Abstract
This paper develops a conceptual framework for addressing questions about reproduction, individuality, and the units of selection in symbiotic associations, with special attention to the origin of the eukaryotic cell. Three kinds of reproduction are distinguished, and a possible evolutionary sequence giving rise to a mitochondrion-containing eukaryotic cell from an endosymbiotic partnership is analyzed as a series of transitions between each of the three forms of reproduction. The sequence of changes seen in this “egalitarian” evolutionary transition is compared with those that apply in “fraternal” transitions, such as the evolution of multicellularity in animals.
Keywords: symbiosis, evolution, reproduction, eukaryote
Symbiosis raises general questions about evolution, cooperation, and “individuality” in living systems. These issues arise in especially important forms in the context of endosymbiotic theories of the evolution of the eukaryotic cell. This family of theories holds that the origins of the mitochondrion lie in a transition that began with the engulfing of a bacterium by an archaeon. The bacterium became first an endosymbiont and eventually an organelle, often playing an essential role in the metabolism of the larger cell. A similar sequence occurred in the history of plastids in photosynthetic eukaryotes, including the lineage leading to land plants (1–4). The endosymbiotic theory holds that the evolutionary transition that produced the eukaryotic cell was one in which a new kind of biological individual arose from the combination and integration of others (5).
This paper develops a conceptual framework for addressing questions about individuality as they arise in symbiotic associations, with the eukaryotic cell as a central case. It does so by focusing especially on reproduction, an evolutionary phenomenon that is reshaped repeatedly in evolutionary transitions. Existing frameworks used in this area often treat reproduction and evolution in purely genetic terms (6). However, all objects that can form parent–offspring lineages can evolve in a Darwinian manner if further conditions are met. Symbiotic associations and the transitions they undergo motivate the development of a general treatment of reproduction, covering diverse kinds of parent–offspring lineages and distinguishing between biological objects that do form such lineages and those that do not. Although this paper is informal, the treatment of reproduction is intended to complement abstract multilevel models of Darwinian evolution, especially those based on the Price equation (7). The paper's framework embraces the importance of intermediate cases and movement between categories.
Conceptual Framework
The framework for thinking about reproduction developed here is designed to work alongside an analysis of evolution by natural selection expressed originally by Lewontin (8, 9). Lewontin gave a schema with three conditions and saw it as a formulation of the necessary and sufficient conditions for change by natural selection. Modified slightly, his summary holds that evolution by natural selection will take place in any population in which there are phenotypic variation, heritability, and differences in fitness (reproductive output) that are caused, at least in part, by that phenotypic variation. This summary has problems of detail when understood as a predictive model of change, especially because the pattern of inheritance and the distribution of fitness differences may cancel, yielding no net change across generations (10, 11), but it still is a lucid summary of how Darwinian evolution works. As Lewontin emphasized, many different kinds of objects—including genes, organelles, cells, demes, and other social groups—can satisfy Darwin's scheme. All can form populations of units with variation, heritability, and fitness differences. However, this use of the concepts of heritability and fitness takes for granted the idea of reproduction and especially the existence of parent–offspring lineages between members of a population. Lewontin did not give an explicit analysis of reproduction but appeared to draw on an informal understanding of the term, i.e., that an entity reproduces when it makes or gives rise to other entities of the same general kind.
Some years later Dawkins and others developed a different abstract description of evolution by natural selection, based on the idea of a replicator (6, 12, 13). Replicators are faithfully copied and have the potential to persist, in the form of copies, over many generations. Interactors or vehicles, such as cells and multicellular organisms, are made by replicators and assist their replication. In most evolutionary contexts, according to this view, genes are the only replicators, although human social behavior may generate cultural replicators (memes) as well. Interactors, such as ourselves, may be linked by parent–offspring relations, but they need not be; reproduction is incidental to their role. What matters to evolution is change in frequencies of rival replicators, or alleles. The replicator/interactor framework encourages a purely genetic accounting of evolutionary change and makes a general analysis of reproduction less important.
The framework used here rejects the replicator/interactor framework and instead develops Lewontin's view. Replicators are not needed for evolution by natural selection, and high-fidelity copying probably evolved from much noisier systems of inheritance. What is needed for evolution by natural selection is heritability, which is a population-relative and statistical concept: Whether a given degree of parent–offspring similarity suffices for evolutionary change depends on the degree of similarity between less closely related individuals in the population. Genes are a very important mechanism by which offspring come to resemble their parents, but they are not necessary in principle, and heritability does not require the existence of replicators. From the viewpoint of a summary such as Lewontin's, genes have two roles in evolution. First, they are a mechanism of inheritance seen in cells and organisms. Second, they are entities that satisfy the criteria needed to form an evolving population in their own right: Gene replication is one form of biological reproduction. The same conception of evolution is embodied in the Price equation, which in recent years has been increasingly recognized as a powerful and abstract representation of evolution by natural selection (6, 14–17). The Price equation uses slightly different organizing concepts—differential production of descendants by individuals, and transmission bias along an ancestor–descendant connection—but it has important features in common with the Lewontin summary: It does not require replicators and allows very imperfect transmission of traits across generations. It does, however, assume the existence of parent–offspring lineages (sexual or asexual) in any population to which it applies (except in the special case of differential persistence in the absence of any reproduction).
Reproduction.
If gene replication is seen as one form of reproduction among many, it is necessary to say more about what reproduction is (18). Before beginning the analysis, I make a terminological note. Ambiguity can arise in this context between talk of types and of particular instances (sometimes called “tokens”) of recurring structures. Your body contains one instance of a heart, but we also can talk of “a heart” or “the heart” in a way that refers to a type or class of these objects (e.g., we can talk of “the human heart”). Below the term instance is used when it is important to indicate reference to a particular physical object that is a member of such a class (your particular heart or a copy of a gene in a particular cell).
Reproduction depends on causal relations between parent and offspring members of a population. Causation will not be analyzed in a general way here (but see ref. 19). I assume that causal responsibility is matter of degree; among the many factors responsible for a new cell or new organism coming to exist, some factors play a greater role than others, and parents have a distinctive kind of causal responsibility for their offspring. This relation usually involves a material overlap between generations (18). The causal relation between parent and offspring is the basis for a transitive relation linking ancestors to descendants.
Much talk of reproduction in biology involves a coarse-graining of a cyclical process, at some spatial and temporal scale, in which each stage gives rise to the next—perhaps to something quite dissimilar from itself —but structures recur in a regular way. Guided by three different paradigm cases, a distinction can be introduced between three general forms of biological reproduction, which I will call simple, collective, and scaffolded reproduction (Fig. 1). A simple reproducer is something that can give rise to more objects of the same kind largely through the operation of resources internal to it—through its own biological machinery, in a broad sense—and, further, is not made of smaller parts that also have this capacity. A paradigm case is a bacterial cell. A collective reproducer is a reproducing object that has parts that are themselves simple or collective reproducers. A paradigm case is a multicellular organism such as a human, which is made of cells that also can reproduce. Third, a scaffolded reproducer is an entity that reproduces (or is reproduced) in a way highly dependent on resources external to itself. Paradigm cases are viruses and also genes; the copying of genes is a form of reproduction, but it is dependent on the machinery of a whole cell. The photocopying of a piece of paper also is scaffolded reproduction in this sense. All three forms of reproduction—simple, collective, and scaffolded—are sufficient to generate parent–offspring lineages in a population of objects, but they have different requirements and different kinds of borderline cases.
Fig. 1.
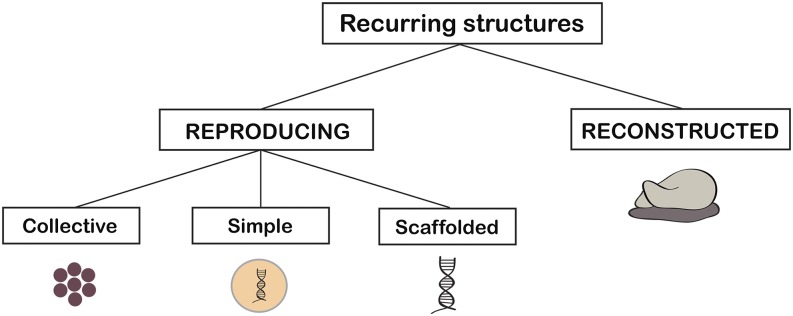
Some distinctions central to this paper. Recurring structures may either be reproducing or reconstructed. Reproducing objects form parent–offspring lineages, whereas reconstructed ones do not. Reconstructed objects in this sense include enzymes, organs, and ribosomes (pictured). Reproduction may be collective, simple, or scaffolded. Collective reproducers include multicellular organisms. An example of a simple reproducer is a prokaryotic cell. Scaffolded reproducers, which rely on external machinery for their reproduction, include genes and viruses. A collective reproducer may contain any of the other kinds of reproducer. A simple reproducer may only contain scaffolded reproducers, such as genes. A scaffolded reproducer also may contain other scaffolded reproducers.
In many biological systems we find a hierarchy of reproducers: A reproducer of one kind contains reproducers of other kinds. A simple reproducer need not be self-contained or simple in a more general sense; it may need a great deal of environmental support, and it might be a biologically complicated object. The term simple applies only to its mode of reproduction. Simple reproducers need not be the lowest-level reproducing entities in a hierarchy: A bacterial cell contains scaffolded reproducers but still qualifies as a simple reproducer. Cell reproduction works in part through the copying (reproduction) of genetic material.
There are intermediate cases and gray areas, one of which is especially important in the present context. Humans are collective reproducers, and genes are scaffolded reproducers. Our cells, like other eukaryotic cells, are a special case. The mitochondria within them have some of the features of simple reproducers, and according to the endosymbiotic theory, eukaryotic cells are at least former collectives. Eukaryotic cells in different taxa today are at different places on a continuum from collective to simple reproduction, and their mitochondria are on a continuum from simple to scaffolded reproduction.
Sex also introduces complications to the framework, especially to the idea of simple reproduction. To say that something contains all or most of the biological machinery for its reproduction seems to imply that it needs no partner. The paradigm simple reproducers—prokaryotes—reproduce asexually (although they also exchange genetic material in various ways), so they are unproblematic. A protist such as Cryptosporidium parvum, which lacks a mitochondrial genome but engages in meiosis and eukaryotic sex, is a more problematic case: It seems to be a sexual but simple reproducer. One response is to treat only the division stages in such a cycle as reproductive and to regard sexual fusion as a different phenomenon, as in prokaryotes. A different response is to see sex in such organisms as creating another intermediate form of reproduction (between simple and scaffolded). Cases of this kind, especially where they involve complex life cycles with many transformations (as in Cryptosporidium), also might be seen as showing limitations in the concept of reproduction itself. In complex life cycles we find causal chains that produce recurring structures separated by many intermediate stages, and these cases put various degrees of pressure on the intuitive idea that parents produce offspring that are similar to themselves. These questions about sex and complex life cycles warrant further investigation but do not affect the issues treated below.
Reproducing objects of all three kinds outlined above can be called “Darwinian individuals.” Individuals in this sense include things that are not at all like organisms. Animal cells are organism-like and have free-living ancestors, but genes and chromosomes are not organism-like. Recent years have seen extensive discussion of the concept of individuality in biology. A variety of views are defensible, but one important move is to distinguish organisms, in a physiological or metabolic sense, from evolutionary units (20). The term individual can be applied reasonably to both organisms and evolutionary units, but the two are quite different sorts of objects. I treat organisms here purely as metabolic units; they are systems that maintain their organization in the face of thermodynamic tendencies toward disorder and decay by taking in raw materials and using sources of energy to control chemical reactions. Sometimes these metabolic units coincide with evolutionary units; sometimes they do not. In this view, viruses, as well as chromosomes and genes, are Darwinian individuals but are not organisms.
Along with organisms and Darwinian individuals, there are other basic kinds of biological objects. One further category comprises objects or structures that recur without reproducing. An example is a heart. Hearts are recurring biological objects, seen in generation after generation, but new hearts are not brought into existence by preexisting hearts in the way new cells are brought into existence by preexisting cells. The hearts of your parents did not have a causal responsibility for your heart that your parents' other organs did not have also. Some of your parents' genes may have had special causal relations to the production of your heart, but genes are not hearts. Hearts recur because they are reconstructed in each generation by a range of developmental resources quite different from themselves. Another example, especially relevant here, is a ribosome. Ribosomes are intracellular structures containing nucleic acid along with protein, but each ribosome does not have a parent ribosome (or a small number of parents), even though preexisting ribosomes may have made proteins that went into the new ribosome. Instead, each ribosome is made by the whole cell.
The main categories above can be organized as follows: Recurrence of structure is a general feature of living systems, seen both in things that reproduce and things that do not. Reproduction and reconstruction are two different causal bases for recurrence (Fig. 1). Structures such as hearts and ribosomes recur because they are reconstructed, from generation to generation or on some other temporal scale. Reproduction generates parent–offspring lineages between instances of recurring structures, whereas reconstruction does not generate such lineages. Reproduction appears in three forms, described above. Organisms are metabolic units. In principle, such units might arise from either reproduction or reconstruction. We usually think of organisms as things that reproduce, but it is possible to put pressure on this idea, in at least some cases.
In some symbioses, the partnership comprises something like an organism without being a Darwinian individual. In these cases there is a tight metabolic association between symbiotic partners, but the partners reproduce separately without an alignment of parent–offspring lineages. Symbioses between animal hosts and microbes provide examples. Acquisition of a microbial symbiont may be vertical (from host parent to host offspring), horizontal (between other host individuals), or environmental (by uptake from a free-living microbial population; in some taxonomies the latter two categories are combined). In deep-sea vestimentiferan tubeworms, for example, the adults depend entirely on intracellular chemoautotrophic bacterial symbionts for nutrition; during metamorphosis the worms lose their functioning digestive tract altogether. However, these bacteria appear to be acquired environmentally during development, not from a host parent, and also are found free-living (21, 22). In this case and others, a multispecies metabolic collective recurs in each generation, but the collectives do not reproduce as a unit. If the resulting partnership is seen as a single organism, as is plausible in the case of vestimentiferan tubeworms, then some organisms are not Darwinian individuals. These organisms instead recur through the actions of several Darwinian individuals that collaborate and coevolve but do not combine into a single reproducing object (23, 24). In many other symbioses the association is not especially tight and is not obligate for either party. Examples include various associations between ants and acacia trees in tropical countries, in which the ants protect the tree from herbivores and the tree provides shelter and sometimes food for the ants (25). Whether a system counts as an organism is a matter of degree, with some cases being clearer and others more marginal (26).
In other forms of symbiosis, microbes and their hosts do evolve a coordinated or fused form of reproduction so that the offspring of the host reliably contain offspring of the host's microbial partners. Then the symbiotic collective is itself a Darwinian individual. The association between aphids and Buchnera is a well-studied example (27). Once again there are mixed and intermediate cases. Hatena arenicola is a protist which mixes vertical and environmental acquisition of an algal symbiont. On cell division, one daughter cell receives the symbiont from the mother, and the other daughter cell must acquire a new one (28). Lichen, which are symbiotic associations between fungi and photosynthetic partners (algae and/or cyanobacteria), are another mixed case. Many lichen combine an asexual mode of reproduction in which propagules are made that contain samples of both partners, with sexual reproduction by the fungus through release of spores which, on germination, must find a compatible photosynthetic partner (29). A lichen then reproduces as a single unit when in asexual mode but not when the fungus reproduces sexually.
Evolutionary Transitions.
Transitions in individuality, in the sense of Michod (30), are events in which evolution produces new kinds of biological individuals. The category of collective reproduction, described above, is important in these transitions. For example, in the evolution of multicellularity a new kind of reproducing individual arises, but the parts of these individuals (their cells, and also their genes) remain able to reproduce. Systems in which there is a hierarchy of objects in which both parts and wholes reproduce are the source of debates and puzzle cases, because it may be unclear which units are the bearers of fitness (31–33). Examples include social insects and their colonies, demes and their constituent organisms, and ramets and genets in botany. It would be possible to insist on the primacy of lower-level reproduction in every case, but if this strong form of reductionism is rejected, it becomes important to distinguish between situations in which the reproduction of higher-level collectives is merely a byproduct of reproduction by lower-level units and situations in which higher-level collectives are reproducers in a more substantial sense in their own right. A bison herd, for example, is a collective that arises as a byproduct of the reproduction of its constituent organisms, and the herd itself is not a plausible bearer of fitness, but integrated multicellular organisms themselves are not merely byproducts of reproduction by their cells.
A gradient thus can be recognized between more marginal and more definite cases of collective reproduction. In earlier work (11), I gave an analysis in which three parameters are used to make this distinction. The first parameter marks the presence or absence of a bottleneck between generations at the level of the collective (6, 34). Human generations, for example, pass through a one-cell bottleneck at the zygote stage, but the narrowing need not be down to one cell. Eusocial insect colonies, in which individual insects are the lower-level units within the collectives, also grow from a quite narrow bottleneck. A second parameter is germ/soma differentiation within a collective. This differentiation prevents within-collective reproductive competition from having longer-term consequences. The third feature is overall integration within the collective, especially coordinated activity and division of labor, over and above the division of labor seen in germ/soma differentiation.
By moving to higher values on each of these gradients, collectives become more definite reproductive units in their own right. Consider, for example, the evolution of multicellularity in animals. As collections of cells become more cohesive, acquire a germ/soma distinction, and maintain clonality within each collective by reproducing through a bottleneck, animals themselves become more definite Darwinian individuals. The cells within animals of this kind continue to be reproducing objects, but they are partly “de-Darwinized” in this transition by having the variation within each organism so greatly curtailed by the bottleneck stage, by having the consequences of within-organism reproductive differences reduced by germ/soma differentiation, and because each cell's survival and reproduction is determined less and less by intrinsic heritable differences and more by control systems within the organism.
Before moving to the case of the eukaryotic cell, I note one further general distinction. Queller (35) distinguished between two different kinds of biological alliances, each of which can be the basis for an evolutionary transition. He called these “fraternal” and “egalitarian” alliances. In a fraternal alliance, the partners are similar and closely related at the initiation of the alliance. The early advantages of the alliance involve scale, and any significant division of labor arises later, through differentiation of the partners. Kinship between the parts of such a collective is important in reducing conflict. The evolution of multicellularity in animals is a fraternal alliance. An egalitarian alliance, in contrast, takes place between partners with different origins and capacities. The initial advantages involve the bringing together of distinct and complementary abilities, so a division of labor is possible from the start, provided that conflict between the partners can be overcome. Symbiotic associations across different taxa are egalitarian alliances, and the evolution of the eukaryotic cell, according to the endosymbiotic theory, was an egalitarian transition.
The Eukaryotic Cell
This section applies the framework described above to the evolution of the eukaryotic cell, assuming an endosymbiotic origin for mitochondria (and plastids). The previous section discussed reproduction in general as it relates to symbioses, and it presented the transition from marginal to definite forms of collective reproduction. However that discussion of different forms of collective reproduction was guided particularly by the cases of multicellularity and eusociality. How does it apply to the evolution of the eukaryotic cell?
This section does not assume a specific version of the endosymbiotic view but rather some scenario that features the engulfing by an archaeon of a bacterium, giving rise to the mitochondrion. This engulfing may have been a one-time event or a to-and-fro happening that gradually became firmly established. When an endosymbiotic history for the mitochondrion is analyzed, we find a series of shifts in which all the categories described above play a role. In most cases, however, this role is quite different from the role they play in fraternal transitions such as the evolution of multicellularity.
I begin with a quick outline. In an endosymbiotic scenario, the system starts with two simple reproducers (prokaryotes). One engulfs the other, and the result is a collective reproducer with two sets of parent–offspring lineages aligned. This collective becomes more integrated over evolutionary time. Then, at least in many cases, an endosymbiont that started as a simple reproducer moves some of the way toward being a scaffolded reproducer, because it loses the ability to reproduce by means of its own internal machinery. As it does so, the whole cell moves toward being a new simple reproducer, rather than a collective. Hypothetically, an endosymbiont could lose its status as reproducing entity entirely, although it may not be easy for this transition to occur.
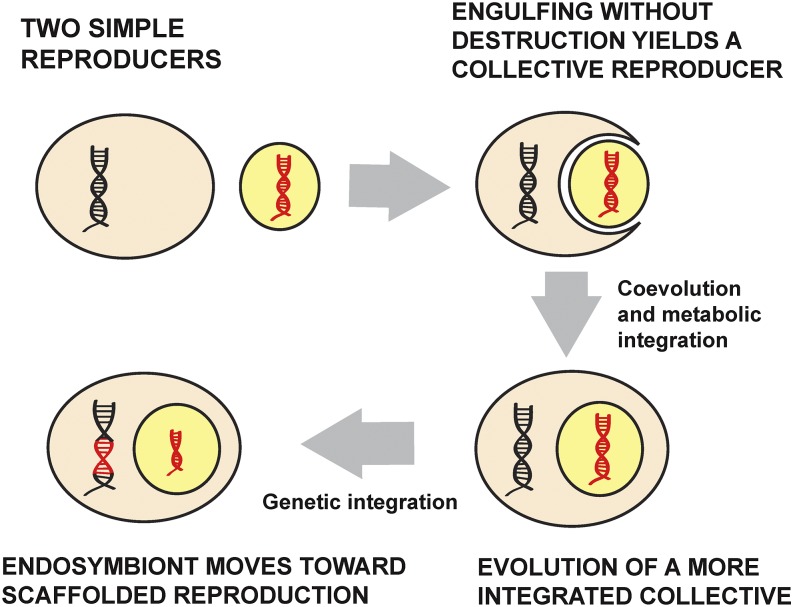
I now discuss each step in more detail (Fig. 2). The process begins with two simple reproducers, one of which engulfs the other. If both persist and reproduce in this new setting, they form a collective reproducer. Initially, however, this collective status pertains only in the most minimal sense: The two simple reproducers and their lineages are linked only by physical containment. They need not show any further coordination or cooperation. Similarly, a harmful parasite might have a reproductive lineage aligned with that of its host if it is able to infect the host's offspring as they are born. The collective (host plus parasite) gives rise to more things of the same kind, but there is no reason to recognize the collective as a reproducing unit, a new kind of individual, in a stronger sense.
Fig. 2.
A breakdown of evolutionary transitions involving endosymbiosis, featuring movement between three kinds of reproduction. A system starts with two simple reproducers. One engulfs the other, yielding a collective reproducer, but initially the collective reproducer is unified only by physical containment. The collective reproducer becomes more metabolically integrated, and eventually, through gene loss, the endosymbiont moves toward scaffolded reproduction.
In the early stages of an endosymbiotic association, then, we have two simple reproducers and a collective in a marginal sense. Eventually, the alliance may become established more firmly. In the previous section I used three features to distinguish marginal cases of collective reproduction from more definite cases: the existence of a bottleneck between the collective's generations, a germ/soma divide, and overall integration. In the case of endosymbionts and the eukaryotic cell, we also find a shift toward a more cohesive reproducing unit, but there is an important difference between this case and the fraternal cases described earlier. In the fraternal cases, the evolution of reproductive cohesion includes a process in which some parts of a collective come to stand in for others in reproduction. Germ cells and queen bees reproduce on behalf of other units in their collective (somatic cells and worker bees, respectively). Queller (35) used the economic term fungible to describe this relation between units in a fraternal alliance. In the case of endosymbionts, in contrast, there is no way for one partner to stand in for another in reproduction; the units are nonfungible. The different capacities of the units are essential to the union, each produces like, and one cannot stand in for another in reproduction.
When a population of endosymbionts exists within a host cell, the factors seen in the fraternal cases are relevant once again. One mitochondrion can reproduce on behalf of another mitochondrion, and one mitochondrion can compete with others in a Darwinian manner. Uniparental inheritance of mitochondria, seen to different degrees in nearly all sexually reproducing multicellular organisms, is widely viewed as an adaptive feature that reduces diversity in the mitochondria within each organism, preventing Darwinian competition that might have adverse effects on the organism (36). Even with uniparental mitochondrial inheritance, evolutionary conflict can arise: High mitochondrial mutation rates make mosaicism common, and hence evolution is possible in a mitochondrial population. Deletions in mitochondrial genomes may lead to a selective advantage through faster replication. A range of mechanisms has been described in mammals that can reduce transmission of deleterious mtDNA mutations from one generation to the next, including a bottleneck that enables a mother with low levels of a pathogenic mitochondrial mutation to produce offspring lacking the mutant (37).
These factors, familiar from the fraternal transitions, are distinct from those that affect the basic mitochondria–host relationship, in which the units in the alliance are nonfungible and initially neither party can reproduce on behalf of the other. Of the three factors I described earlier that bear on the evolution of collective reproduction (bottleneck, germ/soma, integration), only integration plays an essential role. The situation then might be described as one in which even a very cooperative symbiotic union lacks some important marks of collective reproduction and hence is a less definite Darwinian individual, at least until the later stages of the transition described below. Alternatively, it could be argued that bottlenecks and germ/soma differentiation simply are not relevant in the case of egalitarian alliances, so their absence does not affect the status of a collective. Whichever attitude is taken, integration is the most directly relevant of the three features discussed above.
Integration is a broad term that refers to a range of ways in which parts of a collective can come to work together, become mutually dependent in their activities, and divide labor. If an endosymbiont establishes a stable relationship inside a host cell, one outcome may be the evolution of coadapted traits on each side of the partnership, making possible effective metabolic cooperation. As the association continues, however, a form of integration that is specific to these endosymbiotic cases may arise: transfer of genes from endosymbiont to host nucleus. This transfer is seen to different degrees but has occurred in all mitochondrial lineages, with the result that mitochondria have genomes that are greatly reduced from that of their presumed bacterial ancestor. What is referred to as “transfer” of genes involves a combination of physical transfer of DNA by the lysing of mitochondria and incorporation of their genetic material in the nuclear genome, and (probably more often) a coevolutionary loss of mitochondrial genes and evolution of new nuclear genes, without physical transfer (37).
When an endosymbiont begins to lose genes to the host nucleus that are responsible for gene products that go into making a new functioning mitochondrion, this loss is a transition toward scaffolded reproduction on the part of the mitochondrion. In the initial state, an existing mitochondrion gives rise to new mitochondria by fission: Each mitochondrion has a determinate mother. (Mitochondria also fuse, making parent–offspring relations more complex in some cases; 38) Once the process of gene transfer has traveled some distance, fission events still occur, but each new mitochondrion is brought into being in a way that depends on physically external machinery, a way that is not seen in the free-living state or in the earlier stages after absorption. A new mitochondrion contains parts that are not derived from parts in its mother mitochondrion and are not built from raw materials by the new mitochondrion at earlier stages, but that instead are synthesized outside the mitochondrion with the aid of the nuclear genome. Being a scaffolded reproducer in this way is not similar to being a somatic cell in an animal or a member of a sterile caste in a eusocial insect colony. Those somatic cells and sterile eusocial insects become unable to reproduce at all (or, in the case of a somatic cell, have a reproductive dead end imposed). A scaffolded reproducer, in contrast, may be the ancestor of a long line of descendants, but its reproduction is achieved externally.
Simple reproduction and scaffolded reproduction are related by a continuum that reflects how much of this loss has occurred and how dependent reproduction is on external structures. When a mitochondrial genome is highly reduced, and mitochondrial reproduction depends greatly on nuclear genes and their products, the mitochondrion is closer to being a scaffolded reproducer. In some eukaryotes the mitochondrial genome has been lost entirely (although the resulting organelle functions differently from other mitochondria), and in some other cases as few as three protein-coding genes remain (39). When, as in these cases, an organelle is clearly a scaffolded reproducer, the eukaryotic cell as a whole is a simple reproducer. However, there are no sharp cut-offs between these categories.
This kind of scaffolded reproduction, unlike some others (e.g., viral reproduction), curtails the capacity for independent evolution of the endosymbiont. Specifically, heritability from one endosymbiont to another is reduced. Earlier I said that in many evolutionary transitions in individuality, the preexisting units that make up the new collective are de-Darwinized to some extent; that is, their evolutionary capacities are curtailed by the events that give rise to the higher-level individual. In the eukaryotic cell this reduction in evolutionary capacity is brought about by the dependence of mitochondria on nuclear genes. Endosymbionts may come to be dependent on structures other than the host nucleus, as well. In the cryptomonad algae Guillardia theta, four genomes are present, resulting from a series of endosymbiotic events including the engulfing of one eukaryote by another. The nucleus of the engulfed eukaryote lives on in massively reduced form, as a nucleomorph. So these cells contain the genomes of a host nucleus, nucleomorph, chloroplast, and mitochondrion. The chloroplast requires proteins synthesized by its own genome, by the nucleomorph's genome, and by that of the host nucleus (40).
I have described a shift from simple to scaffolded reproduction through gene loss by an endosymbiont. Consider a state that might be reached at the far end of this process. It is possible in principle for an organelle to become a recurring structure that does not reproduce at all (Fig. 1) but instead has new instances made by the whole cell. It could become something more like a heart—an organ in the familiar sense—or a ribosome. This result requires the loss of lineages that would enable us to single out, from all the preexisting objects of the same kind, one or a small number of parents of a newly formed organelle. Once there are no parent–offspring lineages, questions of heritability and fitness do not arise, and the organelle is not a Darwinian individual at all. However, for an endosymbiotically derived organelle to lose its status as a Darwinian individual entirely, it is not enough for all its genes to be lost, as apparently has happened with mitochondria in some cases. In mitochondria and similar entities that arise by fission, the membranes, as well as genes (and perhaps other structures), are a basis for parent–offspring lineages. Each mitochondrion has a single parent with respect to its membrane, even if it received many of its proteins from elsewhere. Thus it is hard for an endosymbiont to follow the complete path from simple reproducer to scaffolded reproducer to reconstructed entity that does not reproduce. As long as membranes are important in organelle function, and new organelle membranes are made by fission of a preexisting membrane, each membrane-bound organelle instance will have a unique parent instance. However, even if the parent–offspring lineage remains intact, as more and more genes are lost from an endosymbiont, fewer and fewer of the important properties of each new instance will derive from the parent. The causal role of the parent can be reduced very far, even though the lineage remains intact. This reduced role of the parent, again, will be reflected in low heritability of traits between parent and offspring.
Concluding Discussion
The preceding analysis of symbiosis and the eukaryotic cell uses a framework that is motivated by the need for a general treatment of reproduction. That treatment, in turn, is motivated by evolutionary theory itself, whose central models imply that all entities that can reproduce may evolve by natural selection. A closer look at biological reproduction shows that it has three forms: simple, collective, and scaffolded. These three forms are unified by their generation of parent–offspring lineages and differ in their relation to biological mechanisms and their roles in evolution. Each of the three categories figures at different stages in the evolutionary transition that yielded the eukaryotic cell. An initial situation with two simple reproducers gives rise to collective reproduction, followed by a loss of reproductive autonomy and the endosymbiont moving toward scaffolded reproduction. The roles of the three forms of reproduction seen here differ significantly from their roles in a fraternal evolutionary transition, such as the evolution of multicellularity in animals. A central difference is the role of scaffolded reproduction in the egalitarian transition. Scaffolded reproduction is unified by the contribution made by external machinery in the reproduction of a structure, but it can arise by different paths, takes diverse forms, and, as evidenced by the case of mitochondria, is distinguished from other kinds of reproduction by degree.
Eventually, an endosymbiont might become a recurring object that does not reproduce at all, like a ribosome, if it came to be reconstructed in a way that did not preserve a parent–offspring lineage from mother instances to daughters. This last event would require the loss not only of genes but also of other determinants of a parent–offspring lineage. This final transition to a recurring but nonreproducing object may not be likely, because membranes are a basis for parent–offspring relations and also play important biological roles. If so, symbiotically derived organelles will tend to remain reproducers of some sort. Mitochondria in various taxa are now at different places on a road between simple and scaffolded reproduction; hence eukaryotic cells in these taxa are more like collectives in some cases and are closer to simple reproducers (albeit very elaborate ones) in others. This mixed status has further effects when a collection of these eukaryotic cells gives rise to a multicellular organism, in another evolutionary transition.
Acknowledgments
I thank Maureen O'Malley, Austin Booth, two anonymous referees, and the participants at the 2014 Sackler Symposium “Symbioses Becoming Permanent: The Origins and Evolutionary Trajectories of Organelles” for discussion and assistance with biological examples and Ford Doolittle for assistance with Fig. 2.
Footnotes
The author declares no conflict of interest.
This paper results from the Arthur M. Sackler Colloquium of the National Academy of Sciences, “Symbioses Becoming Permanent: The Origins and Evolutionary Trajectories of Organelles,” held October 15–17, 2014, at the Arnold and Mabel Beckman Center of the National Academies of Sciences and Engineering in Irvine, CA. The complete program and video recordings of most presentations are available on the NAS website at www.nasonline.org/Symbioses.
This article is a PNAS Direct Submission.
References
- 1.Sagan L. On the origin of mitosing cells. J Theor Biol. 1967;14(3):255–274. doi: 10.1016/0022-5193(67)90079-3. [DOI] [PubMed] [Google Scholar]
- 2.Gray MW, Doolittle WF. Has the endosymbiont hypothesis been proven? Microbiol Rev. 1982;46(1):1–42. doi: 10.1128/mr.46.1.1-42.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kutschera U, Niklas KJ. Endosymbiosis, cell evolution, and speciation. Theory Biosci. 2005;124(1):1–24. doi: 10.1016/j.thbio.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Archibald J. One Plus One Equals One: Symbiosis and the Evolution of Complex Life. Oxford Univ Press; Oxford, UK: 2014. [Google Scholar]
- 5.Maynard Smith J, Szathmáry E. The Major Transitions in Evolution. Oxford Univ Press; Oxford, UK: 1995. [Google Scholar]
- 6.Dawkins R. The Extended Replicator: The Long Reach of the Gene. Oxford Uni Press; Oxford, UK: 1982. [Google Scholar]
- 7.Price GR. Selection and covariance. Nature. 1970;227(5257):520–521. doi: 10.1038/227520a0. [DOI] [PubMed] [Google Scholar]
- 8.Lewontin RC. The units of selection. Annu Rev Ecol Syst. 1970;1:1–18. [Google Scholar]
- 9.Lewontin RC. Adaptation. In: Levins R, Lewontin RC, editors. The Dialectical Biologist. Harvard Univ Press; Cambridge, MA: 1985. [Google Scholar]
- 10.Godfrey-Smith P. Conditions for evolution by natural selection. J Philos. 2007;104(10):489–516. [Google Scholar]
- 11.Godfrey-Smith P. Darwinian Populations and Natural Selection. Oxford Univ Press; Oxford, UK: 2009. [Google Scholar]
- 12.Dawkins R. The Selfish Gene. Oxford Univ Press; Oxford, UK: 1976. [Google Scholar]
- 13.Hull DL. Individuality and selection. Annu Rev Ecol Syst. 1980;11:311–332. [Google Scholar]
- 14.Price GR. Extension of covariance selection mathematics. Ann Hum Genet. 1972;35(4):485–490. doi: 10.1111/j.1469-1809.1957.tb01874.x. [DOI] [PubMed] [Google Scholar]
- 15.Rice SH. Evolutionary Theory: Mathematical and Conceptual Foundations. Sinauer; Sunderland, MA: 2004. [Google Scholar]
- 16.Kerr B, Godfrey-Smith P. Generalization of the Price equation for evolutionary change. Evolution. 2009;63(2):531–536. doi: 10.1111/j.1558-5646.2008.00570.x. [DOI] [PubMed] [Google Scholar]
- 17.Frank SA. Natural selection. IV. The Price equation. J Evol Biol. 2012;25(6):1002–1019. doi: 10.1111/j.1420-9101.2012.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Griesemer J. The informational gene and the substantial body: On the generalization of evolutionary theory by abstraction. In: Jones M, Cartwright C, editors. Idealization XII: Correcting the Model, Idealization and Abstraction in the Sciences. Rodopi; Amsterdam: 2005. [Google Scholar]
- 19.Beebee H, Hitchcock C, Menzies P. Oxford Handbook of Causation. Oxford Univ Press; Oxford, UK: 2009. [Google Scholar]
- 20.Booth A. Symbioses, selection, and individuality. Biol Philos. 2014;29(5):657–673. [Google Scholar]
- 21.Harmer TL, et al. Free-living tube worm endosymbionts found at deep-sea vents. Appl Environ Microbiol. 2008;74(12):3895–3898. doi: 10.1128/AEM.02470-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bright M, Lallie FH. The biology of vestimentiferan tubeworms. Oceanogr Mar Biol. 2010;48:213–226. [Google Scholar]
- 23.Dupré J, O’Malley MA. Varieties of living things: Life at the intersection of lineage and metabolism. Philos Theor Biol. 2009;1:1–24. [Google Scholar]
- 24.Pradeu T. The Limits of the Self: Immunology and Biological Identity. Oxford Univ Press; New York: 2012. [Google Scholar]
- 25.Janzen D. Coevolution of mutualism between ants and acacias in Central America. Evol. 1966;20(3):249–275. doi: 10.1111/j.1558-5646.1966.tb03364.x. [DOI] [PubMed] [Google Scholar]
- 26.Queller DC, Strassmann JE. Beyond society: The evolution of organismality. Philos Trans R Soc Lond B Biol Sci. 2009;364(1533):3143–3155. doi: 10.1098/rstb.2009.0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baumann P, et al. Genetics, physiology, and evolutionary relationships of the genus Buchnera: Intracellular symbionts of aphids. Annu Rev Microbiol. 1995;49:55–94. doi: 10.1146/annurev.mi.49.100195.000415. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto N, Inouye I. A secondary symbiosis in progress? Science. 2005;310(5746):287. doi: 10.1126/science.1116125. [DOI] [PubMed] [Google Scholar]
- 29.Dal Grande F, Widmer I, Wagner HH, Scheidegger C. Vertical and horizontal photobiont transmission within populations of a lichen symbiosis. Mol Ecol. 2012;21(13):3159–3172. doi: 10.1111/j.1365-294X.2012.05482.x. [DOI] [PubMed] [Google Scholar]
- 30.Michod R. Darwinian Dynamics: Evolutionary Transitions in Fitness and Individuality. Princeton Univ Press; Princeton, NJ: 2000. [Google Scholar]
- 31.Okasha S. Evolution and the Levels of Selection. Oxford Univ Press; Oxford, UK: 2006. [Google Scholar]
- 32.Clarke E. In: Plant individuality and multilevel selection theory. The Major Transitions Revisited. Calcott B, Sterelny K, editors. MIT Press; Cambridge, MA: 2011. pp. 227–251. [Google Scholar]
- 33.Herron MD, Rashidi A, Shelton DE, Driscoll WW. Cellular differentiation and individuality in the ‘minor’ multicellular taxa. Biol Rev Camb Philos Soc. 2013;88(4):844–861. doi: 10.1111/brv.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonner JT. On Development: The Biology of Form. Harvard Univ Press; Cambridge, MA: 1974. [Google Scholar]
- 35.Queller DC. Cooperators since life began. Q Rev Biol. 1997;72(20):184–188. [Google Scholar]
- 36.Birky CW., Jr Uniparental inheritance of mitochondrial and chloroplast genes: Mechanisms and evolution. Proc Natl Acad Sci USA. 1995;92(25):11331–11338. doi: 10.1073/pnas.92.25.11331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart JB, Larsson NG. Keeping mtDNA in shape between generations. PLoS Genet. 2014;10(10):e1004670. doi: 10.1371/journal.pgen.1004670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen H, Chan DC. Mitochondrial dynamics in mammals. Curr Top Dev Biol. 2004;59:119–144. doi: 10.1016/S0070-2153(04)59005-1. [DOI] [PubMed] [Google Scholar]
- 39.Adams KL, Palmer JD. Evolution of mitochondrial gene content: Gene loss and transfer to the nucleus. Mol Phylogenet Evol. 2003;29(3):380–395. doi: 10.1016/s1055-7903(03)00194-5. [DOI] [PubMed] [Google Scholar]
- 40.Curtis BA, et al. Algal genomes reveal evolutionary mosaicism and the fate of nucleomorphs. Nature. 2012;492(7427):59–65. doi: 10.1038/nature11681. [DOI] [PubMed] [Google Scholar]