Abstract
After their endosymbiotic acquisition, plastids become intimately connected with the biology of their host. For example, genes essential for plastid function may be relocated from the genomes of plastids to the host nucleus, and pathways may evolve within the host to support the plastid. In this review, we consider the different degrees of integration observed in dinoflagellates and their associated plastids, which have been acquired through multiple different endosymbiotic events. Most dinoflagellate species possess plastids that contain the pigment peridinin and show extreme reduction and integration with the host biology. In some species, these plastids have been replaced through serial endosymbiosis with plastids derived from a different phylogenetic derivation, of which some have become intimately connected with the biology of the host whereas others have not. We discuss in particular the evolution of the fucoxanthin-containing dinoflagellates, which have adapted pathways retained from the ancestral peridinin plastid symbiosis for transcript processing in their current, serially acquired plastids. Finally, we consider why such a diversity of different degrees of integration between host and plastid is observed in different dinoflagellates and how dinoflagellates may thus inform our broader understanding of plastid evolution and function.
Keywords: dinotoms, poly(U) tail, transcript editing, chloroplast genomes, minicircle
Plastids evolve through the endosymbiotic integration of two organisms: a eukaryotic host and a photosynthetic prokaryotic or eukaryotic symbiont. It is generally believed that the host initially consumes the symbiont through phagocytosis. Subsequently, over long evolutionary timescales, pathways evolve within the host to maintain the endosymbiont as a permanent, intracellular organelle (1). At least eight distinct plastid endosymbioses have been documented across the eukaryotes, giving rise to a diverse array of different photosynthetic lineages (reviewed in ref. 2). Understanding what processes underpin the integration of plastids with their hosts may provide valuable insights into the evolution and function of photosynthetic eukaryotes.
Integration in Plastid Evolution
Plastids and their hosts share intricate biological connections. For example, plastids possess transporters that enable them to export photosynthetic and photorespiratory products to the host and to import inorganic nutrients and cofactors essential for plastid metabolism (3, 4). Plastid replication and division are likewise dependent on proteins encoded within the host nucleus (5). Finally, gene expression within the plastid depends on factors expressed within the host, alongside other factors encoded within the plastid genome (6). The host factors may support the plastid, for example, by regulating plastid gene expression and, at an evolutionary level, by correcting mutations in the plastid genome that might otherwise prove deleterious (6, 7).
Each of these examples of integration depends on proteins that are encoded within the nuclear genome but are targeted to the plastid. Some of these proteins were originally of plastid origin, with the genes encoding them having been transferred to the nucleus of the host after endosymbiosis (8, 9). In other cases, genes endogenous to the host may be recruited to support the plastid, changing its biology. It is likely that most extant plastids are supported by a mosaic of pathways, some of symbiont and some of host origin. For example, approximately half of the plant plastid proteome consists of proteins of nonplastid origin, which may thus have been acquired from the host nucleus (10).
Dinoflagellates in the Context of Plastid Integration
Some of the most extreme examples of plastid evolution are found within the dinoflagellate algae. Dinoflagellates are members of the alveolate kingdom, and their nuclei are only distantly related to those of plants (2). Dinoflagellates play important roles in aquatic ecology. Some species (e.g., Amphidinium, Pyrocystis) are principally free-living primary producers and mixotrophs whereas others (e.g., Symbiodinium) are symbionts of marine invertebrates, such as coral (11). Some free-living dinoflagellates (e.g., Ceratium, Lingulodinium) have important economic effects as causative agents of harmful algal blooms, which have major effects on global fisheries (11). The dinoflagellates are closely related to the coral symbiont “chromerid” algae Chromera velia and Vitrella brassicaformis and to the apicomplexans, a lineage that includes the malaria parasite Plasmodium (Fig. 1) (12, 13).
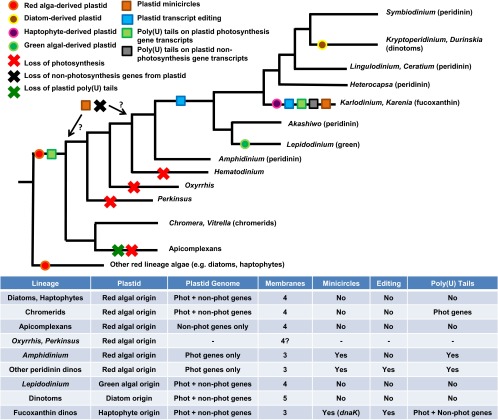
Fig. 1.
Evolution of dinoflagellates and their plastids. (Upper) An evolutionary tree of dinoflagellates and their closest relatives, adapted from ref. 41. The evolutionary relationships in this tree are taken from refs. 13, 48, and 91); for simplicity, only a representative sample of dinoflagellate species is shown. The endosymbiotic acquisition and secondary loss of each individual plastid lineage, the loss of nonphotosynthesis genes from the peridinin plastid lineage, and the origins of minicircles, poly(U) tail addition, and transcript editing in peridinin and fucoxanthin plastids are labeled on the diagram. It is not clear from current data whether the loss of nonphotosynthesis genes and the evolution of minicircle gene organization occurred in the peridinin lineage before or after the divergence of basal dinoflagellates such as Perkinsus, Oxyrrhis, and Hematodinium (which have since lost the capacity for photosynthesis entirely); accordingly, the earliest and latest evolutionary points at which these events can have occurred are shown on the tree, labeled with question marks. (Lower) Tabulates key features of the different plastid lineages discussed in this manuscript.
The cellular organization of dinoflagellates is highly unusual. For example, the dinoflagellate nuclear genome is extremely large, with many genes present in multiple copies (14). This genome is permanently condensed and uses an unusual DNA packaging protein that is evolutionarily distinct from histones (15). The dinoflagellate mitochondrial genome is likewise highly abnormal, containing only three protein-coding genes (cob, coxI, and coxIII), which are present in multiple, fragmented copies (16, 17).
The majority of photosynthetic dinoflagellates possess plastids that contain the accessory carotenoid light harvesting pigment peridinin (18, 19). This plastid is surrounded by three membranes, is of red algal origin, and probably originated through a secondary endosymbiotic event (2, 20). The peridinin plastid branches as a sister group to the plastids found in chromerid algae and to the vestigial, nonphotosynthetic plastids found in apicomplexans, suggesting a common endosymbiotic origin of all three plastid lineages (although chromerid and apicomplexan plastids are surrounded by four membranes and do not seem to contain peridinin) (Fig. 1) (12, 13, 21). The peridinin plastid is also very closely related to other plastid lineages acquired through the secondary endosymbiosis of red algae (for example, those of diatoms and haptophytes) although the current consensus is that the dinoflagellate, diatom, and haptophyte plastids have each been acquired independently by the respective host lineage, rather than all descending from one common endosymbiotic event (Fig. 1) (2, 9, 11).
Dinoflagellates present an ideal model system in which to explore the integration of host and endosymbiont biology, for several reasons. The peridinin dinoflagellate plastid is highly reduced in terms of genome content and thus is particularly dependent on proteins encoded within the host nucleus (18, 22). The peridinin dinoflagellate plastid is supported by several highly unusual pathways that are encoded within the host nucleus: i.e., are likely to have been imposed on the plastid by the host lineage (23–25). Furthermore, some dinoflagellates possess plastids acquired through the serial endosymbiotic replacement of the ancestral peridinin lineage, and these replacement plastids show different degrees of integration with the host dinoflagellate environment (2). In this review, we discuss the integration of different dinoflagellate plastids with their hosts, with a particular focus on plastid genome organization and gene expression pathways. From this discussion, we demonstrate the insights that dinoflagellates may provide into plastid evolution across the eukaryotes.
Unusual Plastid Genome Organization in Dinoflagellates
The peridinin dinoflagellate plastid genome is very different in terms of gene content from the other plastid lineages. Typically, the plastid genomes of plants and algae contain in the region of 60–250 genes (Fig. S1) (22, 26). These genes encode components of the photosynthesis machinery [each photosystem complex, cytochrome b6f complex, ATP synthase, and ribulose bis-phosphate carboxylase (rubisco)], as well as proteins that do not directly function in photosynthesis but perform other essential plastid functions (e.g., cofactor biosynthesis, protein import, and expression of the plastid genome) (22). It has been proposed that some of these genes are retained in the plastid to allow direct regulation of their expression in response to plastid redox state (7, 27).
Studies from multiple dinoflagellate species have indicated that the peridinin plastid, in contrast to other plastids, retains fewer than 20 genes (Fig. S1) (22, 28). These genes form a subset of those found in essentially all other photosynthetic plastids, encoding subunits of the two photosystems, the cytochrome b6f complex, the ATP synthase complex, rRNAs, and a small number of tRNAs (18, 28). Thus, the peridinin dinoflagellate plastid has lost all of the ancestral genes that would have encoded proteins of nonphotosynthetic function (18). There are a small number of genes that are not found in other plastid lineages and are specific to individual peridinin dinoflagellate species (29, 30). It has additionally been suggested that the plastids of the peridinin dinoflagellates Ceratium horridum and Pyrocystis lunula may contain a small number of genes acquired through lateral transfers from bacterial sources although it cannot be excluded that these genes have been misidentified from bacterial contamination in the original sequence datasets (31). Many of the genes that have been lost uniquely from the peridinin plastid genome are known to have relocated to the nucleus and have acquired targeting sequences allowing the import of the expression products into the plastid (28, 32). Thus, the peridinin plastid is particularly dependent on the expression of nuclear genes for its function.
The peridinin dinoflagellate plastid genome also has a highly unusual organization. The plastid genomes of most plant and algal species form a single chromosome, which can be represented as topologically circular (26). There are some exceptions to these features; in some species, including the chromerid alga C. velia, the plastid genome may adopt linear or branched forms (26, 33). However, the plastid genome of the other chromerid species, V. brassicaformis, has a more conventional circular structure (12). Thus, the plastid genomes of early ancestors of the peridinin plastid lineage were likely to be conventionally organized.
In contrast to more conventional plastid genomes, the peridinin dinoflagellate plastid genome is fragmented into multiple coding elements. Zhang et al. showed that a number of plastid genes were contained on plasmid-like “minicircles” in the peridinin dinoflagellate Heterocapsa triquetra (34). Similar organization has since been shown in other dinoflagellate species (18, 35). The minicircles contain one or a few genes and a “core” sequence, which is similar in sequence, although not identical, among the minicircles containing different genes (18). Although the location of these minicircles in the cell was debated (18, 36), recent hybridization studies have confirmed that they are situated in the plastid (37). In peridinin dinoflagellates, the copy numbers of different minicircles vary during different phases of growth and, in log-phase cultures, may reduce to fewer than 10 copies per cell (38). The low copy numbers of individual minicircles in log-phase cells might plausibly lead to minicircle loss, through unequal distribution during plastid division (38). This loss would be disadvantageous unless there were already a copy of the minicircle gene in the nuclear genome that could be expressed and rescue the plastid (8). Thus, the minicircular genome organization of the peridinin plastid may have provided a selective advantage for gene transfer from plastid to nucleus and greater integration of the plastid with its host (39).
Unusual Plastid Biochemistry in Peridinin Dinoflagellates
In addition to the highly reduced nature of the plastid genome, there is evidence for intricate functional relationships between the peridinin plastid and the host dinoflagellate nucleus. Some of the proteins that function in the peridinin plastid are clearly of nuclear or external origin and thus have been secondarily applied to the peridinin plastid by the host. For example, peridinin dinoflagellates lack a conventional form ID rubisco holoenzyme, consisting of eight large and eight small subunits (as found in other plastids descended from red algae, and typically encoded in the plastid genome) and instead use a form II rubisco, consisting of two large subunits, which is encoded in the nucleus (23, 40). The form II large subunit gene is also used by chromerid algae and was acquired via lateral gene transfer from a purple sulfur bacterium into a common ancestor of the dinoflagellate and chromerid lineages (12, 40).
There are several unusual pathways associated with transcript processing in peridinin plastids. These pathways are likely to be dependent on nucleus-encoded proteins, given the absence of nonphotosynthesis genes from peridinin plastid genomes (18, 28). One such pathway is editing, which has been detected in multiple dinoflagellate species (25, 28), although plastid transcript editing does not appear to occur in Amphidinium (29) (Fig. 1 and Table S1). The number of sites edited varies between species and genes, with nearly 1 in 10 sites edited in some Ceratium transcripts (Table S1). The most common editing event in dinoflagellate plastids is A–G, followed by C–U and U–C; however, nine different events, including five different transversion substitutions, have been documented (Table S1) (28). Transcript editing is not found in the plastids of other studied algae, including those of chromerid algae (21, 41) (Fig. 1). Although editing occurs in plant plastids, it is very different from dinoflagellates, with a more restricted range (predominantly C–U) and generally lower frequency of editing events (<1 in 1,000 in angiosperms) (6). Thus, the plastid editing pathways found in peridinin dinoflagellates have evolved specifically within that lineage.
An even more remarkable processing event is the addition of a poly(U) tail to the 3′ end of many transcripts. This transcript processing feature was first reported for Lingulodinium and Amphidinium (24) but has since been reported for other peridinin dinoflagellate species (42). Poly(U) tail addition has also been found in the chromerid algae C. velia and V. brassicaformis, suggesting that it is an ancestral feature of red lineage alveolate plastids although it seems not to occur in apicomplexans (12, 41) (Fig. 1). The role of the poly(U) tail remains unclear, although in chromerids it is principally added to transcripts of genes encoding photosystem subunits, suggesting that it plays a role in the expression of the photosynthesis machinery (33, 41). The role of poly(U) tail addition in dinoflagellate plastids is in contrast to poly(A) tail addition in plant plastids, which principally seems to be involved in the degradation of unwanted transcripts (43). Poly(U) tail addition has been documented in a small number of gene expression pathways in bacteria and in some eukaryotic nuclear and mitochondrial lineages, although not in those of dinoflagellates (44–46). Poly(U) tail addition is not known to occur in any plastids other than those of dinoflagellates and chromerids, indicating that it is a specific evolutionary innovation within this lineage (21).
Serial Endosymbiosis in Dinoflagellates
Not all dinoflagellates possess peridinin plastids. Many (e.g., Perkinsus, Oxyrrhis, Hematodinium) are nonphotosynthetic, including some species that are of ecological importance as free-living predators or as parasites of marine invertebrates (11). These species may possess vestigial plastids but have lost the capacity for photosynthesis (Fig. 1). Some of the lineages that do not possess their own plastids (e.g., Dinophysis) maintain transient symbioses with other photosynthetic organisms. These short-term endosymbioses have been reviewed elsewhere and will not be discussed in further detail here (47). However, other dinoflagellates are photosynthetic and possess permanent plastids that are not of the peridinin type. Because the peridinin plastid was present in the last common dinoflagellate ancestor, these plastid types must have arisen through subsequent serial endosymbioses. Thus far, three major serially acquired plastid lineages have been documented. They are monophyletic, and each arose through independent serial endosymbiosis events (48, 49). As shown in Fig. 1, they are the Karenia/Karlodinium, Kryptoperidinium/Durinskia, and Lepidodinium lineages.
Dinoflagellate species that possess the accessory light-harvesting carotenoid pigment fucoxanthin (e.g., Karenia, Karlodinium) contain plastids that are derived from haptophyte algae (Fig. 1) (50). Many of the fucoxanthin-containing dinoflagellates are implicated in harmful algal blooms (49, 51). Although some early phylogenetic studies of the fucoxanthin plastid indicated that it might be closely related to the peridinin plastid (52), more recent phylogenies have confirmed that the fucoxanthin plastid arose through a subsequent serial endosymbiosis (53–55). The fucoxanthin plastid is surrounded by three membranes, similarly to the peridinin plastid, and there is no evidence for the retention of a nucleus, or mitochondria, from the haptophyte (20).
The “dinotom” algae, typified by Kryptoperidinium and Durinskia, possess complex endosymbionts derived from pennate diatoms (56) (Fig. 1). In contrast to the fucoxanthin dinoflagellates, the dinotom endosymbiont not only consists of a plastid but also contains a nucleus and mitochondria, which retain their own genomes (20, 57). The dinotom plastid is surrounded by four membranes, similarly to the plastids of free-living diatoms, and a final, fifth membrane surrounds the entire endosymbiont (20, 58). Two dinotom lineages—Peridinium quinquecorne, and Peridiniopsis sp.—have been proposed to possess endosymbionts derived from centric, rather than pennate, diatom sources (59, 60). Because relatively little is known about the molecular biology of the centric diatom endosymbionts in these species, the term “dinotom” will be used here to refer to the pennate diatom endosymbiont.
Finally dinoflagellates of the genus Lepidodinium possess plastids derived from green algae (Fig. 1) (61, 62). The Lepidodinium plastid is surrounded by four membranes, of which the innermost two correspond to the plastid membranes of the original endosymbiont lineage, and the third may correspond to the plasma membrane of the endosymbiont (20, 63). Although mitochondria or nuclei have not been documented within the Lepidodinium endosymbiont, membrane-bound bodies and free ribosomes have been observed between the second and third membranes, which may correspond to a highly reduced endosymbiont nucleus (20, 63).
Reductive Evolution of Serially Acquired Dinoflagellate Plastids
The extraordinary diversity of dinoflagellate plastids provides exceptional opportunities for studying the events that occur after plastid acquisition. After their acquisition, the biology of the fucoxanthin, Lepidodinium, and dinotom plastids must have been altered to facilitate productive associations with the host. In each lineage, for example, starch is principally detectable in the host cytoplasm (20). Thus, carbohydrates generated through photosynthesis in the plastid are exported across each of the endosymbiont-derived membranes into the host, including ones derived from the outermost membranes of the endosymbiont, which may not have been involved in carbohydrate transport before the endosymbiotic event.
Thus far, plastid genomes have been sequenced for the fucoxanthin dinoflagellate Karlodinium veneficum (55) and for the dinotoms Kryptoperidinium foliaceum and Durinskia baltica (64). The genomes of the endosymbiont mitochondria of both dinotoms have also been sequenced (17). The dinotom plastid and endosymbiont mitochondrial genomes are similar to those of free-living diatoms, with almost no examples of gene loss (Fig. S1) (17, 64). The genome of the dinotom endosymbiont nucleus has not been fully sequenced, but it retains genes for complex metabolic pathways and for structural proteins (e.g., actin, tubulin) that have been lost from other vestigial nuclei found in association with plastids (e.g., the “nucleomorphs” of chlorarachniophyte and cryptomonad algae) (65–67). In contrast to the dinotoms, the K. veneficum plastid genome has lost over 40 genes that are present in the plastids of free-living haptophytes (Fig. S1) (55, 68). In addition, many of the individual genes contain premature termination codons and may constitute pseudogenes (55, 69).
The different reduction of each serially acquired plastid lineage is reflected by differences in the degree of gene transfer to the host nucleus. EST studies of fucoxanthin dinoflagellates have identified many gene transfers from the plastid to its host (70–73). For example, in a recent study of the fucoxanthin dinoflagellates K. veneficum and Karenia brevis, Burki et al. identified 90 ESTs of predicted haptophyte origin, including 34 that were predicted to encode a plastid targeting sequence, out of a total of 493 ESTs of definable phylogenetic affinity (74). Thus, ∼7% of the fucoxanthin dinoflagellate nuclear genome may encode proteins of haptophyte plastid origin, a figure approaching that found in other plastid lineages derived through secondary or tertiary endosymbiosis (9). In the same study, the authors screened EST libraries of the dinotom algae K. foliaceum and D. baltica. Only 14 ESTs out of a total of 237 of definable phylogenetic origin resolved with diatoms, and none was predicted to encode a plastid-targeting sequence (74). The most recent study of gene transfer in Lepidodinium identified six ESTs, of probable green algal origin, that were predicted to contain a plastid-targeting sequence, from a total dataset of 4,746 sequences of both definable and uncertain phylogenetic origin (75). Whether gene transfer events have occurred from the serially acquired plastids in Lepidodinium to the same extent as in fucoxanthin dinoflagellates awaits further characterization.
Integration of Ancestral and Serially Acquired Endosymbionts
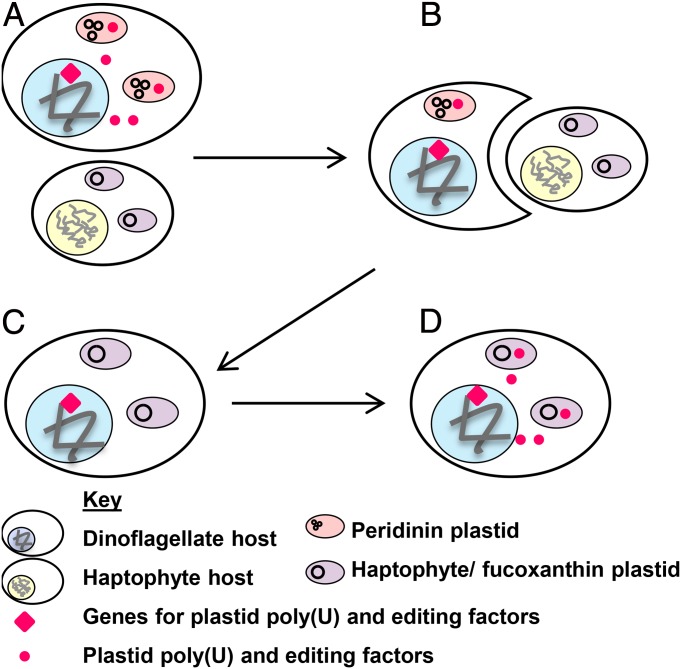
Given that genes have been relocated from serially acquired plastids to the dinoflagellate host nucleus, has there been a more intricate integration of the host and serially acquired plastid genomes? For example, serially acquired dinoflagellate plastids may have benefited from pathways that are endogenous to the host. Any dinoflagellate that undergoes serial endosymbiosis may retain pathways that had been associated with the original peridinin plastid. If these pathways were applied to the incoming replacement plastid, they might facilitate its integration into the host or even change its biology (Fig. 2).
Fig. 2.
Application of ancestral plastid pathways to serially acquired dinoflagellate plastids. This diagram shows how pathways associated with the peridinin plastid may have come to function in serially acquired dinoflagellate plastid lineages. For clarity, only the first membrane around each plastid is shown. Early dinoflagellates possessed a peridinin plastid, which was maintained by pathways [such as poly(U) tail addition and editing] encoded within the nucleus (A). In some lineages, this plastid was replaced by others (such as the fucoxanthin plastid) through serial endosymbiosis (B). Although the ancestral peridinin plastid was lost in these lineages, some of the nucleus-encoded genes associated with its function were retained (C) and, after the serial endosymbiosis event, were applied to the replacement plastid, changing its biology (D).
This hypothesis is consistent with the “shopping bag” model for plastid evolution proposed by Larkum et al. (76). This model argued that the endosymbiotic origin of a plastid is unlikely to have been due to a single event at a particular time and place but instead followed multiple unsuccessful “attempts” at endosymbiosis (1). Although these previous attempts did not lead to extant symbioses, they may have contributed genes that help support present-day plastids. It has been proposed that several major photosynthetic eukaryote lineages possess genes that correspond to the “footprints” of such cryptic endosymbioses. For example, diatoms (which possess red algal plastids) may possess genes retained from an ancestral green algal symbiont and plants, and red and green algae (which possess cyanobacterial plastids) may possess genes from an ancestral chlamydiobacterial symbiont (9, 77–79). These hypotheses remain controversial because of the absence of identifiable extant descendants of the cryptic endosymbiont lineages. Serially acquired dinoflagellate plastids, in contrast, provide a well-defined opportunity to assess the impact of a historical endosymbiont on its successors.
Genes have been identified in the nuclei of fucoxanthin dinoflagellates (70, 72, 80, 81) and of Lepidodinium (62, 75) that encode proteins predicted to be targeted to the plastid and are related to genes from peridinin dinoflagellates, rather than the free-living relatives of the respective serially acquired plastids (Table S2). Thus, the fucoxanthin and Lepidodinium plastids may be supported by pathways retained from the peridinin symbiosis. The dinotom host nucleus has likewise been shown to retain genes for components of several metabolic pathways that were likely to have functioned in the original peridinin plastid (65, 66). However, in each case, components for a second copy of the pathway, of diatom origin, seem to be encoded in the endosymbiont nucleus, and the host-derived copies do not possess targeting sequences appropriate for protein import into diatom plastids (65, 66). Thus, the dinotom plastid is supported by the diatom-derived pathways encoded in the endosymbiont nucleus, rather than the pathways from the peridinin symbiosis.
Transcript Processing in Serially Acquired Plastids
Perhaps the most compelling evidence for pathways retained from the peridinin symbiosis in serially acquired plastids comes from studies of plastid transcript processing. As previously discussed, the peridinin plastid uses two highly unusual transcript-processing pathways—poly(U) tail addition and extensive RNA editing. These pathways are not found in the progenitors of the serial endosymbionts, such as the haptophyte relatives of fucoxanthin dinoflagellates (12, 21, 33). Recently, however, we have demonstrated that plastid transcripts in the fucoxanthin dinoflagellates Karenia mikimotoi and K. veneficum receive poly(U) tails (Fig. 1) (21, 69). Furthermore, we and others have shown that fucoxanthin plastid transcripts undergo high levels of editing, involving both transition and transversion substitutions, as occurs in the peridinin plastid (Fig. 1 and Table S1) (21, 82).
Because neither poly(U) tail addition nor transcript editing is native to free-living haptophytes, the most parsimonious explanation for their occurrence in the fucoxanthin plastid is that they are remnants of the ancestral peridinin plastid symbiosis and were applied to the incoming fucoxanthin plastid after serial endosymbiosis (Fig. 2) (21, 82). Notably, whereas editing and poly(U) addition are found in both the peridinin and fucoxanthin dinoflagellate plastids, they do not occur in dinotom or in Lepidodinium plastids (Fig. 1) (69). Thus, the pathways required for this unusual degree of endosymbiotic integration have been retained through some, but not all, serial endosymbioses.
Functional Consequences of Poly(U) Addition and Editing
Both poly(U) tail addition and editing are widespread features in fucoxanthin dinoflagellate plastid transcript processing. Recently, we profiled the occurrence of each pathway across the plastid genome of the fucoxanthin dinoflagellate K. veneficum (69). We found evidence of poly(U) and editing sites on almost every transcript (69), including those with nonphotosynthesis functions, which are not plastid-encoded in peridinin dinoflagellates (18, 28) and which generally are not polyuridylylated in chromerid algae (41).
Many of the major hypotheses for the origins of transcript processing pathways in other plastid lineages propose they are neutral overall, either compensating for changes in the underlying genomic sequence (6, 83) or having silent effects on plastid transcripts (84). Although the acquisition of foreign RNA processing pathways by the fucoxanthin plastid may have had neutral consequences for the host initially, for transcript editing and poly(U) tail addition to have become such major components of transcript processing in fucoxanthin plastids, it is likely that they conferred some advantageous effects and thus had an adaptive role in fucoxanthin plastid evolution. Poly(U) tail addition and RNA editing may have enabled the fucoxanthin plastid to tolerate the highly divergent sequence evolution of the underlying genome (55). Editing of transcript sequences may enable the compensatory removal of mutations in the genome sequence. For example, premature in-frame termination codons are removed from mRNA sequences by editing in both K. mikimotoi and K. veneficum (21, 69, 82). As detailed above, fucoxanthin plastid genomes are highly divergent from those of free-living haptophytes (55). Transcript editing, by enabling fucoxanthin dinoflagellates to recover regions of sequence that are important for the function of the protein encoded, might allow the plastid to tolerate mutations that would otherwise prove deleterious. Thus, the presence of transcript editing might enable the fucoxanthin plastid to function in a host environment subjected to elevated rates of sequence substitution.
The poly(U) machinery of fucoxanthin dinoflagellates might similarly play a role, alongside editing, in compensating for divergent evolution in the underlying genome sequence. For example, several genes in the K. veneficum plastid are present in multiple copies, one of which is translationally functional whereas others are pseudogenes (55, 68). Remarkably, in these cases where transcripts of the functional gene copy receive poly(U) tails and are highly edited as expected, transcripts of the pseudogene copies do not receive poly(U) tails and undergo only very limited editing (69). A similar discrimination between functional and pseudogene transcript copies by the poly(U) machinery has also been documented in the chromerid alga C. velia (33, 41). Thus, a preferential application of the poly(U) tail might enable the fucoxanthin dinoflagellate plastid to discriminate functional gene copies from pseudogenes in its genome.
Convergence of Peridinin and Fucoxanthin Plastid Genomes
It remains to be determined which other features of serially acquired dinoflagellate plastids, beyond transcript-processing pathways, are derived from the ancestral peridinin plastid. There is a dramatic example of convergence between peridinin and serially acquired dinoflagellate plastids in terms of organization of the plastid genome. As discussed above, the plastid genome of peridinin dinoflagellates is fragmented into small elements termed “minicircles.” Recently, the K. veneficum dnaK gene (encoding the stromal 70-kDa chaperone) has been shown to be located on a minicircle (68, 69). This minicircle gives rise to a complete, polyuridylylated and edited dnaK transcript, confirming that it is located in the plastid (69). This minicircle also contains a secondary structure-rich motif that may constitute an equivalent of the peridinin dinoflagellate minicircle core (68, 69). Similar minicircles have not been reported in Lepidodinium or in dinotoms.
The reason why minicircles are present in fucoxanthin plastids remains to be determined. It is possible that whatever factors caused fragmentation of the peridinin plastid genome have been applied to the fucoxanthin plastid after its endosymbiotic acquisition, leading to the convergent evolution of minicircles from each plastid. The gene order in the K. veneficum plastid genome is highly divergent, with disruptions to gene clusters that are well conserved in other plastids (55). Thus, other rearrangement events may have accompanied the formation of minicircles in fucoxanthin dinoflagellates. The selective consequences of this fragmentation for the fucoxanthin plastid are unclear. As discussed above, the relocation of certain genes to minicircles might have adverse effects on the ability to maintain those genes in the plastid (38). If a similar situation were true in fucoxanthin dinoflagellates, a partial fragmentation of the fucoxanthin plastid genome might have driven the relocation of genes located on plastid minicircles to the nucleus of the host (55, 68).
Why Integration in Some Lineages, and Not Others?
It is apparent, from both the reduced state of the plastid genome (55) and the acquisition of host-derived pathways such as poly(U) addition and transcript editing (21, 82), that the fucoxanthin dinoflagellate plastid has become intricately integrated with that of the host. This integration is likely to have had beneficial consequences. For example, poly(U) tail addition and editing may mitigate against the divergent evolution of the plastid genome (69, 82). Although the Lepidodinium plastid does not use the poly(U) tail addition or editing pathways (69), it is likely that it has become similarly integrated into the host, given the evidence for endosymbiotic gene transfer and the presence of plastid-targeted proteins that are retained from the peridinin symbiosis (62, 63, 75).
In contrast to the situation for the fucoxanthin and Lepidodinium plastids, there is only very limited evidence for integration of the dinotom endosymbiont with its host. Not only is the endosymbiont largely unreduced in terms of genome content (17, 64), there is no significant evidence for the presence of genes in the host nucleus—of any phylogenetic derivation—that are likely to support the plastid (65, 66, 74). It seems instead that the endosymbiont nucleus plays a more significant role in supporting the plastid (65, 66). Why might the dinotom plastid be much less integrated with its host than the plastids of fucoxanthin dinoflagellates and Lepidodinium?
One possible reason for different degree of integration of the dinotom and fucoxanthin plastids with their respective hosts is the relative age of each lineage. The dinotom endosymbiont has been inferred to have been acquired not substantially greater than 50 million years ago whereas the fucoxanthin dinoflagellate plastid may represent a much more ancient acquisition, potentially of the order of 200 million years age or greater (56, 85). The dinotom endosymbiont may thus simply not have had time to have reached as intimate a degree of connection with its host environment. However, plastids of an equivalent age to the dinotom endosymbiont can undergo reduction and integration with the host. For example, gene loss and functional gene transfers have been documented in the independently acquired primary plastids of the photosynthetic amoeba Paulinella chromatophora, which are believed to have originated no more than 60 million years ago (86). Gene loss has even been documented in the cyanobacterial endosymbionts of the diatom Rhopalodia gibba, which are believed to have been acquired by their host as little as 12 million years ago (87). Furthermore, dinotoms do show evidence of postendosymbiotic divergence from one another. For example, the K. foliaceum endosymbiont has acquired a small number of novel coding sequences (encoding DNA recombinases and RNA maturases) in its plastid and endosymbiont mitochondrial genomes that are found neither in free-living diatoms nor in the dinotom D. baltica (17, 64). Thus, the biology of the dinotom endosymbiont may have changed since its initial endosymbiotic uptake; however, it has not become significantly integrated into the biology of its host.
An alternative hypothesis for the lack of integration in some lineages concerns the stages of serial endosymbiosis associated with each plastid lineage. In theory, serial endosymbiosis could occur either via the initial loss of a plastid, then the gain of a replacement, or via the initial gain of a plastid, followed by the loss of the original plastid lineage. In the latter scenario, the two plastids coexist for a certain period, allowing the recruitment of maintenance pathways from one lineage to support the other. Thus, the extreme degree of integration of the fucoxanthin plastid with its host might suggest that, for a period, the fucoxanthin and peridinin plastids coexisted in the host. In contrast, if the dinotom endosymbiont was acquired only a substantial period after loss of the peridinin plastid, plastid-associated pathways that were associated with the peridinin plastid lineage might have been lost before the acquisition of the replacement. However, as detailed above, the host nucleus of dinotom algae still possesses genes for biosynthetic pathways inferred to have functioned in the ancestral peridinin plastid (although subcellular localization predictions suggest that the expression products of these genes function elsewhere from the replacement plastid) (65, 66). Thus, the different degree of integration of fucoxanthin and dinotom plastids with their hosts is not due to a difference in the peridinin-derived genes present in the host lineage at the point of serial endosymbiosis but is due to how these gene complements have been applied to support each serial plastid lineage.
A final possible explanation for the lack of integration in dinotoms concerns the biology of its plastid. There may be specific physiological reasons why the dinotom plastid has not integrated with its host and is instead supported by the mitochondria and nucleus of the endosymbiont. There may be a selective requirement to retain plastid-targeted genes in the endosymbiont nucleus, which might prevent the transfer of these genes to the host, or the cooption of genes within the host nucleus to support the endosymbiont. In dinotoms, the outermost membrane surrounding the plastid is frequently contiguous with the endosymbiont nuclear envelope (58, 88, 89). It will be interesting to determine whether there are particularly intricate cellular connections between the two organelles: for example, in terms of the import of proteins into the plastid or the coordination of gene expression in the plastid and the endosymbiont nucleus.
Similarly, intricate mitochondria–plastid interactions have been characterized in free-living diatoms, including the use of a mitochondrial urea cycle to modulate plastid nitrogen metabolism, and potentially even pathways that redistribute electron potential between mitochondria and plastids to minimize photoinhibition (3, 90). If these mitochondria–plastid interactions also function in the dinotom endosymbiont, they might also provide a selective barrier to elimination of the endosymbiont mitochondria and greater integration of the plastid with the host.
Many questions remain to be answered in the context of serial plastid evolution in dinoflagellates. For example, the exact extent of plastid gene transfer or the number of genes retained from the ancestral peridinin symbiosis to support each serially acquired plastid remain to be determined. In addition, it remains to be determined what the consequences of editing and poly(U) tail addition have been for fucoxanthin plastid evolution. At a broader level, the extreme diversity of integration observed between different plastids with the dinoflagellate host—ranging from the intricate cellular and evolutionary connections between the peridinin and fucoxanthin plastids and the host nucleus to the largely autonomous function of the dinotom endosymbiont—provides insights into the diversity of evolutionary pathways that plastids and other endosymbiotic organelles may undertake. Further exploration of why different dinoflagellate plastids are so differently integrated with their hosts may provide valuable insights into the fundamental processes associated with postendosymbiotic plastid evolution across the eukaryotes.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Symbioses Becoming Permanent: The Origins and Evolutionary Trajectories of Organelles,” held October 15–17, 2014, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/Symbioses.
This article is a PNAS Direct Submission. P.J.K. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1421380112/-/DCSupplemental.
References
- 1.Howe CJ, Barbrook AC, Nisbet RER, Lockhart PJ, Larkum AWD. The origin of plastids. Philos Trans R Soc Lond B Biol Sci. 2008;363(1504):2675–2685. doi: 10.1098/rstb.2008.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keeling PJ. The endosymbiotic origin, diversification and fate of plastids. Philos Trans R Soc Lond B Biol Sci. 2010;365(1541):729–748. doi: 10.1098/rstb.2009.0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prihoda J, et al. Chloroplast-mitochondria cross-talk in diatoms. J Exp Bot. 2012;63(4):1543–1557. doi: 10.1093/jxb/err441. [DOI] [PubMed] [Google Scholar]
- 4.Linka N, Weber APM. Intracellular metabolite transporters in plants. Mol Plant. 2010;3(1):21–53. doi: 10.1093/mp/ssp108. [DOI] [PubMed] [Google Scholar]
- 5.Miyagishima SY. Mechanism of plastid division: From a bacterium to an organelle. Plant Physiol. 2011;155(4):1533–1544. doi: 10.1104/pp.110.170688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fujii S, Small I. The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 2011;191(1):37–47. doi: 10.1111/j.1469-8137.2011.03746.x. [DOI] [PubMed] [Google Scholar]
- 7.Puthiyaveetil S, et al. The ancestral symbiont sensor kinase CSK links photosynthesis with gene expression in chloroplasts. Proc Natl Acad Sci USA. 2008;105(29):10061–10066. doi: 10.1073/pnas.0803928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleine T, Maier UG, Leister D. DNA transfer from organelles to the nucleus: The idiosyncratic genetics of endosymbiosis. Annu Rev Plant Biol. 2009;60:115–138. doi: 10.1146/annurev.arplant.043008.092119. [DOI] [PubMed] [Google Scholar]
- 9.Archibald JM. Genomic perspectives on the birth and spread of plastids. Proc Natl Acad Sci USA. 2015;112:10147–10153. doi: 10.1073/pnas.1421374112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki K, Miyagishima SY. Eukaryotic and eubacterial contributions to the establishment of plastid proteome estimated by large-scale phylogenetic analyses. Mol Biol Evol. 2010;27(3):581–590. doi: 10.1093/molbev/msp273. [DOI] [PubMed] [Google Scholar]
- 11.Walker G, Dorrell RG, Schlacht A, Dacks JB. Eukaryotic systematics: A user's guide for cell biologists and parasitologists. Parasitology. 2011;138(13):1638–1663. doi: 10.1017/S0031182010001708. [DOI] [PubMed] [Google Scholar]
- 12.Janouškovec J, Horák A, Oborník M, Lukes J, Keeling PJ. A common red algal origin of the apicomplexan, dinoflagellate, and heterokont plastids. Proc Natl Acad Sci USA. 2010;107(24):10949–10954. doi: 10.1073/pnas.1003335107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janouškovec J, et al. Factors mediating plastid dependency and the origins of parasitism in apicomplexans and their close relatives. Proc Natl Acad Sci USA. 2015;112:10200–10207. doi: 10.1073/pnas.1423790112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoguchi E, et al. Draft assembly of the Symbiodinium minutum nuclear genome reveals dinoflagellate gene structure. Curr Biol. 2013;23(15):1399–1408. doi: 10.1016/j.cub.2013.05.062. [DOI] [PubMed] [Google Scholar]
- 15.Gornik SG, et al. Loss of nucleosomal DNA condensation coincides with appearance of a novel nuclear protein in dinoflagellates. Curr Biol. 2012;22(24):2303–2312. doi: 10.1016/j.cub.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Nash EA, Nisbet RER, Barbrook AC, Howe CJ. Dinoflagellates: A mitochondrial genome all at sea. Trends Genet. 2008;24(7):328–335. doi: 10.1016/j.tig.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Imanian B, Pombert J-F, Dorrell RG, Burki F, Keeling PJ. Tertiary endosymbiosis in two dinotoms has generated little change in the mitochondrial genomes of their dinoflagellate hosts and diatom endosymbionts. PLoS ONE. 2012;7(8):e43763. doi: 10.1371/journal.pone.0043763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howe CJ, Nisbet RER, Barbrook AC. The remarkable chloroplast genome of dinoflagellates. J Exp Bot. 2008;59(5):1035–1045. doi: 10.1093/jxb/erm292. [DOI] [PubMed] [Google Scholar]
- 19.Haxo FT, Kycia JH, Somers GF, Bennett A, Siegelman HW. Peridinin-chlorophyll A proteins of the dinoflagellate Amphidinium carterae. Plant Physiol. 1976;57(2):297–303. doi: 10.1104/pp.57.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schnepf E, Elbrachter M. Dinophyte chloroplasts and phylogeny: A review. Grana. 1999;38(2-3):81–97. [Google Scholar]
- 21.Dorrell RG, Howe CJ. Functional remodeling of RNA processing in replacement chloroplasts by pathways retained from their predecessors. Proc Natl Acad Sci USA. 2012;109(46):18879–18884. doi: 10.1073/pnas.1212270109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green BR. Chloroplast genomes of photosynthetic eukaryotes. Plant J. 2011;66(1):34–44. doi: 10.1111/j.1365-313X.2011.04541.x. [DOI] [PubMed] [Google Scholar]
- 23.Morse D, Salois P, Markovic P, Hastings JW. A nuclear-encoded form II RuBisCO in dinoflagellates. Science. 1995;268(5217):1622–1624. doi: 10.1126/science.7777861. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Morse D. Rampant polyuridylylation of plastid gene transcripts in the dinoflagellate Lingulodinium. Nucleic Acids Res. 2006;34(2):613–619. doi: 10.1093/nar/gkj438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zauner S, Greilinger D, Laatsch T, Kowallik KV, Maier UG. Substitutional editing of transcripts from genes of cyanobacterial origin in the dinoflagellate Ceratium horridum. FEBS Lett. 2004;577(3):535–538. doi: 10.1016/j.febslet.2004.10.060. [DOI] [PubMed] [Google Scholar]
- 26.Smith DR, Keeling PJ. Mitochondrial and plastid genome architecture: Reoccurring themes, but significant differences at the extremes. Proc Natl Acad Sci USA. 2015;112:10177–10184. doi: 10.1073/pnas.1422049112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Allen JF. The function of genomes in bioenergetic organelles. Philos Trans R Soc Lond B Biol Sci. 2003;358(1429):19–37, discussion 37–38. doi: 10.1098/rstb.2002.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mungpakdee S, et al. Massive gene transfer and extensive RNA editing of a symbiotic dinoflagellate plastid genome. Genome Biol Evol. 2014;6(6):1408–1422. doi: 10.1093/gbe/evu109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbrook AC, et al. Polyuridylylation and processing of transcripts from multiple gene minicircles in chloroplasts of the dinoflagellate Amphidinium carterae. Plant Mol Biol. 2012;79(4-5):347–357. doi: 10.1007/s11103-012-9916-z. [DOI] [PubMed] [Google Scholar]
- 30.Nisbet RER, Koumandou L, Barbrook AC, Howe CJ. Novel plastid gene minicircles in the dinoflagellate Amphidinium operculatum. Gene. 2004;331:141–147. doi: 10.1016/j.gene.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 31.Moszczynski K, Mackiewicz P, Bodyl A. Evidence for horizontal gene transfer from bacteroidetes bacteria to dinoflagellate minicircles. Mol Biol Evol. 2012;29(3):887–892. doi: 10.1093/molbev/msr276. [DOI] [PubMed] [Google Scholar]
- 32.Hackett JD, et al. Migration of the plastid genome to the nucleus in a peridinin dinoflagellate. Curr Biol. 2004;14(3):213–218. doi: 10.1016/j.cub.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 33.Janouškovec J, et al. Split photosystem protein, linear-mapping topology, and growth of structural complexity in the plastid genome of Chromera velia. Mol Biol Evol. 2013;30(11):2447–2462. doi: 10.1093/molbev/mst144. [DOI] [PubMed] [Google Scholar]
- 34.Zhang Z, Green BR, Cavalier-Smith T. Single gene circles in dinoflagellate chloroplast genomes. Nature. 1999;400(6740):155–159. doi: 10.1038/22099. [DOI] [PubMed] [Google Scholar]
- 35.Barbrook AC, Howe CJ. Minicircular plastid DNA in the dinoflagellate Amphidinium operculatum. Mol Gen Genet. 2000;263(1):152–158. doi: 10.1007/s004380050042. [DOI] [PubMed] [Google Scholar]
- 36.Laatsch T, Zauner S, Stoebe-Maier B, Kowallik KV, Maier UG. Plastid-derived single gene minicircles of the dinoflagellate Ceratium horridum are localized in the nucleus. Mol Biol Evol. 2004;21(7):1318–1322. doi: 10.1093/molbev/msh127. [DOI] [PubMed] [Google Scholar]
- 37.Owari S, Hayashi A, Ishida K-i. Subcellular localization of minicircle DNA in the dinoflagellate Amphidinium massartii. Phycol Res. 2014;62(1):1–8. [Google Scholar]
- 38.Koumandou VL, Howe CJ. The copy number of chloroplast gene minicircles changes dramatically with growth phase in the dinoflagellate Amphidinium operculatum. Protist. 2007;158(1):89–103. doi: 10.1016/j.protis.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 39.Lister DL, Bateman JM, Purton S, Howe CJ. DNA transfer from chloroplast to nucleus is much rarer in Chlamydomonas than in tobacco. Gene. 2003;316:33–38. doi: 10.1016/s0378-1119(03)00754-6. [DOI] [PubMed] [Google Scholar]
- 40.Tabita FR, Hanson TE, Satagopan S, Witte BH, Kreel NE. Phylogenetic and evolutionary relationships of RubisCO and the RubisCO-like proteins and the functional lessons provided by diverse molecular forms. Philos Trans R Soc Lond B Biol Sci. 2008;363(1504):2629–2640. doi: 10.1098/rstb.2008.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorrell RG, Drew J, Nisbet RE, Howe CJ. Evolution of chloroplast transcript processing in Plasmodium and its chromerid algal relatives. PLoS Genet. 2014;10(1):e1004008. doi: 10.1371/journal.pgen.1004008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nelson MJ, et al. Identification and transcription of transfer RNA genes in dinoflagellate plastid minicircles. Gene. 2007;392(1-2):291–298. doi: 10.1016/j.gene.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 43.Kudla J, Hayes R, Gruissem W. Polyadenylation accelerates degradation of chloroplast mRNA. EMBO J. 1996;15(24):7137–7146. [PMC free article] [PubMed] [Google Scholar]
- 44.Aphasizhev R. RNA uridylyltransferases. Cell Mol Life Sci. 2005;62(19-20):2194–2203. doi: 10.1007/s00018-005-5198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norbury CJ. 3′ Uridylation and the regulation of RNA function in the cytoplasm. Biochem Soc Trans. 2010;38(4):1150–1153. doi: 10.1042/BST0381150. [DOI] [PubMed] [Google Scholar]
- 46.Otaka H, Ishikawa H, Morita T, Aiba H. PolyU tail of rho-independent terminator of bacterial small RNAs is essential for Hfq action. Proc Natl Acad Sci USA. 2011;108(32):13059–13064. doi: 10.1073/pnas.1107050108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park MG, Kim M, Kim S. The acquisition of plastids/phototrophy in heterotrophic dinoflagellates. Acta Protozool. 2014;53(1):39–50. [Google Scholar]
- 48.Hoppenrath M, Leander BS. Dinoflagellate phylogeny as inferred from heat shock protein 90 and ribosomal gene sequences. PLoS ONE. 2010;5(10):e13220. doi: 10.1371/journal.pone.0013220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nezan E, et al. Genetic diversity of the harmful family Kareniaceae (Gymnodiniales, Dinophyceae) in France, with the description of Karlodinium gentienii sp. nov.: A new potentially toxic dinoflagellate. Harmful Algae. 2014;40:75–91. [Google Scholar]
- 50.Takishita K, Nakano K, Uchida A. Preliminary phylogenetic analysis of plastid-encoded genes from an anomalously pigmented dinoflagellate Gymnodinium mikimotoi (Gymnodiniales, Dinophyta) Phycol Res. 1999;47(4):257–262. [Google Scholar]
- 51.Brand LE, Campbell L, Bresnan E. Karenia: The biology and ecology of a toxic genus. Harmful Algae. 2012;14:156–178. doi: 10.1016/j.hal.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yoon HS, Hackett JD, Bhattacharya D. A single origin of the peridinin- and fucoxanthin-containing plastids in dinoflagellates through tertiary endosymbiosis. Proc Natl Acad Sci USA. 2002;99(18):11724–11729. doi: 10.1073/pnas.172234799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yoon HS, et al. Tertiary endosymbiosis driven genome evolution in dinoflagellate algae. Mol Biol Evol. 2005;22(5):1299–1308. doi: 10.1093/molbev/msi118. [DOI] [PubMed] [Google Scholar]
- 54.Inagaki Y, Simpson A, Dacks J, Roger A. Phylogenetic artifacts can be caused by leucine, serine, and arginine codon usage heterogeneity: Dinoflagellate plastid origins as a case study. Syst Biol. 2004;53(4):582–593. doi: 10.1080/10635150490468756. [DOI] [PubMed] [Google Scholar]
- 55.Gabrielsen TM, et al. Genome evolution of a tertiary dinoflagellate plastid. PLoS ONE. 2011;6(4):e19132. doi: 10.1371/journal.pone.0019132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chesnick JM, Morden CW, Schmieg AM. Identity of the endosymbiont of Peridinium foliaceum (Pyrrophyta): Analysis of the rbcLS operon. J Phycol. 1996;32(5):850–857. [Google Scholar]
- 57.Tomas RN, Cox ER, Steidinger KA. Peridinium balticum (Levander) Lemmermann, an unusual dinoflagellate with a mesocaryotic and an eukaryotic nucleus. J Phycol. 1973;9(1):91–98. [Google Scholar]
- 58.Taylor FJ. Symbionticism revisited: A discussion of the evolutionary impact of intracellular symbioses. Proc R Soc Lond B Biol Sci. 1979;204(1155):267–286. doi: 10.1098/rspb.1979.0027. [DOI] [PubMed] [Google Scholar]
- 59.Takano Y, Hansen G, Fujita D, Horiguchi T. Serial replacement of diatom endosymbionts in two freshwater dinoflagenates, Peridiniopsis spp. (Peridiniales, Dinophyceae) Phycologia. 2008;47(1):41–53. [Google Scholar]
- 60.Horiguchi T, Takano Y. Serial replacement of a diatom endosymbiont in the marine dinoflagellate Peridinium quinquecorne (Peridiniales, Dinophyceae) Phycol Res. 2006;54(3):193–200. [Google Scholar]
- 61.Matsumoto T, et al. Green-colored plastids in the dinoflagellate genus Lepidodinium are of core chlorophyte origin. Protist. 2011;162(2):268–276. doi: 10.1016/j.protis.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Takishita K, et al. Origins of plastids and glyceraldehyde-3-phosphate dehydrogenase genes in the green-colored dinoflagellate Lepidodinium chlorophorum. Gene. 2008;410(1):26–36. doi: 10.1016/j.gene.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 63.Watanabe MM, Suda S, Inouye I, Sawaguchi T, Chihara M. Lepidodinium viride gen et sp-nov (Gymnodiniales, Dinophyta), a green dinoflagellate with a chlorophyll A-containing and B-containing endosymbiont. J Phycol. 1990;26(4):741–751. [Google Scholar]
- 64.Imanian B, Pombert JF, Keeling PJ. The complete plastid genomes of the two ‘dinotoms’ Durinskia baltica and Kryptoperidinium foliaceum. PLoS ONE. 2010;5(5):e10711. doi: 10.1371/journal.pone.0010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hehenberger E, Imanian B, Burki F, Keeling PJ. Evidence for the retention of two evolutionary distinct plastids in dinoflagellates with diatom endosymbionts. Genome Biol Evol. 2014;6(9):2321–2334. doi: 10.1093/gbe/evu182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imanian B, Keeling PJ. Horizontal gene transfer and redundancy of tryptophan biosynthetic enzymes in dinotoms. Genome Biol Evol. 2014;6(2):333–343. doi: 10.1093/gbe/evu014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McEwan ML, Keeling PJ. HSP90, tubulin and actin are retained in the tertiary endosymbiont genome of Kryptoperidinium foliaceum. J Eukaryot Microbiol. 2004;51(6):651–659. doi: 10.1111/j.1550-7408.2004.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 68.Espelund M, et al. Genome fragmentation is not confined to the peridinin plastid in dinoflagellates. PLoS ONE. 2012;7(6):e38809. doi: 10.1371/journal.pone.0038809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Richardson E, Dorrell RG, Howe CJ. Genome-wide transcript profiling reveals the coevolution of plastid gene sequences and transcript processing pathways in the fucoxanthin dinoflagellate Karlodinium veneficum. Mol Biol Evol. 2014;31(9):2376–2386. doi: 10.1093/molbev/msu189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patron NJ, Waller RF, Keeling PJ. A tertiary plastid uses genes from two endosymbionts. J Mol Biol. 2006;357(5):1373–1382. doi: 10.1016/j.jmb.2006.01.084. [DOI] [PubMed] [Google Scholar]
- 71.Ishida K, Green BR. Second- and third-hand chloroplasts in dinoflagellates: Phylogeny of oxygen-evolving enhancer 1 (PsbO) protein reveals replacement of a nuclear-encoded plastid gene by that of a haptophyte tertiary endosymbiont. Proc Natl Acad Sci USA. 2002;99(14):9294–9299. doi: 10.1073/pnas.142091799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nosenko T, et al. Chimeric plastid proteome in the Florida “red tide” dinoflagellate Karenia brevis. Mol Biol Evol. 2006;23(11):2026–2038. doi: 10.1093/molbev/msl074. [DOI] [PubMed] [Google Scholar]
- 73.Hoffman GE, Sanchez Puerta MV, Delwiche CF. Evolution of light-harvesting complex proteins from Chl c-containing algae. BMC Evol Biol. 2011;11:101. doi: 10.1186/1471-2148-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Burki F, et al. Endosymbiotic gene transfer in tertiary plastid-containing dinoflagellates. Eukaryot Cell. 2014;13(2):246–255. doi: 10.1128/EC.00299-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Minge MA, et al. A phylogenetic mosaic plastid proteome and unusual plastid-targeting signals in the green-colored dinoflagellate Lepidodinium chlorophorum. BMC Evol Biol. 2010;10:191. doi: 10.1186/1471-2148-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Larkum AWD, Lockhart PJ, Howe CJ. Shopping for plastids. Trends Plant Sci. 2007;12(5):189–195. doi: 10.1016/j.tplants.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 77.Becker B, Hoef-Emden K, Melkonian M. Chlamydial genes shed light on the evolution of photoautotrophic eukaryotes. BMC Evol Biol. 2008;8:203. doi: 10.1186/1471-2148-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moustafa A, et al. Genomic footprints of a cryptic plastid endosymbiosis in diatoms. Science. 2009;324(5935):1724–1726. doi: 10.1126/science.1172983. [DOI] [PubMed] [Google Scholar]
- 79.Huang J, Gogarten JP. Did an ancient chlamydial endosymbiosis facilitate the establishment of primary plastids? Genome Biol. 2007;8(6):R99. doi: 10.1186/gb-2007-8-6-r99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nosenko T, Bhattacharya D. Horizontal gene transfer in chromalveolates. BMC Evol Biol. 2007;7:173. doi: 10.1186/1471-2148-7-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Waller RF, Slamovits CH, Keeling PJ. Lateral gene transfer of a multigene region from cyanobacteria to dinoflagellates resulting in a novel plastid-targeted fusion protein. Mol Biol Evol. 2006;23(7):1437–1443. doi: 10.1093/molbev/msl008. [DOI] [PubMed] [Google Scholar]
- 82.Jackson CJ, Gornik SG, Waller RF. A tertiary plastid gains RNA editing in its new host. Mol Biol Evol. 2013;30(4):788–792. doi: 10.1093/molbev/mss270. [DOI] [PubMed] [Google Scholar]
- 83.Stoltzfus A. On the possibility of constructive neutral evolution. J Mol Evol. 1999;49(2):169–181. doi: 10.1007/pl00006540. [DOI] [PubMed] [Google Scholar]
- 84.Hirose T, et al. Occurrence of silent RNA editing in chloroplasts: Its species specificity and the influence of environmental and developmental conditions. Plant Mol Biol. 1996;30(3):667–672. doi: 10.1007/BF00049342. [DOI] [PubMed] [Google Scholar]
- 85.Parfrey LW, Lahr DJ, Knoll AH, Katz LA. Estimating the timing of early eukaryotic diversification with multigene molecular clocks. Proc Natl Acad Sci USA. 2011;108(33):13624–13629. doi: 10.1073/pnas.1110633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nowack EC, Grossman AR. Trafficking of protein into the recently established photosynthetic organelles of Paulinella chromatophora. Proc Natl Acad Sci USA. 2012;109(14):5340–5345. doi: 10.1073/pnas.1118800109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nakayama T, et al. Complete genome of a nonphotosynthetic cyanobacterium in a diatom reveals recent adaptations to an intracellular lifestyle. Proc Natl Acad Sci USA. 2014;111(31):11407–11412. doi: 10.1073/pnas.1405222111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pienaar RN, Sakai H, Horiguchi T. Description of a new dinoflagellate with a diatom endosymbiont, Durinskia capensis sp. nov. (Peridiniales, Dinophyceae) from South Africa. J Plant Res. 2007;120(2):247–258. doi: 10.1007/s10265-006-0047-y. [DOI] [PubMed] [Google Scholar]
- 89.Tamura M, Shimada S, Horiguchi T. Galeidiniium rugatum gen. et sp nov (Dinophyceae), a new coccoid dinoflagellate with a diatom endosymbiont. J Phycol. 2005;41(3):658–671. [Google Scholar]
- 90.Allen AE, et al. Evolution and metabolic significance of the urea cycle in photosynthetic diatoms. Nature. 2011;473(7346):203–207. doi: 10.1038/nature10074. [DOI] [PubMed] [Google Scholar]
- 91.Bachvaroff TR, et al. Dinoflagellate phylogeny revisited: Using ribosomal proteins to resolve deep branching dinoflagellate clades. Mol Phylogenet Evol. 2014;70:314–322. doi: 10.1016/j.ympev.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.