Abstract
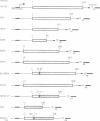
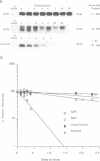
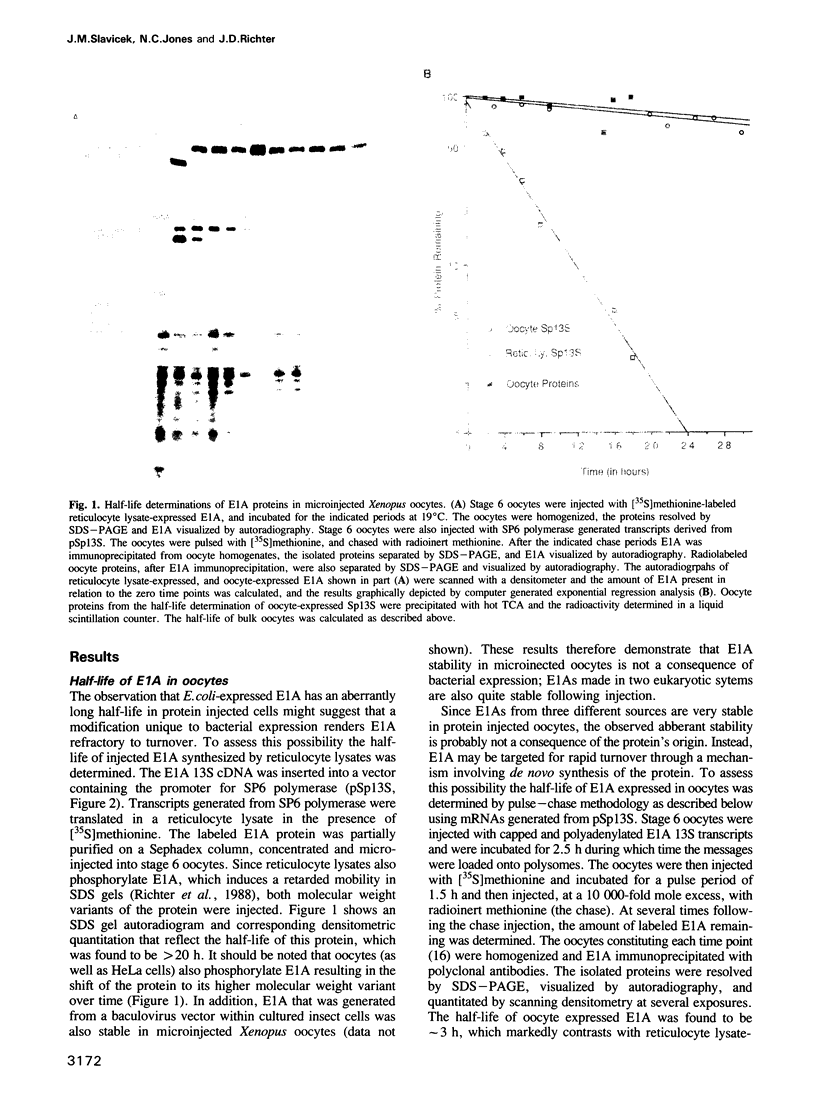
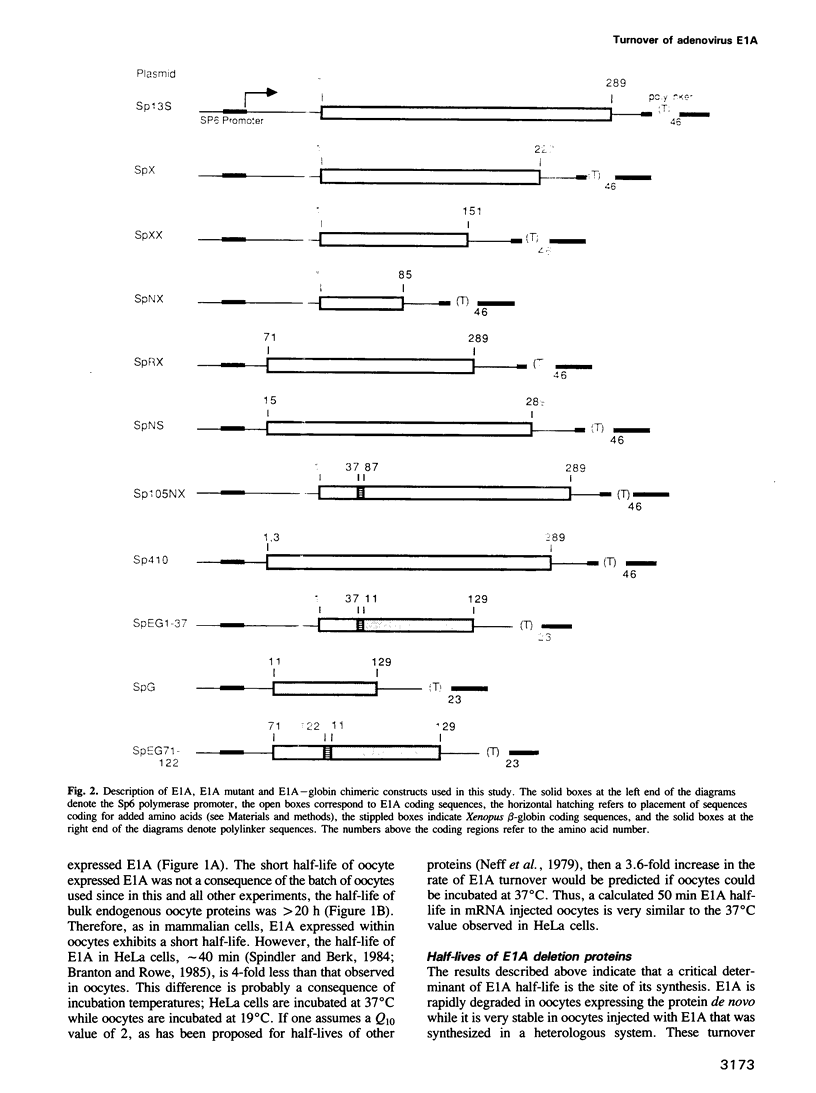
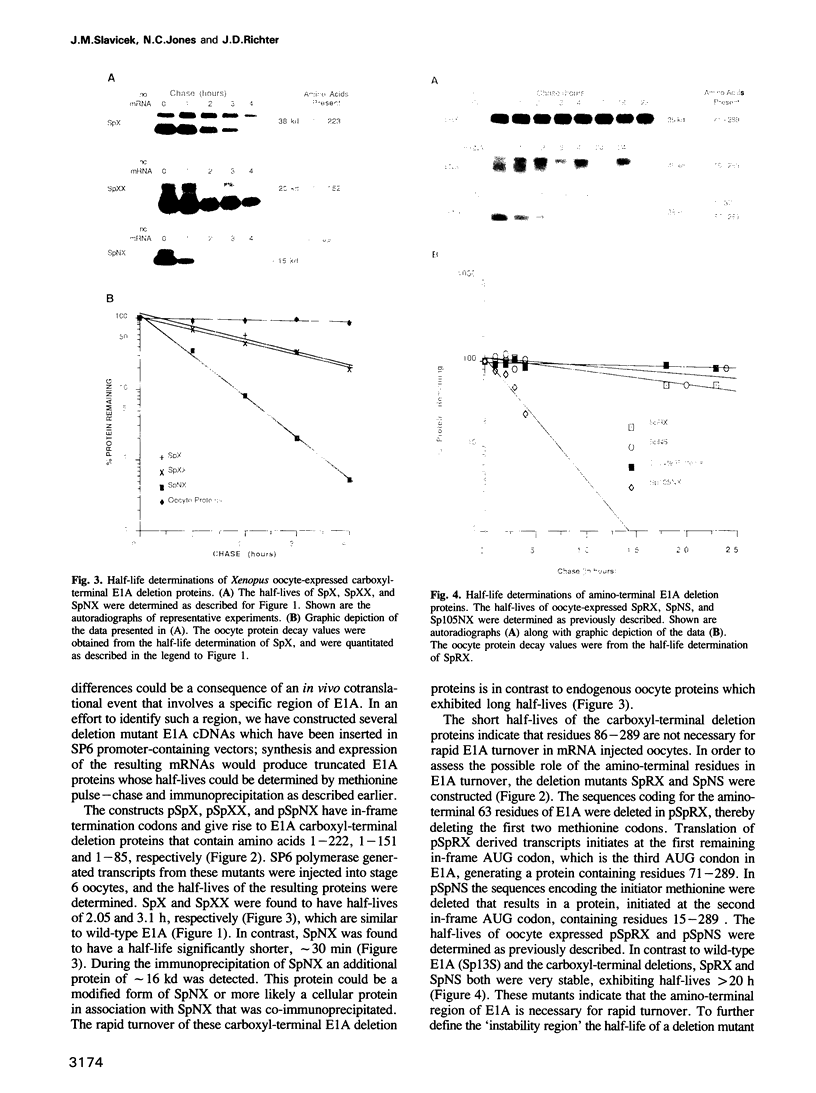
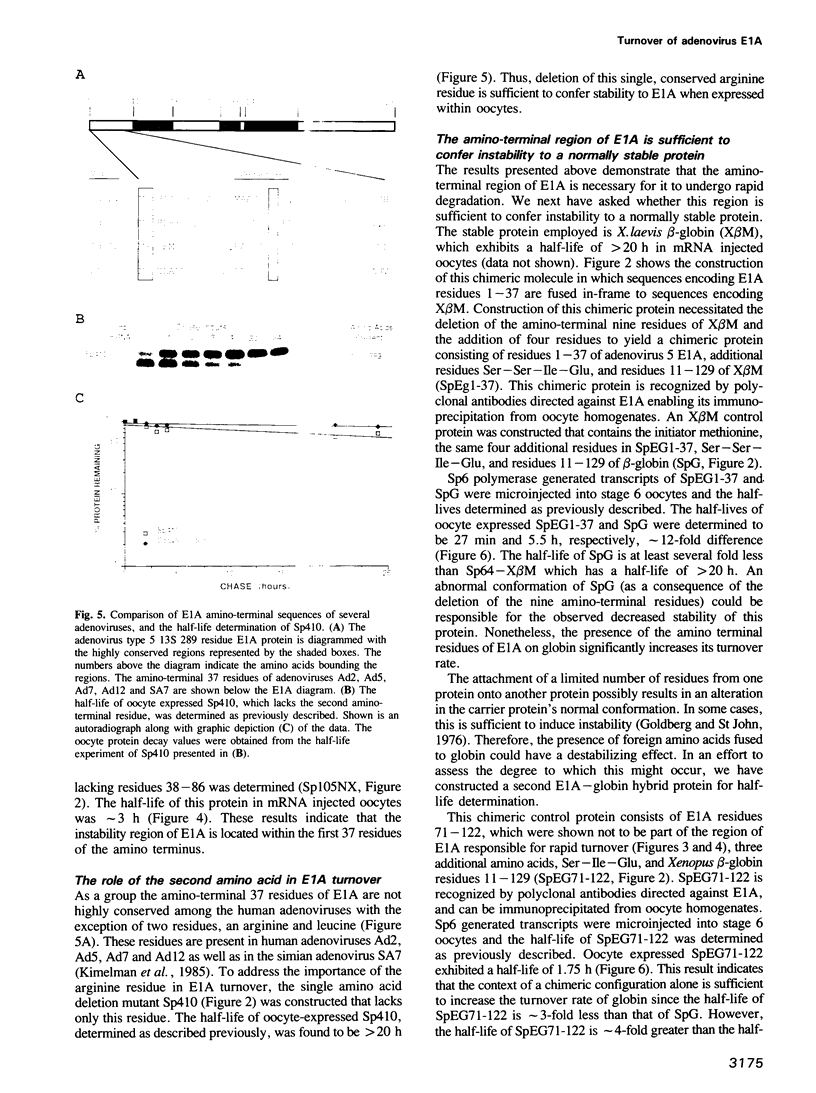
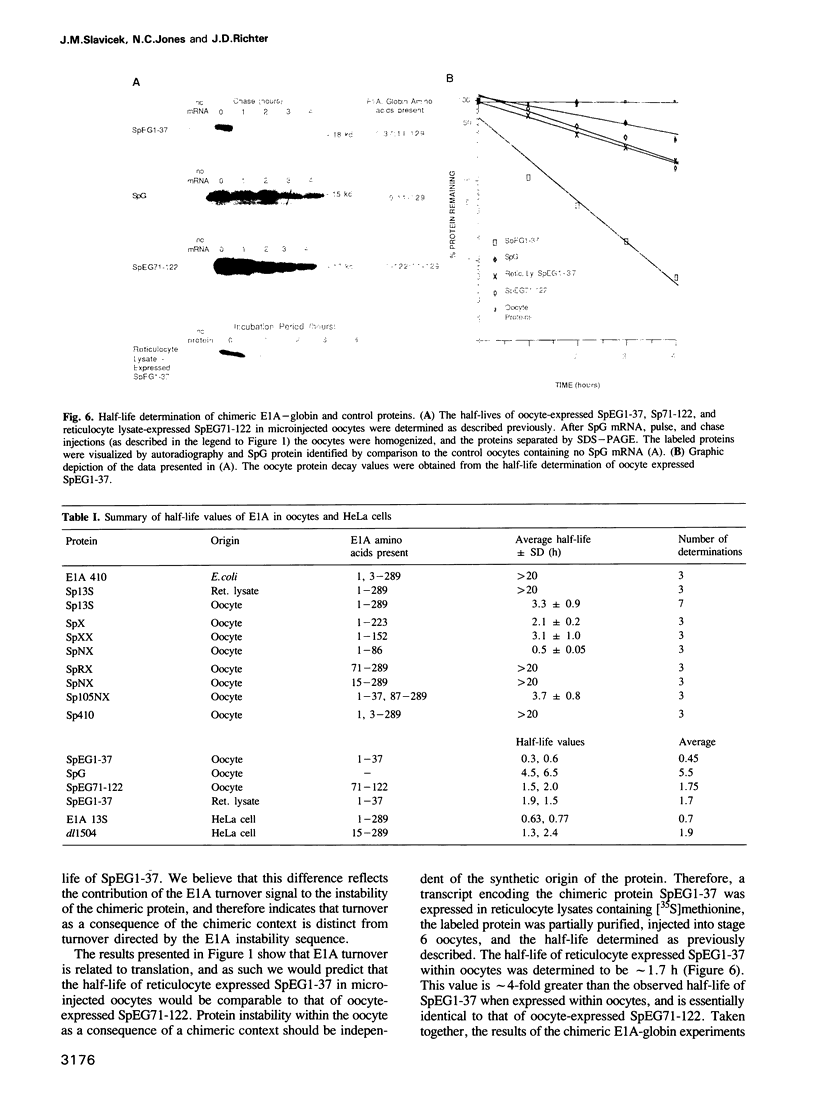
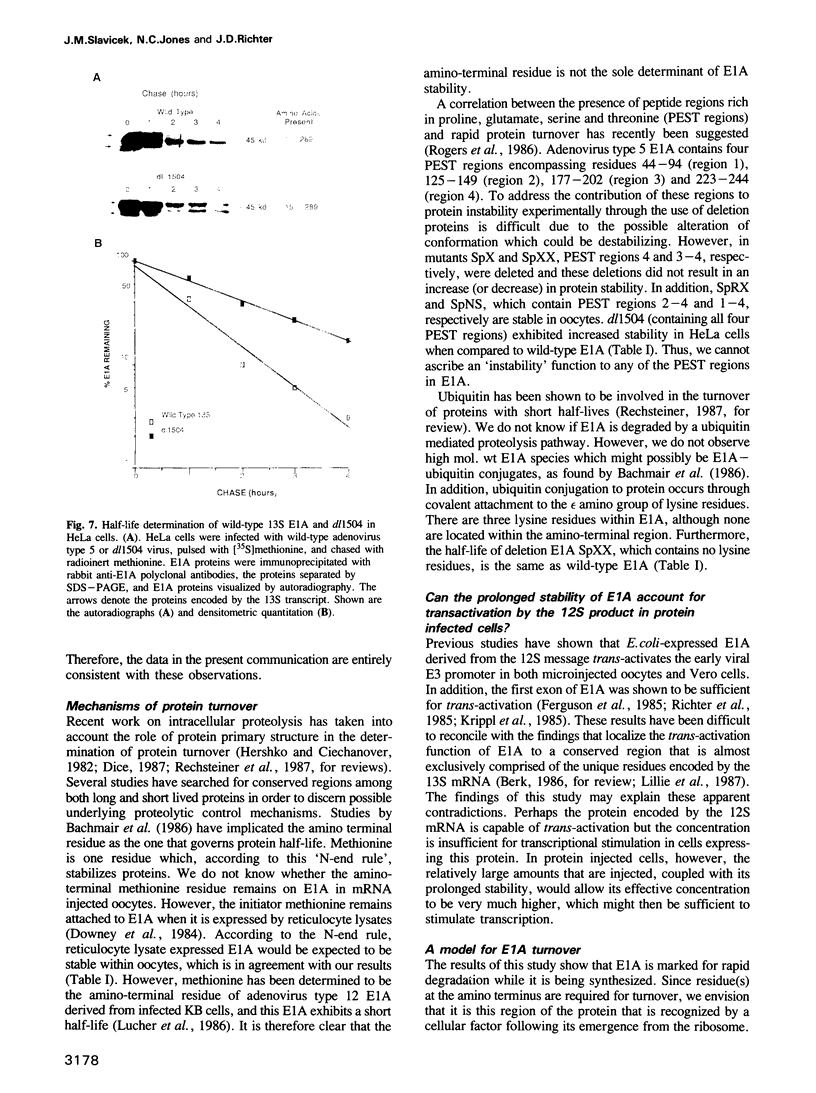
The product of the adenovirus E1A 13S mRNA can both stimulate and repress the expression of certain viral and cellular genes. As with several other regulatory proteins, E1A has a short half-life, approximately 40 min. Although this short half-life is observed in cells expressing the E1A gene, it is not the case with cells injected with E1A protein, where its half-life is very long, generally greater than 15 h. We have sought to reconcile these apparent differences in E1A stability. Using Xenopus oocytes, we find that E1A exhibits its characteristic short half-life when it is synthesized from injected mRNA while it has a very long half-life when it is injected as a protein synthesized originally in Escherichia coli or reticulocyte lysates. In order to delineate the amino acids responsible for rapid E1A turnover, several deletion mRNAs were constructed, injected into oocytes, and E1A half-life determined. Carboxyl-terminal deletions and an internal deletion of residues 38-86 failed to increase the half-life of E1A. In contrast, amino-terminal deletions of 70 and 14 residues resulted in very stable E1A proteins (t1/2 greater than 20 h). Furthermore, deletion of the second amino acid, an arginine, resulted in a stable E1A protein. The amino-terminal region of E1A was able to induce the rapid turnover of a normally stable protein, beta-globin, in oocytes injected with an E1A-globin chimeric mRNA. This E1A-induced instability of globin was abolished, however, when the protein was first synthesized in reticulocyte lysates and then injected into oocytes. The amino-terminal region of E1A is also important in governing halflife in adenovirus-infected HeLa cells. These results demonstrate that the half-life of E1A is established cotranslationally through a mechanism involving sequences within the amino-terminal 37 residues.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmair A., Finley D., Varshavsky A. In vivo half-life of a protein is a function of its amino-terminal residue. Science. 1986 Oct 10;234(4773):179–186. doi: 10.1126/science.3018930. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Borrelli E., Hen R., Chambon P. Adenovirus-2 E1A products repress enhancer-induced stimulation of transcription. Nature. 1984 Dec 13;312(5995):608–612. doi: 10.1038/312608a0. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., Lampert M. A., Li A. C., Baluda M. A. Nuclear compartmentalization of the v-myb oncogene product. Mol Cell Biol. 1985 Nov;5(11):3017–3023. doi: 10.1128/mcb.5.11.3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton P. E., Rowe D. T. Stabilities and interrelations of multiple species of human adenovirus type 5 early region 1 proteins in infected and transformed cells. J Virol. 1985 Nov;56(2):633–638. doi: 10.1128/jvi.56.2.633-638.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Dice J. F. Molecular determinants of protein half-lives in eukaryotic cells. FASEB J. 1987 Nov;1(5):349–357. doi: 10.1096/fasebj.1.5.2824267. [DOI] [PubMed] [Google Scholar]
- Downey J. F., Evelegh C. M., Branton P. E., Bayley S. T. Peptide maps and N-terminal sequences of polypeptides from early region 1A of human adenovirus 5. J Virol. 1984 Apr;50(1):30–37. doi: 10.1128/jvi.50.1.30-37.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson B., Jones N., Richter J., Rosenberg M. Adenovirus E1a gene product expressed at high levels in Escherichia coli is functional. Science. 1984 Jun 22;224(4655):1343–1346. doi: 10.1126/science.6374895. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Hann S. R., Eisenman R. N. Proteins encoded by the human c-myc oncogene: differential expression in neoplastic cells. Mol Cell Biol. 1984 Nov;4(11):2486–2497. doi: 10.1128/mcb.4.11.2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershko A., Ciechanover A. Mechanisms of intracellular protein breakdown. Annu Rev Biochem. 1982;51:335–364. doi: 10.1146/annurev.bi.51.070182.002003. [DOI] [PubMed] [Google Scholar]
- Houweling A., van den Elsen P. J., van der Eb A. J. Partial transformation of primary rat cells by the leftmost 4.5% fragment of adenovirus 5 DNA. Virology. 1980 Sep;105(2):537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Jones N. C., Richter J. D., Weeks D. L., Smith L. D. Regulation of adenovirus transcription by an E1a gene in microinjected Xenopus laevis oocytes. Mol Cell Biol. 1983 Dec;3(12):2131–2142. doi: 10.1128/mcb.3.12.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Miller J. S., Porter D., Roberts B. E. E1a regions of the human adenoviruses and of the highly oncogenic simian adenovirus 7 are closely related. J Virol. 1985 Feb;53(2):399–409. doi: 10.1128/jvi.53.2.399-409.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippl B., Andrisani O., Jones N., Westphal H., Rosenberg M., Ferguson B. Adenovirus type 12 E1A protein expressed in Escherichia coli is functional upon transfer by microinjection or protoplast fusion into mammalian cells. J Virol. 1986 Aug;59(2):420–427. doi: 10.1128/jvi.59.2.420-427.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippl B., Ferguson B., Jones N., Rosenberg M., Westphal H. Mapping of functional domains in adenovirus E1A proteins. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7480–7484. doi: 10.1073/pnas.82.22.7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krippl B., Ferguson B., Rosenberg M., Westphal H. Functions of purified E1A protein microinjected into mammalian cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6988–6992. doi: 10.1073/pnas.81.22.6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff T., Elkaim R., Goding C. R., Jalinot P., Sassone-Corsi P., Perricaudet M., Kédinger C., Chambon P. Individual products of the adenovirus 12S and 13S EIa mRNAs stimulate viral EIIa and EIII expression at the transcriptional level. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4381–4385. doi: 10.1073/pnas.81.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Lucher L. A., Brackmann K. H., Symington J. S., Green M. Posttranslational modification at the N terminus of the human adenovirus type 12 E1A 235R tumor antigen. J Virol. 1986 May;58(2):592–599. doi: 10.1128/jvi.58.2.592-599.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maicas E., Pluthero F. G., Friesen J. D. The accumulation of three yeast ribosomal proteins under conditions of excess mRNA is determined primarily by fast protein decay. Mol Cell Biol. 1988 Jan;8(1):169–175. doi: 10.1128/mcb.8.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff N. T., DeMartino G. N., Goldberg A. L. The effect of protease inhibitors and decreased temperature on the degradation of different classes of proteins in cultured hepatocytes. J Cell Physiol. 1979 Dec;101(3):439–457. doi: 10.1002/jcp.1041010311. [DOI] [PubMed] [Google Scholar]
- Osborne T. F., Gaynor R. B., Berk A. J. The TATA homology and the mRNA 5' untranslated sequence are not required for expression of essential adenovirus E1A functions. Cell. 1982 May;29(1):139–148. doi: 10.1016/0092-8674(82)90098-8. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. Ubiquitin-mediated pathways for intracellular proteolysis. Annu Rev Cell Biol. 1987;3:1–30. doi: 10.1146/annurev.cb.03.110187.000245. [DOI] [PubMed] [Google Scholar]
- Richter J. D., Hurst H. C., Jones N. C. Adenovirus E1A requires synthesis of a cellular protein to establish a stable transcription complex in injected Xenopus laevis oocytes. Mol Cell Biol. 1987 Sep;7(9):3049–3056. doi: 10.1128/mcb.7.9.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. D., Slavicek J. M., Schneider J. F., Jones N. C. Heterogeneity of adenovirus type 5 E1A proteins: multiple serine phosphorylations induce slow-migrating electrophoretic variants but do not affect E1A-induced transcriptional activation or transformation. J Virol. 1988 Jun;62(6):1948–1955. doi: 10.1128/jvi.62.6.1948-1955.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J. D., Young P., Jones N. C., Krippl B., Rosenberg M., Ferguson B. A first exon-encoded domain of E1A sufficient for posttranslational modification, nuclear-localization, and induction of adenovirus E3 promoter expression in Xenopus oocytes. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8434–8438. doi: 10.1073/pnas.82.24.8434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Slavicek J. M., Krider H. M. The organization and composition of the ribosomal RNA gene non-transcribed spacer of D. busckii is unique among the drosophilids. Genet Res. 1987 Dec;50(3):173–180. doi: 10.1017/s0016672300023661. [DOI] [PubMed] [Google Scholar]
- Spindler K. R., Berk A. J. Rapid intracellular turnover of adenovirus 5 early region 1A proteins. J Virol. 1984 Nov;52(2):706–710. doi: 10.1128/jvi.52.2.706-710.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velcich A., Ziff E. Adenovirus E1a proteins repress transcription from the SV40 early promoter. Cell. 1985 Mar;40(3):705–716. doi: 10.1016/0092-8674(85)90219-3. [DOI] [PubMed] [Google Scholar]