Abstract
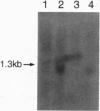
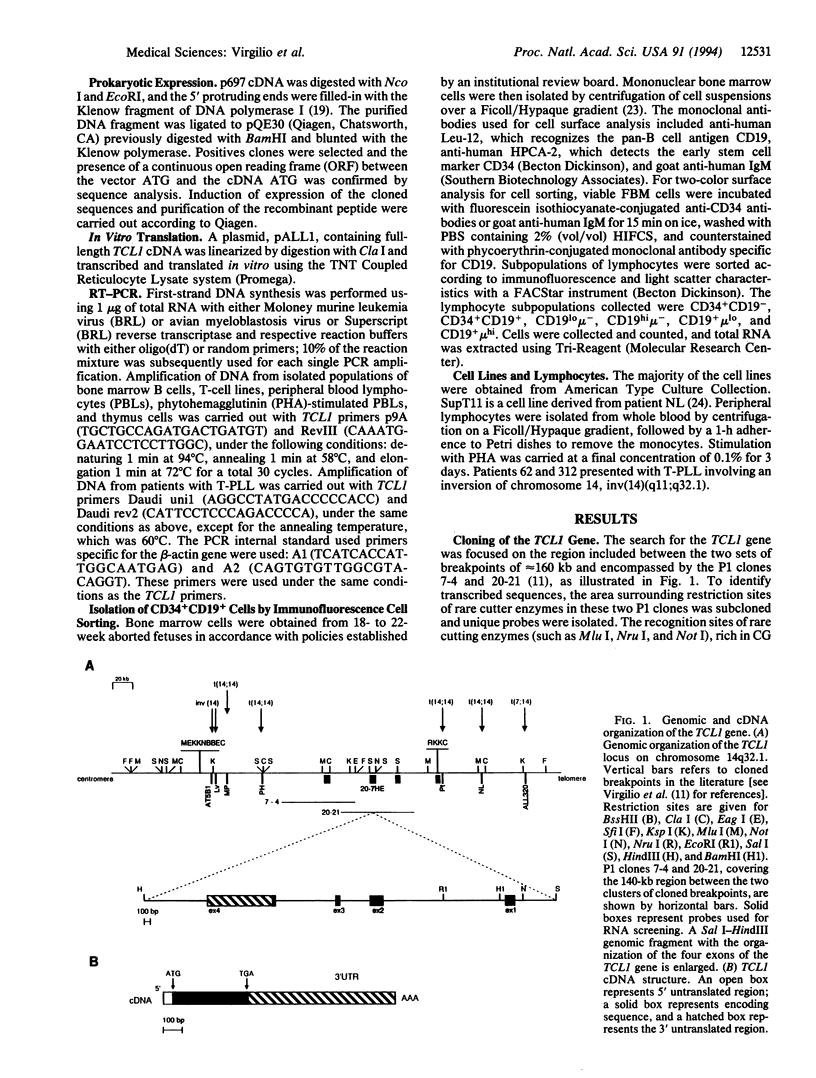
The TCL1 locus on chromosome 14q32.1 is frequently involved in chromosomal translocations and inversions with one of the T-cell receptor loci in human T-cell leukemias and lymphomas. The chromosome 14 region translocated or rearranged involves approximately 350 kb of DNA at chromosome band 14q32.1. Within this region we have identified a gene coding for a 1.3-kb transcript, expressed only in restricted subsets of cells within the lymphoid lineage and expressed at high levels in leukemic cells carrying a t(14;14)(q11;q32) chromosome translocation or a inv(14)(q11;q32) chromosome inversion. The cognate cDNA sequence reveals an open reading frame of 342 nt encoding a protein of 14 kDa. The TCL1 gene sequence, which, to our knowledge, shows no sequence homology with other human genes, is preferentially expressed early in T- and B-lymphocyte differentiation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi M., Cossman J., Longo D., Croce C. M., Tsujimoto Y. Variant translocation of the bcl-2 gene to immunoglobulin lambda light chain gene in chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2771–2774. doi: 10.1073/pnas.86.8.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer R., Chen K. C., Smith S. D., Rabbitts T. H. Fusion of an immunoglobulin variable gene and a T cell receptor constant gene in the chromosome 14 inversion associated with T cell tumors. Cell. 1985 Dec;43(3 Pt 2):705–713. doi: 10.1016/0092-8674(85)90243-0. [DOI] [PubMed] [Google Scholar]
- Baer R., Heppell A., Taylor A. M., Rabbitts P. H., Boullier B., Rabbitts T. H. The breakpoint of an inversion of chromosome 14 in a T-cell leukemia: sequences downstream of the immunoglobulin heavy chain locus are implicated in tumorigenesis. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9069–9073. doi: 10.1073/pnas.84.24.9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertness V. L., Felix C. A., McBride O. W., Morgan R., Smith S. D., Sandberg A. A., Kirsch I. R. Characterization of the breakpoint of a t(14;14)(q11.2;q32) from the leukemic cells of a patient with T-cell acute lymphoblastic leukemia. Cancer Genet Cytogenet. 1990 Jan;44(1):47–54. doi: 10.1016/0165-4608(90)90196-h. [DOI] [PubMed] [Google Scholar]
- Brito-Babapulle V., Catovsky D. Inversions and tandem translocations involving chromosome 14q11 and 14q32 in T-prolymphocytic leukemia and T-cell leukemias in patients with ataxia telangiectasia. Cancer Genet Cytogenet. 1991 Aug;55(1):1–9. doi: 10.1016/0165-4608(91)90228-m. [DOI] [PubMed] [Google Scholar]
- Buckler A. J., Chang D. D., Graw S. L., Brook J. D., Haber D. A., Sharp P. A., Housman D. E. Exon amplification: a strategy to isolate mammalian genes based on RNA splicing. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4005–4009. doi: 10.1073/pnas.88.9.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Isobe M., Palumbo A., Puck J., Ming J., Tweardy D., Erikson J., Davis M., Rovera G. Gene for alpha-chain of human T-cell receptor: location on chromosome 14 region involved in T-cell neoplasms. Science. 1985 Mar 1;227(4690):1044–1047. doi: 10.1126/science.3919442. [DOI] [PubMed] [Google Scholar]
- Croce C. M. Role of chromosome translocations in human neoplasia. Cell. 1987 Apr 24;49(2):155–156. doi: 10.1016/0092-8674(87)90552-6. [DOI] [PubMed] [Google Scholar]
- Croce C. M., Thierfelder W., Erikson J., Nishikura K., Finan J., Lenoir G. M., Nowell P. C. Transcriptional activation of an unrearranged and untranslocated c-myc oncogene by translocation of a C lambda locus in Burkitt. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6922–6926. doi: 10.1073/pnas.80.22.6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Bregni M., Erikson J., Patterson D., Gallo R. C., Croce C. M. Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 1982 Dec;79(24):7824–7827. doi: 10.1073/pnas.79.24.7824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisen L. W., Bird J., West D. C., Soreng A. L., Reynolds T. C., Smith S. D., Sklar J. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991 Aug 23;66(4):649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- Erikson J., Finger L., Sun L., ar-Rushdi A., Nishikura K., Minowada J., Finan J., Emanuel B. S., Nowell P. C., Croce C. M. Deregulation of c-myc by translocation of the alpha-locus of the T-cell receptor in T-cell leukemias. Science. 1986 May 16;232(4752):884–886. doi: 10.1126/science.3486470. [DOI] [PubMed] [Google Scholar]
- Erikson J., Nishikura K., ar-Rushdi A., Finan J., Emanuel B., Lenoir G., Nowell P. C., Croce C. M. Translocation of an immunoglobulin kappa locus to a region 3' of an unrearranged c-myc oncogene enhances c-myc transcription. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7581–7585. doi: 10.1073/pnas.80.24.7581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haluska F. G., Tsujimoto Y., Croce C. M. Oncogene activation by chromosome translocation in human malignancy. Annu Rev Genet. 1987;21:321–345. doi: 10.1146/annurev.ge.21.120187.001541. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Sakamoto H., Tsuruta H., Sasaki H., Muto T., Sugimura T., Terada M. Establishment of a highly sensitive and specific exon-trapping system. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9779–9783. doi: 10.1073/pnas.89.20.9779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe M., Russo G., Haluska F. G., Croce C. M. Cloning of the gene encoding the delta subunit of the human T-cell receptor reveals its physical organization within the alpha-subunit locus and its involvement in chromosome translocations in T-cell malignancy. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3933–3937. doi: 10.1073/pnas.85.11.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay S., Bird A. P. Use of restriction enzymes to detect potential gene sequences in mammalian DNA. 1987 May 28-Jun 3Nature. 327(6120):336–338. doi: 10.1038/327336a0. [DOI] [PubMed] [Google Scholar]
- Magrath I. T., Freeman C. B., Pizzo P., Gadek J., Jaffe E., Santaella M., Hammer C., Frank M., Reaman G., Novikovs L. Characterization of lymphoma-derived cell lines: comparison of cell lines positive and negative for Epstein-Barr virus nuclear antigen. II. Surface markers. J Natl Cancer Inst. 1980 Mar;64(3):477–483. [PubMed] [Google Scholar]
- Mengle-Gaw L., Willard H. F., Smith C. I., Hammarström L., Fischer P., Sherrington P., Lucas G., Thompson P. W., Baer R., Rabbitts T. H. Human T-cell tumours containing chromosome 14 inversion or translocation with breakpoints proximal to immunoglobulin joining regions at 14q32. EMBO J. 1987 Aug;6(8):2273–2280. doi: 10.1002/j.1460-2075.1987.tb02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokura T., Arnold A. PRAD1/cyclin D1 proto-oncogene: genomic organization, 5' DNA sequence, and sequence of a tumor-specific rearrangement breakpoint. Genes Chromosomes Cancer. 1993 Jun;7(2):89–95. doi: 10.1002/gcc.2870070205. [DOI] [PubMed] [Google Scholar]
- Nishikura K., ar-Rushdi A., Erikson J., Watt R., Rovera G., Croce C. M. Differential expression of the normal and of the translocated human c-myc oncogenes in B cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4822–4826. doi: 10.1073/pnas.80.15.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto N., Kubagawa H., Ohno T., Gartland G. L., Stankovic A. K., Cooper M. D. Normal pre-B cells express a receptor complex of mu heavy chains and surrogate light-chain proteins. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6284–6288. doi: 10.1073/pnas.88.14.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C. L., Wong E., Petty E. M., Bale A. E., Tsujimoto Y., Harris N. L., Arnold A. PRAD1, a candidate BCL1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9638–9642. doi: 10.1073/pnas.88.21.9638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo G., Isobe M., Gatti R., Finan J., Batuman O., Huebner K., Nowell P. C., Croce C. M. Molecular analysis of a t(14;14) translocation in leukemic T-cells of an ataxia telangiectasia patient. Proc Natl Acad Sci U S A. 1989 Jan;86(2):602–606. doi: 10.1073/pnas.86.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo G., Isobe M., Pegoraro L., Finan J., Nowell P. C., Croce C. M. Molecular analysis of a t(7;14)(q35;q32) chromosome translocation in a T cell leukemia of a patient with ataxia telangiectasia. Cell. 1988 Apr 8;53(1):137–144. doi: 10.1016/0092-8674(88)90495-3. [DOI] [PubMed] [Google Scholar]
- Smith S. D., McFall P., Morgan R., Link M., Hecht F., Cleary M., Sklar J. Long-term growth of malignant thymocytes in vitro. Blood. 1989 Jun;73(8):2182–2187. [PubMed] [Google Scholar]
- Tsujimoto Y., Cossman J., Jaffe E., Croce C. M. Involvement of the bcl-2 gene in human follicular lymphoma. Science. 1985 Jun 21;228(4706):1440–1443. doi: 10.1126/science.3874430. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Yunis J., Onorato-Showe L., Erikson J., Nowell P. C., Croce C. M. Molecular cloning of the chromosomal breakpoint of B-cell lymphomas and leukemias with the t(11;14) chromosome translocation. Science. 1984 Jun 29;224(4656):1403–1406. doi: 10.1126/science.6610211. [DOI] [PubMed] [Google Scholar]
- Virgilio L., Isobe M., Narducci M. G., Carotenuto P., Camerini B., Kurosawa N., Abbas-ar-Rushdi, Croce C. M., Russo G. Chromosome walking on the TCL1 locus involved in T-cell neoplasia. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9275–9279. doi: 10.1073/pnas.90.20.9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers D. A., Harvey R. C., Faust J. B., Melnyk O., Carey K., Meeker T. C. Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991 Oct;11(10):4846–4853. doi: 10.1128/mcb.11.10.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]