparison group (Novel).
Figure 4.
Three measures involving social interactions during Social Novelty are shown for time spent in proximity to the stimulus animal (A), time exploring the stimulus animal (B), and time spent nose touching (C). In (A), for females the Cohen’s d LARGE effect size was found for DMSO, EB, and A1221 (0.5 mg/kg), but not for A1221 (1 mg/kg). In males, the Cohen’s d LARGE effect size was found for EB and A1221 (1 mg/kg) but not DMSO or A1221 (0.5 mg/kg). In (B) Cohen’s d LARGE effect size was found only in the male EB group. In (C), in females, the Cohen’s d LARGE effect size was found only in the DMSO group, whereas in males, the LARGE effect size was found in DMSO, EB, and A1221 (1 mg/kg) animals but not A1221 (0.5 mg/kg). In addition, total time spent nose touching was significantly different in the male A1221 (0.5 mg/kg) compared to male DMSO or EB groups (p < 0.05 for both). Data shown are mean ± standard error, with individual values shown as circles. *, p < 0.05. Groups with an identified Cohen’s d LARGE effect size are indicated by a, and those without this effect by b. N’s are indicated below the bars for each group and are the same for A, B, and C.
Discussion
This study tested effects of prenatal PCB exposures on suites of behaviors exhibited in tests of sociability and social novelty. Significant sex differences in these behaviors were observed. In the Sociability test, both sexes showed a strong preference for affiliating with a stimulus animal (vs. an empty cage), though in general, experimental males exhibited more interactions with the stimulus males than did experimental females with the stimulus females. In the Social Novelty test, there were several sexually dimorphic responses, but treatment resulted in few differences within each sex.
During the Sociability test, though the preference to spend time associating with the stimulus animal (vs the empty stimulus cage) was present in both sexes, the degree to which the animals interacted differed. Females were quicker to initiate contact with the stimulus animals than males; however, males spent more time investigating and interacting with the stimulus animal. While the increased interactions in male rats has been previously observed (Meaney and Stewart, 1979), to our knowledge the reported differences in latency-to-investigation are novel. Consistent with the literature on social behavior in this particular paradigm (Choleris et al., 2006; Engelmann et al., 1995), there was a strong social preference, with the experimental animals spending more time in the chamber containing the stimulus animal than the chamber containing an empty stimulus cage.
During the Social Novelty test, sexual dimorphisms were again observed; males had longer latencies to initiate interactions, but ultimately interacted with both stimulus animals more so than did the females. Both sexes tended to associate and interact more with the novel conspecific compared to the familiar, in accordance with the literature (Cox and Rissman, 2011; Nadler et al., 2004; Wolstenholme et al., 2011). Our data suggest that (1) treatment does not alter the ability to acquire familiarity during the Sociability Stage, and (2) all animals are able to recognize and distinguish between the familiar and novel animal during the Social Novelty test.
Although most groups exhibited a strong novelty preference, there were exceptions, namely, the A1221 (1 mg/kg) female and the A1221 (0.5 mg/kg) male groups. This suggests that males and females have different sensitivities to A1221. Our laboratory has previously reported other sexually dimorphic changes due to gestational PCB exposure: only females have an increased postnatal body weight; only males exhibit an increased anogenital distance (Dickerson et al., 2011b; Walker et al., 2013). Developmental EDC exposure can also lead to sex-specific changes in behavior (Jacobsen et al., 2012; Kundakovic et al., 2013; Sobolewski et al., 2014; Williams et al., 2013). The male A1221 (0.5 mg/kg) group in our study also showed significantly less nose-to-nose interactions than vehicle males during Social Novelty, which was not seen in any of the female treatment groups. The degree of nose-touching that the A1221 (0.5 mg/kg) males displayed could be due to feminization of the brain areas involved in this behavior, as the behaviors were more similar to those observed in females. This also suggests that the males have an increased sensitivity of A1221-induced changes to adult social behaviors when compared to females. The EB groups were the only animals to lack the strong concordance of effect sizes when comparing the time spent in proximity and nose touching. EB animals in both sexes had the largest novelty preference in the time spent in proximity to the two stimulus animals but not in the nose-to-nose interactions, for which the female EB group lost the LARGE effect size comparison. This suggests that EB treatment, regardless of sex, amplifies the tendency toward social approach, rather than interactive behavior.
Overall, the females showed significantly higher locomotor behaviors (average speed, distance, grooming, and rearing) than the males. The behaviors scored in proximity to the stimulus animals (time near the stimulus animal and cage, time investigating the stimulus animal and cage, time actively nose touching the stimulus animal), indicative of an interaction with the conspecific, were significantly higher in the males. When these same animals were presented with opposite sex stimulus animals during mate preference in a separate study, it was the males that displayed more approach than the females, the latter which were the more interactive sex (Topper, et al., 2014). This discrepancy illuminates how these interactions depend upon the context.
There was a sex- and dose-specific increase in the concentration of serum corticosterone in our experimental animals. Adrenal weights were heavier in females than in males, consistent with observations of Richter (1956) and our previous work using a transgenerational EDC (vinclozolin) exposure and evaluation of F3 descendants (Gillette et al., 2014). Circulating corticosterone concentrations were also higher in females relative to males. Within the females, the A1221 (0.5 mg/kg) group had a significantly higher concentration of this hormone, the latter unlikely to be due to a larger adrenal size as there were no treatment effects on this latter endpoint within each sex. This sex-specificity is parallel to a similar observation made for vinclozolin, another EDC that led to a female-only increase in corticosterone concentration in the F3 descendants (Gillette et al., 2014). Though A1221 is known to be weakly estrogenic, differences with the EB group in the present study suggests an alternate (non-estrogenic) mechanism. It is known that the female rat is most sensitive to stress during proestrus (Viau and Meaney, 1991); thus, the increase observed only in the A1221 (0.5 mg/kg) females may be due to an altered hormonal phenotype at the time of euthanasia, during which all females were in proestrus.
Previous studies indicate that prenatal exposure to Bisphenol A, diethylstilbestrol, and organophosphate insecticides result in changes in the social, anxiety, exploratory, and sex behavior (reviewed in Frye, 2014). However, many of the studies that found alterations in behavior lacked a positive control group. Using large sample sizes and a thorough characterization of the social behavioral phenotype in our experimental rats, we revealed relatively few differences between negative control (DMSO), positive control (EB), and two dosages of A1221. The sexually dimorphic nature of the changes observed, though few, demonstrate that the neurobiological mechanisms underlying the sex differences may present a means of varying vulnerabilities to the organizational processes leading to the acquisition of normal social behavior. This dichotomy must be accounted for when assessing the effects of any environmental toxicant.
Highlights.
PCBs given to a pregnant dam alter social behavior in her adult offspring.
Males affiliated with the stimulus animal more than females.
Gestational exposure to PCBs decreased nose-to-nose investigations in males.
Serum corticosterone was higher in females prenatally exposed to PCBs (0.5 mg/kg).
Behaviors that occurred near the stimulus animals were altered in PCB-exposed rats.
Acknowledgments
We thank Ross Gillette for assistance on statistical analyses and providing the R scripts used in generating figures. Funded by NIH support: 1RO1 ES020662 to Andrea Gore and David Crews, and NIH T32 ES07247 (Michael Reilly).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnold AP. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Horm Behav. 2009;55:570–578. doi: 10.1016/j.yhbeh.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloni V, Dessì-Fulgheri F, Zaccaroni M, Di Consiglio E, De Angelis G, Testai E, Santochirico M, Alleva E, Santucci D. Early exposure to low doses of atrazine affects behavior in juvenile and adult CD1 mice. Toxicology. 2011;279:19–26. doi: 10.1016/j.tox.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Carrier N, Kabbaj M. Sex differences in social interaction behaviors in rats are mediated by extracellular signal-regulated kinase 2 expression in the medial prefrontal cortex. Neuroscience. 2012;212:86–92. doi: 10.1016/j.neuroscience.2012.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choleris E, Ogawa S, Kavaliers M, Gustafsson JÅ, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor α, β and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes, Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- Chung YW, Clemens LG. Effects of perinatal exposure to polychlorinated biphenyls on development of female sexual behavior. Bull Environ Contam Toxicol. 1999;62:664–670. doi: 10.1007/s001289900925. [DOI] [PubMed] [Google Scholar]
- Cox KH, Rissman EF. Sex differences in juvenile mouse social behavior are influenced by sex chromosomes and social context. Genes, Brain Behav. 2011;10:465–472. doi: 10.1111/j.1601-183X.2011.00688.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews D, Gillette R, Scarpino SV, Manikkam M, Savenkova MI, Skinner MK. Epigenetic transgenerational inheritance of altered stress responses. Proc Natl Acad Sci. 2012;109 (23):9143–9148. doi: 10.1073/pnas.1118514109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EC, Popper P, Gorski RA. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 1996;734:10–18. [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Gore AC. Prenatal PCBs disrupt early neuroendocrine development of the rat hypothalamus. Toxicol Appl Pharmacol. 2011a;252:36–46. doi: 10.1016/j.taap.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson SM, Cunningham SL, Patisaul HB, Woller MJ, Gore AC. Endocrine disruption of brain sexual differentiation by developmental PCB exposure. Endocrinology. 2011b;152:581–594. doi: 10.1210/en.2010-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Landgraf R. Social discrimination procedure: An alternative method to investigate juvenile recognition abilities in rats. Physiol Behav. 1995;58:315–321. doi: 10.1016/0031-9384(95)00053-l. [DOI] [PubMed] [Google Scholar]
- Frye CA. Endocrine-disrupting chemicals. Elucidating our understanding of their role in sex and gender-relevant end points. Vitam Horm. 2014;94:41–98. doi: 10.1016/B978-0-12-800095-3.00003-1. [DOI] [PubMed] [Google Scholar]
- Gillette R, Miller-Crews I, Nilsson EE, Skinner MK, Gore AC, Crews D. Sexually dimorphic effects of ancestral exposure to vinclozolin on stress reactivity in rats. Endocrinology. 2014;155:3853–3866. doi: 10.1210/en.2014-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen PR, Axelstad M, Boberg J, Isling LK, Christiansen S, Mandrup KR, Berthelsen LO, Vinggaard AM, Hass U. Persistent developmental toxicity in rat offspring after low dose exposure to a mixture of endocrine disrupting pesticides. Reprod Toxicol. 2012;34:237–250. doi: 10.1016/j.reprotox.2012.05.099. [DOI] [PubMed] [Google Scholar]
- Jacobson CD, Shryne JE, Shapiro F, Gorski RA. Ontogeny of the sexually dimorphic nucleus of the preoptic area. J Comp Neurol. 1980;193:541–548. doi: 10.1002/cne.901930215. [DOI] [PubMed] [Google Scholar]
- Jolous-Jamshidi B, Cromwell HC, McFarland AM, Meserve LA. Perinatal exposure to polychlorinated biphenyls alters social behaviors in rats. Toxicol Lett. 2010;199:136–143. doi: 10.1016/j.toxlet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundakovic M, Gudsnuk K, Franks B, Madrid J, Miller RL, Perera FP, Champagne Fa. Sex-specific epigenetic disruption and behavioral changes following low-dose in utero bisphenol A exposure. Proc Natl Acad Sci U S A. 2013;110:9956–9961. doi: 10.1073/pnas.1214056110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Stewart J. Environmental factors influencing the affiliative behavior of male and female rats (Rattus norvegicus) Anim Learn Behav. 1979;7:397–405. [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: An approach to assess autistic-like behavior in mice. Genes, Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Nadler JJ, Moy SS, Dold G, Trang D, Simmons N, Perez A, Young NB, Barbaro RP, Piven J, Magnuson TR, Crawley JN. Automated apparatus for quantitation of social approach behaviors in mice. Genes, Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- Richter CP. Salt appetite of mammals: Its dependence on instinct and metabolism. In: Grasse PP, editor. L’Instinct Dans Le Comportement DesAnimaux Et De L’Homme. Paris, France: Masson et Cie Editeurs; 1956. pp. 577–632. [Google Scholar]
- Slamberová R, Mikulecká A, Pometlová M, Schutová B, Hrubá L, Deykun K. Sex differences in social interaction of methamphetamine-treated rats. Behav Pharmacol. 2011;22:617–623. doi: 10.1097/FBP.0b013e32834afea4. [DOI] [PubMed] [Google Scholar]
- Sobolewski M, Conrad K, Allen JL, Weston H, Martin K, Lawrence BP, Cory-Slechta Da. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. Neurotoxicology. 2014;45C:121–130. doi: 10.1016/j.neuro.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stack A, Carrier N, Dietz D, Hollis F, Sorenson J, Kabbaj M. Sex differences in social interaction in rats: role of the immediate-early gene Zif268. Neuropsychopharmacology. 2010;35:570–580. doi: 10.1038/npp.2009.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Juenger TE, Gore AC. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm Behav. 2007;51:364–372. doi: 10.1016/j.yhbeh.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg RM, Walker DM, Juenger TE, Woller MJ, Gore AC. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol Reprod. 2008;78:1091–1101. doi: 10.1095/biolreprod.107.067249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AW, Beach EC, Stetzik LA, Perry A, D’Addezio AS, Cushing BS, Patisaul HB. A novel model for neuroendocrine toxicology: Neurobehavioral effects of BPA exposure in a prosocial species, the prairie vole (Microtus ochrogaster) Endocrinology. 2014;155:3867–3881. doi: 10.1210/en.2014-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi Y, Aburada S, Hashimoto K, Kitaura T. Transfer and distribution of accumulated (14C)polychlorinated bipheyls from maternal to fetal and suckling rats. Arch Environ Contam Toxicol. 1986;15:709–715. doi: 10.1007/BF01054917. [DOI] [PubMed] [Google Scholar]
- Topper VY, Reilly MP, Thompson LM, Wagner L, Gore AC. Gestational Exposure to Polychlorinated Biphenyls Alters Sociosexual Behaviors and Ultrasonic Vocalizations in Adult Rats. (Abstract/655.07/XX11). Presented at the Society for Neuroscience annual meeting; November 12, 2013; San Diego, CA. [Google Scholar]
- Veenit V, Cordero MI, Tzanoulinou S, Sandi C. Increased corticosterone in peripubertal rats leads to long-lasting alterations in social exploration and aggression. Front Behav Neurosci. 2013;7:26. doi: 10.3389/fnbeh.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venerosi A, Ricceri L, Tait S, Calamandrei G. Sex dimorphic behaviors as markers of neuroendocrine disruption by environmental chemicals: The case of chlorpyrifos. Neurotoxicology. 2012;33:1420–1426. doi: 10.1016/j.neuro.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Viau V, Meaney MJ. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991;129:2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Walker DM, Goetz BM, Gore AC. Dynamic postnatal developmental and sex-specific neuroendocrine effects of prenatal PCBs in rats. Mol Endocrinol. 2013;28:99–115. doi: 10.1210/me.2013-1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Kermath BA, Woller MJ, Gore AC. Disruption of reproductive aging in female and male rats by gestational exposure to estrogenic endocrine disruptors. Endocrinology. 2013;154:2129–2143. doi: 10.1210/en.2012-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DM, Kirson D, Perez LF, Gore AC. Molecular profiling of postnatal development of the hypothalamus in female and male rats. Biol Reprod. 2012;87:129. doi: 10.1095/biolreprod.112.102798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams SA, Jasarevic E, Vandas GM, Warzak DA, Geary DC, Ellersieck MR, Roberts RM, Rosenfeld CS. Effects of developmental bisphenol A exposure on reproductive-related behaviors in California mice (Peromyscus californicus): A monogamous animal model. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Goldsby JA, Rissman EF. Transgenerational effects of prenatal bisphenol A on social recognition. Horm Behav. 2013;64:833–839. doi: 10.1016/j.yhbeh.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolstenholme JT, Taylor JA, Shetty SRJ, Edwards M, Connelly JJ, Rissman EF. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]