Significance
Most preclinical drug screening systems fail to predict whether a given drug candidate will succeed in clinical trials due to the fact that cells are plated on culture dishes devoid of signaling cues present in vivo. Among other factors, biomechanical stimulation strongly affects tumor behavior. Toward this end, we cultured Ewing sarcoma cells on 3D scaffolds within a flow perfusion bioreactor, which provides mechanical stimulation in the form of flow-derived shear stress. Cells exposed to flow perfusion produced more insulin-like growth factor-1 (IGF-1) ligand and showed shear stress-dependent sensitivity to IGF-1 receptor targeted drugs when compared with static conditions. Collectively, this research provides an improved method to study cancer cells ex vivo and could narrow the gap between preclinical and clinical drug activity.
Keywords: tissue engineering, tumor model, mechanical stimulation, biological therapy
Abstract
Three-dimensional tumor models accurately describe different aspects of the tumor microenvironment and are readily available for mechanistic studies of tumor biology and for drug screening. Nevertheless, these systems often overlook biomechanical stimulation, another fundamental driver of tumor progression. To address this issue, we cultured Ewing sarcoma (ES) cells on electrospun poly(ε-caprolactone) 3D scaffolds within a flow perfusion bioreactor. Flow-derived shear stress provided a physiologically relevant mechanical stimulation that significantly promoted insulin-like growth factor-1 (IGF1) production and elicited a superadditive release in the presence of exogenous IGF1. This finding is particularly relevant, given the central role of the IGF1/IGF-1 receptor (IGF-1R) pathway in ES tumorigenesis and as a promising clinical target. Additionally, flow perfusion enhanced in a rate-dependent manner the sensitivity of ES cells to IGF-1R inhibitor dalotuzumab (MK-0646) and showed shear stress-dependent resistance to the IGF-1R blockade. This study demonstrates shear stress-dependent ES cell sensitivity to dalotuzumab, highlighting the importance of biomechanical stimulation on ES-acquired drug resistance to IGF-1R inhibition. Furthermore, flow perfusion increased nutrient supply throughout the scaffold, enriching ES culture over static conditions. Our use of a tissue-engineered model, rather than human tumors or xenografts, enabled precise control of the forces experienced by ES cells, and therefore provided at least one explanation for the remarkable antineoplastic effects observed by some ES tumor patients from IGF-1R targeted therapies, in contrast to the lackluster effect observed in cells grown in conventional monolayer culture.
The ability to treat cancer patients is critically dependent upon a robust drug discovery pipeline and an efficient method to select the most promising drug candidates for clinical trial advancement. Preclinical drug screening typically relies on the use of 2D culture systems, which are reproducible, fast, and inexpensive. Nevertheless, tumor phenotype is dictated by its interaction with the surrounding 3D microenvironment (1). The inability of 2D systems to mimic this key element has generally resulted in preclinical findings that overstate drug activity when subsequently tested in human clinical trials, therefore undermining the drug discovery process in cancer therapies (2).
To overcome these issues, several 3D tumor models have been proposed, including tumor spheroids and hydrogel systems, which attempt to recapitulate heterotypic interactions either between tumor and stroma or tumor and extracellular matrix (ECM), respectively (3, 4). These systems have begun to bridge the gap between in vitro and in vivo testing in several aspects, such as cell growth (5), gene expression pattern (6), and chemoresistance (7). However, these models are still unable to recapitulate and adequately examine the effects of other cues present in the tumor microenvironment, such as heterotypic cell−cell signaling, cell motility, and tumor angiogenesis. The last element is important for mediating the crosstalk between cancer and stromal cells and for modeling vascular supply effects on tumor cell metabolism. Furthermore, tissues like bone and muscle are continually exposed to physiological mechanical forces, in which specific sensory cells respond by triggering structural and functional changes in mechanosensitive proteins, and ultimately influence the corresponding signaling transduction pathways (8). Additionally, mechanical forces can affect gene expression through direct interactions with the cell nucleus (9). As a result, mechanical stimulation is essential for tissue development, homeostasis, and pathological conditions such as cancer, where it has been shown to drive tumor progression and metastasis (10).
Our laboratory is particularly focused on Ewing sarcoma (ES), a translocation-positive bone tumor mostly prevalent in adolescents and young adults that is rapidly fatal unless effectively treated with chemotherapy and local therapy (11, 12). We recently developed an ex vivo ES model based on the use of electrospun poly(ε-caprolactone) (PCL) 3D scaffolds, which conferred a more physiologically relevant ES cell phenotype compared with conventional monolayer cultures (13). Among other described features, our ES 3D model demonstrated pronounced upregulation of the insulin-like growth factor-1 receptor (IGF-1R) pathway, a receptor commonly expressed in ES tumors and one of the most promising targets in preclinical and early-phase drug development (11–13). However, additional features are still required to mimic in vivo ES biology and drug sensitivity. Other studies show how mechanical stimulation affects IGF1 expression in other cells of mesodermal origin, and chiefly in bones (14, 15). Therefore, using a flow perfusion bioreactor to apply mechanical stimulation to ES cells might enhance our understanding of the role of fluid shear stress on the IGF1/IGF-1R signaling pathway in ES malignancy (16).
The objective of the current work was to investigate the effects of flow perfusion bioreactors in ES 3D PCL constructs. We hypothesized that flow-derived shear stress would influence ES cell phenotype and drug sensitivity, with emphasis on the IGF1/IGF-1R signaling transduction and biological targets against this pathway. We further postulated that perfusion bioreactors would better promote ES cellular proliferation and distribution compared with static conditions through an improved mass transfer. To test these hypotheses, ES cells grown on PCL scaffolds without or within flow perfusion bioreactors were exposed either to chemical drugs or to biologically targeted therapeutics against IGF-1R, analyzed based on their proliferation and apoptosis responsiveness, profiled for biomarkers controlling the IGF-1R crosstalk signaling pathways, and examined by electron microscopy and immunofluorescent microscopy.
Results
Effect of Flow Perfusion on ES Cell Growth and Proliferation.
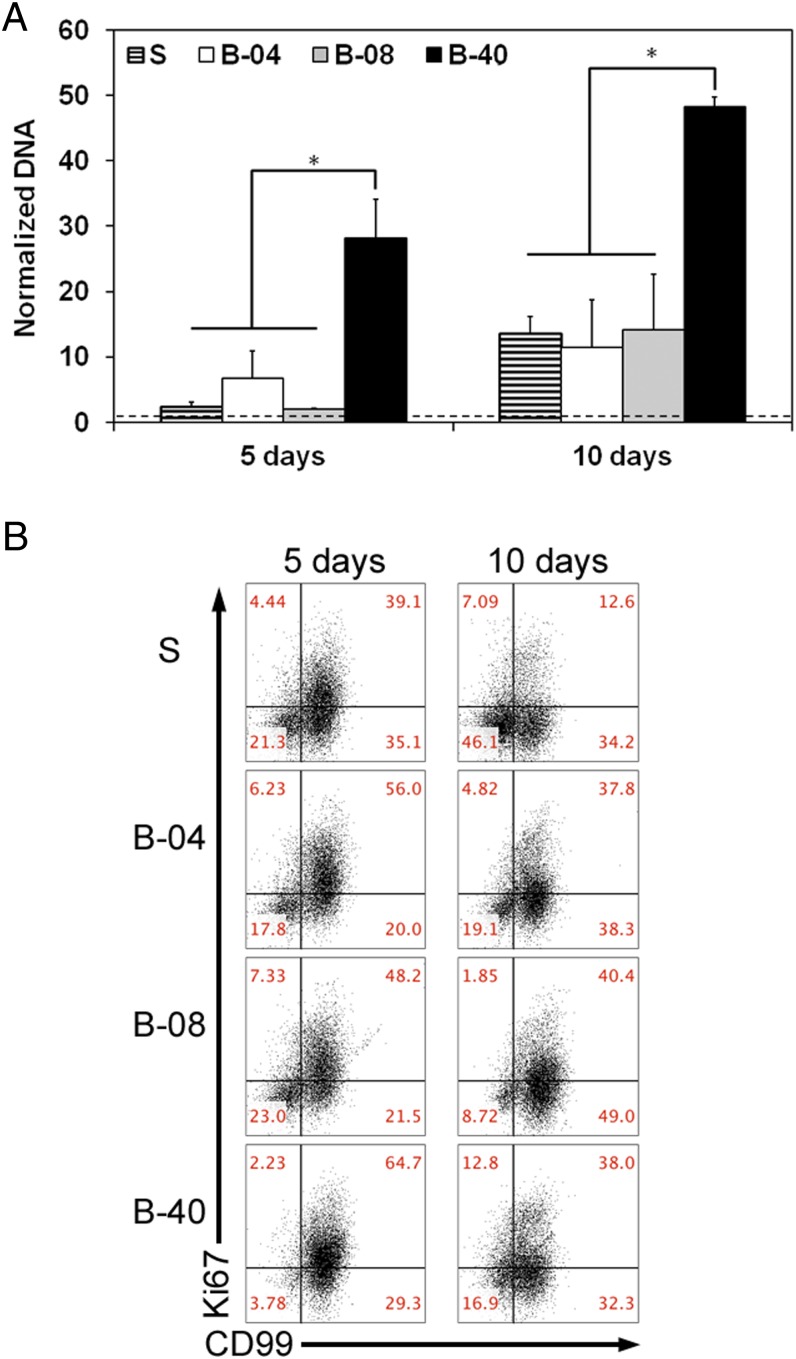
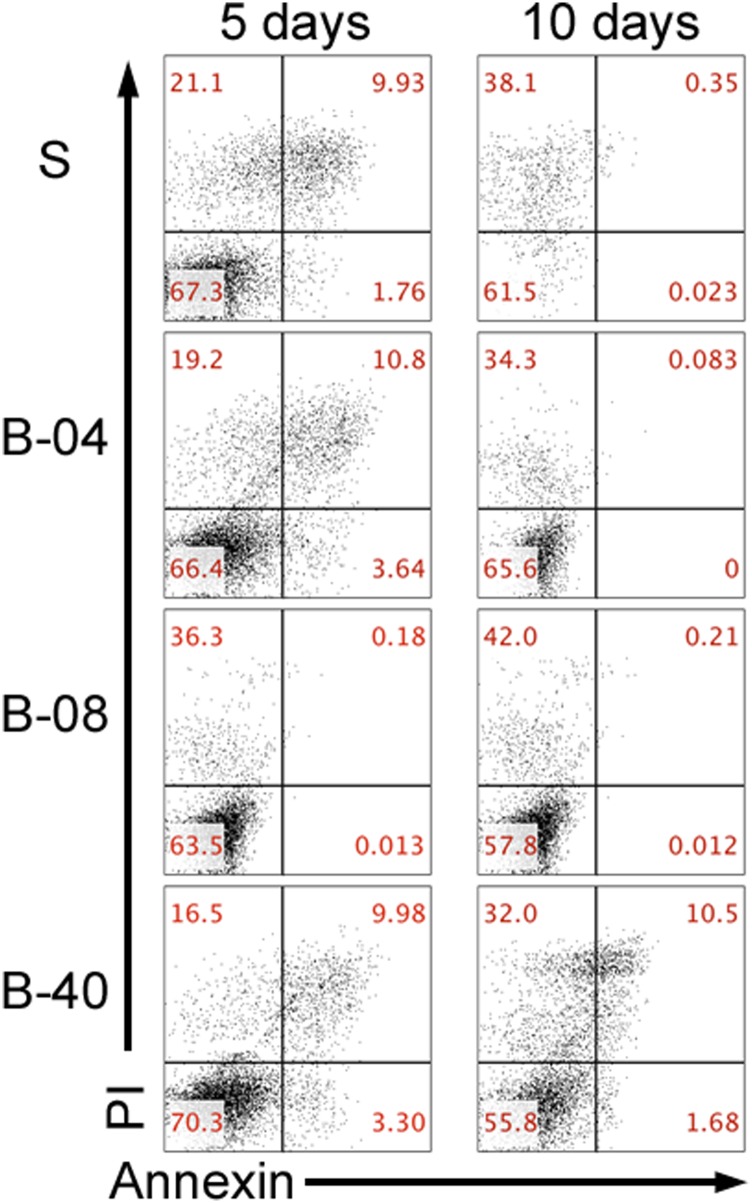
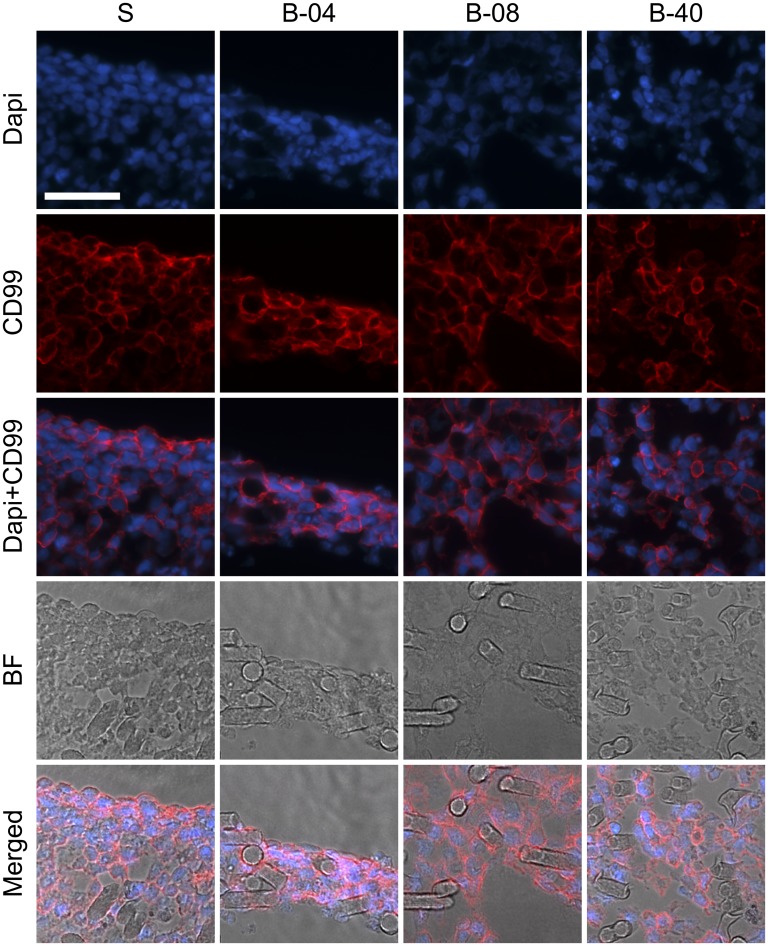
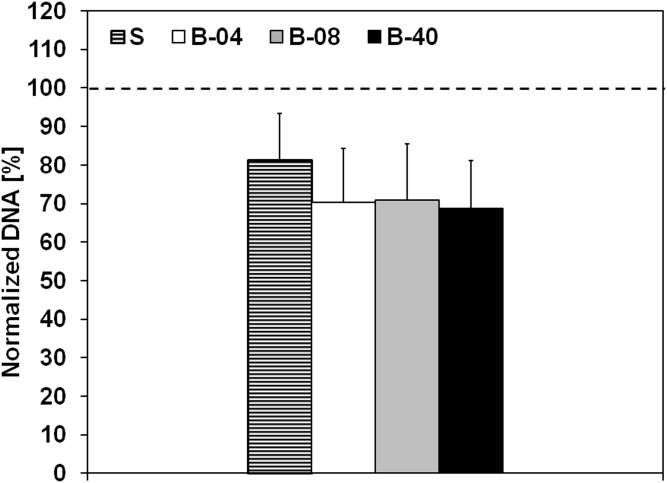
We initially investigated the effect of flow perfusion on ES cellular growth and viability within 3D PCL scaffolds using DNA content as an index of cellular growth. ES cells were seeded on porous 3D PCL scaffolds and cultured for 5 d or 10 d either without flow perfusion (i.e., static culture) or with flow perfusion bioreactors under three different flow rates: 0.04 mL/min (B-04), 0.08 mL/min (B-08), and 0.40 mL/min (B-40). These conditions resulted in a shear stress range of 1.7–17.0 cPa, which correlated to literature data on shear stress in the bone microenvironment (17, 18). DNA content of ES cells increased in all experimental groups in a time-dependent manner (Fig. 1A). The lower (B-04) and intermediate (B-08) flow rates showed no significant improvement in ES cellular growth compared with static culture; however, the highest flow rate (B-40, 0.40 mL/min) induced 28- or 50-fold increase in DNA content after 5 d or 10 d of culture, respectively (Fig. 1A). Moreover, apoptotic ES cell rate was also similar among both static and flow perfusion groups, as confirmed by flow cytometry-based analysis with Annexin and propidium iodide immunostaining (Fig. S1). Further, a flow cytometry-based proliferation assay was performed using Ki67 immunostaining as a second indicator of proliferation; although the static culture showed the lowest fraction of Ki67+ cells, significantly more proliferative cells were identified within the three flow perfusion groups at both 5 d and 10 d (Fig. 1B). Additionally, flow perfusion preserved high cell surface expression of the clinical diagnostic marker CD99 (Fig. 1B and Fig. S2).
Fig. 1.
Effect of flow perfusion on ES cell growth and proliferation. (A) Evolution of DNA content over time, normalized to DNA content in each group at day 0 for the different groups examined (S, static; B-04, 0.04 mL/min; B-08, 0.08 mL/min; and B-40, 0.40 mL/min). Error bars represent the SD for n = 4 samples, and levels not connected by the same letter are statistically different (*P < 0.05). Broken line represents unitary fold change. (B) The 2D plots for ES surface markers CD99 and proliferation marker Ki67.
Fig. S1.
Flow cytometry analysis of ES cell culture under static and flow perfusion conditions. Flow cytometry staining for markers of early (Annexin V) and late (PI) apoptosis after 5 d and 10 d of culture for the different groups examined (S, static; B-04, 0.04 mL/min; B-08, 0.08 mL/min; and B-40, 0.40 mL/min).
Fig. S2.
Immunofluorescent analysis of ES cell culture under static and flow perfusion conditions. After 10 d of culture, constructs for the different experimental groups (S, static; B-04, 0.04 mL/min; B-08, 0.08 mL/min; and B-40, 0.40 mL/min) were stained for surface marker CD99 (red) and nuclei using DAPI stain (blue). Brightfield images show the location of ES cells along PCL fibers (BF). (Scale bar, 50 μm.)
Effect of Flow Perfusion on ES Cell Morphology and Distribution.
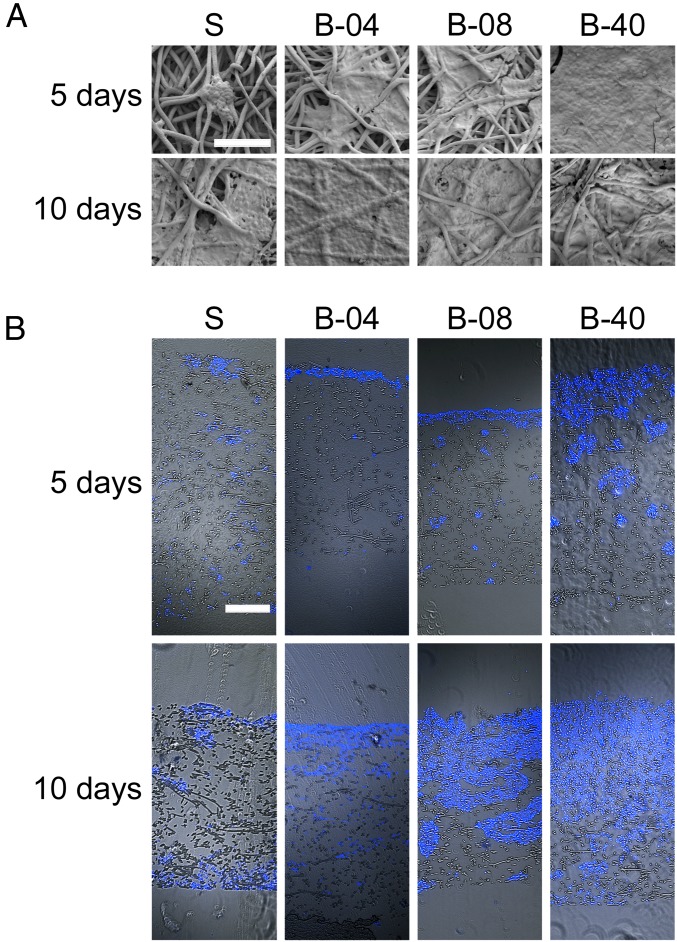
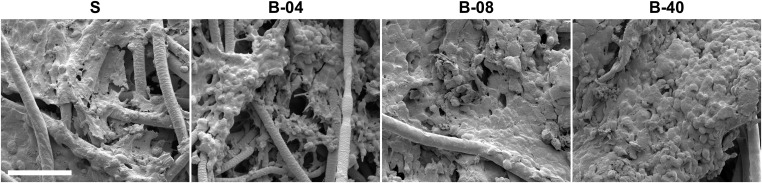
Examination of flow perfusion effects on ES cell morphology and distribution within 3D PCL scaffold was performed using Scanning Electron Microscopy (SEM) and immunofluorescent microscopy. The small round-cell morphology that is characteristic of human ES tumors was present in ES cells cultured under both static and flow perfusion conditions (Fig. 2A and Figs. S2 and S3). Thus, the flow-derived shear stress showed no apparent effect on ES cell morphology. When initially grown (at 5 d) within static 3D scaffolds, ES cells were at low confluency and tended to form clusters where PCL fibers cross, whereas, under flow perfusion, cells became confluent, especially at the highest flow rate (B-40). By day 10, both static and bioreactor groups had saturated the scaffold surface, a phenomenon that occurred more rapidly in ES cell cultures perfused at the highest flow rate (Fig. 2A).
Fig. 2.
Effect of flow perfusion on ES cell morphology and distribution. (A) SEM micrographs of the surface of scaffolds for the different groups examined (S, static; B-04, 0.04 mL/min; B-08, 0.08 mL/min; and B-40, 0.40 mL/min). Scale bar represents 100 μm in all micrographs. (B) Fluorescence microscopy of the cross section of scaffolds. Brightfield images of scaffold fibers are overlapped with ES cells fluorescently stained with DAPI (blue staining). (Scale bar, 200 μm.)
Fig. S3.
SEM micrographs of ES cell culture under static and flow perfusion conditions. After 10 d of culture, scaffold surfaces for the different experimental groups (S, static; B-04, 0.04 mL/min; B-08, 0.08 mL/min; and B-40, 0.40 mL/min) were examined by SEM. (Scale bar, 50 μm.)
The spatial distribution of ES cells within the scaffold constructs was assessed in cross sections using fluorescent microscopy (Fig. 2B). ES cells grown in static culture displayed significant proliferation on scaffold surface and extensive cell migration along the outer edges of the scaffold, but minimal migration occurred within the construct (Fig. 2B). Conversely, the use of flow perfusion bioreactors promoted cell infiltration within the electrospun PCL scaffolds by day 5 (Fig. 2B). This effect was more notable at higher flow rates (groups B-08 and B-40), which showed considerably higher cell infiltration by day 10 (Fig. 2B).
Effect of Flow-Derived Shear Stress on ES Cell Signaling Transduction.
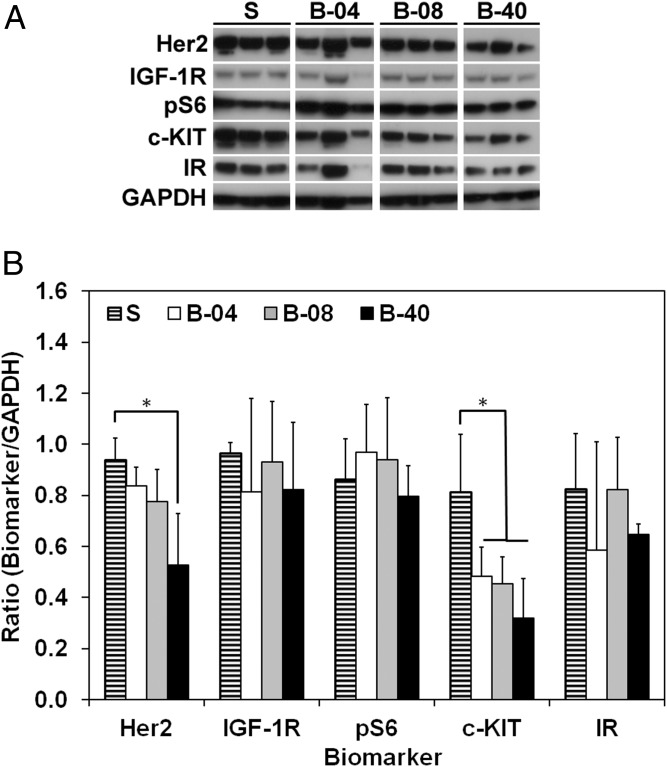
To elucidate the effect of flow-derived shear stress on ES cell, proteomic profiling was performed using Western blotting analysis for key ES-related oncoproteins that might crosstalk with the IGF-1R pathway. Total IGF-1R and insulin receptor (IR) protein expression were consistent among all groups, with no tangible effect of flow perfusion on their regulation (Fig. 3). Expression of protein pS6, a direct downstream marker of IGF-1R/mTORC1 activity, remained constant among flow perfusion states. In contrast, expression of oncoproteins c-KIT and HER2 decreased under flow perfusion conditions compared with static cultures, particularly at the highest flow rate (i.e., B-40) (Fig. 3).
Fig. 3.
Effect of flow-derived shear stress on ES cell signaling transduction. (A) Western blot after 10 d of culture for the different groups examined (S, static; B-04, 0.04 mL/min; B-08, 0.08 mL/min; and B-40, 0.40 mL/min). (B) Histogram analysis for the proteins shown in A. Signal density is normalized to GAPDH content. and error bars represent the SD for n = 3 samples (*P < 0.05).
Effect of Flow-Derived Shear Stress on ES Cell Drug Sensitivity.
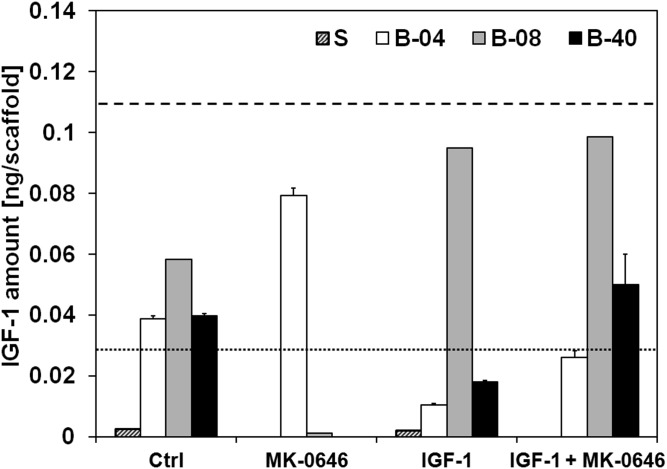
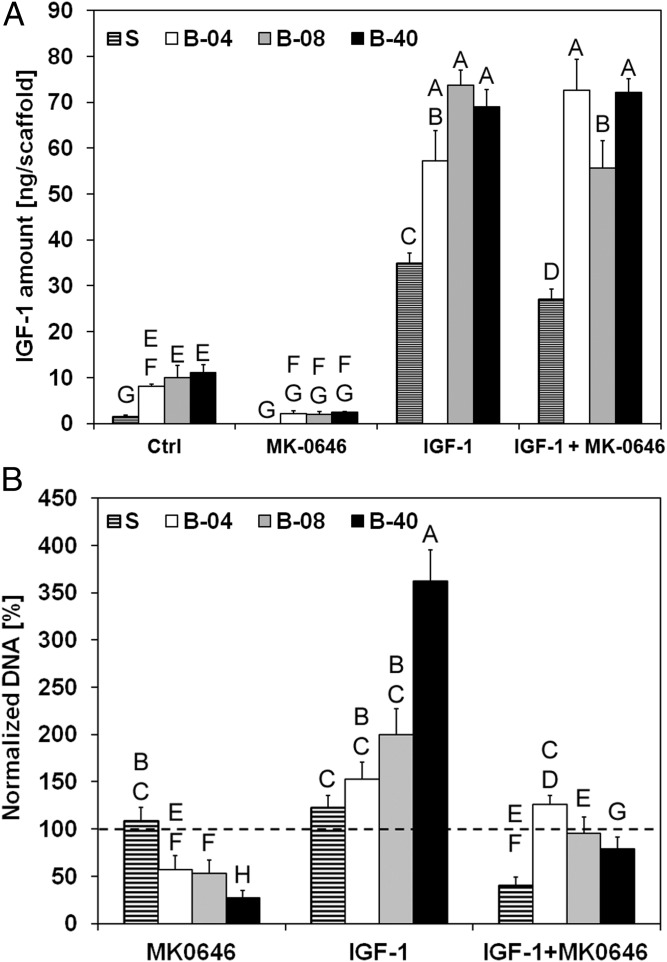
The effect of flow perfusion conditions on ES drug sensitivity was examined using doxorubicin (a conventional cytotoxic drug used in ES treatment) and dalotuzumab (MK-0646), a humanized monoclonal antibody inhibitor of human IGF-1R. ES cells treated with doxorubicin in static and in flow perfusion conditions showed no detectable difference in their cell viability (Fig. S4). Regarding the sensitivity of ES cells to IGF-1R blockade, dalotuzumab was tested in the absence or presence of exogenously supplemented IGF1 ligand. Experimentally, IGF1 amount per scaffold was quantified by ELISA from conditioned media collected at day 10 from ES cells grown in medium (control), single exogenous IGF1 ligand (20 ng/mL), dalotuzumab (100 μg/mL), or the IGF1/ dalotuzumab combination. Within the first 2 d of cell culture, a limited amount of IGF1 was detected in all of the experimental groups (Fig. S5). However, after 10 d, ES cells cultured under flow perfusion conditions had released considerably more IGF1 into the medium than cells grown in static culture (Fig. 4A). Dalotuzumab treatment was effective in all flow perfusion bioreactor conditions, but the strongest flow rate-dependent antiproliferative effect occurred at the highest flow rate of 0.40 mL/min (B-40) while stimulated by physiological levels of exogenous IGF1 (Fig. 4B). ES proliferation was inhibited under static conditions only when dalotuzumab was supplemented together with IGF1 (Fig. 4B). Interestingly, exogenous IGF1 instantiated a feed-forward behavior, noted chiefly in ES grown in flow perfusion, that reinforces autocrine IGF1 secretion by the ES cells (Fig. 4A). This flow rate-dependent effect led to a significant increase in cellularity and IGF1 production (Fig. 4B).
Fig. S4.
ES cell viability upon doxorubicin exposure. ES cells were cultured under static and flow perfusion conditions (S, static; B-04, 0.04 mL/min; B-08, 0.08 mL/min; and B-40, 0.40 mL/min) for 8 d and then exposed to doxorubicin (3 μM) for 2 d. Within each group, cell viability is shown as the DNA content of the drug-treated group normalized to the DNA content of the untreated group. Error bars represent the SD (n = 6), and broken line represents 100% baseline, i.e., no DNA change.
Fig. S5.
Levels of IGF1 ligand after 2 d of culture under static and flow perfusion conditions. The amount of IGF1 ligand per scaffold produced by ES cells under static and flow perfusion conditions (S, static; B-04, 0.04 mL/min; B-08, 0.08 mL/min; and B-40, 0.40 mL/min) is illustrated by the respective histograms. Basal levels of IGF1 ligand in the complete media are shown as a reference for medium prepared with FBS (broken line) or charcoal-stripped FBS (dotted line). Error bars represent the SD (n = 3).
Fig. 4.
Effect of flow-derived shear stress on ES cell drug sensitivity. IGF1 secretion and drug sensitivity for experimental groups tested at different flow rates (S, static; B-04, 0.04 mL/min; B-08, 0.08 mL/min; and B-40, 0.40 mL/min). (A) Amount of IGF1 ligand per scaffold measured in conditioned media after 10 d of culture. Error bars represent the SD (n = 3). (B) Cell viability after 3 d of culture followed by 7 d of exposure to IGF1 ligand, dalotuzumab (MK-0646), or their combinations. Within each group, cell viability is shown as the DNA content of the drug-treated group normalized to the DNA content of the untreated group. Error bars represent the SD (n = 6). Levels not connected by the same letter are statistically different (P < 0.05), and broken line represents 100% baseline, i.e., no DNA change.
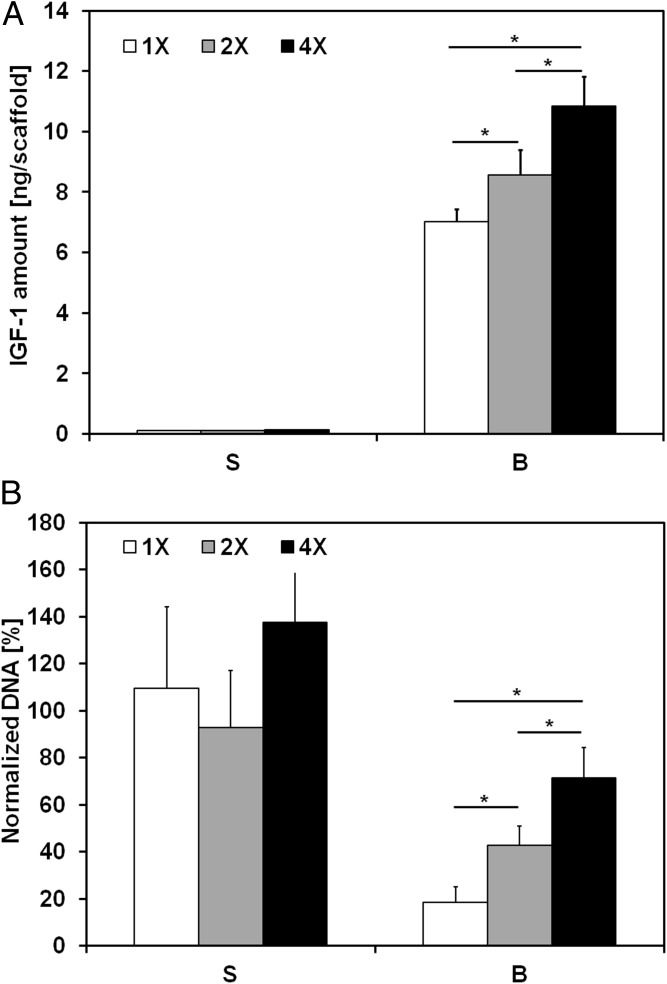
To further assess whether these trends in drug sensitivity and IGF1 production could be ascribed to the variable shear stress or to the enhanced mass transport, we conducted an additional study in which ES cells were cultured for 10 d either in static culture or with flow perfusion bioreactors under three different medium viscosities while maintaining a constant flow rate of 0.2 mL/min: 1× [0.9 ± 0.1 centipoises (cP)], 2× (1.7 ± 0.1 cP), and 4× (3.6 ± 0.3 cP). In this way, we sought to evaluate the effects of increasing shear stress on drug sensitivity and IGF1 secretion, with negligible effects on mass transport under flow perfusion (Table S1). The IGF1 quantification provided evidence that biomechanical stimulation is responsible for the enhanced IGF1 secretion, which was shear stress dependent (Fig. 5A). Under the same conditions, dalotuzumab activity was retested, and the ineffectiveness of this drug in static conditions was confirmed due to low concentrations of IGF1 present (Fig. 5). Conversely, ES cells showed lower sensitivity to IGF-1R blockade under higher shear stress conditions (Fig. 5B).
Table S1.
Experimental parameters for ES cell culture under flow perfusion bioreactor conditions
| Group | B-04 | B-08 | B-40 | B-1x | B-2x | B-4x |
| Flow rate (), mL/min | 0.04 | 0.08 | 0.40 | 0.20 | 0.20 | 0.20 |
| PEG concentration (wt/wt), % | — | — | — | — | 2 | 6 |
| Viscosity (), cP (n = 4) | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.9 ± 0.1 | 1.7 ± 0.1 | 3.6 ± 0.3 |
| Shear stress (), cPa (n = 4) | 1.7 ± 0.2 | 3.4 ± 0.3 | 17.0 ± 1.5 | 8.5 ± 0.8 | 16.0 ± 1.1 | 33.7 ± 2.7 |
Fig. 5.
Effect of flow-derived shear stress on IGF-1R blockade within ES cells. IGF1 secretion and drug sensitivity for the experimental groups tested with different medium viscosities (S, static; B, bioreactor, with 1×: 0.9 ± 0.1 cP, 2×: 1.7 ± 0.1 cP, and 4×: 3.6 ± 0.3 cP) and a constant flow rate of 0.2 mL/min. (A) Amount of IGF1 ligand per scaffold measured in conditioned media after 10 d of culture. Error bars represent the SD (n = 3, *P < 0.05). (B) Cell viability after 3 d of culture followed by 7 d of exposure to dalotuzumab. Within each group, cell viability is shown as the DNA content of the drug-treated group normalized to the DNA content of the untreated group. Error bars represent the SD (n = 6, *P < 0.05).
Discussion
Although monolayer cultures and xenograft animal models will continue to serve the cancer research community as important tools for studying oncogenesis and drug sensitivity, they do not adequately preserve the human in vivo tumor phenotype and often poorly predict clinical drug activity. To overcome these challenges, scientists have turned to innovative ex vivo 3D cell culture systems that better mimic the complexity of human tumors and enable greater control over the cancer microenvironment and its principal components that affect cellular behavior. The National Institutes of Health have recognized growing synergy between the physical sciences and cancer biology fields and have encouraged transdisciplinary collaborative efforts that draw from their respective expertise (19). This convergence has facilitated the design of physiologically relevant and spatially complex cancer microenvironments, improved the resemblance of 3D preclinical models to their human counterparts, and permitted precise management of biophysical stimuli that control tumor interactions with its microenvironment (e.g., shear stress, metabolic stress, and matrix stiffness) (20). From our own experience using tissue-engineered (TE) cancer models, we previously reported that TE ES models are considerably better than 2D models in their mimicry of human tumors, particularly with respect to cell morphology, growth kinetics, proteomic expression patterns, and drug sensitivity (13).
In the context of bone tumors such as ES, which remain the clinical focus of our laboratory, the host microenvironment exposes cells to a range of dynamic biomechanical stimuli. These forces include shear stress associated with interstitial flow, compressive forces under load-bearing conditions, and stresses associated with vasodilatation (8). Although many of the biomechanical forces present in vivo are inherent in any living system (e.g., fluid shear stress in blood vessels and bone cell compression with physical activity), the tumor microenvironment exploits them during tumor growth, cell migration, and contraction. Furthermore, changes in interstitial pressure, signaling transduction, and ECM deposition can participate in modifying the biomechanical and biochemical properties of both the tumor microenvironment and surrounding cells. Although these factors cannot be experimentally controlled in 2D or spheroid culture, TE tumor models lend themselves well to this endeavor (21).
Recognizing the major importance of biomechanical forces in bone tumors (8), and the possible effects on key molecular targets of ES such as the IGF1/IGF-1R pathway (11, 22), we sought to expand upon a well-characterized TE bone tumor model pioneered in our laboratory by inclusion of flow perfusion in this study (13). The use of flow perfusion bioreactors provides mechanical stimulation in the form of flow-derived shear stress, which can be tailored within a physiological range by experimentally regulating flow rate and/or medium viscosity (16–18). Because increased flow perfusion rates are expected to enhance nutrient delivery and promote more uniform cell scaffold distribution, we determined whether physiological levels of flow perfusion could alleviate some of the downsides of 3D systems, including scaffold obstruction by cell-produced ECM and nonuniform cell deposition. Shown in Figs. 1 and 2, flow perfusion improved cell survival and growth. The convective flow promoted nutrient supply within the construct, thus leading to more uniform cell distribution and to higher proliferation when compared with static conditions.
Although our previous data indicated that the simple transition from monolayer culture to a 3D model induces major effects upon IGF-1R due to the altered tumor architecture (13), this investigation demonstrated how mechanical forces also affect ES cell phenotype and drug sensitivity. As shown in Figs. 3–5, we identified striking perfusion-dependent effects upon the IGF1/IGF-1R and other collateral ES signaling pathways, independently of the surrounding microarchitecture. The use of flow perfusion resulted in a downregulation of oncoproteins c-KIT and HER2, with protein expression levels more consistent with in vivo data reported in literature than with 2D monolayer evaluations (13, 23). Interestingly, we observed a robust increase in IGF1 secretion from static to flow perfusion, showing how the extent of this response correlated directly with the level of hydrodynamic shear stress present in the culture rather than that of convective mass transport. Considering the central role of the IGF1/IGF-1R pathway in ES oncogenesis and progression, the use of flow perfusion is superior to static conditions in modeling of IGF1 dysregulated upregulation typical of pediatric sarcomas and demonstrates the importance of mechanotransductive processes during ES progression (24).
Although several studies demonstrated the clinical efficacy of IGF-1R inhibitor dalotuzumab in IGF-1R targeted therapies, little to no activity in vitro has been documented so far (25, 26). In this study, exposure of ES cells to dalotuzumab showed a flow rate-dependent drug response in flow perfusion bioreactors, with greater drug efficacy at higher flow rates. At the same time, varying mechanical stimulation by changing medium viscosity (i.e., different shear stress but negligible effect on mass transport) demonstrated how higher shear stress leads to decreasing dalotuzumab activity. An explanation for this apparent contradiction lies in mass transport differences in the two performed experiments. An increasing flow rate not only implies higher shear stress (and IGF1 amount) but also enhances drug transport within the construct. Accordingly, the observed sensitivity to dalotuzumab increases with flow rate, as the drug is more effectively delivered to ES cells within the construct. Conversely, higher levels of IGF1 ligand counteract dalotuzumab-mediated IGF-1R blockade. Similar effects were not observed in static conditions, highlighting how the mechanical microenvironment affects the efficacy of potential biological therapeutic agents. Therefore, the TE tumor model seems accurately representative of important mechanical cues present in the ES tumor niche that are involved in IGF-1R pathway modulation, and ultimately in the response to dalotuzumab that is observed in the clinic. These findings highlight the importance of recapitulating biomechanical stimuli in vitro toward more accurate preclinical screening of antineoplastic activity of potential drug candidates.
In evaluating the impact of the TE tumor niche upon drug sensitivity, the unexpected finding that shear stress enhances autocrine release of IGF1 is noteworthy, and could have far-reaching implications that alter our understanding of ES tumorigenesis. Most experts agree on a mesodermal origin for ES, and Toretsky and coworkers have shown that IGF1 signaling is required to transform EWS-FLI1+ mouse fibroblasts into ES-like cells (27, 28). In our study, ES cells responded to perfusion analogously to mesenchymal stem cells (MSCs) and other MSC-derived cells, which have been shown to up-regulate IGF1 (and occasionally IGF-2) following mechanical stimulation (14, 15, 22). Although it remains to be proven, our data suggest that autocrine release of IGF1 by MSCs—mediated by physiological levels of shear force—may be sufficient to aid EWS-FLI-1-driven malignant transformation without the need for additional IGF1 in the serum. These findings explain the difficulty of transforming MSCs in monolayer culture.
For years, activation of IGF-1R and its downstream pathways has been considered an essential but insufficient ingredient for initiating tumorigenesis. However, why exogenous IGF1 was required could not be gleaned from monolayer cultures. These findings lend even greater credibility to the mesodermal hypothesis for ES derivation, and contribute to our understanding of the other elements required to initiate and maintain malignancy. Although it remains to be proven, we contend the mechanical forces induced by flow perfusion in culture, and naturally present in vivo, may sufficiently up-regulate autocrine production of IGF1 to levels necessary to complement the oncogenic effects of the EWS-FLI1 protein. Although a mechanistic interpretation for the mechanotransductive modulation of IGF1/IGF-1R axis is still missing in the literature, it can be hypothesized that hydrodynamic shear stress induces a cytoskeletal reorganization (8), with mechanical forces transmitted directly to the nucleus, according to the so-called “hard-wired” cell model (9). These forces might spark nuclear mechanochemical processes that enhance IGF1 promoter recognition by the EWS-FLI1 fusion protein (9, 29), and ultimately lead to upregulation of IGF1 as we observed. Investigating this hypothesis would be beneficial not only for ES but also for understanding the mechanisms behind mechanotransductive IGF1 upregulation in other cells of mesodermal origin (14, 15, 22).
Conclusions
In this research, we investigated how flow perfusion influences tumor cell phenotype, drug sensitivity, and cell proliferation in a 3D ES model compared with a control group in static conditions. In understanding these effects, we focused on a promising class of IGF-1R targeted therapies that have demonstrated remarkable clinical activity in a small subset of ES patients.
Flow perfusion improved cell culture conditions compared with static conditions in terms of cell growth, proliferation, and distribution. Strikingly, mechanical stimulation affected the IGF-1R signaling pathway via upregulation of the IGF1 ligand, with direct consequences on in vitro activity of IGF-1R inhibitor dalotuzumab. Competitive binding between IGF1 and dalotuzumab on IGF-1R was tipped in favor of IGF1, due to the higher amounts of IGF1 ligand in the presence of flow perfusion compared with static conditions. Resistance to dalotuzumab activity thus increased with the extent of shear stress, and this effect was alleviated solely in the presence of convective transport, which was ultimately responsible for higher drug availability to cells. We envision that a similar interplay between mass transport and flow-derived biomechanical stimulation is likely to occur in vivo, where dalotuzumab efficacy within a tumor might depend on the extent of local forces, interstitial flow, and vascularization.
We further showed how flow-derived shear stress resulted in a downregulation of oncoproteins c-KIT and HER2 compared with static conditions, with a physiologically more relevant ES phenotype. Although the mechanistic basis behind these phenomena remains unknown, these findings highlight key oncogenic changes influenced by mechanical forces that can be precisely controlled using a TE bone tumor niche and are a first step in narrowing the gap in IGF-1R targeted drug efficacy that exists between preclinical and clinical settings. Understanding how the microenvironment and biomechanical forces alter a cell’s response to treatment—and important resistance mechanisms—will contribute to the identification of potential biological targets and, consequently, the design of new anticancer drug candidates.
Although this research focused on the role of mechanotransduction on drug sensitivity and on cell phenotype, the ability to perform mechanistic studies within an engineered ES model resulted in the recognition of specific phenomena involved in ES oncogenesis. Accordingly, the system allows for further mechanistic studies on ES biology, and is easily amenable for the incorporation of other components of the tumor microenvironment, namely stromal cells. The present work has improved our knowledge of ES mechanobiology, but further investigations are warranted to study the effects of other components within a systematic framework. Ultimately, the use of flow perfusion bioreactors in concert with 3D TE scaffolds is expected to greatly improve our understanding of the interplay between architectural, biochemical, and mechanical cues and their relevance during tumor progression and acquisition of drug resistance.
Materials and Methods
Electrospun PCL scaffolds (3-mm diameter, 1-mm thickness, 10-μm fiber size) were seeded with 35,000 ES cells, as in previous studies (13). The resulting constructs were cultured in flow perfusion bioreactors at three different flow rates: 0.04 mL/min (B-04), 0.08 mL/min (B-08), and 0.40 mL/min (B-40). In light of the scaffold geometry, the range of chosen flow rates resulted in a shear stress range of 1.7–17.0 cPa, mirroring values of shear stress reported in the literature (17, 18). Time points were taken after 5 d and 10 d, at which time samples were harvested to analyze cell proliferation, protein expression, and drug response.
In an additional study, cell constructs were cultured in complete medium at three different viscosities: 1× (0.9 ± 0.1 cP), 2× (1.7 ± 0.1 cP), and 4× (3.6 ± 0.3 cP). Flow rate was kept constant at 0.2 mL/min, and medium viscosity was approximately doubled (or quadrupled) by adding poly(ethylene glycol) (PEG, Mw 20 kDa; Alpha Aesar) at 2% (or 6%) wt/wt concentration. The viscosity of PEG-containing medium was measured with an AR1000 rheometer (TA Instruments) at 37 °C (Table S1).
Constructs were harvested after 10 d to analyze cell proliferation, IGF1 expression, and drug response. Scaffolds cultured under static conditions (S) without flow perfusion served as controls for both studies. Scaffold preparation and characterization are described in detail in SI Materials and Methods, together with the parameters selected in the two studies for flow perfusion groups (Table S1). Details regarding materials and experimental techniques are comprehensively described in SI Materials and Methods.
SI Materials and Methods
Scaffold Preparation and Characterization.
Nonwoven PCL mats with an average fiber diameter of 10 μm (10.6 ±1.5 μm, n = 90) were fabricated as described in previous studies (13). Briefly, a 5:1 chloroform to methanol solution containing 18% wt PCL (Sigma-Aldrich) was pumped at 25 mL/h through a blunt 18G needle in a horizontal electrospinning setup. The gauge was exposed to a 30-kV voltage, and a grounded collecting plate was positioned normally to the gauge at a distance of 40 cm. Mats were electrospun to a thickness of 1.00 ± 0.01 mm and inspected by SEM (FEI Quanta 400 Environmental; FEI) to determine fiber diameter. Scaffolds of 3-mm diameter were die-punched using dermal biopsy punches and subsequently press-fitted into custom-made scaffold holders, the design of which has been previously illustrated (30). Both scaffolds and scaffold holders were sterilized using ethylene oxide (Anderson Sterilizers) for 12 h, then soaked in a progressive ethanol series (100–25%), rinsed three times in PBS (Gibco), and, finally, incubated overnight in complete Roswell Park Memorial Institute (RPMI) medium 1640 (Mediatech).
Cell Culture and Bioreactor Setup.
The human ES cell line TC71 was available from the repository of sarcoma cell lines at our institution. Before use, cell identity was confirmed using short-tandem repeats fingerprinting (AmpFISTR Identifiler kit according to manufacturer's instructions) (Applied Biosystems) (23), which was compared with known American Type Culture Collection (ATCC) fingerprints (ATCC.org) and to the Integrated Molecular Authentication database (CLIMA) version 0.1.200808 (bioinformatics.hsanmartino.it/clima/) (31). ES cells were cultured in RPMI medium 1640, supplemented with 10% FBS (Gemini Bio-Products) and antibiotics (100 IU/mL penicillin and 100 μg/mL streptomycin; Gibco). Cells were then detached with 0.05% trypsin-EDTA (Gibco) and counted using a hemocytometer. Each scaffold was seeded with 35,000 cells and incubated overnight for cell adhesion. Scaffolds under static conditions were placed in ultralow attachment 24-well plates with 2 mL of complete medium. Scaffolds for the 3D flow perfusion groups were placed in a scaffold holder and transferred into a flow perfusion bioreactor, the design of which has been illustrated previously (30). Each bioreactor unit contained 10 scaffolds and 50 mL of complete medium. Samples were maintained in a heat-jacketed incubator at 37 °C and 5% (vol/vol) CO2 (HeraCell 150i; ThermoScientific) for up to 10 d, and half of the medium was replaced daily.
DNA Quantification.
Samples for DNA quantification (n = 4) were subjected to three cycles of freeze/thawing (5 min in liquid N2/10 min in 37 °C water bath), followed by 10 min of ultrasound sonication to guarantee complete extraction of the DNA from the scaffold into the supernatant solution. The concentration of double-stranded DNA was measured with the Quant-iT PicoGreen dsDNA assay kit (Invitrogen), according to manufacturer’s instructions. Cell lysate, dye solution, and buffer were mixed in a white flat-bottom 96-well plate in triplicates, and the resulting fluorescence was measured (FLx800 Fluorescence Microplate Reader; BioTek Instruments). DNA concentration was calculated using a lambda DNA standard curve. The same protocol was used during drug testing to quantify the number of ES cells (n = 6 in the latter case).
Western Blotting and Flow Cytometry.
Samples were collected at day 10 (n = 3), and protein lysates were extracted via cold incubation using a lysis buffer [1% Triton X-100 (vol/vol), 50 mM Hepes, pH 7.4, 1.5 mM MgCl2, 150 mM NaCl, 100 mM NaF, 1 mM EGTA, 10 mM Na pyrophosphate, 1 mM Na3VO4, 10% (vol/vol) glycerol] containing a fresh mixture of phosphatase and protease inhibitors (Roche Applied Science). Lysates were then stored at −80 °C until analyzed. The protein concentration was quantified using a Micro BCA protein assay kit (Thermo Fisher Scientific). Proteins were resolved by SDS polyacrylamide gel electrophoresis and transferred to PVDF membranes. The membranes were blocked using 5% (wt/vol) milk and hybridized with different primary antibodies against markers involved in the IGF-1R signaling pathway and resistance: insulin-like growth factor-1 receptor β (IGF-1R), human epidermal growth factor receptor-2 (HER2), IR β (IR), protooncogene c-KIT (c-KIT), and phosphorylated ribosomal protein 6 (pS6) (11, 32). GAPDH served as the loading control. All primary antibodies were from Cell Signaling Technology except for c-KIT (Epitomics). Signals were captured using horseradish peroxidase-conjugated secondary anti-rabbit IgG and anti-mouse IgG antibodies (Cell Signaling Technology) and visualized using SuperSignal West Dura chemiluminescent substrate (Thermo Fisher Scientific). The level of immunoreactive protein was measured using chemiluminescent Hyperfilm ECL and quantified using an ImageQuant TL computing densitometer (GE Healthcare).
Flow Cytometry.
Samples were processed immediately after experiment completion. Cells were dissociated from the scaffolds using an enzyme-free cell dissociation buffer (Gibco). Cells were indirectly evaluated by enumerating positive cellular staining to Annexin V (Annexin) and propidium iodide (PI) apoptotic biomarkers and CD99, a diagnostic surface marker of ES cells. Cells were stained with Annexin V-fluorescein isothiocyanate (FITC) and PI (BD Biosciences). In another set of cell staining, the same dissociated ES cells were stained with FITC-CD99 monoclonal antibody. A kit obtained from eBioscience was used to intracellularly label the cells after their permeabilization using a conjugated monoclonal antibody against proliferation biomarker Ki67. Stained cells were then acquired using a flow cytometer (FACSCanto II; BD Biosciences), and data were analyzed using the FlowJo software program 10.0.6 (Tree Star).
SEM.
Samples for imaging were fixed in 2.5% glutaraldehyde for 30 min, dehydrated with serial concentrations of ethanol (70–100%), and, finally, air dried in a laminar airflow cell culture hood overnight. Samples for SEM were then sputter-coated with gold and imaged (FEI Quanta 400 Environmental; FEI).
Immunofluorescent Microscopy.
Constructs at day 10 were fixed in 10% neutral buffered formalin (Fisher Scientific) overnight at room temperature, followed by dehydration in serial concentrations of ethanol (70–100%). Samples were then embedded in Histoprep freezing medium (Fisher Scientific) and sectioned using a cryostat (Leica CM 1850 UV; Leica Biosystems Nussloch GmbH). Nuclei were stained with DAPI, and samples were imaged with a laser Nikon Eclipse TE300 inverted microscope and NIS Elements software (Nikon Instruments).
Drug Testing and ELISA.
Samples for cytotoxicity testing were cultured for 10 d and then exposed for 2 d to complete medium containing 3 μM doxorubicin hydrochloride (Dox; Sigma-Aldrich). Testing of biologically targeted therapeutic dalotuzumab (MK-0646, monoclonal antibody against IGF-1R, Merck) and of IGF1 ligand (R&D Systems) involved three different treatments: (i) stimulation with IGF1 ligand 20 ng/mL, (ii) exposure to IGF-1R inhibitor (dalotuzumab 100 μg/mL), and (iii) a combination of treatments i and ii. Samples were cultured for 3 d in complete medium followed by 7 d of treatment during which FBS was replaced by charcoal-stripped FBS (csFBS; Life Technologies) to minimize the interference of exogenous IGF1 ligand with other growth factors present in the serum. Conditioned media from each group were harvested after 2 d and 10 d of culture to quantify the concentration of IGF1 ligand (ELISA kit DuoSet; R&D Systems). In light of the different volumes of medium and the different number of scaffolds present in static and flow perfusion conditions, comparisons between groups were made considering the amount of soluble IGF1 per scaffold (assuming negligible adsorption of IGF1 on the scaffold). Complete media prepared with either FBS or csFBS were used as controls to estimate the levels of IGF1 normally contained in the sera. A microplate reader (DTX880; Beckman Coulter) was used to measure the optical density in each sample. For both Dox and IGF1/dalotuzumab treatment, half of the medium was replaced every day, and cell viability was calculated by normalizing the average number of cells in each group against the average number of cells in the respective untreated group, similarly to previous studies (13). ES cells exposed to variable medium viscosities were cultured for 3 d in complete medium followed by 7 d of exposure to IGF-1R inhibitor (dalotuzumab 100 μg/mL). They were subsequently analyzed for IGF1 production (n = 3) and DNA quantification (n = 6).
Statistical Analysis.
Where applicable, data are expressed as mean+SD. Statistical analysis was performed using a two-factor ANOVA test followed by Tukey’s honestly significant difference post hoc test. Differences were considered significant for P < 0.05, unless otherwise noted.
Shear Stress Calculations.
To calculate shear stress, we assumed a cylindrical pore model approximation for the scaffold pores (33). This assumption permitted the calculation of average shear stress on the wall () of the scaffold pore (i.e., the shear stress experienced by ES cells) as
where μ is medium viscosity (measured via rheometry at 37 °C), dp is pore diameter [considered 40 μm according to previous studies (34)], and is the average velocity within the pore calculated as
where Q is flow rate, D is scaffold diameter (3 mm), and ϕ is scaffold porosity[considered 0.88 according to previous studies (34)].
Acknowledgments
The authors thank Tiffany N. Vo, Eric R. Molina, and Esther J. Lee for helpful discussions and for assistance with manuscript preparation. This research is supported in part by the University of Texas MD Anderson Cancer Center’s Support Grant CA016672 and the National Institutes of Health Grant R01 CA180279.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1506684112/-/DCSupplemental.
References
- 1.Jacks T, Weinberg RA. Taking the study of cancer cell survival to a new dimension. Cell. 2002;111(7):923–925. doi: 10.1016/s0092-8674(02)01229-1. [DOI] [PubMed] [Google Scholar]
- 2.Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nat Rev Drug Discov. 2004;3(8):711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 3.Shin CS, Kwak B, Han B, Park K. Development of an in vitro 3D tumor model to study therapeutic efficiency of an anticancer drug. Mol Pharm. 2013;10(6):2167–2175. doi: 10.1021/mp300595a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck JN, Singh A, Rothenberg AR, Elisseeff JH, Ewald AJ. The independent roles of mechanical, structural and adhesion characteristics of 3D hydrogels on the regulation of cancer invasion and dissemination. Biomaterials. 2013;34(37):9486–9495. doi: 10.1016/j.biomaterials.2013.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bates RC, Edwards NS, Yates JD. Spheroids and cell survival. Crit Rev Oncol Hematol. 2000;36(2-3):61–74. doi: 10.1016/s1040-8428(00)00077-9. [DOI] [PubMed] [Google Scholar]
- 6.Villasante A, Marturano-Kruik A, Vunjak-Novakovic G. Bioengineered human tumor within a bone niche. Biomaterials. 2014;35(22):5785–5794. doi: 10.1016/j.biomaterials.2014.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chitcholtan K, Sykes PH, Evans JJ. The resistance of intracellular mediators to doxorubicin and cisplatin are distinct in 3D and 2D endometrial cancer. J Transl Med. 2012;10(1):38. doi: 10.1186/1479-5876-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaalouk DE, Lammerding J. Mechanotransduction gone awry. Nat Rev Mol Cell Biol. 2009;10(1):63–73. doi: 10.1038/nrm2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: Mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol. 2009;10(1):75–82. doi: 10.1038/nrm2594. [DOI] [PubMed] [Google Scholar]
- 10.Wozniak MA, Chen CS. Mechanotransduction in development: A growing role for contractility. Nat Rev Mol Cell Biol. 2009;10(1):34–43. doi: 10.1038/nrm2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ludwig JA, Lamhamedi-Cherradi SE, Lee HY, Naing A, Benjamin R. Dual targeting of the insulin-like growth factor and collateral pathways in cancer: Combating drug resistance. Cancers (Basel) 2011;3(3):3029–3054. doi: 10.3390/cancers3033029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subbiah V, et al. Ewing’s sarcoma: Standard and experimental treatment options. Curr Treat Options Oncol. 2009;10(1-2):126–140. doi: 10.1007/s11864-009-0104-6. [DOI] [PubMed] [Google Scholar]
- 13.Fong ELS, et al. Modeling Ewing sarcoma tumors in vitro with 3D scaffolds. Proc Natl Acad Sci USA. 2013;110(16):6500–6505. doi: 10.1073/pnas.1221403110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tahimic CG, Wang Y, Bikle DD. Anabolic effects of IGF-1 signaling on the skeleton. Front Endocrinol (Lausanne) 2013;4:6. doi: 10.3389/fendo.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein-Nulend J, Bakker AD, Bacabac RG, Vatsa A, Weinbaum S. Mechanosensation and transduction in osteocytes. Bone. 2013;54(2):182–190. doi: 10.1016/j.bone.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Sikavitsas VI, Bancroft GN, Holtorf HL, Jansen JA, Mikos AG. Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. Proc Natl Acad Sci USA. 2003;100(25):14683–14688. doi: 10.1073/pnas.2434367100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCoy RJ, O’Brien FJ. Influence of shear stress in perfusion bioreactor cultures for the development of three-dimensional bone tissue constructs: A review. Tissue Eng Part B Rev. 2010;16(6):587–601. doi: 10.1089/ten.TEB.2010.0370. [DOI] [PubMed] [Google Scholar]
- 18.Cowin SC. Mechanosensation and fluid transport in living bone. J Musculoskelet Neuronal Interact. 2002;2(3):256–260. [PubMed] [Google Scholar]
- 19.Schuessler TK, et al. Biomimetic tissue-engineered systems for advancing cancer research: NCI Strategic Workshop report. Cancer Res. 2014;74(19):5359–5363. doi: 10.1158/0008-5472.CAN-14-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lamhamedi-Cherradi SE, et al. 3D tissue-engineered model of Ewing’s sarcoma. Adv Drug Deliv Rev. 2014;79-80:155–171. doi: 10.1016/j.addr.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Villasante A, Vunjak-Novakovic G. Tissue-engineered models of human tumors for cancer research. Expert Opin Drug Discov. 2015;10(3):257–268. doi: 10.1517/17460441.2015.1009442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlegel W, et al. Insulin-like growth factor I (IGF-1) Ec/Mechano Growth factor—A splice variant of IGF-1 within the growth plate. PLoS One. 2013;8(10):e76133. doi: 10.1371/journal.pone.0076133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Scotlandi K, et al. C-kit receptor expression in Ewing’s sarcoma: Lack of prognostic value but therapeutic targeting opportunities in appropriate conditions. J Clin Oncol. 2003;21(10):1952–1960. doi: 10.1200/JCO.2003.11.111. [DOI] [PubMed] [Google Scholar]
- 24.Rikhof B, de Jong S, Suurmeijer AJH, Meijer C, van der Graaf WTA. The insulin-like growth factor system and sarcomas. J Pathol. 2009;217(4):469–482. doi: 10.1002/path.2499. [DOI] [PubMed] [Google Scholar]
- 25.Atzori F, et al. A phase I pharmacokinetic and pharmacodynamic study of dalotuzumab (MK-0646), an anti-insulin-like growth factor-1 receptor monoclonal antibody, in patients with advanced solid tumors. Clin Cancer Res. 2011;17(19):6304–6312. doi: 10.1158/1078-0432.CCR-10-3336. [DOI] [PubMed] [Google Scholar]
- 26.Scartozzi M, et al. Dalotuzumab, a recombinant humanized mAb targeted against IGFR1 for the treatment of cancer. Curr Opin Mol Ther. 2010;12(3):361–371. [PubMed] [Google Scholar]
- 27.Suvà ML, et al. Identification of cancer stem cells in Ewing’s sarcoma. Cancer Res. 2009;69(5):1776–1781. doi: 10.1158/0008-5472.CAN-08-2242. [DOI] [PubMed] [Google Scholar]
- 28.Toretsky JA, Kalebic T, Blakesley V, LeRoith D, Helman LJ. The insulin-like growth factor-I receptor is required for EWS/FLI-1 transformation of fibroblasts. J Biol Chem. 1997;272(49):30822–30827. doi: 10.1074/jbc.272.49.30822. [DOI] [PubMed] [Google Scholar]
- 29.Cironi L, et al. IGF1 is a common target gene of Ewing’s sarcoma fusion proteins in mesenchymal progenitor cells. PLoS One. 2008;3(7):e2634. doi: 10.1371/journal.pone.0002634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dahlin RL, Meretoja VV, Ni M, Kasper FK, Mikos AG. Design of a high-throughput flow perfusion bioreactor system for tissue engineering. Tissue Eng Part C Methods. 2012;18(10):817–820. doi: 10.1089/ten.tec.2012.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano P, et al. Cell Line Data Base: Structure and recent improvements towards molecular authentication of human cell lines. Nucleic Acids Res. 2009;37(Database issue):D925–D932. doi: 10.1093/nar/gkn730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong H, et al. MEDI-573, alone or in combination with mammalian target of rapamycin inhibitors, targets the insulin-like growth factor pathway in sarcomas. Mol Cancer Ther. 2014;13(11):2662–2673. doi: 10.1158/1535-7163.MCT-14-0144. [DOI] [PubMed] [Google Scholar]
- 33.Goldstein AS, Juarez TM, Helmke CD, Gustin MC, Mikos AG. Effect of convection on osteoblastic cell growth and function in biodegradable polymer foam scaffolds. Biomaterials. 2001;22(11):1279–1288. doi: 10.1016/s0142-9612(00)00280-5. [DOI] [PubMed] [Google Scholar]
- 34.Pham QP, Sharma U, Mikos AG. Electrospun poly(ε-caprolactone) microfiber and multilayer nanofiber/microfiber scaffolds: Characterization of scaffolds and measurement of cellular infiltration. Biomacromolecules. 2006;7(10):2796–2805. doi: 10.1021/bm060680j. [DOI] [PubMed] [Google Scholar]