Abstract
One-dimensional ZnO nanowires (NWs) are a promising materials system for a variety of applications. Utilization of ZnO, however, requires a good understanding and control of material properties that are largely affected by intrinsic defects and contaminants. In this work we provide experimental evidence for unintentional incorporation of nitrogen in ZnO NWs grown by rapid thermal chemical vapor deposition, from electron paramagnetic resonance spectroscopy. The incorporated nitrogen atoms are concluded to mainly reside at oxygen sites (NO). The NO centers are suggested to be located in proximity to the NW surface, based on their reduced optical ionization energy as compared with that in bulk. This implies a lower defect formation energy at the NW surface as compared with its bulk value, consistent with theoretical predictions. The revealed facilitated incorporation of nitrogen in ZnO nanostructures may be advantageous for realizing p-type conducting ZnO via N doping. The awareness of this process can also help to prevent such unintentional doping in structures with desired n-type conductivity.
ZnO has a wide and direct band gap (~3.3 eV at room temperature) and a large exciton binding energy (60 meV at room temperature). It can be easily synthesized in both bulk, single-crystal form and also in a diverse group of nanostructure morphologies. In addition, this material is nontoxic, sustainable and cheap. These attributes make ZnO a very promising material for a wide variety of applications including photonics, electronics, sensing and energy harvesting1,2,3,4,5. In particular, one-dimensional ZnO nanowires (NWs) have recently attracted a substantial scientific and technological interest for realization of gas sensors and ultraviolet (UV) optoelectronic devices, such as light emitting diodes (LEDs), solar cells, and photo detectors5,6,7,8. All these applications, however, require a good understanding and precise control of optical and electrical properties of the material that are known to be largely affected by intrinsic defects and impurities. Zinc vacancies (VZn) for instance, which have the lowest formation energy among all intrinsic defects in n-type ZnO9,10,11, can form detrimental complex centers with group III donors (e.g. Al), thus limiting the n-type doping efficiency12,13,14. On the other hand, they can also facilitate energy upconversion in ZnO15,16, attractive for photovoltaic applications. Therefore, depending on the intended outcome, defects can be vital or fatal17,18.
Another group of defects that are important for understanding electrical properties of ZnO involve defects containing nitrogen atoms. Nitrogen has been long considered as one of the most promising candidates for p-type doping in ZnO. Interestingly, even though it has already been proven that nitrogen substituting for oxygen (NO)19,20,21,22,23 does not create a shallow acceptor state, p-type ZnO using nitrogen as a dopant has been commonly reported24,25,26. So far, it is still under discussion how nitrogen doping leads to the formation of shallow acceptors in ZnO. Here one needs to keep in mind that the doping processes can be largely affected not only by growth conditions and type/concentration of the utilized dopants, but also residual contamination of the source material or even the background gases in the growth chamber27. For example, it was reported that p-type conductivity in ZnO could be promoted in nanostructured materials25,28,29. Though the exact physical mechanism responsible for this effect remains unknown, it could be caused by formation of complexes with residual defect/impurities. Indeed, the energy level of the N acceptor can be affected by its local surrounding30, complexing with residual group III impurities31 and defects32,33, as well as formation of N-N molecules34. Moreover, growth conditions utilized during the NW growth may facilitate dopant incorporation leading to higher dopability of these materials. In this letter we employ magnetic resonance spectroscopy35 to investigate defect formation processes in nominally undoped ZnO NWs grown by chemical vapor deposition, aiming to single out chemical origin of incorporated contaminants.
Results and Discussion
Figure 1(a) shows a representative scanning electron microscopy (SEM) image of the investigated ZnO NWs. A magnified image from a single NW is shown in Fig. 1(b). Most of the NWs are found to be vertically aligned along the crystallographic [0001] axis and exhibit a uniform size distribution with an average length and diameter of 30 μm and 100 nm, respectively. Some of the NWs are, however, randomly tilted by up to 20°. The excellent structural quality was further confirmed from performed x-ray diffraction (XRD) experiments, The XRD spectra of the ZnO NWs contain four peaks which can be assigned to ZnO (001) and (002) reflexes, Au and Al2O3 - see Fig. 1(c). The latter two reflexes are expected since the NWs were grown on an Al2O3 substrate and Au was used as a catalyst. The XRD results confirm that the NWs are preferentially oriented along the crystallographic c-axis ([0001] direction), since the calculated d-spacing of the major reflex (2θ = 34.4°, d = 0.261 nm) matches that expected for hexagonal ZnO. The full width at half maximum (FWHM) of the main ZnO reflex was found to be 0.27°, indicative of the high quality ZnO.
Figure 1.
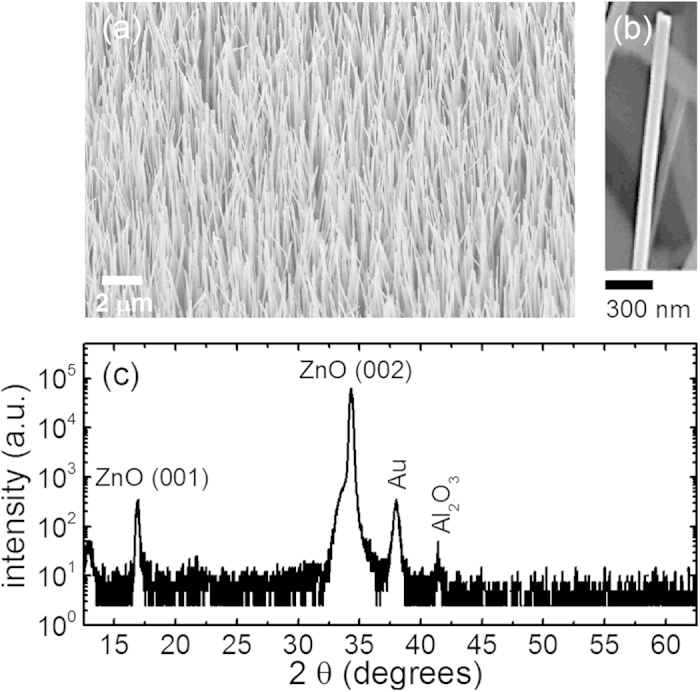
(a) An SEM overview image of the studied ZnO NWs under a tilted view of 45°. (b) a magnified SEM image of a single ZnO NW. (c) XRD diffractogram of the studied ZnO NWs.
Figure 2 depicts EPR spectra of the ZnO NW arrays measured in dark (a) and under white light illumination (b). In the dark, two single-line EPR signals labeled as A and B in Fig. 2 can be observed. White light illumination leads to appearance of another EPR signal (labeled as C) that consists of three equally spaced lines, which is characteristic for a resolved hyperfine interaction involving a nucleus spin I = 1 with natural abundance of 100%.
Figure 2.
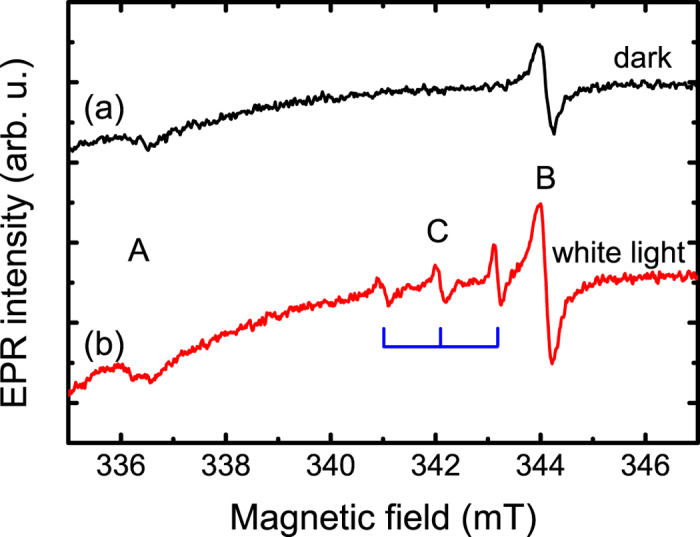
EPR spectra of the studied ZnO NWs measured at 4 K and a microwave frequency of 9.4 GHz in the dark (a) and under white light illumination (b) with an applied magnetic field rotated away from the NW growth axis ([0001], i.e. the c - axis) by Θ = 70°.
In order to identify the observed EPR signals we performed angular dependent measurements under light illumination by rotating the sample from an orientation with the NWs aligned parallel to a static magnetic field (B), i.e. Θ = 0°, towards Θ = 90°. In the latter case B is oriented perpendicularly to the [0001] NWs axis. Figure 3(a) shows representative results from these measurements, taking as an example EPR spectra measured with Θ of 10°, 70° and 90°. An anisotropic behavior for signals B and C can be clearly observed, while signal A is isotropic. By using a spin-Hamiltonian operator in the form of
Figure 3.
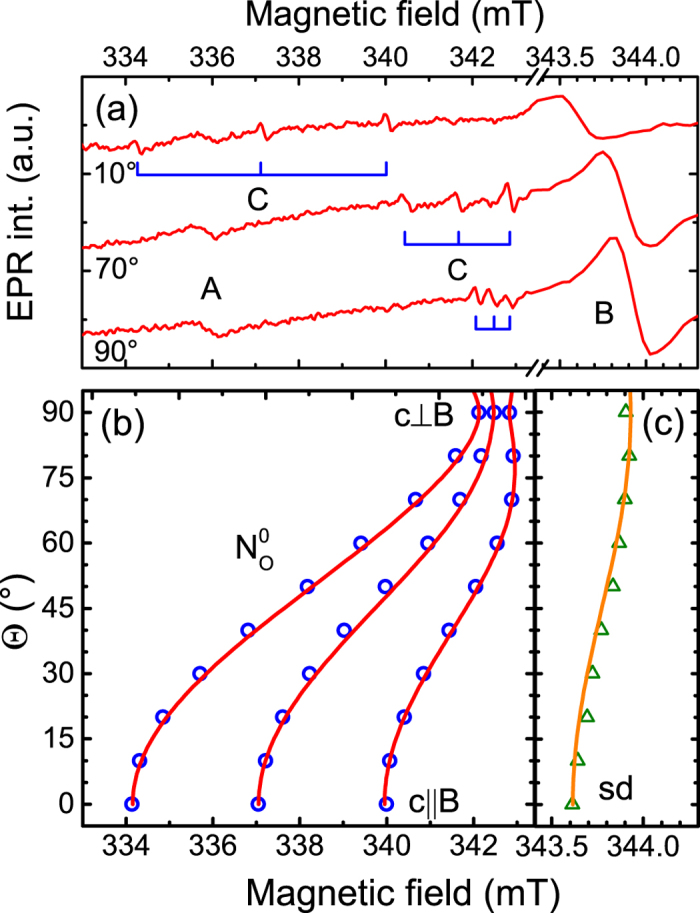
(a) EPR spectra of the ZnO NWs measured at 4 K under white light illumination with an applied magnetic field rotated away from the NW growth axis ([0001], i.e. the c - axis) by Θ = 10°, 70° and 90°. (b) Angular dependent plot of the NO EPR field positions (the open circles) and the best fit using the spin-Hamiltonian described in the text (the solid lines). (c) Anisotropic behavior of signal B, i.e. the shallow donor (sd) EPR field postions (the open triangles) obtained by rotating a magnetic field direction from B parallel to c to B perpendicular to c. The solid line is the best fit obtained by using the spin-Hamiltonian described in the text. (Note different x-axis scales in (b,c)).
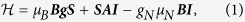 |
i.e. including the Zeeman energy (the first term), the hyperfine interaction (the second term) and the nuclear Zeeman interaction (the third term), we can determine spin-Hamiltonian parameters of the observed EPR signals. Here we neglect the quadrupole interaction for nuclei with I > 1/2, since the EPR signal intensity of the forbidden transitions arising from the quadrupole interaction is too weak to determine its interaction tensor. It is found that signal A has an electron spin S = 1/2 and an isotropic g-value of 2.002. Within the experimental error, this value is identical to the free electron g-value ge = 2.0023. We, therefore, assign this signal to dangling bonds on the surface of the NWs36,37, which seems to be very likely considering a large surface-to-volume ratio in the NWs. Signal B has an electron spin S = 1/2 and a slightly anisotropic g-tensor with g|| = 1.957 and g^ = 1.955 (see Fig. 3(c)), where parallel and perpendicular orientations are given with respect to the crystallographic c-axis. Such g-values are typical for shallow effective mass donors in ZnO35. Judging from the determined g-values, this shallow donor is most likely caused by unintentionally incorporated hydrogen38. Unfortunately the corresponding hyperfine interaction (1.4 MHz) is too small to be resolved in the X-band EPR experiments conducted in this work.
Let us now discuss the chemical origin of signal C. First of all we note that it must arise from a chemical element that has an isotope with a nuclear spin I = 1 and nearly 100% natural abundance. The only element in the periodic table which satisfies this requirement is nitrogen as the 14N isotope has I = 1 and 99.6% natural abundance. Moreover, signal C exhibits strongly anisotropic behavior with an angular dependent g-value and the hyperfine interaction parameter A. This can also be seen from Fig. 3(b) where the dependence of signal C on the orientation of the external magnetic field is depicted. By fitting the experimental data with the spin-Hamiltonian given by Eq. (1) we obtain g|| = 1.996, g^ = 1.963, A|| = 82.3 MHz and A^ = 9.7 MHz. The determined spin-Hamiltonian parameters are very similar to those reported in the literature for the substitutional NO center in bulk ZnO20,39. We therefore identify signal C as arising from a nitrogen atom substituting oxygen. In bulk, a typical linewidth ΔB of the NO EPR lines is about 0.03 mT and slightly deviates between the lines, which can be explained by inhomogeneity of the g-values and hyperfine interaction39. An additional broadening (up to 0.2 mT) of the C lines in the studied NWs is likely caused by imperfect perpendicular alignments of the NWs relative to the substrate surface, evident from their random tilt directions and angles up to 20° from the vertical direction as revealed from the performed scanning electron microscopy measurements40.
The energy level position of the NO center in the ZnO NWs can be determined from photo-EPR measurements. The measured spectral dependence of the NO EPR signal on the excitation photon energy is shown by the open circles in Fig. 4(a). We can see that the NO center can be converted into its paramagnetic state 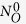 only when the photon energies exceed 1.75 eV. The recharging process can be further understood by analyzing temporal behavior of the NO signal after switching on the light. The corresponding results are shown in Fig. 4(b), where the y-axis displays the difference of the measured EPR intensity (I) from the saturation value (I∞) shown in a logarithmic scale. The linear slope shown by the solid line indicates a mono-exponential process due to direct recharging of the studied center without involvement of other defects. Moreover, since light illumination within the same spectral range also causes an increase of the shallow donor EPR signal (see Fig. 2), the recharging process results in photo-ionization involving the conduction band. This means that the light-induced conversion of the NO center into its paramagnetic charge state occurs as a result of the process
only when the photon energies exceed 1.75 eV. The recharging process can be further understood by analyzing temporal behavior of the NO signal after switching on the light. The corresponding results are shown in Fig. 4(b), where the y-axis displays the difference of the measured EPR intensity (I) from the saturation value (I∞) shown in a logarithmic scale. The linear slope shown by the solid line indicates a mono-exponential process due to direct recharging of the studied center without involvement of other defects. Moreover, since light illumination within the same spectral range also causes an increase of the shallow donor EPR signal (see Fig. 2), the recharging process results in photo-ionization involving the conduction band. This means that the light-induced conversion of the NO center into its paramagnetic charge state occurs as a result of the process
Figure 4.
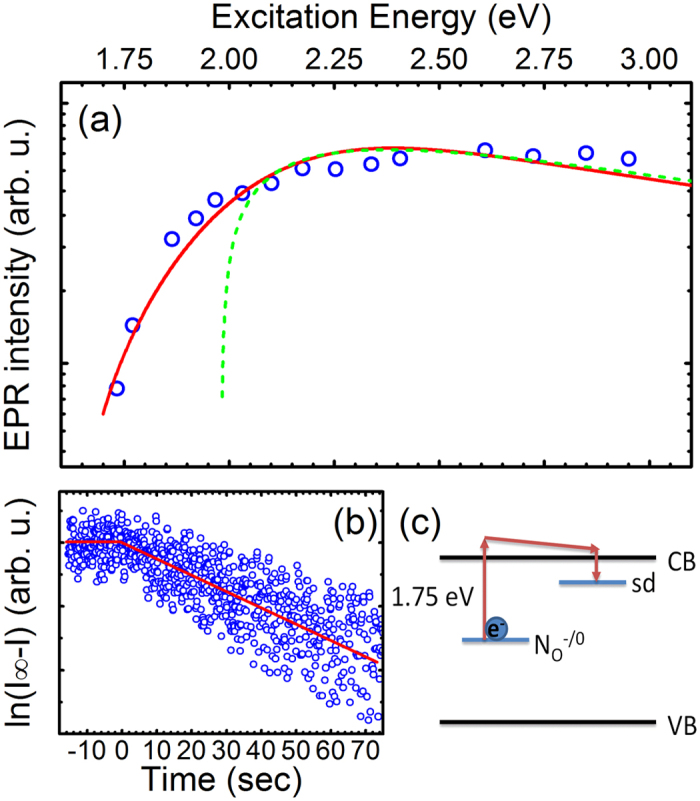
(a) EPR intensity of the NO signal (open circles) as a function of the light excitation energy for the studied ZnO NWs and bulk ZnO (dashed line). The solid line is a guide to the eye. (b) Time dependent behavior of the NO signal after switching on the light. The y-axis displays the difference of the measured EPR intensity (I) from the saturation value (I∞) shown in a logarithmic scale. The linear slope shown by the solid line indicates a mono-exponential process due to direct recharging. (c) Illustration of the NO photo-EPR recharging process. The notation “sd” denotes the shallow donor center.
 |
as schematically shown in Fig. 4(c).
It is interesting to note that the photo-ionization threshold energy in the NWs differs from that in bulk ZnO where the threshold energy is found to be at around 2 eV (see the dashed curve in Fig. 4(a)), which is in agreement with previous reports20. One possible explanation for this deviation is that, unlike in bulk ZnO, the NO centers in the NWs are not distributed uniformly through the volume but are located in proximity to the NW surface. (The possibility of the NO centers to be directly located on the NW surface can be excluded, however, since this would drastically alter the spin-Hamiltonian parameters of the centers, which is not the case). In fact previous theoretical studies41,42 have concluded that the ionization energies of donor and acceptor impurities are strongly enhanced in nanowires with respect to their values in bulk. Moreover, due to surface band bending43 optical ionization of the 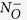 center within the near-surface region can occur not only due to spatially direct, but also spatially indirect optical transitions. In addition, the electron-phonon interactions for the
center within the near-surface region can occur not only due to spatially direct, but also spatially indirect optical transitions. In addition, the electron-phonon interactions for the  center, which significantly affect the photoionization edge, could be altered in the proximity to the surface. Both effects will lead to an overall broadening of the ionization edge, as is observed experimentally. This suggestion is also consistent with the theoretical results of Gutjahr et al.44 and Haffad et al.45, which predicted that incorporation of nitrogen in ZnO is more energetically favorable at (or close to) the surface than in volume regions. This is expected to result in a higher efficiency of N doping in nanostructures with a high surface-to-volume ratio. Gutjahr et al.44 have explained the decrease in the formation energy by charge transfer between Zn dangling bonds on the surface and the NO impurities. Existence of such dangling bonds on the surface of the studied NWs is indeed confirmed by the observation of signal A in our EPR measurements.
center, which significantly affect the photoionization edge, could be altered in the proximity to the surface. Both effects will lead to an overall broadening of the ionization edge, as is observed experimentally. This suggestion is also consistent with the theoretical results of Gutjahr et al.44 and Haffad et al.45, which predicted that incorporation of nitrogen in ZnO is more energetically favorable at (or close to) the surface than in volume regions. This is expected to result in a higher efficiency of N doping in nanostructures with a high surface-to-volume ratio. Gutjahr et al.44 have explained the decrease in the formation energy by charge transfer between Zn dangling bonds on the surface and the NO impurities. Existence of such dangling bonds on the surface of the studied NWs is indeed confirmed by the observation of signal A in our EPR measurements.
Of course the formation energy of NO in bulk and nanostructured ZnO is dependent on the growth conditions (O-rich or Zn-rich) and the Fermi level position, which is strongly affected by the dopant concentration. However, these dependencies are known from ab initio density functional theory (DFT) calculations22,23. For examples, the formation energy scales linearly with the Fermi level position (determined by the dopant concentration) under fixed growth conditions (Zn- or O-rich), since effects of lattice relaxation at doping concentration lower than 6.06% are negligible45. Therefore, our conclusion of the reduced formation energy of the NO centers in the NWs, though obtained for low-doped ZnO, should remain valid even for higher doping concentrations.
The remaining and somewhat puzzling questions are what are the sources and mechanism responsible for the revealed unintentional doping of the ZnO NWs by nitrogen. The studied structures were grown by chemical vapor deposition in an Ar ambient using gold as a catalyst. Therefore, nitrogen atoms were most likely supplied by contaminations in the used Ar2 and O2 source gases and/or by residual background N2 gas. Both source gases had a purity of 6N (99.9999%) and hence can contain up to 1 ppm N2. The contamination of the residual background gas most likely stems from venting the growth chamber with N2 gas after the deposition of the gold catalyst on the sapphire substrate prior to the growth of the NWs. We believe that the N2 contamination from the used Ar2 and O2 source gases dominates, since defect incorporation due to contaminations of the used source gases is a known phenomenon in RTCVD processes46,47 and was shown to prevail over contamination by residual chamber background gases46. According to previous studies27,48, nitrogen molecules can be dissolved easily in molten metals and subsequently split into atomic nitrogen. This process happens when metal is heated to high temperatures (TGrowth = 950 °C) under the presence of molecular nitrogen gas. Under these conditions nitrogen molecules permeate into the metal and nitridation proceeds by dissociation of nitrogen molecules and absorption of nitrogen atoms by the metal48. Taking into account the large surface area of the gold catalyst during the growth, this process can be rather efficient. The dissolved atomic nitrogen could then incorporate and diffuse (comment: diffusion may happen but is not necessary, as the Au droplet is floating on the surface of the growing NWs) into the growing NWs and predominantly reside at oxygen sites giving rise to the NO EPR signal. An illustration of the suggested process of nitrogen incorporation into the ZnO NWs is shown in Fig. 5. We would like to note that considering a limited amount of nitrogen in the growth chamber (only a few ppm), the dopant incorporation efficiency during the revealed doping process must be very high. Indeed, the concentration of the NO centers estimated based on the EPR signal intensity is about 2–4 × 1016 cm−3, which is only by about one order of magnitude lower than the deduced concentration of the residual shallow donors (signal B) of 2–4 × 1017 cm−3 in the NWs. The low formation energy of the substitutional nitrogen center in ZnO nanostructures is advantageous for the synthesis of p-type conductive via nitrogen doping, e.g. due to N-containing defect complexes or nitrogen molecules. The awareness of this process can also help to prevent such unintentional doping in structures where n-type conductivity is desired, as NO can act as a compensation center in n-type ZnO. Unfortunately, it is not possible to directly compare the efficiency of the NO incorporation during the employed growth with that achieved previously in intentionally N-doped ZnO NWs, since the information on the N concentration in the wires was not provided in most of the earlier reports27,49,50. Yuan et al.24 estimated that the carrier concentration in their N-doped ZnO NWs grown by chemical vapor deposition (CVD) is ~1 × 1018 cm−3. However, the exact configuration of the incorporated nitrogen (NO, N-molecules or N-complexes) and its concentration were not determined.
Figure 5.
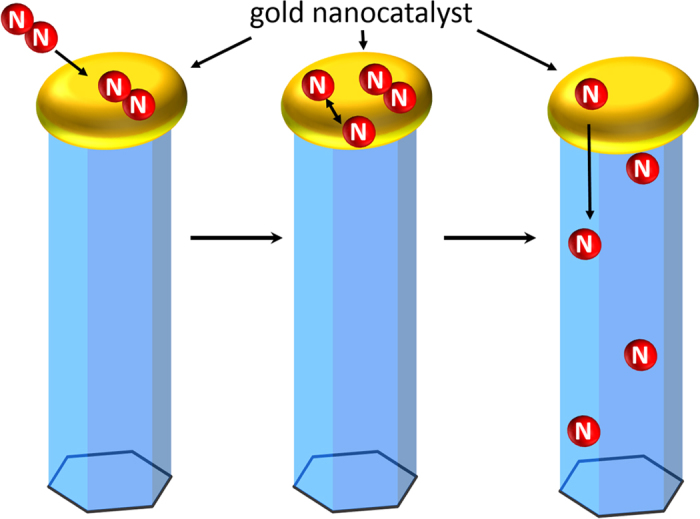
Schematic illustration of the nitrogen incorporation into the ZnO NWs from the residual background gases in the growth chamber that contain N2.
Conclusion
In summary, we have provided an unambiguous experimental proof for efficient unintentional doping with nitrogen in ZnO NWs during the RTCVD growth process. Based on our results from the detailed EPR measurements, incorporated nitrogen is shown to reside at oxygen sites forming a substitutional NO acceptor. Based on the lower photo-ionization threshold energy of the NO center in the ZnO NWs as compared with its value in bulk ZnO, the defect is suggested to be located in proximity to the surface. This assumption is consistent with theoretical predictions44,45 of enhanced N incorporation at or close to the ZnO surface. The revealed doping process is shown to be very efficient leading to a rather high concentration (~2–4 × 1016 cm−3) of unintentional N dopants in the NWs even from the contamination by the source and background gases. Our finding thus underlines the importance of controlling such background contamination as compensation by the NO acceptors may hinder achieving high n-type conductivity in nanostructured ZnO. On the other hand, the lowered formation energy of NO in NWs might be beneficial for achieving p-type conducting ZnO nanostructures via nitrogen doping, e.g. due to N-containing defect complexes or nitrogen molecules.
Methods
The ZnO NWs were grown on gold coated c-plane sapphire substrates by using rapid thermal chemical vapor deposition (RTCVD). The growth was performed at a growth temperature of 950 °C under pressure of 20 Torr with Ar2 and O2 flows of 100 and 2 sccm, respectively40. The Au catalyst had a thickness of about 3 nm and was deposited using e-beam evaporation. Most of the NWs are vertically aligned along the crystallographic [0001] axis and exhibit a uniform size distribution with an average length and diameter of 30 μm and 100 nm, respectively. Some of the NWs are, however, randomly tilted by up to 20°.
EPR experiments were performed at a microwave frequency of 9.4 GHz (X-band) at a temperature of 4.2 K. To perform photo-EPR measurements, a Xenon lamp was used as an excitation source. Appropriate long-pass and short-pass filters were utilized to select specific illumination wavelengths whereas neutral density filters were inserted to ensure the same excitation power at all chosen wavelengths. To avoid effects of ambient illumination and to guarantee the same initial conditions, the sample was cooled down each time in the dark before illumination. Time-dependent photo-EPR measurements were performed at a constant magnetic field corresponding to the EPR peak position and the EPR signal strength was monitored as a function of time after switching on the light.
Additional Information
How to cite this article: Stehr, J. E. et al. Efficient nitrogen incorporation in ZnO nanowires. Sci. Rep. 5, 13406; doi: 10.1038/srep13406 (2015).
Acknowledgments
Financial support by the Swedish Research Council(VR) (Grant No. 621-2010-3971), Linköping Linnaeus Initiative for Novel Functional Materials (LiLI-NFM) supported by the Swedish Research Council (contract number 2008-6582), the ÅForsk Foundation (Grant No. 15-433) and World-Class University Program through NRF grant funded by the Korea government (MEST) (grant # R31-10026) is greatly appreciated.
Footnotes
Author Contributions N.K.R. grew the samples under supervision of C.W.T. J.E.S. performed the EPR experiments and analyzed the data. J.E.S., W.M.C. and I.A.B. wrote the manuscript.
References
- Özgür U. et al. Comprehensive Review of ZnO Materials and Devices. J. Appl. Phys. 98, 41301 (2005). [Google Scholar]
- Pearton S. J. & Ren F. Advances in ZnO-Based Materials for Light Emitting Diodes. Curr. Opin. Chem. Eng. 3, 51–55 (2014). [Google Scholar]
- Xiong H.-M. ZnO Nanoparticles Applied to Bioimaging and Drug Delivery. Adv. Mater. 25, 5329–5335 (2013). [DOI] [PubMed] [Google Scholar]
- Jing Z. & Zhan J. Fabrication and Gas-Sensing Properties of Porous ZnO Nanoplates. Adv. Mater. 20, 4547–4551 (2008). [Google Scholar]
- Law M., Greene L. E., Johnson J. C., Saykally R. & Yang P. Nanowire Dye-Sensitized Solar Cells. Nat. Mater. 4, 455–459 (2005). [DOI] [PubMed] [Google Scholar]
- Xu S. et al. Ordered Nanowire Array Blue/near-UV Light Emitting Diodes. Adv. Mater. 22, 4749–4753 (2010). [DOI] [PubMed] [Google Scholar]
- Gogurla N., Sinha A. K., Santra S., Manna S. & Ray S. K. Multifunctional Au-ZnO Plasmonic Nanostructures for Enhanced UV Photodetector and Room Temperature NO Sensing Devices. Sci. Rep. 4, 6483 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantachaisilp S. et al. Tailoring Deep Level Surface Defects in ZnO Nanorods for High Sensitivity Ammonia Gas Sensing. J. Phys. Chem. C 118, 27150–27156 (2014). [Google Scholar]
- Janotti A. & Van de Walle C. G. Fundamentals of Zinc Oxide as a Semiconductor. Reports Prog. Phys. 72, 126501 (2009). [Google Scholar]
- Clark S. J., Robertson J., Lany S. & Zunger A. Intrinsic Defects in ZnO Calculated by Screened Exchange and Hybrid Density Functionals. Phys. Rev. B 2010, 81, 115311 [Google Scholar]
- Wang X. J., Vlasenko L. S., Pearton S. J., Chen W. M. & Buyanova I. A. Oxygen and Zinc Vacancies in as-Grown ZnO Single Crystals. J. Phys. D. Appl. Phys. 42, 175411 (2009). [Google Scholar]
- Stehr J. E. et al. Zinc-Vacancy–Donor Complex: A Crucial Compensating Acceptor in ZnO. Phys. Rev. Appl. 2, 021001 (2014). [Google Scholar]
- T-Thienprasert J. et al. Compensation in Al-Doped ZnO by Al-Related Acceptor Complexes: Synchrotron X-Ray Absorption Spectroscopy and Theory. Phys. Rev. Lett. 110, 055502 (2013). [DOI] [PubMed] [Google Scholar]
- Look D. C. et al. Self-Compensation in Semiconductors: The Zn Vacancy in Ga-Doped ZnO. Phys. Rev. B 84, 115202 (2011). [Google Scholar]
- Stehr J. E. et al. Turning ZnO into an Efficient Energy Upconversion Material by Defect Engineering. Adv. Funct. Mater. 24, 3760 (2014). [Google Scholar]
- Chen S. L. et al. Efficient Upconversion of Photoluminescence via Two-Photon Absorption in Bulk and Nanorod ZnO. Appl. Phys. B 108, 919–924 (2012). [Google Scholar]
- Queisser H. J. Defects in Semiconductors: Some Fatal, Some Vital. Science 281, 945–950 (1998). [DOI] [PubMed] [Google Scholar]
- McCluskey M. D. Point Defects in ZnO. Semicond. Semimetals (2015). 10.1016/bs.semsem.2014.11.002 [DOI] [Google Scholar]
- Tarun M. C., Iqbal M. Z. & McCluskey M. D. Nitrogen Is a Deep Acceptor in ZnO. AIP Adv. 1, 022105 (2011). [Google Scholar]
- Stehr J. E., Hofmann D. M. & Meyer B. K. Electron Paramagnetic Resonance and Photo-Electron Paramagnetic Resonance Investigation on the Recharging of the Substitutional Nitrogen Acceptor in ZnO. J. Appl. Phys. 112, 103511 (2012). [Google Scholar]
- Philipps J. M. et al. Recharging behavior of nitrogen-centers in ZnO. J. Appl. Phys. 116, 063701 (2014). [Google Scholar]
- Lyons J. L., Janotti A. & Van de Walle C. G. Why nitrogen cannot lead to p-type conductivity in ZnO. Appl. Phys. Lett. 95, 252105 (2009). [Google Scholar]
- Lany S. & Zunger A. Generalized Koopmans density functional calculations reveal the deep acceptor state of NO in ZnO. Phys. Rev. B 81, 205209 (2010). [Google Scholar]
- Tsukazaki A. et al. Repeated Temperature Modulation Epitaxy for P-Type Doping and Light-Emitting Diode Based on ZnO. Nat. Mater. 4, 42–46 (2004). [Google Scholar]
- Yuan G. D. et al. P-Type ZnO Nanowire Arrays. Nano Lett. 8, 2591–2597 (2008). [DOI] [PubMed] [Google Scholar]
- Lautenschlaeger S. et al. Optical Signatures of Nitrogen Acceptors in ZnO. Phys. Rev. B 85, 235204 (2012). [Google Scholar]
- Soudi A., Khan E. H., Dickinson J. T. & Gu Y. Observation of Unintentionally Incorporated Nitrogen-Related Complexes in ZnO and GaN Nanowires. Nano Lett. 9, 1844–1849 (2009). [DOI] [PubMed] [Google Scholar]
- Herring N. P., Panchakarla L. S. & El-Shall M. S. P-Type Nitrogen-Doped ZnO Nanostructures with Controlled Shape and Doping Level by Facile Microwave Synthesis. Langmuir 30, 2230–2240 (2014). [DOI] [PubMed] [Google Scholar]
- Lu M.-P., Lu M.-Y. & Chen L.-J. P-Type ZnO Nanowires: From Synthesis to Nanoenergy. Nano Energy 1, 247–258 (2012). [Google Scholar]
- Li J., Wei S.-H., Li S.-S. & Xia J.-B. Design of Shallow Acceptors in ZnO: First-Principles Band-Structure Calculations. Phys. Rev. B 74, 081201 (2006). [Google Scholar]
- Yamamoto T. & Katayama-Yoshida H. Solution Using a Codoping Method to Unipolarity for the Fabrication of P-Type ZnO. Japanese J. Appl. Physics, Part 2 Lett. 38, L166 (1999). [Google Scholar]
- Liu L. et al. P-Type Conductivity in N-Doped ZnO: The Role of the NZn-VO Complex. Phys. Rev. Lett. 108, 215501 (2012). [DOI] [PubMed] [Google Scholar]
- Stehr J. E. et al. Defects in N, O and N, Zn Implanted ZnO Bulk Crystals. J. Appl. Phys. 113, 103509 (2013). [Google Scholar]
- Lambrecht W. R. L. & Boonchun A. Identification of a N-Related Shallow Acceptor and Electron Paramagnetic Resonance Center in ZnO: N2 on the Zn Site. Phys. Rev. B 87, 195207 (2013). [Google Scholar]
- Stehr J. E., Meyer B. K. & Hofmann D. M. Magnetic Resonance of Impurities, Intrinsic Defects and Dopants in ZnO. Appl. Magn. Reson. 39, 137–150 (2010). [Google Scholar]
- Cartier E., Stathis J. H. & Buchanan D. A. Passivation and Depassivation of Silicon Dangling Bonds at the Si/SiO 2 Interface by Atomic Hydrogen. Appl. Phys. Lett. 63, 1510–1512 (1993). [Google Scholar]
- Miller D. J. & Haneman D. Electron-Paramagnetic-Resonance Study of Clean and Oxygen-Exposed Surfaces of GaAs, AlSb, and Other III-V Compounds. Phys. Rev. B 3, 2918–2928 (1971). [Google Scholar]
- Hofmann D. et al. Hydrogen: A Relevant Shallow Donor in Zinc Oxide. Phys. Rev. Lett. 88, 045504 (2002). [DOI] [PubMed] [Google Scholar]
- Carlos W. E., Glaser E. R. & Look D. C. Magnetic Resonance Studies of ZnO. Phys. B Condens. Matter. 308-310, 976–979 (2001). [Google Scholar]
- Stehr J. E. et al. Defect Properties of ZnO Nanowires Revealed from an Optically Detected Magnetic Resonance Study. Nanotechnology. 24, 015701 (2013). [DOI] [PubMed] [Google Scholar]
- Diarra M., Niquet Y. M., Delerue C. & Allan G. Ionization energy of donor and acceptor impurities in semiconductor nanowires: Importance of dielectric confinement. Phys. Rev. B. 75, 045301 (2007). [Google Scholar]
- Xu H., Rosa A. L., Frauenheim T. & Zhang R. Q. N-doped ZnO nanowires: Surface segregation, the effect of hydrogen passivation and applications in spintronics. Phys. Status Solidi b. 247, 2195–2201 (2010). [Google Scholar]
- Chen C. Y. et al. Probing Surface Band Bending of Surface-Engineered Metal Oxide Nanowires. ACS Nano. 6, 9366–9372 (2012). [DOI] [PubMed] [Google Scholar]
- Gutjahr J., Sakong S. & Kratzer P. Interplay of Hydrogen Treatment and Nitrogen Doping in ZnO Nanoparticles: A First-Principles Study. Nanotechnology 25, 145204 (2014). [DOI] [PubMed] [Google Scholar]
- Haffad S., Samah M. & Cicero G. Effect of nitrogen impurities on the physical properties of ZnO nanowires: First-principles study. Phys. Rev. B. 85, 165207 (2012). [Google Scholar]
- Green M. L. et al. Oxygen and carbon incorporation in low temperature epitaxial Si films grown by rapid thermal chemical vapor deposition (RTCVD). J. Electron. Mater. 19, 1015–1019 (1990). [Google Scholar]
- Sturm J. C., Schwartz P. V., Prinz E. J. & Magee C. W. Control of oxygen incorporation and lifetime measurement in Si1-xGex epitaxial films grown by rapid thermal chemical vapor deposition. Proc. SPIE 1393, 252 (1991). [Google Scholar]
- Lai G. Y. High-Temperature Corrosion and Materials Applications (ed. Lai G. Y.) 67–96 (ASM International, 2007).
- Marzouki A. et al. Structural and optical characterizations of nitrogen-doped ZnO nanowires grown by MOCVD. Mater. Lett. 64, 2112–2114 (2010). [Google Scholar]
- Gao J., Zhang X., Sun Y., Zhao Q. & Yu D. Compensation mechanism in N-doped ZnO nanowires. Nanotechnology. 21, 245703 (2010). [DOI] [PubMed] [Google Scholar]


