Abstract
Bladder cancer is encountered worldwide having been associated with a host of environmental and lifestyle risk factors. The disease has a male to female prevalence of 3 : 1. This disparity has raised the possibility of the androgen receptor (AR) pathway being involved in the genesis of the disease; indeed, research has shown that AR is involved in and is likely a driver of bladder cancer. Similarly, an inflammatory response has been implicated as a major player in bladder carcinogenesis. Consistent with this concept, recent work on anti-inflammatory glucocorticoid signaling points to a pathway that may impact bladder cancer. The glucocorticoid receptor- (GR-) α isoform has an important role in suppressing inflammatory processes, which may be attenuated by AR in the development of bladder cancer. In addition, a GR isoform that is inhibitory to GRα, GRβ, is proinflammatory and has been shown to induce cancer growth. In this paper, we review the evidence of inflammatory mediators and the relationship of AR and GR isoforms as they relate to the propensity for bladder cancer.
1. Introduction
Bladder cancer is the sixth most common cancer in the United States [1]. It was predicted that there would be approximately 75,000 new cases and 16,000 deaths in 2014. There is a recognized predilection for males with an incidence ratio of 3 : 1. Urothelial carcinoma, arising in the mucosa of the bladder, accounts for the majority of cases encountered in the United States. There are three clinicopathologic forms of urothelial carcinomas: papillary, solid/nodular, and carcinoma in situ (CIS). Grading of these cancers is currently defined as either low or high grade depending upon standard histological findings. Patients with low-grade tumors generally have a favorable prognosis; however, the risk of recurrence is high (60–80%) [2]. High-grade tumors are much more aggressive with a proclivity for invasion and metastasis [3]. CIS by definition is high grade and its presence in combination with papillary or solid cancers can alter treatment paradigms and prognosis. Pathological staging is based on depth of bladder wall invasion. High grade and bladder wall muscle invasion are associated with poorer outcomes [4].
Recent investigations have shown that inflammation and proinflammatory cytokine production are correlated with advanced cases of cancer and may be indicators of a poor prognosis [5]. Proinflammatory cytokines, such as tumor necrosis factor alpha (TNFα) and interleukin-6 (IL-6), lead to an inflammatory state that stimulates tumor growth [6]. There is evidence that an isoform of the glucocorticoid receptor (GR), GRβ, which is inhibitory to glucocorticoid action, increased by inflammation and may lead to cancer growth [7, 8]. Further studies have shown that GRβ may also enhance androgen receptor (AR) induced growth in prostate cancer cells [8]. However, the relationship of AR and GRβ has not been established. The importance of anti-inflammatory glucocorticoids in the management of bladder cancer is only now becoming understood. Herein, we will discuss the roles of the androgen and glucocorticoid nuclear receptor signaling pathways as they relate to inflammation and bladder cancer.
2. Factors Leading to Bladder Cancer
Inflammatory pathways and agents that cause inflammation are associated with bladder cancer, which include certain types of infections, environmental/lifestyle factors, and iatrogenic factors.
Inflammation from urinary tract infections caused by Schistosoma haematobium, which is a digenetic trematode found in Africa and the Middle East,is associated with a separate type of bladder cancer, squamous cell bladder cancer (also known as bilharzial bladder cancer) [9]. Heavy egg deposits in the bladder mucosa and submucosa occur during the acute phase of S. haematobium infection in humans [9, 10]. The eggs act as a mechanical irritant to the bladder epithelium, inducing chronic inflammatory lesions thus priming the bladder for inflammation and carcinogenesis [11]. There is a 5.6 : 1 male prevalence for the egg-induced Schistosoma bladder cancers (4.3 : 1 incidence in males for nonegg Schistosoma), which is greater than any other bladder squamous cell carcinoma in patients from Egypt [12]. This may be due to a higher male susceptibility, or more exposure of males to causative agents. Interestingly, the loss of the Y chromosome was observed in 7 of 17 (41%) male cases studied with S. haematobium induced bilharzial bladder cancer [13], indicating a unique male pathway in squamous cell bladder cancer development. However, there have been no investigations in patients with S. haematobium infection on the involvement of the androgen or glucocorticoid receptors.
Both lifestyle factors and environmental agents have been causally related to the development of bladder cancer. Interestingly, there is a male propensity in bladder cancer caused by cigarette smoking, which is estimated to contribute to 50% of cases in men and 35% in women [14]. Regular cystitis is positively associated with bladder cancer risk and may be due to chronic inflammation from carcinogens in the urine of patients that smoke [15]. Environmental or occupational exposure to various chemical carcinogens, such as aromatic amines and polycyclic aromatic hydrocarbons used in the production of aluminum, coal gasification, roofing, and carbon black manufacturing, is one of the agents known to potentially induce bladder cancer [16, 17], which is most likely through induction of inflammation and chronic cystitis in bladder. There is a separation in the amount of occupational chemical exposure in men versus women [18], which may indicate a predisposition of bladder cancer in males. However, the effect of chemical carcinogens on AR signaling activity in bladder is unknown.
Iatrogenic factors that cause bladder cancer include chemotherapeutic agents and radiation. Cyclophosphamide is widely used in a variety of clinical scenarios, which can form metabolites that can contribute to the development of bladder cancer [19]. The inactive metabolite of cyclophosphamide, acrolein, is excreted into urine which induces inflammation of the bladder leading to hemorrhagic cystitis [20]. Bladder epithelial damage occurs because of a reduction of endogenous glutathione and generation of free radicals that initiate lipid peroxidation and other cell damage. There have been no differences found in the treatment of cyclophosphamide and bladder cancer between males and females [21, 22]. In addition, treatment of rats with cyclophosphamide in males showed no significant difference with respect to male reproductive organ weights, serum testosterone, luteinizing hormone or follicle-stimulating hormone, epididymal sperm counts, or fertility [22]. However, cyclophosphamide has been shown to penetrate the male reproductive tract and can be transmitted sexually to a female partner, which may affect progeny outcome [21]. Other iatrogenic factors, such as chronic low-dose radiation, may also lead to bladder cancer through oxidative stress and a reduction in DNA repair by an increase of nitric oxide and reactive oxygen species [23–25]. Therapeutic pelvic radiation used for abnormal uterine bleeding and ovarian, cervical, and prostate cancer is associated with an increase in bladder cancer risk [26]. Altogether, iatrogenic factors insult the bladder, causing inflammation, resulting in DNA damage and mucosal aberrations leading to bladder cancer. However, there is no correlation for sexual prevalence that has been observed.
3. Current Therapies
Treatment of localized bladder cancer can vary from simple fulguration to multimodal therapy including radical extirpative therapy. A number of treatment paradigms exist depending on the clinical situation. Low-grade papillary tumors are handled frequently with simple electrodessication. Inflammation has been shown to play a role in bladder cancer and therapies that are immunomodulators have proven useful in treatment. Adjuvant intravesical therapy with either chemotherapeutic agents such as Mitomycin C or a biologic such as Bacillus Calmette Guerin (BCG) may be employed to prevent recurrences [27]. BCG has proved useful in the management of CIS and superficial high-grade papillary (noninvasive) cancers. BCG is commonly used to prevent bladder cancer recurrence after transurethral resection of the bladder tumor [2, 28, 29]. For high-grade lesions, treatment is based on the depth of invasion. Muscle invasion usually leads to a much more radical treatment including neoadjuvant chemotherapy combined with surgical removal of the bladder or radiation therapy.
4. Inflammatory Pathways and Bladder Cancer
BCG is a weakened vaccine strain of bovine tuberculosis from Mycobacterium bovis that functions as an immunotherapy to redirect the immune system to clear bladder cancer cells (reviewed in [30]). It has been shown that internalization of BCG by urothelial cells enhanced the expression of the major histocompatibility complex (MHC) class II and cluster of differentiation 1 (CD1) proteins [31], thus, indicating that endothelial cells can present more antigens of BCG infection and likely tumor presence. However, up to 40% of patients fail to respond to immunotherapy [32]. In males, BCG treatment has been associated with relatively rare complications of penile edema and meatal ulceration [33], as well as epididymoorchitis [34, 35]. BCG treatment has been shown to be detrimental to healthy sperm development in young men following therapy [36]. In addition, intratesticular injection of BCG in dogs caused a severe granulomatous reaction with widespread degeneration of the tubules, resulting in azoospermia [37]. However, the response to BCG treatment in men and women has shown similar results.
To prevent tumors, macrophages must migrate in the area surrounding the tumor [38]. The exact role that macrophages play depends on which subtype they belong to, as there are pro- and anti-inflammatory types [38] (reviewed in [39, 40]). Insults that induce bladder inflammation without host-derived secreted protein acidic and rich in cysteine (SPARC) cause activation of proinflammatory macrophages and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) [41, 42], which enhances growth of bladder cells. However, proinflammatory macrophages can also regulate cancer growth. Type 1 (proinflammatory) macrophages cocultured with human bladder cancer cells arrested cancer cell growth and increased TNFα expression and phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway activity when compared to cancer cells grown alone and cancer cells cocultured with type 2 (anti-inflammatory) macrophages [43].
AR can be proinflammatory by inducing expression of genes such as TNFα [44], which can enhance immune cell invasion and may contribute to chronic inflammation (Figure 1). Several proteins, including the AR which is found in both bladder stromal cells and the urothelium, are known to contribute to bladder cancer growth [45, 46]. The effect of GRβ on AR guided proinflammatory pathways in bladder cancer remains unknown. However, Ligr et al. recently showed that GRβ can increase AR regulated growth in prostate cancer cells [8]. Future studies on the relationship of GRβ and AR would strengthen our understanding if they work in conjunction to inhibit the anti-inflammatory actions of glucocorticoids.
Figure 1.
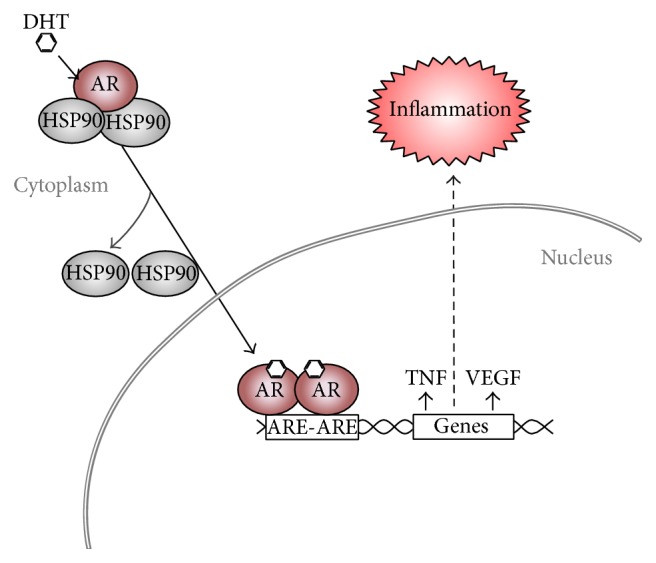
Activation of the androgen receptor leads to inflammation. Activation by androgens causes translocation of AR from the cytoplasm to the nucleus and release of HSP90 chaperone proteins. The AR then binds to androgen-response elements (ARE) in the promoter region of genes resulting in an increase or suppression. TNFα and VEGF are two genes that contain AREs in their promoter and are activated by AR to increase inflammatory signals.
The use of nonsteroidal anti-inflammatory drugs is inversely associated with bladder cancer due to their inhibition of the cyclooxygenase-2 (COX-2) inflammatory pathway [47]. Overexpression of COX-2 is associated with proliferation, angiogenesis, and dysregulation of apoptosis in bladder cancer cells and is upregulated in bladder epithelial cancer [48–50]. Interferon-α (IFN-α) decreased expression of COX-1 and increased COX-2 in bladder cancer cells, suggesting that IFN-α plays a role in COX-2 upregulation in urothelial cancer cells [50]. Glucocorticoids are inhibitors of COX-2 expression (Figure 2) [51], suggesting that they may be useful for inhibition of bladder cancer. Glucocorticoids also have a beneficial anti-inflammatory response by increasing IκBα, an inhibitor of proinflammatory NF-κB [52].
Figure 2.
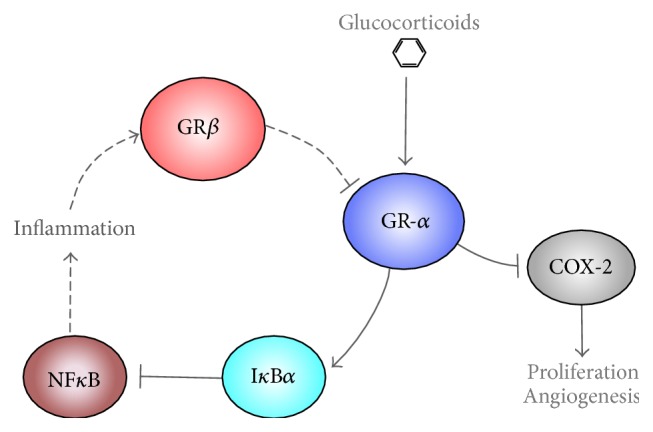
Glucocorticoids suppress inflammation. Glucocorticoids bind and activate GRα, which enhances IκBα and suppresses COX-2 to inhibit inflammation. IκBα binds to inhibit NFκB, the major mediator of inflammation. NFκB increases GRβ to cause glucocorticoid resistance and proliferation.
5. Glucocorticoids and Bladder Cancer
Glucocorticoids are commonly used drugs for treatment of inflammatory and autoimmune disorders. GR is expressed as two alternate major isoforms, GRα and GRβ [53–56]. Glucocorticoids control anti-inflammatory cellular processes by binding to and activating GRα. Antiproliferative properties of glucocorticoids are mediated through GRα, which is a hormone-activated transcription factor [57, 58] that increases cell cycle arrest proteins p27 and p21 [59, 60] as well as the apoptotic-gene phosphatase and tensin homolog deleted on chromosome 10 (PTEN) [61]. In contrast to GRα, GRβ lacks part of the ligand-binding domain, helix 12, of the GR protein and cannot bind glucocorticoids [55]. Although the function of GRβ is not well understood, it has been shown that GRβ acts as an inhibitor to GRα [55, 56, 62–64]. GRβ is induced by inflammatory pathways such as TNFα and NF-κB [7], suggesting that it may have a paramount role in inflammation that is associated with bladder cancer (Figure 2). The inhibitory role of GRβ on glucocorticoid action in the immune system has related it to a variety of immunological diseases, such as ulcerative colitis, asthma, and chronic sinusitis [54, 65–68]. Now, it is also being observed that GRβ may regulate proliferation as well as cancer growth in glioblastoma [65] and leukemia [67]. Potentially, this may occur through the ability of GRβ to augment a chronic inflammatory state by inhibition of GRα and glucocorticoid action.
It has been shown that GR plays a role in bladder cancer. However, the precise mechanism and isoform of the receptor is responsible is unknown. GR expression tends to be weaker in bladder cancer tumors than in normal cells, and strong GR expression tends to be correlated with a better prognosis [69, 70]. Glucocorticoids have been widely used as comedication in patients with advanced bladder cancer [71]. However, recent studies have raised the possibility of an increased risk of bladder cancer from systemic use of glucocorticoids. Recent investigations have demonstrated that glucocorticoids (e.g., corticosterone, dexamethasone, and prednisone) suppress bladder cancer cell invasion, while dexamethasone may induce proliferation via inhibiting apoptosis [72, 73]. However, these effects may be mediated by GRβ, which has been shown to exert a stimulatory effect on proliferation [55], possibly by increasing inflammation in bladder by inhibition of GRα. Glucocorticoids are known to interfere with the transcriptional activity of several immune related transcription factors, including NF-κB [69]. It has been shown that GRα can directly function as a corepressor of NF-κB. Additionally, the synthetic glucocorticoid dexamethasone inactivates NF-κB and downregulates NF-κB-dependent cytokine IL-6, which may be a central mechanism involved in GR-mediated inhibition of bladder cancer cell invasion [69]. We have shown that dexamethasone can increase the expression of GRβ in mouse embryonic fibroblast cells [55]. Interestingly, constant exposure of glucocorticoids in patients leads to elevated GRβ and glucocorticoid resistance, which is due to decreased affinity for GCs and increased total GR proteins [62, 74], suggesting a chronic glucocorticoid resistant state. Furthermore, GRβ can regulate growth through suppression of PTEN, enhancing PI3Kinase/AKT induced proliferation [56]. Suppression of GRβ by siRNA inhibited growth of AR positive prostate cancer cells [8]. This suggests that GRβ may positively affect AR signaling activity and that chronic glucocorticoid treatment in males could result in activation of the GRβ/AR axis leading to bladder cancer (Figure 3).
Figure 3.
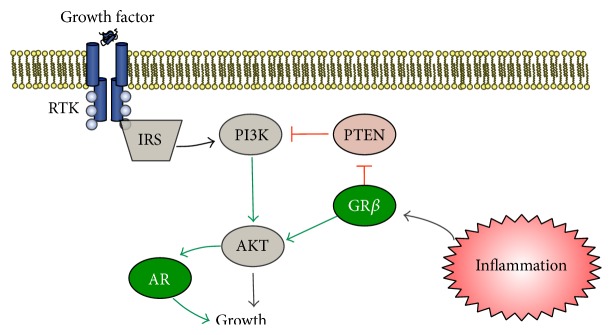
Pathway in males that leads to growth factor activation of PI3K/AKT of AR and GRβ induced growth. Growth factors (e.g., insulin, epidermal growth factor) bind to receptor tyrosine kinases (RTK) increasing phosphorylation and activation of insulin receptor substrate (IRS), resulting in the induction of the PI3Kinase growth pathway. PTEN is a tumor suppressor gene that inhibits PI3K. The PI3K increases activity of AKT resulting in enhanced AR signaling and growth. Proinflammatory mediators increase expression of GRβ, which has been shown to inhibit the tumor suppressor gene, PTEN [122], which leads to enhanced AR induced growth [56].
Several glucocorticoids have been used clinically as cytotoxic agents, predominantly for hematologic malignancies [75]. Evidence suggests a glucocorticoid-induced resistance to cytotoxic effects of the antineoplastic drug cis-diamminedichloroplatinum (CDDP), the most effective agent currently used against urothelial carcinoma [76]. A glucocorticoid is often used as comedication in the standard chemotherapy regimens for bladder cancer, due to its protective factor against toxic chemotherapy drugs. However, prolonged systemic use of glucocorticoids has been shown to increase the subsequent risk of bladder cancer, possibly due to immunosuppression [71] or long-term induction of GRβ causing glucocorticoid resistance leading to inflammation. However, the exact mechanism remains unknown.
6. Androgens and Bladder Cancer
In males, the AR has been shown to play a key role in prostate cancer genesis and progression [77]. However, the role of AR in bladder cancer and the proclivity for males has only recently drawn attention [78, 79]. AR is a ligand-inducible transcription factor that regulates the expression of several genes (Figure 1) [80–82]. AR ligands, the principal being the predominately male hormone, testosterone, enter the target cell and bind to AR directly or after conversion to 5α-dihydrotestosterone (DHT). The ligand-AR complex induces a conformational change in AR, resulting in release of the heat shock proteins (HSPs) and translocation of the complex from the cytoplasm to the nucleus [83]. After translocation, activated AR binds to DNA at androgen-response elements in promoters and recruits additional proteins, leading to specific transcriptional activation or repression of target genes [84]. Several human bladder cancer cell lines have been found to express AR [85–89]. Additionally, AR expression has been detected in human bladder cancer obtained after surgical removal [90–94]. The role of AR in normal bladder is unclear [95].
The role of AR in prostate has been more defined. Investigations have shown that ligand-independent activation of the AR pathway occurs in prostate cancer, which can be enhanced by epidermal growth factor (EGF) through the signal transduction pathway [81]. Connecting AR in prostate and bladder cancers, dysregulation of the epidermal growth factor receptor (EGFR) is associated with bladder cancer [96], suggesting that these cancer pathways may be interconnected. It has been shown that AR can increase expression and activity of EGFR and a protein encoded by the ERBB2 (also known as Her2) gene [96], implying androgen-mediated bladder cancer tumorigenesis and clinical progression via the regulation of the EGFR/ERBB2 pathways. The separation of these pathways in males and females is unknown. However, it may be overly activated in males because of the increased level of androgens.
Earlier investigators have shown that a variety of AR gene alterations are important in the development of bladder cancer, such as allelic loss and gene mutation. This could explain some of the differences between male and female tumors. Allelic loss of the AR locus has been found in cases of muscle-invasive bladder tumors, but not in the adjacent nonneoplastic tissue [97]. Additionally, mRNAs from two human bladder cancer cell lines have revealed AR sequences with short CAG repeat lengths, suggesting that altered mRNA sequences of the AR gene could contribute to bladder cancer [88]. Demonstrating the susceptibility of males, bladder cancer tumors implanted in rats that were treated with androgenic hormones grew more rapidly than rats treated with estrogenic hormones [98]. This was also supported by two studies in mice using AR knockout animals, which indicated a critical role of androgen signaling in bladder carcinogenesis [86, 99]. It is therefore suggested that androgenic hormones stimulate bladder tumor growth, whereas estrogenic hormones may do the opposite (or at least do not stimulate). However, there is evidence of AR induced bladder cancer in females. A study of transitional cell carcinoma showed that AR is expressed in women patients and found that 30% of the bladder cancer tumors are AR positive and that nontumor tissue may also express AR [100]. In addition, the same relationship between AR level and pathological stage was found in men and women.
The estrogen receptor (ER) β has been shown to be highly expressed in bladder cancer, with elevated ERβ expression being correlated with increased bladder cancer stage [101]. In addition, it has been shown that ERβ selective antiestrogen drug, raloxifene, causes bladder cancer cells to undergo apoptosis [102]. This shows that the use of antiestrogen therapy may be useful in treating bladder cancer; however, ERβ has not been shown to be a driver of bladder cancer, while AR's driving capability has been demonstrated. ERα has been shown to interact with GR in breast, where this isoform is dominant [103]. However, no work has been done showing ERβ and GR interaction.
Targeting of AR may potentially be a good therapy for bladder cancer in males. An effective prostate cancer treatment is chemical castration using luteinizing hormone-releasing hormone analogues to ablate testicular androgens or use of antiandrogens (e.g., flutamide), which block androgen signaling at the level of the AR. Typically, antiandrogens are used in early stage prostate cancer. While this therapy is successful at first, the hormonal therapy often fails and patients relapse with “castrate-resistant” prostate cancer. This resistance comes from the selection of cells that bypass androgen requirement by mechanisms including AR gene mutation or receptor amplification [104–108]. Additionally, it has been found that dihydrotestosterone upregulates ERBB2 in androgen receptor positive bladder cancer cells [109]. The communication between the AR and EGFR pathways may play a role in the male prevalence in bladder cancer. While it is known that AR positively correlates with an increased risk of developing prostate cancer, it is unknown whether antiandrogens can have an effect on bladder cancer.
7. Sexual Dimorphism in Cancer Aggressiveness
Males have been shown to develop more high-grade bladder cancer tumors in comparison to females (55.7% males versus 42.0% females) as well as a greater percentage of invasive tumors (26.5% males versus 22.0% females) [96]. Recent research has shown that androgens and AR can induce epithelial-mesenchymal transition (EMT) which is often seen as an indicator for metastasis [110, 111]. The aggressiveness of tumors is derived from oxygen and other nutrients that cancer cells use to induce local neovascularization. Vascular endothelial growth factor (VEGF) is a potent endothelial cell mitogen that stimulates proliferation, migration, and tube formation leading to angiogenic growth of new blood vessels and is essential during development [112]. Neuropilin-1 (NRP-1) and homologue NRP-2 are coreceptors that enhance responses to several growth factors, such as VEGF, and mediators under physiological and pathological conditions [113–116]. NRPs and VEGF receptors are constituently expressed on normal bladder epithelial cells and have been shown to be upregulated in an animal model of chronically inflamed cells, indicating neovascularization [117]. NRPs can regulate the cancer-induced vascular and inflammatory responses. Glucocorticoids have been shown to suppress NRP [118] and VEGF expression [73, 119]. Androgens, on the other hand, increase VEGF expression [120]. In males, GRβ may enhance neovascularization potential through inhibition of GRα, as well as activation of AR and inflammatory pathways that increase VEGF and NRP. We have shown that GRβ does enhance activity of the PI3-kinase and Akt cascade by suppression of PTEN (Figure 3) [121], a known inhibitor of growth and tumor suppressor gene [56]. Ultimately, this may lead to the inflammatory processes that can lead to the progression of bladder cancer in males.
8. Conclusions
Insight into the cellular biology of the bladder cancer disease process offers the opportunity to develop innovative and more targeted therapies. The androgen and the glucocorticoid receptors are both members of the steroid receptor superfamily and appear to offer promise as therapeutic targets for enhanced treatment paradigms. Much research remains to be performed in order to define the roles of glucocorticoids, antiandrogens, and the GR isoforms in the management of bladder cancer. A continuing understanding of the roles of AR and other molecules, such as GRβ, that may directly or indirectly regulate androgens may help reveal better strategies for the management of bladder cancer in males. Additionally, androgen ablation therapy may prove to be useful for treatment in males with bladder cancer. A further understanding of the molecular signaling pathways that cause the predilection for males will aid in the advancement of bladder cancer therapy.
Acknowledgments
Research reported in this paper was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award no. K01HL125445 (Terry D. Hinds Jr.), L32MD009154 (Terry D. Hinds Jr.), and the NIH PRIDE grant [HL106365] (Terry D. Hinds Jr.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported in part by the Stranahan Endowment for Oncologic Research at the University of Toledo Department of Urology (UTCOM) awarded to Terry D. Hinds Jr.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2015. CA: A Cancer Journal for Clinicians. 2015;65(1):5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Herr H. W., Schwalb D. M., Zhang Z.-F., et al. Intravesical bacillus Calmette-Guerin therapy prevents tumor progression and death from superficial bladder cancer: ten-year follow-up of a prospective randomized trial. Journal of Clinical Oncology. 1995;13(6):1404–1408. doi: 10.1200/JCO.1995.13.6.1404. [DOI] [PubMed] [Google Scholar]
- 3.Sievert K. D., Amend B., Nagele U., et al. Economic aspects of bladder cancer: what are the benefits and costs? World Journal of Urology. 2009;27(3):295–300. doi: 10.1007/s00345-009-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chamie K., Litwin M. S., Bassett J. C., et al. Recurrence of high-risk bladder cancer: a population-based analysis. Cancer. 2013;119(17):3219–3227. doi: 10.1002/cncr.28147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trikha M., Corringham R., Klein B., Rossi J.-F. Targeted anti-interleukin-6 monoclonal antibody therapy for cancer: a review of the rationale and clinical evidence. Clinical Cancer Research. 2003;9(13):4653–4665. [PMC free article] [PubMed] [Google Scholar]
- 6.Okamoto M., Kawai K., Reznikoff C. A., Oyasu R. Transformation in vitro of a nontumorigenic rat urothelial cell line by hydrogen peroxide. Cancer Research. 1996;56(20):4649–4653. [PubMed] [Google Scholar]
- 7.Webster J. C., Oakley R. H., Jewell C. M., Cidlowski J. A. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative β isoform: a mechanism for the generation of glucocorticoid resistance. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ligr M., Li Y., Logan S. K., et al. Mifepristone inhibits GRbeta coupled prostate cancer cell proliferation. Journal of Urology. 2012;188(3):981–988. doi: 10.1016/j.juro.2012.04.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheever A. W. Schistosomiasis and neoplasia. Journal of the National Cancer Institute. 1978;61(1):13–18. doi: 10.1093/jnci/61.1.13. [DOI] [PubMed] [Google Scholar]
- 10.Christie J. D., Crouse D., Kelada A. S., Anis-Ishak E., Smith J. H., Kamel I. A. Patterns of Schistosoma haematobium egg distribution in the human lower urinary tract. III. Cancerous lower urinary tracts. The American Journal of Tropical Medicine and Hygiene. 1986;35(4):759–764. doi: 10.4269/ajtmh.1986.35.759. [DOI] [PubMed] [Google Scholar]
- 11.Cheever A. W., Kuntz R. E., Moore J. A., Huang T.-C. Pathology of Schistosoma haematobium infection in the capuchin monkey (Cebus apella) Transactions of the Royal Society of Tropical Medicine and Hygiene. 1988;82(1):107–111. doi: 10.1016/0035-9203(88)90279-9. [DOI] [PubMed] [Google Scholar]
- 12.El-Bolkainy M. N., Mokhtar N. M., Ghoneim M. A., Hussein M. H. The impact of schistosomiasis on the pathology of bladder carcinoma. Cancer. 1981;48(12):2643–2648. doi: 10.1002/1097-0142(19811215)48:12<2643::AID-CNCR2820481216>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 13.Khaled H. M., Aly M. S., Magrath I. T. Loss of Y chromosome in Bilharzial bladder cancer. Cancer Genetics and Cytogenetics. 2000;117(1):32–36. doi: 10.1016/S0165-4608(99)00126-0. [DOI] [PubMed] [Google Scholar]
- 14.Zeegers M. P. A., Tan F. E. S., Dorant E., van den Brandt P. A. The impact of characteristics of cigarette smoking on urinary tract cancer risk: a meta-analysis of epidemiologic studies. Cancer. 2000;89(3):630–639. doi: 10.1002/1097-0142(20000801)89:360;630::aid-cncr1962;3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen S. H., Hanum N., Grotenhuis A. J., et al. Recurrent urinary tract infection and risk of bladder cancer in the Nijmegen bladder cancer study. British Journal of Cancer. 2015;112(3):594–600. doi: 10.1038/bjc.2014.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.IARC Working Group. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 94. Paris, France: International Agency for Research on Cancer (IARC); 2010. (Ingested Nitrate and Nitrite and Cyanobacterial Peptide Toxins). [PMC free article] [PubMed] [Google Scholar]
- 17.Boffetta P., Jourenkova N., Gustavsson P. Cancer risk from occupational and environmental exposure to polycyclic aromatic hydrocarbons. Cancer Causes and Control. 1997;8(3):444–472. doi: 10.1023/A:1018465507029. [DOI] [PubMed] [Google Scholar]
- 18.Arbuckle T. E. Are there sex and gender differences in acute exposure to chemicals in the same setting? Environmental Research. 2006;101(2):195–204. doi: 10.1016/j.envres.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Batista C. K., Brito G., Souza M., Leitão B., Cunha F., Ribeiro R. A model of hemorrhagic cystitis induced with acrolein in mice. Brazilian Journal of Medical and Biological Research. 2006;39(11):1475–1481. doi: 10.1590/s0100-879x2006005000024. [DOI] [PubMed] [Google Scholar]
- 20.Kiuchi H., Takao T., Yamamoto K., et al. Sesquiterpene lactone parthenolide ameliorates bladder inflammation and bladder overactivity in cyclophosphamide induced rat cystitis model by inhibiting nuclear factor-kappaB phosphorylation. Journal of Urology. 2009;181(5):2339–2348. doi: 10.1016/j.juro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Hales B. F., Smith S., Robaire B. Cyclophosphamide in the seminal fluid of treated males: transmission to females by mating and effect on pregnancy outcome. Toxicology and Applied Pharmacology. 1986;84(3):423–430. doi: 10.1016/0041-008x(86)90247-4. [DOI] [PubMed] [Google Scholar]
- 22.Trasler J. M., Hales B. F., Robaire B. Chronic low dose cyclophosphamide treatment of adult male rats: effect on fertility, pregnancy outcome and progeny. Biology of Reproduction. 1986;34(2):275–283. doi: 10.1095/biolreprod34.2.275. [DOI] [PubMed] [Google Scholar]
- 23.Tubiana M. The report of the French Academy of Science: ‘problems associated with the effects of low doses of ionising radiation’. Journal of Radiological Protection. 1998;18(4):243–248. doi: 10.1088/0952-4746/18/4/002. [DOI] [PubMed] [Google Scholar]
- 24.Trosko J. E. Role of low-level ionizing radiation in multi-step carcinogenic process. Health Physics. 1996;70(6):812–822. doi: 10.1097/00004032-199606000-00005. [DOI] [PubMed] [Google Scholar]
- 25.Cardis E., Gilbert E. S., Carpenter L., et al. Effects of low doses and low dose rates of external ionizing radiation: cancer mortality among nuclear industry workers in three countries. Radiation Research. 1995;142(2):117–132. [PubMed] [Google Scholar]
- 26.Boice J. D., Jr., Engholm G., Kleinerman R. A., et al. Radiation dose and second cancer risk in patients treated for cancer of the cervix. Radiation Research. 1988;116(1):3–55. doi: 10.2307/3577477. [DOI] [PubMed] [Google Scholar]
- 27.Morales A., Eidinger D., Bruce A. W. Intracavitary Bacillus Calmette Guerin in the treatment of superficial bladder tumors. Journal of Urology. 1976;116(2):180–183. doi: 10.1016/s0022-5347(17)58737-6. [DOI] [PubMed] [Google Scholar]
- 28.De Jager R., Guinan E., Lamm D., et al. Long-term complete remission in bladder carcinoma in situ with intravesical tice bacillus Calmette guerin. Overview analysis of six phase II clinical trials. Urology. 1991;38(6):507–513. doi: 10.1016/0090-4295(91)80166-5. [DOI] [PubMed] [Google Scholar]
- 29.Cookson M. S., Sarosdy M. F. Management of stage T1 superficial bladder cancer with intravesical bacillus Calmette-Guerin therapy. The Journal of Urology. 1992;148(3):797–801. doi: 10.1016/s0022-5347(17)36724-1. [DOI] [PubMed] [Google Scholar]
- 30.Kapoor R., Vijjan V., Singh P. Bacillus Calmette-Guerin in the management of superficial bladder cancer. Indian Journal of Urology. 2008;24(1):72–76. doi: 10.4103/0970-1591.38608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ikeda N., Toida I., Iwasaki A., Kawai K., Akaza H. Surface antigen expression on bladder tumor cells induced by bacillus Calmette-Guérin (BCG): a role of BCG internalization into tumor cells. International Journal of Urology. 2002;9(1):29–35. doi: 10.1046/j.1442-2042.2002.00415.x. [DOI] [PubMed] [Google Scholar]
- 32.Witjes J. A. Management of BCG failures in superficial bladder cancer: a review. European Urology. 2006;49(5):790–797. doi: 10.1016/j.eururo.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 33.Baniel J., Lev Z., Engelstein D., Servadio C. Penile edema and meatal ulceration after intravesical instillation with Bacillus Calmette-Guérin. Urology. 1996;47(6):932–934. doi: 10.1016/s0090-4295(96)00054-4. [DOI] [PubMed] [Google Scholar]
- 34.Demers V., Pelsser V. ‘BCGitis’: a rare case of tuberculous epididymo-orchitis following intravesical bacillus Calmette-Guerin therapy. Journal of Radiology Case Reports. 2012;6(11):16–21. doi: 10.3941/jrcr.v6i11.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muttarak M., Lojanapiwat B., Chaiwun B., Wudhikarn S. Preoperative diagnosis of bilateral tuberculous epididymo-orchitis following intravesical Bacillus Calmette-Guerin therapy for superficial bladder carcinoma. Australasian Radiology. 2002;46(2):183–185. doi: 10.1046/j.1440-1673.2001.01032.x. [DOI] [PubMed] [Google Scholar]
- 36.Garg M., Sankhwar S. N., Goel A., et al. Effect of intravesical immunotherapy on sperm parameters in young patients with non—muscle-invasive bladder carcinoma: prospective analysis. Clinical Genitourinary Cancer. 2014;12(3):e83–e86. doi: 10.1016/j.clgc.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 37.Naz R. K., Talwar G. P. Immunological sterilization of male dogs by BCG. International Journal of Andrology. 1981;4(1):111–128. doi: 10.1111/j.1365-2605.1981.tb00697.x. [DOI] [PubMed] [Google Scholar]
- 38.Edin S., Wikberg M. L., Dahlin A. M., et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS ONE. 2012;7(10) doi: 10.1371/journal.pone.0047045.e47045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gakis G. The role of inflammation in bladder cancer. Advances in Experimental Medicine and Biology. 2014;816:183–196. doi: 10.1007/978-3-0348-0837-8-8. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Z., Shen Z., Xu C. Inflammatory pathways as promising targets to increase chemotherapy response in bladder cancer. Mediators of Inflammation. 2012;2012:11. doi: 10.1155/2012/528690.528690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Said N., Frierson H. F., Sanchez-Carbayo M., Brekken R. A., Theodorescu D. Loss of SPARC in bladder cancer enhances carcinogenesis and progression. The Journal of Clinical Investigation. 2013;123(2):751–766. doi: 10.1172/jci64782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajili F., Kourda N., Darouiche A., Chebil M., Boubaker S. Prognostic value of tumor-associated macrophages count in human non-muscle-invasive bladder cancer treated by bcg immunotherapy. Ultrastructural Pathology. 2013;37(1):56–61. doi: 10.3109/01913123.2012.728688. [DOI] [PubMed] [Google Scholar]
- 43.Dufresne M., Dumas G., Asselin É., Carrier C., Pouliot M., Reyes-Moreno C. Pro-inflammatory type-1 and anti-inflammatory type-2 macrophages differentially modulate cell survival and invasion of human bladder carcinoma T24 cells. Molecular Immunology. 2011;48(12-13):1556–1567. doi: 10.1016/j.molimm.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 44.Chen H., Yong W., Hinds T. D., Jr., et al. Fkbp52 regulates androgen receptor transactivation activity and male urethra morphogenesis. The Journal of Biological Chemistry. 2010;285(36):27776–27784. doi: 10.1074/jbc.m110.156091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Enkelmann A., Heinzelmann J., Von Eggeling F., et al. Specific protein and miRNA patterns characterise tumour-associated fibroblasts in bladder cancer. Journal of Cancer Research and Clinical Oncology. 2011;137(5):751–759. doi: 10.1007/s00432-010-0932-6. [DOI] [PubMed] [Google Scholar]
- 46.Niu H. T., Yang C. M., Jiang G., et al. Cancer stroma proteome expression profile of superficial bladder transitional cell carcinoma and biomarker discovery. Journal of Cancer Research and Clinical Oncology. 2011;137(8):1273–1282. doi: 10.1007/s00432-011-0995-z. [DOI] [PubMed] [Google Scholar]
- 47.Castelao J. E., Yuan J.-M., Gago-Dominguez M., Yu M. C., Ross R. K. Non-steroidal anti-inflammatory drugs and bladder cancer prevention. British Journal of Cancer. 2000;82(7):1364–1369. doi: 10.1054/bjoc.1999.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Klein R. D., van Pelt C. S., Sabichi A. L., et al. Transitional cell hyperplasia and carcinomas in urinary bladders of transgenic mice with keratin 5 promoter-driven cyclooxygenase-2 overexpression. Cancer Research. 2005;65(5):1808–1813. doi: 10.1158/0008-5472.can-04-3567. [DOI] [PubMed] [Google Scholar]
- 49.Shariat S. F., Kim J.-H., Ayala G. E., Kho K., Wheeler T. M., Lerner S. P. Cyclooxygenase-2 is highly expressed in carcinoma in situ and T1 transitional cell carcinoma of the bladder. Journal of Urology. 2003;169(3):938–942. doi: 10.1097/01.ju.0000043638.89552.ed. [DOI] [PubMed] [Google Scholar]
- 50.Boström P. J., Aaltonen V., Söderström K.-O., Uotila P., Laato M. Expression of cyclooxygenase-1 and -2 in urinary bladder carcinomas in vivo and in vitro and prostaglandin E2 synthesis in cultured bladder cancer cells. Pathology. 2001;33(4):469–474. doi: 10.1080/00313020120083188. [DOI] [PubMed] [Google Scholar]
- 51.Lim W., Park C., Shim M. K., Lee Y. H., Lee Y. M., Lee Y. Glucocorticoids suppress hypoxia-induced COX-2 and hypoxia inducible factor-1α expression through the induction of glucocorticoid-induced leucine zipper. British Journal of Pharmacology. 2014;171(3):735–745. doi: 10.1111/bph.12491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deroo B. J., Archer T. K. Glucocorticoid receptor activation of the I kappa B alpha promoter within chromatin. Molecular Biology of the Cell. 2001;12(11):3365–3374. doi: 10.1091/mbc.12.11.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chrousos G. P., Kino T. Intracellular glucocorticoid signaling: a formerly simple system turns stochastic. Science's STKE. 2005;2005(304):p. pe48. doi: 10.1126/stke.3042005pe48. [DOI] [PubMed] [Google Scholar]
- 54.Lewis-Tuffin L. J., Cidlowski J. A. The physiology of human glucocorticoid receptor beta (hGRbeta) and glucocorticoid resistance. Annals of the New York Academy of Sciences. 2006;1069:1–9. doi: 10.1196/annals.1351.001. [DOI] [PubMed] [Google Scholar]
- 55.Hinds T. D., Jr., Ramakrishnan S., Cash H. A., et al. Discovery of glucocorticoid receptor-beta in mice with a role in metabolism. Molecular Endocrinology. 2010;24(9):1715–1727. doi: 10.1210/me.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stechschulte L. A., Wuescher L., Marino J. S., Hill J. W., Eng C., Hinds T. D., Jr. Glucocorticoid receptor beta stimulates Akt1 growth pathway by attenuation of pten. The Journal of Biological Chemistry. 2014;289(25):17885–17894. doi: 10.1074/jbc.m113.544072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tata J. R. Signalling through nuclear receptors. Nature Reviews Molecular Cell Biology. 2002;3(9):702–710. doi: 10.1038/nrm914. [DOI] [PubMed] [Google Scholar]
- 58.Kumar R., Johnson B. H., Thompson E. B. Overview of the structural basis for transcription regulation by nuclear hormone receptors. Essays in Biochemistry. 2004;40:27–39. doi: 10.1042/bse0400027. [DOI] [PubMed] [Google Scholar]
- 59.Rogatsky I., Trowbridge J. M., Garabedian M. J. Glucocorticoid receptor-mediated cell cycle arrest is achieved through distinct cell-specific transcriptional regulatory mechanisms. Molecular and Cellular Biology. 1997;17(6):3181–3193. doi: 10.1128/mcb.17.6.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yemelyanov A., Czwornog J., Chebotaev D., et al. Tumor suppressor activity of glucocorticoid receptor in the prostate. Oncogene. 2007;26(13):1885–1896. doi: 10.1038/sj.onc.1209991. [DOI] [PubMed] [Google Scholar]
- 61.Ni Z., Tang J., Cai Z., et al. A new pathway of glucocorticoid action for asthma treatment through the regulation of PTEN expression. Respiratory Research. 2011;12, article 47 doi: 10.1186/1465-9921-12-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bamberger C. M., Bamberger A.-M., De Castro M., Chrousos G. P. Glucocorticoid receptor beta, a potential endogenous inhibitor of glucocorticoid action in humans. The Journal of Clinical Investigation. 1995;95(6):2435–2441. doi: 10.1172/jci117943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leung D. Y. M., Hamid Q., Vottero A., et al. Association of glucocorticoid insensitivity with increased expression of glucocorticoid receptor β . Journal of Experimental Medicine. 1997;186(9):1567–1574. doi: 10.1084/jem.186.9.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oakley R. H., Jewell C. M., Yudt M. R., Bofetiado D. M., Cidlowski J. A. The dominant negative activity of the human glucocorticoid receptor β isoform. Specificity and mechanisms of action. The Journal of Biological Chemistry. 1999;274(39):27857–27866. doi: 10.1074/jbc.274.39.27857. [DOI] [PubMed] [Google Scholar]
- 65.Yin Y., Zhang X., Li Z., et al. Glucocorticoid receptor β regulates injury-mediated astrocyte activation and contributes to glioma pathogenesis via modulation of β-catenin/TCF transcriptional activity. Neurobiology of Disease. 2013;59:165–176. doi: 10.1016/j.nbd.2013.07.013. [DOI] [PubMed] [Google Scholar]
- 66.Leung D. Y. M., De Castro M., Szefler S. J., Chrousos G. P. Mechanisms of glucocorticoid-resistant asthma. Annals of the New York Academy of Sciences. 1998;840:735–746. doi: 10.1111/j.1749-6632.1998.tb09612.x. [DOI] [PubMed] [Google Scholar]
- 67.Longui C. A., Vottero A., Adamson P., et al. Low glucocorticoid receptor alpha/beta ratio in T-cell lymphoblastic leukemia. Hormone and Metabolic Research. 2000;32(10):401–406. doi: 10.1055/s-2007-978661. [DOI] [PubMed] [Google Scholar]
- 68.Zhang X., Ognibene C. M., Clark A. F., Yorio T. Dexamethasone inhibition of trabecular meshwork cell phagocytosis and its modulation by glucocorticoid receptor beta. Experimental Eye Research. 2007;84(2):275–284. doi: 10.1016/j.exer.2006.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zheng Y. C., Izumi K., Li Y., Ishiguro H., Miyamoto H. Contrary regulation of bladder cancer cell proliferation and invasion by dexamethasone-mediated glucocorticoid receptor signals. Molecular Cancer Therapeutics. 2012;11(12):2621–2632. doi: 10.1158/1535-7163.mct-12-0621. [DOI] [PubMed] [Google Scholar]
- 70.Ishiguro H., Kawahara T., Zheng Y., Netto G. J., Miyamoto H. Reduced glucocorticoid receptor expression predicts bladder tumor recurrence and progression. The American Journal of Clinical Pathology. 2014;142(2):157–164. doi: 10.1309/ajcpu8ucezyg4wtv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dietrich K., Schned A., Fortuny J., et al. Glucocorticoid therapy and risk of bladder cancer. British Journal of Cancer. 2009;101(8):1316–1320. doi: 10.1038/sj.bjc.6605314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Y., Izumi K., Li Y., Ishiguro H., Miyamoto H. Contrary regulation of bladder cancer cell proliferation and invasion by dexamethasone-mediated glucocorticoid receptor signals. Molecular Cancer Therapeutics. 2012;11(12):2621–2632. doi: 10.1158/1535-7163.mct-12-0621. [DOI] [PubMed] [Google Scholar]
- 73.Ishiguro H., Kawahara T., Zheng Y., Kashiwagi E., Li Y., Miyamoto H. Differential regulation of bladder cancer growth by various glucocorticoids: corticosterone and prednisone inhibit cell invasion without promoting cell proliferation or reducing cisplatin cytotoxicity. Cancer Chemotherapy and Pharmacology. 2014;74(2):249–255. doi: 10.1007/s00280-014-2496-7. [DOI] [PubMed] [Google Scholar]
- 74.Huizenga N. A. T. M., De Lange P., Koper J. W., et al. Five patients with biochemical and/or clinical generalized glucocorticoid resistance without alterations in the glucocorticoid receptor gene. Journal of Clinical Endocrinology and Metabolism. 2000;85(5):2076–2081. doi: 10.1210/jc.85.5.2076. [DOI] [PubMed] [Google Scholar]
- 75.Schlossmacher G., Stevens A., White A. Glucocorticoid receptor-mediated apoptosis: mechanisms of resistance in cancer cells. Journal of Endocrinology. 2011;211(1):17–25. doi: 10.1530/joe-11-0135. [DOI] [PubMed] [Google Scholar]
- 76.Zhang C., Mattern J., Haferkamp A., et al. Corticosteroid-induced chemotherapy resistance in urological cancers. Cancer Biology and Therapy. 2006;5(1):59–64. doi: 10.4161/cbt.5.1.2272. [DOI] [PubMed] [Google Scholar]
- 77.Niu Y., Altuwaijri S., Yeh S., et al. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(34):12188–12193. doi: 10.1073/pnas.0804701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gakis G., Stenzl A., Renninger M. Evolution of the concept of androgen-sensitive bladder cancer. Scandinavian Journal of Urology. 2013;47(3):173–178. doi: 10.3109/00365599.2012.756929. [DOI] [PubMed] [Google Scholar]
- 79.Lucca I., Fajkovic H., Klatte T. Sex steroids and gender differences in nonmuscle invasive bladder cancer. Current Opinion in Urology. 2014;24(5):500–505. doi: 10.1097/MOU.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 80.Heinlein C. A., Chang C. Androgen receptor in prostate cancer. Endocrine Reviews. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 81.Miyamoto H., Messing E. M., Chang C. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate. 2004;61(4):332–353. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- 82.Rahman M., Miyamoto H., Chang C. Androgen receptor coregulators in prostate cancer: mechanisms and clinical implications. Clinical Cancer Research. 2004;10(7):2208–2219. doi: 10.1158/1078-0432.ccr-0746-3. [DOI] [PubMed] [Google Scholar]
- 83.Yeh S., Chang H.-C., Miyamoto H., et al. Differential induction of the androgen receptor transcriptional activity by selective androgen receptor coactivators. Keio Journal of Medicine. 1999;48(2):87–92. doi: 10.2302/kjm.48.87. [DOI] [PubMed] [Google Scholar]
- 84.Chen J. D. Steroid/nuclear receptor coactivators. Vitamins and Hormones. 2000;58:391–448. doi: 10.1016/S0083-6729(00)58032-7. [DOI] [PubMed] [Google Scholar]
- 85.Chen F., Langenstroer P., Zhang G., Iwamoto Y., See W. A. Androgen dependent regulation of bacillus Calmette-Guerin induced interleukin-6 expression in human transitional carcinoma cell lines. Journal of Urology. 2003;170(5):2009–2013. doi: 10.1097/01.ju.0000092238.15685.10. [DOI] [PubMed] [Google Scholar]
- 86.Miyamoto H., Yang Z., Chen Y.-T., et al. Promotion of bladder cancer development and progression by androgen receptor signals. Journal of the National Cancer Institute. 2007;99(7):558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- 87.Johnson A. M., O'Connell M. J., Miyamoto H., et al. Androgenic dependence of exophytic tumor growth in a transgenic mouse model of bladder cancer: a role for thrombospondin-1. BMC Urology. 2008;8, article 7 doi: 10.1186/1471-2490-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boorjian S. A., Heemers H. V., Frank I., et al. Expression and significance of androgen receptor coactivators in urothelial carcinoma of the bladder. Endocrine-Related Cancer. 2009;16(1):123–137. doi: 10.1677/erc-08-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kauffman E. C., Robinson B. D., Downes M. J., et al. Role of androgen receptor and associated lysine-demethylase coregulators, LSD1 and JMJD2A, in localized and advanced human bladder cancer. Molecular Carcinogenesis. 2011;50(12):931–944. doi: 10.1002/mc.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosenzweig B. A., Bolina P. S., Birch L., Moran C., Marcovici I., Prins G. S. Location and concentration of estrogen, progesterone, and androgen receptors in the bladder and urethra of the rabbit. Neurourology and Urodynamics. 1995;14(1):87–96. doi: 10.1002/nau.1930140114. [DOI] [PubMed] [Google Scholar]
- 91.Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. Histology and Histopathology. 2000;15(4):1261–1270. doi: 10.14670/HH-15.1261. [DOI] [PubMed] [Google Scholar]
- 92.Wilson C. M., McPhaul M. J. A and B forms of the androgen receptor are expressed in a variety of human tissues. Molecular and Cellular Endocrinology. 1996;120(1):51–57. doi: 10.1016/0303-7207(96)03819-1. [DOI] [PubMed] [Google Scholar]
- 93.Salmi S., Santti R., Gustafsson J.-Å., Mäkelä S. Co-localization of androgen receptor with estrogen receptor beta in the lower urinary tract of the male rat. Journal of Urology. 2001;166(2):674–677. doi: 10.1016/s0022-5347(05)66041-7. [DOI] [PubMed] [Google Scholar]
- 94.Celayir S., Ilçe Z., Dervisoglu S. The sex hormone receptors in the bladder in childhood—I: preliminary report in male subjects. European Journal of Pediatric Surgery. 2002;12(5):312–317. doi: 10.1055/s-2002-35951. [DOI] [PubMed] [Google Scholar]
- 95.Litman H. J., Bhasin S., O'Leary M. P., Link C. L., McKinlay J. B. An investigation of the relationship between sex-steroid levels and urological symptoms: results from the Boston Area Community Health survey. BJU International. 2007;100(2):321–326. doi: 10.1111/j.1464-410x.2007.06938.x. [DOI] [PubMed] [Google Scholar]
- 96.Zheng Y., Izumi K., Yao J. L., Miyamoto H. Dihydrotestosterone upregulates the expression of epidermal growth factor receptor and ERBB2 in androgen receptor-positive bladder cancer cells. Endocrine-Related Cancer. 2011;18(4):451–464. doi: 10.1530/ERC-11-0010. [DOI] [PubMed] [Google Scholar]
- 97.Cheng L., MacLennan G. T., Pan C. X., et al. Allelic loss of the active X chromosome during bladder carcinogenesis. Archives of Pathology & Laboratory Medicine. 2004;128(2):187–190. doi: 10.5858/2004-128-187-ALOTAX. [DOI] [PubMed] [Google Scholar]
- 98.Reid L. M., Leav I., Kwan P. W. L. Characterization of a human, sex steroid-responsive transitional cell carcinoma maintained as a tumor line (R 198) in athymic nude mice. Cancer Research. 1984;44(10):4560–4573. [PubMed] [Google Scholar]
- 99.Hsu J.-W., Hsu I., Xu D., et al. Decreased tumorigenesis and mortality from bladder cancer in mice lacking urothelial androgen receptor. American Journal of Pathology. 2013;182(5):1811–1820. doi: 10.1016/j.ajpath.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Boorjian S., Ugras S., Mongan N. P., et al. Androgen receptor expression is inversely correlated with pathologic tumor stage in bladder cancer. Urology. 2004;64(2):383–388. doi: 10.1016/j.urology.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 101.Tan W., Boorjian S., Advani P., et al. The estrogen pathway: estrogen receptor-α, progesterone receptor, and estrogen receptor-β expression in radical cystectomy urothelial cell carcinoma specimens. Clinical Genitourinary Cancer. 2015 doi: 10.1016/j.clgc.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 102.Shen S. S., Smith C. L., Hsieh J.-T., et al. Expression of estrogen receptors-α and -β in bladder cancer cell lines and human bladder tumor tissue. Cancer. 2006;106(12):2610–2616. doi: 10.1002/cncr.21945. [DOI] [PubMed] [Google Scholar]
- 103.Kim H. T., Kim B. C., Kim I. Y., et al. Raloxifene, a mixed estrogen agonist/antagonist, induces apoptosis through cleavage of BAD in TSU-PR1 human cancer cells. The Journal of Biological Chemistry. 2002;277(36):32510–32515. doi: 10.1074/jbc.m202852200. [DOI] [PubMed] [Google Scholar]
- 104.Karmakar S., Jin Y., Nagaich A. K. Interaction of glucocorticoid receptor (GR) with estrogen receptor (ER) alpha and activator protein 1 (AP1) in dexamethasone-mediated interference of ERalpha activity. The Journal of Biological Chemistry. 2013;288(33):24020–24034. doi: 10.1074/jbc.m113.473819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsai M.-J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annual Review of Biochemistry. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 106.Brinkmann A. O., Trapman J. Prostate cancer schemes for androgen escape. Nature Medicine. 2000;6(6):628–629. doi: 10.1038/76194. [DOI] [PubMed] [Google Scholar]
- 107.Brinkmann A. O. Molecular basis of androgen insensitivity. Molecular and Cellular Endocrinology. 2001;179(1-2):105–109. doi: 10.1016/s0303-7207(01)00466-x. [DOI] [PubMed] [Google Scholar]
- 108.Isaacs J. T., Isaacs W. B. Androgen receptor outwits prostate cancer drugs. Nature Medicine. 2004;10(1):26–27. doi: 10.1038/nm0104-26. [DOI] [PubMed] [Google Scholar]
- 109.Brooke G. N., Bevan C. L. The role of androgen receptor mutations in prostate cancer progression. Current Genomics. 2009;10(1):18–25. doi: 10.2174/138920209787581307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gupta P., Jain M., Kapoor R., Muruganandham K., Srivastava A., Mandhani A. Impact of age and gender on the clinicopathological characteristics of bladder cancer. Indian Journal of Urology. 2009;25(2):207–210. doi: 10.4103/0970-1591.52916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jing Y., Cui D., Guo W., et al. Activated androgen receptor promotes bladder cancer metastasis via Slug mediated epithelial-mesenchymal transition. Cancer Letters. 2014;348(1-2):135–145. doi: 10.1016/j.canlet.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 112.Jitao W., Jinchen H., Qingzuo L., et al. Androgen receptor inducing bladder cancer progression by promoting an epithelial-mesenchymal transition. Andrologia. 2014;46(10):1128–1133. doi: 10.1111/and.12203. [DOI] [PubMed] [Google Scholar]
- 113.Ferrara N., Carver-Moore K., Chen H., et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380(6573):439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 114.Bielenberg D. R., Pettaway C. A., Takashima S., Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Experimental Cell Research. 2006;312(5):584–593. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- 115.Wild J. R. L., Staton C. A., Chapple K., Corfe B. M. Neuropilins: expression and roles in the epithelium. International Journal of Experimental Pathology. 2012;93(2):81–103. doi: 10.1111/j.1365-2613.2012.00810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jubb A. M., Strickland L. A., Liu S. D., Mak J., Schmidt M., Koeppen H. Neuropilin-1 expression in cancer and development. Journal of Pathology. 2012;226(1):50–60. doi: 10.1002/path.2989. [DOI] [PubMed] [Google Scholar]
- 117.Jubb A. M., Sa S. M., Ratti N., et al. Neuropilin-2 expression in cancer. Histopathology. 2012;61(3):340–349. doi: 10.1111/j.1365-2559.2012.04224.x. [DOI] [PubMed] [Google Scholar]
- 118.Saban M. R., Sferra T. J., Davis C. A., et al. Neuropilin-VEGF signaling pathway acts as a key modulator of vascular, lymphatic, and inflammatory cell responses of the bladder to intravesical BCG treatment. The American Journal of Physiology—Renal Physiology. 2010;299(6):F1245–F1256. doi: 10.1152/ajprenal.00352.2010. [DOI] [PubMed] [Google Scholar]
- 119.Stamer W. D., Hoffman E. A., Luther J. M., Hachey D. L., Schey K. L. Protein profile of exosomes from trabecular meshwork cells. Journal of Proteomics. 2011;74(6):796–804. doi: 10.1016/j.jprot.2011.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mirabelli P., Peebo B. B., Xeroudaki M., Koulikovska M., Lagali N. Early effects of dexamethasone and anti-VEGF therapy in an inflammatory corneal neovascularization model. Experimental Eye Research. 2014;125:118–127. doi: 10.1016/j.exer.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 121.Eisermann K., Broderick C. J., Bazarov A., Moazam M. M., Fraizer G. C. Androgen up-regulates vascular endothelial growth factor expression in prostate cancer cells via an Sp1 binding site. Molecular Cancer. 2013;12, article 7 doi: 10.1186/1476-4598-12-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gericke A., Munson M., Ross A. H. Regulation of the PTEN phosphatase. Gene. 2006;374(1-2):1–9. doi: 10.1016/j.gene.2006.02.024. [DOI] [PubMed] [Google Scholar]


