Abstract
Background
Recently, a lot of cases with microcalcifications of the breast are pointed by the images of mammography (MG), because breast screening using MG become common. Although MG is a gold standard modality for detecting microcalcifications, images of ultrasonography (US) are now feasible to detect microcalcifications with recent improvements to ultrasound diagnostic devices. In this report, we analyzed clinical significance of microcalcifications detected with US images in invasive breast carcinoma.
Methods
Eighty-eight patients with invasive breast carcinoma who underwent MG and US before surgery at the Division of Breast and Endocrine Surgery of Tottori University Hospital between January 2012 and August 2013. After reviewing US images, the association between the presence of echogenic spots that indicate microcalcifications and images of MG or pathological findings was assessed.
Results
Patients without microcalcifications on US images were significantly more likely to have the Luminal A subtype and a lower nuclear grading. Conversely, patients with microcalcifications on US images were significantly more likely to have higher level of MIB-1 index, lymphovascular invasion, comedonecrosis and lymph node metastasis. The rate of detecting microcalcifications on US images was relatively good, with 81.8% of sensitivity, 94.5% of specificity and 89.8% of diagnostic accuracy. Among the calcifications detected by MG images, detected rate of calcifications with US images was higher in necrotic type (92.6%) than secretory type (33.3%).
Conclusion
This study suggest that microcalcifications of tumors detected by US images could serve as an useful prediction to evaluate the degree of malignancy for patients with invasive breast carcinoma.
Keywords: breast carcinoma, microcalcification, ultrasonography
Recently, a lot of cases with microcalcifications of the breast are pointed by the images of mammography (MG), because breast screening using MG become common. Although MG is a gold standard modality for detecting microcalcifications, images of ultrasonography (US) are now feasible to detect microcalcifications with recent improvements to ultrasound diagnostic devices.1-4 There have been several studies on the performance of US for detecting microcalcifications, but most reports focused on ductal carcinoma in situ (DCIS). 1-3, 5 Microcalcifications are seen in invasive carcinomas as well as DCIS (Fig. 1), but there have been few detailed studies about the significance of microcalcifications in such carcinomas. In this study, we compared the presence of microcalcifications on US images with findings from MG images and clinical pathology, to interpret the significance of detection of microcalcifications on US images.
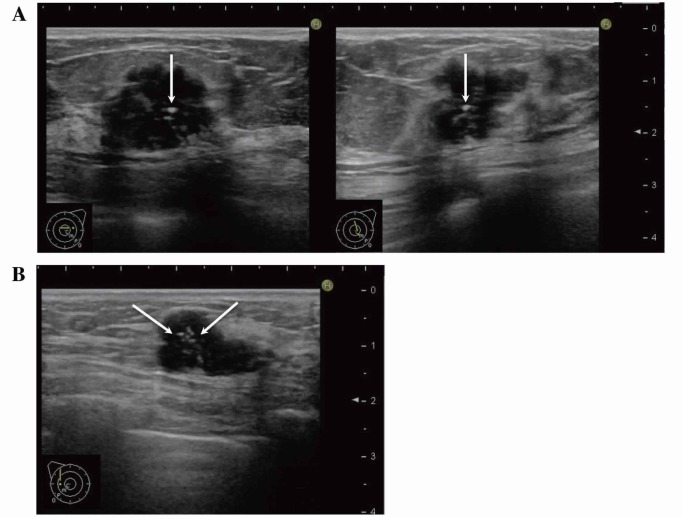
Fig. 1.
US images show microcalcifications (arrows) observed not only in DCIS (left) but also in invasive carcinoma (right).
DCIS, ductal carcinoma in situ; US, ultrasonography.
SUBJECTS AND METHODS
Patients
Eighty-eight patients with invasive breast carcinoma who underwent preoperative MG and US in the Division of Breast and Endocrine Surgery of Tottori University Hospital between January 2012 and August 2013 were retrospectively investigated. Patients with DCIS and those who received neoadjuvant chemotherapy were excluded.
Methods
US examinations were performed using a HI VISION Preirus (Hitachi Aloka Medical, Tokyo, Japan) with an EUP-L74M probe (linear, center frequency: 7.5 MHz). Echogenic spots indicative of microcalcifications were defined as discontinuous echogenic spots within a mass or hypoechoic area, or clustered echogenic spots clearly different from the mammary glands (Fig. 2). In this study, we defined microcalcifications detected by MG images as positive cases of microcalcifications. Preoperative MG and US images were retrospectively reviewed individually without patients information by a medical breast cancer specialist who had over 25 years experiences in breast examinations and a medical sonographer (superficial organs) with over 3 years experiences. When mutual evaluation of findings was not matched, consensuses were got in each other. The association between the presence of echogenic spots within a tumor on US images and findings from MG images and pathological features of the resected specimen (subtype, histologic type, lymphovascular invasion, comedonecrosis, nuclear grading, MIB-1 index and lymph node metastases) were investigated. Fischer’s exact test and the Mann-Whitney U test were used for statistical analysis. P values of less than 0.05 were considered to indicate statistically significant differences. This study was conducted with the approval of the Ethics Committee of Tottori University Faculty of Medicine.
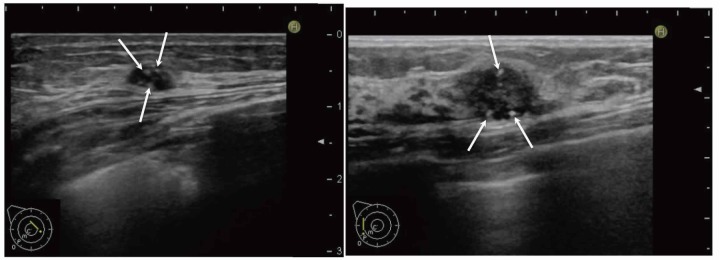
Fig. 2.
Microcalcifications on US images.
A: Transverse (left) and longitudinal (right) US images of the AC area of the left breast. Noncontinuous echogenic spots (arrows) were observed on orthogonal tomographic images.
B: Longitudinal image of the C area of the left breast. Punctuate echogenic spots (arrows) with a tissue composition clearly different from that of the mammary glands were observed inside the mass.
US, ultrasonography.
RESULTS
All patients were female and mean age was 61.2 years (range: 28–87 years). Table 1 shows the results of relationship between US and MG images for detecting microcalcifications in all 88 patients. Microcalcifications were detected in 33 patients but not in 55 patients with MG images. Twenty-seven of the 33 patients appeared as echogenic spots on US images. US examination showed 81.8% sensitivity, 94.5% specificity and 89.8% diagnostic accuracy for detecting microcalcifications. There were 6 false negatives where microcalcifications were detected with MG but not US images. Conversely, there were 3 false positives where microcalcifications were not detected with MG but echogenic spots were seen on US images. In such false positive cases, pathological specimens revealed no microcalcifications and no characteristic features.
Table 1.
Detection of microcalcifications with MG and US
| MG | Total | |||
| + | − | |||
| US | + | 27 | 3 | 30 |
| − | 6 | 52 | 58 | |
| Total | 33 | 55 | 88 | |
US examination showed 81.8% sensitivity, 94.5% specificity and 89.8% diagnostic accuracy for detecting microcalcifications.
MG, mammography; US, ultrasonography.
US detectivity for microcalcifications was evaluated (Table 2). It was found that US examination detected a higher rate of pleomorphic or linear/branching calcifications that appeared to be necrotic calcifications (25/27) compared with amorphous calcifications that appeared to be secretory calcifications (2/6). And US examination showed 92.6% sensitivity, 66.7% specificity for detecting necrotic calcifications. In six false negative cases, 4 had secretary types and 2 had necrotic types.
Table 2.
US detectivity for microcalcifications
| Type of microcaplcification with MG | P value | |||
| Necrotic (pleomorphic or linear/branching) | Secretory (amorphous) | |||
| US | + | 25 | 2 | < 0.01 |
| − | 2 | 4 | ||
US examination showed 92.6% sensitivity, 66.7% specificity for detecting necrotic calcifications.
MG, mammography; US, ultrasonography.
Table 3 shows a correlation between the presence of microcalcifications and clinicopathological findings in 79 patients, with the 3 false positives and 6 false negatives excluded. Patients without microcalcifications were significantly more likely to have the Luminal A subtype (P < 0.01) and a lower nuclear grading (P < 0.05). Conversely, patients with microcalcifications were significantly more likely to have lymphatic invasion (P < 0.05), comedonecrosis (P < 0.01), more lymph node metastases (P < 0.01) and higher level of MIB-1 index (34.8% vs. 22.0%; P < 0.01) (Fig. 3). Histologic type and vascular invasion were not associated with the presence of microcalcifications.
Table 3.
Microcalcifications and pathological findings
| Microcalcification | P value | |||
| + | − | |||
| (n=27) | (n=52) | |||
| Subtype | Luminal A | 0 | 17 | < 0.01 |
| Luminal B(HER2−) | 19 | 25 | ||
| Luminal B(HER2+) | 3 | 2 | ||
| HER2 enriched | 2 | 3 | ||
| Triple negative | 3 | 5 | ||
| Histological classification | Scirrhous carcinoma | 8 | 20 | NS |
| Solid-tubular carcinoma | 7 | 8 | ||
| Papillotubular carcinoma | 11 | 14 | ||
| Special types | 1 | 10 | ||
| Lymphatic invasion | + | 16 | 15 | < 0.05 |
| − | 11 | 36 | ||
| Vascular invasion | + | 2 | 1 | NS |
| − | 25 | 50 | ||
| Comedonecrosis | + | 21 | 17 | < 0.01 |
| − | 5 | 33 | ||
| Nuclear atypia | 1 | 1 | 11 | < 0.05 |
| 2 | 20 | 20 | ||
| 3 | 5 | 11 | ||
| Number of mitotic counts | 1 | 3 | 19 | < 0.05 |
| 2 | 13 | 16 | ||
| 3 | 10 | 7 | ||
| Nuclear grading | 1 | 3 | 19 | < 0.05 |
| 2 | 11 | 10 | ||
| 3 | 12 | 13 | ||
| Lymph node metastases | + | 14 | 8 | < 0.01 |
| − | 13 | 44 | ||
HER2, human epidermal growth factor receptor 2; NS, not significant.
Fig. 3.
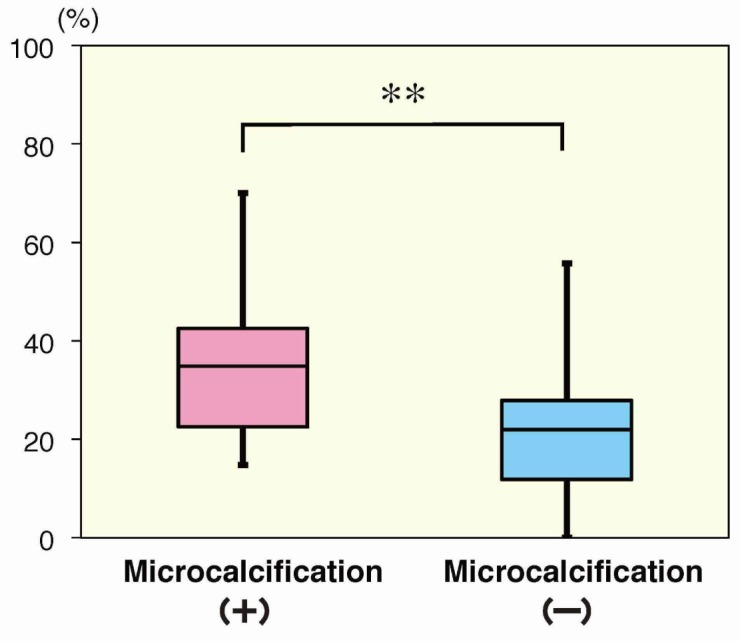
The average value of MIB-1 index was 34.8% for the patients with microcalcifications (n = 27), whereas 22.0% for those without microcalcifications (n = 52). **P < 0.01.
DISCUSSION
In this study, there were some cases without confirmation of microcalcifications on pathological specimens, though microcalcifications were found clearly on MG images. Sensitivity and specificity to detect microcalcifications on MG images were good with 89.7% and 90.7% respectively.6 Therefore, we defined microcalcifications detected by MG images as positive cases of microcalcifications.
Most studies conducted on the performance of US for detecting microcalcifications have focused on DCIS.1–3, 5 Nagashima et al.1 found that the detection rate of microcalcifications with US images was higher for comedo type of DCIS than non comedo type. However, there have been few studies on the significance of microcalcifications in invasive carcinoma. In this study, we compared the presence of microcalcifications on US images to findings of MG images and clinical pathology, to interpret the significance of detection of microcalcifications on US images.
In our study, patients without microcalcifications on US images were significantly more likely to have the Luminal A subtype and a lower nuclear grading. Many previous studies have shown that the patients with Luminal A subtype have a good outcome compared with the other subtypes due to high hormone sensitivity and low expression of proliferative genes.7–10 The nuclear grading of invasive ductal carcinoma has also been shown to be useful as an indicator of degree of malignancy and as a clinical prognostic factor, with higher nuclear grading having a less favorable prognosis.11, 12 Seo et al.13 found that overexpression of human epidermal growth factor receptor 2 (HER2) is associated with microcalcifications, but there is no association in our study. The reason for this may be the different rate of patients with HER2 overexpression. Seo et al.13 reported approximately 30% of all patients with HER2 overexpression, whereas our study included a small number of patients (13%).
In contrast, patients with microcalcifications on US images were significantly more likely to have lymphatic invasion, comedonecrosis, more lymph node metastases and higher level of MIB-1 index. Previous studies have shown that lymphovascular invasion,14 comedonecrosis15 and MIB-1 index16 could be useful prognostic factors of the patients with breast carcinoma. Additionally, axillary lymph node metastasis is a major prognostic factor and play an important role in selecting adjuvant therapy.17–19 As described above, lymphatic invasion, comedonecrosis, lymph node metastases and higher level of MIB-1 index are considered indicators of high grade malignancy and poor prognosis. These findings suggest that breast carcinoma with microcalcifications may be high grade malignant and the patients with them have an unfavorable prognosis.
The results of US images for detecting microcalcifications in this study were inferior to those of MG images, but still relatively good of sensitivity, specificity and diagnostic accuracy. Calcifications of the breast can be conceptually divided into three major types: stromal, secretory and necrotic. The stromal type develops through hyalinization of the stroma and appears on MG images as coarse calcifications. Secretory calcifications develop through crystallization of secretions and are seen in benign mastopathy as well as low grade breast carcinoma. On MG images, they appear as small round or amorphous calcifications. Necrotic calcifications are caused by comedonecrosis. Studies have shown that in the tumor of breast carcinoma, necrosis occurs as a result of poor blood flow caused by rapid growth and calcium deposits form in those areas.20–22 On MG images, necrotic type appear as pleomorphic or linear/branching calcifications. The stromal type of calcifications is diagnosed as benign, the necrotic type as malignant, and the secretory type are seen in a variety lesions of both benign and malignant conditions. Among the calcifications detected by MG images, detected rate of calcifications with US images was higher in necrotic type than secretory type. And US examination showed 92.6% sensitivity for detecting necrotic calcifications. Secretary type calcifications are smaller and paler than necrotic type, therefore, it could be closed in the surrounding mammary gland composition, and it seemed to be false negative on US images. These findings indicate that necrotic calcifications could be detected easier with US images in invasive carcinoma.
In conclusion, this study suggest that the detection ability of microcalcifications on US was inferior to MG, but microcalcifications of tumors detected by US images could easily serve as an useful prediction to evaluate the degree of malignancy, furthermore, it may be possible to decide the selection of adjuvant chemotherapy. In addition, US examination can check it repeatedly without a radiation exposure, and may be useful method to detect the tumor for the patients with dense breast.23 We considered that preoperative US is useful diagnostic tool for the patients with breast disease.
The authors declare no conflict of interest.
REFERENCES
- 1.Nagashima T , Hashimoto H , Oshida K , Nakano S , Tanabe N , Nikaido T , et al. Ultrasound Demonstration of Mammographically Detected Microcalcifications in Patients with Ductal Carcinoma in situ of the Breast. Breast Cancer. 2005; 12: 216-20. . [DOI] [PubMed] [Google Scholar]
- 2.Moon WK , Im JG , Koh YH , Noh DY , Park IA . US of mammographically detected clustered microcalcifications. Radiology. 2000; 217: 849-54. . [DOI] [PubMed] [Google Scholar]
- 3.Soo MS , Baker JA , Rosen EL . Sonographic detection and sonographically guided biopsy of breast microcalcifications. AJR Am J Roentgenol. 2003; 180: 941-8. . [DOI] [PubMed] [Google Scholar]
- 4.Kamiyama N , Okamura Y , Kakee A , Hashimoto H . Investigation of ultrasound image processing to improve perceptibility of microcalcifications. J Med Ultrasonics. 2008; 35: 97-105. [DOI] [PubMed] [Google Scholar]
- 5.Gufler H , Buitrago-TĚllez CH , Madjar H , Allmann KH , Uhl M , Rohr-Reyes A . Ultrasound demonstration of mammographically detected microcalcifications. Acta Radiol. 2000; 41: 217-21. . [DOI] [PubMed] [Google Scholar]
- 6.Okazaki H , Tsujimoto F , Maeda I , Ohta T , Kanemaki Y , Okamoto K , et al. Radiologic-pathological correlation of punctate hyperechoic foci by ultrasound in stereotactic vacuum-assisted breast biopsy samples. Jpn J Radiol. 2009; 27: 438-43. . [DOI] [PubMed] [Google Scholar]
- 7.Loi S . Molecular analysis of hormone receptor positive (luminal) breast cancers: what have we learnt?. Eur J Cancer. 2008; 44: 2813-8. . [DOI] [PubMed] [Google Scholar]
- 8.Buzdar AU , Ibrahim NK , Francis D , Booser DJ , Thomas ES , Theriault RL , et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005; 23: 3676-85. . [DOI] [PubMed] [Google Scholar]
- 9.Goldhirsch A , Wood WC , Coates AS , Gelber RD , Thürlimann B , Senn HJ , et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011; 22: 1736-47. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wirapati P , Sotiriou C , Kunkel S , Farmer P , Pradervand S , Haibe-Kains B , et al. Meta-analysis of gene expression profiles in breast cancer: toward a unified understanding of breast cancer subtyping and prognosis signatures. Breast Cancer Res. 2008; 10: R65. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsuda H , Hirohashi S , Shimosato Y , Hirota T , Tsugane S , Watanabe S , et al. Correlation between histologic grade of malignancy and copy number of c-erbB-2 gene in breast carcinoma. A retrospective analysis of 176 cases. Cancer. 1990; 65: 1794-800. . [DOI] [PubMed] [Google Scholar]
- 12. The Japanese Breast Cancer Society, editor. General Rules for Clinical and Pathological Recording of Breast Cancer. 17th ed Tokyo: Kanehara-shuppan; 2012. p.64-67. Japanese. [Google Scholar]
- 13.Seo BK , Pisano ED , Kuzimak CM , Koomen M , Pavic D , Lee Y , et al. Correlation of HER-2/neu overexpression with mammography and age distribution in primary breast carcinomas. Acad Radiol. 2006; 13: 1211-8. . [DOI] [PubMed] [Google Scholar]
- 14.Kato T , Kameoka S , Kimura T , Nishikawa T , Kobayashi M . Blood vessel invasion as a predictor of long-term survival for Japanese patients with breast cancer. Breast Cancer Res Treat. 2002; 73: 1-12. . [DOI] [PubMed] [Google Scholar]
- 15.Yagata H , Harigaya K , Suzuki M , Nagashima T , Hashimoto H , Ishii G , et al. Comedonecrosis is an unfavorable marker in node-negative invasive breast carcinoma. Pathol Int. 2003; 53: 501-6. . [DOI] [PubMed] [Google Scholar]
- 16.de Azambuja E , Cardoso F , de Castro G , Colozza M , Mano MS , Durbecq V , et al. Ki-67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer. 2007; 96: 1504-13. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silverstein H , Skinner K , Lomis M . Predicting axillary nodal positivity in 2282 patients with breast carcinoma. World J Surg. 2001; 25: 767-72. . [DOI] [PubMed] [Google Scholar]
- 18.Cummings MC , Walsh MD , Hohn BG , Bennett IC , Wright RG , McGuckin MA , et al. Occult axillary lymph node metastases in breast cancer do matter: results of 10-year survival analysis. Am J Surg Pathol. 2002; 26: 1286-95. . [DOI] [PubMed] [Google Scholar]
- 19.Hansen NM , Grube B , Ye X , Turner RR , Brenner RJ , Sim MS , et al. Impact of micrometastases in the sentinel node of patients with invasive breast cancer. J Clin Oncol. 2009; 27: 4679-84. . [DOI] [PubMed] [Google Scholar]
- 20. Japan Radiological Society, Japanese Society of Radiological Technology editors. [Mammography guidelines]. Tokyo: Igaku-shoin; 2011. p.69-74. Japanese. [Google Scholar]
- 21.Tse GM , Tan PH , Cheung HS , Chu WC , Lam WW . Intermediate to highly suspicious calcification in breast lesions: a radio-pathologic correlation. Breast Cancer Res Treat. 2008; 110: 1-7. . [DOI] [PubMed] [Google Scholar]
- 22.Kurebayashi J , Kurosumi M , Sonoo H . A new human breast cancer cell line, KPL-3C, secretes parathyroid hormone-related protein and produces tumours associated with microcalcifications in nude mice. Br J Cancer. 1996; 74: 200-7. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bae MS , Han W , Koo HR , Cho N , Chang JM , Yi A , et al. Characteristics of breast cancers detected by ultrasound screening in women with negative mammograms. Cancer Sci. 2011; 102: 1862-7. . [DOI] [PubMed] [Google Scholar]