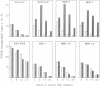
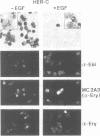
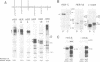Abstract
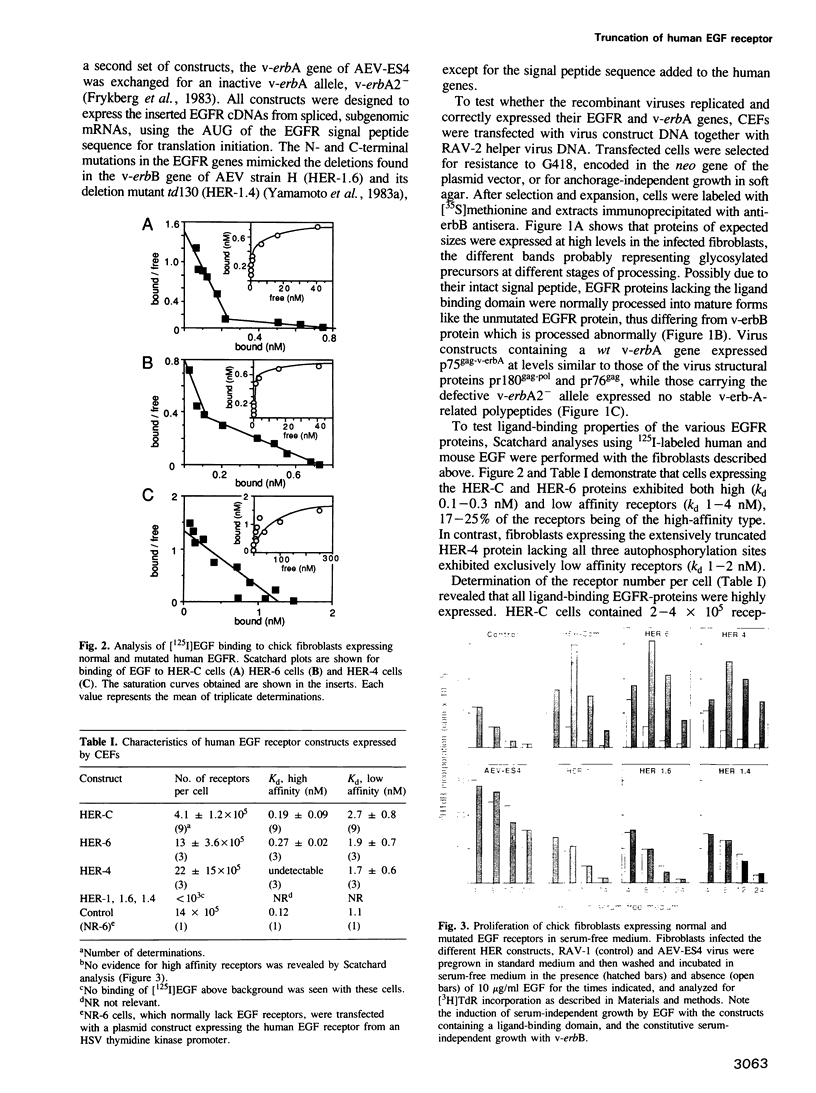
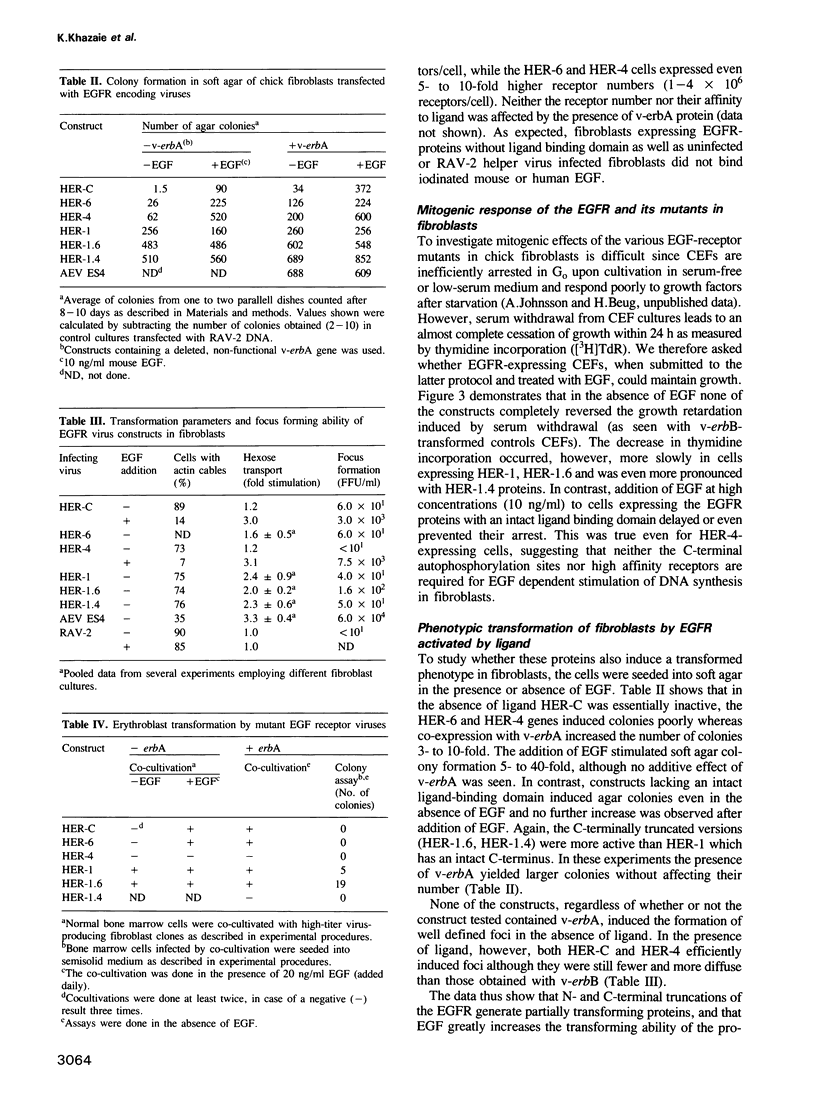
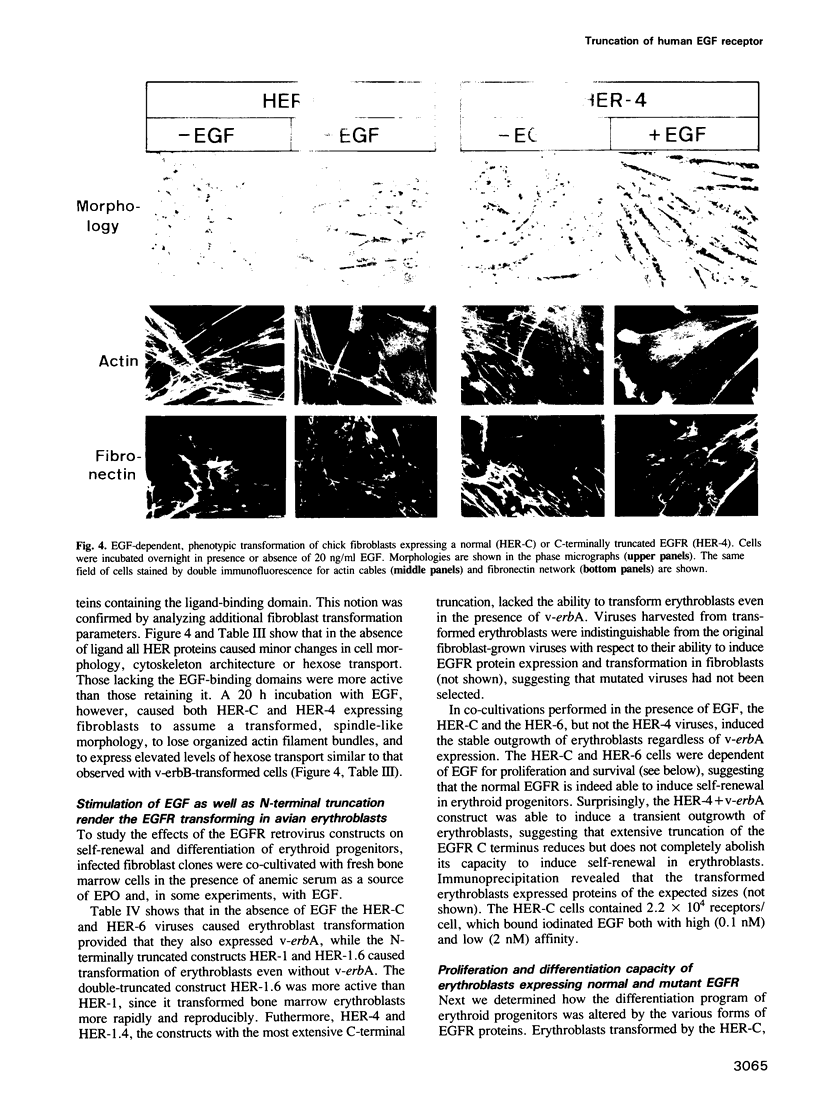
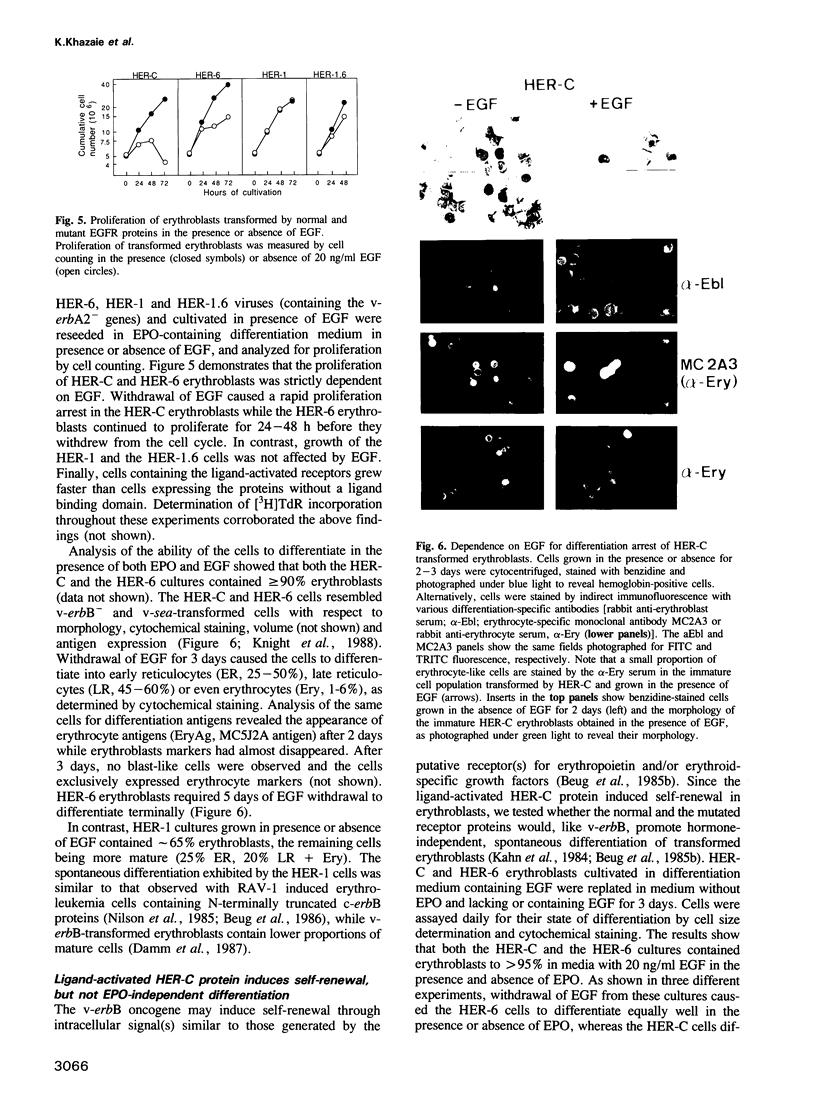
The transforming capacity of the normal and mutant human EGF receptor (EGFR) was investigated in primary chicken cells. In fibroblasts, both N- and C-terminal truncations resulted in a weak, additive oncogenic activity. However, not even double truncations caused a v-erbB-like phenotype. Upon EGF-binding, on the other hand, both normal and C-terminally truncated EGFRs resembled v-erbB in their fibroblast transforming potential. In erythroblasts, N-terminal truncation was sufficient to induce constitutive self-renewal, which was enhanced by deletion of 32 C-terminal amino acids but abolished by a larger truncation of 202 amino acids. In contrast to the normal EGFR, the receptor lacking 32 C-terminal amino acids resembled v-erbB in conferring erythropoietin independence for spontaneous differentiation to the transformed erythroblasts. Our results indicate that the C-terminal domain of the EGFR is non-essential in fibroblast transformation, but seems to be crucial for both self renewal induction and specificity of receptor function in erythroblasts.
Full text
PDF
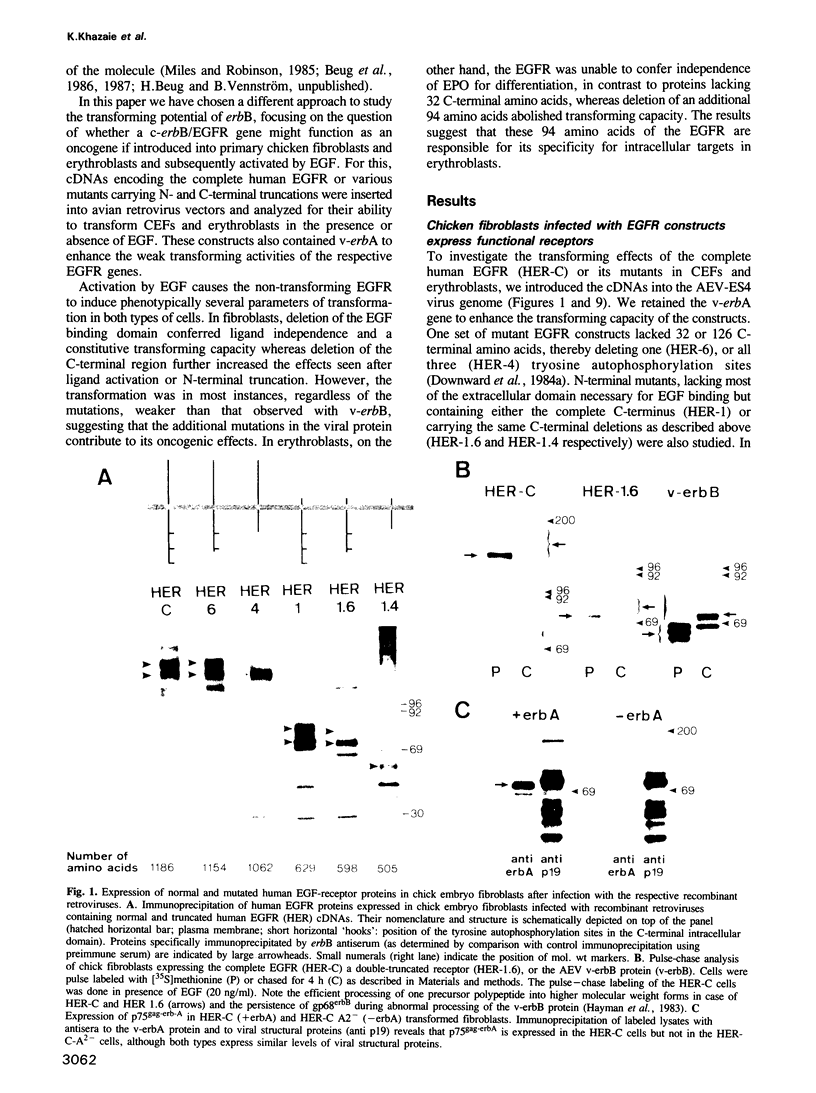




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassiri M., Privalsky M. L. Mutagenesis of the avian erythroblastosis virus erbB coding region: an intact extracellular domain is not required for oncogenic transformation. J Virol. 1986 Aug;59(2):525–530. doi: 10.1128/jvi.59.2.525-530.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Westermark B. Growth factor-induced proliferation of human fibroblasts in serum-free culture depends on cell density and extracellular calcium concentration. J Cell Physiol. 1984 Feb;118(2):203–210. doi: 10.1002/jcp.1041180213. [DOI] [PubMed] [Google Scholar]
- Beug H., Hayman M. J., Raines M. B., Kung H. J., Vennström B. Rous-associated virus 1-induced erythroleukemic cells exhibit a weakly transformed phenotype in vitro and release c-erbB-containing retroviruses unable to transform fibroblasts. J Virol. 1986 Mar;57(3):1127–1138. doi: 10.1128/jvi.57.3.1127-1138.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Hayman M. J. Temperature-sensitive mutants of avian erythroblastosis virus: surface expression of the erbB product correlates with transformation. Cell. 1984 Apr;36(4):963–972. doi: 10.1016/0092-8674(84)90046-1. [DOI] [PubMed] [Google Scholar]
- Beug H., Müller H., Grieser S., Doederlein G., Graf T. Hematopoietic cells transformed in vitro by REVT avian reticuloendotheliosis virus express characteristics of very immature lymphoid cells. Virology. 1981 Dec;115(2):295–309. doi: 10.1016/0042-6822(81)90112-4. [DOI] [PubMed] [Google Scholar]
- Beug H., Palmieri S., Freudenstein C., Zentgraf H., Graf T. Hormone-dependent terminal differentiation in vitro of chicken erythroleukemia cells transformed by ts mutants of avian erythroblastosis virus. Cell. 1982 Apr;28(4):907–919. doi: 10.1016/0092-8674(82)90070-8. [DOI] [PubMed] [Google Scholar]
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Choi O. R., Trainor C., Graf T., Beug H., Engel J. D. A single amino acid substitution in v-erbB confers a thermolabile phenotype to ts167 avian erythroblastosis virus-transformed erythroid cells. Mol Cell Biol. 1986 May;6(5):1751–1759. doi: 10.1128/mcb.6.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm K., Beug H., Graf T., Vennström B. A single point mutation in erbA restores the erythroid transforming potential of a mutant avian erythroblastosis virus (AEV) defective in both erbA and erbB oncogenes. EMBO J. 1987 Feb;6(2):375–382. doi: 10.1002/j.1460-2075.1987.tb04765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fiore P. P., Pierce J. H., Fleming T. P., Hazan R., Ullrich A., King C. R., Schlessinger J., Aaronson S. A. Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell. 1987 Dec 24;51(6):1063–1070. doi: 10.1016/0092-8674(87)90592-7. [DOI] [PubMed] [Google Scholar]
- Downward J., Parker P., Waterfield M. D. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984 Oct 4;311(5985):483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Frykberg L., Graf T., Vennström B. The transforming activity of the chicken c-myc gene can be potentiated by mutations. Oncogene. 1987;1(4):415–422. [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Fung Y. K., Lewis W. G., Crittenden L. B., Kung H. J. Activation of the cellular oncogene c-erbB by LTR insertion: molecular basis for induction of erythroblastosis by avian leukosis virus. Cell. 1983 Jun;33(2):357–368. doi: 10.1016/0092-8674(83)90417-8. [DOI] [PubMed] [Google Scholar]
- Gamett D. C., Tracy S. E., Robinson H. L. Differences in sequences encoding the carboxyl-terminal domain of the epidermal growth factor receptor correlate with differences in the disease potential of viral erbB genes. Proc Natl Acad Sci U S A. 1986 Aug;83(16):6053–6057. doi: 10.1073/pnas.83.16.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Benchaibi M., Xiao J. H., Ghysdael J., Samarut J. Expression of the v-erbA oncogene in chicken embryo fibroblasts stimulates their proliferation in vitro and enhances tumor growth in vivo. Cell. 1987 Jun 5;49(5):687–697. doi: 10.1016/0092-8674(87)90545-9. [DOI] [PubMed] [Google Scholar]
- Gilmore T., DeClue J. E., Martin G. S. Protein phosphorylation at tyrosine is induced by the v-erbB gene product in vivo and in vitro. Cell. 1985 Mar;40(3):609–618. doi: 10.1016/0092-8674(85)90209-0. [DOI] [PubMed] [Google Scholar]
- Graf T., Royer-Pokora B., Schubert G. E., Beug H. Evidence for the multiple oncogenic potential of cloned leukemia virus: in vitro and in vitro studies with avian erythroblastosis virus. Virology. 1976 Jun;71(2):423–433. doi: 10.1016/0042-6822(76)90370-6. [DOI] [PubMed] [Google Scholar]
- Graf T. Two types of target cells for transformation with avian myelocytomatosis virus. Virology. 1973 Aug;54(2):398–413. doi: 10.1016/0042-6822(73)90152-9. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Beug H. Identification of a form of the avian erythroblastosis virus erb-B gene product at the cell surface. 1984 May 31-Jun 6Nature. 309(5967):460–462. doi: 10.1038/309460a0. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Kitchener G., Knight J., McMahon J., Watson R., Beug H. Analysis of the autophosphorylation activity of transformation defective mutants of avian erythroblastosis virus. Virology. 1986 Apr 15;150(1):270–275. doi: 10.1016/0042-6822(86)90287-4. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Jansson M., Beug H., Gray C., Graf T., Vennström B. Defective v-erbB genes can be complemented by v-erbA in erythroblast and fibroblast transformation. Oncogene. 1987 May;1(2):167–173. [PubMed] [Google Scholar]
- Johnsson A., Betsholtz C., Heldin C. H., Westermark B. Antibodies against platelet-derived growth factor inhibit acute transformation by simian sarcoma virus. Nature. 1985 Oct 3;317(6036):438–440. doi: 10.1038/317438a0. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Betsholtz C., Heldin C. H., Westermark B. The phenotypic characteristics of simian sarcoma virus-transformed human fibroblasts suggest that the v-sis gene product acts solely as a PDGF receptor agonist in cell transformation. EMBO J. 1986 Jul;5(7):1535–1541. doi: 10.1002/j.1460-2075.1986.tb04394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P., Adkins B., Beug H., Graf T. src- and fps-containing avian sarcoma viruses transform chicken erythroid cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7122–7126. doi: 10.1073/pnas.81.22.7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn P., Frykberg L., Brady C., Stanley I., Beug H., Vennström B., Graf T. v-erbA cooperates with sarcoma oncogenes in leukemic cell transformation. Cell. 1986 May 9;45(3):349–356. doi: 10.1016/0092-8674(86)90320-x. [DOI] [PubMed] [Google Scholar]
- Kawai S., Nishizawa M., Yamamoto T., Toyoshima K. Cell transformation by a virus containing a molecularly constructed gag-erbB fused gene. J Virol. 1987 May;61(5):1665–1669. doi: 10.1128/jvi.61.5.1665-1669.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lax I., Kris R., Sasson I., Ullrich A., Hayman M. J., Beug H., Schlessinger J. Activation of c-erbB in avian leukosis virus-induced erythroblastosis leads to the expression of a truncated EGF receptor kinase. EMBO J. 1985 Dec 1;4(12):3179–3182. doi: 10.1002/j.1460-2075.1985.tb04062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutz A., Beug H., Graf T. Purification and characterization of cMGF, a novel chicken myelomonocytic growth factor. EMBO J. 1984 Dec 20;3(13):3191–3197. doi: 10.1002/j.1460-2075.1984.tb02278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles B. D., Robinson H. L. High-frequency transduction of c-erbB in avian leukosis virus-induced erythroblastosis. J Virol. 1985 May;54(2):295–303. doi: 10.1128/jvi.54.2.295-303.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen T. W., Maroney P. A., Goodwin R. G., Rottman F. M., Crittenden L. B., Raines M. A., Kung H. J. c-erbB activation in ALV-induced erythroblastosis: novel RNA processing and promoter insertion result in expression of an amino-truncated EGF receptor. Cell. 1985 Jul;41(3):719–726. doi: 10.1016/s0092-8674(85)80052-0. [DOI] [PubMed] [Google Scholar]
- Palmieri S., Kahn P., Graf T. Quail embryo fibroblasts transformed by four v-myc-containing virus isolates show enhanced proliferation but are non tumorigenic. EMBO J. 1983;2(12):2385–2389. doi: 10.1002/j.1460-2075.1983.tb01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Ruggiero M., Fleming T. P., Di Fiore P. P., Greenberger J. S., Varticovski L., Schlessinger J., Rovera G., Aaronson S. A. Signal transduction through the EGF receptor transfected in IL-3-dependent hematopoietic cells. Science. 1988 Feb 5;239(4840):628–631. doi: 10.1126/science.3257584. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Bishop J. M. Subcellular localization of the v-erb-B protein, the product of a transforming gene of avian erythroblastosis virus. Virology. 1984 Jun;135(2):356–368. doi: 10.1016/0042-6822(84)90192-2. [DOI] [PubMed] [Google Scholar]
- Radke K., Beug H., Kornfeld S., Graf T. Transformation of both erythroid and myeloid cells by E26, an avian leukemia virus that contains the myb gene. Cell. 1982 Dec;31(3 Pt 2):643–653. doi: 10.1016/0092-8674(82)90320-8. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Miles B. D., Catalano D. E., Briles W. E., Crittenden L. B. Susceptibility to erbB-induced erythroblastosis is a dominant trait of 151 chickens. J Virol. 1985 Sep;55(3):617–622. doi: 10.1128/jvi.55.3.617-622.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer-Pokora B., Beug H., Claviez M., Winkhardt H. J., Friis R. R., Graf T. Transformation parameters in chicken fibroblasts transformed by AEV and MC29 avian leukemia viruses. Cell. 1978 Apr;13(4):751–760. doi: 10.1016/0092-8674(78)90225-8. [DOI] [PubMed] [Google Scholar]
- Samarut J., Gazzolo L. Target cells infected by avian erythroblastosis virus differentiate and become transformed. Cell. 1982 Apr;28(4):921–929. doi: 10.1016/0092-8674(82)90071-x. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Schmidt J. A., Marshall J., Hayman M. J., Doderlein G., Beug H. Monoclonal antibodies to novel erythroid differentiation antigens reveal specific effects of oncogenes on the leukaemic cell phenotype. Leuk Res. 1986;10(3):257–272. doi: 10.1016/0145-2126(86)90023-8. [DOI] [PubMed] [Google Scholar]
- Scotting P., Vennstrom B., Jansen M., Graf T., Beug H., Hayman M. J. Common site of mutation in the erbB gene of avian erythroblastosis virus mutants that are temperature sensitive for transformation. Oncogene Res. 1987 Aug;1(3):265–278. [PubMed] [Google Scholar]
- Sealy L., Privalsky M. L., Moscovici G., Moscovici C., Bishop J. M. Site-specific mutagenesis of avian erythroblastosis virus: erb-B is required for oncogenicity. Virology. 1983 Oct 15;130(1):155–178. doi: 10.1016/0042-6822(83)90125-3. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Velu T. J., Beguinot L., Vass W. C., Willingham M. C., Merlino G. T., Pastan I., Lowy D. R. Epidermal-growth-factor-dependent transformation by a human EGF receptor proto-oncogene. Science. 1987 Dec 4;238(4832):1408–1410. doi: 10.1126/science.3500513. [DOI] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Hihara H., Nishida T., Kawai S., Toyoshima K. A new avian erythroblastosis virus, AEV-H, carries erbB gene responsible for the induction of both erythroblastosis and sarcomas. Cell. 1983 Aug;34(1):225–232. doi: 10.1016/0092-8674(83)90153-8. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nishida T., Miyajima N., Kawai S., Ooi T., Toyoshima K. The erbB gene of avian erythroblastosis virus is a member of the src gene family. Cell. 1983 Nov;35(1):71–78. doi: 10.1016/0092-8674(83)90209-x. [DOI] [PubMed] [Google Scholar]
- Zenke M., Kahn P., Disela C., Vennström B., Leutz A., Keegan K., Hayman M. J., Choi H. R., Yew N., Engel J. D. v-erbA specifically suppresses transcription of the avian erythrocyte anion transporter (band 3) gene. Cell. 1988 Jan 15;52(1):107–119. doi: 10.1016/0092-8674(88)90535-1. [DOI] [PubMed] [Google Scholar]