Abstract
Background
Cardiopulmonary exercise testing allows for assessment of cardiac and respiratory limitation, but is often affected by patient effort. Indices of oxygen kinetics, including the oxygen uptake efficiency slope (OUES), oxygen uptake–work-rate slope (VO2–WR slope) and the heart rate–oxygen uptake slope (HR–VO2 slope) are relatively effort independent but may be affected by patient characteristics.
The objective of this study is to identify the impact of factors, such as age, gender, body size, respiratory function, smoking and beta-blockade on these parameters, as well as generate predictive equations.
Methods
1708 volunteers from the population-based Study of Health in Pomerania underwent an incremental bicycle exercise protocol. Markers of oxygen kinetics were calculated. Participants with structural heart disease, echocardiographic or lung function pathology were excluded, leaving 577 males and 625 females. Age, height, weight, smoking, forced expiratory volume in 1 s (FEV1) and beta-blockers were analysed for their influencing power by gender. Quantile regression analysis determined the reference equations for each parameter.
Results
Age, gender, height, weight and FEV1 (but not percent predicted FEV1) are strongly related to OUES. Participants using beta-blockers and male smokers had significantly lower OUES values. VO2–WR slope was minimally affected by age, gender, weight and FEV1. Gender, height, weight and beta-blocker use, but not FEV1 and smoking status, were related to the HR–VO2 slope whilst age was only related in females.
Conclusions
Markers of oxygen kinetics are differentially affected by patient characteristics. This study provides normal reference values for these variables thereby facilitating interpretation of oxygen uptake kinetics in health and disease.
Keywords: Cardiopulmonary exercise testing, Oxygen uptake efficiency slope, Reference values, Study of Health in Pomerania
1. Background
Recently new cardiopulmonary exercise test (CPET) parameters, beyond peak oxygen uptake (peakVO2) have demonstrated an improved ability to predict mortality, in various forms of chronic circulatory (i.e. heart) failure [1–4].
The oxygen uptake efficiency slope (OUES) has greater prognostic power than the established parameters peakVO2 and the minute ventilation to carbon dioxide production (VE/VCO2) slope [1]. OUES is the slope of the relationship between oxygen uptake (VO2) and minute ventilation logarithmically transformed (log10VE) [5,6]. Little is known about the impact of common clinical factors on OUES. Whilst OUES appears to be a simple ratio, it is, in reality, an absolute change in VO2 during a relative change in ventilation (as this is logarithmically corrected) and therefore will be dependent on the absolute VO2. OUES might therefore be related to variables like weight that affect resting and exercise VO2.
The Study of Health in Pomerania (SHIP) is a large population cohort study and recently published the influence of factors such as age, gender, body size, smoking and beta-blockade on many of the more commonly used CPET parameters such as peakVO2 [7,8]. A sample of participants within the SHIP population also provides predictive equations for a European population, and, unlike previous studies [9–11], the distribution of data was across both genders and a wide age range.
The objective of the present study is to identify the impact of factors, such as age, gender, body size, respiratory function, smoking and beta-blockade, on newer parameters of oxygen kinetics that can easily be calculated from a standard incremental exercise test, as well as generate predictive equations. These parameters include the OUES, the slope of the relationship between oxygen uptake and work-rate (VO2–WR slope) and the slope of the relationship between heart rate and oxygen uptake (HR–VO2 slope).
2. Methods
2.1. Study population
SHIP is a population-based project in the north-east of Germany. Study details are given elsewhere [12,13]. In brief, a sample from 212,157 inhabitants living in the area was selected from the population registration offices, where all German inhabitants are registered. A two-stage cluster sampling method was adopted from the World Health Organization Monitoring Cardiovascular Disease Project in Augsburg, Germany. A representative sample, comprising 7008 adults aged 20–79 years with 292 persons of each sex in each of the twelve 5-year age strata, was drawn. The net sample (without migrated or deceased people) consisted of 6267 eligible subjects, of whom 4308 individuals participated in the baseline study of SHIP (SHIP-0). Data collection started in 1997 and finished in 2001, and from March 2003 until July 2006, a 5-year follow-up examination was performed (SHIP-1). The sample (without migrated, deceased or non-responding people) then comprised 3300 subjects (1589 males, 1711 females) aged 25–85 years. Of those, 1708 individuals (834 males and 874 females) volunteered for a standardised progressive incremental cycle exercise test. All participants gave written informed consent. The study conformed to the principles of the Declaration of Helsinki as reflected by an a priori approval of the Ethics Committee of the University of Greifswald (Greifswald, Germany).
2.2. Pre-exercise diagnostics and exclusion criteria
The definition of cardiopulmonary disorders was based on self-reported physician's diagnosis, use of specific medication, electro- or echocardiographic pathological findings, and lung function abnormalities measured by spirometry and body plethysmography [14,15]. Reasons for exclusion are reported elsewhere [7]. Initial certification of all physicians and technicians involved in the study was awarded after a minimum of 3 months of training. The data collection phase was monitored by a Data Safety and Monitoring Committee.
Lung function testing was performed as part of the SHIP protocol, but only forced expiration in 1 s (FEV1) was used in this analysis to assess impact on oxygen kinetic parameters.
2.3. Exercise testing
A symptom-limited, physician supervised exercise test using one calibrated electromagnetically braked cycle-ergometer (Ergoselect100; Ergoline, Bitz, Germany) was performed according to a modified Jones protocol [9] (stepwise increase in work-rate of 16 W/min, starting with unloaded cycling). Gas exchange variables were analysed breath-by-breath using a VIASYS Healthcare system (Oxycon Pro or Rudolph's mask; VIASYS GmbH, Hoechberg, Germany). Prior to each test, equipment was calibrated in standard fashion with reference gas and volume calibration. Each test was preceded by a ≥ 3 min resting period. Participants were encouraged to reach maximal exhaustion. All tests were performed according to current guidelines [16,17], with continuous 12-lead ECG monitoring and blood pressure measurements with a standard cuff sphygmomanometer. Minute ventilation (VE), tidal volume (VT), VO2 and VCO2 were averaged over 10-second intervals.
Off-line calculations of OUES, VO2–WR slope and HR–VO2 slope were conducted using Matlab (Mathworks, Natick, Mass, USA) using only data points during exercise. OUES was defined as the slope of the regression line of VO2 plotted against log10VE. VO2–WR slope was defined as the slope of the regression line of VO2 plotted against work-rate. HR–VO2 slope was defined as the slope of the regression line of heart rate plotted against VO2.
For OUES, the current predictive equations were compared to those previously published [6] using the median male and female within each 10-year age group and BMI ≤ 25 vs > 25 kg/m2.
2.4. Statistical analysis
Age, height, weight and BMI were assessed as the independent continuous variables using regression analysis with the CPET parameters as the dependent variables. The cohort was divided into 5 age ranges and 2 body mass index (BMI) groups for graphical data representation. ANOVA was used for categorical potential confounders such as gender, smoking and beta-blocker use. We used quantile regression to construct median, 5th and 95th percentile predictive equations [18]. Based on the results of the regression analysis for each parameter individually, age, gender, height and weight, beta-blocker use, smoking status (graded as current or non-smoker — former smokers are graded with non-smokers) and FEV1 could be included as co-variates in the model (in keeping with the previous reference ranges published for peakVO2, anaerobic threshold and O2-pulse). Each variable was only added to the regression equation for each parameter individually following a significant contribution in the univariate analyses. These co-variates were also included in a multivariate model and the beta co-efficients calculated to show the impact of each co-variate on the model.
A p-value of < 0.05 was considered significant throughout.
2.5. Results
Characteristics of study participants are shown in Table 1. There were 1203 participants following clinical exclusions, and one further participant's exercise data was non-interpretable. Age range was 25–84 years (males) and 25–80 years (females, n = 625).
Table 1.
Baseline characteristics of all participants and the final 1202 following exclusions. All 1708 participants who undertook CPET testing are included in the first 5 columns, whilst only those included in the final analysis (1202) following exclusions (as described in the text) are shown in the latter columns. Subjects are divided into 10 year age categories and by gender, with information shown on body mass index (BMI), presence of hypertension (HTN), beta-blocker use (percentage using), current smokers (final cohort only) and peak VO2.
| All CPET participants |
Final 1202 participants |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Subjects | BMI (kg/m2) | HTN (%) | Beta-blockers (%) | PeakVO2 (ml/min/kg) | Subjects | BMI (kg/m2) | HTN (%) | Diabetics (%) | Smokers (%) | Beta-blockers (%) | HbA1c | Total cholesterol | LDL | HDL | PeakVO2 (ml/min/kg) |
| 25–34 | 210 | 170 | ||||||||||||||
| M | 98 | 26.1 ± 3.6 | 29.6 | 5.1 | 34.3 ± 6.8 | 79 | 25.7 ± 3.2 | 26.6 | 0 | 41.8 | 0 | 5.0 ± 0.4 | 5.1 ± 1.0 | 3.3 ± 0.9 | 1.1 ± 0.4 | 34.5 ± 6.3 |
| F | 112 | 24.4 ± 5.0 | 26.8 | 6.25 | 27.9 ± 6.3 | 91 | 24.3 ± 4.8 | 26.4 | 0 | 35.1 | 1.1 | 4.8 ± 0.4 | 4.7 ± 0.9 | 2.8 ± 0.8 | 1.4 ± 0.4 | 28.1 ± 6.2 |
| 35–44 | 381 | 307 | ||||||||||||||
| M | 191 | 26.9 ± 3.6 | 43.4 | 5.8 | 32.1 ± 7.0 | 154 | 26.8 ± 3.5 | 42.9 | 0.6 | 31.2 | 1.9 | 5.1 ± 0.4 | 5.7 ± 1.0 | 3.7 ± 1.0 | 1.0 ± 0.4 | 32.2 ± 7.0 |
| F | 190 | 25.0 ± 4.3 | 36.8 | 4.2 | 25.8 ± 5.1 | 153 | 24.8 ± 4.0 | 63.6 | 1.3 | 38.6 | 1.3 | 4.8 ± 0.5 | 5.2 ± 1.0 | 3.2 ± 0.9 | 1.4 ± 0.4 | 26.1 ± 5.0 |
| 45–54 | 411 | 286 | ||||||||||||||
| M | 189 | 28.3 ± 4.2 | 45.0 | 13.2 | 27.7 ± 6.2 | 129 | 27.8 ± 3.7 | 44.2 | 7.0 | 35.9 | 3.9 | 5.4 ± 0.8 | 5.9 ± 1.1 | 3.8 ± 1.0 | 1.1 ± 0.4 | 28.4 ± 6.4 |
| F | 222 | 27.4 ± 5.2 | 41.4 | 18.5 | 22.7 ± 4.3 | 157 | 27.2 ± 5.2 | 39.5 | 1.3 | 25.4 | 7.0 | 5.1 ± 0.5 | 5.7 ± 1.2 | 3.6 ± 1.1 | 1.4 ± 0.4 | 22.8 ± 4.1 |
| 55–64 | 426 | 257 | ||||||||||||||
| M | 196 | 27.9 ± 3.5 | 39.3 | 28.1 | 26.5 ± 5.5 | 117 | 27.6 ± 3.4 | 37.6 | 5.1 | 19.7 | 8.5 | 5.5 ± 0.8 | 5.9 ± 1.0 | 3.9 ± 0.9 | 1.0 ± 0.3 | 27.5 ± 5.6 |
| F | 230 | 28.2 ± 4.9 | 46.5 | 27.4 | 21.2 ± 4.5 | 140 | 27.6 ± 4.6 | 50.0 | 2.9 | 15.7 | 11.4 | 5.4 ± 0.6 | 6.2 ± 1.0 | 4.0 ± 0.9 | 1.4 ± 0.4 | 22.0 ± 3.9 |
| > 65 | 384 | 183 | ||||||||||||||
| M | 216 | 28.1 ± 4.2 | 32.4 | 37.5 | 22.1 ± 5.4 | 99 | 27.7 ± 4.5 | 39.4 | 10.1 | 9.1 | 21.2 | 5.7 ± 0.8 | 5.4 ± 1.2 | 3.6 ± 1.0 | 1.0 ± 0.3 | 23.7 ± 5.4 |
| F | 168 | 28.2 ± 4.5 | 41.7 | 41.1 | 19.0 ± 4.0 | 84 | 26.9 ± 4.1 | 45.2 | 10.7 | 8.3 | 28.6 | 5.6 ± 0.6 | 6.1 ± 1.1 | 3.9 ± 0.9 | 1.4 ± 0.5 | 19.7 ± 3.6 |
| Total | 1812 | 1202 | ||||||||||||||
| M | 890 | 27.6 ± 3.9 | 38.5 | 19.9 | 27.8 ± 7.4 | 577 | 27.2 ± 3.8 | 39.3 | 4.5 | 27.6 | 6.8 | 5.3 ± 0.7 | 5.7 ± 1.1 | 3.7 ± 1.0 | 1.0 ± 0.3 | 29.3 ± 7.1 |
| F | 922 | 26.9 ± 5.0 | 40.0 | 20.4 | 22.9 ± 5.5 | 625 | 26.3 ± 4.8 | 40.0 | 2.7 | 25.6 | 8.6 | 5.1 ± 0.6 | 5.6 ± 1.2 | 3.5 ± 1.0 | 1.4 ± 0.4 | 23.8 ± 5.3 |
Beta-blocker use is specifically indicated within the tables, however other anti-hypertensive agents were also used including ACE inhibitors or angiotensin-receptor blockers (13% of participants), calcium channel blockers (6%), diuretics (6%), alpha-blockers for hypertension (1%) and other agents (2%).
2.6. Confounding factors on cardiopulmonary exercise testing
The impact of gender, age and BMI is shown in Table 2.
Table 2.
Median values (5th, 95th percentiles) for the 3 CPET parameters. Shown for males and females in the 5 age categories and by weight (as divided into 2 categories — normal weight BMI ≤ 25 kg/m2 and overweight BMI > 25 kg/m2).
| Age | OUES | VO2–WR slope | HR–VO2 slope | |
|---|---|---|---|---|
| Males | 25–34 | 2841 (1861, 3675) | 10.2 (8.1, 11.6) | 0.041 (0.03, 0.057) |
| Normal weight | 35–44 | 2665 (1770, 4330) | 10.1 (7.1, 11.1) | 0.043 (0.029, 0.057) |
| 45–54 | 2524 (1703, 3255) | 9.9 (8.8, 11.1) | 0.041 (0.026, 0.061) | |
| 55–64 | 2391 (1758, 3565) | 9.9 (9, 11.4) | 0.042 (0.034, 0.061) | |
| > 65 | 2249 (1478, 3172) | 9.9 (4.8, 11.1) | 0.043 (0.027, 0.07) | |
| Overweight | 25–34 | 3094 (2380, 4304) | 10.4 (8.7, 12) | 0.035 (0.025, 0.049) |
| 35–44 | 2951 (2160, 3963) | 10.2 (8.5, 11.6) | 0.036 (0.024, 0.049) | |
| 45–54 | 2764 (1909, 4019) | 10 (8.3, 11.7) | 0.034 (0.022, 0.054) | |
| 55–64 | 2520 (1862, 3833) | 9.9 (8.1, 11.3) | 0.037 (0.022, 0.054) | |
| > 65 | 2322 (1534, 2971) | 10.1 (7.5, 11.5) | 0.035 (0.02, 0.052) | |
| Females | ||||
| Normal weight | 25–34 | 1958 (1505, 2907) | 9.3 (8.2, 11.3) | 0.058 (0.04, 0.084) |
| 35–44 | 1908 (1266, 2672) | 9.3 (7.4, 10.5) | 0.058 (0.038, 0.082) | |
| 45–54 | 1823 (1236, 2431) | 9.2 (7.4, 11.3) | 0.062 (0.043, 0.092) | |
| 55–64 | 1620 (1166, 2211) | 9.5 (7.7, 11.3) | 0.059 (0.04, 0.08) | |
| > 65 | 1594 (1209, 2060) | 10 (7.5, 11.7) | 0.053 (0.035, 0.074) | |
| Overweight | 25–34 | 2090 (1516, 2965) | 9.5 (8.3, 10.4) | 0.05 (0.031, 0.07) |
| 35–44 | 2152 (1642, 3058) | 9.4 (7.9, 10.9) | 0.049 (0.033, 0.075) | |
| 45–54 | 2065 (1403, 2680) | 9.4 (7.6, 11.4) | 0.053 (0.028, 0.075) | |
| 55–64 | 1814 (1294, 2515) | 9.7 (7.9, 11.2) | 0.048 (0.024, 0.071) | |
| > 65 | 1571 (1101, 2232) | 10.1 (7.4, 12.2) | 0.046 (0.028, 0.077) | |
Gender was a determinant of OUES (R2 = 0.38, p < 0.0001) and HR–VO2 slope (R2 = 0.30, p < 0.0001), with a weaker interaction with VO2–WR slope (R2 = 0.04, p < 0.0001). Further determinants were assessed for males and females separately. The relationships between determinants and parameters are shown in Table 3.
Table 3.
Relationship between patient characteristics and three cardiopulmonary exercise test parameters. Table A) males and B) females. The R2 value indicates the rough contribution of the independent determinant (age, height, weight, body mass index (BMI), FEV1, beta-blocker use (BB) and current smoking status) to the variability of the three dependent CPET parameters. A p < 0.05 indicates statistical significance. The R2 value is calculated using linear regression analysis (for continuous variables) or ANOVA (for categorical variables). R2 values with a preceding (−) indicate an inverse relationship (i.e. an increase in the magnitude of a continuous determinant causes a reduction in the CPET parameter, or the presence of the determinant, for example with beta-blockers, also causes a reduction in the parameter). Significant co-variates on univariate analysis were added to a multivariate model with the resulting beta co-efficient within this model indicated.
| A |
OUES |
VO2–WR slope |
HR–VO2 slope |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Determinant | Strength of relationship (R2) | p-value | Beta co-efficient | Strength of relationship (R2) | p-value | Beta co-efficient | Strength of relationship (R2) | p-value | Beta co-efficient |
| Age (years) | (−)0.17 | < 0.001 | − 12.0 | (−)0.02 | 0.001 | − 0.006 | 0.00 | 0.66 | − 0.00007 |
| Height (cm) | 0.18 | < 0.001 | + 8.8 | 0.00 | 0.77 | (−)0.06 | < 0.001 | − 0.00007 | |
| Weight (kg) | 0.12 | < 0.001 | + 11.2 | 0.01 | 0.046 | + 0.005 | (−)0.23 | < 0.001 | − 0.00033 |
| BMI (kg/m2) | 0.02 | < 0.001 | 0.01 | 0.032 | (−)0.17 | < 0.001 | |||
| FEV1 (L) | 0.22 | < 0.001 | + 161.0 | 0.03 | < 0.001 | + 0.156 | 0.01 | 0.031 | − 0.00067 |
| % FEV1 (%) | 0.01 | 0.037 | 0.01 | 0.003 | 0.00 | 0.48 | |||
| BB (yes/no) | (−)0.01 | 0.010 | − 112.7 | 0.00 | 0.36 | (−)0.02 | 0.003 | − 0.00442 | |
| Smoker (yes/no) | (−)0.01 | 0.011 | − 253.5 | 0.00 | 0.86 | 0.00 | 0.48 | ||
| B | OUES | VO2–WR slope | HR–VO2 slope | ||||||
| Determinant | Strength of relationship (R2) | p-value | Beta co-efficient | Strength of relationship (R2) | p-value | Beta co-efficient | Strength of relationship (R2) | p-value | Beta co-efficient |
| Age (years) | (−)0.12 | < 0.001 | − 9.9 | 0.02 | 0.001 | + 0.007 | (−)0.02 | < 0.001 | − 0.00006 |
| Height (cm) | 0.14 | < 0.001 | + 12.5 | 0.00 | 0.37 | (−)0.02 | < 0.001 | − 0.00031 | |
| Weight (kg) | 0.10 | < 0.001 | + 9.7 | 0.01 | 0.015 | + 0.008 | (−)0.21 | < 0.001 | − 0.00045 |
| BMI (kg/m2) | 0.03 | < 0.001 | 0.01 | 0.003 | (−)0.17 | < 0.001 | |||
| FEV1 (L) | 0.12 | < 0.001 | + 3.3 | 0.01 | 0.003 | − 0.135 | 0.01 | 0.054 | − 0.00303 |
| % FEV1 (%) | 0.00 | 0.54 | 0.00 | 0.19 | 0.01 | 0.012 | |||
| BB (yes/no) | (−)0.01 | 0.004 | − 127.6 | 0.00 | 0.90 | (−)0.02 | < 0.001 | − 0.00457 | |
| Smoker (yes/no) | 0.01 | 0.006 | + 1.2 | 0.00 | 0.17 | 0.00 | 0.43 | ||
In both males and females, age was strongly inversely related to OUES. The VO2–WR slope was only weakly, though significantly, related to age; in males this relationship is positive and inverse in females. The HR–VO2 slope had a weak, albeit significant, relation to age in females only.
Height had a strong, positive relationship to OUES in males and females. The VO2–WR slope was not related to height. The HR–VO2 slope was weakly, inversely related to height in both genders. Weight was strongly positively related to OUES. The VO2–WR slope was weakly related to weight in both genders. The HR–VO2 slope was strongly, inversely related to weight in males and females. Importantly BMI did not relate as strongly to any of the 3 parameters as height and weight as co-variates, and the addition of BMI to a simple model of height and weight did not increase the predictive power of the model (i.e. no change in R2) suggesting that height and weight, but not BMI should be used in the more complex regression models.
FEV1 was strongly related to OUES and less strongly to the VO2–WR slope, however when percentage of predicted FEV1 was used these were only weakly related in males and not in females.
OUES was significantly lower in males and females taking beta-blockers (p < 0.001). However beta-blocker use increased with age, which may explain this difference. Only in the age group of over 65 did beta-blocker use significantly decrease OUES. The VO2–WR slope was not different in people who took beta-blockers and those that didn't. The HR–VO2 slope was lower (mean = 0.041) in people taking beta-blockers than in those who didn't (mean = 0.047). There appeared to be a more noticeable effect on females (mean = 0.055 versus 0.046) compared to males (mean = 0.038 versus 0.032).
Smoking did not relate to VO2–WR slope or the HR–VO2 slope, and had a weak inverse relationship with OUES in males and a weak positive relationship in females.
2.7. Reference distribution
The effect of age, gender, height, weight, beta-blocker use, current smoking, and FEV1 on the predictive equations for these three parameters is shown in Table 4.
Table 4.
Reference ranges predictive equations for three CPET parameters. A subject's predicted average (50th percentile) and range of normality (5th and 95th percentiles) are calculated using age (A) in years, height (H) in cm, weight (W) in kg, and FEV1 (f) in L. Beta blocker intake (bb) is coded as ‘0’ for no and ‘1’ for yes. Current smoking (cs) is coded as ‘0’ for no and ‘1’ for yes.
| Parameter | Percentile | Value | Equation |
|---|---|---|---|
| Females | |||
| OUES | 5th | 1272.9 | − 727 − 6.98A + 11.82H + 6.56 W − 47.65bb − 10.65cs + 15.29f |
| 50th | 1876.3 | − 182.4 − 8.89A + 10.12H + 10.51W − 117.65bb − 21.45cs + 40.31f | |
| 95th | 2613.6 | 36.5 − 18.47A + 15.55H + 12.63W − 317.24bb − 91.98cs + 13.47f | |
| VO2–WR slope | 5th | 7.66 | 7.28 − 0.01A + 0.02 W − 0.04f |
| 50th | 9.47 | 8.82 + 0.01A + 0.01W − 0.1f | |
| 95th | 11.3 | 10.61 + 0.02A + 0.01W − 0.23f | |
| HR–VO2 slope | 5th | 0.0320 | 0.0897 − 0.0001A − 0.0002H − 0.0004 W − 0.0021bb + 0.0027f |
| 50th | 0.0544 | 0.1327 − 0.0001A − 0.0003H − 0.0004 W − 0.0042bb + 0.0027f | |
| 95th | 0.0796 | 0.2279 + 0.0001A -0.0008H-0.0005 W − 0.01bb + 0.0038f | |
| Males | |||
| OUES | 5th | 1783.5 | 54.7 − 9.82A + 4.42H + 11.74 W − 227bb − 169cs + 180.72f |
| 50th | 2703.5 | 907.7 − 11.51A + 5.67H + 8.62W − 49.99bb − 214.53cs + 172.97f | |
| 95th | 3951.4 | − 2380 − 13.97A + 34.48H + 9.54W − 251.11bb − 523.24cs − 0.47f | |
| VO2–WR slope | 5th | 8.06 | 9.31 − 0.02A − 0.01W + 0.17f |
| 50th | 10.06 | 9.63 − 0.01A + 0.01W + 0.07f | |
| 95th | 11.47 | 9.98 − 0.003A + 0.01W + 0.17f | |
| HR–VO2 slope | 5th | 0.0236 | 0.0686 − 0.0001A − 0.0001H − 0.0002 W − 0.0016bb + 0.0004f |
| 50th | 0.0368 | 0.0743 − 0.0001A − 0.0003W − 0.0039bb − 0.0006f | |
| 95th | 0.0548 | 0.0795 − 0.0001A + 0.00023H − 0.0005 W − 0.002bb − 0.0051f | |
3. Discussion
Here we demonstrate, for three novel CPET parameters, the influence of common variables, as well as a set of contemporary predictive equations.
Understanding the impact of individual characteristics on parameters is important when interpreting the results, rather than using a “one-size fits all” approach. Gender, age, height and weight are variables known or measured in all subjects, and are expected to influence CPET parameters, however we can see from these results that their impact is variable. The parameters here described focus on oxygen kinetics, and as such may tell us more about the heart's role in gas exchange rather than the lungs'. This is supported by the influence of beta-blockade on most of these parameters, with the relative lack of influence from percentage of predicted FEV1 and smoking.
Understanding the impact of variables on CPET parameters allows the generation of predictive equations, which are necessary in order to establish boundaries of normality for patients. Previous predictive equations should be revisited as populations change. As CPET availability has grown, the number of commonly reported parameters has also expanded. In many cases predictive values are either based on small studies or on studies where participants recruited may not necessarily be representative of the whole population. It is expected that over 50% of patients will have values below average, therefore a measure of the spread of normality is more important than a measure of the average. Quantile regression allows the calculation of the 5th and 95th percentiles without any effect from significant outliers beyond these boundaries.
3.1. Oxygen uptake efficiency slope
OUES is accepted as a largely effort-independent parameter [5]. Therefore in patients who stop prematurely the OUES may remain applicable to diagnosing the extent of cardiorespiratory limitation. It has excellent intra-test and test–retest reliability [6,19], and can discriminate severity and prognosis in heart failure patients [1,20,21].
In our study, gender, age, height and weight influenced OUES, with lower values in females, and older, shorter, less heavy individuals. This can be explained by total oxygen uptake which, unlike a relative change in ventilation (i.e. logarithmically corrected), is dependent on the mass of metabolising tissue. The use of beta-blockers also significantly lowered OUES, although after correction for age this relationship only persisted in the oldest group of patients (on average patients taking beta-blockers were 13 years older). FEV1 was strongly related to OUES, however this is largely due to the very strong relationship FEV1 has with height and age, such that once corrected for these, FEV1 was no longer strongly associated. Smoking displays a weak, albeit significant, relationship with OUES, which is different in direction between males and females. Although these reach significance, the small R2 value indicates that the magnitude of influence is very small, i.e. unlikely to be clinically relevant. These observations suggest that OUES may be largely independent of respiratory function. However within healthy individuals the lungs are almost never the limiting organ [22,23], and because we excluded participants with significant respiratory abnormalities, even those with the worst respiratory function retained within the analysis may still have significant redundancy within the lungs to allow for normal oxygen uptake. Overall we show a strong relationship between the independent variables age, height and weight and the measured OUES, whilst the other described variables are of limited relevance.
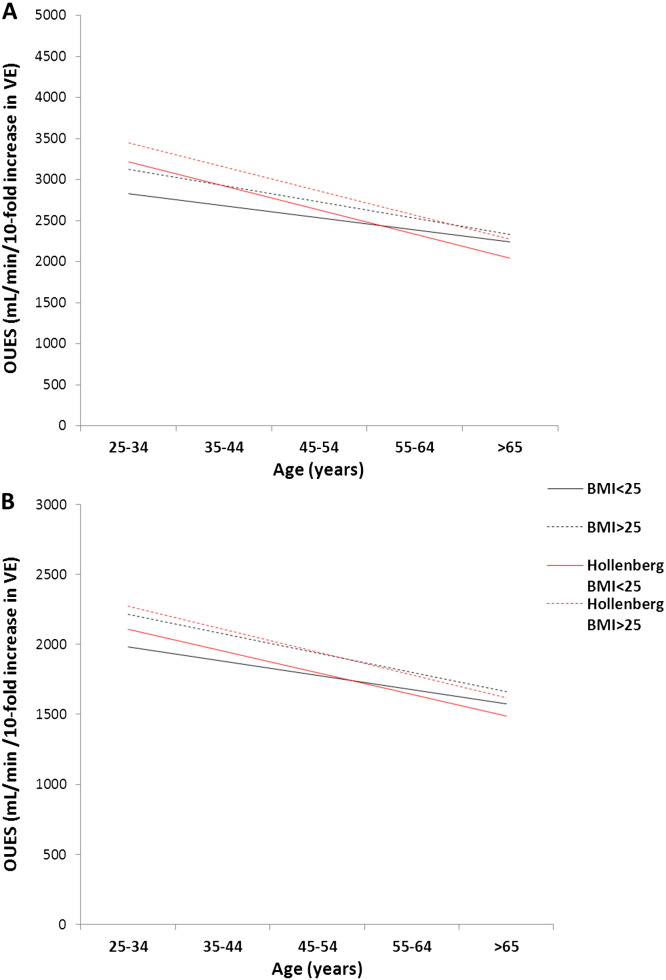
The established adult predictive equations for OUES have two significant limitations [6]. Firstly the patients were all older (the youngest is 53 years). As the effect of age on OUES was extrapolated from this data it may not be applicable for younger patients. Secondly calculations for the average, rather than reference ranges, are shown. We therefore feel that our current predictive equations supersede these. The difference between Hollenberg's equation [6] and the current study's can be seen graphically in Fig. 1. Unsurprisingly these previous equations appear to over-predict the average in younger patients.
Fig. 1.
A comparison of oxygen uptake efficiency slope (OUES) predictive equations. We compare our current equation to those from a previously accepted reference range for OUES, for A) males and B) females with relation to age and subdivided according to body mass index (BMI) groups  BMI ≤ 25 kg/m2,
BMI ≤ 25 kg/m2,  BMI > 25 kg/m2. The median participant within each age decile had their predicted OUES calculated using both references ranges. The lines represent the regression slope of these median subjects only and for the Hollenberg equation use the body surface area and age.
BMI > 25 kg/m2. The median participant within each age decile had their predicted OUES calculated using both references ranges. The lines represent the regression slope of these median subjects only and for the Hollenberg equation use the body surface area and age.
3.2. Oxygen uptake to work-rate relationship
The linear slope of work-rate against VO2 is felt to reflect aerobic work efficiency, relating to oxygen delivery and utilisation at the muscle [24]. It is thus believed to be primarily influenced by cardiac rather than respiratory function. What is interesting about this parameter is its relative independence from gender, age and weight where the magnitude of influence is small despite statistical significance.
Previous studies identified almost identical average values of 10 mL/min/W in healthy subjects with a standard deviation of 0.7–1.0 mL/min/W [10,25]. In patients with heart failure, peripheral vascular disease and myopathies a reduced slope occurs. In contrast, extremely fit cyclists have been found to have an increased slope of 11.5 mL/min/W [26]. These studies however have been small or limited to male gender; accurate confidence intervals will therefore be limited in their applicability. We therefore believe that this new predictive equation should supersede previous data. In our cohort age barely affects median predicted values, however there does appear to be widening of the 95% percentiles of normality with advancing age. BMI has little effect on the slope, which is consistent with previous studies [10,25].
3.3. Heart rate to oxygen uptake relationship
In the absence of significant pulmonary disease there are three main components influencing oxygen uptake: heart rate, stroke volume (together measured as cardiac output) and oxygen extraction at the tissue; the interaction of these three can be assessed. Typically the relationship of VO2/heart rate, the O2-pulse, is used. Given that for the majority of subjects peak oxygen extraction is believed to be near equal under standard conditions [27], the O2-pulse is used largely as a surrogate for stroke volume. However this has a similar limitation to peakVO2; sub-maximal effort may fail to produce a true reflection of peak exercise stroke volume. An alternative is the heart rate–VO2 relationship or slope, with the typical response a linear progression of heart rate and VO2. A comparable parameter discussed previously in the literature is the heart rate response (HRR) equal to (HRpeak − HRrest) / (VO2peak − VO2rest). The slope of the regression line of HR versus VO2 will be very similar but will incorporate all data points, and we therefore believe will be more robust (resting values are especially susceptible to variability). Although a value of 50 beats/L as the upper limit of normal for HRR has been proposed, the authors are unaware of any studies validating a normal range of either the HRR or the HR–VO2 slope. Importantly there are multiple clinical utilities of this parameter. A healthy subject stopping prematurely through non-cardiac limitation will display a normal slope; both maximum heart rate and peakVO2 are similarly reduced from predicted maxima and this makes it a more useful parameter than the O2-pulse in these cases. In patients with heart failure the slope may either be elevated (when heart rate responses are maintained) or decreased (when there is associated chronotropic incompetence). The latter is more commonly seen, especially in the modern era of beta-blockade. Another cause of an elevated slope is mitochondrial myopathies [28] where HRR values above 50 beats/L were found, reflecting the increased dependency of oxygen uptake on heart rate to compensate for reduced oxygen extraction at the muscle.
The results of the present study show markedly higher median values amongst females compared to males, with minimal impact from ageing. Generally the slope is reduced in overweight patients (although the intercept of the HR–VO2 relationship is higher, so that, for any given VO2, the heart rate may actually be higher — results not shown). Certainly within a female population, a HR–VO2 slope of 0.05 beats/mL (equivalent to a HRR of 50 beats/L), would be considered a normal finding based upon our data.
3.4. Limitations
SHIP employed a standardised protocol that was the same for all subjects. In clinical practice it is common to adjust the protocol so that exercise duration might last 8–12 min. Four hundred and three participants did not achieve 8 min; 258 participants achieved > 12 min. It is established that shorter, more intense exercise protocols may decrease the VO2–WR slope [24,29]. Therefore we may see a greater range of normal values for this parameter given the large spread of maximum exercise times.
This study was conducted in a single region. Typical values in other countries may be different; however for other industrialised countries they are likely to be similar to our results. It is possible that there is selection bias; the participants were volunteers, and individuals who are adverse to physical activity would be less likely to volunteer. However, participants with subclinical cardiorespiratory disorders and clinical diseases were excluded from the present analysis, and the sample undergoing exercise testing was comparable to the general SHIP cohort with the exception of having fewer hypertensive subjects and fewer smokers [7]. It is possible that by principally using FEV1 to define respiratory disease we may have included some participants with preserved FEV1 but early, asymptomatic lung disease. However we used FEV1 because it was felt that this was more likely to be affected than other spirometric variables in the majority of cases of lung pathology.
With a recruitment age between 25 and 84 these results may not be applicable for younger individuals. It is likely that maximum exercise capacity occurs somewhere between 20–35 years of age; when testing adults aged 18–25, an age of 25 would seem prudent to enter into the equation, although this has not been validated.
We have only denoted beta-blocker and other anti-hypertensive agents use as yes/no. Data recorded did not specify dose or type of beta-blocker which could potentially affect the results but given the vast range of doses and beta-blockers statistical analysis of the impact of this on CPET parameters would be complex.
4. Conclusions
Over recent years cardiopulmonary exercise testing is gaining acceptance for a multitude of clinical functions. Here we present determinants of CPET parameters and contemporary reference ranges from a large European cross-sectional population study for three potentially effort-independent parameters associated with the kinetics of oxygen uptake. These parameters will be most useful in patients unable to attain a cardiopulmonary maxima from their test, but also appear to have specific clinical diagnostic utilities. Our study reports not only the median value but also the lower 95% confidence limit, in order to be maximally helpful in clinical practice.
Funding sources
DPF was supported by the Senior Clinical Fellowship programme of the British Heart Foundation (FS/10/038).
AB and RW were supported by a British Heart Foundation Project Grant (PG/11/36/28883).
SHIP is part of the Community Medicine Net of the University of Greifswald, which is funded by grants from the German Federal Ministry of Education and Research for SHIP (BMBF, grant 01ZZ96030, 01ZZ0701) and German Asthma and COPD Network (COSYCONET; BMBF grant 01GI0883); the Ministry for Education, Research, and Cultural Affairs; and the Ministry for Social Affairs of the Federal State of Mecklenburg — West Pomerania.
Conflict of interest statement
The authors have no conflicts of interest to declare.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agreed to the manuscript as written.
Acknowledgements
The contribution to data collection made by field workers, technicians, interviewers, and computer assistants is gratefully acknowledged. Furthermore we would like to thank the participants of SHIP.
References
- 1.Davies L.C., Wensel R., Georgiadou P. Enhanced prognostic value from cardiopulmonary exercise testing in chronic heart failure by non-linear analysis: oxygen uptake efficiency slope. Eur Heart J. 2006;27:684–690. doi: 10.1093/eurheartj/ehi672. [DOI] [PubMed] [Google Scholar]
- 2.Francis D.P., Shamim W., Davies L.C. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2) Eur Heart J. 2000;21:154–161. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 3.Robbins M., Francis G., Pashkow F.J. Ventilatory and heart rate responses to exercise: better predictors of heart failure mortality than peak oxygen consumption. Circulation. 1999;100:2411–2417. doi: 10.1161/01.cir.100.24.2411. [DOI] [PubMed] [Google Scholar]
- 4.Chua T.P., Ponikowski P., Harrington D. Clinical correlates and prognostic significance of the ventilatory response to exercise in chronic heart failure. J Am Coll Cardiol. 1997;29:1585–1590. doi: 10.1016/s0735-1097(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 5.Baba R., Nagashima M., Goto M. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol. 1996;28:1567–1572. doi: 10.1016/s0735-1097(96)00412-3. [DOI] [PubMed] [Google Scholar]
- 6.Hollenberg M., Tager I.B. Oxygen uptake efficiency slope: an index of exercise performance and cardiopulmonary reserve requiring only submaximal exercise. J Am Coll Cardiol. 2000;36:194–201. doi: 10.1016/s0735-1097(00)00691-4. [DOI] [PubMed] [Google Scholar]
- 7.Koch B., Schaper C., Ittermann T. Reference values for cardiopulmonary exercise testing in healthy volunteers: the SHIP study. Eur Respir J Off J Eur Soc Clin Respir Physiol. 2009;33:389–397. doi: 10.1183/09031936.00074208. [DOI] [PubMed] [Google Scholar]
- 8.Glaser S., Koch B., Ittermann T. Influence of age, sex, body size, smoking, and beta blockade on key gas exchange exercise parameters in an adult population. Eur J Cardiovasc Prev Rehab Off J Eur Soc Cardiol Work Groups on Epidemiol Prev Card Rehab Exerc Physiol. 2010;17:469–476. doi: 10.1097/HJR.0b013e328336a124. [DOI] [PubMed] [Google Scholar]
- 9.Jones N.L., Makrides L., Hitchcock C., Chypchar T., McCartney N. Normal standards for an incremental progressive cycle ergometer test. Am Rev Respir Dis. 1985;131:700–708. doi: 10.1164/arrd.1985.131.5.700. [DOI] [PubMed] [Google Scholar]
- 10.Hansen J.E., Sue D.Y., Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129:S49–S55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 11.Storer T.W., Davis J.A., Caiozzo V.J. Accurate prediction of VO2max in cycle ergometry. Med Sci Sports Exerc. 1990;22:704–712. doi: 10.1249/00005768-199010000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Volzke H., Alte D., Schmidt C.O. Cohort profile: the Study of Health in Pomerania. Int J Epidemiol. 2011;40:294–307. doi: 10.1093/ije/dyp394. [DOI] [PubMed] [Google Scholar]
- 13.John U., Greiner B., Hensel E. Study of Health in Pomerania (ship): a health examination survey in an east German region: objectives and design. Soz Praventivmed. 2001;46:186–194. doi: 10.1007/BF01324255. [DOI] [PubMed] [Google Scholar]
- 14.Miller M.R., Hankinson J., Brusasco V. Standardisation of spirometry. Eur Respir J Off J Eur Soc Clin Respir Physiol. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 15.Hankinson J.L., Odencrantz J.R., Fedan K.B. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 16.Ross R.M. Ats/accp statement on cardiopulmonary exercise testing. Am J Respir Crit Care Med. 2003;167:1451. doi: 10.1164/ajrccm.167.10.950. [author reply 1451] [DOI] [PubMed] [Google Scholar]
- 17.Palange P., Ward S.A., Carlsen K.H. Recommendations on the use of exercise testing in clinical practice. Eur Respir J Off J Eur Soc Clin Respir Physiol. 2007;29:185–209. doi: 10.1183/09031936.00046906. [DOI] [PubMed] [Google Scholar]
- 18.Gannoun A., Girard S., Guinot C., Saracco J. Reference curves based on non-parametric quantile regression. Stat Med. 2002;21:3119–3135. doi: 10.1002/sim.1226. [DOI] [PubMed] [Google Scholar]
- 19.Van Laethem C., De Sutter J., Peersman W., Calders P. Intratest reliability and test–retest reproducibility of the oxygen uptake efficiency slope in healthy participants. Eur J Cardiovasc Prev Rehab Off J Eur Soc Cardiol Work Groups on Epidemiol Prev Card Rehab Exerc Physiol. 2009;16:493–498. doi: 10.1097/HJR.0b013e32832c88a8. [DOI] [PubMed] [Google Scholar]
- 20.Baba R., Tsuyuki K., Kimura Y. Oxygen uptake efficiency slope as a useful measure of cardiorespiratory functional reserve in adult cardiac patients. Eur J Appl Physiol Occup Physiol. 1999;80:397–401. doi: 10.1007/s004210050610. [DOI] [PubMed] [Google Scholar]
- 21.Van Laethem C., Bartunek J., Goethals M., Nellens P., Andries E., Vanderheyden M. Oxygen uptake efficiency slope, a new submaximal parameter in evaluating exercise capacity in chronic heart failure patients. Am Heart J. 2005;149:175–180. doi: 10.1016/j.ahj.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Blackie S.P., Fairbarn M.S., McElvaney N.G., Wilcox P.G., Morrison N.J., Pardy R.L. Normal values and ranges for ventilation and breathing pattern at maximal exercise. Chest. 1991;100:136–142. doi: 10.1378/chest.100.1.136. [DOI] [PubMed] [Google Scholar]
- 23.Olafsson S., Hyatt R.E. Ventilatory mechanics and expiratory flow limitation during exercise in normal subjects. J Clin Invest. 1969;48:564–573. doi: 10.1172/JCI106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hansen J.E., Sue D.Y., Oren A., Wasserman K. Relation of oxygen uptake to work rate in normal men and men with circulatory disorders. Am J Cardiol. 1987;59:669–674. doi: 10.1016/0002-9149(87)91190-8. [DOI] [PubMed] [Google Scholar]
- 25.Wasserman K., Whipp B.J. Exercise physiology in health and disease. Am Rev Respir Dis. 1975;112:219–249. doi: 10.1164/arrd.1975.112.2.219. [DOI] [PubMed] [Google Scholar]
- 26.Riley M., Wasserman K., Fu P.C., Cooper C.B. Muscle substrate utilization from alveolar gas exchange in trained cyclists. Eur J Appl Physiol Occup Physiol. 1996;72:341–348. doi: 10.1007/BF00599695. [DOI] [PubMed] [Google Scholar]
- 27.Stringer W.W., Hansen J.E., Wasserman K. Cardiac output estimated noninvasively from oxygen uptake during exercise. J Appl Physiol Respir Environ Exerc Physiol. 1997;82:908–912. doi: 10.1152/jappl.1997.82.3.908. [DOI] [PubMed] [Google Scholar]
- 28.Flaherty K.R., Wald J., Weisman I.M. Unexplained exertional limitation: characterization of patients with a mitochondrial myopathy. Am J Respir Crit Care Med. 2001;164:425–432. doi: 10.1164/ajrccm.164.3.2005110. [DOI] [PubMed] [Google Scholar]
- 29.Agostoni P., Bianchi M., Moraschi A. Work-rate affects cardiopulmonary exercise test results in heart failure. Eur J Heart Fail. 2005;7:498–504. doi: 10.1016/j.ejheart.2004.06.007. [DOI] [PubMed] [Google Scholar]