Significance
Although model organisms have been used to understand the underlying basis of hypoxia and how to treat it, it has always been useful to learn from natural experiments in humans, such as in high-altitude dwellers. In this study, we demonstrate that decreased Endothelin receptor type B (EdnrB), a gene associated with altitude adaptation from our human study, improves cardiac tolerance to hypoxia. We show that heterozygote EdnrB mice maintain higher cardiac output and peripheral perfusion and better O2 delivery to vital organs, even in severe hypoxia. Furthermore, the transcriptome profile revealed three atria-specific genes, natriuretic peptide type A (Nppa), sarcolipin (Sln), and myosin light polypeptide 4 (Myl4), which were down-regulated by hypoxia and possibly have a role in improving cardiac performance during normoxia and hypoxia.
Keywords: Endothelin receptor type B, hypoxia, high altitude, cardiac output, lactate
Abstract
To better understand human adaptation to stress, and in particular to hypoxia, we took advantage of one of nature’s experiments at high altitude (HA) and studied Ethiopians, a population that is well-adapted to HA hypoxic stress. Using whole-genome sequencing, we discovered that EDNRB (Endothelin receptor type B) is a candidate gene involved in HA adaptation. To test whether EDNRB plays a critical role in hypoxia tolerance and adaptation, we generated EdnrB knockout mice and found that when EdnrB−/+ heterozygote mice are treated with lower levels of oxygen (O2), they tolerate various levels of hypoxia (even extreme hypoxia, e.g., 5% O2) very well. For example, they maintain ejection fraction, cardiac contractility, and cardiac output in severe hypoxia. Furthermore, O2 delivery to vital organs was significantly higher and blood lactate was lower in EdnrB−/+ compared with wild type in hypoxia. Tissue hypoxia in brain, heart, and kidney was lower in EdnrB−/+ mice as well. These data demonstrate that a lower level of EDNRB significantly improves cardiac performance and tissue perfusion under various levels of hypoxia. Transcriptomic profiling of left ventricles revealed three specific genes [natriuretic peptide type A (Nppa), sarcolipin (Sln), and myosin light polypeptide 4 (Myl4)] that were oppositely expressed (q < 0.05) between EdnrB−/+ and wild type. Functions related to these gene networks were consistent with a better cardiac contractility and performance. We conclude that EDNRB plays a key role in hypoxia tolerance and that a lower level of EDNRB contributes, at least in part, to HA adaptation in humans.
Oxygen (O2) is often referred to as a biosignature, a chemical marker in the atmosphere closely associated with life, and in a complex organism, such as humans, various physiological systems have evolved to maintain an optimal O2 homeostasis. Arguably, humans living at high altitude (HA) for thousands of years ought to have undergone a significant level of natural selection to adjust to the challenging hypoxic condition. Human adaptation to HA hypoxia, which can be protective to tissues, can potentially be harnessed for better therapeutic modalities for sea-level diseases that involve hypoxia and ischemia in their pathogenesis. Indeed, lessons from such an “experiment in nature” can be derived from HA adaptation and can advance low-altitude medicine (1). With the advent of newer technology including next-generation sequencing (seq), this idea has recently led to intensive efforts, and a number of publications have appeared on studies of human populations living at HA (2–4). These studies also draw added significance not only to sea-level human diseases but also to more than 140 million people living at an altitude above 2,500 m, where the hypoxic condition presents a major challenge for survival (5). Although a number of laboratory methods that mimic hypoxia adaptation using model organisms (6) have been used as tools to identify causative genetic pathways (7, 8), studies in human populations living at different HA regions with distinct genetic backgrounds can provide direct insight for identifying mechanisms regulating hypoxia responses in humans.
Over the last few decades, much research has been done on humans permanently living at high altitude. Although there is still debate about the mechanisms of adaptation in HA populations, especially in relation to those populations that are best-studied (9), the Ethiopians are considered to be the best-adapted because the prevalence of chronic mountain sickness, a maladaptation syndrome to HA, has never been reported (10, 11). In our recent effort to understand the basis for adaptation to HA in humans, we analyzed the whole genome for genetic variation in HA Ethiopians (4). Using cross-population tests of selection, we searched for genomic regions with a significant loss of genetic diversity indicative of selective sweeps (4). We discovered several regions on different chromosomes that were significantly associated with HA adaptation. To elucidate the potential role of each of the individual genes in hypoxia adaptation and tolerance, we experimentally evaluated whether these genes affected hypoxia tolerance in Drosophila by manipulating respective orthologs in the fly. Indeed, we succeeded in functional evaluation of some of these candidate genes in flies (4). However, Endothelin receptor B (EDNRB), which is one of these significant candidate genes [chromosome (chr)13], had no ortholog in flies (4).
To functionally validate and study the role of this candidate gene, we resorted to experiments in mice. Given that HA exposure has long been recognized as a cardiac stress (12) and endothelin (ET) receptor blockade with bosentan ameliorates an increase in pulmonary artery pressure (13) with hypoxia, we hypothesized that a decreased level of EDNRB would play a protective role in HA hypoxia. In addition, it appears that EdnrA-specific antagonism affects only pulmonary hypertension with no effect on cardiac performance (14). This supported the notion that EdnrB-specific antagonism of bosentan would improve cardiac performance at high altitude. Because EdnrB is widely expressed (15), we generated an EdnrB global knockout mouse model and used the heterozygous (EdnrB−/+) to evaluate its role in cardiovascular response to various degrees of hypoxia from mild to severe. We report here, to our knowledge for the first time, a major heterozygous advantage of EdnrB under hypoxic conditions and its cross-talk with three specific genes that were differentially expressed in the ventricles.
Results
Genomic Analysis of EDNRB Reveals Fixation of SNPs in the HA Population.
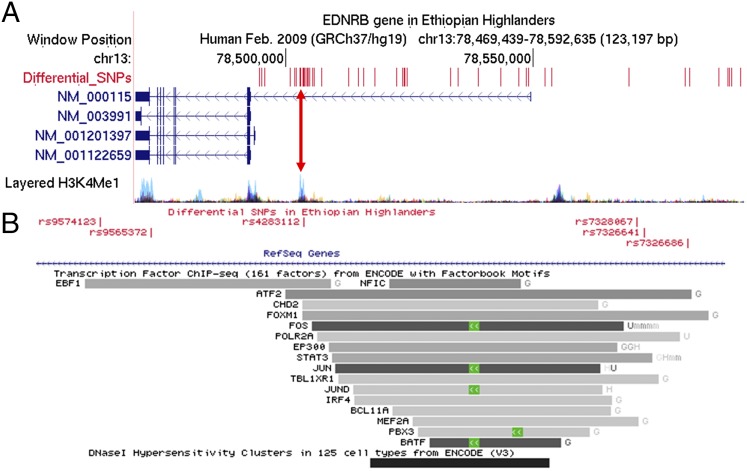
Earlier, we reported EDNRB as one of our top candidates for genes underlying hypoxia adaptation in Ethiopian highlanders (4). This HA population showed a strong signature of selection in the EDNRB region, with a large block of fixed SNPs that was observed at significantly lower frequencies in lowlander controls (Table S1). Upon reanalyzing the sequenced data of the region containing EDNRB, we noted a large block of 52 “differential” SNPs upstream of the promoter region of EDNRB (Fig. 1A), 20 of which were located near a cluster of ENCODE project (genome.ucsc.edu/ENCODE/index.html) transcription-factor binding sites (TFBSs). This region is also depicted with a stronger signal for the layered H3K4Me1 (H3 histone protein, lysine 4, monomethylation; Fig. 1A, pink line) histone mark across the genome as determined by ChIP-seq assay. Indirectly, H3K4Me1 is associated with enhancers and with DNA regions downstream of the transcription start site. The signal peak was observed primarily in the HUVEC (human umbilical vein endothelial cell) tissue type, represented in Fig. 1A as light blue peaks, implying that potential disruption of this site would be consistent with blood vessel-related phenotypes. Five of these SNPs overlapped with the ENCODE TFBSs (Table S2). This includes binding sites for transcription factors such as ATF2, FOXM1, STAT3, and MEF2A (Fig. 1B), which are reported to have roles in cardiac function either independently (16, 17) or in cross-talk with the endothelin system (18, 19). Also, within this cluster, the strongest signal was a 400-bp Chip-seq peak for the FOS/JUN (chr13:78503006–78503405; Fig. 1B) transcription factors reported as related to hypoxia and angiogenesis (20). Although we did not find any SNPs lying within this stretch of sequence, three SNPs were flanking FOS/JUN and were at 100% in the Amhara population and roughly 66% in the Oromo population, both HA populations. Furthermore, this peak appears to overlap a DNaseI-hypersensitive region, observed mainly in blood and lymphatic endothelial cells. These observations, and the fact that this region is in the 5′ region of EDNRB or within the first intron of one of the EDNRB transcripts, reference sequence (RefSeq) accession no. NM_000115 (Fig. 1A), makes it unlikely that the strong signature of selection in the EDNRB region was purely by chance. Although the SNPs in the EdnrB promoter region play a role (21), our rationale for hypothesizing that they render EdnrB hypofunctional are data obtained from the clinical use of bosentan in patients at high altitude.
Table S1.
Selected SNPs in the EDNRB region on chromosome 13 of Ethiopian highlanders describing the conserved haplotype in the promoter region of the EDNRB gene
| Serial number | Reference SNP ID | Alleles, ancestral/alternate* | 1000 Genomes | Luhya† | Ethiopian highlander‡ |
| 1 | rs7330412 | C/T | 0.51/0.49 | 0.58/0.42 | 0.07/0.93 |
| 2 | rs3759475 | A/G | 0.52/0.48 | 0.67/0.33 | 0.07/0.93 |
| 3 | rs7989185 | T/A | 0.93/0.07 | 0.91/0.09 | 1/0 |
| 4 | rs7989190 | C/A | 0.93/0.07 | 0.91/0.09 | 1/0 |
| 5 | rs6563023 | C/A | 0.51/0.49 | 0.58/0.42 | 0.07/0.93 |
| 6 | rs8000166 | T/G | 0.52/0.48 | 0.67/0.33 | 0.07/0.93 |
| 7 | rs6563024 | T/C | 0.52/0.48 | 0.67/0.33 | 0.07/0.93 |
| 8 | rs2329047 | T/G | 0.48/0.52 | 0.58/0.42 | 0/1 |
| 9 | rs4275748 | G/A | 0.53/0.47 | 0.45/0.55 | 1/0 |
| 10 | rs9574123 | A/T | 0.52/0.48 | 0.42/0.58 | 1/0 |
| 11 | rs9565372 | G/A | 0.53/0.47 | 0.45/0.55 | 1/0 |
| 12 | rs4283112 | T/A | 0.52/0.48 | 0.42/0.58 | 1/0 |
| 13 | rs7328067 | C/T | 0.53/0.47 | 0.42/0.58 | 1/0 |
| 14 | rs7326641 | A/G | 0.48/0.52 | 0.58/0.42 | 0/1 |
| 15 | rs7326686 | T/A | 0.52/0.48 | 0.42/0.58 | 1/0 |
| 16 | rs7333359 | G/T | 0.52/0.48 | 0.42/0.58 | 1/0 |
| 17 | rs7333372 | C/T | 0.52/0.48 | 0.42/0.58 | 1/0 |
| 18 | rs7332215 | G/A | 0.52/0.48 | 0.42/0.58 | 1/0 |
| 19 | rs7333015 | A/G | 0.48/0.52 | 0.58/0.42 | 0/1 |
| 20 | rs9544638 | A/G | 0.52/0.48 | 0.67/0.33 | 0.07/0.93 |
| 21 | rs2329050 | C/T | 0.48/0.52 | 0.58/0.42 | 0/1 |
| 22 | rs9544640 | T/C | 0.48/0.52 | 0.58/0.42 | 0/1 |
| 23 | rs9574124 | G/C | 0.48/0.52 | 0.58/0.42 | 0/1 |
| 24 | rs9318502 | C/G | 0.51/0.49 | 0.58/0.42 | 0.07/0.93 |
| 25 | rs12859148 | C/T | 0.47/0.53 | 0.64/0.36 | 0.07/0.93 |
| 26 | rs1924916 | C/A | 0.47/0.53 | 0.64/0.36 | 0.07/0.93 |
| 27 | rs9544643 | G/A | 0.53/0.47 | 0.69/0.31 | 0.07/0.93 |
| 28 | rs7332993 | T/A | 0.48/0.52 | 0.67/0.33 | 0.07/0.93 |
| 29 | rs1924914 | A/G | 0.61/0.39 | 0.5/0.5 | 1/0 |
| 30 | rs7319342 | G/A | 0.48/0.52 | 0.66/0.34 | 0.07/0.93 |
| 31 | rs7324245 | C/T | 0.41/0.59 | 0.5/0.5 | 0/1 |
| 32 | rs7325878 | T/C | 0.41/0.59 | 0.5/0.5 | 0/1 |
| 33 | rs1924938 | G/C | 0.48/0.52 | 0.66/0.34 | 0.07/0.93 |
| 34 | rs1924937 | G/T | 0.48/0.52 | 0.66/0.34 | 0.07/0.93 |
| 35 | rs7317154 | C/T | 0.48/0.52 | 0.66/0.34 | 0.07/0.93 |
| 36 | rs9574126 | G/A | 0.4/0.6 | 0.5/0.5 | 0/1 |
| 37 | rs9544650 | A/G | 0.48/0.52 | 0.66/0.34 | 0.07/0.93 |
| 38 | rs1537065 | A/G | 0.48/0.52 | 0.66/0.34 | 0.07/0.93 |
| 39 | rs7339077 | T/C | 0.48/0.52 | 0.32/0.68 | 0.07/0.93 |
| 40 | rs1854800 | G/A | 0.48/0.52 | 0.66/0.34 | 0.07/0.93 |
| 41 | rs9574129 | T/G | 0.52/0.48 | 0.69/0.31 | 0.07/0.93 |
| 42 | rs9544661 | G/A | 0.53/0.47 | 0.69/0.31 | 0.07/0.93 |
| 43 | rs9544662 | G/A | 0.48/0.52 | 0.66/0.34 | 0.07/0.93 |
| 44 | rs9574130 | T/C | 0.48/0.52 | 0.66/0.34 | 0.07/0.93 |
| 45 | rs1970036 | C/G | 0.99/0.01 | 0.96/0.04 | 1/0 |
| 46 | rs1537067 | A/G | 0.47/0.53 | 0.65/0.35 | 0.07/0.93 |
| 47 | rs1330911 | G/A | 0.47/0.53 | 0.65/0.35 | 0.07/0.93 |
| 48 | rs4482161 | A/C | 0.47/0.53 | 0.65/0.35 | 0.07/0.93 |
| 49 | rs9565375 | A/G | 0.52/0.48 | 0.68/0.32 | 0.07/0.93 |
| 50 | rs9544672 | C/T | 0.47/0.53 | 0.65/0.35 | 0.07/0.93 |
| 51 | rs7997143 | T/G | 0.47/0.53 | 0.65/0.35 | 0.07/0.93 |
| 52 | rs8001692 | C/T | 0.47/0.53 | 0.65/0.35 | 0.07/0.93 |
The bold highlighted allele in the Alleles column is the high-frequency variant that makes the dominant haplotype in Ethiopian highlanders.
The alternate allele is the second-most common allele.
The Luhya in Webuye from Kenya are the genetically closest population from the 1000 Genomes data to the Ethiopian highlander [Udpa et al. (4)].
All variants with frequency >90% were included.
Fig. 1.
EDNRB region in Ethiopian highlanders. (A) Four known transcripts of human EDNRB with RefSeq accession numbers. Note that EDNRB is transcribed from the negative strand (i.e., right to left in this figure). Overlaid above (in red) are the genomic positions of 52 SNPs deemed differential by Udpa et al. (4). These SNPs show a strong signal of frequency differentiation between the Ethiopian highlander population (Amhara) and a nearby lowlander control population (Luhya population from the 1000 Genomes project, www.1000genomes.org), indicative of strong positive selection in the region. A large number of SNPs are condensed in a region with high H3K4Me1 track [web-accessible directories of genomic data viewable on the University of California, Santa Cruz (UCSC), genome browser (the pink line overlaid is further resolved in B)] and its associated regulatory role. (B) Many of these SNPs are in the cluster of ENCODE transcription-factor binding sites. These SNPs are within the TFBSs (Table S1). This site also overlaps a DNaseI-hypersensitive region, observed mainly in blood and lymphatic endothelial cells.
Table S2.
SNPs and overlapping transcription factors within the TF binding site cluster located in the promoter region of EDNRB
| SNPs | Genome position | Transcription factors |
| rs9574123 | 78502735 | EBF1 |
| rs9565372 | 78502800 | EBF1 |
| rs4283112 | 78502996 | EBF1, ATF2, FOXM1, CHD2 |
| rs7328067 | 78503423 | ATF2, FOXM1, POLR2A, STAT3, TBL1XR1, MEF2A |
| rs7326641 | 78503433 | ATF2, FOXM1, POLR2A, STAT3, TBL1XR1 |
| rs7326686 | 78503488 | ATF2, FOXM1 |
The table provides the list of TFs that have a binding site overlapping a cluster of SNPs that were differential in the Ethiopian highlanders.
Generation of EdnrB Heterozygous Mice.
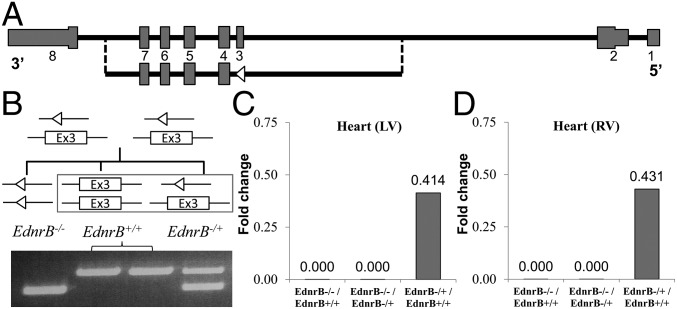
We hypothesized that decreasing functional EDNRB would be advantageous in hypoxia, and hence we knocked out this gene in a mouse model and then studied its phenotype in both normoxia and hypoxia. Using a Cre-Lox recombination system, exon 3 from EdnrBflox/flox was completely removed (Fig. 2A). A detailed strategy is provided in SI Materials and Methods and Fig. S1. This resulted in an out-of-frame transcript of exon 2–exon 4 splicing, making the gene nonfunctional (22). As we expected, the complete knockout of EdnrB (EdnrB−/−) led to a phenotype observed in the Hirschsprung mouse model and accordingly dies at around weaning age (∼21 d). Considering that the homozygous EdnrB mutant is lethal and believing that a partial knockout would be pharmacologically relevant, we used heterozygous (EdnrB−/+) mice for all of our experiments (Fig. 2B). The body weights of EdnrB−/+ were similar to those of age-matched littermate controls (EdnrB+/+). Both male and female EdnrB−/+ mice were fertile, and therefore were used as breeding pairs (Fig. 2B). To make sure that the gene is expressed as assumed by its genotype, we measured the expression level of EdnrB in left and right cardiac ventricles. Quantitative (q)RT-PCR confirmed no expression of the EdnrB gene in EdnrB−/− mice, and the ratio of expression in EdnrB−/+ was about half (0.414-fold change) of that in EdnrB+/+ (Fig. 2 C and D).
Fig. 2.
Generation of EdnrB knockout mice confirms removal of exon 3, and its expression is reduced to approximately half in the EdnrB−/+. (A) Diagram representing the complete EdnrB gene from exons 1–8. (Lower) EdnrB with exon 3 removed (the open triangle represents a loxP site). (B) Breeding strategy for generating EdnrB−/+ and EdnrB+/+ (box) mice used for the experiments. Lines with open triangles represent loxP sites after the removal of exon 3. The gel picture shows the three genotypes whereby the upper PCR band size of 481 bp depicts the intact exon 3 (EdnrB+/+), whereas the lower band size of 368 bp results from the removal of the 113-bp exon 3 (EdnrB−/−). Both bands are seen in EdnrB−/+. (C and D) qRT-PCR from RNA isolated from heart left ventricle (LV) (C) and right ventricle (RV) (D) of EdnrB−/−, EdnrB−/+, and EdnrB+/+. The y axis depicts the fold-change ratio.
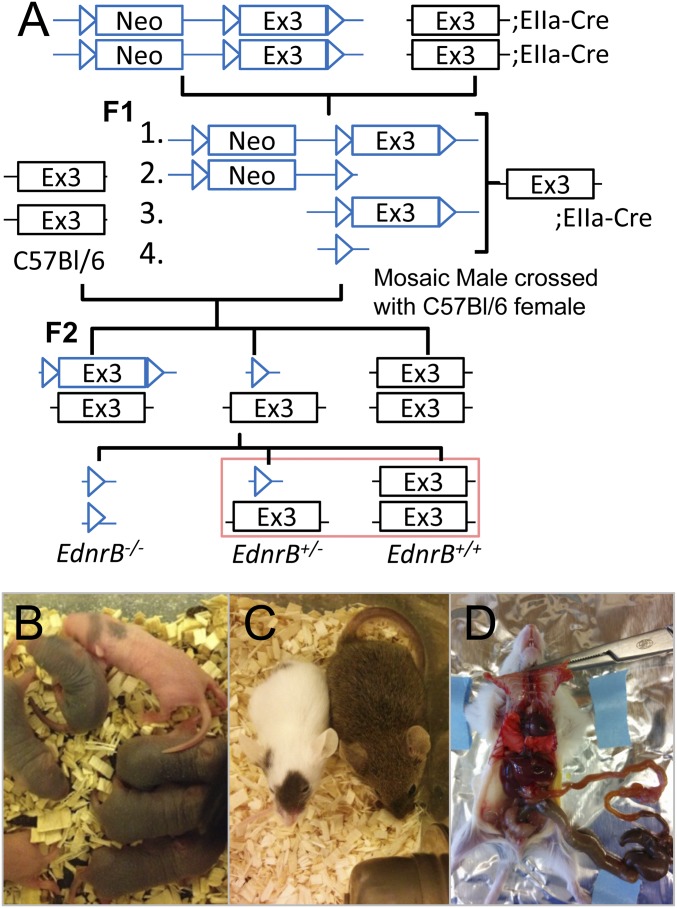
Fig. S1.
Generation of endothelin receptor type B (EdnrB) heterozygous mice. (A) Schematic representation of the strategy used to generate EdnrB−/+ using EIIa-cre, the adenovirus EIIa promoter that expresses cre recombinase during the early mouse embryo. (B) P3 pups, where the EdnrB−/− (homozygous) can be easily detected, as they have black spots due to a melanin pigmentation defect. (C) The EdnrB−/− (white with black spots) is significantly smaller compared with its littermate (brown; EdnrB−/+ or EdnrB+/+). (D) The dissection of EdnrB−/− depicts megacolon due to abnormal dilation of the colon. This is primarily because of the lack of EDNRB whereby ET3/EdnrB has an important role in neural crest migration and thus enteric nervous system formation.
Better Performance of EdnrB−/+ Under Extreme Hypoxia.
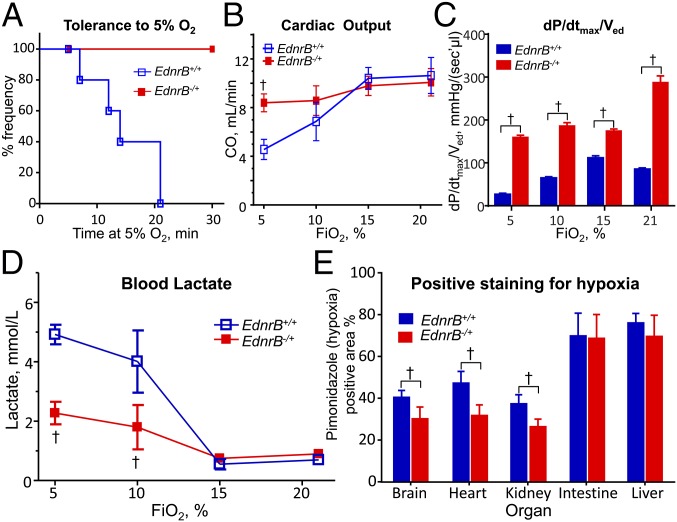
To directly investigate the role of EdnrB in hypoxia adaptation, we examined how EdnrB−/+ responded to 30 min of four levels of O2 concentration compared with EdnrB+/+ controls. Because the phenotype could be more prominent under extreme conditions, we first investigated whether severe hypoxia (5% O2) induces hypotension, defined as mean arterial pressure (MAP) <40 mmHg. Notably, all of the EdnrB−/+ had MAP >60 mmHg (Fig. 3A and Table S3), but none of the controls could maintain MAP >40 mmHg for the whole 30-min period. This suggests that EdnrB−/+ mice are potentially more resistant to a severe hypoxic challenge. When we investigated further the differences in cardiovascular performance between EdnrB−/+ and EdnrB+/+, EdnrB−/+ mice clearly had a great advantage over EdnrB+/+, especially under extreme hypoxia (5% O2). Cardiac output (CO) was remarkably different between the two groups (Fig. 3B). Whereas there was no difference in the CO between EdnrB+/+ and EdnrB−/+ mice at baseline and in mild hypoxia, namely at 21% and 15%, the difference was significant (P < 0.05) at 10% and 5% O2, with CO being stable in the heterozygote but decreased to about half of baseline in the wild type in severe hypoxia. To further our understanding of the differences in cardiovascular function between the two groups of mice, we measured individual pressure–volume parameters. The maximum rate of pressure change (dP/dtmax) per ventricular end-diastolic volume (Ved), which is a measure of contractility, was higher in the EdnrB−/+ at baseline as well as at all of the subsequent hypoxia exposures (Fig. 3C).
Fig. 3.
Enhanced hypoxia performance of EdnrB−/+. (A) Tolerance (defined as MAP >40 mmHg) of EdnrB−/+ and EdnrB+/+ kept in 5% O2 for a period of 30 min. The average MAP for EdnrB−/+ was 60.5 ± 53 mmHg at 5% O2, and all mice from this group had MAP >40 mmHg. None of the EdnrB+/+ littermates could maintain MAP >40 mmHg for the entire 30-min time. (B) Cardiac output measured in EdnrB−/+ and EdnrB+/+ at different O2 concentrations (21%, 15%, 10%, and 5% O2). The CO is similar in both groups of mice when kept under room air condition. A slight decrease in O2 to 15% does not indicate any change in CO; however, under extreme hypoxia, namely 10% and 5% O2, a significant drop in CO could be seen in EdnrB+/+ but no change in EdnrB−/+. (C) The dP⋅dtmax−1⋅Ved−1 distinctly indicates a higher value for EdnrB−/+ at baseline as well as during 15%, 10%, and 5% hypoxic exposures. (D) The blood lactate levels in EdnrB−/+ and EdnrB+/+ at 21% and 15% O2 are similar in both groups. Below 15% O2, the blood lactate levels, although increased in both groups, the increase is mild in EdnrB−/+ (1.8 ± 0.75 mmol/L at 10%; 2.28 ± 0.38 mmol/L at 5% O2), compared with a significant increase in EdnrB+/+ (4.01 ± 1.05 mmol/L at 10%; 4.92 ± 0.33 mmol/L at 5% O2). (E) The relative percentage hypoxic area using pimonidazole dye in brain, heart, and kidney is significantly lower in EdnrB−/+ compared with its littermate controls (EdnrB+/+). The difference appears more significant in heart tissue. No difference in the % hypoxic area is detected in the intestine and liver. †P < 0.05; error bar represents ± SD.
Table S3.
Mean arterial pressure in control and heterozygote mice at different oxygen treatment
| FiO2, % | EdnrB+/+, | EdnrB−/+, |
| MAP ± SD | MAP ± SD | |
| 21 | 88.5 ± 5.6 | 119.3 ± 10.4 |
| 15 | 74.6 ± 5.2 | 93.6 ± 6.9 |
| 10 | 47.2 ± 4.3 | 77.8 ± 6.2 |
| 5 | 43.8 ± 1.5 | 70.2 ± 5.3 |
Control, EdnrB+/+; heterozygote, EdnrB−/+. FiO2, fraction of inspired oxygen; MAP, mmHg. n = 5 mice were used in each group and none was excluded from the study.
Comparison of lactate levels also reveals an interesting difference: Whereas the levels were similar between the two groups at room air and 15% O2, the difference was prominent as we gradually decreased the O2 level. At 10% the blood lactate increased significantly in the EdnrB+/+ compared with the EdnrB−/+, and at 5% O2 the levels in EdnrB+/+ were more than double that of EdnrB−/+ (Fig. 3D). There was no significant difference in the arterial O2 partial pressure (PaO2) between EdnrB−/+ and EdnrB+/+ mice. PaCO2 in EdnrB−/+ was significantly lower (P < 0.05) in room air but not when O2 was lowered in inspired air. Interestingly, pH was almost identical between the two groups, except in severe hypoxia, when the blood became more acidic (a significant difference of 0.11 pH units) in the controls than in EdnrB−/+.
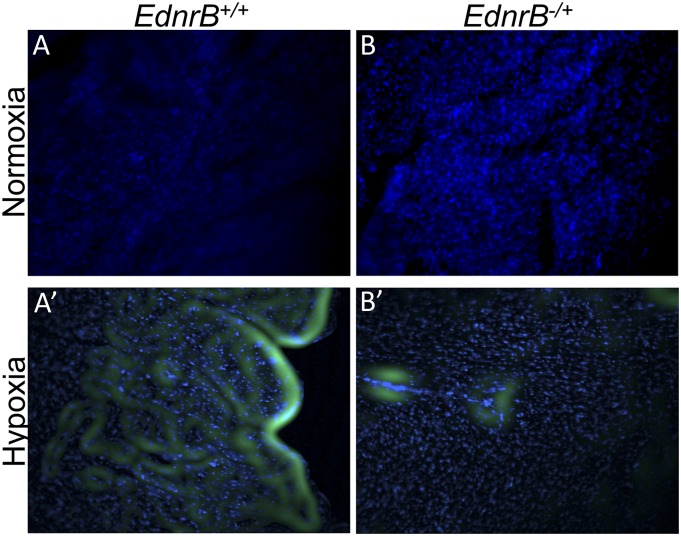
Interesting observations were also noted at organ/tissue levels. When the tissues were harvested after the final 5% O2 exposure and stained using pimonidazole dye, a hypoxia indicator (23), the relative hypoxic area (%) in brain, heart, and kidney was significantly lower in the EdnrB−/+ compared with controls (Fig. 3E and Fig. S2). The difference was more significant in heart tissue. In contrast, no difference was detected in the intestine and liver.
Fig. S2.
Representative images of hypoxia indicator pimonidazole dye staining. Heart tissue stained from EdnrB+/+ (A and A′) and EdnrB−/+ (B and B′) mice kept in normoxia (A and B) and hypoxia (A′ and B′). Green, pimonidazole dye; blue, Hoechst.
Differential Gene Expression in EdnrB−/+ Under Hypoxia.
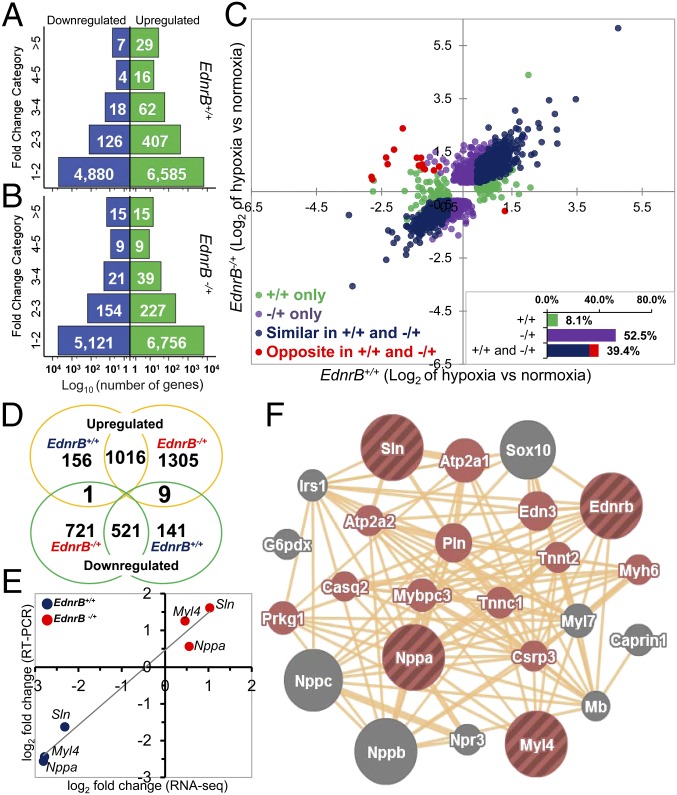
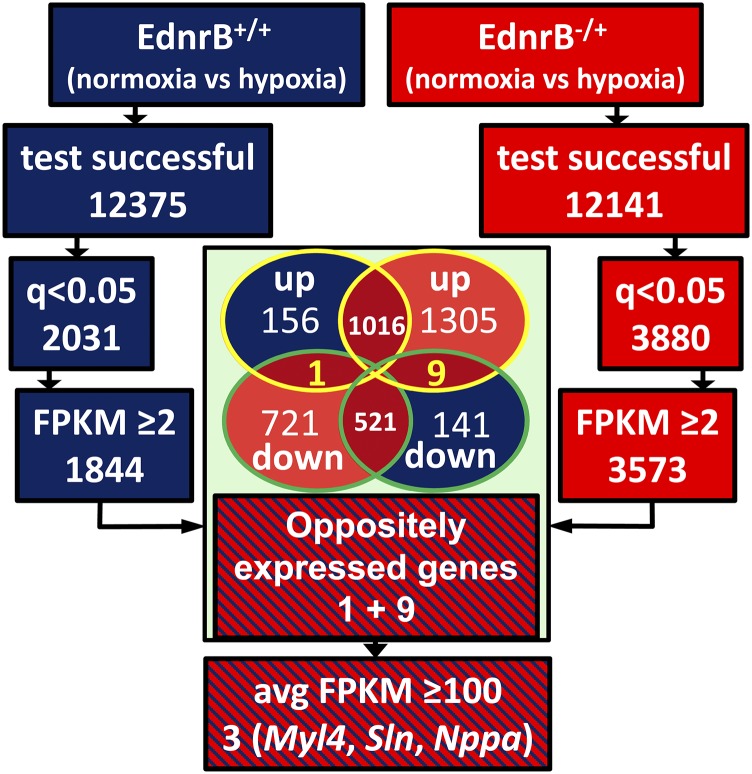
Transcriptome analysis of left ventricles revealed that there were more genes that were differentially expressed in the EdnrB−/+ (Fig. 4 A and B) under hypoxia than in the controls. The baseline comparison between the normoxic EdnrB+/+ vs. EdnrB−/+ depicts only 34 genes differentially expressed at q < 0.05 [q value is the false discovery rate (FDR)-corrected P value to account for multiple testing]. The number rose to 80 in hypoxia EdnrB+/+ vs. EdnrB−/+, of which 12 genes were in common with the normoxia EdnrB+/+ vs. EdnrB−/+. However, when we compared within each group, namely EdnrB+/+ normoxia vs. hypoxia and EdnrB−/+ normoxia vs. hypoxia, the number of genes for which q <0.05 was 4,252. This included 342 genes (8%; Fig. 4C, green circles and Inset) that were differentially expressed only in EdnrB+/+ and 2,207 (52%; Fig. 4C, mauve circles and Inset) in EdnrB−/+. In addition to this, 39% of differentially expressed genes (1,659; Fig. 4C, blue and red circles and Inset) were in common in both groups. On closer examination, it was interesting to note that there were 16 genes (Fig. 4C, red circles) that were counterregulated by hypoxia (q < 0.05); that is, under hypoxia, these genes were up-regulated in EdnrB+/+ and down-regulated in EdnrB−/+ or vice versa. At fragments per kilobase of exon per million reads mapped (FPKMs) ≥2 there were 10 genes oppositely expressed (Fig. 4D and Fig. S3) and, as highlighted in Table 1, only Pygl (liver glycogen phosphorylase) was down-regulated in hypoxic EdnrB−/+. The remaining nine genes were all up-regulated in EdnrB−/+ and down-regulated in EdnrB+/+. Of the counterregulated genes, we decided to focus on three genes, namely natriuretic peptide type A (Nppa), sarcolipin (Sln), and myosin light polypeptide 4 (Myl4), because their average FPKM values were >100 and because we validated these three genes (Fig. 4E) by RT-PCR. Interestingly, these three genes were also differentially expressed at baseline (normoxia) when EdnrB+/+ and EdnrB−/+ were compared. We then used GeneMANIA (24) to identify the final composite networks of these three oppositely expressed genes (Fig. 4F). The functions related to these networks were primarily related to Ca2+ homeostasis, regulation of ATPase activity, regulation of the force of heart contraction, and blood circulation, consistent with the better cardiovascular performance of EdnrB−/+ under extreme hypoxia. Simultaneously, we also studied the genes closely interacting with EdnrB. Most importantly, the ligand Edn-1 (or ET-1) under normoxia had similar Edn-1 expression in both groups, and this was also the case at the protein level when measured in circulating plasma (2.64 ± 0.39 and 2.88 ± 0.26 pg/mL in EdnrB+/+ and EdnrB−/+, respectively, at room air; 2.94 ± 0.90 and 3.46 ± 1.50 pg/mL in EdnrB+/+ and EdnrB−/+, respectively, at 11% O2).
Fig. 4.
Transcriptome analysis from RNA isolated from left ventricles of EdnrB+/+ and EdnrB−/+ mice kept in normoxia and hypoxia. (A and B) Hypoxia vs. normoxia fold change categorized as down-regulated (blue) and up-regulated (green) genes in EdnrB+/+ (A) and EdnrB−/+ (B) mice. The numbers within the bars represent the number of genes. (C) Hypoxia vs. normoxia log2 fold-change values between the genes in EdnrB+/+ and EdnrB−/+ plotted on the x and y axes, respectively. Only those genes for which there was a significant fold change (q < 0.05) in either of the groups alone or similarly and oppositely expressed in the two groups are displayed and color-coded. (Inset) The % genes that are differentially expressed in different groups. The bar “+/+ and −/+” includes similarly (blue) and oppositely (red) expressed genes. (D) Venn diagram depicting the number of genes in common in different groups of hypoxia treatment. The oppositely expressing genes after conditioning with different criteria for mining specific genes and pathways are 1+9 (up-regulated in EdnrB+/+ and down-regulated in EdnrB−/+, 1; down-regulated in EdnrB+/+ and up-regulated in EdnrB−/+, 9). (E) Log2 fold-change comparison between RNA-seq and RT-PCR for the three genes (Nppa, Sln, and Myl4) with FPKM >100. (F) Network analysis of Nppa, Sln, and Myl4 along with EdnrB (striped brown circles) reveals the top-scoring networks (colored circles) all related to cardiovascular function ranging from heart contraction, blood circulation, and the cGMP metabolic process to Ca2+ homeostasis.
Fig. S3.
Schematic representation of gene prioritization from the whole-transcriptome profile of hypoxia-treated EdnrB+/+ (blue) and EdnrB−/+ (red). The Venn diagram depicts the common genes up/down-regulated in hypoxia-treated EdnrB+/+ and EdnrB−/+. The yellow-highlighted genes are genes oppositely expressed (up-regulated in EdnrB+/+ and down-regulated in EdnrB−/+, 1; down-regulated in EdnrB+/+ and up-regulated in EdnrB−/+, 9). Nppa, Sln, and Myl4 are the final selection.
Table 1.
Genes counterregulated by hypoxia
| Symbol | Description [mouse genome informatic (MGI) ID] |
| Pygl | Liver glycogen phosphorylase [MGI:97829] |
| Gm1078 | Predicted gene 1078 [MGI:2685924] |
| Mybphl | Myosin-binding protein H-like [MGI:1916003] |
| Myl4 | Myosin, light polypeptide 4 [MGI:97267] |
| Sln | Sarcolipin [MGI:1913652] |
| Upk3b | Uroplakin 3B [MGI:2140882] |
| Msln | Mesothelin [MGI:1888992] |
| Dkk3 | Dickkopf homolog 3 (Xenopus laevis) [MGI:1354952] |
| Cfd | Complement factor D (adipsin) [MGI:87931] |
| Nppa | Natriuretic peptide type A [MGI:97367] |
Discussion
In Udpa et al. (4), we reported that EDNRB is one of the top candidate genes underlying hypoxia adaptation in Ethiopian highlanders. Indeed, there was a strong signature of positive selection in the DNA region containing the EDNRB gene, in the form of a large block of (differential) SNPs that were fixed in the highlanders but at a significantly lower frequency in nearby lowlander populations. Although none of the SNPs were in the coding regions, many of these SNPs were overlying a cluster of transcription-factor binding sites identified in ENCODE (Fig. 1 A and B), where the underlying transcription factors were reported to have roles in endothelin-mediated cardiac function (16, 18, 19). Promoter luciferase assay of the EDNRB gene indicated an 89-fold increased activity with an intact 1,258-bp promoter sequence and a further boost of 2.7- and 3.1-fold with overexpression of c-Jun or C/EBPb, respectively (21). Additionally, the promoter region was also found to be rich in CpG dinucleotide repeats, and methylation of this CpG-riched region reportedly regulates EDNRB gene expression (25). It is therefore important to note that the “differential” SNPs in the DNA region containing the EDNRB gene may have a regulatory role, still unexplored. Although these observations do not unequivocally characterize EDNRB to be of a higher or lower level in the Ethiopian highlanders, they do imply the presence of a regulatory connection between transcription and the level of EDNRB.
We believe that understanding HA adaptation (or maladaptation) is important not only for better treatment or prevention of disease at HA but also for better understanding and therapy of sea-level diseases that involve hypoxia as a major etiologic factor in pathogenesis. Here we show, to our knowledge for the first time, that indeed, EDNRB, a candidate gene we identified in HA adaptation, proves to be critical in protecting cardiac function in moderate to severe hypoxia at sea level. The receptor itself is one of two known endothelin receptors, the other being EDNRA. The protein encoded by EDNRB is a G protein-coupled receptor that activates a phosphatidylinositol/calcium second-messenger system (26). Whereas EDNRA is present on the cell membrane, EDNRB is also present on the nuclear membrane and is associated with regulation of nuclear Ca2+ signaling (27). The two receptors usually have opposing actions, EDNRA in vasoconstriction and EDNRB in vasodilatation. However, due to the tissue-specific vasoconstrictor role of EDNRB, the reports on this receptor are often contradictory (28). Interestingly, in humans, the EDNRB gene is well-known for its role in neural crest cell migration, proliferation, and differentiation, where a mutation in this gene may lead to phenotypes such as the aganglionosis of the entire gut (Hirschsprung’s disease, megacolon) (29) and hearing loss (30, 31). Studies have also shown down-regulation of EDNRB in human melanoma (32, 33), colorectal cancer (25), bladder cancer (34), and renal cell carcinoma (35).
In the heart, specifically in the ventricles, the ratio of EDNRA to EDNRB density is reportedly 4:1 (36). However, studies on cardiac-specific knockout of EdnrA indicated that type A receptors were unnecessary in baseline cardiac function and also under stressful conditions (37). This clearly indicates a more significant role of EDNRB in cardiac tissues and would correlate with bosentan-related type A and type B receptor antagonism, improvement in cardiac performance, and microcirculatory blood flow during septic shock (38, 39). When a type A receptor-specific antagonist was administered during porcine endotoxin shock, it only antagonized pulmonary hypertension and had no effect on the deteriorated cardiac performance (14). This would suggest that the type B-specific antagonist property of bosentan was involved in improving cardiac performance. Evidence has been accumulating to implicate EdnrB in cardiac function, where an increased expression of vascular EDNRB in patients with ischemic heart disease (40, 41) is seemingly beneficial when the expression is reduced. In addition, pharmacological agents blocking the intracellular loop 2 of EdnrB also appear beneficial under hypoxic conditions (42). We demonstrate in this work that EdnrB−/+ mice withstand various levels of hypoxia, even severe hypoxia, by maintaining cardiac performance close to that at baseline. For example, cardiac function in EdnrB−/+ was better-maintained under moderate and severe hypoxia than in control mice, and our data (Fig. 3) are consistent with an increase in contractile force and contraction and relaxation velocities of the left ventricle (43, 44). Previous hypoxia studies on an EdnrB-deficient (EdnrBsl/sl) rat model missed this very important phenotype in the heterozygote (EdnrB+/sl), as they treated rats at 10% O2 (45, 46). Indeed, we know from our results that the significant drop of CO in EdnrB+/+ and sustenance of CO among EdnrB−/+ are more profoundly visible at 5% O2 (Fig. 3B). It is worth noting that a complete knockout of EdnrB even in specific cells may be deleterious (47).
Because EdnrB−/+ mice maintain better cardiac performance under various levels of hypoxia, we argue that a down-regulated level of EDNRB receptor in EdnrB−/+ mice is sufficient for normal functioning, as seen in normoxia (P > 0.05). However, in severe hypoxic conditions, a lower EDNRB modulates certain genes/pathways that would increase cardiac contractility, thus achieving higher CO.
Cardiovascular or metabolic parameters (such as the dP⋅dtmax−1⋅Ved−1 and lactate level) started to change at a level of O2 of ∼10–13%, altitudes that our Ethiopian subjects came from. Previous studies have also demonstrated indeed that HA dwellers have a lower chance of dying from ischemic heart disease (48), which may relate to better cardiac function. In our heterozygous mice, the remarkable cardiac contractility was associated with a higher peripheral blood flow and better O2 delivery to vital organs (Fig. 3) especially under extreme hypoxic stress, as evidenced by a lower blood lactate level (Fig. 3D) and reduced hypoxic areas in vital organs (Fig. 3E).
To gain insight into the regulatory mechanisms that might explain the phenotypic advantage that we observed in EdnrB−/+, we used a series of measures to shortlist specific candidate genes from the whole-genome transcriptome profile. In particular, we used a Bayesian approach, conditioning the criteria for mining specific genes and pathways. For example, because the phenotype is so different between the wild-type and the heterozygote, we were interested in focusing on genes that were statistically significant at baseline (normoxia) and during hypoxia and also on genes that changed in different directions when wild type and heterozygote were compared. As depicted, three counterregulated genes (Nppa, Sln, and Myl4; q < 0.05) stand apart from the rest by having an average FPKM >100 and by RT-PCR validation. These three genes are differentially expressed in normoxia as well as hypoxia, and we believe that they are involved in the phenotypic difference between the EdnrB+/+and EdnrB−/+. Of interest, we note that these genes are expressed in the fetal heart (49, 50), possibly adding some evidence to a role in hypoxia, because gene expression of fetal transcripts can often show up during stress (51). As anticipated, the top-scoring networks for these genes were all related to cardiovascular function ranging from heart contraction, blood circulation, and the cGMP metabolic process to Ca2+ homeostasis (Fig. 4F). It is known that the endothelin system not only stimulates the secretion of Nppa (52) but that they also counteract each other in some cardiovascular, renal, and endocrine functions (53). Furthermore, previous studies have also demonstrated that Nppa secretion occurs via an EDNRB-mediated pathway involving the MAPK signaling pathway (54), and MAPK along with PI3K pathways regulate the hypoxia-induced Nppa secretion by controlling HIF-1α (55). Studies using Nppa null (Nppa−/−) mice also provide evidence that Nppa plays an important role in pulmonary vascular adaptation to chronic hypoxia (56). Similarly, Sln regulates Ca2+ uptake through interaction with sarcoplasmic reticulum Ca2+ ATPase (SERCA). Like Nppa, Sln is also normally expressed at high levels in atria, and its expression is at very low/undetectable levels in ventricles (57), except in some conditions when it is increased by several-fold, such as in patients with preserved left ventricular ejection fraction and chronic isolated mitral regurgitation, where there is >10-fold increased expression of both Nppa and Sln (58). Therefore, altered Nppa and Sln expression may contribute to impaired Ca2+ handling, and our study indicates that their cross-talk with EdnrB could lead to better cardiac performance under extreme hypoxia. Myl4, the third prioritized gene, is also atria-specific in the adult. Interestingly, it is reexpressed in the ventricles of the diseased heart, suggesting a role in repair mechanisms (59). The up-regulation of Myl4 in EdnrB−/+ indicated cross-talk between the two genes (EdnrB and Myl4). This notion is supported by a recent study showing that Myl4 in the left ventricle was differentially regulated under the protective influence of endothelin receptor inhibitors, including BQ788 (EdnrB-specific), which protects against doxorubicin-induced cardiomyopathy (60).
One question that can be raised is whether our results are relevant to understanding mechanisms of HA hypoxia adaptation. Although this question is difficult to answer, our observation that decreasing EdnrB expression (a gene we identified in HA dwellers) is beneficial for cardiovascular performance is in itself very illuminating. This is of particular interest especially because the results that we obtained are in acute hypoxia. Indeed, these data become relevant to the treatment of many more patients suffering from diseases that have acute hypoxia as part of their etiology at sea level.
In summary, we have demonstrated, for the first time to our knowledge, how an experiment in nature at high altitude in human adaptation could provide insight into disease mechanisms in acute forms involving hypoxia and ischemia at sea level. This is supported by our observation in knockout mice where EdnrB−/+ mice were resistance to various levels of hypoxia, maintaining higher CO and peripheral perfusion and better DO2 to vital organs, even in severe hypoxia. The idea that reducing the expression of functional EDNRB helps cells/tissues endure extreme hypoxia is appealing, and may forage novel therapy for cardiac failure in the near future. A transcriptome profile from ventricular tissue revealed three atria-specific genes, Nppa, Sln, and Myl4, which likely play a role in the improved cardiac performance during hypoxia in the heterozygote mouse.
SI Materials and Methods
Genomic Reanalysis of the Whole-Genome Seq Data.
We systematically searched and compared published/publicly available data with our seq data (4) that may further support the regulation or disregulation of EDNRB (ideally via our observed SNPs as a significant eQTL for EDNRB) in human studies. For this, we compared our seq data with (i) GTeX project data (www.gtexportal.org/home/), dataset/portal for searching eQTL in different tissues; (ii) GeuVadis project data (www.geuvadis.org/web/geuvadis/home), dataset/portal for genetic variation affecting gene expression; and (iii) ENCODE project data. We also used the UCSC Genome Browser (genome.ucsc.edu/cgi-bin/hgGateway) to see SNPs overlying TFBSs and drew information relevant to the regulation of transcription from the ENCODE project. Furthermore, information on modification of histone proteins and the regions where chromatin is hypersensitive to cutting by the DNase enzyme is available on the UCSC Genome Browser, suggestive of enhancer and, to a lesser extent, other regulatory activity.
Generation of EdnrB KO and Its Validation.
The transgenic mice that have loxP flanking exon 3 of the EdnrB gene have been described previously (22). A pair of these mice (male and female) was kindly provided by M. Epstein, University of Wisconsin, Madison, WI. As mentioned in Druckenbrod et al. (22), the neo cassette in these floxed mice was not deleted, and we crossed them with EIIa-cre (provided by the Jackson Laboratory; 003724) (62), the cre transgene under the control of the adenovirus EIIa promoter that expresses cre recombinase during the early mouse embryo. In the first filial (F1) generation, cre-mediated recombination will occur in a wide range of tissues (mosaic pattern of expression), including germ cells. A schematic of the breeding strategy used is provided in Fig. S1A. As depicted, when these mosaic EIIa-cre ‘+’ve mice were mated with C57BL/6 mice, the F2 mice would be in all possible loxP combinations, namely (i) all three loxPs unchanged; (ii) loxP flanking exon 3 (neo cassette removed; can be used for tissue-specific knockout studies); (iii) loxP flanking neo cassette (exon 3 removed); and (iv) only one loxP (both exon 3 and neo cassette removed; used for global knockout studies). At the F2 heterozygote (where only one allele is coming from the mosaic F1 mice) stage, only cre-negative pups with both exon 3 and the neo cassette removed on one allele were selected and used as breeding pairs. The genotyping to confirm the removal of exon 3 and the neo cassette was done using primers mentioned earlier (22). The heterozygous (EdnrB−/+), when inbred as expected, would also produce the homozygote (Ednrb−/−) and wild type (EdnrB+/+), along with the EdnrB−/+. The Ednrb−/− represents the classical phenotype (white coat color with black patches) and can be identified as early as postnatal day 3 (P3) (Fig. S1B). These mice grew weaker compared with their littermates (Fig. S1C) and do not survive beyond 3 wk due to megacolon (Fig. S1D), and therefore could not be used for our experiments. However, the EdnrB−/+ were normal. Only male EdnrB−/+ mice were used for the experiments and age-matched EdnrB+/+ littermates as controls. The mice were always housed in a 12-h light–dark cycle and had free access to food and water.
qRT-PCR of EdnrB Expression in the Three Genotypes.
RNA was isolated from left and right ventricles of 18-d-old EdnrB−/−, EdnrB−/+, and EdnrB+/+ mice. cDNA was generated from the total RNA of the right and left ventricles by RT-PCR using the SuperScript III First-Strand Synthesis System (Invitrogen). RT-PCR was followed by real-time PCR on the 7900HT Fast Real-Time PCR System using Power SYBR Green Master Mix (Applied Biosystems). The primers were designed using the software tool Primer3 available online (primer3.ut.ee/). The sequences were 5′-TGTAAGCTGGTGCCCTTCA-3′ for the forward primer and 5′-TGCTGTCCATTTTGGAACC-3′ for the reverse primer. The expression levels were normalized to mouse β-actin. Fold-change calculations were calculated for EdnrB−/−/EdnrB−/+, EdnrB−/−/EdnrB+/+, and EdnrB−/+/EdnrB+/+.
Mouse Preparation for Awake, Unanesthetized Hypoxia.
Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals (61). Mice were anesthetized to implant arterial catheters (PE-50) into the carotid artery. Catheters were tunneled under the skin, exteriorized at the dorsal side of the neck, and secured under the skin on the animal’s back. Mice recovered from catheter implantation for 24 h before the hypoxia protocol. Mice were suitable for the experiments if systemic parameters were within normal range, namely heart rate (HR) >450 beats per min, mean arterial blood pressure (MAP) >90 mmHg, systemic hematocrit (Hct) >42%, and arterial O2 partial pressure (PaO2) >80 mmHg.
Hypoxia Protocol.
The awake animals were placed in a restraining tube within a sealed acrylic box (4′′ × 4′′ × 10′′). The inlet of the box was connected to gas tanks with different O2 concentrations: (i) compressed air; (ii) 15% O2 balance N2; (iii) 10% O2 balance N2; and (iv) 5% O2 balance N2. The gas flow rate into the box was kept at 0.2 L/min using a gas flow meter. Air coming into the box was diffused using a cotton filter. O2 concentration in the box was measured continuously using an O2 gas sensor (Vernier). Mice were first exposed to compressed air and given 20 min to adjust to the experimental environment before baseline measurements were completed. Then, the compressed air was replaced by 15% O2, and mice were given 15 min to adjust to the change in the gas environment before measurements. Similarly, the 15% O2 gas was replaced by 10% O2 gas, and another 15 min was allowed before measurements. Last, the 10% O2 gas was replaced by 5% O2 gas, and another 10–15 min was allowed before measurements. At each time point, systemic parameter and blood gas analyses were performed. To prevent animal stress or discomfort, hypoxia was stopped if blood pressure dropped below 40 mmHg, and the animal was excluded from the study.
Mouse Preparation for Cardiac Function Measurements.
Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals (61). The experimental protocol was approved by the University of California, San Diego Institutional Animal Care and Use Committee (protocol S04052). Anesthesia was induced by i.p. injection of sodium pentobarbital (50 mg/kg), and core body temperature was maintained using a heating pad. Animal preparation included (i) left femoral artery catheterization, (ii) tracheotomy (polyethylene-90 tube to facilitate spontaneous breathing), and (iii) left ventricle conductance catheter introduction through the right carotid artery. Animals were placed in the supine position on the heating pad before the experimental procedure. A toe-pinching test was performed at least every 5 min, and animals who responded received a small dose of sodium pentobarbital (10 mg/kg) to prevent animal discomfort. Mice were suitable for the experiments if systemic parameters were within normal range, namely HR >300 beats per min, MAP >90 mmHg, and PaO2 >80 mmHg.
Hypoxia Protocol for Cardiac Function.
The anesthetized animals were placed on a heating pad within a sealed acrylic box. As described before for awake animals, the O2 concentrations in the box were changed using compressed air, 15% O2 balance N2, 10% O2 balance N2, and 5% O2 balance N2. Each hypoxic step was maintained for 30 min, and measurements were completed 15 min after the animal’s acclimatization. Hypoxia was stopped if blood pressure dropped below 40 mmHg, and the animal was excluded from the study.
Systemic Parameters.
MAP and HR were recorded continuously (MP150; BIOPAC Systems). Hct was measured from arterial blood samples taken in heparinized capillary tubes centrifuged at 13,000 × g for 3 min at 20 °C (Sol-Bat microhematocrit centrifuge M-600, Mexico). Hb content was determined spectrophotometrically (B-Hemoglobin; HemoCue). Arterial blood was collected in heparinized glass capillaries (50 µL) and immediately analyzed for pO2, pCO2, base excess (BE), and pH (RAPIDLab 248; Bayer). Arterial Hb saturations were measured on the IL482 CO-Oximeter System (Instrumentation Laboratory).
Cardiac Function.
The closed chest method was used to study cardiac function. The right common carotid artery was exposed to insert a 1.4F pressure–volume conductance catheter (PV catheter; SPR-839, Millar Instruments). The pressure–volume catheter was advanced passing through the aortic valve into the LV (63). At baseline and the end of the experiment, a bolus of 15% (wt/vol) hypertonic saline (10 µL) was i.v. injected to determine parallel volume (64). The pressure and volume signals were continuously acquired (MPVS300, Millar Instruments; PowerLab 8/30, AD Instruments). Left ventricular volume was measured continuously in conductance units (relative volume unit; RVU) and converted to actual blood volume (μL) at the end of the experiment.
Cardiac Pressure–Volume Indices.
Cardiac function data were analyzed with PVAN software (Millar Instruments). All cardiac function parameters were averaged from 8–12 cardiac cycles at each time point. End systolic pressure (Pes) was directly measured. Maximum rate of pressure change (dP/dtmax), minimum rate of pressure change (dP/dtmin), maximum filling volume rate (dV/dtmax), cardiac output (CO), stroke work (SW), and stroke volume (SV) were calculated. Vascular resistance (VR) was calculated by using the MAP divided by the CO (VR = MAP/CO). Oxygen delivery (DO2) was calculated as the product of the total Hb by the O2-carrying capacity of saturated Hb (1.34 mLO2/gHb) by the arterial blood O2 saturation (SaO2) and CO (DO2 = [RBCHb × 1.34 × SaO2] × CO).
Estimation of Left Ventricular Blood Volume.
LV blood volume was measured continuously in conductance units (RVUs) and converted to actual blood volume (μL) at the end of the experiment (63).
Hypoxic Areas.
The hypoxia protocol for awake mice was followed as mentioned earlier. Vital organ hypoxic areas were measured using immunohistochemistry staining for pimonidazole bound to hypoxic zones. Mice received an infusion of the hypoxic marker Hypoxyprobe-1 [pimonidazole; 40 mg/kg (Hypoxyprobe Incorporation)] and 5 mg/kg Hoechst 33342 (Invitrogen) diluted in PBS (total volume 100 μL) when they were exposed to 10% O2. Then, the mice received a second infusion of pimonidazole (40 mg/kg) and Hoechst 33342 (5 mg/kg) diluted in PBS (100 μL) when they were exposed 5% O2. Finally, mice were euthanized and their vital organs (brain, heart, kidney, intestine, and liver) were removed. Tissues were fixed by immersion in formalin for 24 h at room temperature before transfer to 70% (vol/vol) ethanol. Last, tissues were cut into 100-μm-thick sections.
Pimonidazole Immunohistochemistry.
Sections were cleaned and rehydrated according to standard procedures. A monoclonal antibody directed against pimonidazole (included in the Hypoxyprobe-1 Green Kit) was used for immunohistochemical staining of the tissue sections. Fluorescence microscopy was performed using an Olympus BX51WI equipped with a high-resolution digital CCD ORCA-285 (Hamamatsu) illuminated with a mercury burner and the appropriate fluorescent cubes (XF100-2 and XF02-2; Omega Optical). Images for pimonidazole antibody-stained areas and Hoechst were prepared using Wasabi imaging software (Hamamatsu). The ratio of pixels stained for pimonidazole in each region to the total cellular area of the image was calculated. Ten images were analyzed, by sections, and the results were pooled to determine the mean and SD. To indicate the colocalization of pimonidazole and Hoechst in cells, images were superimposed.
ELISA.
We also measured the ET-1 protein level in the serum of EdnrB+/+ and EdnrB−/+ mice using the ELISA method using Quantikine ELISA for ET-1 (R&D Systems; DET100). The steps are as mentioned in the kit manual. The concentrations are measured as pg/mL.
Data Analysis.
Results are presented as mean ± SD. The Grubbs method was used to assess closeness for all measured parameter values at baseline. Data comparison between groups was analyzed using two-way analysis of variance (ANOVA) with genotype as a between-factor measure and hypoxia treatment as a repeated measure. Data within each group were analyzed using Kruskal–Wallis one-way ANOVA. When appropriate, post hoc analyses were performed with the Dunn multiple comparison test and Bonferroni posttest comparison. All statistics were calculated using Prism 4.01 (GraphPad). Changes were considered statistically significant if P < 0.05.
RNA-Seq Data Generation and Analysis.
The RNA from the left ventricles of EdnrB+/+ and EdnrB−/+ mice kept in normoxia and the littermates exposed to 2 d of hypoxia (10% O2) was submitted for RNA-seq. We used 500 ng of RNA with an RNA integrity number (RIN) of 8 or greater to generate libraries using the TruSeq Stranded mRNA Sample Prep Kit (Illumina). Library preparation and RNA-seq were conducted at the IGM Genomics Center, University of California, San Diego. The manufacturer’s protocol was followed, with the exception that the RNA was fragmented for 5 min. Libraries were multiplexed and sequenced with 100-bp single-end reads (SRs) to a depth of ∼25 million reads per sample on an Illumina HiSeq 2500. Sequences were aligned with TopHat (https://ccb.jhu.edu/software/tophat/index.shtml) by default settings for Mus musculus using the mm9 genome index as provided by Illumina (iGenome package). The aligned reads in BAM format [(.bam) the binary version of a tab-delimited text file, also called a SAM format (.sam), that contains sequence alignment data] were then used to determine differentially expressed genes between samples obtained from various conditions using the mm9 gene transfer format (GTF). The expression was statistically significant, that is, q value (adjusted P value for multiple testing) <0.05 when absolute fold change was >1.5. During initial shortlisting, we considered genes with fragments per kilobase of exon per million reads mapped (FPKMs) ≥2 (Fig. S3). The common genes that were differentially expressed (FPKM ≥2; q < 0.05) in different conditions were sorted using an Excel sheet and Venn diagram-plotted (Fig. 4D). From oppositely expressed genes, the genes with average FPKM >100 were picked for RT-PCR validation.
Materials and Methods
Genomic Reanalysis of the Whole-Genome Seq Data.
We systematically searched and compared published/publicly available data [Genotype-Tissue Expression (GTEx), Genetic European Variation in Health and Disease (GeuVadis), and ENCODE project] with our seq data (4) that may further support the regulation or disregulation of EDNRB [ideally via our observed SNPs as a significant expression quantitative trait loci (eQTL) for EDNRB] in human studies. See SI Materials and Methods for full methods.
Generation of the EdnrB Knockout and Its Validation.
All animal care and handling were performed according to the protocols approved by the Animal Care Committee of the University of California, San Diego. We generated global knockout mice for EdnrB, where exon 3 of the EdnrB gene was completely removed, resulting in an out-of-frame transcript of exon 2–4 splicing. A schematic of the breeding strategy and its validation using RT-PCR are provided in SI Materials and Methods and Fig. S1A.
Mouse Preparation for Hypoxia Treatment.
Animal handling and care followed the NIH Guide for the Care and Use of Laboratory Animals (61). Detailed steps are provided in SI Materials and Methods. Briefly, the mice were anesthetized to implant arterial catheters (PE-50) into the carotid artery and used for hypoxia treatment after a 24-h recovery. The inclusion and exclusion criteria are as mentioned in SI Materials and Methods. Further experimental details for the immunohistochemistry, ELISA, and statistical analysis are presented in SI Materials and Methods.
RNA-Seq Data Generation and Analysis.
We used 500 ng of RNA with an RNA integrity number of 8 or greater to generate libraries using Illumina’s TruSeq Stranded mRNA Sample Prep Kit. Details are in SI Materials and Methods.
Acknowledgments
We thank Prof. Miles Epstein for providing the EdnrBflex mice. We are grateful to Travis Smith and Orit Poulsen for technical assistance and F. Barra and C. Walser for surgical preparation of the animals. This work was supported by NIH Grants 1P01-HL098053 (to G.G.H.), P01-HL110900, R56HL123015, and R01-HL52684 (to P.C.), and NSF-IIS-1318386 (in part to V.B. and R.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 10080.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1507486112/-/DCSupplemental.
References
- 1.Allemann Y, Scherrer U. High-altitude medicine: Important for trekkers and mountaineers, essential for progress in medicine. Prog Cardiovasc Dis. 2010;52(6):449–450. doi: 10.1016/j.pcad.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 2.Bigham A, et al. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 2010;6(9):e1001116. doi: 10.1371/journal.pgen.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonson TS, et al. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329(5987):72–75. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- 4.Udpa N, et al. Whole genome sequencing of Ethiopian highlanders reveals conserved hypoxia tolerance genes. Genome Biol. 2014;15(2):R36. doi: 10.1186/gb-2014-15-2-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore LG, Niermeyer S, Zamudio S. Human adaptation to high altitude: Regional and life-cycle perspectives. Am J Phys Anthropol. 1998;(Suppl 27):25–64. doi: 10.1002/(sici)1096-8644(1998)107:27+<25::aid-ajpa3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 6.Zhou D, et al. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 2008;4(10):e1000221. doi: 10.1371/journal.pgen.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou D, et al. Experimental selection of hypoxia-tolerant Drosophila melanogaster. Proc Natl Acad Sci USA. 2011;108(6):2349–2354. doi: 10.1073/pnas.1010643108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou D, et al. Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. Am J Hum Genet. 2013;93(3):452–462. doi: 10.1016/j.ajhg.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beall CM. Andean, Tibetan, and Ethiopian patterns of adaptation to high-altitude hypoxia. Integr Comp Biol. 2006;46(1):18–24. doi: 10.1093/icb/icj004. [DOI] [PubMed] [Google Scholar]
- 10.Beall CM, et al. An Ethiopian pattern of human adaptation to high-altitude hypoxia. Proc Natl Acad Sci USA. 2002;99(26):17215–17218. doi: 10.1073/pnas.252649199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xing G, et al. Adaptation and mal-adaptation to ambient hypoxia; Andean, Ethiopian and Himalayan patterns. PLoS One. 2008;3(6):e2342. doi: 10.1371/journal.pone.0002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naeije R. Physiological adaptation of the cardiovascular system to high altitude. Prog Cardiovasc Dis. 2010;52(6):456–466. doi: 10.1016/j.pcad.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Kojonazarov B, et al. Bosentan reduces pulmonary artery pressure in high altitude residents. High Alt Med Biol. 2012;13(3):217–223. doi: 10.1089/ham.2011.1107. [DOI] [PubMed] [Google Scholar]
- 14.Wanecek M, et al. Endothelin(A)-receptor antagonism attenuates pulmonary hypertension in porcine endotoxin shock. Eur Respir J. 1999;13(1):145–151. doi: 10.1183/09031936.99.13114599. [DOI] [PubMed] [Google Scholar]
- 15.Hori S, Komatsu Y, Shigemoto R, Mizuno N, Nakanishi S. Distinct tissue distribution and cellular localization of two messenger ribonucleic acids encoding different subtypes of rat endothelin receptors. Endocrinology. 1992;130(4):1885–1895. doi: 10.1210/endo.130.4.1312429. [DOI] [PubMed] [Google Scholar]
- 16.Bolte C, et al. Postnatal ablation of Foxm1 from cardiomyocytes causes late onset cardiac hypertrophy and fibrosis without exacerbating pressure overload-induced cardiac remodeling. PLoS One. 2012;7(11):e48713. doi: 10.1371/journal.pone.0048713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Oort RJ, et al. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurin-induced heart failure. Circulation. 2006;114(4):298–308. doi: 10.1161/CIRCULATIONAHA.105.608968. [DOI] [PubMed] [Google Scholar]
- 18.Clerk A, Sugden PH. Phosphorylation c-Jun and ATF2 in ventricular myocytes by endothelin and phenylephrine. Biochem Soc Trans. 1997;25(2):222S. doi: 10.1042/bst025222s. [DOI] [PubMed] [Google Scholar]
- 19.Marchant D, et al. Bosentan enhances viral load via endothelin-1 receptor type-A-mediated p38 mitogen-activated protein kinase activation while improving cardiac function during coxsackievirus-induced myocarditis. Circ Res. 2009;104(6):813–821. doi: 10.1161/CIRCRESAHA.108.191171. [DOI] [PubMed] [Google Scholar]
- 20.Tulchinsky E. Fos family members: Regulation, structure and role in oncogenic transformation. Histol Histopathol. 2000;15(3):921–928. doi: 10.14670/HH-15.921. [DOI] [PubMed] [Google Scholar]
- 21.He S, et al. Involvement of AP-1 and C/EBPβ in upregulation of endothelin B (ETB) receptor expression in a rodent model of glaucoma. PLoS One. 2013;8(11):e79183. doi: 10.1371/journal.pone.0079183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Druckenbrod NR, Powers PA, Bartley CR, Walker JW, Epstein ML. Targeting of endothelin receptor-B to the neural crest. Genesis. 2008;46(8):396–400. doi: 10.1002/dvg.20415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arteel GE, Thurman RG, Raleigh JA. Reductive metabolism of the hypoxia marker pimonidazole is regulated by oxygen tension independent of the pyridine nucleotide redox state. Eur J Biochem. 1998;253(3):743–750. doi: 10.1046/j.1432-1327.1998.2530743.x. [DOI] [PubMed] [Google Scholar]
- 24.Warde-Farley D, et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38(Web Server issue):W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen C, et al. Hypermethylation of EDNRB promoter contributes to the risk of colorectal cancer. Diagn Pathol. 2013;8:199. doi: 10.1186/1746-1596-8-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tykocki NR, Watts SW. The interdependence of endothelin-1 and calcium: A review. Clin Sci (Lond) 2010;119(9):361–372. doi: 10.1042/CS20100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merlen C, et al. Intracrine endothelin signaling evokes IP3-dependent increases in nucleoplasmic Ca2+ in adult cardiac myocytes. J Mol Cell Cardiol. 2013;62:189–202. doi: 10.1016/j.yjmcc.2013.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider MP, Boesen EI, Pollock DM. Contrasting actions of endothelin ET(A) and ET(B) receptors in cardiovascular disease. Annu Rev Pharmacol Toxicol. 2007;47:731–759. doi: 10.1146/annurev.pharmtox.47.120505.105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heanue TA, Pachnis V. Enteric nervous system development and Hirschsprung’s disease: Advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8(6):466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 30.Lee HO, Levorse JM, Shin MK. The endothelin receptor-B is required for the migration of neural crest-derived melanocyte and enteric neuron precursors. Dev Biol. 2003;259(1):162–175. doi: 10.1016/s0012-1606(03)00160-x. [DOI] [PubMed] [Google Scholar]
- 31.Hosoda K, et al. Targeted and natural (piebald-lethal) mutations of endothelin-B receptor gene produce megacolon associated with spotted coat color in mice. Cell. 1994;79(7):1267–1276. doi: 10.1016/0092-8674(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 32.Eberle J, et al. Downregulation of endothelin B receptor in human melanoma cell lines parallel to differentiation genes. J Invest Dermatol. 1999;112(6):925–932. doi: 10.1046/j.1523-1747.1999.00598.x. [DOI] [PubMed] [Google Scholar]
- 33.Cruz-Muñoz W, et al. Roles for endothelin receptor B and BCL2A1 in spontaneous CNS metastasis of melanoma. Cancer Res. 2012;72(19):4909–4919. doi: 10.1158/0008-5472.CAN-12-2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zuiverloon TC, et al. A methylation assay for the detection of non-muscle-invasive bladder cancer (NMIBC) recurrences in voided urine. BJU Int. 2012;109(6):941–948. doi: 10.1111/j.1464-410X.2011.10428.x. [DOI] [PubMed] [Google Scholar]
- 35.Wuttig D, et al. CD31, EDNRB and TSPAN7 are promising prognostic markers in clear-cell renal cell carcinoma revealed by genome-wide expression analyses of primary tumors and metastases. Int J Cancer. 2012;131(5):E693–E704. doi: 10.1002/ijc.27419. [DOI] [PubMed] [Google Scholar]
- 36.Kuc RE, Maguire JJ, Davenport AP. Quantification of endothelin receptor subtypes in peripheral tissues reveals downregulation of ET(A) receptors in ET(B)-deficient mice. Exp Biol Med (Maywood) 2006;231(6):741–745. [PubMed] [Google Scholar]
- 37.Kedzierski RM, et al. Cardiomyocyte-specific endothelin A receptor knockout mice have normal cardiac function and an unaltered hypertrophic response to angiotensin II and isoproterenol. Mol Cell Biol. 2003;23(22):8226–8232. doi: 10.1128/MCB.23.22.8226-8232.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wanecek M, Weitzberg E, Alving K, Rudehill A, Oldner A. Effects of the endothelin receptor antagonist bosentan on cardiac performance during porcine endotoxin shock. Acta Anaesthesiol Scand. 2001;45(10):1262–1270. doi: 10.1034/j.1399-6576.2001.451015.x. [DOI] [PubMed] [Google Scholar]
- 39.Krejci V, Hiltebrand LB, Erni D, Sigurdsson GH. Endothelin receptor antagonist bosentan improves microcirculatory blood flow in splanchnic organs in septic shock. Crit Care Med. 2003;31(1):203–210. doi: 10.1097/00003246-200301000-00031. [DOI] [PubMed] [Google Scholar]
- 40.Dimitrijevic I, et al. Increased expression of vascular endothelin type B and angiotensin type 1 receptors in patients with ischemic heart disease. BMC Cardiovasc Disord. 2009;9:40. doi: 10.1186/1471-2261-9-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dagassan PH, et al. Up-regulation of endothelin-B receptors in atherosclerotic human coronary arteries. J Cardiovasc Pharmacol. 1996;27(1):147–153. doi: 10.1097/00005344-199601000-00023. [DOI] [PubMed] [Google Scholar]
- 42.Green DS, et al. A cell permeable peptide targeting the intracellular loop 2 of endothelin B receptor reduces pulmonary hypertension in a hypoxic rat model. PLoS One. 2013;8(11):e81309. doi: 10.1371/journal.pone.0081309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meerson FZ, Pshennikova MG. Effects of adaptation to high altitude hypoxia on the contractile function and adrenoreactivity of the heart. Basic Res Cardiol. 1979;74(2):142–154. doi: 10.1007/BF01907817. [DOI] [PubMed] [Google Scholar]
- 44.Meerson FZ, Larionov NP, Makarova EK, Bogomolov AF. [Contractile function and the effectiveness of oxygen utilization by the heart during adaptation to hypoxia] Kardiologiia. 1975;15(7):70–78. Russian. [PubMed] [Google Scholar]
- 45.Ivy D, et al. Endothelin B receptor deficiency potentiates ET-1 and hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol. 2001;280(5):L1040–L1048. doi: 10.1152/ajplung.2001.280.5.L1040. [DOI] [PubMed] [Google Scholar]
- 46.Ivy DD, et al. Exaggerated hypoxic pulmonary hypertension in endothelin B receptor-deficient rats. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L703–L712. doi: 10.1152/ajplung.00272.2001. [DOI] [PubMed] [Google Scholar]
- 47.Kelland NF, et al. Endothelial ET(B) limits vascular remodelling and development of pulmonary hypertension during hypoxia. J Vasc Res. 2010;47(1):16–22. doi: 10.1159/000231717. [DOI] [PubMed] [Google Scholar]
- 48.Ezzati M, et al. Altitude, life expectancy and mortality from ischaemic heart disease, stroke, COPD and cancers: National population-based analysis of US counties. J Epidemiol Community Health. 2012;66(7):e17. doi: 10.1136/jech.2010.112938. [DOI] [PubMed] [Google Scholar]
- 49.Zeller R, Bloch KD, Williams BS, Arceci RJ, Seidman CE. Localized expression of the atrial natriuretic factor gene during cardiac embryogenesis. Genes Dev. 1987;1(7):693–698. doi: 10.1101/gad.1.7.693. [DOI] [PubMed] [Google Scholar]
- 50.Arnold HH, Lohse P, Seidel U, Bober E. A novel human myosin alkali light chain is developmentally regulated. Expression in fetal cardiac and skeletal muscle and in adult atria. Eur J Biochem. 1988;178(1):53–60. doi: 10.1111/j.1432-1033.1988.tb14428.x. [DOI] [PubMed] [Google Scholar]
- 51.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: A strong hypothesis. Heart Fail Rev. 2007;12(3-4):331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 52.Thibault G, Doubell AF, Garcia R, Larivière R, Schiffrin EL. Endothelin-stimulated secretion of natriuretic peptides by rat atrial myocytes is mediated by endothelin A receptors. Circ Res. 1994;74(3):460–470. doi: 10.1161/01.res.74.3.460. [DOI] [PubMed] [Google Scholar]
- 53.Ota K, et al. Interaction of ANP with endothelin on cardiovascular, renal, and endocrine function. Am J Physiol. 1992;262(2 Pt 1):E135–E141. doi: 10.1152/ajpendo.1992.262.2.E135. [DOI] [PubMed] [Google Scholar]
- 54.Liu LP, et al. Ouabain stimulates atrial natriuretic peptide secretion via the endothelin-1/ET(B) receptor-mediated pathway in beating rabbit atria. Life Sci. 2012;90(19-20):793–798. doi: 10.1016/j.lfs.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Zhang QL, et al. MAPK and PI3K pathways regulate hypoxia-induced atrial natriuretic peptide secretion by controlling HIF-1 alpha expression in beating rabbit atria. Biochem Biophys Res Commun. 2013;438(3):507–512. doi: 10.1016/j.bbrc.2013.07.106. [DOI] [PubMed] [Google Scholar]
- 56.Chen YF, et al. Atrial natriuretic peptide-dependent modulation of hypoxia-induced pulmonary vascular remodeling. Life Sci. 2006;79(14):1357–1365. doi: 10.1016/j.lfs.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 57.Vangheluwe P, et al. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389(Pt 1):151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zheng J, et al. Increased sarcolipin expression and adrenergic drive in humans with preserved left ventricular ejection fraction and chronic isolated mitral regurgitation. Circ Heart Fail. 2014;7(1):194–202. doi: 10.1161/CIRCHEARTFAILURE.113.000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am J Physiol Heart Circ Physiol. 2007;292(4):H1643–H1654. doi: 10.1152/ajpheart.00931.2006. [DOI] [PubMed] [Google Scholar]
- 60.Schwebe M, et al. Protective effects of endothelin receptor A and B inhibitors against doxorubicin-induced cardiomyopathy. Biochem Pharmacol. 2015;94(2):109–129. doi: 10.1016/j.bcp.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 61. National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals (2011) Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC), 8th Ed. [Google Scholar]
- 62.Lakso M, et al. Efficient in vivo manipulation of mouse genomic sequences at the zygote stage. Proc Natl Acad Sci USA. 1996;93(12):5860–5865. doi: 10.1073/pnas.93.12.5860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pacher P, Nagayama T, Mukhopadhyay P, Bátkai S, Kass DA. Measurement of cardiac function using pressure-volume conductance catheter technique in mice and rats. Nat Protoc. 2008;3(9):1422–1434. doi: 10.1038/nprot.2008.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baan J, et al. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 1984;70(5):812–823. doi: 10.1161/01.cir.70.5.812. [DOI] [PubMed] [Google Scholar]