Abstract
Periodontitis and type 2 diabetes mellitus are known to be associated. The relationship between periodontal microbiota and early diabetes risk has not been studied. We investigated the association between periodontal bacteria and prediabetes prevalence among diabetes-free adults. ORIGINS (the Oral Infections, Glucose Intolerance and Insulin Resistance Study) cross sectionally enrolled 300 diabetes-free adults aged 20 to 55 y (mean ± SD, 34 ± 10 y; 77% female). Prediabetes was defined as follows: 1) hemoglobin A1c values ranging from 5.7% to 6.4% or 2) fasting plasma glucose ranging from 100 to 125 mg/dL. In 1,188 subgingival plaque samples, 11 bacterial species were assessed at baseline, including Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, and Actinomyces naeslundii. Full-mouth clinical periodontal examinations were performed, and participants were defined as having no/mild periodontitis vs. moderate/severe periodontitis per the definition of the Centers for Disease Control and Prevention / American Academy of Periodontology. Modified Poisson regression evaluated prediabetes prevalence across bacterial tertiles. Prevalence ratios and 95% confidence intervals for third vs. first tertiles are presented. All analyses were adjusted for cardiometabolic risk factors. All results presented currently arise from the baseline cross section. Prediabetes prevalence was 18%, and 58% of participants had moderate/severe periodontitis. Prevalence ratios (95% confidence intervals) summarizing associations between bacterial levels and prediabetes were as follows: A. actinomycetemcomitans, 2.48 (1.34, 4.58), P = 0.004; P. gingivalis, 3.41 (1.78, 6.58), P = 0.0003; T. denticola, 1.99 (0.992, 4.00), P = 0.052; T. forsythia, 1.95 (1.0, 3.84), P = 0.05; A. naeslundii, 0.46 (0.25, 0.85), P = 0.01. The prevalence ratio for prediabetes among participants with moderate/severe vs. no/mild periodontitis was 1.47 (0.78, 2.74), P = 0.23. Higher colonization levels of specific periodontal microbiota are associated with higher prediabetes prevalence among diabetes-free adults.
Keywords: periodontitis, microbiota, diabetes, impaired glucose regulation, infection, epidemiology
Type 2 diabetes (T2D) causes 450,000 annual deaths, representing 19% of all deaths among individuals aged >25 y (Engelgau et al. 2004). At the preclinical level, impaired glucose regulation in the prediabetic state (i.e., prediabetes) is also a growing public health concern, affecting 38% of U.S. adults (Go et al. 2013). The prediabetic state is a strong predictor of future conversion to overt T2D (Nathan et al. 2007) and cardiovascular disease (Coutinho et al. 1999; Fonseca 2009; Shaye et al. 2012) and is an indicator of suboptimal cardiovascular health according to the American Heart Association 2020 goals (Go et al. 2013).
Dysbiotic microbial communities commonly observed in gingivitis and periodontitis (Socransky and Haffajee 2005; Kistler et al. 2013; Cekici et al. 2014; Palmer 2014) have been hypothesized as cardiometabolic risk factors (Kebschull et al. 2010; Lalla and Papapanou 2011; Lockhart et al. 2012). In regard to T2D and periodontal status, a large body of evidence suggests that these associations are bidirectional and potentially causal (Lalla and Papapanou 2011). Diabetes is associated with increased risk for developing periodontitis, and classical bidirectional hypotheses posit subgingival dysbiosis, a hallmark of periodontitis, as a risk factor for poor glycemic control among patients with established diabetes.
Recently, it has also been hypothesized that subgingival dysbiosis might contribute to diabetogenesis among diabetes-free individuals (Demmer, Jacobs, et al. 2008; Demmer, Desvarieux, et al. 2010; Demmer et al. 2012; Arora et al. 2014). This hypothesis is biologically plausible (Lalla and Papapanou 2011; Demmer et al. 2013), and a limited number of studies among diabetes-free populations have linked periodontal infections cross sectionally to insulin resistance (Demmer et al. 2012), elevated hemoglobin A1c (HbA1c; Wolff et al. 2009), and prediabetes (Zadik et al. 2010; Choi et al. 2011; Arora et al. 2014), as well as to longitudinal changes in HbA1c (Demmer, Desvarieux, et al. 2010), and incident prediabetes (Saito et al. 2004) and T2D (Demmer, Jacobs, et al. 2008).
However, all previous studies have used clinical signs of periodontal inflammation and tissue destruction, without concomitant assessment of the subgingival bacterial communities that are hypothesized as the major etiologic exposure for increased T2D risk, thereby limiting validation of the hypothesis and missing the opportunity for early risk assessment. While clinical periodontal measures are valid and clinically relevant indicators of exposure to dysbiotic biofilms, levels of select bacteria are possibly altered prior to the development of clinically manifest disease. Therefore, it is plausible that periodontitis might mark exposure to dysbiosis and not be directly involved in the biological processes linking subgingival dysbiosis and early diabetes risk. Consequently, bacterial levels might represent earlier biomarkers of future risk for local and systemic pathologies. A better understanding of these dynamics could prove valuable in terms of not only prevention—if causal linkage were established in the future—but also risk stratification regardless of causal linkage.
We examine the cross-sectional relationship between levels of 11 periodontal bacteria and prediabetes prevalence among diabetes-free adults enrolled in the Oral Infections, Glucose Intolerance, and Insulin Resistance Study (ORIGINS). We hypothesized that periodontopathic bacteria would be associated with higher prediabetes prevalence.
Methods
ORIGINS is an occupation-based cohort study among members of the Service Employees International Union 1199 investigating the relationship between subgingival microbial community composition and impaired glucose metabolism. The results herein arise from the baseline cross section. From February 2011 to May 2013, 300 men and women were enrolled. Inclusion criteria were as follows: 1) aged 20 to 55 y; 2) no diabetes mellitus (T1 or T2; based on participant self-report of no previously diagnosed disease), HbA1c values <6.5%, and fasting plasma glucose <126 mg/dL; and 3) no history of myocardial infarction, congestive heart failure, stroke, or chronic inflammatory conditions (participant self-report). The Columbia University Institutional Review Board approved the study protocol. All participants provided informed consent.
Periodontal Examination
Trained calibrated dental examiners assessed bleeding on probing, probing depth, and clinical attachment loss at 6 sites per tooth (mesiobuccal, midbuccal, distobuccal, mesiolingual, midlingual, distolingual) with a UNC-15 manual probe (Hu-Friedy). Periodontal examination reliability studies were performed, and results are summarized in the Appendix.
Subgingival Plaque Collection and Bacterial Assessments
In total, 1,188 subgingival plaque samples (4 samples from 297 participants) were collected from the most posterior tooth per quadrant (excluding third molars) via sterile curettes after removal of the supragingival plaque (Desvarieux et al. 2005). Digoxigenin-labeled whole genomic probes were prepared by random priming from the following microbial strains: Aggregatibacter actinomycetemcomitans ATCC 43718, Porphyromonas gingivalis ATCC 33277, Tannerella forsythia ATCC 43037, Treponema denticola ATCC 35404, Prevotella intermedia ATCC 25611, Fusobacterium nucleatum ATCC 10953, Parvimonas micra ATCC 33270, Campylobacter rectus ATCC 33238, Eikenella corrodens ATCC 23834, Veillonella parvula ATCC 10790, and Actinomyces naeslundii ATCC 49340. Further processing was performed via checkerboard DNA-DNA hybridization to derive relative bacterial counts for each bacterial species (Socransky et al. 1994; Appendix Methods).
Laboratory Measures
Blood was collected following an overnight fast. Plasma glucose, serum lipids, and HbA1c from whole blood were measured. Adiponectin, tumor necrosis factor α, interleukin 6, and high-sensitivity C-reactive protein were measured and values combined to create a summary inflammatory score (SIS) as previously described (Behle et al. 2009). For additional details regarding laboratory methods, see Appendix.
Risk Factors
Cardiometabolic risk factors were measured by trained research assistants in space provided by a Center for Translational Science Award (CTSA). Seated systolic and diastolic blood pressures were measured in triplicate and the last 2 measurements averaged. Participant body mass index was calculated as weight in kilograms / height in meters squared. Questionnaires were administered to obtain information on the following: age, sex, race/ethnicity (non-Hispanic black, non-Hispanic white, Hispanic, other), educational level (high school completion, college or vocational training, advanced degrees), and cigarette smoking (current, former or never smoking, and duration/intensity of smoking). Leisure-time physical activity was assessed and converted to metabolic equivalents. As previously described (Thai et al. 2014), metabolic equivalents categorized participants into 4 leisure-time physical activity categories. See Appendix for additional information on risk factor operationalization.
Statistical Analysis
Periodontal bacterial levels were the primary exposures. Standardized bacterial values were derived for each species via the following steps: 1) the within-mouth average of laboratory-derived values was calculated; 2) values from step 1 were natural log transformed; and 3) values from step 2 were divided by the overall sample standard deviation for the species to compute standardized values (Desvarieux et al. 2005). These standardized values enable more direct comparison of results across species, as a 1-unit change is equivalent to a 1–standard deviation change of the ln-transformed bacterial levels. Standardization also enables bacterial score creation by summation of standardized values across species; without standardization, the influence of low abundance organisms may be overshadowed by more abundant species.
To minimize the potential for false-positive findings due to multiple comparisons, we computed a “bacterial burden score” (BBS) as our primary exposure by summing the standardized values of A. actinomycetemcomitans, P. gingivalis, T. forsythia, and T. denticola as previously published (Desvarieux et al. 2005; Demmer, Papapanou, et al. 2010; Desvarieux et al. 2013) and described in the Appendix. Additionally, participants were classified as having no/mild periodontitis vs. moderate/severe periodontitis per the CDC/AAP guidelines (i.e., Centers for Disease Control and Prevention and American Academy of Periodontology; Page and Eke 2007).
Prediabetes and fasting glucose values were the primary outcomes. Prediabetes was defined according to the definition of the American Diabetes Association: HbA1c ranging from 5.7% to 6.4% or impaired fasting glucose defined as fasting plasma glucose ranging from 100 to 125 mg/dL (American Diabetes 2014).
A multivariable modified Poisson regression with robust error variance regressed prediabetes prevalence across either tertile of bacterial exposure levels or category of CDC/AAP-defined periodontitis; prevalence is defined as the probability of having prediabetes. Prevalence ratios and 95% confidence intervals (95% CIs) are presented for the second and third tertiles of bacterial exposure levels relative to the first tertile (i.e., the lowest bacterial levels). Regression models were adjusted for cardiometabolic risk factors as previously described (Arora et al. 2014) and summarized in the Appendix. A. naeslundii was added to multivariable models as an internal bacterial “control” because this species is not considered periodontopathic and is typically overly abundant in states of periodontal health (Desvarieux et al. 2005; Socransky and Haffajee 2005). Therefore, A. naeslundii adjustment enables interpretation of results across species in terms of relative—rather than absolute—abundance as previously discussed (Desvarieux et al. 2005).
Note that involving bacterial data are based on a sample size of 297.
Results
General Characteristics of the Cohort
The mean ± SD age of the participants was 34 ± 10 y; the sample was 77% female, 47% Hispanic, 23% white, 17% black, and 13% other. Nearly 70% of participants had a college degree, and only 4% had less than a high school education; 90% of participants were nonsmokers, and ~80% were never smokers. The prevalence estimates of none/mild, moderate, and severe periodontitis were 42%, 52%, and 6%, respectively. Table 1 and Appendix Table 1 summarize additional participant characteristics.
Table 1.
Participant Characteristics Overall and According to Prediabetes Status: Results among Participants Enrolled in ORIGINS, 2011–2013
| All (N = 300) | No Prediabetes (n = 246) | Prediabetes (n = 54) | P Value | |
|---|---|---|---|---|
| Age, y | 34 ± 10 | 32 ± 1 | 42 ± 1 | <0.0001 |
| Female | 77 | 76 | 83 | 0.22 |
| Race/ethnicity | 0.0004 | |||
| Hispanic | 47 | 44 | 57 | |
| Non-Hispanic white | 23 | 27 | 2 | |
| Black | 17 | 15 | 28 | |
| Other | 13 | 13 | 13 | |
| Education | <0.0001 | |||
| <High school degree | 4 | 2 | 13 | |
| High school degree | 7 | 5 | 15 | |
| Some college or vocational degree | 22 | 20 | 31 | |
| Bachelor’s degree | 45 | 49 | 28 | |
| >Bachelor’s degree | 22 | 24 | 13 | |
| Smoking status | 0.49 | |||
| Never | 78 | 79 | 72 | |
| Former | 12 | 11 | 17 | |
| Current | 10 | 9 | 11 | |
| Pack-years of smoking | 1.0 ± 3.6 | 1 ± 0.2 | 2 ± 0.7 | 0.02 |
| Activity level | 0.01 | |||
| None | 31 | 28 | 44 | |
| Low | 12 | 11 | 15 | |
| Moderate | 16 | 15 | 19 | |
| High | 41 | 45 | 21 | |
| Body mass index, kg/m2 | 27.1 ± 6.1 | 26.5 ± 0.4 | 29.7 ± 0.9 | 0.0004 |
| Body mass index category | 0.001 | |||
| Normal | 44 | 49 | 24 | |
| Overweight | 33 | 32 | 37 | |
| Obese | 23 | 19 | 39 | |
| Family history of diabetes | 53 | 51 | 63 | 0.39 |
| Mean blood pressure, mm Hg | ||||
| Systolic | 117.7 ± 12.4 | 116.0 ± 0.7 | 125.6 ± 1.9 | <0.0001 |
| Diastolic | 75.2 ± 9.6 | 74.2 ± 0.6 | 79.9 ± 1.6 | <0.0001 |
| Cholesterol, mg/dL | ||||
| LDL | 97.9 ± 27.8 | 95.1 ± 1.7 | 110.9 ± 4.0 | 0.0001 |
| HDL | 59.2 ± 16.1 | 60.6 ± 1.0 | 52.8 ± 1.8 | 0.0012 |
| Triglycerides, mg/dL | 77.4 ± 45.4 | 75.1 ± 2.9 | 88.1 ± 5.8 | 0.06 |
| Tumor necrosis factor α, pg/mL | 2.0 ± 2.3 | 2.1 ± 0.2 | 1.7 ± 0.1 | 0.24 |
| Interleukin 6, pg/mL | 1.6 ± 5.7 | 1.5 ± 0.3 | 2.3 ± 1.1 | 0.35 |
| C-reactive protein, mg/L | 3.4 ± 8.5 | 3.4 ± 0.6 | 3.4 ± 0.7 | 0.98 |
| Adiponectin, ng/mL | 9,342 ± 4,850 | 9,691 ± 310 | 7,754 ± 616 | 0.007 |
| Inflammatory z score, standard normal units | −0.0008 ± 2.4 | −0.2 ± 0.2 | 0.9 ± 0.3 | 0.0033 |
| Mean probing depth, mm | 2.4 ± 0.3 | 2.3 ± 0.02 | 2.4 ± 0.04 | 0.21 |
| Mean attachment loss, mm | 1.5 ± 0.6 | 1.5 ± 0.04 | 1.8 ± 0.1 | 0.0007 |
| Periodontal bleeding on probing, % of sites/mouth | 50 ± 0.3 | 44 ± 0.02 | 54 ± 0.04 | 0.007 |
| Tooth loss, n | 4 ± 2.8 | 4 ± 0.2 | 4 ± 0.5 | 0.24 |
| Brushing frequency, % | 0.99 | |||
| <1/d | 1 | 1 | 0 | |
| 1/d | 14 | 14 | 13 | |
| ≥2/d | 85 | 85 | 87 | |
| Flossing frequency, % | 0.25 | |||
| Never | 8 | 9 | 7 | |
| <Weekly | 17 | 18 | 11 | |
| Once a week | 14 | 15 | 9 | |
| More than once a week | 24 | 24 | 22 | |
| Everyday | 37 | 34 | 50 |
Values presented in mean ± SD or percentages. n = 2, missing for frequency of flossing or brushing; n = 6, missing values for activity level; n = 1, missing clinical periodontal measures; n = 1, missing for LDL cholesterol; n = 3, missing tumor necrosis factor α; n = 11, with undetectable C-reactive protein; n = 9 missing pack-years information.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; ORIGINS, Oral Infections, Glucose Intolerance, and Insulin Resistance Study.
Validation of Bacterial Measures
Unstandardized bacterial counts showed clear differences in the absolute abundance across species. Standardization of bacterial values produced similar distributions across species (Fig. 1). Correlation patterns among microbiota were consistent with previous research (Socransky et al. 1998). All bacterial species were positively correlated (P < 0.0001 for all pairwise comparisons in Fig. 2), and bacterial levels were related to periodontal inflammation among participants with moderate/severe periodontitis (see Appendix Results and Appendix Table 2).
Figure 1.
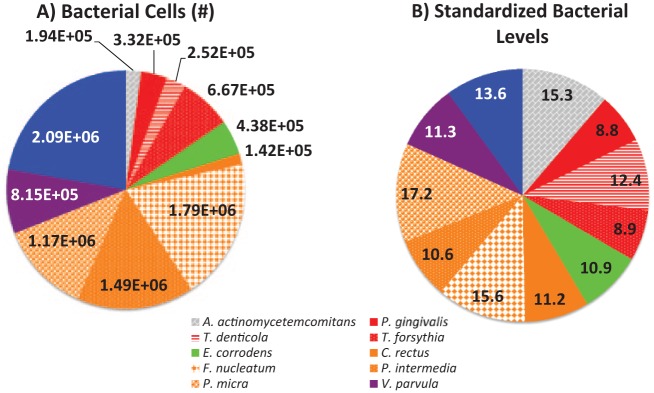
(A) Average nonstandardized bacterial cell counts and (B) standardized bacterial levels (in standard deviation units) among 1,188 subgingival plaques collected from 297 particiapants enrolled in the Oral Infections, Glucose Intolerance and Insulin Resistance Study, 2011–2013.
Figure 2.
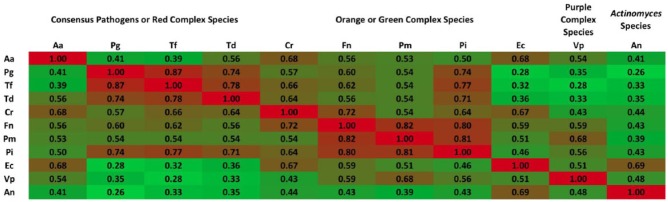
Matrix of Pearson correlations among 11 periodontal bacteria grouped according to evidence for pathogenesis and/or membership in microbial community complexes as previously described (Socransky et al. 1998). Consensus periodontal pathogens defined by the 1996 World Workshop in Periodontics: Aggregatibacter actinomycetemcomitans (Aa), Porphyromonas gingivalis (Pg), and Tannerella forsythia (Tf); red complex species include Pg, Tf, and Treponema denticola (Td). Orange complex species include Campylobacter rectus (Cr), Fusobacterium nucleatum (Fn), Parvimonas micra (Pm), and Prevotella intermedia (Pi). Eikenella corrodens (Ec) has green complex membership. Actinomyces naeslundii (An) clusters with Actinomyces species. Veillonella parvula (Vp) has purple complex membership. For pairwise correlations, all P values <0.0001. Results from 297 participants (1,188 dental plaques) enrolled in the Oral Infections, Glucose Intolerance and Insulin Resistance Study, 2011–2013.
Predictors of Prediabetes Prevalence
Mean ± SD fasting plasma glucose and HbA1c values were 85 ± 8 mg/dL and 5.4% (0.3%). The correlation between fasting plasma glucose and HbA1c was 0.3 (P < 0.0001). The prevalence of impaired fasting glucose was 4%, and 16% of participants had HbA1c ³5.7%; an additional 18% had prediabetes according to at least 1 definition. Participant characteristics according to prediabetes status are presented in Table 1. After multivariable adjustment, the following nonmicrobial factors were associated with prediabetes: age, sex, race/ethnicity, systolic blood pressure, and high-density lipoprotein (HDL) cholesterol (Appendix Table 3). Overweight and obesity were associated with prediabetes, but the association was removed after multivariable adjustments including the potential mediators HDL cholesterol and systolic blood pressure (Appendix Table 3). This was consistent when body mass index was modeled continuously; the crude prevalence ratio for a 5-kg/m2 increase in body mass index was 1.32 (95% CI: 1.14, 1.51), P < 0.0001. After age, sex, and race/ethnicity adjustment, the prevalence ratio was attenuated but statistically significant at 1.17 (95% CI: 1.01, 1.37), P = 0.04; after additional adjustment for systolic blood pressure and HDL cholesterol, the prevalence ratio was 1.00 (95% CI: 0.80, 1.26), P = 0.99.
Association between Periodontal Microbiota and Prediabetes Prevalence
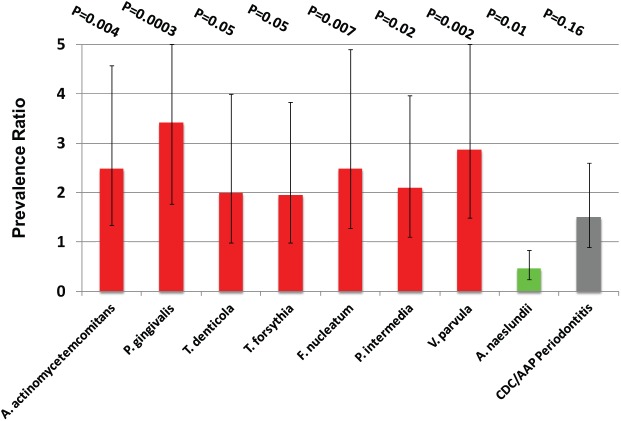
Higher levels of 4 species (A. actinomycetemcomitans, F. nucleatum, P. gingivalis, and V. parvula) were associated with elevated prediabetes prevalence. Multivariable-adjusted prevalence ratios (95% CIs) comparing third vs. first tertiles for these species were as follows: A. actinomycetemcomitans, 2.34 (1.31, 4.18), P = 0.008; F. nucleatum, 2.43 (1.32, 4.48), P = 0.02; P. gingivalis, 2.33 (1.33, 4.10), P = 0.004; V. parvula, 3.03 (1.54, 5.98), P = 0.002. Results for the remaining 7 species are presented in Table 2. When periodontitis and A. naeslundii were simultaneously adjusted for, results were stronger and statistically significant for 8 species (Fig. 3). Similarly, prediabetes prevalence was elevated among individuals with a high BBS. Adjusted prevalence ratios for second and third (vs. first) tertiles of BBS were 2.69 (1.27, 5.69), P = 0.01, and 2.61 (1.30, 5.26), P = 0.01, respectively.
Table 2.
Prevalence Ratios (95% Confidence Intervals) for Prediabetes According to Periodontal Bacterial Levels among 297 Participants Enrolled in ORIGINS, 2011–2013
| Variable | Tertile 2a (n = 99) | Tertile 3a (n = 99) | P Value |
|---|---|---|---|
| Porphyromonas gingivalis | |||
| M1 | 1.29 (0.68, 2.44) | 1.43 (0.77, 2.67) | 0.50 |
| M2 | 1.78 (1.01, 3.15) | 1.83 (1.05, 3.17) | 0.04 |
| M3 | 1.86 (1.02, 3.38) | 1.99 (1.12, 3.53) | 0.03 |
| M4 | 2.37 (1.24, 4.54) | 2.78 (1.49, 5.16) | 0.003 |
| M5 | 2.37 (1.24, 4.54) | 2.78 (1.49, 5.16) | 0.003 |
| Aggregatibacter actinomycetemcomitans | |||
| M1 | 1.12 (0.62, 2.02) | 0.94 (0.50, 1.76) | 0.85 |
| M2 | 1.45 (0.87, 2.41) | 1.58 (0.91, 2.78) | 0.18 |
| M3 | 1.34 (0.74, 2.43) | 1.67 (0.94, 2.99) | 0.23 |
| M4 | 1.76 (0.97, 3.19) | 2.18 (1.23, 3.88) | 0.03 |
| M5 | 1.74 (0.96, 3.17) | 2.16 (1.22, 3.82) | 0.03 |
| Tannerella forsythia | |||
| M1 | 1.06 (0.57, 1.98) | 1.19 (0.65, 2.17) | 0.85 |
| M2 | 1.13 (0.64, 2.00) | 1.29 (0.75, 2.22) | 0.65 |
| M3 | 1.18 (0.63, 2.19) | 1.37 (0.76, 2.46) | 0.58 |
| M4 | 1.08 (0.58, 2.00) | 1.73 (0.95, 3.15) | 0.16 |
| M5 | 1.07 (0.58, 1.98) | 1.71 (0.94, 3.12) | 0.18 |
| Treponema denticola | |||
| M1 | 1.02 (0.55, 1.88) | 1.07 (0.59, 1.95) | 0.98 |
| M2 | 1.26 (0.72, 2.21) | 1.29 (0.76, 2.20) | 0.58 |
| M3 | 1.32 (0.75, 2.34) | 1.38 (0.78, 2.44) | 0.48 |
| M4 | 1.67 (0.87, 3.20) | 1.74 (0.93, 3.26) | 0.15 |
| M5 | 1.68 (0.88, 3.22) | 1.73 (0.93, 3.22) | 0.16 |
| Bacterial burden scoreb | |||
| M1 | 1.20 (0.64, 2.24) | 1.27 (0.68, 2.35) | 0.73 |
| M2 | 1.60 (0.92, 2.77) | 1.60 (0.93, 2.77) | 0.12 |
| M3 | 1.41 (0.76, 2.60) | 1.76 (1.00, 3.10) | 0.14 |
| M4 | 1.99 (0.94, 4.20) | 2.46 (1.29, 4.66) | 0.02 |
| M5 | 1.99 (0.95, 4.17) | 2.42 (1.28, 4.58) | 0.02 |
| Campylobacter rectus | |||
| M1 | 0.90 (0.51, 1.60) | 0.70 (0.38, 1.31) | 0.50 |
| M2 | 1.12 (0.67, 1.86) | 0.94 (0.52, 1.70) | 0.82 |
| M3 | 1.16 (0.66, 2.04) | 1.05 (0.56, 1.97) | 0.86 |
| M4 | 1.22 (0.71, 2.10) | 1.10 (0.56, 2.17) | 0.77 |
| M5 | 1.22 (0.71, 2.09) | 1.10 (0.56, 2.17) | 0.76 |
| Eikenella corrodens | |||
| M1 | 0.95 (0.54, 1.67) | 0.65 (0.34, 1.23) | 0.33 |
| M2 | 1.05 (0.62, 1.78) | 1.00 (0.57, 1.76) | 0.98 |
| M3 | 1.03 (0.61, 1.74) | 1.00 (0.54, 1.84) | 0.99 |
| M4 | 1.10 (0.63, 1.93) | 1.16 (0.61, 2.24) | 0.90 |
| M5 | 1.10 (0.63, 1.92) | 1.16 (0.61, 2.21) | 0.90 |
| Fusobacterium nucleatum | |||
| M1 | 1.07 (0.56, 2.04) | 1.40 (0.77, 2.55) | 0.51 |
| M2 | 1.21 (0.66, 2.23) | 1.70 (0.98, 2.98) | 0.17 |
| M3 | 1.10 (0.57, 2.13) | 1.76 (1.00, 3.12) | 0.12 |
| M4 | 1.18 (0.58, 2.37) | 2.13 (1.12, 4.06) | 0.04 |
| M5 | 1.18 (0.59, 2.39) | 2.12 (1.11, 4.06) | 0.04 |
| Parvimonas micra | |||
| M1 | 1.33 (0.73, 2.45) | 1.13 (0.60, 2.14) | 0.65 |
| M2 | 1.49 (0.84, 2.65) | 1.32 (0.76, 2.31) | 0.36 |
| M3 | 1.15 (0.62, 2.15) | 1.24 (0.71, 2.17) | 0.75 |
| M4 | 1.22 (0.66, 2.23) | 1.62 (0.90, 2.93) | 0.28 |
| M5 | 1.21 (0.66, 2.22) | 1.59 (0.88, 2.88) | 0.31 |
| Prevotella intermedia | |||
| M1 | 1.13 (0.60, 2.14) | 1.33 (0.73, 2.45) | 0.65 |
| M2 | 1.23 (0.67, 2.23) | 1.47 (0.86, 2.52) | 0.37 |
| M3 | 1.17 (0.59, 2.11) | 1.41 (0.82, 2.42) | 0.44 |
| M4 | 1.30 (0.68, 2.47) | 1.84 (0.98, 3.46) | 0.15 |
| M5 | 1.31 (0.69, 2.51) | 1.82 (0.97, 3.41) | 0.17 |
| Veillonella parvula | |||
| M1 | 1.63 (0.82, 2.94) | 2.09 (1.08, 4.05) | 0.07 |
| M2 | 2.09 (1.10, 3.96) | 2.31 (1.25, 4.28) | 0.008 |
| M3 | 2.07 (1.11, 3.85) | 2.44 (1.31, 4.53) | 0.006 |
| M4 | 1.96 (1.05, 3.68) | 2.39 (1.28, 4.46) | 0.01 |
| M5 | 1.97 (1.04, 3.75) | 2.42 (1.27, 4.62) | 0.02 |
| Actinomyces naeslundii | |||
| M1 | 1.06 (0.59, 1.89) | 0.83 (0.45, 1.56) | 0.73 |
| M2 | 1.01 (0.59, 1.74) | 0.96 (0.55, 1.68) | 0.98 |
| M3 | 0.85 (0.46, 1.57) | 0.93 (0.54, 1.60) | 0.87 |
| M4 | 0.90 (0.49, 1.67) | 0.82 (0.47, 1.42) | 0.78 |
| M5 | 0.90 (0.49, 1.65) | 0.83 (0.48, 1.45) | 0.81 |
Additional adjustment for frequency of brushing and frequency of flossing in M3 did not produce any meaningful changes in results for any species (data not shown). Additional adjustment for family history of diabetes did not meaningfully influence results (data not shown).
M1, crude model; M2, age, sex, race/ethnicity, education; M3, M2 + body mass index, smoking status, physical activity level; M4, M3 + systolic blood pressure, HDL cholesterol; M5, M4 + inflammatory score (sum of standardized values of tumor necrosis factor α, interleukin 6, C-reactive protein minus standardized adiponectin values).
Tertile 1 is the reference (n = 99).
Bacterial burden score is defined as the combined level of A. actinomycetemcomitans, P. gingivalis, T. forsythia, and T. denticola.
Figure 3.
Prevalence ratios summarizing prediabetes prevalence among third- vs. first-tertile bacterial levels. All species (except Actinomyces naeslundii) are mutually adjusted for A. naeslundii levels and CDC/AAP-defined periodontitis. Results for A. naeslundii are mutually adjusted for Porphyromonas gingivalis levels and periodontitis. Periodontitis is mutually adjusted for A. naeslundii and P. gingivalis levels. All prevalence ratios additionally adjusted for age, sex, race/ethnicity, education, smoking, physical activity level, body mass index, systolic blood pressure, HDL cholesterol, and summary inflammatory z score. Results from 297 participants enrolled in the Oral Infections, Glucose Intolerance and Insulin Resistance Study, 2011–2013. AAP, American Academy for Periodontology; CDC, Centers for Disease Control and Prevention.
The multivariable-adjusted prevalence ratio for prediabetes among participants with moderate/severe vs. those with no/mild periodontitis was 1.47 (95% CI: 0.78, 2.74), P = 0.23.
Discussion
We demonstrated that select subgingival bacteria are associated with prediabetes prevalence. Most notably, A. actinomycetemcomitans, P. gingivalis, T. denticola, T. forsythia, and the BBS were all associated with an ~2- to 3-fold higher prevalence of prediabetes, while A. naeslundii levels were inversely related to prediabetes prevalence.
These data advance current knowledge about associations between the clinical entities of T2D and periodontitis by demonstrating the emergence of associations between abnormal glucose metabolism and periodontal microbiota prior to diabetes development and overt hyperglycemia. This substantially advances knowledge about the temporality of associations as previously discussed (Demmer, Jacobs, et al. 2008; Demmer, Desvarieux, et al. 2010; Demmer et al. 2012; Arora et al. 2014).
These data also inform hypotheses linking periodontitis to diabetogenesis by examining whether the most relevant exposure in this context is the clinical periodontal status or the microbial communities associated with periodontitis. If bacterial dysbiosis can in fact contribute to prediabetes development in susceptible individuals, it is possible that periodontitis and prediabetes (or diabetes) may be comorbid conditions due to shared microbial risk factors. By studying a population with mostly moderate or no periodontitis and by considering the clinical and bacterial measures simultaneously in our statistical models, we can inform this question. Our findings show that associations between bacterial measures and prediabetes were consistently stronger than associations between periodontitis and prediabetes. This suggests that bacterial communities are likely to be highly relevant to the hypothesis despite the fact that causal conclusions cannot be drawn on the basis of cross-sectional results. It is also noteworthy that a recent report found that prediabetes status was not associated with higher levels of periodontitis (Kowall et al. 2015), which supports the notion that bacterial exposures might be more relevant among population-based samples without overt hyperglycemia.
The role of positive confounding appeared to be minimal in this population—particularly in regard to smoking, as 90% of participants were nonsmokers, and environmental tobacco exposures were likely minimal because smoking rates in the New York City area are low (Mbamalu et al. 2011) and participants work in a smoke-free medical center. Negative confounding was more influential, as previously observed in the context of prediabetes outcomes (Arora et al. 2014). Presently, negative confounding was due to factors such as age and smoking intensity being inversely related to bacterial levels but positively related to prediabetes. Accordingly, associations between microbiota and prediabetes were strengthened after comprehensive multivariable adjustment.
Our findings were generally consistent with a priori notions about how the bacterial species measured might relate to systemic outcomes. Importantly, results showing the bacterial score (based on combined levels of A. actinomycetemcomitans, P. gingivalis, T. denticola, and T. forsythia) to be positively associated with prediabetes are consistent with previous research showing the score to be associated with carotid atherosclerosis (Desvarieux et al. 2005; Desvarieux et al. 2013) and clinical periodontal disease (Demmer, Papapanou, et al. 2008, 2010) in INVEST (Oral Infections and Vascular Disease Epidemiology Study). Of note, ORIGINS is a distinct sample of young adults (~30 y younger than INVEST participants), with enrollment separated by over a decade. The 4 species included in the BBS definition are not sufficient causes of periodontal disease, and other, more recently identified potential pathogens were not assessed in this study. Nevertheless, we believe that this construct still provides a meaningful biomarker of “dysbiotic communities” (Demmer, Papapanou, et al. 2010; Palmer 2014) and is therefore consistent with new models of periodontal pathogenesis suggesting that periodontitis arises from polymicrobial synergy in dysbiotic biofilms among susceptible individuals (Hajishengallis 2014). Moreover, the use of a bacterial score enabled the formation of a priori hypotheses using cost-effective methods available for epidemiologic studies when ORIGINS was designed.
Systemic inflammation is hypothesized as a potential intermediate linking periodontal infections and systemic outcomes. In these data, although the inflammatory score was elevated in prediabetes, there was no apparent relationship between the inflammatory score and bacterial burden. However, we used a very limited panel of only 4 inflammatory mediators in a cross-sectional study that cannot reveal the temporality of the interrelationships among microbial exposures, systemic inflammation, and the development of prediabetes. Ongoing longitudinal follow-up among individuals in ORIGINS free of prediabetes will be informative in this regard.
The magnitude of observed associations between bacterial levels and prediabetes is clinically relevant in the context of risk assessment for both future diabetes development and clinical cardiovascular disease. The prediabetic state is a strong predictor of future conversion to overt diabetes, as up to 70% of prediabetic individuals may eventually convert to diabetes (Nathan et al. 2007). Fasting glucose levels in the normal and prediabetic range have also been shown to predict increased risk for future cardiovascular disease (Coutinho et al. 1999; Muntner et al. 2004; Fonseca 2009; Shaye et al. 2012), which is consistent with the strong risk prediction of overt diabetes for cardiovascular disease.
A number of limitations of this study should be noted. We collected dental plaque samples from only 4 index teeth rather than from sites with clinically manifest periodontal disease. While this might have underestimated associations, it makes the results more relevant to real-world clinical and research settings by demonstrating that sampling strategies without a priori knowledge of periodontal status can yield meaningful results. It is also an important design characteristic that maximizes the potential predictive value of combined clinical and bacterial measures; that is, plaque sampling dependent on clinical status might reduce between-participant variability in microbial measures and reduce their predictive value.
In an attempt to balance biological relevance against feasibility, we measured only 11 bacterial species among >700 subgingival species discovered to date (Wade 2013). In addition, assessment of bacterial levels using DNA-DNA checkerboard hybridizations is subject to errors due to cross reactivity among phylogenetically similar taxa. However, these errors likely occurred nondifferentially by prediabetes status and most likely underestimated the observed strength of association. Future research is necessary to consider a broader assessment of the subgingival microbiota, now possible with next-generation sequencing methods. This work has been initiated in ORIGINS, based on a purposeful (as opposed to representative) sample, thereby limiting the generalizability of our findings to other populations. For example, the prevalence of moderate/severe periodontitis in ORIGINS (58%) is higher than the national average (Eke et al. 2012) despite being substantially younger. In populations with a lower prevalence of periodontitis (and presumably lower levels of subgingival dysbiosis), the currently observed relationships might not be evident, therefore necessitating replication of our current findings in different populations.
As these data are cross sectional in nature, reverse causality is possible (i.e., features of the prediabetes state contributed to shifts in the subgingival microbiota). Longitudinal results from ORIGINS will be necessary to inform this possibility.
One notable inconsistency with prior publications is the finding for V. parvula, which was originally grouped with A. naeslundii and considered a “health-associated” internal control species (Desvarieux et al. 2005). In contrast, V. parvula levels in ORIGINS were positively associated with prediabetes prevalence. Whether this is a chance finding or due to other dynamics that make V. parvula a poor choice as an internal control in the context of prediabetes risk requires further study.
To our knowledge, this is the first study to investigate and demonstrate an association between periodontal microbiota and prediabetes prevalence among young diabetes-free adults. Whether these findings represent truly causal interrelationships remains to be determined in future longitudinal studies, but they nevertheless demonstrate associations between potentially periodontopathic subgingival bacteria and impaired glucose metabolism preceding diabetes development. Therefore, while the temporality of the association cannot be addressed in these cross-sectional data, the observation that levels of key subgingival bacteria are overly abundant in and predictive of prevalent prediabetes in itself constitutes new knowledge. If periodontal microbiota do influence early risk for diabetogenesis, the public health implications could be significant given the high population prevalence of periodontal infections in the United States (Eke et al. 2015) and globally (Demmer and Papapanou 2010) and the substantial global burden of cardiometabolic disease.
Author Contributions
R.T. Demmer, contributed to conception, design, data acquisition, analysis and interpretation, drafted and critically revised the manuscript; D.R. Jacobs Jr, contributed to conception and design, critically revised the manuscript; R. Singh, contributed to data analysis, drafted the manuscript; A. Zuk, M. Rosenbaum, contributed to data acquisition, critically revised the manuscript; P.N. Papapanou, M. Desvarieux, contributed to conception and design, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Acknowledgments
We thank the following individuals for their invaluable contributions to this research: the 1199 SEIU–HS-3/SSA area leadership, including Consuelo Mclaughin, Bennett Batista, and Victor Rivera; Romanita Celenti for her efforts in performing phlebotomy and processing and analyzing plaque samples; Drs. Nidhi Arora, Ashwata Pokherel, Publio Silfa, and Thomas Spinell for their skilled examinations and essential participant engagement. We are also profoundly grateful to the ORIGINS participants for their participation in this research.
Footnotes
This research was supported by grants from the National Institutes of Health (R00 DE018739, R21 DE022422) to Dr. Demmer. Dr. Demmer also received funding from a Calderone Research Award, Mailman School of Public Health, and a Pilot & Feasibility Award from the Diabetes and Endocrinology Research Center, College of Physicians and Surgeons (DK-63608). This publication was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (grant UL1 TR000040), formerly the National Center for Research Resources (grant UL1 RR024156). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- American Diabetes Association. 2014. Diagnosis and classification of diabetes mellitus. Diabetes Care. 37 Suppl 1:S81–S90. [DOI] [PubMed] [Google Scholar]
- Arora N, Papapanou PN, Rosenbaum M, Jacobs DR, Jr, Desvarieux M, Demmer RT. 2014. Periodontal infection, impaired fasting glucose and impaired glucose tolerance: results from the Continuous National Health and Nutrition Examination Survey 2009–2010. J Clin Periodontol. 41(7):643–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behle JH, Sedaghatfar MH, Demmer RT, Wolf DL, Celenti R, Kebschull M, Belusko PB, Herrera-Abreu M, Lalla E, Papapanou PN. 2009. Heterogeneity of systemic inflammatory responses to periodontal therapy. J Clin Periodontol. 36(4):287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekici A, Kantarci A, Hasturk H, Van Dyke TE. 2014. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol 2000. 64(1):57–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YH, McKeown RE, Mayer-Davis EJ, Liese AD, Song KB, Merchant AT. 2011. Association between periodontitis and impaired fasting glucose and diabetes. Diabetes Care. 34(2):381–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho M, Gerstein HC, Wang Y, Yusuf S. 1999. The relationship between glucose and incident cardiovascular events: a metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care. 22(2):233–240. [DOI] [PubMed] [Google Scholar]
- Demmer RT, Desvarieux M, Holtfreter B, Jacobs DR, Jr, Wallaschofski H, Nauck M, Volzke H, Kocher T. 2010. Periodontal status and A1C change: longitudinal results from the Study of Health in Pomerania (SHIP). Diabetes Care. 33(5):1037–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Jacobs DR, Jr, Desvarieux M. 2008. Periodontal disease and incident type 2 diabetes: results from the First National Health and Nutrition Examination Survey and its epidemiologic follow-up study. Diabetes Care. 31(7):1373–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Papapanou PN. 2010. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontol 2000. 53:28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Papapanou PN, Jacobs DR, Jr, Desvarieux M. 2008. Bleeding on probing differentially relates to bacterial profiles: the Oral Infections and Vascular Disease Epidemiology Study. J Clin Periodontol. 35(6):479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Papapanou PN, Jacobs DR, Jr, Desvarieux M. 2010. Evaluating clinical periodontal measures as surrogates for bacterial exposure: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). BMC Med Res Methodol. 10(2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Squillaro A, Papapanou PN, Rosenbaum M, Friedewald WT, Jacobs DR, Jr, Desvarieux M. 2012. Periodontal infection, systemic inflammation, and insulin resistance: results from the continuous National Health and Nutrition Examination Survey (NHANES) 1999–2004. Diabetes Care. 35(11):2235–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer RT, Trinquart L, Zuk A, Fu BC, Blomkvist J, Michalowicz BS, Ravaud P, Desvarieux M. 2013. The influence of anti-infective periodontal treatment on C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. PLoS One. 8(10):e77441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Jacobs DR, Papapanou PN, Sacco RL, Rundek T. 2013. Changes in clinical and microbiological periodontal profiles relate to progression of carotid intima-media thickness: the oral infections and vascular disease epidemiology study. J Am Heart Assoc. 2(6):e000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr, Sacco RL, Papapanou PN. 2005. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 111(5):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. 2015. Update on prevalence of periodontitis in adults in the United States: NHANES 2009–2012. J Periodontol. 86(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ; CDC Periodontal Disease Surveillance workgroup. 2012. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 91(10):914–920. [DOI] [PubMed] [Google Scholar]
- Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF, Rios-Burrows N, Mokdad AH, Ford ES, et al. 2004. The evolving diabetes burden in the United States. Ann Intern Med. 140(11):945–950. [DOI] [PubMed] [Google Scholar]
- Fonseca VA. 2009. Defining and characterizing the progression of type 2 diabetes. Diabetes Care. 32 Suppl 2:S151–S156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, et al. 2013. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation. 127(1):e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G. 2014. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol. 15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebschull M, Demmer RT, Papapanou PN. 2010. “Gum bug, leave my heart alone!” Epidemiologic and mechanistic evidence linking periodontal infections and atherosclerosis. J Dent Res. 89(9):879–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistler JO, Booth V, Bradshaw DJ, Wade WG. 2013. Bacterial community development in experimental gingivitis. PLoS One. 8(8):e71227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowall B, Holtfreter B, Volzke H, Schipf S, Mundt T, Rathmann W, Kocher T. 2015. Prediabetes and well controlled diabetes are not associated with periodontal disease: the SHIP Trend Study. J Clin Periodontol. 42(5):422–430. [DOI] [PubMed] [Google Scholar]
- Lalla E, Papapanou PN. 2011. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 7(12):738–748. [DOI] [PubMed] [Google Scholar]
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, et al. 2012. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 125(20):2520–2544. [DOI] [PubMed] [Google Scholar]
- Mbamalu IG, Coady MH, Johns M, Kansagra SM. 2011. Trends in cigarette use among adults in New York City, 2002–2010: New York City Department of Health and Mental Hygiene, Epi Data Brief. [accessed on 2015 May 18]. http://www.nyc.gov/html/doh/downloads/pdf/epi/databrief12.pdf.
- Muntner P, He J, Chen J, Fonseca V, Whelton PK. 2004. Prevalence of non-traditional cardiovascular disease risk factors among persons with impaired fasting glucose, impaired glucose tolerance, diabetes, and the metabolic syndrome: analysis of the Third National Health and Nutrition Examination Survey (NHANES III). Ann Epidemiol. 14(9):686–695. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B. 2007. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 30(3):753–759. [DOI] [PubMed] [Google Scholar]
- Page RC, Eke PI. 2007. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 78(7 Suppl):1387–1399. [DOI] [PubMed] [Google Scholar]
- Palmer RJ., Jr. 2014. Composition and development of oral bacterial communities. Periodontol 2000. 64(1):20–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Shimazaki Y, Kiyohara Y, Kato I, Kubo M, Iida M, Koga T. 2004. The severity of periodontal disease is associated with the development of glucose intolerance in non-diabetics: the Hisayama study. J Dent Res. 83(6):485–490. [DOI] [PubMed] [Google Scholar]
- Shaye K, Amir T, Shlomo S, Yechezkel S. 2012. Fasting glucose levels within the high normal range predict cardiovascular outcome. Am Heart J. 164(1):111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD. 2005. Periodontal microbial ecology. Periodontol 2000. 38:135–187. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RLJ. 1998. Microbial complexes in subgingival plaque. J Clin Periodontol. 25(2):134–144. [DOI] [PubMed] [Google Scholar]
- Socransky SS, Smith C, Martin L, Paster BJ, Dewhirst FE, Levin AE. 1994. “Checkerboard” DNA-DNA hybridization. Biotechniques. 17(4):788–792. [PubMed] [Google Scholar]
- Thai A, Papapanou PN, Jacobs DR, Jr, Desvarieux M, Demmer RT. 2014. Periodontal infection and cardiorespiratory fitness in younger adults: results from continuous national health and nutrition examination survey 1999–2004. PLoS One. 9(3):e92441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade WG. 2013. The oral microbiome in health and disease. Pharmacol Res. 69(1):137–143. [DOI] [PubMed] [Google Scholar]
- Wolff RE, Wolff LF, Michalowicz BS. 2009. A pilot study of glycosylated hemoglobin levels in periodontitis cases and healthy controls. J Periodontol. 80(7):1057–1061. [DOI] [PubMed] [Google Scholar]
- Zadik Y, Bechor R, Galor S, Levin L. 2010. Periodontal disease might be associated even with impaired fasting glucose. Br Dent J. 208(10):E20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.