Abstract
SnO2 has been studied intensely for applications to sensors, Li-ion batteries and solar cells. Despite this, comparatively little attention has been paid to the changes in morphology and crystal phase that occur on the metal oxide surface during chemical reactions. This paper reports anaerobic and aerobic ethanol and CO oxidation reactions over SnO2 nanoparticles (NPs), as well as the subsequent changes in the nature of the NPs. Uniform SnO2@C core-shells (10 nm) were formed by an aerobic ethanol oxidation reaction over SnO2 NPs. On the other hand, metallic Sn spheres were produced by an anaerobic ethanol oxidation reaction at 450 °C, which is significantly lower than that (1200 °C) used in industrial Sn production. Anaerobic and aerobic CO oxidation reactions were also examined. The novelty of the methods for the production of metallic Sn and SnO2@C core-shells including other anaerobic and aerobic reactions will contribute significantly to Sn and SnO2-based applications.
Tin (IV) oxide (SnO2) has been studied widely because of its potential applicability to lithium-ion batteries, gas-sensors, solar cells, and catalysts1,2,3. Considerable efforts have been made to control the exposed crystal facets, and synthesize pure and hybridized SnO2 materials with a range of morphologies, including hollow nanoparticles, wires, nanorods, nanotubes, nanoparticles, nanosheets, and cubes4,5,6,7,8,9,10,11,12,13,14,15,16. C/SnO2 hybrid materials were reported to have potential applicability to Li-ion batteries17,18,19,20,21,22. Recently, Liang et al. synthesized bowl-like SnO2@C hollow nanoparticles to retain the advantages of hollow structures, and showed high performance as an anode material for Li-ion batteries17. The SnO2 materials reported for applications to Li-ion batteries include hollow SnO223, SnO2 nanotubes9,24, nanosheets12,25, nanoboxes26, NiCo2O4@SnO2 hetero-nanostructures27, capped Sn/SnO2 nanocrystals28, sandwich-stacked SnO2/Cu nanosheets29, graphene/SnO2 nanoribbon composites30, and SnO2@TiO2 core-shells31, Gas-sensing is an active research area for SnO2 nanomaterials32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47. A wide range of materials have been prepared and tested as sensor materials. These include Cu-doped SnO2 film for H2S sensing33, multi-layer SnO2 nanoplates34 and flower-like SnO2 for ethanol sensing35, aligned epitaxial SnO2 nanowires for ppb-level NO2 sensing36, p-Te/n-SnO2 hierarchical heterostructures37 and SnO2 hollow spheres for ppm-level CO sensing38, hollow SnO2 nanofibers39 and graphene/SnO2 hybrids40 for H2 sensing, clustered SnO2 NPs for toluene detection41, and SnO2 NP-coated ZnO nanotubes for electrochemical dopamine sensing42, For solar cell applications48,49,50,51, Dong et al. reported that quintuple-shelled SnO2 hollow microspheres showed superior light scattering suitable for dye-sensitized solar cells50. The (thermal and photo) catalytic activity of SnO2 has also been studied actively52,53,54,55,56,57. Several examples include the inactivation of bacteria using fluorinated SnO2 hollow nanospheres52, rhodamine B treatment using flower-like hollow SnO/Sn3O4 microspheres53, Rhodamine 6G photodegradation using hollow supersymmetric SnO2 microspheres54. SnO2 nanorods with exposed (110) facets were reported to have high CO oxidation activity following a Mars–van Krevelen mechanism, even though the nanorods have a low surface area and a less active surface oxygen species55,56. Studying the surface reaction on SnO2 is extremely important to better understand the sensing and catalytic mechanism and for fabricating the nanostructures. Adsorption is a common first step for both sensing and catalytic reactions. Jeong et al. reported that nanotextured SnO2 surfaces could be produced using a self-catalytic growth method with different oxygen concentrations and annealing temperatures58. Müller et al. reported showed that the precursor chemistry was important for controlling the morphology and composition of SnO2 nanowires59.
The specific aim of this study was to identify the changes in morphology and crystal phase of SnO2 NPs after aerobic and anaerobic oxidation reactions. This paper reports a new methodology for the production of metallic Sn spheres and SnO2@C core-shells. The core-shell structures have very high potential applicability to gas sensing and Li-ion batteries. Metallic Sn spheres were produced from SnO2 by an anaerobic ethanol oxidation reaction below 600 °C, which is a significantly lower temperature than that used in the high temperature (>1000 °C) carbothermal reduction method in industry. In addition, the high CO oxidation activity of SnO2 NPs has potential applications to catalysis.
Methods
SnO2 NPs were synthesized using a facial hydrothermal method. Briefly, 10.0 mL of 0.1 M SnCl4·5H2O and 20 mL of deionized water (18.2 MΩ-cm resistivity) were fully mixed in a Teflon bottle. An appropriate amount of 0.1 M NaOH solution was then added to induce precipitation. The bottle was tightly capped and placed in an oven at 120 °C for 12 hours. The reaction bottle was cooled naturally and the white precipitate was collected by centrifugation. The precipitates were washed several times with deionized water and ethanol, and dried in an ambient oven at 70 °C. The morphology and size of the dried powder samples were examined by transmission electron microscopy (TEM, Hitachi H-7600) operated at 100.0 kV. A high resolution TEM image was obtained using a FEI Tecnai G2 F20 S-TWIN at an applied voltage of 200.0 kV. The surface morphology and the chemical composition were examined by scanning electron microscopy (SEM) and energy dispersive X-ray (EDX) analysis, respectively using a Hitachi S-4100 SEM/EDX. The X-ray diffraction (XRD) patterns for the powder samples were obtained using a PANalytical X’Pert Pro MPD diffractometer using Cu Kα radiation. The crystal structures of the metallic Sn spheres were tested by both powder and single crystal X-ray diffraction experiments. Powder X-ray diffraction was conducted using a Bruker AXS D8 diffractometer with a Cu Kα radiation source (40 kV and 40 mA) using a Linxeye 1-D detector. Single crystal X-ray diffraction was conducted using a Bruker AXS APEX II CCD-single crystal diffractometer with a Mo Kα radiation source (50 kV and 30 mA, point beam) and a CCD detector system. The diffuse reflectance absorption spectra for the powder samples were measured using a double beam Neosys-2000 UV–Vis spectrophotometer (Scinco). X-ray photoelectron spectroscopy (XPS) was performed using a Thermo-VG Scientific MultiLab 2000 with a monochromatic Al Kα X-ray source (1486.6 eV) equipped with a hemispherical energy analyzer. The photoluminescence spectra were obtained using a SCINCO (Seoul, South Korea) FluoroMate FS-2 spectrometer. Raman spectra were obtained using a Bruker Senterra Raman spectrometer at an excitation laser wavelength of 532 nm. The attenuated total reflection (ATR) Fourier transform infrared (FTIR) spectra were obtained using a Thermo scientific Nicolet iS10 spectrometer. The Brunauer-Emmett-Teller (BET) surface areas of the as-prepared powder samples were measured using a Quantachrome ChemBET TPR/TPD analyzer equipped with a thermal conductivity detector. Fig. S1 in the Supporting Information presents the schematics of the experimental setup for aerobic and anaerobic oxidation reactions. Briefly, for aerobic ethanol oxidation reaction, ethanol vapor was introduced into the catalyst by flowing 5% O2/N2 gas at a flow rate of 40 mL/min. Pure N2 gas was used for the anaerobic ethanol oxidation reaction. The reaction products were examined using a SRS RGA200 quadrupole mass spectrometer. The temperature-programmed aerobic CO oxidation experiments were performed by flowing (40 mL/min) a CO (1%) and O2 (2.5%) in N2 gas mixture to the catalyst powder sample. For anaerobic CO oxidation, 5% CO/N2 gas was flowed instead. The sample (20 mg) was placed in a U-quartz tube (4 mm inner diameter) for each oxidation experiment.
Results and Discussion
Figure 1 shows a photograph and optical microscopy images of the samples produced after the ethanol oxidation reaction under aerobic (with O2) and anaerobic (without O2) conditions. The morphology changed significantly after the reactions. Upon the ethanol oxidation reaction under aerobic conditions, the white powder sample changed to a black powder. Interestingly, under anaerobic conditions, all the power samples changed to silvery-white spheres, indicating complete reduction to Sn metal. Several of the spheres were a few mm in size. To clearly show the metallic Sn spheres, a larger photograph is supplied in the Supporting information, Fig. S2.
Figure 1. Photograph (top) and optical microscopy (bottom) images for the production of SnO2@C core-shells (left, black in color) and metallic Sn spheres (right, silvery-white in color) by aerobic (left) and anaerobic (right) ethanol oxidation reactions over SnO2 NPs (middle, white in color), respectively.
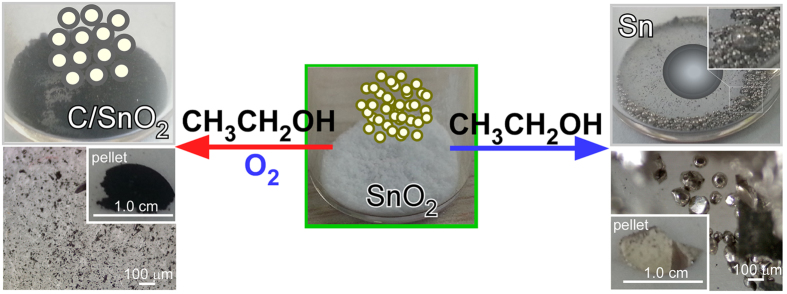
The insets (left and right bottom two) in the optical microscopy images show photographs of the corresponding pelletized samples.
Figure 2 presents the XRD patterns of the SnO2 NPs before and after the aerobic ethanol oxidation reaction. All XRD peaks matched the standard crystal planes of tetragonal SnO2 (JCPDS 1–0657), as displayed in Fig. 2. SnO2 was likely obtained via Sn4+ + OH− → Sn hydroxides → SnO2 + H2O during the hydrothermal reaction10,15. The three major XRD peaks at 2θ = 26.5, 33.8 and 51.7° were assigned to the (110), (101), and (211) reflection planes of tetragonal SnO2, respectively. The XRD peaks of the as-prepared sample were quite broad, indicating smaller particle sizes. The NP size was estimated using the Scherer formula, d = Kλ/L cos θ, where K is the Scherer constant and L is the full width at half maximum of a reflection. The calculated particle size was in good agreement with the mean size (~2 nm) obtained from the TEM images. Li et al. also employed a similar hydrothermal (180 °C for 24 hrs) method to synthesize SnO2 with reaction recipes of 1.0 mmol SnCl4·5H2O, 20 mL deionized water, 10 mL NaOH (1.5 M) solution, and 10 ML ethanol60. They obtained nanoparticle-aggregated hierarchical SnO2 hollow microspheres (600–900 nm) and understood by dissolution–recrystallization process60,61 with two major controlling factors of pH and reaction temperature.
Figure 2. XRD patterns (left) and TEM/HRTEM (right) images of SnO2 NPs before and after the aerobic ethanol oxidation reaction.
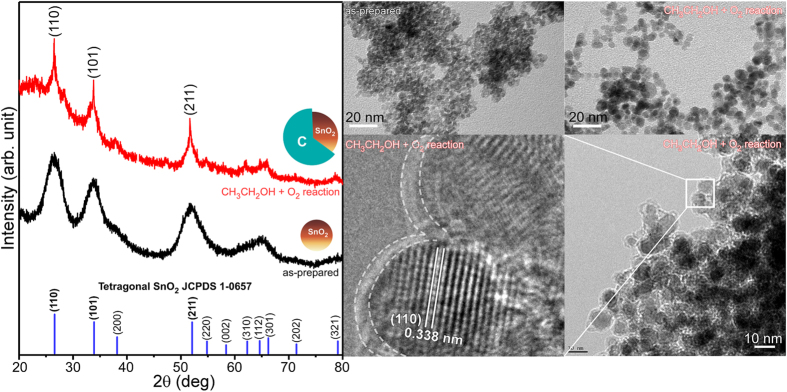
The SnO2@C core-shells were formed upon the aerobic reaction.
For the SnO2@C core-shells, the XRD peak became sharper, indicating an increase in crystallinity and/or particle size. The TEM images illustrate the high uniformity and size of the SnO2 NPs before and after the aerobic ethanol oxidation reaction. A clear lattice spacing of 3.38 Å was obtained, which is indicative of the single crystal nature. The spacing corresponds to the distance between the adjacent (110) planes of tetragonal SnO225,30. Alaf et al. and Uysal et al. employed an alternative method to obtain core-shell type structures, which were tested as anode materials for Li-ion batteries62,63,64. They thermal-evaporated metallic Sn on multiwalled carbon nanotube (MWCNT), and obtained Sn/SnO2/MWCNT and Sn/MWCNT nanocomposites with and without plasma oxidation treatment, respectively. The grain sizes of Sn and SnO2 were controlled by varying thermal evaporation and plasma oxidation conditions.
The as-prepared SnO2 NPs were characterized by UV-Vis absorption spectroscopy, photoluminescence spectroscopy, FT-IR spectroscopy, and BET surface area measurements. A direct band gap of 3.4 eV was measured from the reflectance absorption spectrum and a plot of (αhν)2 versus hν (Supporting Info. Fig. S3)53. The photoluminescence spectrum was recorded for the as-prepared SnO2 NPs at an excitation wavelength of 285 nm (Supporting Info. Fig. S4). Broad emission peaks were observed between 350 and 600 nm, which have commonly been attributed to oxygen defects (or vacancies)45,65,66. The as-prepared SnO2 NPs exhibited a large amount of adsorbed water, based on the broad FT-IR peak at 3400 cm−1 (Supporting Info. Fig. S5)38. The broad peak was almost completely diminished after the aerobic ethanol oxidation reaction at temperatures up to 600 °C. The SnO2 NPs (~2 nm) showed a BET surface area of 197.5 m2/g, which is significantly larger than the surface area of 38.3 m2/g for a reference (>100 nm, Sigma-Aldrich) SnO2 powder. For comparison, Xi et al.5 and Wang et al.25 reported surface areas of 191.5 m2/g for ultra-small (2nm) SnO2 nanorods and 180.3 m2/g for SnO2 nanosheets, respectively.
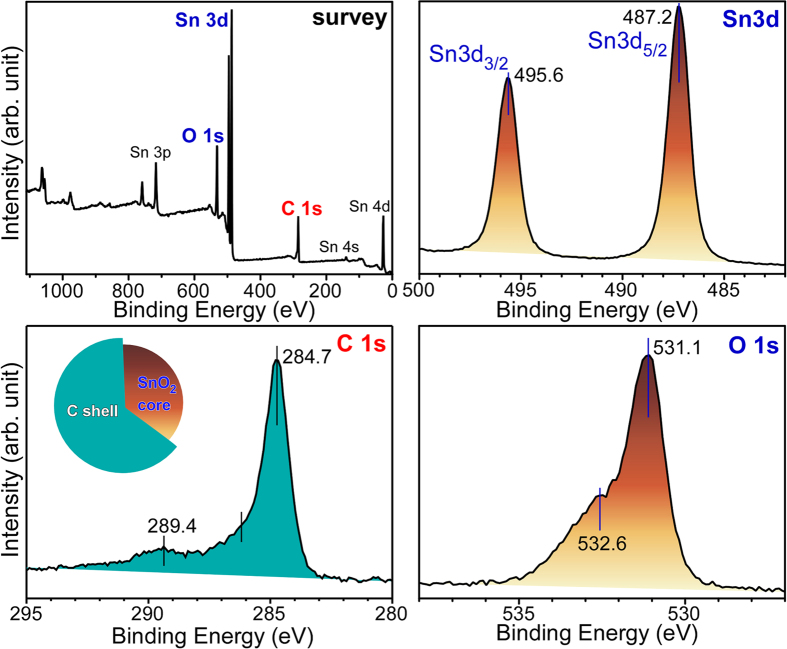
The X-ray photoelectron spectra (Fig. 3) were recorded to further examine the chemical nature of the SnO2@C core-shell nanostructures. The survey spectrum revealed the chemical information of Sn, O and carbon with no other elements. The prominent Sn 3d3/2 and Sn 3d5/2 XPS peaks were observed at 487.2 ( ± 0.1) eV and 495.6 ( ± 0.1) eV, respectively, with spin-orbit splitting of 8.4 eV, due to the Sn4+ oxidation state of SnO214,31,55. These binding energies (BEs) showed no critical difference from those of the as-prepared SnO2 nanoparticles. This suggests that the overlayer carbon had been physisorbed on the SiO2 surface. A major C 1s XPS peak was observed at 284.7 eV, due to elemental carbon. The two smaller C 1s peaks at 289.4 and 286 eV were assigned to O = C-O and C-OH/C-O-C species, respectively. These chemical species are related to the formation of ethylacetate and aldehyde after the aerobic ethanol oxidation reaction, as further discussed in detail below. Two O 1s XPS peaks were observed at 531.1 and 532.6 eV due to lattice oxygen (O2−) of SnO2 and adsorbed oxygen (O− abd O2−) species (e.g., H2O and OH), respectively14. The adsorbed oxygen species were confirmed by FT-IR spectroscopy (Supporting Information, Fig. S5). The thickness of the carbon overlayer was estimated using a well-known equation67, I = I0exp(−d/λ), where d is the shell thickness, λ is the electron inelastic mean-free path (IMFP)68, I and Io is the Sn 3d XPS intensities before and after carbon shell formation, respectively. The thickness of the carbon overlayer was calculated to be 1.2 nm using an IMFP (λ) of 2.7 nm68.
Figure 3. Survey (top left) and high resolution XP spectra of Sn 3d, C 1s and O 1s regions for SnO2@C core-shells prepared by an aerobic ethanol oxidation reaction over SnO2 NPs.
The SnO2@C core-shells were briefly tested as an oxygen sensor material (Supporting Info. Fig. S6) using a two-point probe resistance measurement method43. Using a SnO2@C pellet, a surface resistance of 6 kΩ was measured at room temperature, which is an extremely low resistance compared to that of bare SnO2. The resistance decreased gradually with increasing temperature and was 3 kΩ at 80 °C. The resistance increased upon exposure to oxygen, and showed a good response to the changing oxygen concentration (0.5–5.0%).
The CCD-SCD and PXRD analyses of the Sn spheres produced by the anaerobic ethanol oxidation reaction were obtained in Fig. 4. The very sharp XRD patterns matched those of tetragonal Sn (JCPDS 04-0673)58,62,63,64. The diffraction image (inset in Fig. 4) showed single and polycrystalline natures of Sn spheres. Various crystal patterns of Phi 360 degrees obtained for other Sn spheres are provided in the Supporting Information (Fig. S7).
Figure 4. XRD patterns of metallic Sn formed by the anaerobic ethanol oxidation reaction.
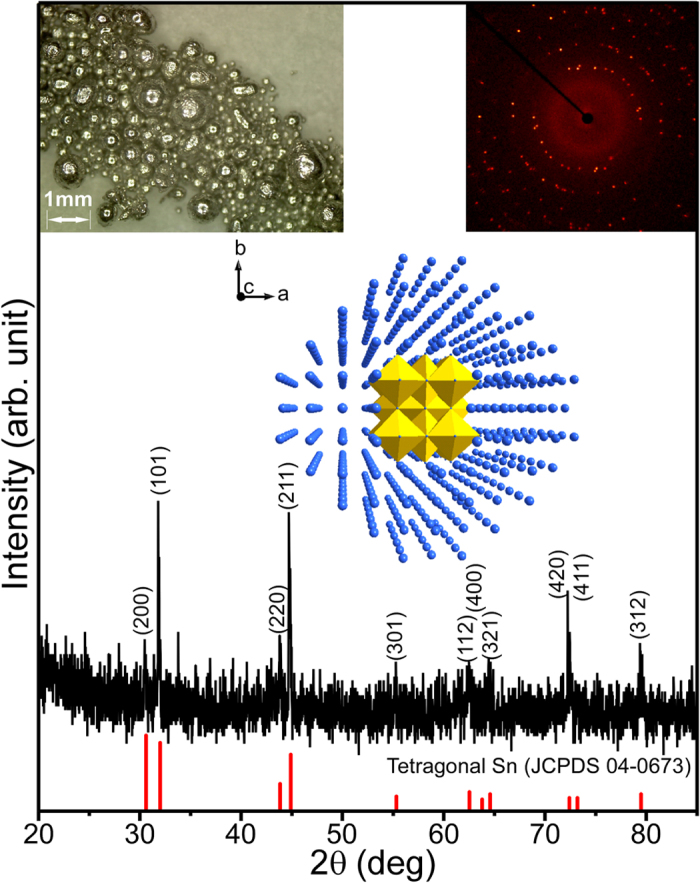
Optical microscopy image (top left inset), body centered tetragonal Sn crystal structure (middle inset), and crystal pattern of Phi 360 image (top right inset).
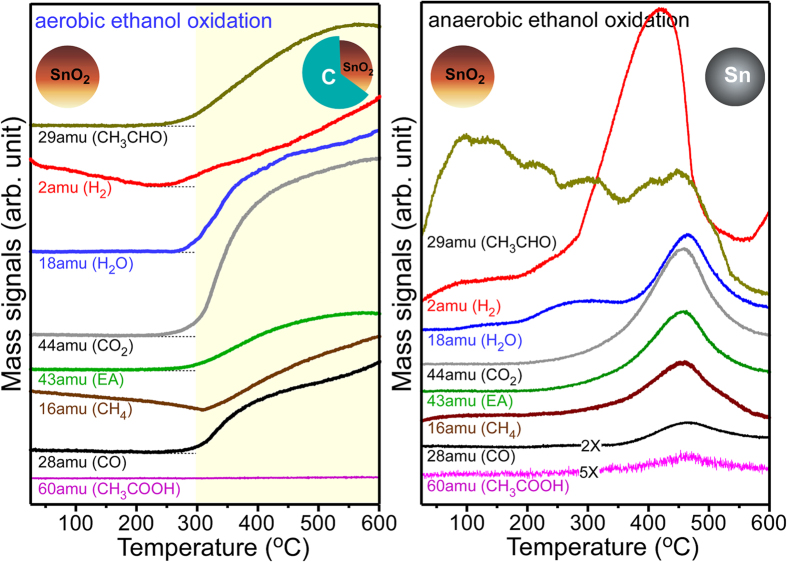
The reaction products produced during the aerobic and anaerobic ethanol oxidation reactions at different temperatures were examined by temperature-programmed mass spectrometry, as shown in Fig. 5. The quantity of reaction products was not measured, and only the chemical species are discussed. Under aerobic conditions, the mass signals commonly began to increase at ~250 °C, and the intensities kept increasing with in temperature. Based on the mass profiles, the oxidation gas products include hydrogen (H2), water (H2O), carbon dioxide (CO2), carbon monoxide (CO), aldehyde, (CH3CHO), methane (CH4), acetaldehyde (CH3CHO), and ethylacetate (CH3COOCH2CH3, EA) with no acetic acid (CH3COOH). On the basis of the literatures69,70,71 and the present results, we propose the following simplified mechanism.
Figure 5. Mass profiles of the chemical species detected during the aerobic (left) and anaerobic (right) ethanol oxidation reactions over the SnO2 nanoparticles.
SnO2@C core-shells were formed under aerobic conditions, whereas metallic Sn spheres were produced under anaerobic conditions.
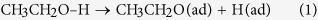 |
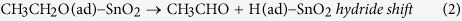 |
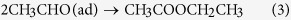 |
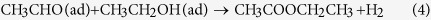 |
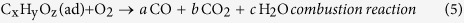 |
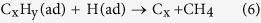 |
In reaction (1), the O–H bond of ethanol dissociates and adsorbs as H and CH3CH2O. The adsorbed CH3CH2O desorbs as CH3CHO (acetaldehyde) by a hydride shift reaction in (2). The acetaldehyde in (3) and (4) further proceeds to form CH3COOCH2CH3 (ethylacetate). In reaction (5), CO, CO2, and H2O are formed by a combustion reaction. Elemental carbon and CH4 are formed in reaction (6).
Under anaerobic conditions, although the reaction products were similar, the mass profile curves showed remarkable difference with temperature. Hydrogen and aldehyde were evolved earlier than the other reaction products, possibly due to CH3CH2O–H → CH3CHO + H2. At above 450 °C, the mass signals (or the catalytic activity) decreased suddenly and showed the maximum intensities at approximately 450 °C. This suggests that SnO2 changes metallic Sn at above 450 °C via the reactions of CxHy (ad)−SnO2 → a CO2 + b H2O + c H2 + Sn, as discussed above. Because the reaction was performed in the absence of oxygen the oxidation of metallic Sn was less likely. Because the melting point (~230 °C)58 of Sn is lower than the reaction temperature of ~450 °C (Fig. 5, right), the reduced Sn may form a spherical droplets of liquid Sn. Upon cooling, the liquid droplet would change to solid Sn spheres, as shown in Fig. 1. A very weak acetic acid signal was also observed under anaerobic conditions, possibly due to the reaction, CH3CHO + Os → CH3COO(ad) + H(ad) → CH3COOH, where Os is the adsorbed oxygen species. The anaerobic ethanol oxidation reaction was also performed with 50× larger SnO2 NPs (<100 nm) (Supporting Info. Fig. S8). Although the maximum peaks were observed at a slightly higher temperature of 500 °C, the mass profiles were similar to those of the reactions with 2 nm SnO2 NPs. metallic Sn spheres were also obtained after the anaerobic ethanol oxidation reaction with the larger NPs.
In industry, metallic Sn is commonly produced from SnO2 via a carbothermal reaction (SnO2 + C → Sn + CO2) at temperatures above 1200 °C, where carbon is used as the reducing agent. As a green method, a hydrogen reduction [SnO2 + 2H2 (g) → Sn (s, l) + 2H2O (g)] method is used at high hydrogen pressures (>30 kPa) and temperature (>700 °C)72. Zhang et al. prepared metal Sn nanobelts from SnO2 by a substitution reaction using Zn powders in a furnace temperature of 1200 °C73. Compared to these two methods, the present anaerobic ethanol oxidation reaction requires a much lower temperature under ambient pressure.
To demonstrate the changes in morphology and crystal phase, another chemical reaction over the NPs was also tested, e.g., aerobic and anaerobic CO oxidation reactions. CO initially adsorbs on the oxide surface, then reacts with surface oxygen and desorbs as CO2 (g). The surface oxygen vacancy is replenished by the adsorption of residual (molecular) oxygen; CO (g) + Sn-Os → CO2 (g) + Sn-□vac Sn-□vac + 1/2O2 (g) → Sn-Os, where Sn-□vac = oxygen vacancy and Sn-Os = surface oxygen species15. The morphology and/or crystal structure is expected to change differently according to whether there is sufficient replenishment of surface oxygen. Figure 6 shows the XRD patterns and corresponding photographs and optical microscopy images. The color and morphology showed significant changes. Larger brown semitransparent crystals appeared to be formed under aerobic conditions, whereas the aggregated power form was formed under anaerobic conditions. The corresponding SEM images are provided in the Supporting Information Fig. S9. The corresponding XRD peaks became very weak, indicating poor crystallinity, but still showed the reflection planes of tetragonal SnO2.
Figure 6. XRD profiles of SnO2 after the anaerobic (5% CO in N2) and aerobic (1% CO + 2.5% O2 in N2) CO oxidation reactions.
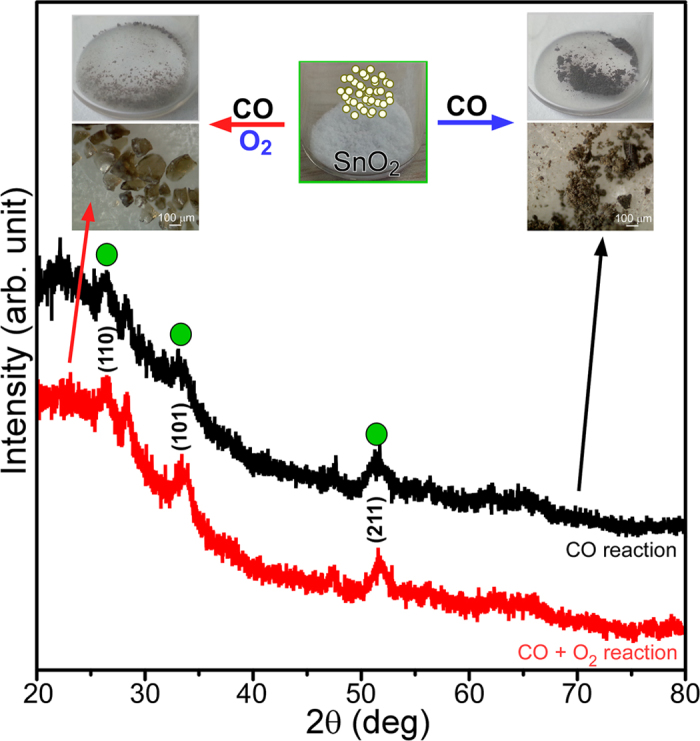
The inset shows photographs and optical microscopy images of SnO2 (brown semitransparent crystals and clusters) by the aerobic (left) and anaerobic (right) CO oxidation reactions. The as-prepared SnO2 nanoparticles (white in color) are shown in the middle.
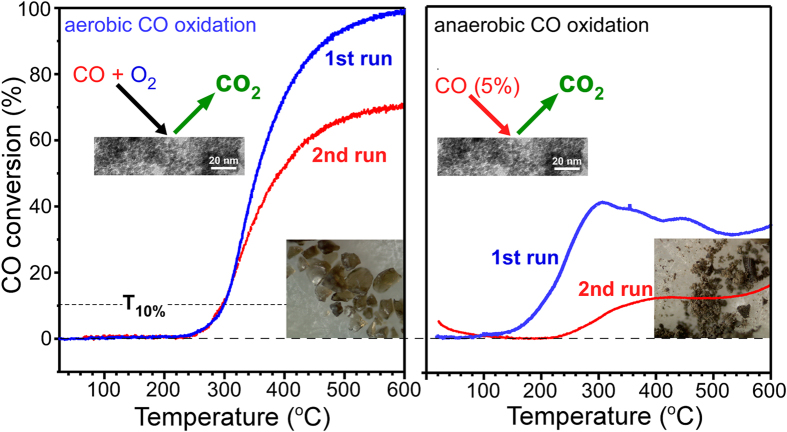
Figure 7 displays the CO conversion (to CO2) profiles of the aerobic and anaerobic CO oxidation reactions. For the first CO oxidation run under aerobic conditions, the oxidation onset temperature and T10% were observed at 230 °C and 300 °C, respectively. This study showed that the SnO2 NPs have comparable CO oxidation activity to that reported in the literature55. Compared to the first run, the reaction temperatures in the second run were the same between CO conversion of 0% and 20%. Above 20%, however, the conversion differed according to the reaction temperature. This suggests that the CO conversion is critically affected by heat and/or mass transfer limitations at a higher temperatures74,75. The CO conversion was lower for the second run at higher temperatures. At 600 °C, the catalytic activity was degraded by approximately 30% compared to that of the first run. The degraded activity was attributed to the change in surface area and crystallinity, based on the microscopy images and the XRD patterns. An activation energy (Ea) of 104.7 kJ/mol was obtained in the CO conversion range of 10–15% from the Arrhenius plot (Supporting Info. Fig. S10). Under anaerobic conditions, CO conversion (%) was much lower than that under aerobic conditions, as expected. The conversion efficiency was more degraded in the second run. Compared to the anaerobic ethanol oxidation, the CO oxidation showed no critical change in crystal phase. This suggests that CO is not a good reducing agent for SnO2. A water signal was also detected during the first runs for both the aerobic and anaerobic reactions (Supporting Info. Fig. S11), but not during the second runs. This was expected because the as-prepared SnO2 NPs contain water (based on the FT-IR spectrum). For the weaker water signal during the aerobic reaction, it was assumed that SnO2 was dehydrated by a stream of oxygen before running the CO oxidation.
Figure 7. First and second runs temperature programmed CO oxidation conversion (%) profiles of the aerobic (left) and anaerobic (right) CO oxidation reactions over SnO2 nanoparticles, where CO conversion (%) = {([CO]in-[CO]out)/[CO]in}×100, T10% = the temperature at 10% CO conversion.
CO (1%)/O2 (2.5%) in N2 and 5% O2 in N2 were used for the aerobic and anaerobic CO oxidation reactions, respectively.
The BET surface area was measured to be 81.2 m2/g after anaerobic CO oxidation. The surface area was decreased to 70.0 m2/g after aerobic CO oxidation reaction. We measured a surface area of 92.5 m2/g for SnO2@C core-shells formed after aerobic ethanol oxidation reaction. Compared with the surface area of 197. 5 m2/g for the as-prepared SnO2 NPs, the substantial decrease is due to an increase in particle size. Energy dispersive X-ray (EDX) analysis was performed for SnO2 NPs after the anaerobic and aerobic CO and ethanol oxidation reactions (Supporting Info. Fig. S12). For metallic Sn formed after anaerobic ethanol oxidation, the EDX spectrum showed mainly Sn with minor impurity C signal. For SnO2@C core-shells formed after aerobic ethanol oxidation, significant C, O and Sn EDX signals were observed as we expected. For the two samples after CO oxidation reactions, the EDX spectra showed similar Sn and O signals, but the sample after anaerobic CO oxidation showed a presence of carbon.
Raman spectroscopy was further employed to examine the structural and chemical states displayed in Fig. 8. For tetragonal SnO2 with a space group of D4h, the vibrational modes were ascribed to 1A1g + 1A2g + 1A2u + 1B1g + 1B2g + 2B1u + 1Eg + 3Eu, where A1g, B1g, B2g, and Eg are Raman active modes15. For the as-prepared SnO2 NPs, three peaks were found at 467, 630 and 772 cm−1, assigned to Eg, A1g and B2g vibrational modes, respectively. Interestingly, a broad and strong peak was observed at 569 cm−1, which was attributed to surface-related defects15,76. The peak has commonly been observed and well known to increase with decreasing particle size4,5,15,44. Upon anaerobic CO oxidation, the peaks were substantially decreased and showed no clear vibrational modes, indicating formation of very poor crystalline structure. However, after aerobic CO oxidation reaction the A1g peak was more clearly observed as generally expected for SnO2 NPs15,44. Upon anaerobic ethanol oxidation, no Raman signal was observed, in good consistent with the literature for metallic Sn77. For SnO2@C core-shells formed after aerobic ethanol oxidation, new strong Raman signals was observed, attributed to the shell-carbon species. The smaller peaks at 467 and 630 cm−1 were attributed to the Eg and A1g vibrational modes of the core SnO2, respectively.
Figure 8. Raman spectra of SnO2 NPs before and after the anaerobic and aerobic CO and ethanol oxidation reactions.
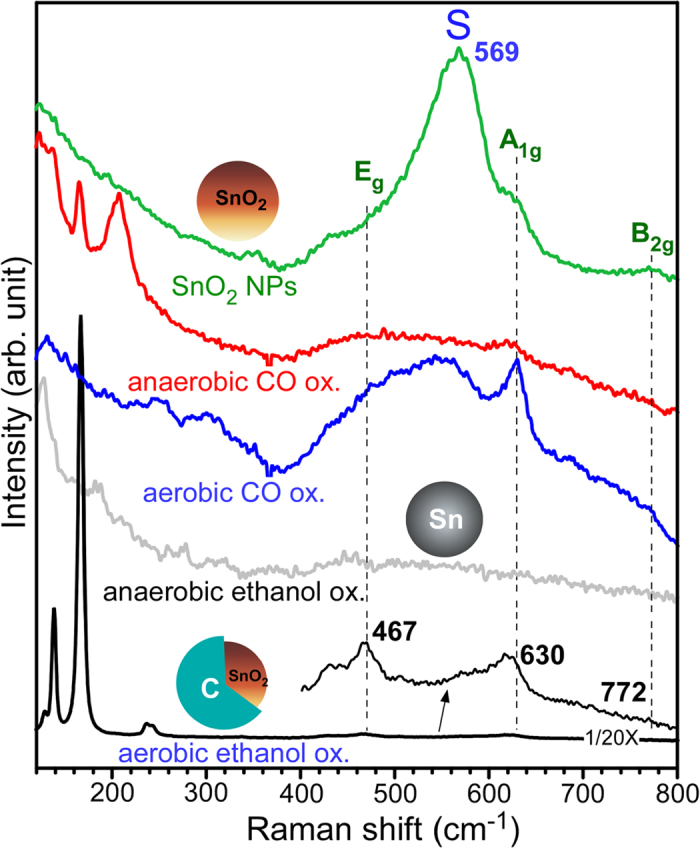
A laser intensity of 0.25 mW was used for SnO2@C core-shells, and 5 mW was used for other samples.
Conclusion
Metallic Sn spheres can be produced by a simple anaerobic ethanol oxidation reaction over SnO2 (2 nm size, BET surface area = 197.5 m2/g, band gap = 3.4 eV, and broad photoluminescence peaks between 350 and 600 nm) NPs. The conversion (SnO2 → Sn) reaction temperature was ~450 °C, which is significantly lower than the reaction temperature (1200 °C) of carbothermal Sn production used in industry. The ethanol oxidation products included H2, H2O, CO, CO2, CH4, aldehyde, and ethylacetate. On the other hand, uniform nanosized SnO2@C core-shells could be prepared by aerobic ethanol oxidation reaction over SnO2 NPs. The carbon overlayer was estimated to be ~1 nm on <10 nm size SnO2 nanoparticles based on TEM. The core-shell structure showed a good oxygen sensing response and potential applicability to a gas sensor. Under aerobic conditions, the CO oxidation activity of the SnO2 NPs showed T10% = 300 °C. This suggests that SnO2 NPs have potential applications to an oxidation catalyst.
Overall, the aerobic and anaerobic oxidation reaction could be a versatile method for the fabrication of various nanostructures and provide new insights for understanding the sensing and catalytic reaction mechanism. Uniform nanosized SnO2@C core-shells are produced in a much simpler manner and the carbon-support material has very high potential applicability to electrode and sensor materials. The transformation from SnO2 to metallic Sn under anaerobic conditions provides a new insights to better understanding the alcohol sensing mechanism. In addition, the unique anaerobic ethanol (alcohol) oxidation reaction could be a promising method for the industrial production of high quality metallic Sn from SnO2.
Additional Information
How to cite this article: Kim, W. J. et al. Metallic Sn spheres and SnO2@C core-shells by anaerobic and aerobic catalytic ethanol and CO oxidation reactions over SnO2 nanoparticles. Sci. Rep. 5, 13448; doi: 10.1038/srep13448 (2015).
Supplementary Material
Acknowledgments
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF- 2014R1A1A2055923). The authors greatly acknowledge Mr. Seungwon Lee and Ms. Young In Choi for CO oxidation, BET and EDX experimental assistance.
Footnotes
Author Contributions Y.S. designed the main experimental concepts and prepared the manuscript. W.J.K. mainly performed the material synthesis and oxidation experiments. S.W.L. contributed to XRD measurements and analysis.
References
- Wang H. & Rogach A. L. Hierarchical SnO2 Nanostructures: Recent Advances in Design, Synthesis, and Applications. Chem. Mater. 26, 123−133 (2014). [Google Scholar]
- Kar A. & Patra A. Recent development of core–shell SnO2 nanostructures and their potential applications. J. Mater. Chem. C 2, 6706–6722 (2014). [Google Scholar]
- Chen J. S. & Lou X. W. SnO2-Based Nanomaterials: Synthesis and Application in Lithium-Ion Batteries. Small 9, 1877–1893 (2013). [DOI] [PubMed] [Google Scholar]
- Cheng B., Russell J. M., Shi W., Zhang L. & Samulski E. T. Large-Scale, Solution-Phase Growth of Single-Crystalline SnO2 Nanorods. J. Am. Chem. Soc. 126, 5972–5973 (2004). [DOI] [PubMed] [Google Scholar]
- Xi G. & Ye J. Ultrathin SnO2 Nanorods: Template- and Surfactant-Free Solution Phase Synthesis, Growth Mechanism, Optical, Gas-Sensing, and Surface Adsorption Properties. Inorg. Chem. 49, 2302–2309 (2010). [DOI] [PubMed] [Google Scholar]
- Zhuang Z., Huang F., Lin Z. & Zhang H. Aggregation-Induced Fast Crystal Growth of SnO2 Nanocrystals. J. Am. Chem. Soc. 134, 16228−16234 (2012). [DOI] [PubMed] [Google Scholar]
- Wu J. et al. Mechanistic Insights into Formation of SnO2 Nanotubes: Asynchronous Decomposition of Poly(vinylpyrrolidone) in Electrospun Fibers during Calcining Process. Langmuir 30, 11183–11189 (2014). [DOI] [PubMed] [Google Scholar]
- Jiang L. et al. Size-Controllable Synthesis of Monodispersed SnO2 Nanoparticles and Application in Electrocatalysts. J. Phys. Chem. B 109, 8774–8778 (2005). [DOI] [PubMed] [Google Scholar]
- Ye J., Zhang H., Yang R., Li X. & Qi L. Morphology-Controlled Synthesis of SnO2 Nanotubes by Using 1D Silica Mesostructures as Sacrificial Templates and Their Applications in Lithium-Ion Batteries. Small 6, 296–306 (2010). [DOI] [PubMed] [Google Scholar]
- Sain S., Kar A., Patra A. & Pradhan. S. K. Structural interpretation of SnO2 nanocrystals of different morphologies synthesized by microwave irradiation and hydrothermal methods. CrystEngComm 16, 1079–1090 (2014). [Google Scholar]
- Ding S. et al. Formation of SnO2 Hollow Nanospheres inside Mesoporous Silica Nanoreactors. J. Am. Chem. Soc. 133, 21–23 (2011). [DOI] [PubMed] [Google Scholar]
- Wang C. et al. Ultrathin SnO2 Nanosheets: Oriented Attachment Mechanism, Nonstoichiometric Defects, and Enhanced Lithium-Ion Battery Performances. J. Phys. Chem. C 116, 4000–4011 (2012). [Google Scholar]
- Tian W. et al. Flexible SnO2 hollow nanosphere film based high-performance ultraviolet photodetector. Chem. Commun. 49, 3739–3741 (2013). [DOI] [PubMed] [Google Scholar]
- Hu D. et al. Novel Mixed Phase SnO2 Nanorods Assembled with SnO2 Nanocrystals for Enhancing Gas-Sensing Performance toward Isopropanol Gas. J. Phys. Chem. C 118, 9832–9840 (2014). [Google Scholar]
- Sohn Y. Structural/Optical Properties and CO Oxidation Activities of SnO2 Nanostructures. J. Am. Cer. Soc. 97, 1303–1310 (2014). [Google Scholar]
- Choi Y. I. & Sohn Y. Photoluminescence profile mapping of Eu (III) and Tb (III → IV)-embedded in quantum size SnO2 nanoparticles. RSC. Adv. 4, 31155–31161 (2014). [Google Scholar]
- Liang J. et al. Bowl-like SnO2@Carbon Hollow Particles as an Advanced Anode Material for Lithium-Ion Batteries. Angew. Chem. 126, 1–6 (2014). [DOI] [PubMed] [Google Scholar]
- Choi S. H. & Kang Y. C. Kilogram-Scale Production of SnO2 Yolk–Shell Powders by a Spray-Drying Process Using Dextrin as Carbon Source and Drying Additive. Chem. Eur. J. 20, 5835–5839 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang L., Zhang G., Wu H. B., Yu L. & Lou X. W. Hierarchical Tubular Structures Constructed by Carbon-Coated SnO2 Nanoplates for Highly Reversible Lithium Storage. Adv. Mater. 25, 2589–2593 (2013). [DOI] [PubMed] [Google Scholar]
- Lin Y.-S., Duh J.-G. & Hung M.-H. Shell-by-Shell Synthesis and Applications of Carbon-Coated SnO2 Hollow Nanospheres in Lithium-Ion Battery. J. Phys. Chem. C 114, 13136–13141 (2010). [Google Scholar]
- Lou X. W., Chen J. S., Chen P. & Archer L. A. One-Pot Synthesis of Carbon-Coated SnO2 Nanocolloids with Improved Reversible Lithium Storage Properties. Chem. Mater. 21, 2868–2874 (2009). [Google Scholar]
- Wang Y., Su F., Lee J. Y. & Zhao X. S. Crystalline Carbon Hollow Spheres, Crystalline Carbon-SnO2 Hollow Spheres, and Crystalline SnO2 Hollow Spheres: Synthesis and Performance in Reversible Li-Ion Storage. Chem. Mater. 18, 1347–1353 (2006). [Google Scholar]
- Lou X. W., Wang Y., Yuan C., Lee J. Y. & Archer L. A. Template-Free Synthesis of SnO2 Hollow Nanostructures with High Lithium Storage Capacity. Adv. Mater. 18, 2325–2329 (2006). [Google Scholar]
- Liu Z. et al. Ultrafast and scalable laser liquid synthesis of tin oxide nanotubes and its application in lithium ion batteries. Nanoscale, 6, 5853–5858 (2014). [DOI] [PubMed] [Google Scholar]
- Wang C. et al. Large-Scale Synthesis of SnO2 Nanosheets with High Lithium Storage Capacity. J. Am. Chem. Soc. 132, 46–47 (2010). [DOI] [PubMed] [Google Scholar]
- Wang Z., Luan D., Boey F. Y. C. & Lou X. W. Fast Formation of SnO2 Nanoboxes with Enhanced Lithium Storage Capability. J. Am. Chem. Soc. 133, 4738–4741 (2011). [DOI] [PubMed] [Google Scholar]
- Gao G., Wu H. B., Ding S. & Lou X. W. Preparation of Carbon-Coated NiCo2O4@SnO2 Hetero-nanostructures and Their Reversible Lithium Storage Properties. Small 10.1002/smll.201400152 (2014). [DOI] [PubMed] [Google Scholar]
- Kravchyk K. et al. Monodisperse and Inorganically Capped Sn and Sn/ SnO2 Nanocrystals for High-Performance Li-Ion Battery Anodes. J. Am. Chem. Soc. 135, 4199–4202 (2013). [DOI] [PubMed] [Google Scholar]
- Deng J. et al. Sandwich-Stacked SnO2/Cu Hybrid Nanosheets as Multichannel Anodes for Lithium Ion Batteries. ACS Nano 7, 6948–6954 (2013). [DOI] [PubMed] [Google Scholar]
- Lin J. et al. Graphene Nanoribbon and Nanostructured SnO2 Composite Anodes for Lithium Ion Batteries. ACS Nano 7, 6001–6006 (2013). [DOI] [PubMed] [Google Scholar]
- Guan C. et al. Highly Stable and Reversible Lithium Storage in SnO2 Nanowires Surface Coated with a Uniform Hollow Shell by Atomic Layer Deposition. Nano Lett. 14, 4852–4858 (2014). [DOI] [PubMed] [Google Scholar]
- D’Arienzo M., Cristofori D., Scotti R. & Morazzoni F. New Insights into the SnO2 Sensing Mechanism Based on the Properties of Shape Controlled Tin Oxide Nanoparticles. Chem. Mater. 25, 3675–3686 (2013). [Google Scholar]
- Zhang S. et al. Facile Fabrication of a Well-Ordered Porous Cu-Doped SnO2 Thin Film for H2S Sensing. ACS Appl. Mater. Interfaces 6, 14975–14980 (2014). [DOI] [PubMed] [Google Scholar]
- Li K.-M., Li Y.–J., Lu M.-Y., Kuo C. I. & Chen L.-J. Direct Conversion of Single-Layer SnO Nanoplates to Multi-Layer SnO2 Nanoplates with Enhanced Ethanol Sensing Properties. Adv. Funct. Mater. 19, 2453–2456 (2009). [Google Scholar]
- Wang H. et al. Preparation of Flower-like SnO2 Nanostructures and Their Applications in Gas-Sensing and Lithium Storage. Cryst. Growth Des. 11, 2942–2947 (2011). [Google Scholar]
- Wang X. et al. Aligned Epitaxial SnO2 Nanowires on Sapphire: Growth and Device Applications. Nano Lett. 14, 3014−3022 (2014). [DOI] [PubMed] [Google Scholar]
- Her Y. -C., Yeh B.-Y. & Huang S.-L. Vapor–Solid Growth of p-Te/n-SnO2 Hierarchical Heterostructures and Their Enhanced Room-Temperature Gas Sensing Properties. ACS Appl. Mater. Interfaces 6, 9150–9159 (2014). [DOI] [PubMed] [Google Scholar]
- Gyger F., Hubner M., Feldmann C., Barsan N. & Weimar U. Nanoscale SnO2 Hollow Spheres and Their Application as a Gas-Sensing Material. Chem. Mater. 22, 4821–4827 (2010). [Google Scholar]
- Kadir R. A. et al. Electrospun Granular Hollow SnO2 Nanofibers Hydrogen Gas Sensors Operating at Low Temperatures. J. Phys. Chem. C 118, 3129−3139 (2014). [Google Scholar]
- Russo P. A. et al. Room-Temperature Hydrogen Sensing with Heteronanostructures Based on Reduced Graphene Oxide and Tin Oxide. Angew. Chem. Int. Ed. 51, 11053–11057 (2012). [DOI] [PubMed] [Google Scholar]
- Suematsu K. et al. Nanoparticle Cluster Gas Sensor: Controlled Clustering of SnO2 Nanoparticles for Highly Sensitive Toluene Detection. ACS Appl. Mater. Interfaces 6, 5319−5326 (2014). [DOI] [PubMed] [Google Scholar]
- She G. et al. Electrochemical Sensors: SnO2 Nanoparticle-Coated ZnO Nanotube Arrays for High-Performance Electrochemical Sensors. Small 10, 4685–1692 (2014). [DOI] [PubMed] [Google Scholar]
- Kolmakov A., Zhang Y., Cheng G. & Moskovits M. Detection of CO and O2 Using Tin Oxide Nanowire Sensors. Adv. Mater. 15, 997–1000 (2003). [Google Scholar]
- Jiang C., Zhang G., Wu Y., Li L. & Shi K. Facile synthesis of SnO2 nanocrystalline tubes by electrospinning and their fast response and high sensitivity to NOx at room temperature. Cryst Eng Comm 14, 2739–2747 (2012). [Google Scholar]
- Epifani M. et al. The Role of Surface Oxygen Vacancies in the NO2 Sensing Properties of SnO2 Nanocrystals. J. Phys. Chem. C 112, 19540–19546 (2008). [Google Scholar]
- Xu X., Zhuang J. & Wang X. SnO2 quantum dots and quantum wires: controllable synthesis, self-assembled 2D architectures, and gas-sensing properties. J. Am. Chem. Soc. 130, 12527–12535 (2008). [DOI] [PubMed] [Google Scholar]
- Chen X. et al. Templating Synthesis of SnO2 Nanotubes Loaded with Ag2O Nanoparticles and Their Enhanced Gas Sensing Properties. Adv. Funct. Mater. 21, 2049–2056 (2011). [Google Scholar]
- Gubbala S. et al. Surface properties of SnO2 nanowires for enhanced performance with dye-sensitized solar cells. Energy Environ. Sci. 2, 1302–1309 (2009). [Google Scholar]
- Wang Y. et al. Microwave-Assisted Synthesis of SnO2 Nanosheets Photoanodes for Dye-Sensitized Solar Cells. J. Phys. Chem. C 118, 25931–25938 (2014). [Google Scholar]
- Dong Z. et al. Quintuple-Shelled SnO2 Hollow Microspheres with Superior Light Scattering for High-Performance Dye-Sensitized Solar Cells. Adv. Mater. 26, 905–909 (2014). [DOI] [PubMed] [Google Scholar]
- Snaith H. J. & Ducati C. SnO2-Based Dye-Sensitized Hybrid Solar Cells Exhibiting Near Unity Absorbed Photon-to-Electron Conversion Efficiency. Nano Lett. 10, 1259–1265 (2010). [DOI] [PubMed] [Google Scholar]
- Liu S. et al. Porous Fluorinated SnO2 Hollow Nanospheres: Transformative Self-assembly and Photocatalytic Inactivation of Bacteria. ACS Appl. Mater. Interfaces 6, 2407−2414 (2014). [DOI] [PubMed] [Google Scholar]
- Xia W. et al. High-efficiency photocatalytic activity of type II SnO/Sn3O4 heterostructures via interfacial charge transfer. Cryst Eng Comm 16, 6841–6847 (2014). [Google Scholar]
- You H. et al. Gold nanoparticle doped hollow SnO2 supersymmetric nanostructures for improved photocatalysis. J. Mater. Chem. A 1, 4097–4104 (2013). [Google Scholar]
- Wang X., Xiao L., Peng H., Liu W. & Xu X. SnO2 nano-rods with superior CO oxidation performance. J. Mater. Chem. A 2, 5616–5619 (2014). [Google Scholar]
- Lu Z. et al. Direct CO oxidation by lattice oxygen on the SnO2 (110) surface: a DFT study. Phys. Chem. Chem. Phys. 16, 12488–12494 (2014). [DOI] [PubMed] [Google Scholar]
- Du W. et al. Platinum-Tin Oxide Core–Shell Catalysts for Efficient Electro-Oxidation of Ethanol. J. Am. Chem. Soc. 136, 10862−10865 (2014). [DOI] [PubMed] [Google Scholar]
- Jeong S., McDowell M. T. & Cui Y. Low-Temperature Self-Catalytic Growth of Tin Oxide Nanocones over Large Areas. ACS Nano 5, 5800–5807 (2011). [DOI] [PubMed] [Google Scholar]
- Müller R. et al. Influence of Precursor Chemistry on Morphology and Composition of CVD-Grown SnO2 Nanowires. Chem. Mater. 24, 4028–4035 (2012). [Google Scholar]
- Li Y. et al. Synthesis of self-assembled 3D hollow microspheres of SnO2 with an enhanced gas sensing performance. Appl. Surf. Sci. 285P, 130–135 (2013). [Google Scholar]
- Zhao Q. et al. Morphology-modulation of SnO2 Hierarchical Architectures by Zn Doping for Glycol Gas Sensing and Photocatalytic Applications. Sci. Rep. 5, 7874, 10.1038/srep07874 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaf M., Gultekin D. & Akbulut H. Electrochemical properties of free-standing Sn/SnO2/multi-walled carbon nano tube anode papers for Li-ion batteries. Appl. Surf. Sci. 275, 244–251 (2013). [Google Scholar]
- Uysal M., Cetinkaya T., Alp A. & Akbulut H. Production of Sn/MWCNT nanocomposite anodes by pulse electrodeposition for Li-ion batteries. Appl. Surf. Sci. 290, 6–12 (2014). [Google Scholar]
- Alaf M. & Akbulut H. Electrochemical energy storage behavior of Sn/SnO2 double phase nanocomposite anodes produced on the multiwalled carbon nanotube buckypapers for lithium-ion batteries. J. Power Sources 247, 692–702 (2014). [Google Scholar]
- Kar A., Kundu S. & Patra A. Surface Defect-Related Luminescence Properties of SnO2 Nanorods and Nanoparticles. J. Phys. Chem. C 115, 118–124 (2011). [Google Scholar]
- Fang M., Tan X., Cheng B. & Zhang L. SnO2 hierarchical nanostructure and its strong narrow-band photoluminescence. J. Mater. Chem. 19, 1320–1324 (2009). [Google Scholar]
- Sohn Y. Interfacial electronic structure and ion beam induced effect of anatase TiO2 surface modified by Pd nanoparticles. Appl. Surf. Sci. 257, 1692–1697 (2010). [Google Scholar]
- Powell C. J. & Jablonski A. NIST Electron Inelastic-Mean-Free-Path Database - Version 1.2, National Institute of Standards and Technology, Gaithersburg, MD (2010). [Google Scholar]
- Abad A., Concepcion P., Corma A. & Garcia H. A Collaborative Effect between Gold and a Support Induces the Selective Oxidation of Alcohols. Angew. Chem. Int. Ed. 44, 4066–4069 (2005). [DOI] [PubMed] [Google Scholar]
- Guzman J., Carrettin S. & Corma A. Spectroscopic evidence for the supply of reactive oxygen during CO oxidation catalyzed by gold supported on nanocrystalline CeO2. J. Am. Chem. Soc. 127, 3286–3287 (2005). [DOI] [PubMed] [Google Scholar]
- Li M., Wu Z. & Overbury S. H. Surface Structure Dependence of Selective Oxidation of Ethanol on Faceted CeO2 Nanocrystals. J. Catal. 306, 164–176 (2013). [Google Scholar]
- Kim B.-S., Lee J.–C., Yoon H.–S. & Kim S.-K. Reduction of SnO2 with Hydrogen. Mater. Trans. 52, 1814- 1817 (2011). [Google Scholar]
- Zhang J. et al. Synthesis of metal Sn nanobelts from SnO2 nanopowders by a substitution reaction. Nanotechnology 16, 2887–2891 (2005). [Google Scholar]
- Park Y., Kim S. K., Pradhan D. & Sohn Y. Thermal H2-treatment effects on CO/CO2 conversion over Pd-doped CeO2 comparison with Au and Ag-doped CeO2. React. Kinet. Mech. Cat. 113, 85–100 (2014). [Google Scholar]
- Park Y., Kim S. K., Pradhan D. & Sohn Y. Surface treatment effects on CO oxidation reactions over Co, Cu, and Ni-doped and codoped CeO2 catalysts. Chem. Eng. J. 250, 25–34 (2014). [Google Scholar]
- Xu J. et al. Synthesis, characterizations and improved gas-sensing performance of SnO2 nanospike arrays. J. Mater. Chem. 21, 19086–19092 (2011). [Google Scholar]
- Sinha A. K., Sil A., Sasmal A. K., Pradhan M. & Pal T. Synthesis of active tin: an efficient reagent for allylation reaction of carbonyl compounds. New J. Chem. 39, 1685–1690 (2015). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.