Abstract
Lactobacilli have been associated with dental caries for over a century. Here, we review the pertinent literature along with findings from our own study to formulate a working hypothesis about the natural history and role of lactobacilli. Unlike most indigenous microbes that stably colonize a host, lactobacilli appear to be planktonic, opportunistic settlers that can gather and multiply only in certain restrictive niches of the host, at least within the oral cavity. We postulate that the following essential requirements are necessary for sustained colonization of lactobacilli in humans: 1) a stagnant, retentive niche that is mostly anaerobic; 2) a low pH milieu; and 3) ready access to carbohydrates. Three sites on the human body meet these specifications: caries lesions, the stomach, and the vagina. Only a handful of Lactobacillus species is found in caries lesions, but they are largely absent in caries-free children. Lactobacilli present in caries lesions represent both a major contributor to caries progression and a major reservoir to the gastrointestinal (GI) tract. We extend the assertion from other investigators that lactobacilli found in the GI tract originate in the oral cavity by proposing that lactobacilli in the oral cavity arise from caries lesions. This, in turn, leads us to reflect on the health implications of the lactobacilli in the mouth and downstream GI and to ponder whether these or any of the Lactobacillus species are truly indigenous to the human GI tract or the oral cavity.
Keywords: Lactobacillus, mutans streptococci, colonization, mouth, gastrointestinal tract, natural history
Introduction
The prevailing ecological view of the etiology of dental caries is more attuned to the polymicrobial nature of the dental plaque biofilm. However, only a limited number of bacteria are consistently recovered from caries lesions and have thus been recognized to be specifically associated with dental caries (Marsh 2003). The association between lactobacilli and dental caries dates back to a century (Kligler 1915). In fact, lactobacilli were the leading candidate in the causation of dental caries prior to the 1950s, when the mutans streptococci (MS) started to dominate the literature (Badet and Thebaud 2008). Despite numerous studies linking lactobacilli to caries in both adults and children, our understanding about their role in this disease remains incomplete. Some of the major gaps in knowledge span the transmission and colonization of the oral cavity, as well as the genetic basis for the adaptation to this niche.
After several decades of near-exclusive focus on the MS paradigm as the major etiological agent of caries, the compelling role of lactobacilli warrants renewed attention to piece together the pathogenesis of dental caries. The aim of this critical review is to integrate studies from the dental and medical literature into a working model describing the contribution of lactobacilli to the caries process. We also provide a compelling argument showing that the caries lesion provides not only the necessary conditions for sustained presence of lactobacilli in the oral cavity but also the source of most lactobacilli to the gastrointestinal (GI) tract.
Natural History of the Genus Lactobacillus
The lactobacilli comprise a diverse collection of gram-positive bacilli, with the genomes ranging in size from 1.23 to 4.91 Mb and in GC content from 31.93% to 57.02% (Sun et al. 2014). This study also indicated that the genetic diversity of the Lactobacillus genus is larger than that of a typical family. The lactobacilli are aero-tolerant or anaerobic and commonly found in food, water, soil, sewage, humans, and many animals. These habitats contain carbohydrates and nutrients, where the strictly fermentative lactobacilli can eke out their catabolic needs, producing lactic acid as the main by-product. In this way, they generate a low pH environment that they can tolerate but is inhospitable for most other competing microbes.
The ability of lactobacilli to adapt to a wide variety of ecological niches is likely tied to their genomic plasticity. The adaptation to new hosts or niches is often accompanied by a reductive genome coupled with the acquisition of key additional genes by gene duplication and horizontal gene transfer (Makarova et al. 2006). The phylogenetic relationships and taxonomy of the Lactobacillus genus have been complicated by this loss or replacement of large segments of their genomes. The most recent phylogenetic reconstruction revealed that Lactobacillus is paraphyletic and that 5 other genera are grouped within the lactobacilli as subclades (Sun et al. 2014).
Due to the intimate association of lactobacilli with food, definitive statements about their indigenous (autochthonous) versus transient (allochthonous) nature must be viewed with caution. Although recent reports present compelling support for lactobacilli being stably maintained in the postmenses vagina (Ravel et al. 2011; Mendes-Soares et al. 2014) and the oral cavity (Dal Bello and Hertel 2006), which species of lactobacilli are actually indigenous to the GI tract remains contentious (Walter 2008). A case has been made that a few Lactobacillus species are thought to be part of the indigenous biota of the GI tract (Reuter 2001; Frese et al. 2011). Among the voids in defining lactobacilli as indigenous to humans is the lack of clear evidence of sustained colonization, via tissue-specific adhesins or other means.
An important clue as to the ecological requirements for colonization by lactobacilli comes from their isolation from the stomach (Ryan et al. 2008). The lining of the stomach contains retentive areas in the form of crypts and rugal folds. However, lactobacilli are not commonly found in other retentive niches of the GI tract, like the caecum and appendix (Walter 2008). The key feature that distinguishes the stomach from the rest of the GI tract is the low pH. Just outside the stomach, in the duodenum, the pH rises to around 7 and continues at or slightly above pH 7 to the distal end of the colon. We propose that the necessary colonization requirements for lactobacilli in the human GI tract, in the absence of specific adhesins, are retentive areas and low pH.
Natural History of Lactobacilli in the Oral Cavity
The relationship between lactobacilli and dental caries is well established. But can the oral cavity sustain them in the absence of caries? Several investigators posit that the oral cavity is the source of lactobacilli to the downstream GI tract (Dal Bello and Hertel 2006; Walter 2008). If so, which attributes of the oral cavity sustain their colonization? Vaginally delivered neonates have been shown to harbor lactobacilli in the oral cavity at the time of birth, which can be traced to the mother’s vagina (Carlsson and Gothefors 1975). However, they are transients and not sustained in the baby’s mouth after 1 mo. Lactobacilli are also found in the mouths of breastfed infants, while they are seldom found in bottle-fed infants (Holgerson et al. 2013; Vestman et al. 2013). After weaning and prior to tooth emergence, lactobacilli are rarely found in the oral cavity of infants (Carlsson et al. 1975).
Once teeth emerge, the occlusal fissures would seem suitable retentive sites for the colonization of lactobacilli (Loesche et al. 1984). However, the key ecological determinant for the sustained colonization of lactobacilli in the oral cavity seems to be the presence of caries. It is of historical note to mention that this relationship was established by Harrison and Opal (1944), who isolated lactobacilli from 93% of saliva samples and 66% of fecal samples of children with dental caries but not from those who were caries free. Furthermore, the phenotypic similarity between oral and fecal strains led them to postulate that the GI tract was continually seeded with lactobacilli-contaminated saliva. Modern studies of infants younger than age 6 y also showed a correlation between the presence of lactobacilli in the oral cavity and dental caries, with few caries-free children positive for lactobacilli (Leverett et al. 1993; Marchant et al. 2001; Teanpaisan et al. 2007; Piwat et al. 2010). In a previous study (Yang et al. 2010), we also failed to detect lactobacilli in the plaque or occlusal fissures of caries-free children. This observation was recently extended to 38 caries-free children 3 to 6 y of age, despite deep sampling of intact fissures (our unpublished results). In this current study, we detected signal for the presence of lactobacilli in the plaque of some caries-free children by selective amplification of the 16S ribosomal RNA (rRNA) gene using Lactobacillus-specific primers (Byun et al. 2004) but not universal primers. This finding suggests to us and others (Walter 2008) that lactobacilli may be present but in very low levels compared with the overall microbiome and may be simply contamination from food.
The composition of the oral microbiome varies greatly as individuals age (Xu et al. 2015). Nevertheless, the evidence indicates that the presence of lactobacilli in the oral cavity from childhood to adulthood is a function of whether an individual develops caries or not. In 2012, the World Health Organization stated that 60% to 90% of children and nearly 100% of adults have dental cavities (http://www.who.int/mediacentre/factsheets/fs318/en/). Given this almost universal presence of caries in modern humans, it would be interesting to monitor the levels of lactobacilli in the oral cavity and GI tract of caries-free teenagers and adults. A recent survey of healthy adults 18 to 40 y old and free of untreated caries showed a stark absence of lactobacilli in oral and stool samples (Segata et al. 2012). This suggests that lactobacilli are not present in adults without active caries lesions or that they exist below the detection level of a 16S rRNA gene survey with bacterial universal primers. In this respect, surveys with Lactobacillus-specific primers could be more informative.
In summary, lactobacilli levels seem to fluctuate in the oral cavity of young children as a result of various developmental events from birth to tooth eruption. Lactobacilli from milk may be the main source to the oral cavity of young children, at least until teeth emerge. Children who remain caries-free generally do not harbor remarkable levels of lactobacilli in the mouth, while those with caries clearly do. The presence or absence of lactobacilli in the oral cavity seems to, in turn, determine their presence or absence downstream in stool samples, pointing to the oral cavity as the main reservoir of lactobacilli for the GI tract. This has profound implications not only for overall health but also for diagnostics.
Lactobacilli and Dental Caries
Caries-specific Species of Lactobacillus
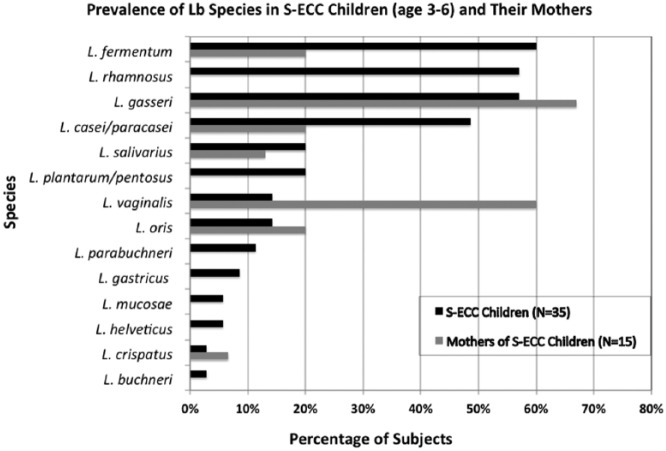
The prevalence data from different studies of Lactobacillus species associated with caries show a remarkable overall concordance in the distribution and diversity despite different caries groups and geographic locations (Tables 1, 2). One of the challenges in comparing species across studies resides in the method employed to determine species affiliations. In Tables 1 and 2, we present data only from studies in which species identification was based on the 16S rRNA gene sequence. We detected a similar constellation of Lactobacillus species in our survey of 38 children with severe early childhood caries (S-ECC) based on culture (Fig. 1). The dominant species in both adult and childhood caries include Lactobacillus fermentum, Lactobacillus rhamnosus, Lactobacillus gasseri, Lactobacillus casei/paracasei, Lactobacillus salivarius, Lactobacillus plantarum, and, in lesser prevalence, Lactobacillus oris and Lactobacillus vaginalis. Less common species included Lactobacillus mucosae, Lactobacillus crispatus, Lactobacillus ultunesis, Lactobacillus reuteri, Lactobacillus gastricus, and Lactobacillus parabuchneri. Most Lactobacillus species found in caries lesions cohabitate with other lactobacilli; only L. fermentum, L. casei/paracasei, and L. salivarius were found as the single Lactobacillus occupant of caries lesions in our study (unpublished results). All those species with prevalence below 10% in our cohort of S-ECC children (i.e., Lactobacillus buchneri, L. parabuchneri, L. gastricus, L. mucosae, and L. crispatus) (Fig. 1) were always coisolated with other lactobacilli. Moreover, based on the original isolation plates, they always comprised less than 20% of the isolates per subject. Thus, they can be presumed to be transient contaminants from food or other sources with no major role in caries, at least in our study population.
Table 1.
Lactobacillus Species Isolated from the Dentitions of Adults with Caries, Ranked by Abundance
| Ahrne et al. (1998) | Byun et al. (2004) | Munson et al. (2004) | Chhour et al. (2005) | Dal Bello and Hertel (2006) | Caufield et al. (2007) | Obata et al. (2014) |
|---|---|---|---|---|---|---|
| Oral and rectal mucosa samples from 42 adults | Adult extracted teeth | Adult caries lesions | Adult caries lesions | Saliva and feces from 3 adults | Caries-active mothers’ saliva | 22 dentin caries from subjects ages 4 to 76 y |
| Goteborg, Sweden | Sydney, Australia | London, United Kingdom | Sydney, Australia | Stuttgart, Germany | Birmingham, AL, United States | Fukuoka, Japan |
| L. plantarum | L. gasseri | L. gasseri/johnsonii | L. casei/rhamnosus | L. gasseri | L. fermentum | L. gasseri |
| L. rhamnosus | L. rhamnosus | L. rhamnosus | L. panis/reuteri | L. casei/paracasei | L. gasseri | L. paracasei |
| L. casei | unknown Lacto | L. casei | L. fermentum | L. vaginalis | L. vaginalis | L. salivarius |
| L. salivarius | L. casei/paracasei | L. pentosus/ plantarum | L. gasseri | L. rhamnosus | L. salivarius | L. vaginalis |
| L. crispatus | L. oris | L. salivarius | L. oris | L. crispatus | ||
| L. ultunensis | L. fermentum | L. ultunensis | L. fermentum | |||
| L. salivarius | L. vaginalis |
Table 2.
Lactobacillus Species Isolated from the Dentitions of Children with Caries, Ranked by Abundance
| Marchant et al. (2001) | Piwat et al. (2010) | Tanner et al. (2011) | Yang et al. (2012) | Teanpaisan et al. (2012) |
|---|---|---|---|---|
| Extracted teeth from 3- to 5-y-old children with S-ECC | Saliva from 2- to 5-y-old caries-prone children | Plaque sample from 2- to 6-y-old S-ECC children | Saliva and caries lesions from S-ECC children | 2- to 5-y-old caries-prone mother-child pairs |
| London, United Kingdom | Songkhla, Thailand | Boston, MA, United States | New York, NY, United States | Songkhla, Thailand |
| L. casei | L. fermentum | L. fermentum | L. rhamnosus | L. fermentum |
| L. fermentum | L. salivarius | L. gasseri | L. gasseri | L. casei/paracasei |
| L. rhamnosus | L. casei/paracasei | L. casei/paracasei | L. fermentum | L. rhamnosus |
| L. salivarius | L. rhamnosus | L. casei | L. salivarius | |
| L. plantarum | L. mucosae | L. oris | L. mucosae | |
| L. buchneri | L. oris | L. salivarius | L. plantarum | |
| L. brevis | L. gasseri | L. vaginalis |
S-ECC, severe early childhood caries.
Figure 1.
Prevalence of the different Lactobacillus species isolated from children with severe early childhood caries (S-ECC) (n = 35) and their mothers (n = 15); for description of study cohort, see the Appendix (Li et al. 2015).
The dominant repertoire of oral Lactobacillus species shows little overlap with that of the human vagina, composed mainly of Lactobacillus iners, L. gasseri, L. crispatus, and L. jensenii (Ravel et al. 2011; Mendes-Soares et al. 2014). Unsurprisingly, perhaps, the oral data are more consistent with the species isolated from human breast milk, comprising L. rhamnosus, L. plantarum, and L. fermentum (Martin et al. 2007), and from biopsy specimens of the stomach and intestine, consisting mainly of L. gasseri, L. reuteri, L. salivarius, L. fermentum, and L. vaginalis (Ryan et al. 2008). An oral origin has been proposed for most of the GI species (Dal Bello and Hertel 2006; Walter 2008). To our knowledge, comprehensive studies of the lactobacilli present in the mouth, GI tract, and vagina of the same population are lacking. Nevertheless, the evidence hitherto positions L. gasseri as the only species consistently found in all 3 sites.
Only a handful of Lactobacillus species out of the over 150 species currently recognized are found in the oral cavity, more specifically, in caries lesions. In addition, these species constitute a distinct repertoire, different from that of the vagina. This suggests that the oral species display a niche specificity, as yet not fully undefined. The phylogenetic relationships between the dominant oral Lactobacillus species inferred based on the sequence of the 16S rRNA gene (Salvetti 2012) or the 73 core genes (Sun et al. 2014) indicate that they belong to different clades or phylogroups. This suggests that the lactobacilli associated with dental caries did not arise from a recent common ancestor but that the adaptation to the caries niche appeared independently in different lineages. What do these Lactobacillus species have in common that makes them suited to the oral cavity and dental caries? Our group is actively engaged in determining whether there are niche-specific attributes common to caries-associated species in contrast to nonoral species of Lactobacillus. Niche-specific genes have been identified in Lactobacillus species associated with dairy and gut environments (O’Sullivan et al. 2009). On the other hand, the search for common niche-specific genetic loci for vaginal Lactobacillus species remains unresolved (Ravel et al. 2011) despite their close phylogenetic relationships (Salvetti 2012; Sun et al. 2014).
Retentive Niche: The Relationship between MS and Lactobacilli
The current caries paradigm holds that dental caries is caused by acidogenic bacteria that produce lactic acid as a result of the anaerobic fermentation of carbohydrates, coupled with their aciduric properties that allow their survival in a low pH milieu. The ability of lactobacilli and MS to ferment a variety of carbohydrates and to survive in a low pH environment is the major hallmark of the caries paradigm. Before the discovery of MS, lactobacilli were considered the major etiological agent of dental caries because of the high correlation between the Lactobacillus salivary counts and the caries scores (Loesche et al. 1984). However, the interpretation of this correlation as causation was erroneous. It is likely that the existing caries lesions harbored lactobacilli and shed them into the saliva, rather than high levels of lactobacilli predating caries. Indeed, lactobacilli have been regarded as secondary invaders rather than the primary initiator of caries, a role traditionally reserved for the MS (van Houte 1980; Badet and Thebaud 2008). van Houte and coworkers recognized that the relatively low affinity of lactobacilli for teeth implied that mechanical retention may play an important role in their colonization of the tooth surface (van Houte et al. 1972). Taken together, these observations suggest that the MS and other acidogenic oral bacteria create the necessary niche (a precaries lesion) capable of mechanically retaining lactobacilli. In our model, we will refer to this as the “retentive niche,” a necessary prerequisite for sustained colonization of lactobacilli in the oral cavity. The precaries retentive niche also becomes a site of stagnation, capable of not only retaining the otherwise planktonic lactobacilli but also providing a physical containment area that supports low pH and an anaerobic environment. An additional feature of the retentive niche is the mechanical trapping of food, a source of carbohydrates. Thus, not unlike the environment of silage and other food fermentations, the caries lesion constitutes a retentive, stagnant, acidic environment rich in carbohydrates, where lactobacilli can thrive.
Sucrose Connection and Other Cariogenic Attributes
Sucrose is the most important dietary contributor to dental caries in modern humans. Streptococcus mutans exemplifies the cariogenic properties of sucrose metabolism, acidogenity via fermentation, and adherence and biofilm formation via synthesis of extracellular glucans (Banas 2004). Lactobacilli, like the MS, have the ability to metabolize sucrose (Almståhl et al. 2013), but details as to the specific genetic mechanism are limited to a few species associated with food processing or probiotics. We found that many of the Lactobacillus genomes isolated from the S-ECC children in our study harbor a sucrose-specific phosphotransferase system (PTS) EII component, sucrose-6-phosphate hydrolase and sucrose phosphorylase (unpublished results), although the genetic repertoire varies between species and strains. Likewise, glucosyltransferases (Gtf) capable of synthesizing different types of glucans have been described for strains of L. reuteri, L. fermentum, L. parabuchneri, and Lactobacillus sakei (Kralj et al. 2004). We found gtf genes in about half of our L. fermentum oral genomes, but they were remarkably absent from the other species associated with S-ECC. This suggests that sucrose-dependent glucan synthesis is a strain-specific attribute in lactobacilli, rather than a widespread mechanism of adhesion as in streptococci (Argimon et al. 2013).
Unlike enamel, which is mainly mineral, dentin is composed of both apatite mineral and an extracellular organic matrix of mostly type I collagen. Bacterial acids alone are sufficient for enamel demineralization, but they are not as disruptive to dentin, where proteolytic activity is also required. Weak acids that lower the pH of the dentinal matrix are thought to activate human MMP-8 dormant in the matrix or tubercles (Hedenbjörk-Lager et al. 2015) and this, in turn, promotes caries progression. Once the collagen in dentin is exposed, it becomes a target for bacterial collagenases and collagen-binding proteins. Several Lactobacillus species have showed type I collagen binding activity, a feature that could help sustain them in the caries lesion (McGrady et al. 1995). Consistent with this, we found putative collagen-binding proteins in the genomes of L. casei/paracasei, L. gasseri, L. rhamnosus, L. fermentum, L. salivarius, L. oris, and L. vaginalis strains isolated from S-ECC children (unpublished results). However, we did not find evidence of collagenase genes in these same genomes, suggesting that lactobacilli might be more prone to binding to collagen than degrading it. This is in contrast with their cariogenic partner S. mutans, where the cnm gene encoding a type I collagen binding protein is not very prevalent among strains (Nomura et al. 2009; Argimon and Caufield 2011).
Another difference of clinical significance is the inherent tolerance to fluoride of some lactobacilli (Hamilton et al. 1985; Milnes et al. 1985; Bradshaw et al. 1990), up to 10-fold more resistant than S. mutans. This difference has been attributed in part to the glycolytic enzyme enolase, since purified enolase from L. rhamnosus was less sensitive to fluoride than that of S. mutans (Guha-Chowdhury et al. 1997). In addition, bacterial cells are highly permeable to hydrogen fluoride (HF), the protonated form of fluoride found at low pH, which reduces acid tolerance by inhibiting the extrusion of protons by the cell membrane F1F0 ATPase. However, L. casei seems to be able to maintain proton translocation by virtue of greater amounts of membrane ATPase compared with S. mutans (Bender and Marquis 1987). If fluoride resistance applies to all the Lactobacillus species associated with dental caries, it may be that fluoride therapy could promote dominance of lactobacilli in lesions over other F− sensitive microbes such as S. mutans. Lactobacilli have been shown to be sensitive to high concentrations of fluoride, however, in a mixed-species cariogenic biofilm (Mei et al. 2013).
In addition to their tolerance to fluoride, 36%, 50%, and 52% of Lactobacillus strains isolated from caries-prone adults could metabolize xylitol, mannitol, and sorbitol, respectively (Almståhl et al. 2013). Since these sugar alcohols are often recommended for caries control as noncariogenic sweeteners (Burt 2006) and anticaries agents, they could conceivably promote caries progression in those lesions dominated by lactobacilli.
Where Do Lactobacilli in the Oral Cavity Come From?
Given the proposal that caries lesions are necessary and sufficient for sustained colonization of lactobacilli in the oral cavity, what is the source? The most obvious sources for lactobacilli would be food or other infected humans. Vertical transmission from the mother, as in the case of the indigenous S. mutans, is certainly an attractive possibility. Unlike the transient character of the oral lactobacilli acquired by vaginally delivered neonates or breastfed infants, lactobacilli transmitted from the mother to a child could potentially persist in the oral cavity once a retentive niche is present. Among young children with caries, Teanpaisan and coworkers (2012) suggested that the mothers are the source of L. fermentum, since they show 50% of genotypes common to both mother and child. However, the presence of the same genotypes does not prove vertical transmission, as children and their mothers typically have a similar diet and could therefore be seeded with the same strains of lactobacilli. In our study cohort, S-ECC children and their mothers have a similar distribution of Lactobacillus species, with the notable exception of L. rhamnosus and L. plantarum—none of the mothers harbored these 2 species. L. vaginalis was also more common among mothers than their S-ECC children. We found commonality of species between mothers and their child in 23% of 15 pairs (data not shown). Our findings, although from a single, cross-sectional sampling, suggest that most children harbor Lactobacillus species and/or genotypes not found in their mothers.
Lactobacilli are key players in the fermentation and preservation of food. They are present in yogurt, cheese, coffee, and a wide variety of fermented foods, depending on cultural and geographic preferences. Several of the Lactobacillus species found in the oral cavity (Tables 1, 2 and Fig. 1) are also found in food. For example, L. casei/paracasei, L. rhamnosus, and L. plantarum are common in dairy products (Bernardeau et al. 2008). L. fermentum, L. plantarum, and Lactobacillus pentosus are commonly isolated from Asian fermented fruits and vegetables (Swain et al. 2014). Population-focused studies of oral and food Lactobacillus genotypes are required to single out food as a source of lactobacilli to the oral cavity.
Model for the Role of Lactobacilli in Dental Caries
This review supports the notion that lactobacilli colonizing the oral cavity are opportunistic invaders of precaries or existing caries lesions, rather than members of the indigenous biota that have coevolved with their human host. We propose that the colonization of the oral cavity by lactobacilli requires 3 essential conditions, as shown in Figure 2: 1) a retentive niche that allows lactobacilli to accumulate, which in turn creates 2) a low pH milieu and anaerobic environment, combined with 3) access to a ready source of carbohydrates. Two of these requirements involve niche creation by earlier colonizers, such as the MS and/or other cariogens. The concept that “S. mutans may be necessary for fissure caries, though not a sufficient condition” (Burt et al. 1983) is consistent with our model (Fig. 2) because MS are present in both caries-free and caries-active sites. However, under cariogenic conditions, MS play the critical role required for the colonization of lactobacilli in the oral cavity (i.e., creating a retentive niche). MS begin colonizing teeth as they enter the oral cavity, especially fissured-primary molars and any teeth with surface defects or enamel hypoplasia (EHP) (Li et al. 1994; Caufield et al. 2012). In the presence of fermentable carbohydrates, MS and other acidogenic microbes demineralize the enamel to form precaries lesions. These areas become a retentive, low pH niche for lactobacilli accumulation, which take advantage of their proclivity for making and surviving in an increasingly reduced pH environment. In some cases, the lactobacilli can outcompete and exclude the same bacteria that created the retentive niche (Chhour et al. 2005).
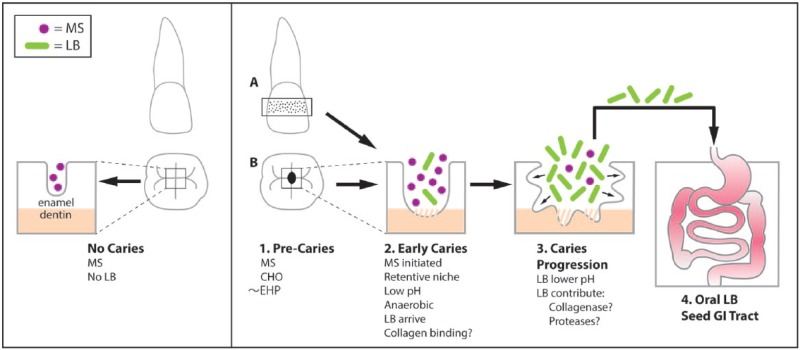
Figure 2.
Proposed model showing caries lesions as the major or only source of Lactobacillus (LB) from the oral cavity to the GI tract. Left panel: Primary teeth (molar and incisor) in a caries-free child in the absence of LB. Most children harbor mutans streptococci (MS) as commensal bacteria of the oral cavity. Right Panel: 1. An early caries lesion with MS in the presence of simple carbohydrates (CHO; e.g., sucrose). Some teeth of children with severe early childhood caries (S-ECC) have retentive niches in the form of enamel hypoplasia (~EHP) as well as pits and fissures found on the occlusal and buccal surfaces of the molars. 2. MS and other acidogenic microbes coalesce and form a retentive niche characterized by low pH and anaerobic conditions. Select species of LB from food or other humans accumulate in the retentive, low pH niche. If early caries continues to dentin, LB may be able to bind and/or degrade exposed dentinal collagen. 3. Caries has progressed due to lower pH contributed by both MS and LB. LB can dominate lesions to the exclusion of MS. 4. LB from caries lesions spill over into the saliva and are swallowed, seeding the downstream gastrointestinal (GI) tract, including retentive sites in the low pH stomach. We hypothesize that in the absence of dental caries, the oral cavity does not harbor LB, and as a result, the downstream GI tract no longer has a source of LB except those present in food.
Perhaps the most profound implication of this model, if true, is that the isolation of lactobacilli in sufficient numbers from either the oral cavity or GI tract is an indication of dental caries. Even though caries affects most individuals, their presence is not consistent with health. In the past, clinicians have gauged caries risk via a surrogate measure for lactobacilli counts in saliva, final medium pH (e.g., the Snyder Test; Snyder 1940). The shortcoming of this and other diagnostic tests is that they are not specific to just lactobacilli or to the species listed in Tables 1 and 2. More precise tests could be devised for diagnostic use. Elimination of all retentive sites, leading to absence of the caries-associated lactobacilli from the oral cavity, might indicate a therapeutic endpoint. In fact, a past study showed that restoration of caries resulted in significant reductions of lactobacilli in saliva (Wright et al. 1992). Clinicians should also be mindful that some lactobacilli are fluoride tolerant and capable of producing acid from xyiltol and other sucrose substitutes.
On a broader and more theoretical note, the collective evidence presented here strongly suggests that the lactobacilli of the oral cavity and downstream GI tract are not indigenous to humans as a result of a long-term host-commensal bacterial coevolution. Rather, lactobacilli represent opportunistic microbes that have adapted to a specific niche created by the destabilization of a healthy oral microbiome to one conducive to dental caries. We believe that the advent of modern, intense agriculture principally centered on harvesting of carbohydrates along with their companion lactobacilli may directly correlate with the introduction of dental caries in modern humans. The administration of lactobacilli supplements (probiotics) in the name of health will need to be weighed against their possible contribution to dental caries.
Author Contributions
P.W. Caufield, S. Argimón, contributed to conception, design, and data analysis, drafted and critically revised the manuscript; C.N. Schön, contributed to data acquisition and data analysis, critically revised the manuscript; Y. Li, contributed to conception, design, data acquisition, and analysis, drafted the manuscript; P. Saraithong, contributed to conception, design, data acquisition, and analysis, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank Peter Catapano, Untray Brown, and Charles Larsen for their clinical expertise and obtaining microbiological samples. Johanna Warshaw prepared the Figure 2 illustration.
Footnotes
This research was supported by grant R01 DE019455 from the National Institute of Dental and Craniofacial Research (NIDCR).
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Ahrne S, Nobaek S, Jeppsson B, Adlerberth I, Wold AE, Molin G. 1998. The normal Lactobacillus flora of healthy human rectal and oral mucosa. J Appl Microbiol. 85(1):88–94. [DOI] [PubMed] [Google Scholar]
- Almståhl A, Lingström P, Eliasson L, Carlén A. 2013. Fermentation of sugars and sugar alcohols by plaque Lactobacillus strains. Clin Oral Investig. 17(6):1465–1470. [DOI] [PubMed] [Google Scholar]
- Argimon S, Alekseyenko AV, DeSalle R, Caufield PW. 2013. Phylogenetic analysis of glucosyltransferases and implications for the coevolution of mutans streptococci with their mammalian hosts. PLoS One. 8(2):e56305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argimon S, Caufield PW. 2011. Distribution of putative virulence genes in Streptococcus mutans strains does not correlate with caries experience. J Clin Microbiol. 49(3):984–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badet C, Thebaud NB. 2008. Ecology of lactobacilli in the oral cavity: a review of literature. Open Microbiol J. 2:38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banas JA. 2004. Virulence properties of Streptococcus mutans. Front Biosci. 9:1267–1277. [DOI] [PubMed] [Google Scholar]
- Bender GR, Marquis RE. 1987. Membrane ATPases and acid tolerance of Actinomyces viscosus and Lactobacillus casei. Appl Environ Microbiol. 53(9):2124–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardeau M, Vernoux JP, Henri-Dubernet S, Guéguen M. 2008. Safety assessment of dairy microorganisms: the Lactobacillus genus. Int J Food Microbiol. 126(3):278–285. [DOI] [PubMed] [Google Scholar]
- Bradshaw DJ, McKee AS, Marsh PD. 1990. Prevention of population shifts in oral microbial communities in vitro by low fluoride concentrations. J Dent Res. 69(2):436–441. [DOI] [PubMed] [Google Scholar]
- Burt BA. 2006. The use of sorbitol- and xylitol-sweetened chewing gum in caries control. J Am Dent Assoc. 137(2):190–196. Published erratum in J Am Dent Assoc. 2006;137(4):447. [DOI] [PubMed] [Google Scholar]
- Burt BA, Loesche WJ, Eklund SA, Earnest RW. 1983. Stability of Streptococcus mutans and its relationship to caries in a child population over 2 years. Caries Res. 17(6):532–542. [DOI] [PubMed] [Google Scholar]
- Byun R, Nadkarni MA, Chhour KL, Martin FE, Jacques NA, Hunter N. 2004. Quantitative analysis of diverse Lactobacillus species present in advanced dental caries. J Clin Microbiol. 42(7):3128–3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J, Gothefors L. 1975. Transmission of Lactobacillus jensenii and Lactobacillus acidophilus from mother to child at time of delivery. J Clin Microbiol. 1(2):124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J, Grahnen H, Jonsson G. 1975. Lactobacilli and streptococci in the mouth of children. Caries Res. 9(5):333–339. [DOI] [PubMed] [Google Scholar]
- Caufield PW, Li Y, Bromage TG. 2012. Hypoplasia-associated severe early childhood caries—a proposed definition. J Dent Res. 91(6):544–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caufield PW, Li Y, Dasanayake A, Saxena D. 2007. Diversity of lactobacilli in the oral cavities of young women with dental caries. Caries Res. 41(1):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhour KL, Nadkarni MA, Byun R, Martin FE, Jacques NA, Hunter N. 2005. Molecular analysis of microbial diversity in advanced caries. J Clin Microbiol. 43(2):843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dal Bello F, Hertel C. 2006. Oral cavity as natural reservoir for intestinal lactobacilli. Syst Appl Microbiol. 29(1):69–76. [DOI] [PubMed] [Google Scholar]
- Frese SA, Benson AK, Tannock GW, Loach DM, Kim J, Zhang M, Oh PL, Heng NC, Patil PB, Juge N, Mackenzie DA, et al. 2011. The evolution of host specialization in the vertebrate gut symbiont Lactobacillus reuteri. PLoS Genet. 7(2):e1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha-Chowdhury N, Clark AG, Sissons CH. 1997. Inhibition of purified enolases from oral bacteria by fluoride. Oral Microbiol Immunol. 12(2):91–97. [DOI] [PubMed] [Google Scholar]
- Hamilton IR, Boyar RM, Bowden GH. 1985. Influence of pH and fluoride on properties of an oral strain of Lactobacillus casei grown in continuous culture. Infect Immun. 48(3):664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R, Opal Z. 1944. Comparative studies on lactobacilli isolated from the mouth and intestine. J Dent Res. 23(1):1–22. [Google Scholar]
- Hedenbjörk-Lager A, Bjørndal L, Gustafsson A, Sorsa T, Tjäderhane L, Akerman S, Ericson D. 2015. Caries correlates strongly to salivary levels of MMP-8. Caries Res. 49(1):1–8. [DOI] [PubMed] [Google Scholar]
- Holgerson PL, Vestman NR, Claesson R, Ohman C, Domellöf M, Tanner AC, Hernell O, Johansson I. 2013. Oral microbial profile discriminates breast-fed from formula-fed infants. J Pediatr Gastroenterol Nutr. 56(2):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kligler IJ. 1915. Chemical studies of the relations of oral microorganisms to dental caries. J Allied Dental Soc. 10:141–166. [Google Scholar]
- Kralj S, van Geel-Schutten GH, Dondorff MM, Kirsanovs S, van der Maarel MJ, Dijkhuizen L. 2004. Glucan synthesis in the genus Lactobacillus: isolation and characterization of glucansucrase genes, enzymes and glucan products from six different strains. Microbiology. 150(Pt 11):3681–3690. [DOI] [PubMed] [Google Scholar]
- Leverett DH, Proskin HM, Featherstone JD, Adair SM, Eisenberg AD, Mundorff-Shrestha SA, Shields CP, Shaffer CL, Billings RJ. 1993. Caries risk assessment in a longitudinal discrimination study. J Dent Res. 72(2):538–543. [DOI] [PubMed] [Google Scholar]
- Li Y, Argimón S, Schön CN, Saraithong P, Caufield PW. 2015. Characterizing diversity of Lactobacilli associated with severe early childhood caries: a study protocol. Adv Microbiol. 5:9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Navia JM, Caufield PW. 1994. Colonization by mutans streptococci in the mouths of 3- and 4-year-old Chinese children with or without enamel hypoplasia. Arch Oral Biol. 39(12):1057–1062. [DOI] [PubMed] [Google Scholar]
- Loesche WJ, Eklund S, Earnest R, Burt B. 1984. Longitudinal investigation of bacteriology of human fissure decay: epidemiological studies in molars shortly after eruption. Infect Immun. 46(3):765–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova K, Slesarev A, Wolf Y, Sorokin A, Mirkin B, Koonin E, Pavlov A, Pavlova N, Karamychev V, Polouchine N, et al. 2006. Comparative genomics of the lactic acid bacteria. Proc Natl Acad Sci U S A. 103(42):15611–15616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant S, Brailsford SR, Twomey AC, Roberts GJ, Beighton D. 2001. The predominant microflora of nursing caries lesions. Caries Res. 35(6):397–406. [DOI] [PubMed] [Google Scholar]
- Marsh PD. 2003. Are dental diseases examples of ecological catastrophes? Microbiology. 149(Pt 2):279–294. [DOI] [PubMed] [Google Scholar]
- Martin R, Heilig GH, Zoetendal EG, Smidt H, Rodriguez JM. 2007. Diversity of the Lactobacillus group in breast milk and vagina of healthy women and potential role in the colonization of the infant gut. J Appl Microbiol. 103(6):2638–2644. [DOI] [PubMed] [Google Scholar]
- McGrady JA, Butcher WG, Beighton D, Switalski LM. 1995. Specific and charge interactions mediate collagen recognition by oral lactobacilli. J Dent Res. 74(2):649–657. [DOI] [PubMed] [Google Scholar]
- Mei ML, Li QL, Chu CH, Lo EC, Samaranayake LP. 2013. Antibacterial effects of silver diamine fluoride on multi-species cariogenic biofilm on caries. Ann Clin Microbiol Antimicrob. 12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Soares H, Suzuki H, Hickey RJ, Forney LJ. 2014. Comparative functional genomics of Lactobacillus spp. reveals possible mechanisms for specialization of vaginal lactobacilli to their environment. J Bacteriol. 196(7):1458–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milnes AR, Bowden GH, Hamilton IR. 1985. Effect of NaF and pH on the growth and glycolytic rate of recently isolated strains of oral Lactobacillus species. J Dent Res. 64(3):401–404. [DOI] [PubMed] [Google Scholar]
- Munson MA, Banerjee A, Watson TF, Wade WG. 2004. Molecular analysis of the microflora associated with dental caries. J Clin Microbiol. 42(7):3023–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura R, Nakano K, Taniguchi N, Lapirattanakul J, Nemoto H, Grönroos L, Alaluusua S, Ooshima T. 2009. Molecular and clinical analyses of the gene encoding the collagen-binding adhesin of Streptococcus mutans. J Med Microbiol. 58(Pt 4):469–475. [DOI] [PubMed] [Google Scholar]
- Obata J, Takeshita T, Shibata Y, Yamanaka W, Unemori M, Akamine A, Yamashita Y. 2014. Identification of the microbiota in carious dentin lesions using 16S rRNA gene sequencing. PloS One. 9(8):e103712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan O, O’Callaghan J, Sangrador-Vegas A, McAuliffe O, Slattery L, Kaleta P, Callanan M, Fitzgerald GF, Ross RP, Beresford T. 2009. Comparative genomics of lactic acid bacteria reveals a niche-specific gene set. BMC Microbiol. 9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwat S, Teanpaisan R, Thitasomakul S, Thearmontree A, Dahlen G. 2010. Lactobacillus species and genotypes associated with dental caries in Thai preschool children. Mol Oral Microbiol. 25(2):157–164. [DOI] [PubMed] [Google Scholar]
- Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, et al. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci U S A. 108(Suppl 1):4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter G. 2001. The Lactobacillus and bifidobacterium microflora of the human intestine: composition and succession. Curr Issues Intest Microbiol. 2(2):43–53. [PubMed] [Google Scholar]
- Ryan KA, Jayaraman T, Daly P, Canchaya C, Curran S, Fang F, Quigley EM, O’Toole PW. 2008. Isolation of lactobacilli with probiotic properties from the human stomach. Lett Appl Microbiol. 47(4):269–274. [DOI] [PubMed] [Google Scholar]
- Salvetti E, Torriani S, Felis GE. 2012. The genus Lactobacillus: a taxonomic update. Probiotics Antimicro Prot. 4(4):217–226. [DOI] [PubMed] [Google Scholar]
- Segata N, Haake SK, Mannon P, Lemon KP, Waldron L, Gevers D, Huttenhower C, Izard J. 2012. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 13(6):R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. 1940. Colorimetric method for estimation of the relative number of lactobacilli in saliva. J Dent Res. 19(4):349–355. [Google Scholar]
- Sun Z, Harris HM, McCann A, Yang X, Argimon S, Zhang W, Guo C, Jeffery IB, Cooney JC, Kagawa TF, et al. 2014. Expanding the biotechnology potential of lactobacilli through comparative genomics. Nat Communications. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain MR, Anandharaj M, Ray RC, Parveen Rani R. 2014. Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnol Res Int. 2014:250424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner AC, Mathney JM, Kent RL, Chalmers NI, Hughes CV, Loo CY, Pradhan N, Kanasi E, Hwang J, Dahlan MA, et al. 2011. Cultivable anaerobic microbiota of severe early childhood caries. J Clin Microbiol. 49(4):1464–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teanpaisan R, Chaethong W, Piwat S, Thitasomakul S. 2012. Vertical transmission of mutans streptococci and lactobacillus in Thai families. Pediatr Dent. 34(2):e24–e29. [PubMed] [Google Scholar]
- Teanpaisan R, Thitasomakul S, Piwat S, Thearmontree A, Pithpornchaiyakul W, Chankanka O. 2007. Longitudinal study of the presence of mutans streptococci and lactobacilli in relation to dental caries development in 3-24 month old Thai children. Int Dent J. 57(6):445–451. [DOI] [PubMed] [Google Scholar]
- van Houte J. 1980. Bacterial specificity in the etiology of dental caries. Int Dent J. 30(4):305–326. [PubMed] [Google Scholar]
- van Houte J, Gibbons R, Pulkkinen A. 1972. Ecology of human oral lactobacilli. Infect Immun. 6(5):723–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestman NR, Timby N, Holgerson PL, Kressirer CA, Claesson R, Domellöf M, Öhman C, Tanner AC, Hernell O, Johansson I. 2013. Characterization and in vitro properties of oral lactobacilli in breastfed infants. BMC Microbiol. 13:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J. 2008. Ecological role of lactobacilli in the gastrointestinal tract: implications for fundamental and biomedical research. Appl Environ Microbiol. 74(16):4985–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JT, Cutter GR, Dasanayake AP, Stiles HM, Caufield PW. 1992. Effect of conventional dental restorative treatment on bacteria in saliva. Community Dent Oral Epidemiol. 20(3):138–143. [DOI] [PubMed] [Google Scholar]
- Xu X, He J, Xue J, Wang Y, Li K, Zhang K, Guo Q, Liu X, Zhou Y, Cheng L, et al. 2015. Oral cavity contains distinct niches with dynamic microbial communities. Environ Microbiol. 17(3):699–710. [DOI] [PubMed] [Google Scholar]
- Yang R, Argimon S, Li Y, Gu H, Zhou X, Caufield PW. 2010. Determining the genetic diversity of lactobacilli from the oral cavity. J Microbiol Methods. 82(2):163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.