Abstract
Background
In recent years, neoadjuvant chemotherapy (NAC) is often performed for patients with unresectable breast carcinoma or without indication of breast conserving therapy. However, it is currently difficult to predict response to NAC with diagnostic imaging of breast carcinoma. In this study, we investigated imaging findings that could serve as a predictor of the response to NAC for patients with invasive breast carcinoma.
Methods
Twenty-six patients with invasive breast carcinoma who received NAC at the Division of Breast and Endocrine Surgery of Tottori University Hospital between January 2010 and May 2014 were retrospectively investigated. Their imaging findings from mammograms and ultrasonograms were reviewed. The association between findings on mammograms and ultrasonograms captured before NAC and response to treatment after NAC was examined.
Results
Of the 26 patients with invasive breast carcinoma, 19 (73%) responded well to treatment and 7 (27%) did not. Most notably, all 10 patients who had microcalcifications on mammogram responded well to treatment (53% of responders), and all patients who did not respond to treatment had no microcalcifications (P < 0.05). Of these 10 patients, 9 (90%) had microcalcifications of comedo type and one (10%) had non comedo type. As a distribution, 8 of the 10 (80%) had a clustered type of microcalcifications and the remaining 2 (20%) had a segmental type of them.
Conclusion
Microcalcifications of tumor observed in mammogram (particularly comedo type) could be a predictor of response to NAC for patients with invasive breast carcinoma.
Keywords: breast carcinoma, microcalcification, neoadjuvant chemotherapy
Neoadjuvant chemotherapy (NAC) is used to treat invasive breast carcinoma when the carcinoma is unresectable or when breast-conserving surgery is not indicated. Some advantages of NAC include downstaging due to tumor shrinkage and improved survival in patients showing a pathological complete response (pCR).1–4 However, changes in medications or adjustments to the course of treatment (e.g., surgery) could be necessary if a patient does not respond.
In this study, we investigated imaging findings of invasive breast carcinoma that could serve as a predictor of response to NAC with the aim of reducing the frequency of unnecessary treatment.
SUBJECTS AND METHODS
Twenty-six patients with invasive breast carcinoma who received NAC at the Division of Breast and Endocrine Surgery of Tottori University Hospital between January 2010 and May 2014 were retrospectively investigated. Their imaging findings from mammograms and ultrasonograms were reviewed individually without patients information by two medical breast cancer specialists who had over 25 years experiences in breast examinations. When mutual evaluation of findings was not matched, consensuses were got in each other. The association between findings on mammograms [tumor (size, shape, and border/margin), microcalcifications (morphology and distribution)] and ultrasonograms [tumor size, shape, border area, halo, internal echo, posterior echo, and high echogenic foci] before NAC and response to NAC was assessed.
Of the 26 patients, 14 received DTX (docetaxel hydrate) followed by FEC (F: fluorouracil, E: epirubicin hydrochloride, C: cyclophosphamide), 11 received DTX plus trastuzumab followed FEC, and one received trastuzumab alone. The typical number of cycles was 4 of FEC and 4 of DTX. Response to NAC was determined according to the Response Evaluation Criteria in Solid Tumors (RECIST)5 using contrast computed tomography (CT), and well response was defined as either a CR or partial response (PR). All patients besides one patient who had progressive disease (PD) after NAC underwent mastectomy or breast-conserving surgery. Fischer’s exact test was used for statistical analysis and P < 0.05 was considered significant. This study was approved by the ethics review board of Tottori University Faculty of Medicine.
RESULTS
All patients were women and mean age was 55 years (range: 27–70 years). Of the 26 patients, 19 (73%; CR: 6, PR: 13) responded well to treatment and 7 [27%; stable disease (SD): 6, PD: 1] did not. All 10 patients who had microcalcifications on mammogram responded well to treatment (53% of responders), and all patients who did not respond to treatment had no microcalcifications (P < 0.05) (Fig. 1). Of these 10 patients with microcalcifications on mammogram, 9 (90%) had microcalcifications of comedo type and one (10%) had non comedo type (Fig. 2). Eight of the 10 (80%) had a clustered distribution of microcalcifications and the remaining 2 (20%) had a segmental distribution of them (Fig. 3). Additionally, 7 of the 10 patients with microcalcifications, including 6 of the 9 patients with microcalcifications of comedo type, had overexpressed human epidermal growth factor receptor type2 (HER2). But there was no association between microcalcifications and HER2 overexpression (Table 1). When the association between response to treatment and the regimen of NAC was examined, it was found that all 11 patients who received trastuzumab in combination therapy (all of them had HER2 overexpression) responded well to treatment. Furthermore, 7 of the 11 had microcalcifications and 6 of the 7 with microcalcifications were comedo type. The patient who received trastuzumab alone did not respond well to treatment. No other findings on mammograms or ultrasonograms were associated with response to treatment.
Fig. 1.
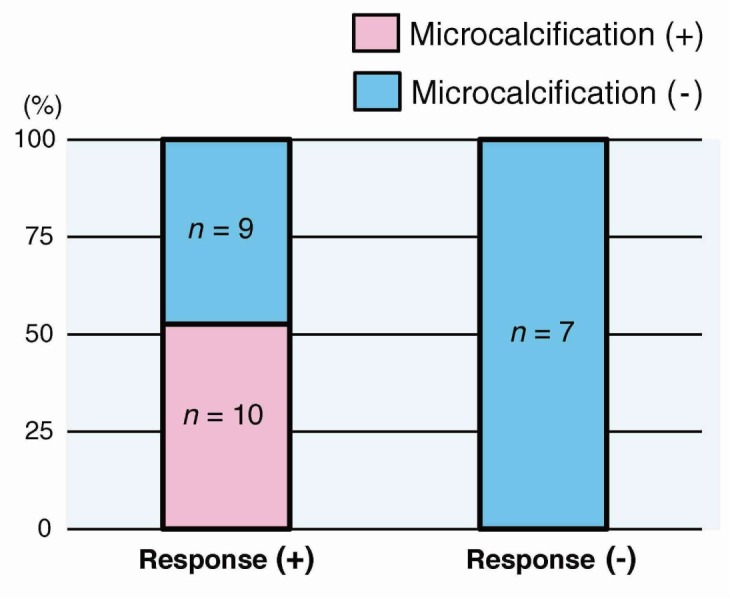
Microcalcifications and response to treatment. All breast carcinomas with microcalcifications responded well to treatment (P < 0.05).
Fig. 2.
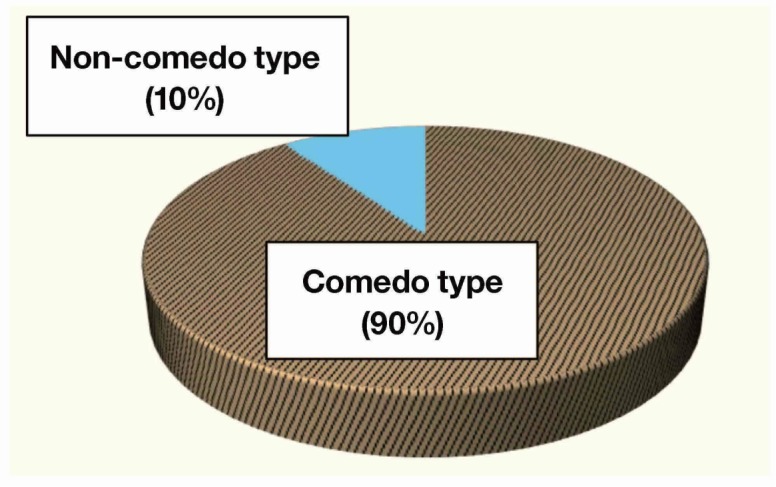
Type of microcalcifications. Most microcalcifications were comedo type.
Fig. 3.
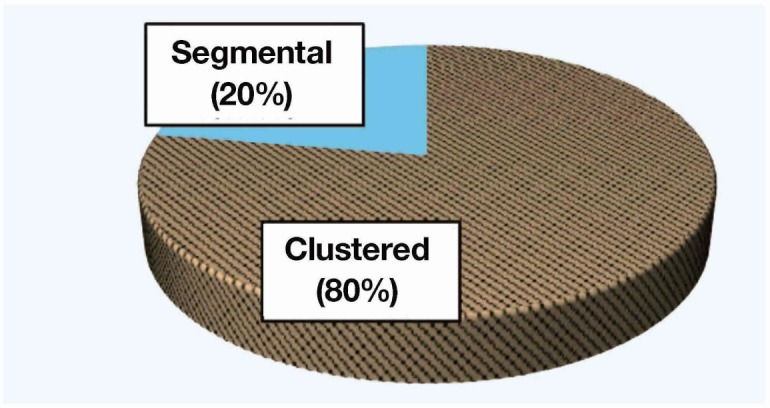
Distribution of microcalcifications. Most microcalcifications had a clustered distribution.
Table 1.
Microcalcifications and HER2 overexpression
| Microcalcifications (+) | Microcalcifications (−) | Total | |
| HER2 (+) | 7 | 5 | 12 |
| HER2 (−) | 3 | 11 | 14 |
| Total | 10 | 16 | 26 |
There was no association between microcalcifications and HER2 overexpression (P = 0.105).
Values within table indicate number of patients.
HER2, human epidermal growth factor receptor type2.
DISCUSSION
Few studies have discussed microcalcifications on mammogram in the context of NAC,6–8 and there is no consensus regarding the utility of microcalcifications as a predictor of response to NAC. Li et al. found that breast carcinoma patients with microcalcifications were more likely to exhibit an evaluable category of CR to neoadjuvant chemotherapy, although there was no significant difference.7 In our study, all breast carcinoma patients who had microcalcifications on mammogram responded well to NAC (i.e., exhibited a CR or PR). Furthermore, the fact that there was no association between HER2 overexpression and microcalcification, indicates that breast carcinoma with microcalcifications should exhibit at least PR (tumor shrinkage of ≥ 30%) to NAC regardless of HER2 overexpression.
In contrast, Weiss et al. found that many of breast carcinoma patients with microcalcifications exhibit SD or PD after NAC, whereas breast carcinoma patients without microcalcifications exhibit CR.8 In their study, responses to NAC were evaluated to measuring the diameter of breast carcinoma by using mammograms. They reported that the size of tumor with microcalcifications was 4.4 cm average in diameter evaluated by mammogram after NAC, while acutual histological size of tumor with microcalcifications was 2.3 cm average in diameter. Furthermore, the diameter of breast carcinoma measured by mammogram was not correlated with histological tumor size. It was thought that overestimation of the tumor diameter causes poor evaluation of the responses to NAC.
Whereas we evaluated response using contrast CT. Akashi-Tanaka et al. found that for all breast carcinomas besides invasive lobular carcinoma and inflammatory breast carcinoma, tumor diameter measured with contrast CT was most highly correlated with histological tumor diameter.9
In this study, characteristic feature of almost all microcalcifications on mammograms were comedo type. Breast carcinoma with microcalcifications of comedo type is considered to be highly proliferative activity.10 Furthermore, Brower et al. found that Ki-67 is high value in breast carcinoma with microcalcifications of comedo type.11 As to association between Ki-67 and chemotherapy, Criscitiello et al. reported that the addition of chemotherapy to endocrine therapy is useful for estrogen receptor (ER) positive, node positive breast carcinoma with high Ki-67 value.12 And, Petit et al. found that breast carcinoma patients with low ER expression and high Ki-67, responded well to NAC.13 Moreover, several studies have shown that patients with high Ki-67 are more likely to show a pathological CR.14, 15
The above findings demonstrate that microcalcifications of comedo type reflect the proliferative potential of breast carcinoma. Additionally, the fact that NAC is effective against breast carcinomas with highly proliferative activity, may explain that breast carcinoma patients with microcalcifications on mammogram responded well to treatment in this study.
In conclusion, the present study suggests that microcalcifications on mammogram (particularly comedo type) could be a predictor of response to NAC for patients with invasive breast carcinoma.
The authors declare no conflict of interest.
REFERENCES
- 1.Rastogi P , Anderson SJ , Bear HD , Geyer CE , Kahlenberg MS , Robidoux A , et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26: 778-85. . [DOI] [PubMed] [Google Scholar]
- 2.Gralow JR , Burstein HJ , Wood W , Hortobagyi GN , Gianni L , von Minckwitz G , et al. Preoperative therapy in invasive breast carcinoma : pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008; 26: 814-9. . [DOI] [PubMed] [Google Scholar]
- 3.Chollet P , Charrier S , Brain E , Cure H , van Praagh I , Feillel V , et al. Clinical and pathological response to primary chemotherapy in operable breast carcinoma. Eur J Cancer. 1997; 33: 862-6. [DOI] [PubMed] [Google Scholar]
- 4.Makris A , Powles TJ , Ashley SE , Chang J , Hickish T , Tidy VA , et al. A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast carcinoma. Ann Oncol. 1998; 9: 1179-84. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauera EA , Therasseb P , Bogaertsc J , Schwartzd LH , Sargente D , Fordf R , et al. New response evaluation criteria in solid tumours : revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228-47. . [DOI] [PubMed] [Google Scholar]
- 6.Esserman LE , d’Almeida M , Da Costa D , Gerson DM , Poppiti RJ Jr. . Mammographic appearance of microcalcifications: can they change after neoadjuvant chemotherapy?. Breast J. 2006; 12: 86-7. . [DOI] [PubMed] [Google Scholar]
- 7.Li JJ , Chen C , Gu Y , Di G , Wu J , Liu G , et al. The role of mammographic calcification in the neoadjuvant therapy of breast carcinoma imaging evaluation. PLoS One. 2014; 233: 830-49. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss A , Lee KC , Romero Y , Ward E , Kim Y , Ojeda-Fournier H , et al. Calcifications on Mammogram do not correlate with tumor size after neoadjuvant chemotherapy. Ann Surg Oncol. 2014; 21: 3310-6. . [DOI] [PubMed] [Google Scholar]
- 9.Akashi-Tanaka S , Fukutomi T , Watanabe T , Katsumata N , Nanasawa T , Matsuo K , et al. Accuracy of contrast-enhanced computed tomography in the prediction of residual breast carcinoma after neoadjuvant chemotherapy. Int J Cancer. 2001; 96: 66-73. . [DOI] [PubMed] [Google Scholar]
- 10.Yagata H , Harigaya K , Suzuki M , Nagashima T , Hashimoto H , Ishii G , et al. Comedonecrosis is an unfavorable marker in node-negative invasive breast carcinoma. Pathol Int. 2003; 53: 501-6. . [DOI] [PubMed] [Google Scholar]
- 11.Brower ST , Ahmed S , Tartter PI , Bleiweiss I , Amberson JB . Prognostic variables in invasive breast carcinoma: contribution of comedo versus noncomedo in situ component. Ann Surg Oncol. 1995; 2: 440-4. . [DOI] [PubMed] [Google Scholar]
- 12.Criscitiello C , Disalvatore D , de Laurentiis M , Gelao L , Fumagalli L , Locatelli M , et al. High Ki-67 score is indicative of a greater benefit from adjuvant chemotherapy when added to endocrine therapy in luminal B HER2 negative and node-positive breast carcinoma. Breast. 2014; 23: 69-75. . [DOI] [PubMed] [Google Scholar]
- 13.Petit T , Wilt M , Velten M , Rodier JF , Fricker JP , Dufour P , et al. Semi-quantitative evaluation of estrogen receptor expression is a strong predictive factor of pathological complete response after anthracycline-based neo-adjuvant chemotherapy in hormonal sensitive breast carcinoma. Breast Carcinoma Res Treat. 2010; 124: 387-91. . [DOI] [PubMed] [Google Scholar]
- 14.Petit T , Wiltb M , Veltenc M , Millond R , Rodiere JF , Borela C . Comparative value of tumour grade hormonal receptors Ki-67, HER-2 and topoisomerase II alpha status as predictive markers in breast carcinoma patients treated with neoadjuvant anthracycline-based chemotherapy. Eur J Cancer. 2004; 40: 205-11. . [DOI] [PubMed] [Google Scholar]
- 15.Prisack HB , Karreman C , Karreman O , Audretsch W , Danae M , Rezai M , et al. Predictive biological markers for response of invasive breast carcinoma to anthracycline / cyclophosphamide-based primary (radio-)chemotherapy. Anticancer Res. 2005; 25: 4615-21. . [PubMed] [Google Scholar]


