Abstract
Parity has been shown to inversely associate with cardiovascular disease (CVD) mortality, but the evidence of epidemiological studies is still controversial. Therefore, we quantitatively assessed the relationship between parity and CVD mortality by summarizing the evidence from prospective studies. We searched MEDLINE (PubMed), EMBASE and ISI Web of Science databases for relevant prospective studies of parity and CVD mortality through the end of March 2015. Fixed- or random-effects models were used to estimate summary relative risks (RRs) and 95% confidence intervals (CIs). Heterogeneity among studies was assessed using the I2 statistics. All statistical tests were two-sided. Ten prospective studies were included with a total of 994,810 participants and 16,601 CVD events. A borderline significant inverse association was observed while comparing parity with nulliparous, with summarized RR = 0.79 (95% CI: 0.60–1.06; I2 = 90.9%, P < 0.001). In dose-response analysis, we observed a significant nonlinear association between parity number and CVD mortality. The greatest risk reduction appeared when the parity number reached four. The findings of this meta-analysis suggests that ever parity is inversely related to CVD mortality. Furthermore, there is a statistically significant nonlinear inverse association between parity number and CVD mortality.
Cardiovascular disease (CVD) is one of the dominant causes of death and disability. It accounts for millions deaths worldwide each year and leads to be an important public issue1. Apart from age, hypertension, hyperlipidemia, diabetes, obesity, and smoking, which are the most established risk factors for CVD, recent studies have hypothesized that sex hormone may play a role in the etiology of CVD1,2. Several studies have demonstrated that males are two to three times more likely to die prematurely from cardiovascular disease than females. This ratio persists in all countries independent of their overall death rate from this condition2. This theory may be affected by complex influences from sex hormone.
Pregnancy and parturition are important events in the life of a woman. Fluctuations of serum sex hormone levels during the process of pregnancy and delivery, perinatal hemodynamic changes, oxidative stress and other gestational factors exert complex influences on the cardiovascular system. Although previous studies have devoted great efforts to evaluate the relationship between childbearing history and mortality of CVD, the aforementioned association is still inconsistent. The association between parity and risk of CVD was first studied in the 1980s, which demonstrated the increased prevalence of CVD with parity number3. However, subsequent studies found minimal or no evidence for the aforementioned association3,4,5,6,7,8,9. Given the inconsistency of previous findings, we therefore carried out this systematic review and meta-analysis to summarize the evidence of the relationship between parity and CVD mortality.
Results
Study characteristics and quality assessment
Table 1 demonstrated the characteristics of the ten included prospective studies10,11,12,13,14,15,16,17,18,19, in which 16,601 cases and 978,209 non-cases was represented. Among the ten included studies, five were carried out in the United States11,15,17,18,19, and one for each in Israel10, Australia12, China14, Korea13, and Finland16. Cohort sizes ranged from 86718 to 585,45517, and the number of CVD cases varied from 4518 to 712514.
Table 1. Characteristics of studies of parity and CVD mortality.
| Authors | Year | Location | Studydesign | Gender | No. ofcases | No. ofsubject | Age (years) | Duration offollow-up(years) | Meanage atfirstbirth(year) | Exposure categories(exposure/caseassessment) | HR/RR (95% CI) | Matched/Adjustedfactors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dior et al.10† | 2013 | Israel | CS | Female | 386 | 40,454 | 23.8–60.9y | 37y | 23.8y | CHD: ≥10 vs. 1 (Questionnaire/Population registry) | 5.40 (1.97–14.85) | Age at first birth, mother’s origin, socioeconomic status, diabetes mellitus, gestational diabetes mellitus, toxemia, hypertension, smoking, Cesarean sections |
| Simons et al.12 | 2012 | Australia | CS | Female | N/A | 1571 | Mean, 69.6y | 16y | N/A | CHD: ≥6 vs. Nulliparous (Questionnaire/Death records) | 1.34 (0.68–2.66) | Alcohol intake, smoking, peak expiratory flow, physical disability, self-rated health and atrial fibrillation |
| Jacobs et al.11† | 2012 | United States | CS | Female | 523 | 1294 | 50–69y | 19.3y | N/A | CVD: Ever parous vs. Nulliparous ≥4 vs. Nulliparous CHD: Ever parous vs. Nulliparous ≥4 vs. Nulliparous (Questionnaire/Death registry) | 0.99 (0.74–1.33) 0.63 (0.40–0.99) 1.29 (0.84–1.98) 0.87 (0.45–1.71) | Age, years postmenopause, BMI, and high-density lipoprotein cholesterol |
| Jaffe et al.19§ | 2011 | United States | CS | Female | 1857 | 62,822 | 45–89y | 9y | N/A | CVD: Ever parous vs. Nulliparous ≥8 vs. Nulliparous (Questionnaire/Death registry) | 0.45 (0.39–0.55) 0.77 (0.59–0.98) | N/A |
| Jacobsen et al.15† | 2011 | United States | CS | Female | 1149 | 19,688 | ≥25y | 10.7y | N/A | IHD: Ever parous vs. Nulliparous ≥5 vs. Nulliparous (Questionnaire/Death registry) | 0.93 (0.77–1.12) 1.12 (0.78–1.59) | Marital status, level of education, and age at first delivery (parity number) |
| Gallagher et al.14† | 2011 | China | CS | Female | 7125 | 267,400 | ≥30y | 11y | N/A | IHD: Ever parous vs. Nulliparous ≥5 vs. Nulliparous (Questionnaire/Death registry) | 0.89 (0.59–1.34) 1.05 (0.55–2.01) | Age |
| Chang et al.13 | 2011 | Korea | CS | Female | 478 | 3257 | Mean, 66.8y | 20y | 21.3y | CVD: ≥8 vs. 0–4 CHD: ≥8 vs. 0–4 (Questionnaire/Death records) | 1.20 (0.94–1.54) 1.80 (0.82–3.98) | Age at entry, BMI, hypertension, drinking, smoking, education, and occupation |
| Koski-Rahikkala et al.16† | 2006 | Finland | CS | Female | 251 | 12002 | 49–83y | 35y | 22.8y | CVD: Parity: ≥10 vs. 1 (Questionnaire/Death records) | 2.33 (0.69–7.87) | Age, socioeconomic position, pre-pregnancy BMI, smoking before pregnancy, age at menarche and age at first birth |
| Cooper et al.18 | 1999 | United States | CS | Female | 45 | 867 | 63–81y | 5y | N/A | IHD: Ever parous vs. Nulliparous Parity: ≥4 vs. Nulliparous (Questionnaire/Death records) | 0.78 (0.40–1.51) 0.88 (0.40–1.97) | Age |
| Steenland et al.17† | 1996 | United States | CS | Female | 4787 | 585,455 | ≥30y | 8y | N/A | CHD: Ever parous vs. Nulliparous Parity: ≥6 vs. Nulliparous (Questionnaire/Death records) | 0.90 (0.83–0.98) 0.94 (0.83–1.08) | Age, race, smoking, baseline health status, blue collar status, education, exercise, hypertension medication use, BMI, estrogen use, and vegetable consumption. |
BMI: body mass index; CHD: coronary heart disease; CI: confidence interval; CS: cohort study; CVD: cardiovascular disease; IHD: ischemic heart disease; N/A: not available; RR: relative risk.
†Recalculate the RR by the method proposed by Hamling et al.
§Odds ratio and 95% CI calculated from published data using EpiCalc 2000.
The information of study quality is described in Supplementary Table S1. Briefly, three studies13,14,15 were not assigned a star in the column of “representativeness of the exposed cohort” because these studies utilized special populations. Three studies13,16,17 were assigned two stars in the column of “control for important factor or additional factor” because they adjusted for more than two important confounders in the primary analyses. Two studies17,18 were not assigned a star in the column of “follow-up long enough for outcomes to occur” because the follow-up periods were less than ten years. Three studies12,13,19 were not assigned a star in the column of “adequacy of follow-up of cohorts” because the follow-up rates were less than 75%.
Ever parity versus nulliparous
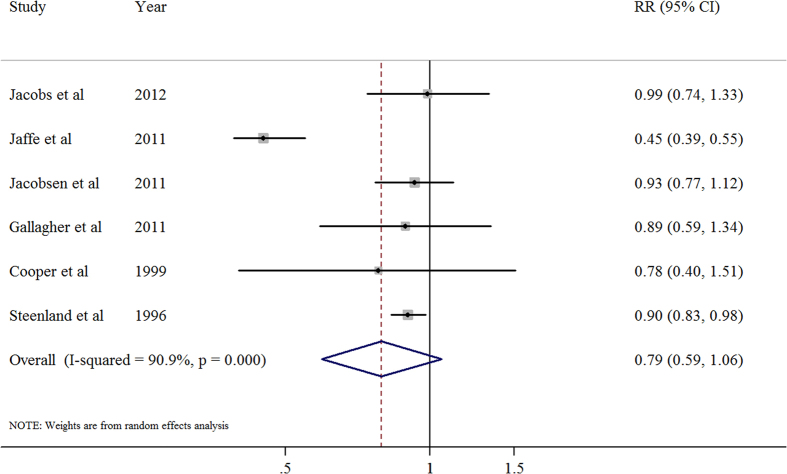
Six studies11,14,15,17,18,19 focused on the relationship between ever parity and CVD mortality. The summary relative risk of CVD for the ever parity compared with nulliparous was 0.79 (95% CI, 0.60–1.06), with significant heterogeneity (I2 = 90.9%; P < 0.001; Fig. 1). We observed no publication bias through Egger’s test (P = 0.760) or Begg’s test (P = 0.260) (Supplementary Figure S1).
Figure 1. Association between ever parity and cardiovascular disease mortality.
Dose-response analysis of parity number
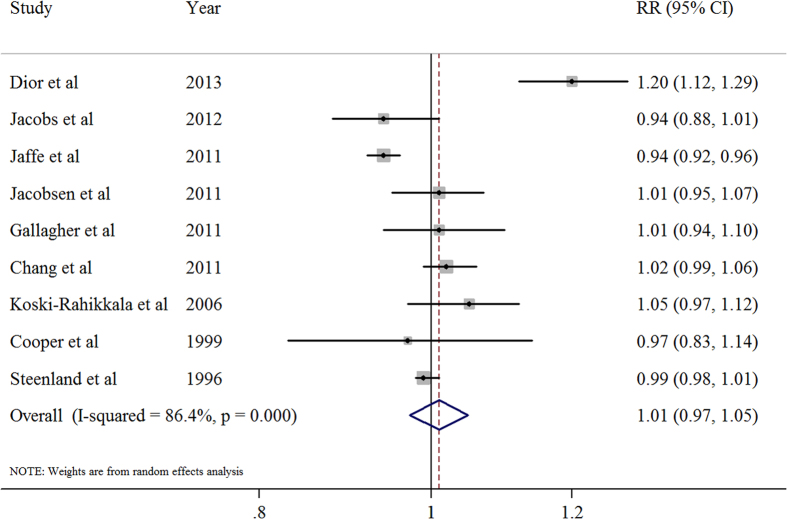
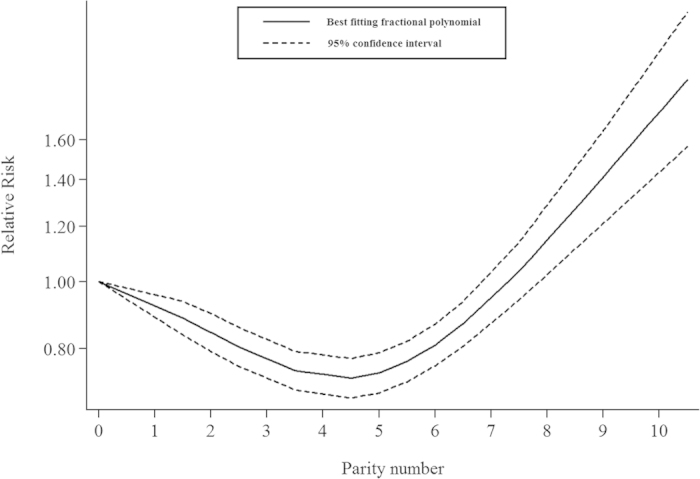
We included nine prospective studies10,11,13,14,15,16,17,18,19 in the dose-response analysis. The summary risk estimates for per live birth was 1.01 (95% CI, 0.97–1.05), with significant heterogeneity (I2 = 86.4%; P < 0.001; Fig. 2). In addition, we observed a significant nonlinear relationship between parity number and CVD mortality (P < 0.001). There was evidence of a J-shaped association in the non-linear dose-response meta-analysis of parity number and CVD mortality. The association of parity number with CVD mortality appears to follow an inversely linear dose-response pattern until the parity number reaches four live births. After this point, the association appears to rebound (Fig. 3).
Figure 2. The relative risk(RR) for per live birth.
Figure 3. Dose-response analysis of parity number.
Subgroup and sensitivity analysis
In the subgroup analysis of per one live birth and CVD mortality, we found non-significant results in the majority of the strata. However, there was a significant difference among the summarized results of the studies whether adjustment for cigarette smoking (Table 2), which might be partially responsible for the significant heterogeneity of the main result.
Table 2. Summary risk estimates of the association between parity number and cardiovascular disease mortality (per 1 live birth).
| No. of | Summary RR | I2 Value | Ph* | |
|---|---|---|---|---|
| studies | (95% CIs) | (%) | ||
| Overall | 9 | 1.01 (0.97–1.05) | 86.4 | <0.001 |
| Subgroup analyses | ||||
| Number of cases | ||||
| ≤500 | 4 | 1.07 (0.98–1.16) | 82.8 | 0.001 |
| >500 | 5 | 0.97 (0.94–1.01) | 78.3 | 0.001 |
| Geographic Location | ||||
| North America | 5 | 0.97 (0.94–1.01) | 77.3 | 0.001 |
| Europe | 1 | 1.05 (0.98–1.12) | N/A | N/A |
| Asia | 3 | 1.07 (0.97–1.19) | 88.5 | <0.001 |
| Adjustment for potential confounders | ||||
| Age | ||||
| Yes | 7 | 1.03 (0.98–1.07) | 82.2 | <0.001 |
| No | 2 | 0.97 (0.90–1.04) | 79.9 | 0.026 |
| BMI | ||||
| Yes | 4 | 1.00 (0.97–1.03) | 58.5 | 0.065 |
| No | 5 | 1.02 (0.93–1.12) | 91.3 | <0.001 |
| DM | ||||
| Yes | 1 | 1.20 (1.12–1.29) | N/A | N/A |
| No | 8 | 0.99 (0.96–1.02) | 75.2 | <0.001 |
| Hypertension | ||||
| Yes | 3 | 1.06 (0.98–1.14) | 93.0 | <0.001 |
| No | 6 | 0.98 (0.94–1.03) | 65.2 | 0.013 |
| Alcohol drinking | ||||
| Yes | 1 | 1.02 (0.99–1.06) | N/A | N/A |
| No | 8 | 1.01 (0.97–1.05) | 87.2 | <0.001 |
| Cigarette smoking | ||||
| Yes | 4 | 1.05 (0.99–1.12) | 90.0 | <0.001 |
| No | 5 | 0.95 (0.93–0.97) | 46.7 | 0.112 |
BMI: body mass index; CI: confidence interval; DM: diabetes mellitus; N/A: not available; RR: relative risk.
*P value for heterogeneity within each subgroup.
In the sensitivity analysis, the six study-specific RR of the ever parity versus nulliparous ranged from a low of 0.76 (95% CI, 0.54–1.06; I2 = 92.5%; P < 0.001) after omission of the study by Jacobs and colleagues11 to a high of 0.91 (95% CI, 0.85–0.98; I2 = 0%; P = 0.957) after omission of the study by Jaffe and colleagues19. Additionally, The 9 study-specific RR of the parity number ranged from a low of 0.99 (95% CI, 0.96–1.02; I2 = 77.1%; P < 0.001) after omission of the study by Dior and colleagues10 to a high of 1.02 (95% CI, 0.98–1.06; I2 = 80.0%; P < 0.001) after omission of the study by Jaffe and colleagues19,20.
Discussion
Findings from this meta-analysis demonstrate a borderline significant reduced risk of CVD mortality in ever parity versus nulliparous women (RR = 0.79, 95% CI: 0.60–1.06). Additionally, non-linear dose-response analysis displays a potential J-shaped relationship between parity number and CVD mortality. We firstly comprehensively and quantitatively evaluate the relationship between parity and CVD mortality.
Several potential mechanisms might have been proposed but the exact biologic mechanisms are not fully understood. Previous studies showed that complicated metabolic changes, such as dyslipidemia, abnormal glucose tolerance and increased body mass index, developed with increasing parity number20,21. Besides, as pregnancy itself can be regarded as a state of increased insulin resistance, recurrent pregnancies may result in an additive effect on the later insulin resistance, which may give an explanation to the positive association between high (>4) parity and cardiovascular mortality16. The protective effect from moderate parity for CVD may link with the enhanced endothelial function in pregnancy, which results in greater bioavailable nitric oxide11. It is worth mentioning that increased endothelial function from pregnancy, unlike its concurrent metabolic change and other temporary disorders, may continue postpartum22.
As for the associations between endogenous estrogen exposure and CVD mortality, however, former studies have reported conflicting results13. Numerous pregnancies always result in prolonged exposure to high levels of estrogen and progesterone, which may reduce the risk of CVD. However, numerous pregnancies also relate to older maternal age, inflammation and oxidative stress, which are tightly associated with adverse predictors to CVD. It is also possible that higher fertility may reflect women who are better in general and therefore at relatively lower risk for CVD mortality11. However, increased risk of CVD was more likely to be attributable to lifestyle factors such as anxiety, stress, even fear of raising children6,13. Socioeconomic factors may also enhance the relationship between parity and CVD mortality, because both CVD and high parity have higher frequency in lower social classes10.
Compared to nulliparous, our meta-analysis shows that ever parity has a significantly protective effect against the death caused by CVD within a limited number of parity. Interestingly, as the 10 included cohorts displayed different viewpoints of parity and CVD mortality, the published information on this issue is scarce and inconsistent. Some studies reported a non-linear association between parity and CVD mortality10,19. For example, Dior et al.10 reported that higher mortality rates were observed for mothers of 1 child (HR = 1.18; 95% CI, 1.04–1.4), mothers of 5–9 children (HR = 1.21; 95% CI, 1.09–1.33), and mothers of ≥10 children (HR = 1.49; 95% CI,1.12–1.99) than mothers of 2–4, which was similar to the findings of present study. Additionally, Gallagher et al.14 showed slightly increased risk of CVD mortality associated with more than five births while Jaffe confirmed that the risk estimates of CVD mortality were higher among women with no children (HR 2.43, CI 1.49, 3.96) and women with more than 8 children (HR 1.64, CI 1.02, 2.65) than those with two children19,20. In contrast, a cohort study reported by Jacobs et al. suggested that women with more than 4 pregnancies were at lower CVD mortality risk11, with further reduction of mortality as parity increases12. Conversely, several studies denied the direct association between parity and CVD mortality13,15. These conflicting results from previous investigations make the present study more meaningful. Possible reasons for this discrepancy include a variation of cohort size, different races, subgroup analysis and so on. The results of the present study suggest that women with 4 or 5 children have the lowest risk of CVD induced mortality.
When we carried out the analysis of parity and CVD mortality by geographic location, significant positive association were observed in Israelite10. Considering limited studies from other European countries(Table 1), the interpretation of the results should be taken restrainedly. Although more American studies focused on this topic11,15,17,18,19, the results were still confusing. Nevertheless, we found few studies from Asia and only two of them focused on this topic from China14 and Korea13, which both showed borderline relationship between parity and CVD mortality without statistical significance. However, comparing with the reports from northern hemisphere, Simons and colleagues12, from Australia, reported that increased parity was associated with a tendency for decreased risk of CVD mortality, but without notably statistical significance either.
Our meta-analysis has several strengths. The included studies showed conflicting results, which may result from their limited statistical range and power. Our study, however, was conducted based on approximately 994,810 study participants and 16,601 CVD cases from 10 cohort studies. This massive database generated a stronger statistical power for us to detect and verify this putative association. Moreover, prospective design also helped to make the present study more robust by eliminating selection bias and recall bias. At the same time, our approach with meta-analysis toward the studies was one of the powerful tools to assess the role of parity in the risk of CVD mortality. To guarantee the analysis quality, this meta-analysis had a big sample size, and the follow-up duration was considerably long. Although outcome evidence from long-term randomized trials is ideal, these studies are too difficult to implement on a practical basis, especially regarding reproductive factors.
Meanwhile, several limitations should also be acknowledged. First, the discovered information toward the relationship between parity and mortality from subtypes of CVD and each diagnosis criteria was limited, which remained this topic open to further research in the field of observational cohort studies. Second, parity is associated with several other factors, such as gestational hypertension, gestational diabetes mellitus, gynecological tumor and so on, which are established risk factors for CVD. Third, higher deliveries was generally associated with unhealthy factors such as a higher possibility of abortion, oral contraceptive and so on. The observed association between moderate parity and a lower risk of CVD is unlikely to consider these confounders. Fourth, delivery mode was not taken into account in our study., natural labor and caesarean section might lead to different prognoses. Last, the quality of individual original studies varied. Quality scoring might ignore some important information or introduce somewhat arbitrary subjective factors into the analysis because of combining disparate study features into a single score. Although we used the Newcastle-Ottawa Scale (NOS)23 to assess the quality of included studies, we did not score these studies or describe them as high or low quality quantitatively. Studies included in this review were all prospective studies and met most validity criteria, but three of them13,14,15 utilized special populations, four studies14,15,18,19 adjusted for limited confounders in the primary analyses, two studies17,18 didn’t follow up for at least ten years, and the follow-up rates of three studies12,13,19 were less than 75%. These flaws might bring some bias, which should be paid more attention by investigators in the future. We have observed a significant heterogeneity across studies in our analysis pool, showing notable clinical relevance. We believe this may result from our large sample size, which can confer greater statistical power to heterogeneity tests.
In conclusion, the present meta-analysis suggests a potential non-linear J-shaped relationship between the number of parity and CVD mortality. Since the number of included studies was limited, further studies are warranted to confirm our findings as well as to stratify the results by the types of CVD and other risk factors to eliminate the residual confounding.
Methods
Search Strategy
We applied the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) as the guidance to conduct and report this study24. A holistic review of the published articles (through the end of March 2015) with limitation to humans was performed by using MEDLINE (PubMed), EMBASE and ISI Web of Science databases. We used the following search strategy and keywords: (reproduction or reproductive factors or livebirth or pregnancy or parity) and (cardiovascular diseases or coronary heart disease) and (cohort study or prospective study). Furthermore, the listed article references were also examined and analyzed for additional studies.
Study Selection
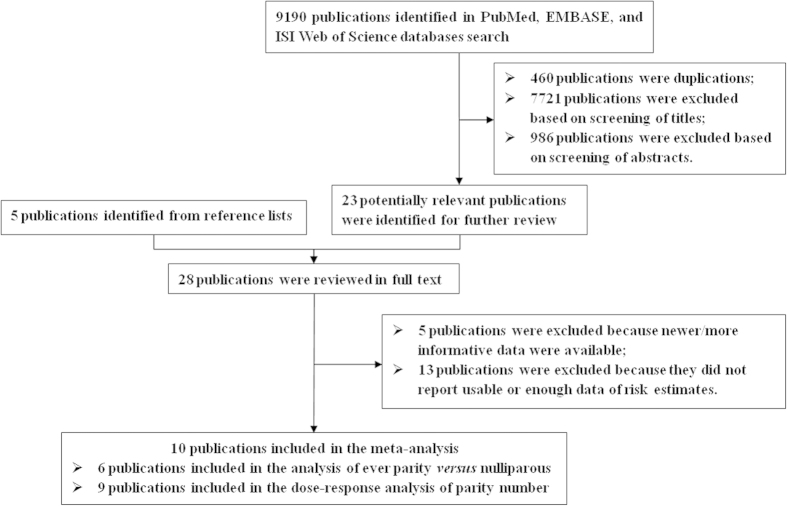
The literature was selected by 4 criteria: (1) the study had a prospective design; (2) the exposure was either parity or the number of livebirth; (3) the outcome was mortality from CVD, coronary heart diseases (CHD), or ischemic heart disease (IHD); and (4) risk estimates with 95% confidence intervals (CIs). If multiple literatures were based on the same population, we chose the literature with larger sample size. We identified 10 potentially relevant prospective studies10,11,12,13,14,15,16,17,18,19 from 7973 articles (Fig. 4). Four studies10,12,13,16 only reported the results of highest compared with lowest number of livebirth, which were only brought into the analysis of parity number.
Figure 4. Selection of studies for inclusion in meta-analysis.
Data Extraction
Two independent investigators (Haichen Lv and Hongyi Wu) evaluated the eligibility and abstracted the data of each study. Discrepancies were settled by discussion. We summarized the following data from the selected studies: name of first author, year of publication, study’s country and design, sample size, follow-up year, exposure and outcome method, adjusted risk estimates and their 95%CIs of each included study for ever parity compared with nulliparous, and potential confounders adjusted for in the primary analysis. We also calculated the risk estimate from the raw data demonstrated in the literature19 when it was not presented in the study.
Quality Assessment
The Newcastle-Ottawa Scale (NOS)23 included 3 quality parameters for cohort studies: the selection of study groups, comparability of groups and ascertainment of either the exposure or outcome of interest was used by two independent researchers (Haichen Lv and Hongyi Wu) to assess study quality.
Statistical Analysis
Considering heterogeneity between the studies, we used fixed-effects25 or the random-effects model26 to calculate summary risk estimate and 95% CIs for the ever parity compared with nulliparous and for the dose-response analysis. The method proposed by Hamling et al.27 was used to recalculate RRs for studies10,11,14,15,16,17 that did not use the category with the lowest number of parity as the reference or those that reported the risk estimates of each exposed category instead of combined estimates.
The methods described by Greenland et al.28 and Orsini et al.29 were used for the dose-response analysis, which require the distribution of cases and person-years or non-cases and the RRs with the variance estimates for at least three quantitative exposure categories30. We assigned the median or mean level of parity number in each category to the corresponding RR for each study demonstrated in the literature. We estimated the mean duration in each category by calculating the average of the lower and upper bound for studies that reported parity number by ranges of duration. We made the assumption that the open-ended interval length was the same as the adjacent interval when the highest category was open-ended. We set the lower bound to zero. We examined a potential nonlinear dose-response relationship between parity number and CVD risk using fractional polynomial models31, when the lowest category did not have a lower bound. We determined the best-fitting second-order fractional polynomial regression model, defined as the one with the lowest deviance. We used a likelihood ratio test to assess the difference between the nonlinear and linear models to test for nonlinearity32. The dose-response results are presented at one live birth increments. Finally, we carried out a sensitivity analysis by excluding each study in turn to evaluate the impact of individual data set on the overall estimate.
In the studies, heterogeneity was assessed by using the I2 statistics25. Subgroup analyses were carried out based on the CVD case number (≤500 versus >500), geographic location (North America, Europe, and Asia), and whether adjustment for potential confounders. Small study bias, such as publication bias, was evaluated via Egger’s test33, Begg’s test34, and funnel plots. All statistical analyses were conducted via Stata software (version 11.2; StataCorp). P values were two sided with a significance level of 0.05.
Additional Information
How to cite this article: Lv, H. et al. Parity and Cardiovascular Disease Mortality: a Dose-Response Meta-Analysis of Cohort Studies. Sci. Rep. 5, 13411; doi: 10.1038/srep13411 (2015).
Supplementary Material
Acknowledgments
Thanks to the authors who kindly provided the data necessary for our meta-analysis. Our work was supported by National Natural Science Foundation of China[81300152].
Footnotes
Author Contributions This study was conceived and designed by H.L. and J.G. H.L., H.W. and J.Y. did the data collection and data analysis, and wrote the main manuscript text along with preparing the tables and figures. H.L., J.Q. and J.G. supervised the data collection and data analysis and critically revised the manuscriptdid. All authors reviewed the manuscript.
References
- Bonow R. O., Smaha L. A., Smith S. J., Mensah G. A. & Lenfant C. World Heart Day 2002: The International Burden of Cardiovascular Disease: Responding to the Emerging Global Epidemic. Circulation. 106, 1602–1605 (2002). [DOI] [PubMed] [Google Scholar]
- Jones T. H. Testosterone Deficiency: A Risk Factor for Cardiovascular Disease? Trends Endocrinol Metab. 21, 496–503 (2010). [DOI] [PubMed] [Google Scholar]
- Colditz G. A. et al. A Prospective Study of Age at Menarche, Parity, Age at First Birth, and Coronary Heart Disease in Women. Am J Epidemiol. 126, 861–870 (1987). [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E. et al. Factors Associated with Glucose and Insulin Levels in Healthy Postmenopausal Women. Diabetes Care. 19, 333–340 (1996). [DOI] [PubMed] [Google Scholar]
- Skilton M. R., Serusclat A., Begg L. M., Moulin P. & Bonnet F. Parity and Carotid Atherosclerosis in Men and Women: Insights Into the Roles of Childbearing and Child-Rearing. Stroke. 40, 1152–1157 (2009). [DOI] [PubMed] [Google Scholar]
- Lawlor D. A. et al. Is the Association Between Parity and Coronary Heart Disease Due to Biological Effects of Pregnancy Or Adverse Lifestyle Risk Factors Associated with Child-Rearing? Findings From the British Women’s Heart and Health Study and the British Regional Heart Study. Circulation. 107, 1260–1264 (2003). [DOI] [PubMed] [Google Scholar]
- Atsma F. et al. Reproductive Factors, Metabolic Factors, and Coronary Artery Calcification in Older Women. Menopause. 15, 899–904 (2008). [DOI] [PubMed] [Google Scholar]
- Catov J. M. et al. Parity and Cardiovascular Disease Risk Among Older Women: How Do Pregnancy Complications Mediate the Association? Ann Epidemiol. 18, 873–879 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertuccio P., Tavani A., Gallus S., Negri E. & La Vecchia C. Menstrual and Reproductive Factors and Risk of Non-Fatal Acute Myocardial Infarction in Italy. Eur J Obstet Gynecol Reprod Biol. 134, 67–72 (2007). [DOI] [PubMed] [Google Scholar]
- Dior U. P. et al. Association Between Number of Children and Mortality of Mothers: Results of a 37-Year Follow-Up Study. Ann Epidemiol. 23, 13–18 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. B., Kritz-Silverstein D., Wingard D. L. & Barrett-Connor E. The Association of Reproductive History with All-Cause and Cardiovascular Mortality in Older Women: The Rancho Bernardo Study. Fertil Steril. 97, 118–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L. A., Simons J., Friedlander Y. & McCallum J. Childbearing History and Late-Life Mortality: The Dubbo Study of Australian Elderly. Age Ageing. 41, 523–528 (2012). [DOI] [PubMed] [Google Scholar]
- Chang H. S., Odongua N., Ohrr H., Sull J. W. & Nam C. M. Reproductive Risk Factors for Cardiovascular Disease Mortality Among Postmenopausal Women in Korea: The Kangwha Cohort Study, 1985–2005. Menopause. 18, 1205–1212 (2011). [DOI] [PubMed] [Google Scholar]
- Gallagher L. G. et al. Reproductive History and Mortality From Cardiovascular Disease Among Women Textile Workers in Shanghai, China. Int J Epidemiol. 40, 1510–1518 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen B. K., Knutsen S. F., Oda K. & Fraser G. E. Parity and Total, Ischemic Heart Disease and Stroke Mortality. The Adventist Health Study, 1976–1988. Eur J Epidemiol. 26, 711–718 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski-Rahikkala H., Pouta A., Pietilainen K. & Hartikainen A. L. Does Parity Affect Mortality Among Parous Women? J Epidemiol Community Health. 60, 968–973 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenland K., Lally C. & Thun M. Parity and Coronary Heart Disease Among Women in the American Cancer Society CPS II Population. Epidemiology. 7, 641–643 (1996). [DOI] [PubMed] [Google Scholar]
- Cooper G. S. et al. Menstrual and Reproductive Risk Factors for Ischemic Heart Disease. Epidemiology. 10, 255–259 (1999). [PubMed] [Google Scholar]
- Jaffe D. H., Eisenbach Z. & Manor O. The Effect of Parity On Cause-Specific Mortality Among Married Men and Women. Matern Child Health J. 15, 376–385 (2011). [DOI] [PubMed] [Google Scholar]
- Jaffe D. H., Neumark Y. D., Eisenbach Z. & Manor O. Parity-Related Mortality: Shape of Association Among Middle-Aged and Elderly Men and Women. Eur J Epidemiol. 24, 9–16 (2009). [DOI] [PubMed] [Google Scholar]
- Zhang X. et al. Pregnancy, Childrearing, and Risk of Stroke in Chinese Women. Stroke. 40, 2680–2684 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarelainen H. et al. Flow Mediated Vasodilation and Circulating Concentrations of High Sensitive C-reactive Protein, Interleukin–6 and Tumor Necrosis Factor-Alpha in Normal pregnancy–The Cardiovascular Risk in Young Finns Study. Clin Physiol Funct Imaging. 29, 347–352 (2009). [DOI] [PubMed] [Google Scholar]
- Wells G. A. et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. (2015) http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Date of access: 1/April/2015).
- Stroup D. F. et al. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA. 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying Heterogeneity in a Meta-Analysis. Stat Med. 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-Analysis in Clinical Trials. Control Clin Trials. 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Hamling J., Lee P., Weitkunat R. & Ambuhl M. Facilitating Meta-Analyses by Deriving Relative Effect and Precision Estimates for Alternative Comparisons From a Set of Estimates Presented by Exposure Level Or Disease Category. Stat Med. 27, 954–970 (2008). [DOI] [PubMed] [Google Scholar]
- Greenland S. & Longnecker M. P. Methods for Trend Estimation From Summarized Dose-Response Data, with Applications to Meta-Analysis. Am J Epidemiol. 135, 1301–1309 (1992). [DOI] [PubMed] [Google Scholar]
- Orsini N., Li R., Wolk A., Khudyakov P. & Spiegelman D. Meta-Analysis for Linear and Nonlinear Dose-Response Relations: Examples, an Evaluation of Approximations, and Software. Am J Epidemiol. 175, 66–73 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan H. B., Wu L., Wu Q. J., Zhu J. & Gong T. Parity and Pancreatic Cancer Risk: A Dose-Response Meta-Analysis of Epidemiologic Studies. PLoS One. 9, e92738 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P. A Strategy for Modelling the Effect of a Continuous Covariate in Medicine and Epidemiology. Stat Med. 19, 1831–1847 (2000). [DOI] [PubMed] [Google Scholar]
- Bagnardi V., Zambon A., Quatto P. & Corrao G. Flexible Meta-Regression Functions for Modeling Aggregate Dose-Response Data, with an Application to Alcohol and Mortality. Am J Epidemiol. 159, 1077–1086 (2004). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey S. G., Schneider M. & Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ. 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics. 50, 1088–1101 (1994). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.