Abstract
High-altitude residents have lower mortality rates for ischaemic heart disease and this is ascribed to cardiac gene remodelling by chronic hypoxia. SUR2A is a cardioprotective ABC protein serving as a subunit of sarcolemmal ATP-sensitive K+ channels. The purpose of this study was to determine whether SUR2A is regulated by mild hypoxia in vivo and to elucidate the underlying mechanism. Mice were exposed to either 21% (control) or 18% (mild hypoxia) oxygen for 24 h. Exposure to 18% oxygen did not affect partial pressure of O2 (PO2) and CO2 (PCO2) in the blood, haematocrit or level of ATP in the heart. However, hypoxia increased myocardial lactate dehydrogenase (LDH) and lactate as well as NAD+ without affecting total NAD. SUR2A levels were significantly increased as well as myocardial resistance to ischaemia–reperfusion. Exposure to 18% oxygen did not phosphorylate extracellular signal regulated kinases (ERK1/2) or AMP activated protein kinase (AMPK), but it phosphorylated protein kinase B (Akt). An inhibitor of phosphoinositide 3-kinases (PI3K), LY294002 (0.2 mg/mouse), abolished all observed effects of hypoxia. LDH inhibitors, galloflavin (50 μM) and sodium oxamate (80 mM) significantly decreased levels of SUR2A in heart embryonic H9c2 cells, while inactive mutant LDH form, gly193-M-LDH increased cellular sensitivity towards stress induced by 2,4-dinitrophenol (10 mM). Treatment of H9c2 cells with sodium lactate (30 mM) increased intracellular lactate, but did not affect LDH activity or SUR2A levels. We conclude that PI3K/Akt signalling pathway and LDH play a crucial role in increase of cardiac SUR2A induced by in vivo exposure to 18% oxygen.
Keywords: Hypoxia, LDH, Oxygen, SUR2A, Heart, Akt
Highlights
-
•
Mild hypoxia increases levels of cardioprotective SUR2A in the heart.
-
•
Phosphorylation of Akt mediates mild hypoxia-induced increase in SUR2A.
-
•
Phosphorylation of ERK1/2 and AMPK is not involved in observed increase in SUR2A.
-
•
PI3K/Akt target LDH to regulate SUR2A levels in the myocardium.
-
•
LDH mediates regulation of SUR2A in a lactate-independent manner.
1. Introduction
SUR2A is an “atypical” ABC protein that binds to inward rectifier Kir6.2 to form ATP-sensitive K+ (KATP) channels in the sarcolemma [1]. Increase in level of SUR2A is associated with increased myocardial resistance to metabolic stress in both healthy and diseased hearts [2–4]. A SUR2A-mediated increase in myocardial resistance to stress increases physical endurance, decreases size of myocardial infarction induced by ischaemia–reperfusion and counteracts ageing-induced decline in physical performance [2–5]. The mechanism of cardioprotection afforded by SUR2A is still under investigation. SUR2A is the least expressed KATP channel-forming protein and its levels seems to be a rate limiting factor in KATP channel formation [2,6]. It has been reported that increased expression of SUR2A results in increased numbers of fully assembled KATP channels resulting in their earlier activation when the heart is exposed to ischemia. In turn, this shortens action membrane potential and prevents influx of Ca2 + and Ca2 + overload, which is cardioprotective [2]. However, there are more recent studies suggesting that cardioprotection afforded by KATP channels could be also mediated, at least in part, independently from the channel opening [7–9]. It has been proposed that sarcolemmal KATP channel protein complex controls subsarcolemmal levels of ATP by activity-independent mechanism, which promotes cardioprotection during stress conditions [7–9]. Manipulation with SUR2A levels has been suggested to be a promising therapeutic strategy against ischaemic heart diseases and other diseases where increased heart resistance to stress is beneficial [10–12]. Some years ago, it has been reported that high-altitude residents have lower mortality rates for ischaemic heart disease [13–15] and this has been confirmed by later studies [16]. In agreement with such studies were also findings that exposure to moderate hypoxia confers cardioprotection in experimental animals in vivo [17,18]. As SUR2A is an important factor in regulating heart resistance to metabolic stress we thought that it is worthwhile to assess whether mild hypoxia in vivo regulates cardiac levels of this protein. If it does, it could positively modify the outcome of a range of cardiovascular diseases, which is what was observed in clinical and experimental studies [13–18].
Therefore, in this study we have tested whether exposure to mild hypoxia (18% oxygen), which is equivalent to oxygen tension occurring at ~ 1200 m above sea level, would have any effect on SUR2A expression. Not only did we find that this concentration of oxygen increases level of SUR2A, but that it does that by activating a previously unknown signalling cascade.
2. Materials and methods
2.1. Mice and in vivo exposure to hypoxia
C57BL/6J male mice (6–8 weeks old) were exposed to either ambient oxygen (detected to be 21%) or fractional concentration of oxygen of 18% oxygen (normobaric) using integral Animal Hypoxia Chamber System; oxygen levels were controlled by ProOx Model 110 version 2.2 (Biospherix, Lacona, NY, USA). Mice, in groups of 5, were placed in a plexiglass chamber for 24 h in either 21% or 18% oxygen, which level was continuously monitored. All manipulations with animals including heart harvesting were performed inside the chamber. For hearts harvesting mice were sacrificed using a schedule 1 procedure of cervical dislocation. Some animals were injected i.p. with inhibitor of phosphatidylinositol 3-kinases (PI3K), LY294002 (0.2 mg/mouse; volume was 200 μl and vehicle was saline; Sigma-Aldrich, Gillingham, UK). For this series of experiments, control animals were injected with only vehicle (ie. 200 μl of saline i.p. injection) and subjected to the same protocol as LY294002-treated animals. All experiments have been approved by the appropriate ethical committee in agreement with the 1964 Declaration of Helsinki and its later amendments and the UK Home Office. The experiments have been done under authority of Project Licences 60/3925 and 70/7796.
2.2. H9c2 cells
Some experiments were performed on rat embryonic heart-derived female H9c2 cells (ECACC, Salisbury, UK). Cells were cultured in a tissue flask containing DMEM medium and were supplemented with 2 mM glutamine and 10% FCS in a 96-well plate. The cells were stored at 37 °C at 5% CO2. Either galloflavine (50 μM; Tocris Bioscience, Bristol, UK), sodium oxamate (80 mM; Sigma Aldrich, Gillingham, UK) or sodium lactate (30 mM; Sigma Aldrich, Gillingham, UK) was added into the culture media and solvent was added to the control group. The cultures were then left for a 24 hour incubation period before experimentation. For the experiments with inactive mutant of muscle form of LDH (gly193-M-LDH) H9C2 cells were infected with adenoviral constructs containing either luciferase (cells infected with luciferase have served as control cells in this study) or gly193-M-LDH. To infect H9C2 cells, a solution of recombinant adenovirus was mixed with culture medium, and cells were exposed to the virus with a multiplicity of 10 viral particles/cell for 48 h. Experiments were performed 48 h after the infection.
2.3. Cell survival assay
The survival of H9C2 cells were assayed using Multitox-Fluor Multiplex Cytotoxicity Assay (Promega). Briefly, H9C2 cells were plated in complete media (DMEM containing 10% FCS) in a 96-well plate, the recombinant adenovirus (luciferase or gly193-M-LDH) was added to the wells. After 48 h infection, the DNP was added to each well at the final concentration of 10 mM. To measure cell survival 6 h later, the peptide substrate (GF-AFC) that can be cleaved only by live cells was added to the each well. Following 30 min-long incubation at 37 °C, plates were measured using 1420 Multibabel Counter (Victor) plate reader, with excitation at 370 nm and emissions of 480 nm. The percentage of live cells was calculated based on the intensity of fluorescence according to the manufacturer's instructions.
2.4. Blood gas and haematocrit analysis
Blood gas (PO2 and PCO2) and haematocrit were measured in samples (500–700 μl) taken directly from the heart using pre-heparinised (1000 IU/ml) syringes and Rapidlab 348EX blood Gas System (Siemens, Frimley, UK).
2.5. Western blotting
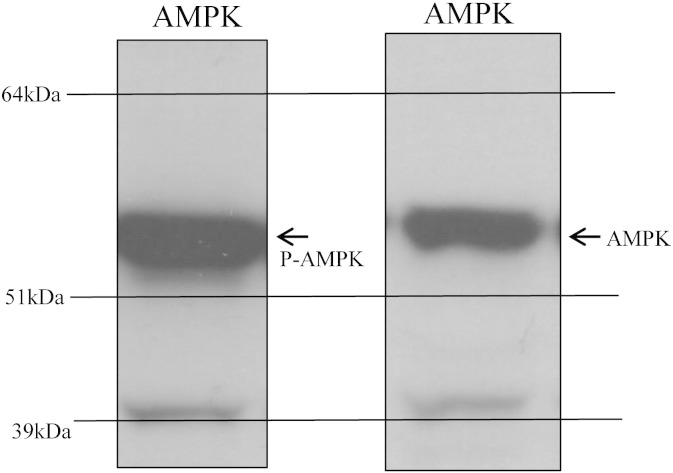
For Western blotting hearts/H9c2 cells were harvested and snap-frozen and homogenised in lysis buffer (50 mM Tris–HCl, pH 7.5; 1 mM EDTA; 1 mM EGTA; 1% (w/v) Triton X-100; 0.1% (v/v) mercaptoethanol; 1 mM sodium orthovanadate; 50 mM sodium fluoride; 5 mM sodium pyrophosphate; 1 μM microcystin-LR; and one tablet of “complete” proteinase inhibitor per 50 ml of buffer). A 10-fold mass excess of ice-cold lysis buffer was added to the powdered tissue, briefly vortexed, and then centrifuged at 4 °C for 10 min at 13,000 g to remove insoluble material. The supernatant was snap-frozen in aliquots in liquid nitrogen and stored at − 80 °C. Protein concentration was determined by Bradford Assay. From each sample, 20 μg of protein was subjected to SDS/PAGE and transferred to nitrocellulose. For all blots, the nitrocellulose membranes were incubated at 4 °C for 16 h using the antibodies against SUR2A, Kir6.2 (both from Santa Cruz Biotechnology, Heidelberg, Germany), Akt, AMPK, ERK1/2, phospho-Akt antibodies (Thr308 and Ser473), phosphor-AMPK and phospho-ERK1/2 (all from Millipore, Watford, UK). All antibodies were applied in 1:1000 dilution. The blots were incubated in 50 mM Tris/HCl, pH 7.5; 0.15 M NaCl; and 0.2% (by vol) Tween containing 5% (by mass) skimmed milk. Detection of total or phosphorylated protein was performed using horse radish peroxidase conjugated secondary antibodies (Pierce, Rockford, IL, USA) and enhanced chemiluminescence (ECL) reagent (Upstate, Dundee, UK). All antibodies apart from anti-AMPK and anti-phospho-AMPK have been characterised in detail and this characterisation has been published in our recent paper [18]. Thus, SUR2A antibody produced a single signal in the expected region, while Kir6.2 antibody produced several signals, but one of those was the exact size of this protein. Antibodies against ERK1/2, phospho-ERK1/2, Akt, S473 phospho-Akt, T308 phospho-Akt, AMPK and phospho-AMPK yielded solitary or double signals (where appropriate) in expected regions (see ref. [19]; Fig. 1). The band intensities were analysed using Quantiscan software (Cambridge, UK).
Fig. 1.
Western blot signals obtained by anti-AMPK antibodies were of appropriate molecular weight and clearly distinguishable from any other signals appearing on blots. Typical examples of original Western blots in this study when using anti-AMPK (AMPK) and anti-phospho-AMPK (P-AMPK) antibodies.
2.6. Measurement of ATP
ATP concentration in heart tissue was measured using luciferase-based ATP determination kit (Invitrogen, Paisley, UK) according to the manufacturer's instructions. Luminescence was measured at 560 nm using microplate reader/multidetection reader (SPECTRAMAX M2, Molecular Devices, Wokingham, UK).
2.7. Measurement of NAD/NADH
NAD/NADH was measured in heart tissue using NAD/NADH kit (Abcam, Cambridge, UK) according to the manufacturer's instruction. Absorbance was measured at 450 nm using microplate reader/multidetection reader (SPECTRAMAX M2, Molecular Devices, Wokingham, UK). Total NAD (NADt) and NADH were estimated directly while the value of NAD+ was estimated by subtracting NADH from NADt.
2.8. Measurement of lactate and LDH in the heart
Lactate was measured in heart tissue lysates using ADVIA Chemistry Lactate Enzymatic Assay and ADVIA Chemistry System 1200 (Siemens, Frimley, UK). Lactate is oxidised by lactate oxidase to pyruvate and hydrogen peroxide and it was measured by the formation of dye from hydrogen peroxide and a chromogen in the presence of a peroxidase. Absorbance was measured at 545/694 nm. LDH was measured using ADVIA Chemistry System 2400 (Siemens, Frimley, UK). Lactate dehydrogenase catalyses the conversion of lactate to pyruvate in the presence of NAD. The enzymatic activity of lactate dehydrogenase is proportional to the rate of production of NADH. The amount of NADH produced is determined by increase in absorbance at 340/410 nm.
2.9. Ischaemia–reperfusion and measurement of size of myocardial infarction, creatine kinase (CK) and LDH
The heart collection and ischaemia–reperfusion injury was performed as described in detail in our previous papers [3,19]. In brief, mice were killed by cervical dislocation (according to UK Home Office procedures), the hearts rapidly removed, the aorta cannulated, secured and attached to a custom-made Langendorff perfusion apparatus. Hearts were perfused at a constant flow rate of 5 ml/min at 37 °C with oxygenated (95% O2, 5% CO2; the PO2 in perfusate was ~ 600 mm Hg) Tyrode's solution (in mM: NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose (Glc) 5.5, HEPES-NaOH 5.5, pH 7.4) for a stabilisation period of 30 min. The heart was then subjected to 30 min of ischaemia by placing it into degassed Tyrode's (the solution was degassed with argon for 60 min and the PO2 in this solution was ~ 20 mm Hg) and switching off perfusion. A second 60 minute reperfusion with oxygenated Tyrode's followed the ischaemia. In some experiments during this period effluent was collected for later measurement of CK and LDH. CK and LDH in effluent were measured using ADVIA Chemistry System 2400 (Siemens, Frimley, UK). LDH was measured as described in above section. CK measurement was based on reaction with creatine phosphate and ADP, which form ATP coupled to the hexokinase–G6PD reaction, generating NADPH. The concentration of NADPH as a measure of CK activity was determined by changes in absorbance at 340/410 nm. After reperfusion, some hearts were snap-frozen in liquid nitrogen and stored at − 80 °C. Infarct size was determined using triphenyltetrazolium chloride (TTC) as described previously [3].
2.10. Statistical analysis
Data are presented as mean ± S.E.M., with n representing the number of analysed mice. Mean values were compared by Student's t-test using SigmaStat program (Jandel Scientific, Chicago, Illinois). P < 0.05 was considered statistically significant.
3. Results
3.1. 24 h-long exposure to 18% oxygen up-regulates SUR2A in the heart
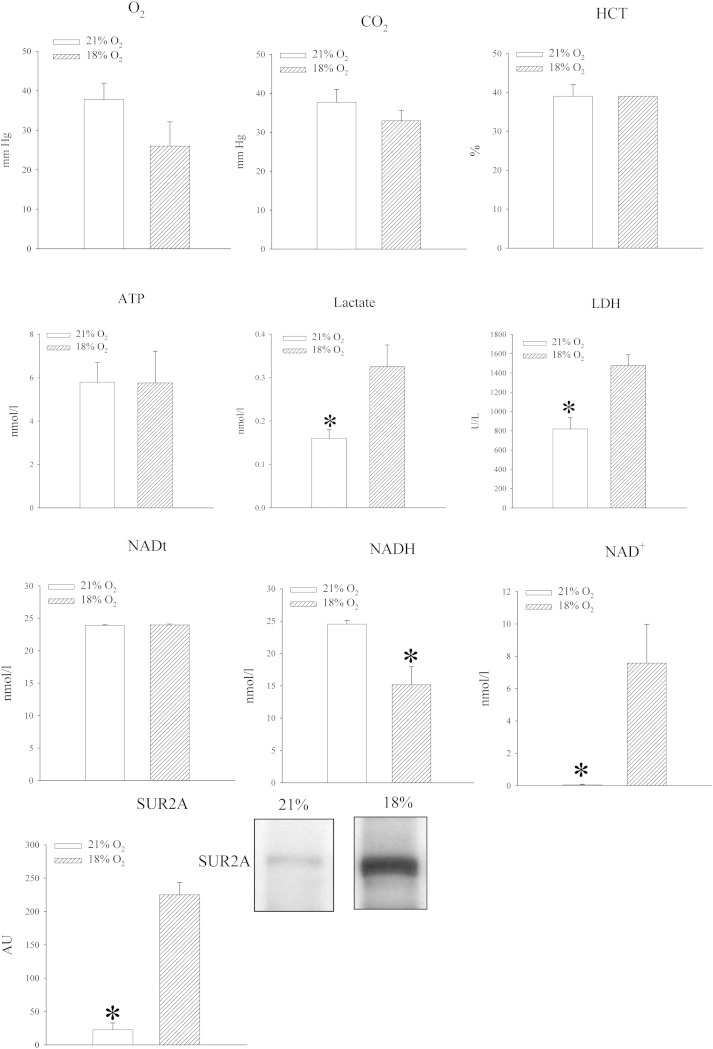
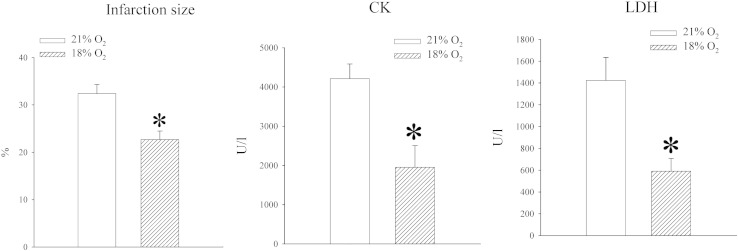
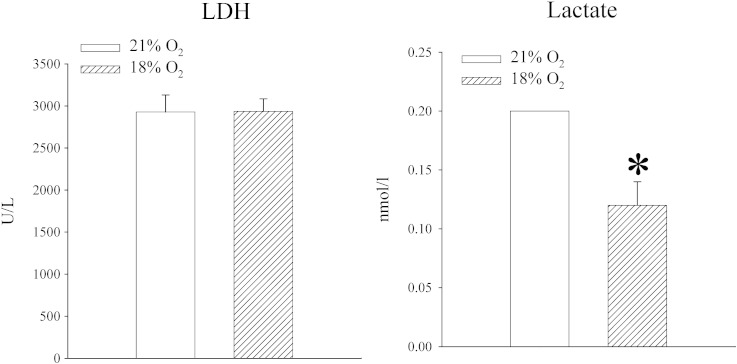
Mice were exposed to either atmospheric oxygen that was measured to be 21% or to 18% oxygen. Oxygen tension in the blood was decreased in mice exposed to 18% oxygen, but the difference was not statistically significant (PO2 in the blood was 37.8 ± 5.8 mm Hg in mice exposed to 21% oxygen and 26.0 ± 6.1 mm Hg in mice exposed to 18% oxygen, n = 5 for each, P = 0.211; Fig. 2). No differences were observed between blood levels of CO2 (PCO2 in the blood was 33.7 ± 6.2 mm Hg in mice exposed to 21% oxygen and 32.9 ± 2.7 mm Hg in mice exposed to 18% oxygen, n = 5 for each, P = 0.753; Fig. 2) and haematocrit did not differ between experimental groups (haematocrit was 39.0 ± 3.0% in mice exposed to 21% oxygen and 39.0 ± 0% in mice exposed to 18% oxygen, n = 5 for each, P = 1.000; Fig. 2). The level of ATP in heart tissue was not affected (5.8 ± 0.9 nmol/l in mice exposed to 21% oxygen and 5.8 ± 1.5 nmol/l in mice exposed to 18% oxygen, n = 5 for each, P = 0.983; n = 5 for each; Fig. 2), but exposure to 18% oxygen significantly increased lactate levels in the heart (from 0.16 ± 0.02 mmol/l to 0.33 ± 0.05 mmol/l, P = 0.013, n = 5; Fig. 2). An increased level of lactate was accompanied by increased level of LDH (from 817.6 ± 117.0 U/l to 1477.2 ± 112.7, n = 5, P < 0.01; Fig. 2). In addition to that, exposure to 18% oxygen was associated with significant decrease in myocardial NADH (24.5 ± 0.6 nmol/l vs 15.1 ± 2.8 nmol/l, P = 0.030, n = 3–4; Fig. 2) and increase in NAD+ (0.1 ± 0.05 nmol/l vs 7.6 ± 2.4 nmol/l, P = 0.045; Fig. 2). Total NAD was not affected (23.9 ± 0.1 nml/l vs 24.0 ± 0.2 nmol/l, P = 0.755, n = 3-4; Fig. 2). At the same time, exposure to 18% oxygen dramatically increased level of SUR2A (Fig. 2; signal intensity was 22.7 ± 10.1 AU in mice exposed to 21% oxygen and 224.9 ± 18.6 AU in mice exposed to 18% oxygen, n = 4, P < 0.001), while the levels of Kir6.2 were just slightly, but significantly, increased (signal intensity was 53.8 ± 1.9 AU in mice exposed to 21% oxygen and 64.0 ± 1.1 AU in mice exposed to 18% oxygen, n = 3, P = 0.006; data not shown). We have tested whether exposure to 18% oxygen would affect myocardial resistance to ischaemia–reperfusion by measuring the size of myocardial infarction as well as amounts of CK and LDH released from the muscle during this challenge. We have found that exposure to 18% oxygen significantly decreased the size of myocardial infarction (from 32.4 ± 1.9% in mice exposed to 21% oxygen to 22.7 ± 1.8% in mice exposed to 18% oxygen, n = 3, P = 0.044) and hearts from animals exposed to 18% oxygen released significantly less CK and LDH than animals exposed to 21% oxygen (CK: 4381 ± 823 U/l in 21% and 1956 ± 553 in 18%, n = 5, P = 0.04; LDH: 1424 ± 210 U/l in 21% and 591 ± 117 in 18%, n = 5, P < 0.001; Fig. 3).
Fig. 2.
Systemic exposure to 18% oxygen regulates levels of NAD +, NADH, lactate, LDH and SUR2A in the heart. Bar graphs depicting PO2 (O2), PCO2 (CO2) and haematocrit (HCT) in the blood as well as ATP (ATP), lactate (Lactate), LDH, total NAD (NADt), NADH (NADH), NAD + (NAD +) and SUR2A (SUR2A) in the myocardial tissue; inset in SUR2A bar graph represents original Western blots for SUR2A under labelled conditions. Each bar is a mean ± SEM (n = 3–5). *P < 0.01. AU = arbitrary units.
Fig. 3.
Exposure to 18% oxygen increases heart resistance to ischaemia–reperfusion. Bar graphs depicting size of myocardial infarction, CK and LDH concentration in heart effluent following ischaemia–reperfusion. Each bar is a mean ± SEM (n = 3–5). *P < 0.05.
3.2. 24 h-long exposure to 18% oxygen does not activate ERK or AMPK signalling in the heart
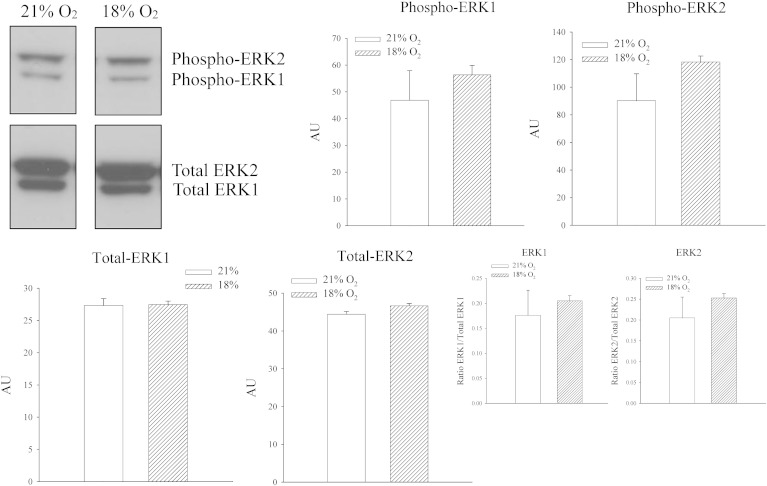
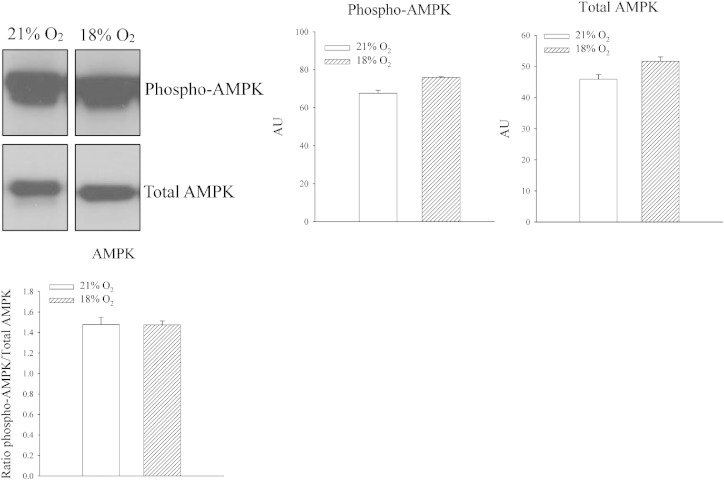
In H9C2 heart embryonic cells, it has been previously shown that in vitro chronic mild hypoxia activates ERK leading to increase in SUR2A levels [20]. Here we have tested whether exposure to 18% oxygen would phosphorylate ERK. Exposure to 18% oxygen did not have any effect on either ERK1 or ERK2 (signal intensity: ERK1: 46.9 ± 11.0 AU in mice exposed to 21% oxygen and 56.3 ± 3.5 AU in mice exposed to 18% oxygen, n = 4–5, P = 0.393; ERK2: 90.2 ± 19.6 AU in mice exposed to 18% oxygen and 118.3 ± 4.3 AU in mice exposed to 21% oxygen, n = 4–5, P = 0.158; Fig. 4). The ratio of phospho vs total ERK was also not affected for both ERK1 (0.18 ± 0.05 for mice exposed to 21% oxygen and 0.20 ± 0.01 for mice exposed to 18% oxygen, n = 4–5, P = 0.520; Fig. 4) and ERK2 (0.21 ± 0.05 for mice exposed to 21% oxygen to 0.25 ± 0.01 in mice exposed to 18% oxygen, n = 4–5, P = 0.291; Fig. 4). Activation of AMPK is known to stimulate trafficking of KATP channels subunits, including SUR2A [21]. Therefore, we have examined whether exposure to 18% oxygen phosphorylates AMPK. An increase in both phosphorylated (signal intensity: 67.6 ± 1.6 AU in mice exposed to 21% oxygen and 76.0 ± 1.6 AU in mice exposed to 18% oxygen, n = 4–5, P < 0.001; Fig. 5) and total (signal intensity: 45.9 ± 1.5 AU in mice exposed to 21% oxygen and 51.7 ± 1.4 AU in mice exposed to 18% oxygen, n = 4–5, P = 0.03; Fig. 5) AMPK was observed (Fig. 5). However, the ratio of phospho vs total AMPK was not affected at all (1.48 ± 0.07 for mice exposed to 21% oxygen and 1.47 ± 0.04 for mice exposed to 18% oxygen, n = 4–5, P = 0.958; Fig. 5).
Fig. 4.
Systemic exposure to 18% oxygen does not phosphorylate ERK1/2 in the heart. Original Western blots with phospho-ERK1, phospho-ERK2 and total ERK1 and ERK2 antibodies applied on extracts from hearts under depicted conditions and corresponding graphs. Each bar is a mean ± SEM (n = 4-5). AU = arbitrary units.
Fig. 5.
Systemic exposure to 18% oxygen does not phosphorylate AMPK in the heart. Original Western blots with phospho-AMPK and total AMPK antibodies applied on extracts from hearts under depicted conditions and corresponding graphs. Each bar is a mean ± SEM (n = 4–5). AU = arbitrary units.
3.3. 24 h-long exposure to 18% oxygen activates PI3K/Akt signalling pathway
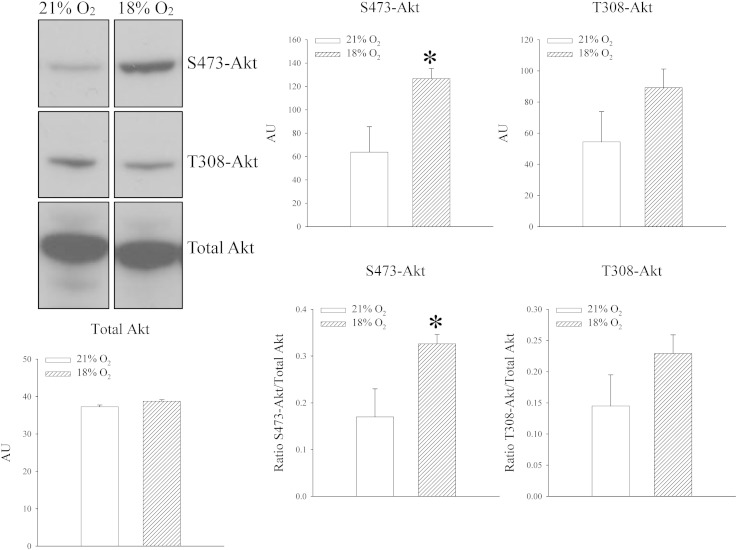
Antibodies targeting phosphorylation of Akt at S473 and T308 were used as well as an antibody targeting Akt non-selectively to examine whether exposure to 18% oxygen phosphorylates Akt in the heart. Exposure to 18% oxygen was associated with phosphorylation of S473 Akt site (signal intensity: 63.8 ± 21.8 AU in mice exposed to 21% oxygen and 126.7 ± 8.6 AU in mice exposed to 18% oxygen, n = 4–5, P = 0.022; Fig. 6; the ratio of phospho S473 vs total Akt was 0.17 ± 0.06 for mice exposed to 21% oxygen and 0.33 ± 0.02 for mice exposed to 18% oxygen, n = 4–5, P = 0.024; Fig. 6). On the other hand, exposure of mice to 18% oxygen did not affect phosphorylation on T308 site (signal intensity: 54.4 ± 19.5 AU in mice exposed to 21% oxygen and 89.2 ± 11.9 AU in mice exposed to 18% oxygen, n = 4–5, P = 0.142; Fig. 6; the ratio of phospho T308 vs total Akt was 0.15 ± 0.06 for mice exposed to 21% oxygen and 0.23 ± 0.03 for mice exposed to 18% oxygen, n = 4–5, P = 0.157; Fig. 6).
Fig. 6.
Systemic exposure to 18% oxygen phosphorylates S473Akt in the heart. Original Western blots with phospho-S473-Akt, phospho-T308-Akt and total Akt antibodies applied on extracts from hearts under depicted conditions and corresponding graphs. Each bar is a mean ± SEM (n = 4–5). *P < 0.05. AU = arbitrary units.
3.4. Phosphorylation of S473 Akt site is responsible for up-regulation of SUR2A in the heart induced by 24 h-long exposure to 18% oxygen
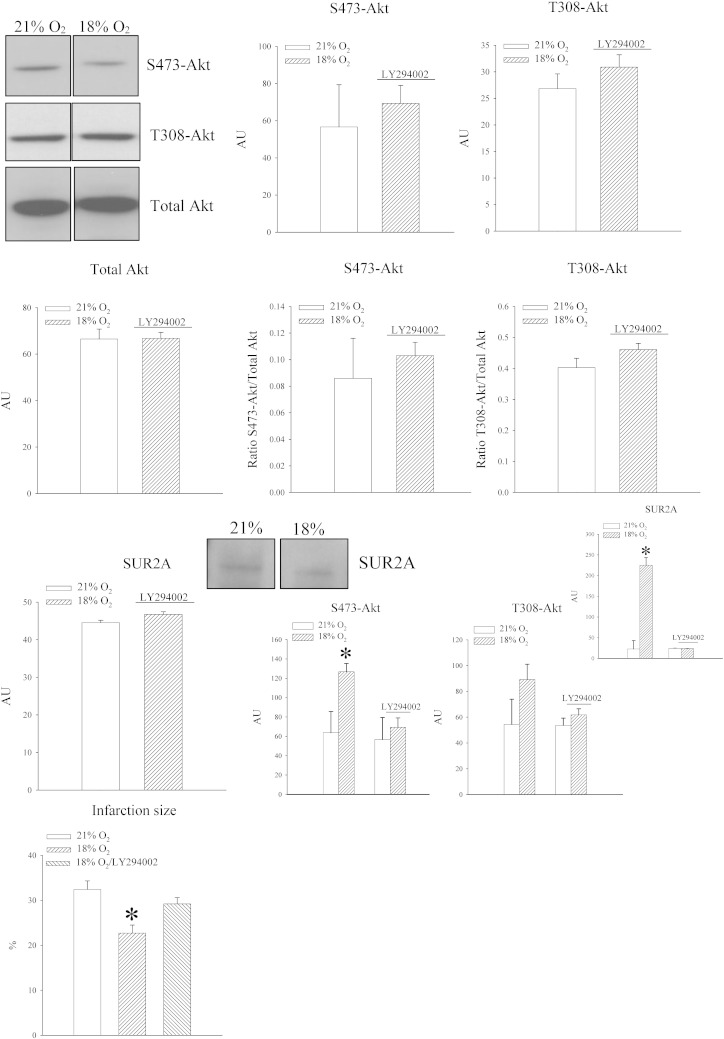
Akt S473 phosphorylation could have been a process independent from regulation of SUR2A. In order to find out a causal relationship between phosphorylation of Akt at S473 site and SUR2A, we have treated animals with LY294002, an inhibitor of PI3K (22). When treated animals exposed to 18% oxygen were compared with untreated animals exposed to 21% oxygen no differences in Akt phosphorylation was observed regardless of the site (signal intensity: S473Akt: 56.7 ± 22.7 AU in mice exposed to 21% oxygen and 69.3 ± 9.7 AU in mice exposed to 18% oxygen, n = 3 for each, P = 0.635; T308Akt: 26.8 ± 2.8 AU in mice exposed to 21% oxygen and 30.9 ± 2.3 AU in mice exposed to 18% oxygen, n = 3 for each, P = 0.372; Fig. 7). The ratio of phospho vs total Act was not changed for both S473 (0.09 ± 0.03 in untreated mice exposed to 21% oxygen and 0.10 ± 0.01 in treated mice exposed to 18% oxygen, n = 3, P = 0.653; Fig. 7) and T308 (0.40 ± 0.03 in untreated mice exposed to 21% oxygen and 0.46 ± 0.02 in treated mice exposed to 18% oxygen, n = 3, P = 0.208; Fig. 7) site.
Fig. 7.
LY294002 inhibits phosphorylation of S473Akt, increase of SUR2A in the heart and cardioprotection in response to 18% oxygen. Original Western blots with phospho-S473-Akt, phospho-T308-Akt and total Akt antibodies applied on extracts from hearts under depicted conditions (21% refers to untreated mice exposed to 21% oxygen and 18% to mice treated with LY294002 and exposed to 18% oxygen) and corresponding graphs as well as a graph showing the size of myocardial infarction under depicted conditions. Each bar is a mean ± SEM (n = 3–5). *P < 0.05. AU = arbitrary units.
Western blot did not reveal any difference in signal intensity between animals exposed to 21% oxygen and those exposed to 18% oxygen and treated with LY294002 (Fig. 7; signal intensity: 44.5 ± 6.6 AU in untreated mice exposed to 21% oxygen and 46.8 ± 6.8 AU in treated mice exposed to 18% oxygen, n = 3 for each, P = 0.825). When untreated and treated mice exposed to 18% oxygen were compared, LY294002-treatment prevented both Akt S473 phosphorylation and increase in myocardial SUR2A (Fig. 7). In addition, treatment with LY294002 abolished cardioprotection afforded by 18% oxygen (Fig. 7; the size of myocardial infarction was 32.4 ± 1.9% in mice exposed to 21% of oxygen, 22.7 ± 1.8% in mice exposed to 18% oxygen and 29.2 ± 1.4% in mice pretreated with LY294002 and exposed to 18% oxygen, P = 0.0469 when LY294002-treated mice are compared with 18% oxygen-untreated mice and P = 0.426 when they are compared with 21% oxygen-untreated mice, n = 3 for each).
3.5. Phosphorylation of S473 Akt site is also responsible for up-regulation of LDH and increased lactate production in the heart induced by 24 h-long exposure to 18% oxygen
One of the important features of exposure to 18% oxygen was up-regulation of heart LDH and increased levels of lactate (Fig. 2). If up-regulation of SUR2A is S473 Akt-dependent and SUR2A expression is regulated by LDH and lactate, it would be logical to expect that phosphorylation of S473 Akt also regulates LDH and lactate levels. Therefore, we measured levels of LDH and lactate in mice treated with LY294002 and exposed to 18% oxygen. LY294002 prevented increase in lactate (lactate levels were 0.20 ± 0.00 mmol/l in untreated mice exposed to 21% and 0.12 ± 0.02 mmol/l in treated mice exposed to 18%, P < 0.001, n = 4–5; Fig. 8) and LDH (2729.6 ± 201.8 U/l in untreated mice exposed to 21% and 2936.0 ± 149.1 in treated mice exposed to 18%, n = 4–5, P = 0.434; Fig. 8).
Fig. 8.
Akt regulates LDH/lactate, which, in turn, regulates SUR2A. LY294002 inhibits increase in LDH and lactate in the heart in response to 18% oxygen. Bar graphs depicting LDH (LDH) and lactate (Lactate) levels in extracts from hearts under depicted conditions (21% refers to untreated mice exposed to 21% oxygen and 18% to mice treated with LY294002 and exposed to 18% oxygen). Each bar is a mean ± SEM (n = 4–5).
3.6. LDH regulates expression of SUR2A
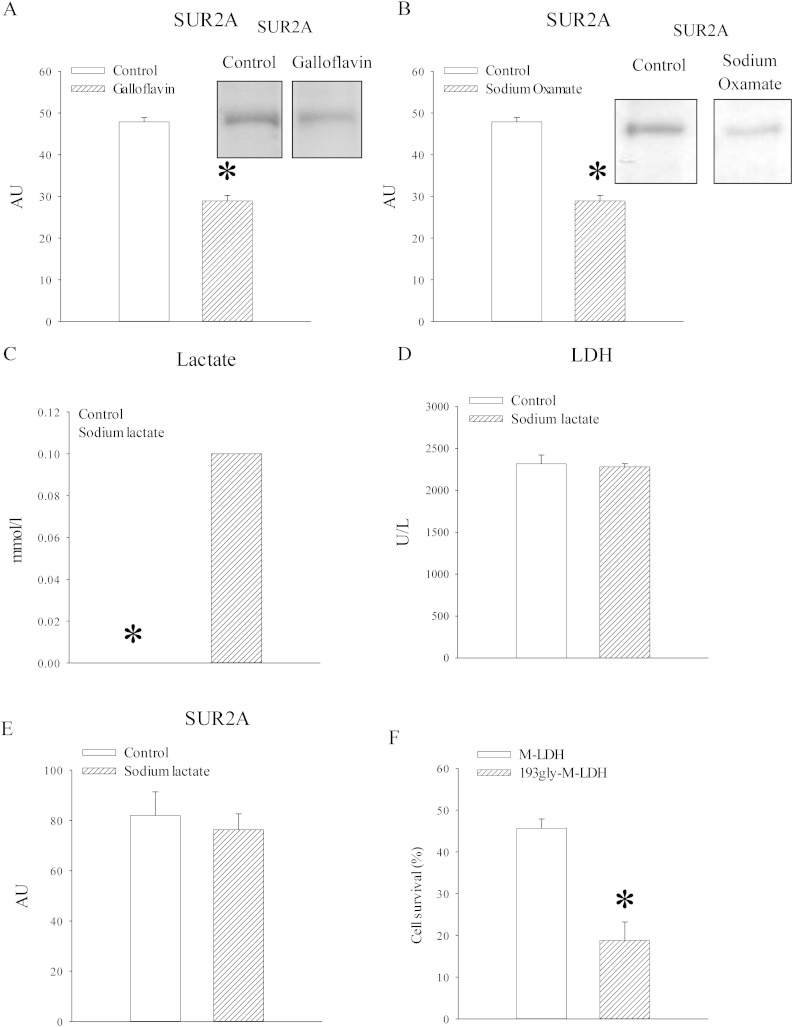
To test further a relationship between LDH/lactate and SUR2A expression we have used galloflavin and sodium oxamate, inhibitors of LDH. In rat heart embryonic H9c2 cells, 24 h-long treatment with galloflavin (50 μM) and sodium oxamate (80 mM) significantly decreased levels of SUR2A (Fig. 9; galloflavin: signal intensity was 47.9 ± 1.0 AU in the absence and 28.9 ± 1.3 AU in the presence of galloflavin, n = 3, P < 0.001; sodium oxamate: signal intensity was 17.8 ± 1.8 AU in the absence and 10.9 ± 1.7 AU in the presence of sodium oxamate, n = 3, P = 0.049). To determine whether increase in lactate alone is sufficient to up-regulate SUR2A, we have treated H9c2 cells with sodium lactate (30 mM). This treatment significantly increased levels of lactate in these cells (lactate levels were 0.00 ± 0.00 mmol/l in untreated H9c2 cells and 0.10 ± 0.00 mmol/l, n = 3, P = 0.00; Fig. 9), but it did not affect levels/activity of LDH (2317.7 ± 104.9 U/l in untreated cells and 2279.7 ± 38.9 in cells treated with sodium lactate, n = 3, P = 0.751; Fig. 9). No difference was observed in SUR2A levels between untreated cells and cells treated with sodium lactate (30 mM; Fig. 9; signal intensity was 82.0 ± 9.4 AU in untreated cells and 76.3 ± 6.3 AU in cells treated with sodium lactate, n = 3, P = 0.638). 193glyM-LDH is a mutant form of muscle lactate dehydrogenase (M-LDH) that has lost its catalytic activity that has been shown to have major effect on LDH activity in H9c2 cells [8]. Infection of H9c2 cells with 193glyM-LDH significantly increased cellular susceptibility towards severe metabolic stress induced by DNP (10 mM; Fig. 9; survival of control cells was 45.6 ± 2.3% and 18.8 ± 4.4% for cells infected with 193glyM-LDH, n = 3–5, P < 0.001).
Fig. 9.
LDH activity, but not lactate, seems to regulate SUR2A levels. A–B. Original Western blots with SUR2A antibody applied on extracts from H9c2 cells in the absence (control) and presence of galloflavin (A) or sodium oxamate (B) and corresponding graphs. Each bar is a mean ± SEM (n = 3). C–E. Graphs showing levels of lactate (C), LDH (D) and SUR2A (E) and in untreated cells and cells treated with sodium lactate (30 mM). Each bar is a mean ± SEM (n = 3). F. A graph showing cell survival in H9c2 cells infected by luciferase (control cells labelled as M-LDH) and cell infected by 193gly-M-LDH. Each bar is a mean ± SEM (n = 3–5). *P < 0.05. AU = arbitrary units.
4. Discussion
Normobaric 18% oxygen corresponds to oxygen tension at ~ 1200 m above sea level. When effects of several hours-long hypoxia is studied in vivo, usually lower concentration of oxygen is applied (typically 6–16%) than the one we applied in the present study [17,23]. In order to determine whether 18% oxygen induced any sign of hypoxia we have measured a string of parameters. PO2 in the blood was lower than those in mice at ambient oxygen, but the difference was not statistically significant. ATP levels in the heart were also not changed, which is probably not entirely surprising when considering that mice were exposed to very mild hypoxia. However, NAD+/NADH ratio in the heart did change and lactate increased. A change in NAD+/NADH ratio is one of the hallmarks of myocardial response to hypoxia [24] and these findings suggest that systemic exposure to 18% oxygen induced hypoxia in mouse hearts in vivo. An increase in lactate is additional sign of hypoxia in the heart as lactate is a product of anaerobic glycolysis [23].
Decades ago, it has been suggested that high-altitude residents have lower mortality rates for ischaemic heart disease [13–15]. Later experiments on animals have demonstrated that exposure to moderate hypoxia confers cardioprotection [16,17]. It has been suggested that adaptive metabolic reorganisation, in particular increase in utilisation of carbohydrates as cardiac fuel substrate (versus fatty acids) and augmented mitochondrial respiratory capacity mediate observed cardioprotection [23]. Also, chronic hypoxia is associated with metabolic gene remodelling [23]. SUR2A is a significant regulator of myocardial resistance to stress whose expression is regulated by mild hypoxia in vitro [20]. In fact, it has been shown that SUR2A was particularly sensitive to changes in oxygen tensions as 13% of oxygen in vitro affected expression exclusively of this protein [20]. In the present study, systemic exposure to 18% resulted in substantial increase in SUR2A levels in the heart, which is in agreement with the notion that SUR2A expression is highly sensitive to hypoxia. It is well established that increased SUR2A levels confer cardioprotection [12] and our findings can explain, at least in part, why residents living at high altitude would have lower mortality rates for ischaemic heart disease. Size of myocardial infarction, CK and LDH are well established markers of myocardial injury [24], and we have measured them to determine whether exposure to 18% oxygen is cardioprotective. Indeed, exposure to 18% oxygen decreased the size of myocardial infarction as well as amounts of CK and LDH released from the myocardium in response to ischaemia–reperfusion suggesting an increase in myocardial resistance to injury. These results fit into the notion that mild hypoxia increases levels of SUR2A in the heart resulting in cardioprotection. In addition to SUR2A, we have also found that exposure to 18% oxygen increased levels of LDH in the heart, which is in agreement with previous studies showing up-regulation of LDH induced by chronic hypoxia [25]. This can also explain increased lactate levels in the heart tissue we observed.
In vitro, it has been shown that activation of ERK is the main event in hypoxia-mediated up-regulation of SUR2A [20]. We have also recently found that sub-hypoxic drop in oxygen activates ERK to up-regulate SUR2A [19]. However, in the present study, we have shown that in vivo exposure to 18% hypoxia did not affect phosphorylation of ERK1/2 meaning that this kinase does not mediate the effect on SUR2A that we observed. This suggests that different signalling pathway(s) regulate expression of SUR2A in sub-hypoxic decrease in oxygen tension and mild hypoxia.
It has been previously demonstrated that phosphorylation of AMPK and PKB (Akt) mediates cardioprotection. AMPK-mediated cardioprotection was associated with regulation of sarcolemmal KATP channel number and activity [21], while phosphorylation of PKB/Akt was associated with regulation of mitochondrial function [26,27]. None of these kinases were associated with regulation of SUR2A expression so far. Here, we have found that exposure to 18% oxygen did not phosphorylate AMPK, but it phosphorylated Akt. The activation of PI3K/Akt signalling cascade by hypoxia has been previously reported [28] and from this prospective phosphorylation of Akt by hypoxia in vivo was not entirely unexpected. What is interesting is that phosphorylation of S473 was observed alone, while T308 site was not phosphorylated. Phosphorylation of site T308 was previously associated with 3-phosphoinositide-dependent protein kinase-1 (PDK1; ref. [29]), while phosphorylation of the S473 site is a more controversial issue. It has been suggested that mammalian target of rapamycin (mTOR) complex 2 (mTORC2) is responsible for S473 phosphorylation and that this requires assistance of PKCε (epsilon isoform of protein kinase-C; ref. [30]). PKCε has been already shown to be activated by hypoxia in vivo where it acts in a cardioprotective way [17], which is in agreement with our notion that hypoxia phosphorylates Akt at S473. A lack of phosphorylation at the T308 site would suggest that PDK1 is probably not involved in the signalling pathway we observed here, which is also compatible with our previous study showing that the primary target of PDK1-mediated cardioprotection are mitochondria [26]. A simultaneous phosphorylation of S473 Akt and up-regulation of SUR2A did not necessarily mean that these two events are connected. Therefore, to test a causal relationship between Akt and SUR2A, we have used an inhibitor of PI3K, LY294002 [22]. We found that this compound inhibited S473 phosphorylation as well as up-regulation of SUR2A and cardioprotection induced by 18% oxygen in vivo. This demonstrates a causal relationship between S473Akt phosphorylation and SUR2A levels.
We had also taken advantage of LY294002 to assess whether up-regulation of LDH and increase in lactate level has anything to do with Akt phosphorylation. LY294002 inhibited the effect of 18% oxygen on LDH and lactate suggesting that 1) activation of PI3K/Akt signalling cascade is required for up-regulation of LDH and that 2) increased level of lactate is most likely a consequence of increased level of LDH. These findings provided a link between PI3K/Akt and LDH expression. Taking these into account, the question whether LDH/lactate and SUR2A are linked naturally arises.
To address this question we have tested whether LDH/lactate could regulate levels of SUR2A in a different cellular system: rat heart embryonic H9c2 cell line. These cells have been used with success to study expression of SUR2A [7–9,31]. We have used sodium oxamate (structural analogue of pyruvate) and galloflavin (a novel highly selective inhibitor of LDH), two compounds known to inhibit LDH [32,33]. We found that these inhibitors significantly decreased the level of SUR2A in H9c2 cells showing that LDH activity regulates SUR2A levels. Based on these findings we supposed that lactate levels controlled by LDH regulate SUR2A expression. To test this hypothesis we have treated cells with a concentration of sodium lactate that increased lactate levels without affecting LDH activity. Surprisingly, this treatment did not affect SUR2A levels suggesting that lactate does not mediate the effect of LDH. That the LDH activity is crucial in cellular response to stress was further confirmed by our finding that an inactive mutant form of LDH significantly increased sensitivity of H9c2 towards DNP. So, how is it possible that LDH could regulate levels of SUR2A independently from the lactate production? Recently, it has been suggested that LDH can bind to DNA and regulate RNA synthesis in a process that is unrelated to LDH property to catalyse lactate production [34]. Such effect of LDH could mediate regulation of SUR2A levels by LDH.
Taking all these together, it is possible to suggest a signalling cascade that mediates up-regulation of SUR2A in the heart of mice exposed to 18% oxygen in vivo. 18% oxygen activates PI3K/Akt signalling cascade that up-regulates LDH. Increase in LDH increases level of SUR2A independently from increased lactate production. This is the first account of SUR2A-mediated regulation by Akt and LDH.
5. Conclusion
In conclusion, this study has shown that mild hypoxia (18%) up-regulates cardioprotective SUR2A by activating PI3K/Akt signalling cascade that targets LDH to increase SUR2A independently from its property to catalyse lactate production.
Conflict of interest
The authors have declared that there is no conflict of interest.
Funding
This research was supported by the British Heart Foundation (PG/11/106/29235).
References
- 1.Burke M.A., Mutharasan R.K., Ardehali H. The sulfonylurea receptor, an atypical ATP-binding cassette protein, and its regulation of the KATP channel. Circ. Res. 2008;102:164–176. doi: 10.1161/CIRCRESAHA.107.165324. [DOI] [PubMed] [Google Scholar]
- 2.Du Q., Jovanović S., Clelland A., Sukhodub A., Budas G.R., Phelan K., Murray-Tait V., Malone L., Jovanović A. Overexpression of SUR2A generates a cardiac phenotype resistant to ischaemia. FASEB J. 2006;20:1131–1141. doi: 10.1096/fj.05-5483com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukhodub A., Du Q., Jovanović S., Jovanović A. Nicotinamide-rich diet protects the heart against ischaemia–reperfusion in mice: a crucial role for cardiac SUR2A. Pharmacol. Res. 2010;61:564–570. doi: 10.1016/j.phrs.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sukhodub A., Sudhir R., Du Q., Jovanović S., Reyes S., Jovanović A. Nicotinamide-rich diet improves physical endurance by upregulating SUR2A in the heart. J. Cell. Mol. Med. 2011;15:1703–1712. doi: 10.1111/j.1582-4934.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudhir R., Sukhodub A., Du Q., Jovanović S., Jovanović A. Ageing-induced decline in physical endurance in mice is associated with decrease in cardiac SUR2A and increase in cardiac susceptibility to metabolic stress: therapeutic prospects for up-regulation of SUR2A. Biogerontology. 2011;12:147–155. doi: 10.1007/s10522-010-9306-3. [DOI] [PubMed] [Google Scholar]
- 6.Ranki H.J., Budas G.R., Crawford R.M., Jovanović A. Gender-specific difference in cardiac ATP-sensitive K+ channels. J. Am. Coll. Cardiol. 2001;38:906–915. doi: 10.1016/s0735-1097(01)01428-0. [DOI] [PubMed] [Google Scholar]
- 7.Jovanović S., Du Q., Sukhodub A., Jovanović A. M-LDH physically associated with sarcolemmal KATP channels mediates cytoprotection in heart embryonic H9C2 cells. Int. J. Biochem. Cell Biol. 2009;41:2295–2301. doi: 10.1016/j.biocel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jovanović S., Du Q., Sukhodub A., Jovanović A. Dual mechanism of cytoprotection afforded by M-LDH in embryonic heart H9C2 cells. Biochem. Biophys. Acta Mol. Cell. Res. 2009;1793:1379–1386. doi: 10.1016/j.bbamcr.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Q., Jovanović S., Sukhodub A., Jovanović A. Infection with AV-SUR2A protects H9C2 cells against metabolic stress: a mechanism of SUR2A-mediated cytoprotection independent from the KATP channels activity. Biochem. Biophys. Acta Mol. Cell. Res. 2010;1803:405–415. doi: 10.1016/j.bbamcr.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curtis M.J. Emerging druggable targets for cardiovascular disease. Curr. Opin. Pharmacol. 2009;9:81–83. doi: 10.1016/j.coph.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Jovanović A., Jovanović S. SUR2A targeting for cardioprotection? Curr. Opin. Pharmacol. 2009;9:189–193. doi: 10.1016/j.coph.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Jovanović S., Mohammed Abdul K.S., Jovanović A. Cardioprotection afforded by SUR2A: an update. J. Cardiobiol. 2013;1:4. [Google Scholar]
- 13.Mortimer E.A., Monson R.R., MacMahon B. Reduction in mortality from coronary heart disease in men residing at high altitude. N. Engl. J. Med. 1977;296:581–585. doi: 10.1056/NEJM197703172961101. [DOI] [PubMed] [Google Scholar]
- 14.Ezzati M., Horwitz M.E., Thomas D.S., Friedman A.B., Roach R., Clark T., Murray C.J.L., Honigman B. Altitude, life expectancy and mortality from ischaemic heart disease, stroke, COPD and cancers: national population-based analysis of US counties. J. Epidemiol. Community Health. 2012;66:e17. doi: 10.1136/jech.2010.112938. [DOI] [PubMed] [Google Scholar]
- 15.Hurtado A., Escudero E., Pando J., Sharma S., Johnson R.J. Cardiovascular and renal effects of chronic exposure to high altitude. Nephrol. Dial. Transplant. 2012;27(Suppl. 4):iv11–iv16. doi: 10.1093/ndt/gfs427. [DOI] [PubMed] [Google Scholar]
- 16.Faeh D., Gutzwiller F., Bopp M. Lower mortality from coronary heart disease and stroke at higher altitudes in Switzerland. Circulation. 2009;120:495–501. doi: 10.1161/CIRCULATIONAHA.108.819250. [DOI] [PubMed] [Google Scholar]
- 17.Tajima M., Katayose D., Bessho M., Isoyama S. Acute ischaemic preconditioning and chronic hypoxia independently increase myocardial tolerance to ischaemia. Cardiovasc. Res. 1994;28:312–319. doi: 10.1093/cvr/28.3.312. [DOI] [PubMed] [Google Scholar]
- 18.Berger M.M., Huhn R., Oei G.T., Heinen A., Winzer A., Bauer I., Preckel B., Weber N.C., Schlack W., Hollmann M.W. Hypoxia induces late preconditioning in the rat heart in vivo. Anesthesiology. 2010;113:1351–1360. doi: 10.1097/ALN.0b013e3181fce7ea. [DOI] [PubMed] [Google Scholar]
- 19.Mohammed Abdul K.S., Jovanovic S., Du Q., Sukhodub A., Jovanovic A. Upregulation of cardioprotective SUR2A by sub-hypoxic drop in oxygen. Biochem. Biophys. Acta Mol. Cell. Res. 2014;1843:2424–2431. doi: 10.1016/j.bbamcr.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crawford R.M., Jovanović S., Budas G.R., Davies A.M., Lad H., Wenger R.H., Robertson K.A., Roy D.J., Ranki H.J., Jovanović A. Chronic mild hypoxia protects heart-derived H9c2 cells against acute hypoxia/reoxygenation by regulating expression of the SUR2A subunit of the ATP-sensitive K+ channels. J. Biol. Chem. 2003;278:31444–31455. doi: 10.1074/jbc.M303051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sukhodub A., Jovanović S., Du Q., Budas G.R., Clelland A., Shen M., Sakamoto K., Tian R., Jovanović A. AMP-activated protein kinase mediates preconditioning in cardiomyocytes by regulating activity and trafficking of sarcolemmal ATP-sensitive K+ channels. J. Cell. Physiol. 2007;210:224–236. doi: 10.1002/jcp.20862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlahos C.J., Matter W.F., Hui K.Y., Brown R.F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J. Biol. Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 23.Essop M.F. Cardiac metabolic adaptations in response to hypoxia. J. Physiol. Lond. 2007;584:715–726. doi: 10.1113/jphysiol.2007.143511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jovanović S., Jovanović A. Models of cardioprotection. Drug Discov. Today Dis. Models. 2007;4:185–190. [Google Scholar]
- 25.McClelland G.B., Brooks G.A. Changes in MCT 1, MCT 4, and LDH expression are tissue specific in rats after long-term hypobaric hypoxia. J. Appl. Physiol. 2004;92:1573–1584. doi: 10.1152/japplphysiol.01069.2001. [DOI] [PubMed] [Google Scholar]
- 26.Budas G.R., Sukhodub A., Alessi D.R., Jovanovic A. 3′phosphoinositide-dependent kinase-1 (PDK1) is essential for ischaemic preconditioning of the myocardium. FASEB J. 2006;20:2556–2558. doi: 10.1096/fj.06-6252fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hausenloy D.J., Yellon D.M. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc. Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 28.Alvarez-Tejado M., Naranjo-Suarez S., Jimenez C., Carrera A.C., Landazuri M.O., del Peso L. Hypoxia induces the activation of the phosphatidylinositol 3-kinase/Akt cell survival pathway in PC12 cells. Protective role in apoptosis. J. Biol. Chem. 2001;276:22368–22374. doi: 10.1074/jbc.M011688200. [DOI] [PubMed] [Google Scholar]
- 29.Hemmings B.A., Restuccia D.F. PI3K-PKB/Akt pathway. Cold Spring Harb. Perspect. Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vadlakonda L., Dash A., Pasupuleti M., Anil Kumar K., Reddanna P. The paradox of Akt-mTOR interactions. Front. Oncol. 2013;3:165. doi: 10.3389/fonc.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballantyne T., Du Q., Jovanovic S., Neemo A., Holmes R., Shiba S., Jovanovic A. Testosterone protects female embryonic heart H9C2 cells against severe metabolic stress by activating estrogen receptors and up-regulating IES SUR2B. Int. J. Biochem. Cell Biol. 2013;45:283–291. doi: 10.1016/j.biocel.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson J.H., Walter S.J. Oxamate as a differential inhibitor of lactate dehydrogenase isoenzymes. Enzyme. 1972;13:170–176. doi: 10.1159/000459658. [DOI] [PubMed] [Google Scholar]
- 33.Farabegoli F., Vettraino M., Manerba M., Fiume L., Roberti M., Di Stefano G. Galloflavin, a new lactate dehydrogenase inhibitor, induces the death of human breast cancer cells with different glycolytic attitude by affecting distinct signaling pathways. Eur. J. Pharm. Sci. 2012;47:729–738. doi: 10.1016/j.ejps.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 34.Fiume L., Vettriano M., Carnicelli D., Arfilli V., Di Stefano G., Brigotti M. Galloflavin prevents the binding of lactate dehydrogenase A to single stranded DNA and inhibits RNA synthesis in cultured cells. Biochem. Biophys. Res. Commun. 2013;430:466–469. doi: 10.1016/j.bbrc.2012.12.013. [DOI] [PubMed] [Google Scholar]