Significance
IFN-γ is a proinflammatory cytokine and stimulates induction of ∼2,000 genes, including IFN-γ–inducible GTPases, such as immunity-related GTPases (IRGs) and guanylate-binding proteins (GBPs), that are critically required for cell-autonomous host defense against the vacuolar pathogen Toxoplasma gondii. Mechanisms of how recruitment of these GTPases to the vacuoles is positively regulated have been gradually elucidated. However, the negative regulation remains unknown. Here, we show that Rab GDP dissociation inhibitor α (RabGDIα) acts as a suppressor of IFN-γ–inducible GTPases, such as Gbp2 and Irga6. RabGDIα deficiency resulted in enhanced IFN-γ–mediated T. gondii clearance in vitro and in vivo. Furthermore, RabGDIα inhibited the act of Gbp2 and Irga6 through the lipid-binding pocket. Thus, our current study demonstrates a negative regulatory mechanism for IFN-γ–inducible GTPase-dependent cell-autonomous immunity.
Keywords: IFN-γ–inducible GTPase, cell-autonomous immunity, negative regulation, Toxoplasma gondii
Abstract
IFN-γ orchestrates cell-autonomous host defense against various intracellular vacuolar pathogens. IFN-γ–inducible GTPases, such as p47 immunity-related GTPases (IRGs) and p65 guanylate-binding proteins (GBPs), are recruited to pathogen-containing vacuoles, which is important for disruption of the vacuoles, culminating in the cell-autonomous clearance. Although the positive regulation for the proper recruitment of IRGs and GBPs to the vacuoles has been elucidated, the suppressive mechanism is unclear. Here, we show that Rab GDP dissociation inhibitor α (RabGDIα), originally identified as a Rab small GTPase inhibitor, is a negative regulator of IFN-γ–inducible GTPases in cell-autonomous immunity to the intracellular pathogen Toxoplasma gondii. Overexpression of RabGDIα, but not of RabGDIβ, impaired IFN-γ–dependent reduction of T. gondii numbers. Conversely, RabGDIα deletion in macrophages and fibroblasts enhanced the IFN-γ–induced clearance of T. gondii. Furthermore, upon a high dose of infection by T. gondii, RabGDIα-deficient mice exhibited a decreased parasite burden in the brain and increased resistance in the chronic phase than did control mice. Among members of IRGs and GBPs important for the parasite clearance, Irga6 and Gbp2 alone were more frequently recruited to T. gondii-forming parasitophorous vacuoles in RabGDIα-deficient cells. Notably, Gbp2 positively controlled Irga6 recruitment that was inhibited by direct and specific interactions of RabGDIα with Gbp2 through the lipid-binding pocket. Taken together, our results suggest that RabGDIα inhibits host defense against T. gondii by negatively regulating the Gbp2–Irga6 axis of IFN-γ–dependent cell-autonomous immunity.
IFN-γ is an important T-helper 1 (Th1) cytokine that inhibits the survival and growth of a wide range of intracellular pathogens, such as viruses, bacteria, and parasites (1). Stimulation of innate immune cells, such as macrophages, by IFN-γ up-regulates almost 2,000 effector genes encoding various IFN-γ–inducible proteins, including immunity-related GTPases such as the MX proteins, p47 immunity-related GTPases (IRGs), and p65 guanylate-binding proteins (GBPs) (2). MX proteins and GBPs have been shown to restrict replication of viruses (3). Moreover, IRGs and GBPs play roles in host defense against vacuole-forming bacteria, including Salmonella, Chlamydia, Mycobacteria, and Listeria, by induction of antibacterial responses involving autophagic effectors, inflammasomes, and phagocytic oxidases (4–6).
Not only viruses and bacteria but also the vacuolar parasite Toxoplasma gondii is targeted by IFN-γ–inducible GTPases. T. gondii is an obligatory protozoan parasite that causes a life-threatening toxoplasmosis in humans and animals (7). After the active invasion of host cells, T. gondii forms a nonfusogenic cytoplasmic membranous structure called the parasitophorous vacuole (PV), in which the parasite efficiently proliferates (8, 9). In terms of cellular host defense against T. gondii, interleukin-12 (IL-12) is mainly produced from macrophages and dendritic cells, in which Toll-like receptors and the chemokine receptor CCR5 recognize T. gondii-derived ligands. Also, IL-12 promotes development of IFN-γ–producing Th1 cells (10–15). IFN-γ is critically required for suppression of T. gondii replication inside PVs and cell-autonomous clearance. Nitric oxide that is produced by inducible nitric oxide synthase (iNOS) in the infected cells mainly inhibits the replication (16, 17). On the other hand, T. gondii survival within infected cells is suppressed by cooperative action between IRGs and GBPs (18). Indeed, various types of cells (such as macrophages, fibroblasts, and astrocytes) derived from mice lacking IRGs [such as Irgm1 (also known as LRG-47), Irgm3 (IGTP), and Irga6 (IIGP1)] or GBPs [such as Gbp1, Gbp2, and a cluster of GBPs on murine chromosome 3 (GBPchr3; Gbp1, Gbp2, Gbp3, Gbp5, and Gbp7)] were defective for IFN-γ–mediated intracellular killing of T. gondii (19–25). After the formation of PVs, GBPs and a subfamily of IRG members called GKS-IRGs [such as Irga6, Irgb6 (TGTP), and Irgb10] are shown to accumulate on PV membrane (PVM) and oligomerize dependently on GTP binding to destroy PV membrane integrity and structure (26, 27), resulting in cell-autonomous clearance by intracellular digestive pathways (20, 21, 28). The IFN-γ–mediated clearance by these GTPases is T. gondii strain-specific. Most T. gondii in North America and Europe belong to type I, type II, and type III (29). Virulent type I strain inactivates IFN-γ–inducible GTPases by effectors, such as ROP18 and ROP5, during the parasite infection (30). On the other hand, avirulent type II and type III strains are susceptible to IFN-γ–dependent clearance due to polymorphisms or reduced expression of the effectors (31–34).
The regulatory mechanism of how IFN-γ–induced GTPases are recruited to PVs has gradually been elucidated. In the absence of essential autophagy-related proteins Atg3, Atg5, Atg7, and Atg16L1 and of another subfamily of IRGs called GMS-IRGs, such as Irgm1 and Irgm3, the recruitment of IFN-γ–inducible GTPases and the killing of T. gondii are severely impaired (35–39). Thus, Atg3/Atg5/Atg7/Atg16L1 and Irgm1/Irgm3 are required for proper targeting of GKS-IRGs and GBPs to T. gondii PVM and play positive roles in the cell-autonomous resistance to the pathogen. On the other hand, the inhibitory mechanism for the IFN-γ–inducible GTPase-dependent immunity remains unclear.
To explore the molecular mechanism to control the action of IFN-γ–inducible GTPases, we have attempted to identify binding partners of Gbp2 because a single deletion of Gbp2 in mice has been shown to result in impaired in vitro and in vivo resistance to type II T. gondii (22). In the present study, we identify Rab GDP dissociation inhibitor α (RabGDIα) as a Gbp2-interacting protein. We have an interest in this protein for two reasons: One is because RabGDIα has been shown to participate in the regulation of Rab proteins, which, like GBPs, belong to another family of GTPases (40, 41), and the other is because we demonstrate that overexpression of RabGDIα in cells impairs IFN-γ–induced reduction of T. gondii numbers. We have tested whether RabGDIα acts as a regulator of IFN-γ–inducible GTPases under physiological conditions. Macrophages and fibroblasts from RabGDIα-deficient mice exhibit enhanced IFN-γ–dependent clearance of T. gondii. Moreover, the enhanced clearance by RabGDIα deficiency is accompanied by increased recruitment of Irga6 and Gbp2 to the parasite. Notably, Gbp2 is required for Irga6 recruitment, which is suppressed by direct and specific interactions of RabGDIα with Gbp2 through a lipid-binding pocket. Furthermore, a high dose of type II T. gondii infection in RabGDIα-deficient mice results in increased resistance, which is characterized by a decreased parasite burden in the brain. Taken together, our data indicate that RabGDIα plays a negative role in the Gbp2–Irga6 axis of IFN-γ–inducible GTPase-dependent cell-autonomous resistance to T. gondii.
Results
RabGDIα, but Not RabGDIβ, Associates with Gbp2.
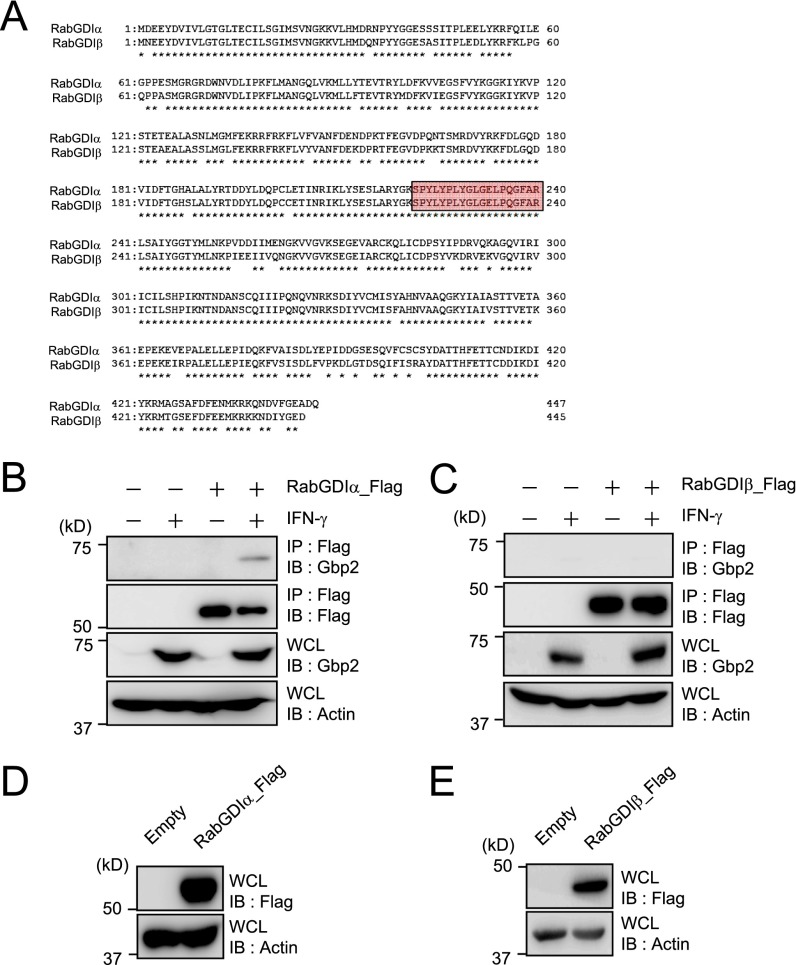
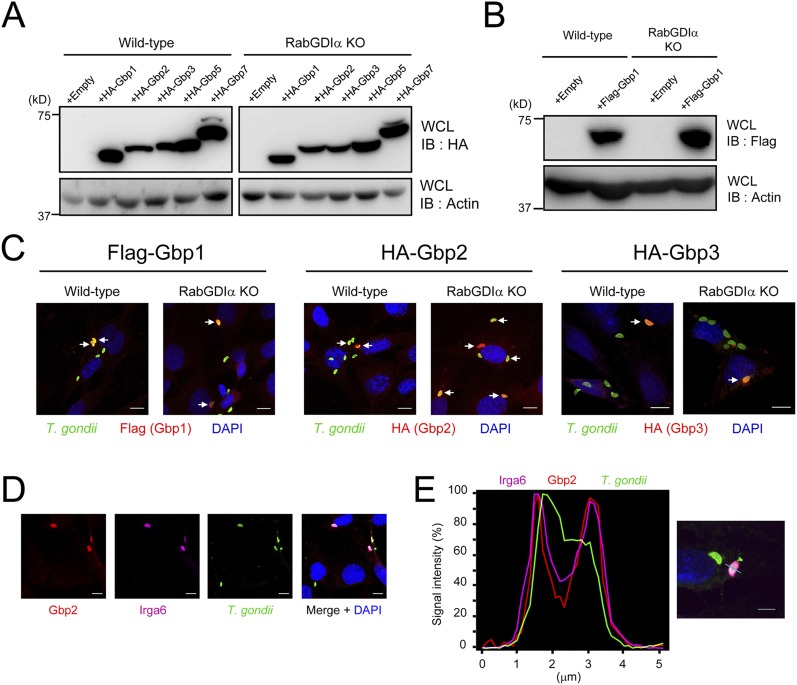
To elucidate the molecular mechanisms of Gbp2-dependent host defense against type II T. gondii, which is susceptible to Gbp2-dependent cell-autonomous immunity (22), we attempted to identify binding partners of Gbp2. Immunoprecipitants of Flag-tagged Gbp2 in IFN-γ–stimulated macrophages infected with type II T. gondii were submitted for mass spectrometry analysis. We recovered a peptide fragment shared by RabGDIα and RabGDIβ that functioned as a Rab small GTPase (Rabs) inhibitor (Fig. S1A and Table S1) (42). Gdi1 and Gdi2 genes encode RabGDIα and RabGDIβ, respectively. An immunoprecipitation assay to assess whether Gbp2 associated with RabGDIα and/or RabGDIβ demonstrated that Flag-tagged RabGDIα, but neither RabGDIβ nor Gbp1, coprecipitation with endogenous Gbp2 was dependent on IFN-γ (Fig. 1A and Fig. S1 B and C).
Fig. S1.
Identification of RabGDIα as a Gbp2-interacting protein. (A) The sequences for RabGDIα and RabGDIβ are shown on the first and the second line, respectively. An asterisk indicates the identity of the amino acid sequence. The portion with the red square was recovered in the mass spectrometric analysis. (B and C) MEFs overexpressing RabGDIα (B) or RabGDIβ (C) untreated or treated with 10 ng/mL IFN-γ were lysed. The lysates were immunoprecipitated with anti-Flag and detected by protein immunoblot with the indicated antibodies. (D and E) Lysates of MEFs stably transfected with retroviral vectors encoding the Flag-tagged RabGDIα (D) or RabGDIβ (E) were subjected to Western blot using indicated Abs. Data are representative of two independent experiments (B–E).
Table S1.
Primers used in this study
| Primer name | Enzyme | Sequence | Resulting plasmids and descriptions |
| RabGDIα_F | BamHI | 5′-GGATCCACCATGGATGAGGAATACGATGTGATTG-3′ | pRabGDIα_Flag |
| RabGDIα_R | XhoI | 5′-GCGGCCGCTCACTCGAGCTGATCAGCTTCTCCAAAGACATCA-3′ | pRabGDIα_Flag |
| RabGDIα_M132I_F | — | 5′-AAGATCTACAAAGTGCCATCCACCGAGACTGAGGCCTTGGCTTCTAATCTGATCGGCATG-3′ | pRabGDIα_Μ132Ι_Flag |
| RabGDIα_M132I_R | — | 5′-CGGTGGATGGCACTTTGTAGATCTTG-3′ | pRabGDIα_Μ132Ι_Flag |
| RabGDIα_Y39V_R | — | 5′-AAGAGCTCTCACCCCCAACGTAGGGGTTT-3′ | pRabGDIα_Y39V_Flag |
| RabGDIα_R218A/Y219A_F | — | 5′-AGCGAGTCCCTGGCCGCGGCTGGCAAGAGCCCCTAT-3′ | pRabGDIα_R218A/Y219A_Flag |
| RabGDIα_R218A/Y219A_R | — | 5′-ATAGGGGCTCTTGCCAGCCGCGGCCAGGGACTCGCT-3′ | pRabGDIα_R218A/Y219A_Flag |
| RabGDIα_E233S/R240A_F | — | 5′-GGCCTGGGTTCGCTGCCCCAGGGCTTTGCCGCATTGAGTGCC-3′ | pRabGDIα_E233S/R240A_Flag |
| RabGDIα_E233S/R240A_R | — | 5′-GGCACTCAATGCGGCAAAGCCCTGGGGCAGCGAACCCAGGCC-3′ | pRabGDIα_E233S/R240A_Flag |
| RabGDIβ_F | BamHI | 5′-GGATCCACCATGAATGAGGAATACGACGTGATCGTGCTGGGC-3′ | pRabGDIβ_Flag |
| RabGDIβ_R | XhoI | 5′-GCGGCCGCTTACTCGAGGTCTTCTCCATAAATGTCATTCTTCTTGCGC-3′ | pRabGDIβ_Flag |
| Gbp1_F | BamHI | 5′-GGATCCACCATGGCCTCAGAAATCCACATGAAAG-3′ | pHA_Gbp1 |
| Gbp1_R | NotI | 5′-GCGGCCGCTTAAAGTATGGTGCATGATCGAGGTGG-3′ | pHA_Gbp1 |
| Gbp2_F | BglII | 5′-AGATCTACCATGGCCTCAGAGATCCACATGTCG-3′ | pHA_Gbp2 |
| Gbp2_R | NotI | 5′-GCGGCCGCTCAGAGTATAGTGCACTTCCCAGACG-3′ | pHA_Gbp2 |
| Gbp2_C586S_R | NotI | 5′-GCGGCCGCTCAGAGTATAGTGCTCTTCCCAGACG-3′ | pHA_Gbp2_C586S |
| Gbp2_K51A_R | — | 5′-CAGCTAGCTTGTTCATCAGGTAGGATGCGCCTGTGCGGTAGAGGCCCACG-3′ | pHA_Gbp2_K51A |
| Gbp3_F | BamHI | 5′-GGATCCACCATGGAGGCACCCATTTGTCTGGTGGAA-3′ | pHA_Gbp3 |
| Gbp3_R | NotI | 5′-GCGGCCGCCTAACTACTTAGTGAGCCGAGG-3′ | pHA_Gbp3 |
| Gbp5_F1 | EcoRI | 5′-GAATTCACCATGGCCCCAGAGATTCACATGCC-3′ | pHA_Gbp5 |
| Gbp5_R1 | — | 5′-CATCTGATGTGATGGCATGCCCATTGG-3′ | pHA_Gbp5 |
| Gbp5_F2 | — | 5′-CCAATGGGCATGCCATCACATCAGATG-3′ | pHA_Gbp5 |
| Gbp5_R2 | NotI | 5′-GCGGCCGCTTAGCTTATAACACAGTCATGATG-3′ | pHA_Gbp5 |
| Gbp7_F | BamHI | 5′-GGATCCACCATGGCATCTGGTCCCAACATGGAG-3′ | pHA_Gbp7 |
| Gbp7_R | NotI | 5′-GCGGCCGCTTAGAGTTTTCTAACTTTGTCTGAA-3′ | pHA_Gbp7 |
| Rab10_F | EcoRI | 5′-GAATTCATGGCGAAGAAGACGTACGACCTG-3′ | pHA_Rab10 |
| Rab10_R | NotI | 5′-GCGGCCGCTCAGCAGCACTTGCTCTTCCAGCC -3′ | pHA_Rab10 |
| RabGDIαKO_SA_F | SacII | 5′-CCGCGGCTTCGTGTCACTCATTTCTGCACTG-3′ | pKO-RabGDIα |
| RabGDIαKO_SA_R | SalI | 5′-GTCGACGTACTAGAGTGCAGGAACACAGGAC-3′ | pKO-RabGDIα |
| RabGDIαKO_MA_F | XhoI | 5′-CTCGAGTCCTGTGTTCCTGCACTCTAGTAC-3′ | pKO-RabGDIα |
| RabGDIαKO_MA_R | BamHI | 5′-GGATCCGTAAGGGTCCTCTAGGACTACACAG-3′ | pKO-RabGDIα |
| RabGDIαKO_LA_F | MluI | 5′-ACGCGTCTGTGTAGTCCTAGAGGACCCTTAC-3′ | pKO-RabGDIα |
| RabGDIαKO_LA_R | NotI | 5′-GCGGCCGCGATATGCAAGTTGTGCACACCCACC-3′ | pKO-RabGDIα |
| RabGDIα_screening_F | — | 5′-ATGCAGAGTCCGGTATCCGATCCAT-3′ | PCR screening |
| RabGDIα_WT_R | — | 5′-CACCTCGGTGATAGAGATAGGGAAG-3′ | PCR screening |
| RabGDIα_KO_R | — | 5′-TACGTGCCAGCCATCTTAGAGATACAG-3′ | PCR screening |
| RabGDIβ_WT_F | — | 5′-GATGCCATTGCTGATACCTGGACTG-3′ | PCR screening |
| RabGDIβ_WT_R | — | 5′-GAGTGAAATACCACTCTGATGGCTGG-3′ | PCR screening |
| RabGDIβ_KO_F | — | 5′-CTGTCGCTGTCTTCAAATGCACCAG-3′ | PCR screening |
| RabGDIβ_KO_R | — | 5′-GCCTGGCGCACTATTCAATACCATC-3′ | PCR screening |
| RabGDIα_qpF | — | 5′-GCTGATCTGTGATCCCAGTTACATCCC-3′ | Quantitative RT-PCR |
| RabGDIα_qpR | — | 5′-TTCTGCAGTCTCTACAGTGGTGCTGG-3′ | Quantitative RT-PCR |
| Gdi2_gRNA1_F | — | 5′-TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCgcttttctaagcattcacta-3′ | Gdi2_gRNA1 |
| Gdi2_gRNA1_R | — | 5′-GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACtagtgaatgcttagaaaagc-3′ | Gdi2_gRNA1 |
| Gdi2_gRNA2_F | — | 5′-TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCgtttgacataaacacgcccc-3′ | Gdi2_gRNA2 |
| Gdi2_gRNA2_R | — | 5′-GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACggggcgtgtttatgtcaaac-3′ | Gdi2_gRNA2 |
| Gbp2_gRNA1_F | — | 5′-TTTCTTGGCTTTATATATCTTGTGGAAAGGACGAAACACCgtcatcacctgaccagagtg-3′ | Gbp2_gRNA1 |
| Gbp2_gRNA1_R | — | 5′-GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAACcactctggtcaggtgatgac-3′ | Gbp2_gRNA1 |
| Gdi2_T7gRNA1_F | — | 5′-TTAATACGACTCACTATAGGgcttttctaagcattcactaGTTT-3′ | Gdi2_gRNA1 |
| Gdi2_T7gRNA2_F | — | 5′-TTAATACGACTCACTATAGGgtttgacataaacacgccccGTTT -3′ | Gdi2_gRNA2 |
| Gbp2_T7gRNA2_F | — | 5′-TTAATACGACTCACTATAGGgtcatcacctgaccagagtgGTTT-3′ | Gbp2_gRNA1 |
| gRNA_common_R | — | 5′-AAAAGCACCGACTCGGTGCCACTTTT-3′ | Gdi2_gRNA1, Gdi2_gRNA2, Gbp2_gRNA1 |
| T7Cas9_IVT_F | — | 5′-taatacgactcactatagggagaATGGACAAGAAGTACTCCATT-3′ | Cas9 mRNA |
| Cas9_R | — | 5′-ggatccGCTATGGCAGGGCCTGCCGCCCCGA -3′ | Cas9 mRNA |
| Gbp2_indel_F | — | 5′-catagggatggcaccacatgttgtg-3′ | Screening the mutation of the Gbp2 locus |
| Gbp2_indel_R | — | 5′-aggagtccaagtgattctgctaagc-3′ | Screening the mutation of the Gbp2 locus |
—, no restriction enzyme sites in primers.
Fig. 1.
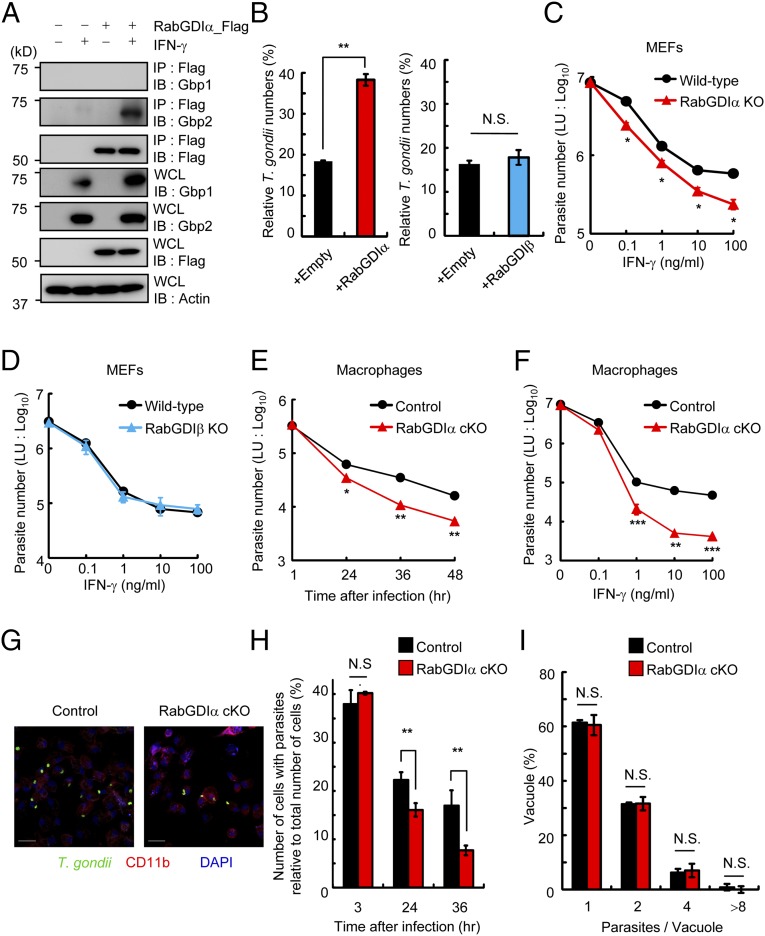
Enhanced IFN-γ–dependent T. gondii clearance in RabGDIα-deficient cells. (A) MEFs stably transfected with empty or Flag-tagged RabGDIα expression plasmids were untreated or treated with IFN-γ and lysed. The lysates were immunoprecipitated with anti-Flag and detected with the indicated Abs by Western blot. (B) T. gondii numbers at 36 h postinfection in control and MEFs overexpressing RabGDIα (Left) or RabGDIβ (Right) untreated or treated with IFN-γ were analyzed by luciferase assay. Percentages of parasite numbers (calculated by luciferase counts) in IFN-γ–stimulated control or cells overexpressing RabGDIα or RabGDIβ relative to those in the unstimulated respective cells are shown as “Relative T. gondii numbers.” (C and D) MEFs lacking RabGDIα (C) or RabGDIβ (D), and WT cells were untreated or treated with the indicated concentrations of IFN-γ. Untreated or IFN-γ–treated cells were infected with ME49 T. gondii expressing luciferase [multiplicity of infection (moi) = 1] and harvested at 36 h postinfection. The luciferase units (LUs) were assayed with the lysates. Error bars represent means ± SD of triplicates. (E) Control and RabGDIα conditional KO (cKO) macrophages stimulated with 10 ng/mL IFN-γ were infected with ME49 T. gondii expressing luciferase (moi = 0.5) and harvested at the indicated points postinfection. The luciferase units (LU) were assayed with the lysates. Indicated values are means ± SD of triplicates. (F) Control and RabGDIα cKO macrophages were untreated or treated with the indicated concentrations of IFN-γ. Untreated or IFN-γ–treated cells were infected with ME49 T. gondii expressing luciferase (moi = 0.5) and harvested at 36 h postinfection. The LUs were assayed with the lysates. Indicated values are means ± SD of triplicates. (G) IFN-γ–stimulated control and RabGDIα cKO peritoneal macrophages were infected with ME49 T. gondii (moi = 0.5), fixed at 36 h postinfection, and stained with rabbit anti-T. gondii (green) or rat anti-CD11b (red). (Scale bars: 20 μm.) (H) The percentage (number of cells with parasites relative to total number of cells) of control and RabGDIα cKO macrophages containing at least one parasite at the indicated points postinfection. Indicated values are means ± SD of triplicates. (I) The number of T. gondii parasites per vacuole in control or RabGDIα cKO macrophages at 36 h postinfection. Indicated values are means ± SD of triplicates. N.S., not significant; *P < 0.05, **P < 0.01, ***P < 0.001. Data are representative of three (A and C–F) and two (B and G) independent experiments. Data in H and I are pooled from two independent experiments in which almost 200 cells and 100 vacuoles were counted, respectively.
RabGDIα, but Not RabGDIβ, Plays a Role in IFN-γ–Dependent Responses to T. gondii.
Next we examined the effect of overexpression of RabGDIα or RabGDIβ in anti-T. gondii response. RabGDIα or RabGDIβ overexpression in IFN-γ–mediated suppression of T. gondii proliferation in mouse embryonic fibroblasts (MEFs) showed that retroviral ectopic expression of RabGDIα, but not of RabGDIβ, significantly impaired IFN-γ–dependent reduction of T. gondii numbers (Fig. 1B and Fig. S1 D and E). To assess the physiological roles of RabGDIα and RabGDIβ, we generated MEFs derived from embryos lacking RabGDIα- or RabGDIβ-deficient mice by conventional ES cell-based gene targeting (Fig. S2 A–D) or CRISPR/Cas9-mediated genome editing (Fig. S3), respectively, and tested the IFN-γ–dependent inhibition of T. gondii proliferation (Fig. 1 C and D). RabGDIα-deficient MEFs showed enhanced dose-dependent IFN-γ–dependent reduction of parasite numbers compared with WT cells (Fig. 1C). In contrast, RabGDIβ-deficient MEFs were comparable with WT cells (Fig. 1D). Taken together, these data demonstrate that RabGDIα, but not RabGDIβ, interacted with Gbp2 and presumably plays a negative role in IFN-γ–dependent suppression of T. gondii proliferation in MEFs.
Fig. S2.
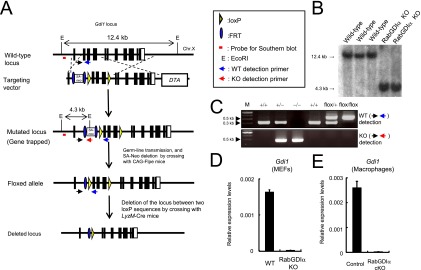
Generation of conditional RabGDIα-deficient mice. (A) The gene-targeting strategy for Gdi1 locus by chromosome engineering. (B) Southern blot analysis of total genomic DNA extracted from the WT or Gdi1-/Y ES cells. Genomic DNA was digested with EcoRI, electrophoresed, and hybridized with the radio-labeled probe indicated in A. Southern blotting resulted in a 12.4-kb band for WT locus, a 4.3 kb band for mutated locus. (C) PCR detection of mice with mutated or floxed Gdi1 locus. Primers used are denoted in A. Because FRT and loxP sequences remain in the floxed Gdi1 allele after the deletion of the neo cassette, the band size for the floxed allele seems to be higher than that of WT allele. (D and E) Relative mRNA levels of RabGDIα in MEFs (D) or macrophages (E) compared with the β-actin level are shown in the y axis. Indicated values are means ± SD of triplicates. Error bars represent means ± SD of triplicates. Data are representative of two independent experiments (B–E).
Fig. S3.
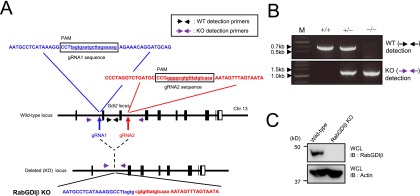
Generation of conventional RabGDIβ-deficient mice. (A) The gene-targeting strategy for Gdi2 locus by Cas9-mediated genome editing. Target sequences for gRNA1 (blue) and gRNA2 (red) were designed in the first and fourth introns of the Gdi2 gene. The sequence of the PCR band for the RabGDIβ-deficient MEFs harboring a junction with the proximal (blue) and distal (red) ends of Gdi2 gene is shown. (B) PCR detection of cells with deletion of Gdi2 locus. Primers used are denoted in A. Data are representative of two independent experiments. (C) WT and RabGDIβ-deficient MEFs were lysed, and the lysates were detected by Western blot with the indicated Abs. Data are representative of two independent experiments (B and C).
Enhanced IFN-γ–Dependent Clearance of T. gondii by RabGDIα Deficiency in Macrophages.
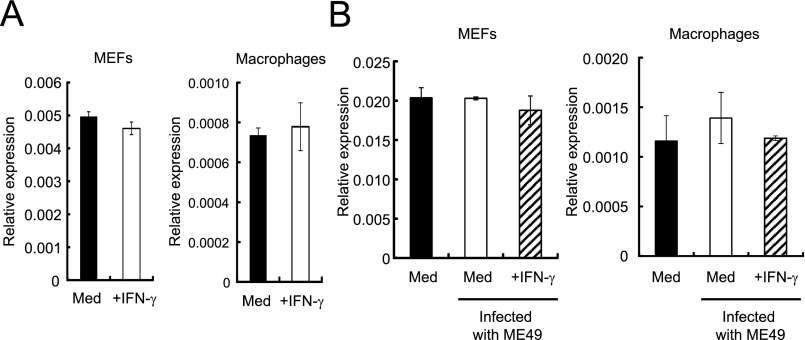
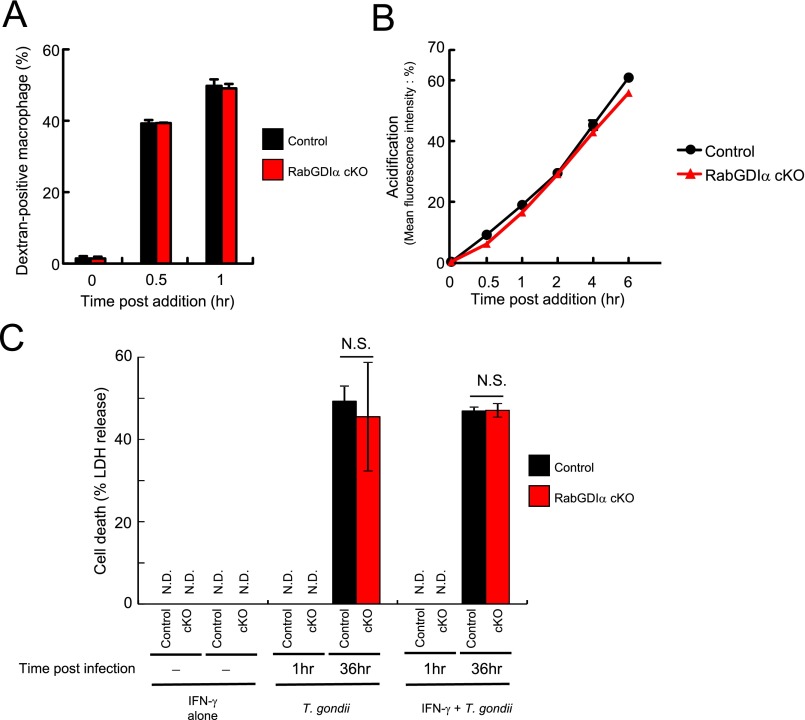
Innate immune cells, such as macrophages, play a vital role in IFN-γ–mediated cellular innate immunity against T. gondii in vivo (43). The expression levels of RabGDIα mRNA in macrophages as well as MEFs were unchanged after IFN-γ stimulation or T. gondii infection (Fig. S4). To analyze the physiological role of RabGDIα in macrophages, we generated mice harboring loxP-flanked alleles of Gdi1 (Gdi1fl/fl) and deleted the floxed allele by Cre recombinase under control of the myeloid-specific lysozyme LysM (LysM-Cre) gene (Fig. S2A). RabGDIα mRNA expression was abolished in peritoneal macrophages from LysM-Cre Gdi1fl/fl mice (Fig. S2E). Rab proteins are involved in phagocytosis and phagosome maturation (44). Therefore, we first tested whether RabGDIα deficiency in macrophages affects the phagocytic activity or the phagosomal acidification by analyzing the macrophage uptake of FITC-labeled or pH-sensitive dye-conjugated dextran, respectively (Fig. S5 A and B). RabGDIα-deficient macrophages normally incorporated FITC-labeled dextran (Fig. S5A). Furthermore, the phagosomal acidification in RabGDIα-deficient cells was comparable with that in control cells (Fig. S5B), suggesting that RabGDIα is dispensable for the regulation of phagocytosis and phagosomal maturation in macrophages.
Fig. S4.
Unaltered expression of RabGDIα mRNA after IFN-γ stimulation and/or T. gondii infection. (A) MEFs or macrophages were untreated or treated with 10 ng/mL IFN-γ for 24 h. Relative mRNA levels of RabGDIα in untreated or IFN-γ–stimulated cells compared with the β-actin level are shown in the y axis. Indicated values are means ± SD of triplicates. (B) MEFs or macrophages were untreated or treated with 10 ng/mL IFN-γ for 24 h, and/or followed by the infection of the T. gondii ME49 (moi = 2). Relative mRNA levels of RabGDIα at 24 h postinfection compared with the β-actin level are shown in the y axis. Indicated values are means ± SD of triplicates. Data are representative of two independent experiments (A and B).
Fig. S5.
Dextran uptake, phagosome acidification, and T. gondii infection-induced cell death are comparable between control and RabGDIα-deficient macrophages. (A and B) Control and RabGDIα cKO peritoneal macrophages were mixed with dextran conjugated with FITC (A) or pH sensor (B) and harvested at the indicated time points. The percentage of macrophages positive for fluorescence is shown. Indicated values are means ± variations of duplicates. (C) Control and RabGDIα cKO macrophages were untreated or treated with IFN-γ for 24 h and/or infected with ME49 T. gondii-expressing luciferase (moi = 0.5). At 1 or 36 h postinfection, the concentrations of LDH in the culture supernatants were measured. Indicated values are means ± SD of triplicates. N.D., not detected; N.S., not significant. Data are representative of two independent experiments (A–C).
Next, we assessed whether RabGDIα deficiency affected IFN-γ–mediated suppression of parasite replication or survival in macrophages. Similar to MEFs, RabGDIα-deficient macrophages exhibited an augmented IFN-γ–dependent reduction of parasite numbers that was time- or dose-dependent (Fig. 1 E and F). Stimulation of macrophages by IFN-γ efficiently reduces T. gondii numbers in cells by suppression of parasite replication and survival. IFN-γ–dependent growth inhibition is mainly mediated by nitric oxide dependently on iNOS in the infected cells (16, 17), leading to reduction of the parasite number per vacuole. On the other hand, T. gondii survival within infected cells is suppressed by cooperative action between IRGs and GBPs, decrementing the number of cells with parasites (17, 20, 21). The degree of parasite infection and growth in macrophages from control or LysM-Cre Gdi1fl/fl mice by confocal microscopy showed that the percentage of T. gondii-infected cells was comparable between control or RabGDIα-deficient cells at 3 h postinfection (Fig. 1H). In contrast, percentages for number of RabGDIα-deficient macrophages with parasites relative to total number of cells in visual fields at later time points were significantly lower than those of controls (Fig. 1 G and H). Parasite replication in control or RabGDIα-deficient macrophages was determined by counting parasite numbers in PVs and demonstrated that T. gondii numbers in PVs of RabGDIα-deficient cells were similar to controls (Fig. 1 G and I). In addition, cell death in response to IFN-γ has been shown to restrict parasite growth (45). However, RabGDIα-deficient macrophages showed comparable cell death in the parasite infection in comparison with control macrophages (Fig. S5C). Thus, loss of RabGDIα in macrophages and MEFs resulted in the augmented IFN-γ–dependent suppression of T. gondii survival whereas the inhibition of replication and cell death was unaffected.
Increased in Vivo Resistance to T. gondii by Myeloid-Specific Ablation of RabGDIα.
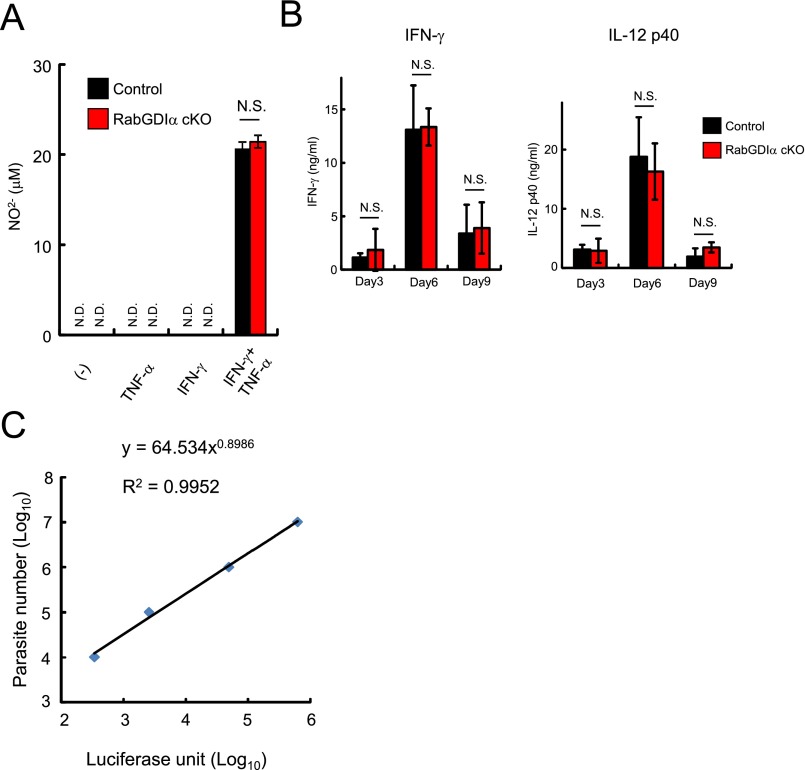
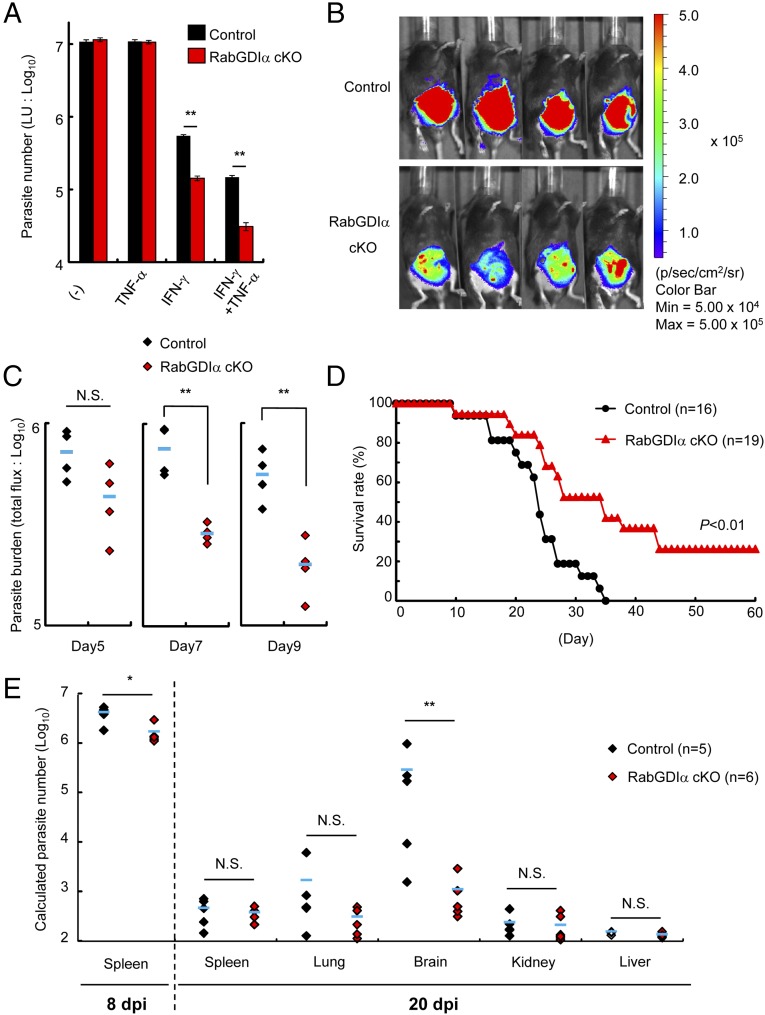
Tumor necrosis factor-α (TNF-α) plays a critical role in the in vivo resistance to T. gondii and is shown to strongly enhance anti-T. gondii activity in macrophages mainly by the synergistic production of nitric oxide (NO) (46). Both control and RabGDIα-deficient macrophages produced comparable levels of nitrite ion (NO2-) in costimulation of IFN-γ and TNF-α (Fig. S6A). Furthermore, RabGDIα-deficient macrophages still exhibited augmented IFN-γ–inducible reduction of T. gondii numbers even in the presence of TNF-α (Fig. 2A). Therefore, we tested whether increased T. gondii clearance activity in RabGDIα-deficient macrophages results in in vivo resistance to the parasite. Control or LysM-Cre Gdi1fl/fl mice were intraperitoneally infected with a high dose of type II T. gondii, to which all control mice succumbed around 30 d postinfection (dpi), and parasite spread and burden in organs and mouse survival were assessed (Fig. 2 B–E). In vivo imaging analysis indicated reduced abdominal parasite signal in LysM-Cre Gdi1fl/fl mice compared with control mice at 7 or 9 dpi (Fig. 2 B and C). Although control and LysM-Cre Gdi1fl/fl mice showed an initially comparable mortality up to 16 dpi and serum levels of proinflammatory cytokines, such as IFN-γ and IL-12 p40, in the acute phase (Fig. S6B), LysM-Cre Gdi1fl/fl mice were less susceptible in the chronic phase of the infection (Fig. 2D). In terms of parasite burdens in organs, high numbers in spleens at 8 dpi in both control and LysM-Cre Gdi1fl/fl mice, albeit slightly lower in spleens of LysM-Cre Gdi1fl/fl mice, were observed. In sharp contrast, the parasite numbers in spleens from both groups were dramatically reduced at 20 dpi (Fig. 2E and Fig. S6C). When other organs were tested at 20 dpi, we found that brains of control mice contained strikingly higher parasite numbers than those from LysM-Cre Gdi1fl/fl mice (Fig. 2E). However, no significant differences in the parasite burdens in lungs, kidneys, and livers were measured in LysM-Cre Gdi1fl/fl mice compared with control mice (Fig. 2E). Thus, these results demonstrated that myeloid cell-specific deletion of RabGDIα leads to a lower parasitic burden in the brain and confers increased in vivo resistance to T. gondii, which is because of neither IL-12 p40 nor IFN-γ production defects.
Fig. S6.
NO production in macrophages and in vivo serum cytokine levels during the acute phase are normal in myeloid-specific RabGDIα-deficient mice. (A) Control and RabGDIα cKO macrophages were untreated or treated with TNF-α and/or IFN-γ for 24 h. The concentrations of NO2- in the culture supernatant were measured. N.D., not detected. Indicated values are means ± SD of triplicates. (B) Sera were obtained from control or RabGDIα cKO mice (n = 4 per group) at the indicated time postinfection of 5 × 103 T. gondii. Serum concentrations of the indicated cytokines were measured by ELISA. Data are mean ± SD of triplicates. (C) A standard curve of luciferase activity (x axis) and parasite number (y axis) was generated according to luciferase activities of serial dilutions of ME49-expressing luciferase (104 to 107). N.S., not significant. Data are representative of two independent experiments (A–C).
Fig. 2.
Myeloid-specific RabGDIα-ablated mice are resistant to T. gondii infection. (A) Control and RabGDIα cKO macrophages were untreated or treated with 10 ng/mL IFN-γ and/or TNF-α for 24 h, and followed by the infection of the luciferase expressing T. gondii (moi = 0.5). T. gondii numbers at 36 h postinfection were analyzed by luciferase assay. Indicated values are means ± SD of triplicates. (B) Control (Gdi1flox/flox) or RabGDIα conditional KO (cKO; LyzM-Cre Gdi1flox/flox) mice (n = 4 per each group) were intraperitoneally infected with 5 × 103 ME49 T. gondii-expressing luciferase, and the progress of infection was assessed by bioluminescence imaging at day 7 postinfection. The color scales indicate photon emission during a 60-s exposure. (C) Total photon emission analysis from mice (n = 4 per group) in B at indicated days postinfection. Abdominal photon emission was assessed during a 60-s exposure. The flux (photons/s/cm2/sr) was determined as a measure of parasite burden. The statistical significance was determined by Mann–Whitney U test. (D) Control (n = 16) or RabGDIα cKO (n = 19) mice were infected with 5 × 103 T. gondii, and the survival rates were monitored for 60 d. The statistical significance was determined by log-rank test. (E) Quantification of parasites in indicated tissues from mice at day 8 or 20 postinfection by the standard curve (in Fig. S6C). The statistical significance was determined by Mann–Whitney U test. N.S., not significant; *P < 0.05, **P < 0.01. Data are representative of two independent experiments (A–C and E). Data in D are pooled from three independent experiments.
Increased Loading of Irga6 onto T. gondii PVs by RabGDIα Deficiency.
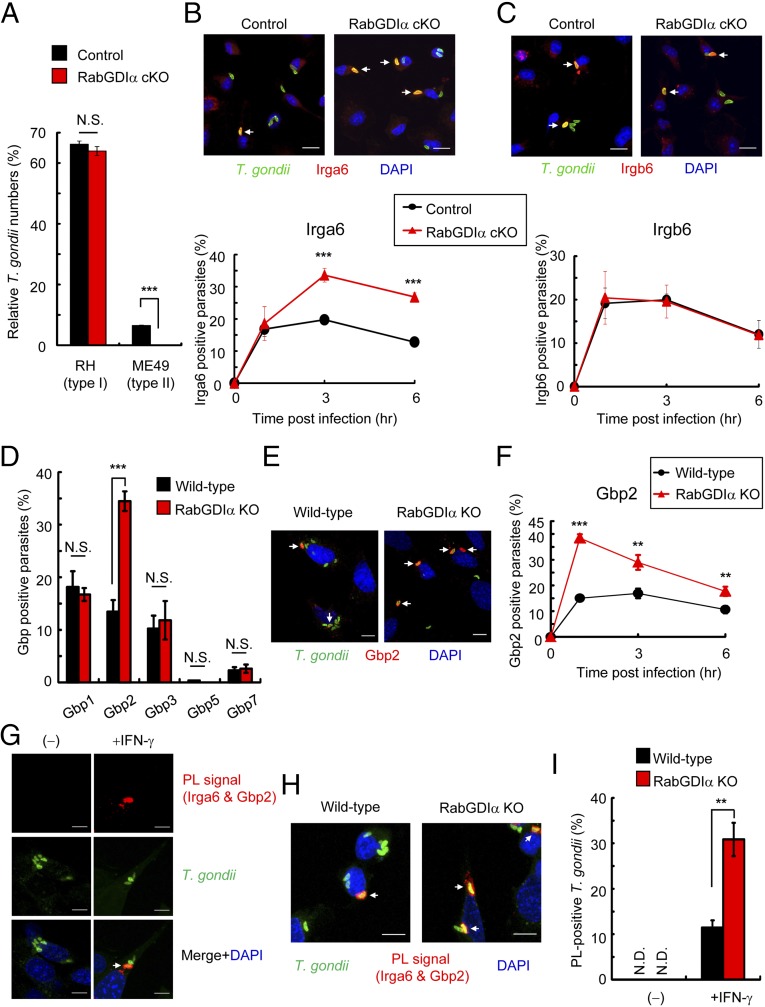
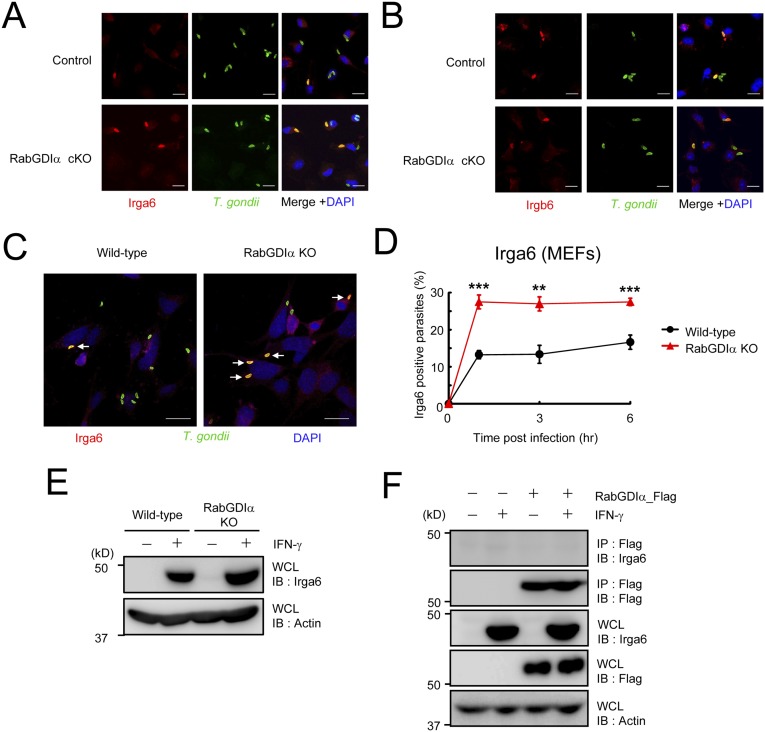
IFN-γ–induced cellular clearance of T. gondii is dependent on IRGs (17). The virulent type I T. gondii parasite is resistant to the IRG-dependent clearance due to ROP18, a parasite-secreted kinase that acts as a virulence effector molecule (33, 34). Consistently, IFN-γ–stimulated reduction of type I T. gondii was not as efficient as that of the avirulent IRG-susceptible type II strain (Fig. 3A). Moreover, although type II parasite numbers were significantly more decreased in RabGDIα-deficient macrophages after IFN-γ stimulation, those of type I parasite were unchanged between control and RabGDIα-deficient macrophages (Fig. 3A), suggesting that the phenotype caused by the RabGDIα deficiency may be associated with IRGs. Analysis of the recruitment of two different IRG family members, Irga6 and Irgb6, to T. gondii in IFN-γ–stimulated macrophages by confocal microscopy indicated a significantly higher percentage of Irga6 recruitment to parasites in RabGDIα-deficient macrophages at 3 or 6 h postinfection compared with controls (Fig. 3B and Fig. S7A). In contrast, the rates of Irgb6-positive parasites were comparable between control and RabGDIα-deficient macrophages (Fig. 3C and Fig. S7B). Furthermore, RabGDIα-deficient MEFs also showed increased Irga6 loading onto parasites compared with WT cells (Fig. S7 C and D), indicating that RabGDIα deficiency leads to a more frequent recruitment of Irga6, but not Irgb6, to T. gondii. Furthermore, the negative effect of RabGDIα on Irga6 loading was not determined by protein levels because Irga6 proteins were similarly induced and expressed in WT and RabGDIα-deficient cells (Fig. S7E). Moreover, we did not detect an association between RabGDIα and Irga6 by coprecipitation (Fig. S7F), suggesting that the higher rates of Irga6 loading to T. gondii might be indirectly caused by the RabGDIα deficiency.
Fig. 3.
Increased accumulation of Irga6 and Gbp2 in RabGDIα-deficient cells. (A) Control and RabGDIα cKO macrophages were untreated or treated with IFN-γ. Untreated or IFN-γ–treated cells were infected with RH (type I) or ME49 (type II) T. gondii expressing luciferase (moi = 0.5) and harvested at 36 h postinfection. Percentages of parasite numbers (calculated by luciferase counts) in IFN-γ–stimulated control or RabGDIα-deficient cells relative to those in the unstimulated respective cells are shown as “Relative T. gondii numbers.” Indicated values are means ± SD of triplicates. (B and C) IFN-γ–stimulated control and RabGDIα-deficient macrophages were infected with ME49 T. gondii (moi = 0.5), fixed at 3 h postinfection, and incubated with rabbit anti-T. gondii (green) and mouse anti-Irga6 (red in B) or goat anti-Irgb6 (red in C). (Scale bars: 10 μm.) Arrows indicate colocalization of Irga6 or Irgb6 with T. gondii (Upper). The percentage of parasites positive for Irga6 or Irgb6 staining at the indicated points postinfection in IFN-γ–stimulated control and RabGDIα-deficient macrophages (Lower). Indicated values are means ± SD of triplicates. (D) The percentage of WT or RabGDIα-deficient MEFs positive for indicated Gbps staining at 1 h postinfection. Indicated values are means ± SD of triplicates. (E) IFN-γ–stimulated WT or RabGDIα-deficient MEFs were infected with T. gondii (moi = 4), fixed at 1 h postinfection, and incubated with rabbit anti-Gbp2 (red), and goat anti-T. gondii (green). (Scale bars: 10 μm.) Arrows indicate colocalization of endogenous Gbp2 with T. gondii. (F) The percentage of WT or RabGDIα-deficient MEFs positive for endogenous Gbp2 costaining with T. gondii at the indicated time points postinfection. Indicated values are means ± SD of triplicates. (G and H) Proximity-ligation (PL) assay of both endogenous Irga6 and Gbp2 in IFN-γ–stimulated or unstimulated (G), and WT or RabGDIα-deficient (H) MEFs. Cells were treated with IFN-γ, infected with ME49 T. gondii (moi = 4), and fixed at 3 h postinfection: [red, PL-positive (PL+) signals; green, T. gondii; blue, nuclei]. Arrows indicate colocalization of PL signal with T. gondii. (I) The percentage of parasites positive for PL signals (in H) at 3 h postinfection. Indicated values are means ± SD of triplicates. N.D., not detected; N.S., not significant; **P < 0.01, ***P < 0.001. Data are representative of two (A, B, Upper, C, Upper, D, E, G, and H) independent experiments. Data in B, Lower, C, Lower, D, F, and I are pooled from three independent experiments, in which almost 150 cells at each time point were counted.
Fig. S7.
Increased loading of Irga6, but not of Irgb6, in RabGDIα-deficient cells albeit no interaction between Irga6 and RabGDIα. (A and B) IFN-γ–stimulated control and RabGDIα-deficient macrophages were infected with ME49 T. gondii (moi = 0.5), fixed at 3 h postinfection, and incubated with rabbit anti-T. gondii (green) and mouse anti-Irga6 (red in A) or goat anti-Irgb6 (red in B). (Scale bars: 10 μm.) The merged images are shown in Fig. 3 B and C. (C) WT or RabGDIα-deficient MEFs treated with IFN-γ were infected with ME49 T. gondii (moi = 4), fixed at 3 h postinfection, and incubated with rabbit anti-T. gondii (green), mouse anti-Irga6 (red), and DAPI (blue). (Scale bars: 10 μm.) Arrows indicate colocalization of Irga6 with T. gondii. (D) The percentage of parasites positive for Irga6 staining at the indicated points postinfection in IFN-γ–stimulated WT or RabGDIα-deficient MEFs. Data are mean ± SD of triplicates. (E) Peritoneal macrophages untreated or treated with IFN-γ were lysed and detected by Western blot with the indicated antibodies. (F) RabGDIα-deficient MEFs were stably transfected with empty or RabGDIα expression plasmids, untreated or treated with IFN-γ, and lysed. The lysates were immunoprecipitated with anti-Flag and detected by protein immunoblot with the indicated antibodies. **P < 0.01, ***P < 0.001. Data are representative of two independent experiments (A–C, E, and F). Data in D are pooled from three independent experiments, in which almost 150 cells at each time point were counted.
Gbp2 Is Specifically More Recruited onto T. gondii PVs in RabGDIα-Deficient Cells.
In the first place, we identified RabGDIα as a Gbp2-interacting protein (Fig. S1A). To test whether RabGDIα specifically interacted with Gbp2 alone or with other GBP family members, we performed an immunoprecipitation assay. Flag-tagged RabGDIα coprecipitated with endogenous Gbp2 but not Gbp1 (Fig. 1A). Next, to compare the recruitment of individual GBPs in WT or RabGDIα-deficient MEFs, Flag- or HA-tagged GBPs (such as Gbp1, Gbp2, Gbp3, Gbp5, and Gbp7) were stably expressed in WT or RabGDIα-deficient MEFs (Fig. S8 A and B). The percentages of accumulation of Gbp1, Gbp3, Gbp5, and Gbp7 on T. gondii were comparable between WT and RabGDIα-deficient cells (Fig. 3D and Fig. S8C). In sharp contrast, the rate of loading of HA-tagged Gbp2 alone in RabGDIα-deficient MEFs was significantly greater than in WT cells (Fig. 3D and Fig. S8C). Furthermore, endogenous Gbp2 proteins in RabGDIα-deficient cells were more recruited to T. gondii than in WT cells (Fig. 3 E and F), indicating that specific association of RabGDIα with Gbp2, but not with other GBPs, may account for enhanced Gbp2 recruitment observed in RabGDIα deficiency.
Fig. S8.
Stable expression of GBPs in MEFs and signal intensity profile of Gbp2 and Irga6 in IFN-γ–stimulated macrophages. (A and B) Lysates of MEFs stably transfected with empty or indicated HA-tagged Gbps (A) or Flag-tagged Gbp1 (B) expression vectors were subjected to Western blot using indicated Abs. (C) IFN-γ–stimulated WT or RabGDIα-deficient MEFs stably expressing indicated Gbps were infected with T. gondii (moi = 4), fixed at 1 h postinfection, and incubated with rabbit anti-T. gondii (green) and mouse anti-HA (for Gbp2 and Gbp3) (red) or mouse anti-Flag (for Gbp1) (red). (Scale bars: 10 μm.) Arrows indicate colocalization of Gbps with T. gondii. (D and E) MEFs treated with IFN-γ were infected with ME49 T. gondii (moi = 4), fixed at 3 h postinfection, and incubated with goat anti-T. gondii (green), rabbit anti-Gbp2 (red), or mouse anti-Irga6 (magenta). The samples were analyzed by confocal laser microscopy and subsequently by intensity profiling (E). (Scale bars: D, 10 μm; E, 5 μm.) The signal intensities of each color on the 5-μm light blue line on the parasites are shown. Data are representative of two independent experiments (A–E).
Gbp2 and Irga6 on T. gondii Are Colocalized in the Very Proximity.
These results suggested that, among IRGs and GBPs, the behavior of Irga6 and Gbp2 were similar in RabGDIα-deficient cells. We previously demonstrated that GBPchr3 regulated Irgb6 and Irgb10 loading to T. gondii (21). Furthermore, a previous study showed that Gbp1 was important for Irgb6 recruitment on T. gondii-containing vacuoles (20). Thus, a molecular link between IRGs and GBPs has been suggested; however, a specific association between Irga6 and Gbp2 is unclear. Thus, we tested whether Irga6 and Gbp2 were colocalized on T. gondii-containing vacuoles in IFN-γ–stimulated MEFs. Confocal microscopy and intensity profiling of fluorescent signals showed that both endogenous Irga6 and Gbp2 proteins were detected at similar sites (Fig. S8 D and E). Although we examined the association of Irga6 and Gbp2, we failed to detect an interaction by coprecipitation analysis. To test whether Irga6 and Gbp2 proteins were in close proximity (<16 nm) (47), we performed a proximity ligation (PL) assay. No PL signals were observed in unstimulated cells (Fig. 3G). In sharp contrast, strong PL signals between endogenous Gbp2 and Irga6 were observed in IFN-γ–stimulated cells (Fig. 3G). Moreover, markedly higher numbers of T. gondii with PL signals between Gbp2 and Irga6 were also detected in RabGDIα-deficient cells than in WT cells (Fig. 3 H and I), which is consistent with more frequent accumulation of Irga6 and Gbp2 in RabGDIα-deficient cells (Fig. 3 B and E). Together, these results indicated that Irga6 and Gbp2 were located in close proximity to allow a physical association with the parasites.
Gbp2 Plays a Positive Role in Irga6 Recruitment to T. gondii.
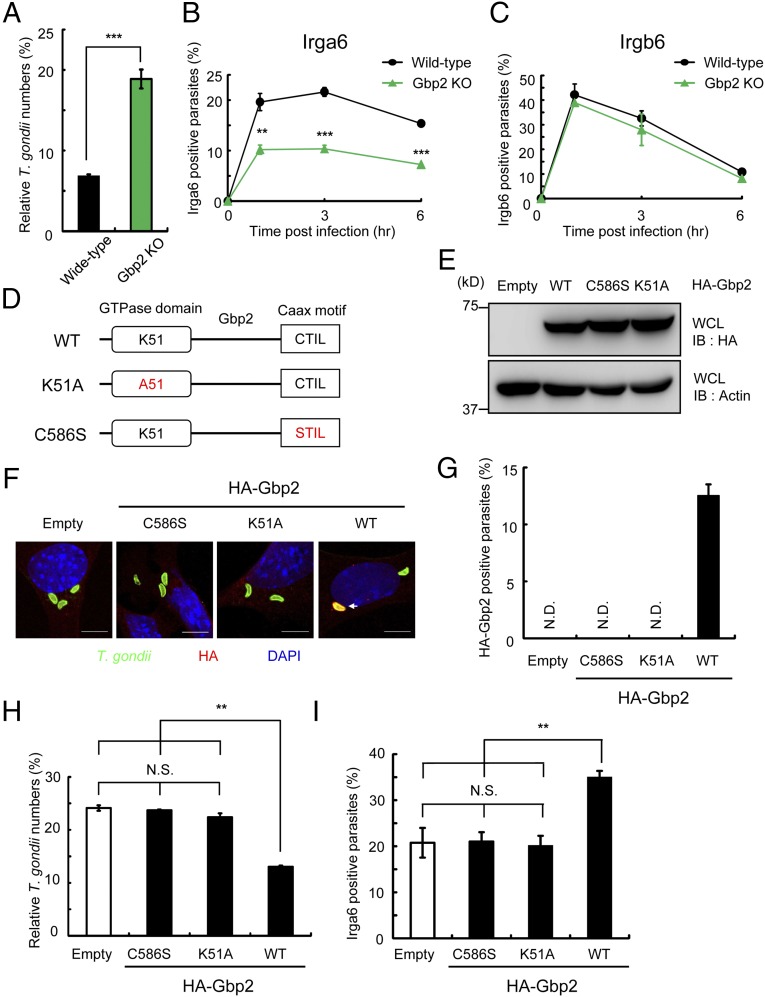
To assess the relationship between Irga6 and Gbp2 directly, we created MEFs derived from embryos of Gbp2-deficient mice, generated by CRISPR/Cas9-mediated genome editing (Fig. S9A). Although Irga6 proteins were similarly induced upon IFN-γ stimulation, Gbp2 proteins were not detected in Gbp2-deficient MEFs (Fig. S9B). Compared with WT cells, Gbp2-deficient cells had a defective suppression of T. gondii proliferation in response to IFN-γ (Fig. 4A). In addition, the percentages of Irga6-positive parasites in Gbp2-deficient MEFs were significantly lower than in WT cells (Fig. 4B), which is opposite to that observed in RabGDIα-deficient cells (Fig. 3B and Fig. S7D). On the other hand, the percentages of Irgb6-positive T. gondii were comparable between WT and Gbp2-deficient MEFs (Fig. 4C). Thus, Gbp2 positively regulated the recruitment of Irga6 to PVs of T. gondii.
Fig. S9.
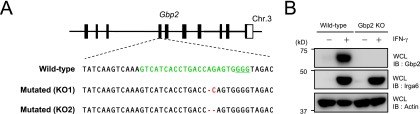
Generation of Gbp2-deficient MEFs. (A) The Cas9/gRNA-targeted site in the fourth exon of Gbp2 gene. (Top) Structure of the Gbp2 gene. Black and white boxes denote the coding and noncoding exons, respectively. (Middle) The target sequence for gRNA was labeled with green, and the PAM sequence was underlined. (Bottom) Sequences of the mutated allele contained 1 bp deletion and substitution of guanine to cytosine (KO1) or of a deletion of 2 bp (KO2) are shown. Both KO1 and KO2 cause a frameshift mutation. (B) IFN-γ–stimulated WT and Gbp2-deficient MEFs were lysed and detected with the indicated antibodies by Western blot. Data are representative of two independent experiments (B).
Fig. 4.
Gbp2 positively regulates Irga6 recruitment to T. gondii through prenylation. (A) T. gondii numbers at 36 h postinfection in WT and Gbp2-deficient MEFs untreated or treated with IFN-γ were analyzed by luciferase assay. Percentages of parasite numbers (calculated by luciferase counts) in IFN-γ–stimulated WT or Gbp2-deficient cells relative to those in the unstimulated respective cells are shown as “Relative T. gondii numbers.” Indicated values are means ± SD of triplicates. (B and C) The percentage of parasites positive for Irga6 (B) or Irgb6 (C) staining at the indicated points postinfection in IFN-γ–stimulated WT and Gbp2-deficient MEFs. Indicated values are means ± SD of triplicates. (D) Gbp2 mutants possessing amino acid substitutions in the GTPase domain or Caax motif. (E) Lysates of Gbp2-deficient MEFs stably transfected with empty or indicated HA-tagged Gbp2 expression plasmids were detected with indicated Abs by Western blot. (F and G) IFN-γ–stimulated Gbp2-deficient MEFs stably transfected with empty or indicated HA-tagged Gbp2 expression plasmids were infected with ME49 T. gondii (moi = 4), fixed at 1 h postinfection, and incubated with rabbit anti-T. gondii (green), mouse anti-HA (red), and DAPI (blue). (Scale bars, 10 μm.) Arrows indicate colocalization of Gbp2 with T. gondii. Percentage of parasites positive for Gbp2 staining is shown G. Indicated values are means ± SD of triplicates. (H) T. gondii numbers at 36 h postinfection in Gbp2-deficient MEFs stably transfected with empty (control) or indicated HA-tagged Gbp2 expression plasmids untreated or treated with IFN-γ were analyzed by luciferase assay. Percentages of parasite numbers (calculated by luciferase counts) in IFN-γ–stimulated control or indicated Gbp2 variants expressing cells relative to those in the unstimulated respective cells are shown as “Relative T. gondii numbers.” Indicated values are means ± SD of triplicates. (I) The percentage of parasites positive for Irga6 staining in Gbp2-deficient MEFs expressing indicated Gbp2 variants at 3 h postinfection. Indicated values are means ± SD of triplicates. N.D., not detected; N.S., not significant; **P < 0.01, ***P < 0.001. Data are representative of two (E and F) or three (A and H) independent experiments. Data in B, C, G, and I are pooled from two independent experiments, in which almost 150 cells at each time point were counted.
Prenylation of Gbp2 Is Required for Its Positive Function on IFN-γ–Dependent Clearance and Irga6 Recruitment.
GBPs, including Gbp2, comprise two structural domains, an N-terminal globular nucleotide-binding domain, where the GTP-binding motif is located, and a C-terminal helical effector domain. Gbp2 contains a C-terminal isoprenylated Caax motif (22, 48, 49). To assess the role of GTP-binding status and Gbp2 prenylation in anti-T. gondii responses, we generated K51A and C586S mutants, where either lysine 51 or cysteine 586 was substituted to alanine or serine, respectively (Fig. 4D). K51A causes the defective binding of human GBP1 with nucleotides, including GTP and GDP (50). The C586S mutation abolishes prenylation by geranylgeranyltransferase I (51, 52). We introduced these mutations into Gbp2-deficient MEFs and tested the IFN-γ–dependent killing of T. gondii and Irga6 loading (Fig. 4E). Gbp2-deficient MEFs expressing WT Gbp2, but neither K51A nor C586S, restored the IFN-γ–dependent reduction of parasite numbers and recruitment of Irga6 and Gbp2 (Fig. 4 F–I). These data indicate that both nucleotide binding and prenylation of Gbp2 are essential for the IFN-γ–dependent cellular immunity.
The Lipid-Binding Pocket in RabGDIα Is Required for Its Negative Function.
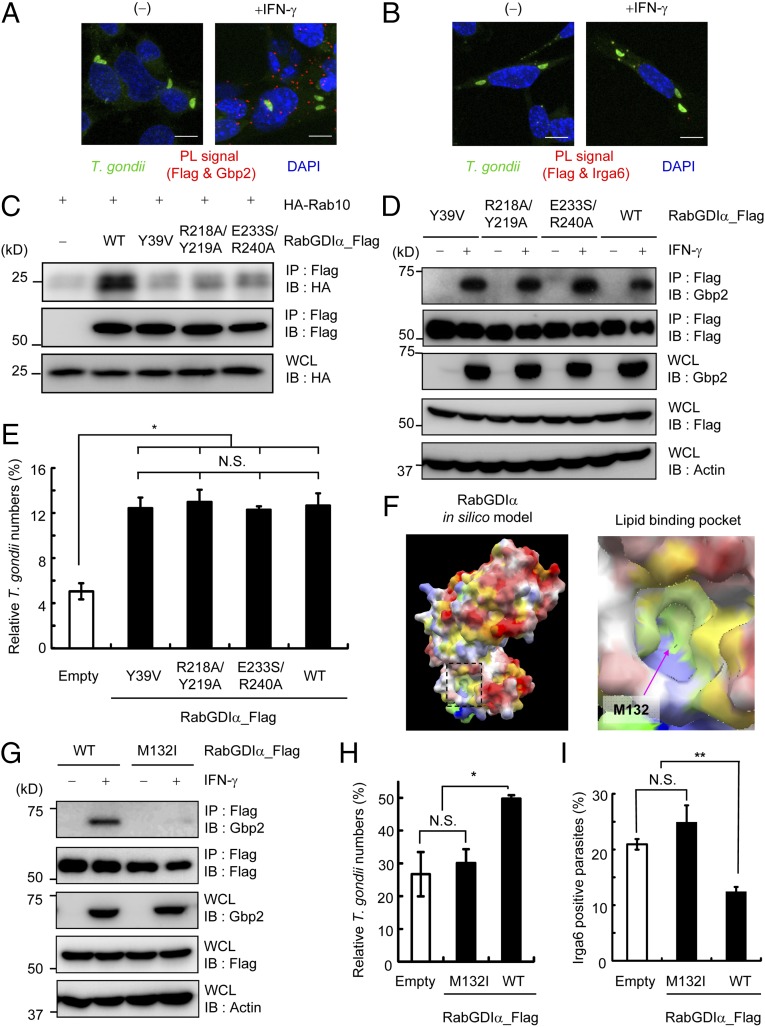
RabGDI functions as a negative regulator of Rabs by sequestering Rabs in an inactive GDP-bound form in the cytoplasm (53). The extraction of prenylated Rabs from membranes by RabGDI is achieved by the direct interaction of Rabs with RabGDIα through the Rab-binding platform and the lipid-binding pocket, into which the prenyl group of Rabs is inserted (54, 55). The significance of the two domains of RabGDIα was originally characterized in the context of Rabs; however, their function in the context of IFN-γ–induced cell-autonomous immunity is unknown. Therefore, we analyzed the localization of interactions between RabGDIα and Gbp2 by PL assay. In contrast to interactions between Irga6 and Gbp2, which were strongly detected on parasites (Fig. 3 G and H), IFN-γ–dependent PL signals for the association of Flag-tagged RabGDIα with endogenous Gbp2 were not detected on parasites, but only in the cytoplasm (Fig. 5A). In addition, PL signals between endogenous Irga6 and Flag-tagged RabGDIα were not detected in IFN-γ–stimulated cells (Fig. 5B), suggesting that RabGDIα and Irga6 are not localized in the proximity. Next, we characterized the Rab-binding platform of RabGDIα in IFN-γ–mediated immunity. Consistent with previous reports (54, 56), RabGDIα Y39V, R218A/Y219A, and E233S/E240A mutants nearly abolished the interaction with HA-tagged Rab10 (Fig. 5C). However, when these Rab-binding platform mutants of RabGDIα were stably expressed in RabGDIα-deficient MEFs, the RabGDIα mutants coprecipitated with endogenous Gbp2 and restored the suppressive activity of RabGDIα in the IFN-γ–induced reduction of parasite numbers (Fig. 5 D and E). Thus, the negative effect of RabGDIα on Gbp2-mediated responses might be independent of Rabs. To verify the importance of the lipid-binding pocket of RabGDIα (Fig. 5F), we generated an M132I mutant of RabGDIα and tested an association with Gbp2. Notably, the Flag-tagged M132I mutant of RabGDIα failed to coprecipitate with endogenous Gbp2 (Fig. 5G). Moreover, introduction of the RabGDIα M132I mutant in RabGDIα-deficient MEFs did not restore suppression of IFN-γ–mediated killing activity of T. gondii and reduced Irga6 loading onto PVs (Fig. 5 H and I). Taken together, these results indicate that lipid-binding activity, but not the Rab-binding function, of RabGDIα is critical for IFN-γ–induced Gbp2-dependent cell-autonomous immunity against T. gondii.
Fig. 5.
RabGDIα suppresses Gbp2-dependent responses through the lipid-binding pocket. (A and B) PL assay of Flag-tagged RabGDIα and endogenous Gbp2 (A) or Irga6 (B) in IFN-γ–stimulated or unstimulated MEFs. Cells were infected with ME49 T. gondii (moi = 4), fixed at 3 h postinfection; red, PL-positive (PL+) signals; green, T. gondii; blue, nuclei. (C) Lysates of 293T cells transiently cotransfected with indicated Flag-tagged RabGDIα vectors and/or HA-tagged Rab10 were immunoprecipitated with anti-Flag and detected using the indicated Abs by Western blot. (D) RabGDIα-deficient MEFs expressing indicated Flag-tagged RabGDIα were untreated or treated with IFN-γ and lysed. The lysates were immunoprecipitated with anti-Flag and detected using with the indicated Abs by Western blot. (E and H) T. gondii numbers at 36 h postinfection in RabGDIα-deficient MEFs stably transfected with empty (control) or indicated Flag-tagged RabGDIα expression plasmids untreated or treated with IFN-γ were analyzed by luciferase assay. Percentages of parasite numbers (calculated by luciferase counts) in IFN-γ–stimulated control or indicated RabGDIα variants-expressing cells relative to those in the unstimulated respective cells are shown as “Relative T. gondii numbers.” Indicated values are means ± SD of triplicates. (F) In silico profiling of the molecular surface of RabGDIα, colored by electrostatic potential, with red (or blue) representing the most negative (or positive) values, and by hydrophobicity, with yellow representing the most hydrophobic. The lipid-binding pocket is framed by a dashed line, and its close-up view is shown Right. The approximate location of the methionine residue 132 is indicated by an arrow. (G) Lysates of RabGDIα-deficient MEFs stably transfected with empty or indicated Flag-tagged RabGDIα expression plasmids were detected with indicated Abs by Western blot. (I) The percentage of parasites positive for Irga6 staining in RabGDIα-deficient MEFs expressing indicated RabGDIα variants at 3 h postinfection. Indicated values are means ± SD of triplicates. N.S., not significant; *P < 0.05, **P < 0.01. Data are representative of two (A–D and G) or three (E and H) independent experiments and two independent calculations (F). Data in I are pooled from two independent experiments, in which almost 150 cells at each time point were counted.
Discussion
In the present study, we have found that RabGDIα plays a suppressive role in cell-autonomous host defense against T. gondii by IFN-γ–inducible GTPases. RabGDIα-deficient cells exhibited increased IFN-γ–mediated Gbp2/Irga6-depedent in vitro clearance of T. gondii. Furthermore, mice specifically lacking RabGDIα in myeloid cell linage showed increased resistance to a high dose of type II T. gondii infection due to a decreased parasite burden in the brain, which is an opposite phenotype of Gbp2-deficient mice showing increased parasite numbers in the brain and decreased resistance in the chronic phase (22).
The function of RabGDIα, as well as yeast RabGDI, has been well-characterized for the regulation of Rabs, which play important roles in membrane vesicular trafficking. Rabs cycle between a GDP-bound inactive state and a GTP-bound active state (57). Inactive Rabs are activated by an exchange of GDP for GTP on donor membranes that generate a carrier vesicle (58). Active Rabs on carrier vesicles facilitate docking and fusion with target membranes, culminating in the inactivation of Rabs by hydrolysis of GTP to GDP. RabGDI associates with inactive Rabs on target membranes through the Rab binding platform, which contains a prenyl group of Rabs in the lipid-binding pocket. This association between Rabs and RabGDI allows the retrieval of Rabs from target membranes to the cytosolic pool for recycling for the next round of vesicular transport (59). In the context of IFN-γ–induced responses, the Rab-binding platform of RabGDIα was dispensable for the negative function, indicating that RabGDIα might function in a Rab-independent manner. In contrast, the lipid-binding pocket of RabGDIα was indispensable for the suppressive effect. In addition, prenylation of Gbp2 in the C-terminal CTIL motif was essential for both the recruitment to PV membranes of T. gondii and the IFN-γ–dependent killing of parasites (22). Similarly, prenylation of Rabs was critical for membrane anchoring and function (54). In terms of RabGDIα and Gbp2 localization, PL signals indicative of direct interactions between both proteins were detected mainly in the cytoplasm. Thus, by analogy with the relationship between RabGDIα and Rabs in membrane vesicular trafficking, RabGDIα might retrieve Gbp2 from target PV membranes to the cytoplasm, resulting in termination of Gbp2-dependent Irga6 loading on PV membranes.
Compared with the two isoforms of RabGDI in mammals, yeast has only one copy of the GDI gene that encodes yeast RabGDI, and its disruption results in lethality (57). Although conventional RabGDIα-deficient mice are viable and healthy except for impaired cognitive functions (60), we failed to obtain postnatal RabGDIβ-deficient mice due to unknown reasons. In this regard, the RabGDIβ deficiency could not be compensated for by RabGDIα in vivo. In addition, the differential roles of RabGDIα and RabGDIβ in the regulation of Rabs as well as embryonic development are still unclear. Both RabGDIα and RabGDIβ bind and solubilize similar members of GDP-formed inactive Rabs in vitro (61). Of note, we demonstrated that RabGDIα, but not RabGDIβ, specifically associated with Gbp2 and suppressed Gbp2-dependent cell-autonomous immune responses. Currently, the specific function of RabGDIβ in IFN-γ–induced responses is unknown. RabGDIβ might associate with Gbp family members other than Gbp2, or be totally unrelated to this immune response. In terms of phagocytosis, RabGDIβ rather than RabGDIα may mainly regulate the activity and the acidification.
Genes encoding murine GBPs consist of 13 family members (11 active members and 2 pseudo genes), are organized in two clusters on chromosomes 3 and 5 in mice, and share a high degree of homology (62). We previously generated mice lacking GBPchr3 and demonstrated that GBPchr3 participated in the recruitment and/or retention of IRGs to T. gondii or Chlamydia trachomatis (21, 39). A subsequent study revealed that Gbp1 plays a role in the recruitment and/or retention of Irgb6, but not of Irga6, to T. gondii (20). Here, we found that Gbp2 positively regulated the recruitment of Irga6, but not Irgb6, to the parasite. Gbp2-deficient cells displayed almost 50% loss of Irga6 loading to T. gondii. Furthermore, the defect in the Irga6 accumulation in GBPchr3-deleted cells was almost comparable with that in Gbp2-deficient cells, indicating that IFN-γ–induced Irga6 recruitment to the parasite may be controlled in a Gbp2 (GBPchr3)-dependent and -independent manner. Although recruitment of all IRGs, comprising 23 members in mice, was not tested in Gbp1-, Gbp2-, or GBPchr3-deficient cells, these results suggest that individual GBPs may differentially regulate the recruitment and/or retention of IRGs. We did not detect an interaction between Gbp2 and Irga6 by coprecipitation; however, both proteins were localized in close proximity as demonstrated by PL assay. The detailed molecular mechanism by which GBPs associate with IRGs and positively regulate the recruitment and/or retention should be analyzed in the future.
GMS-IRGs (Irgm1, Irgm2, and Irgm3) are shown to prevent activation of GKS-IRGs, including Irga6 and Irgb6, by limiting the GTP nucleotide exchange from GDP in the detailed biochemical analysis (27). Together with our current data, cell-autonomous immunity dependent on IRGs or GBPs may possess differential inhibitory circuits by GMS-IRGs and RabGDIα, respectively. It is of note that mice lacking Irgm1 or Irgm3 showed high susceptibility to T. gondii, probably due to dysregulation of GKS-IRGs; in stark contrast, RabGDIα-deficient mice displayed resistance to the pathogen.
In conclusion, we have identified RabGDIα as a negative regulator in the Irga6-Gbp2 axis of IFN-γ–inducible GTPase-dependent cell-autonomous immunity to T. gondii. By close analysis of each step of IFN-γ–inducible GTPase-dependent T. gondii PV disruption, other positive and negative regulators for this cell-autonomous immune response might be further identified in the future.
Materials and Methods
Mice, Cells, and Parasites.
All animal experiments were conducted with the approval of the Animal Research Committee of the Research Institute for Microbial Diseases in Osaka University. Detailed information for mice, cells, and T. gondii are provided in SI Materials and Methods.
Reagents.
Detailed information for antibodies and other reagents is provided in SI Materials and Methods.
Immunofluorescence.
Detailed methods for indirect immunofluorescence are provided in SI Materials and Methods.
Generation of Conditional Gdi1-Deficient Mice, Conventional Gdi2-Deficient Mice, and Gbp2-Deficient MEFs.
Detailed protocols for the generation of these knockout mice and MEFs are provided in SI Materials and Methods.
Statistical Analysis.
The unpaired Student’s t test or ANOVA plus post hoc Tukey was used to determine the statistical significance of the in vitro experimental data. Animal experiments in vivo were evaluated with the Mann–Whitney U test or log-rank test. All data were analyzed with Prism software (GraphPad).
SI Materials and Methods
Mice, Cells, and Parasites.
C57BL/6 and B6C3F1 mice were obtained from SLC. ME49 expressing luciferase of T. gondii was maintained in Vero cells by biweekly passage in RPMI 1640 (Nacalai Tesque) supplemented with 2% (vol/vol) heat-inactivated FBS (Nichirei Bioscience), 100 U/mL penicillin, and 0.1 mg/mL streptomycin (Nacalai Tesque). Bone marrow-derived macrophages were differentiated in RPMI 1640 with 10% (vol/vol) FBS, 10% (vol/vol) L929 cell supernatant, 100 U/mL penicillin, and 0.1 mg/mL streptomycin. Peritoneal macrophages were collected from peritoneal cavities 72 h after thioglycolate injection. MEFs and macrophages were maintained in DMEM (Nacalai Tesque) and RPMI 1640 supplemented with 10% (vol/vol) heat-inactivated FBS, 100 U/mL penicillin, and 0.1 mg/mL streptomycin, respectively. All animal experiments were conducted with the approval of the Animal Research Committee of the Research Institute for Microbial Diseases in Osaka University.
Reagents.
Antibodies against Irgb6 (TGTP; sc-11079), Gbp1 (sc-10586), Gbp2 (sc-10588), RabGDIβ (sc-133939), and HA (sc-7392) were purchased from Santa Cruz Biotechnology, Inc. Anti-CD11b (M1/70) PE was obtained from eBioscience. Antibodies against Flag (F3165, F7425), HA (H6908), and Actin (A1978) were purchased from Sigma. Anti-HA antibody (HA.11; MMS-101R) was obtained from Covance. Anti-GAP45 Ab to stain T. gondii was kindly provided by D. Soldati-Favre, University of Geneva, Geneva. Anti-Irga6 (10D7) Ab has been described previously (26). Anti-T. gondii (no. 0281) antibodies were obtained from ViroStat. The rabbit anti-Gbp2 antibody (11854-1-AP) for indirect immunofluorescence study was obtained from Proteintech. Recombinant mouse IFN-γ and TNF-α were obtained from PeproTech. Dextran conjugated with FITC (D22910) or pH-sensor (pHRODO-Green; P35368) was obtained from Life Technologies.
Large-Scale Immunoprecipitation and Mass Spectrometry.
RAW264.7 cells overexpressing HA-mGBP2 were stimulated with 200 U/mL IFN-γ overnight and then infected with T. gondii Pru for 1 h at an moi of 5. Cells were lysed for 30 min at 4 °C in 0.5% Nonidet P-40 lysis buffer [25 mM Tris⋅HCl, pH 7.4, 150 mM NaCl, 5 mM MgCl2, cOmplete EDTA-Free Protease Inhibitor Mixture (Roche) and 0.5 mM GTP], and the nuclear fraction was removed by centrifugation (48). Supernatants were precleared for 3 h at 4 °C with Protein G beads and subsequently incubated for 2 h at 4 °C with a rat monoclonal anti-HA antibody (Roche Applied Science) and Protein G beads. Bound proteins were eluted from the washed beads by boiling at 95 °C in Laemmli buffer and subjected to SDS/PAGE and silver staining. Single bands were excised from each lane of a silver-stained SDS/PAGE gel encompassing the entire molecular weight range. Trypsin-digested extracts were analyzed by reversed phase HPLC and a Thermo-Fisher LTQ linear ion trap mass spectrometer. Peptides were identified from the MS data using SEQUEST algorithms44, which searched a species-specific database generated from National Center for Biotechnology Information’s nonredundant (nr.fasta) database.
Generation of Conditional Gdi1-Deficient Mice.
The Gdi1 gene was isolated from genomic DNA extracted from ES cells (V6.5) by PCR using KOD FX NEO (Toyobo). The targeting vector was constructed by replacement of a 3.0-kb fragment of Gdi1 with a neomycin-resistance gene (neo) cassette driven by a PGK promoter, which follows a splice acceptor (SA) sequence to trap the gene. In addition, a 3.0-kb fragment of Gdi1 genomic DNA containing exon 2, 3, and 4 was inserted between two loxP sites in the targeting vector pKS-SApGKneo-DTA, which contains a diphtheria toxin A (DTA) fragment driven by a PGK promoter. After the targeting vector was transfected into ES cells using electroporation, colonies resistant to G418 were selected and screened by PCR and Southern blotting as described previously (63). Correctly targeted ES cells were injected into C57BL/6 blastocysts, and the chimeric male mice were tested for germ-line transmission by crossing them with female C57BL/6 mice to generate F1 mice heterozygous for Gdi1. Heterozygote Gdi1flox(+neo)/Y male mice were crossed with transgenic CAG-Flpe female mice to remove the neo-cassette franked by two FRT sequences. Mice were genotyped by PCR analysis of DNA extracted from the tail using primers RabGDIα_screening_F and RabGDIα_WT_R. Heterozygote Gdi1flox/+ female mice lacking neo were crossed with a LysM-Cre transgenic mouse expressing the Cre recombinase under the control of the mouse LysM promoter. The deficiency of RabGDIα in macrophages was confirmed by quantitative RT-PCR. Sequences of the primers are showed in Table S1.
Generation of Gdi2- or Gbp2-Deficient MEFs by Cas9/CRISPR Genome Editing.
The insert fragments of Gdi2 and Gbp2 guide RNA (gRNA) were amplified using KOD FX NEO (Toyobo) and primers (Table S1): Gdi2_gRNA1_F and Gdi2_gRNA1_R, Gdi2_gRNA2_F and Gdi2_gRNA2_R, and Gbp2_gRNA1_F and Gbp2_gRNA1_R. The fragment for gRNA was inserted into the gRNA cloning vector (Plasmid 41824) using Gibson Assembly mix (New England Biolabs) to generate gRNA-expressing plasmids. T7 promoter was added to the gRNA template using KOD FX NEO and primers: Gdi2_T7gRNA_F, Gbp2_T7gRNA_F, and gRNA_common_R. The T7-Gdi2 or -Gbp2 gRNA PCR product was gel purified and used as the subsequent generation of gRNA. MEGAshortscript T7 (Life Technologies) was used for the generation of the gRNA. Cas9 mRNA was generated by in vitro transcription (IVT) using an mMESSAGE mMACHINE T7 ULTRA kit (Life technologies) and the template that was amplified by PCR using pEF6-hCas9-Puro and primers (T7Cas9_IVT_F and Cas9_R) (37), and gel-purified. The synthesized gRNA and Cas9 mRNA were purified using a MEGAclear kit (Life Technologies) and eluted in RNase-free water (Nacalai Tesque). To obtain RabGDIβ-deficient MEFs, B6C3F1 (C57BL/6 × C3H) female mice (6 wk old) were superovulated and mated to B6C3F1 stud males. Fertilized one-cell-stage embryos were collected from oviducts and injected into the pronuclei or the cytoplasm with the Cas9 mRNA (100 ng/μL) and the gRNA (50 ng/μL) in accordance with the previous report (64). The injected live embryos were transferred into oviducts of pseudopregnant Institute of Cancer Research (ICR) females at 0.5 days post coitum (dpc) as described previously (65). The Gdi2 loci of the resulting pups were screened using primers: RabGDIβ_KO_F and RabGDIβ_KO_R. The male pup harboring the mutation was mated to C57BL/6 female mice and tested for the germ-line transmission. Heterozygotic mice were intercrossed to generate homozygotic RabGDIβ-deficient embryos, which were used to obtain primary MEFs.
To obtain Gbp2-deficient MEFs, C57BL/6 female mice (6 wk old) were superovulated and mated to C57BL/6 stud males. Fertilized one-cell-stage embryos were collected from oviducts and injected into the pronuclei or the cytoplasm with the Cas9 mRNA (100 ng/μL) and the gRNA (50 ng/μL). The injected live embryos were transferred into oviducts of pseudopregnant ICR females at 0.5 dpc, and 13.5-day-old embryos were collected to generate primary MEFs. The Gbp2 loci of the resulting MEFs were screened using primers: Gbp2_indel_F and Gbp2_indel_R. The Gbp2 protein expression was analyzed by Western blot using anti-Gbp2 antibody.
Expression Plasmids.
To construct the mammalian expression vectors, cDNA fragments were amplified using primer sets: RabGDIα_F and RabGDIα_R; RabGDIβ_F and RabGDIβ_R; Gbp1_F and Gbp1_R; Gbp2_F and Gbp2_R; Gbp3_F and Gbp3_R; Gbp5_F and Gbp5_R; Gbp5_F2 and Gbp_R2; Gbp7_F and Gbp7_R (Table S1). RabGDIs cDNA fragments were ligated into the pcDNA and pMRX-Puromycin vector for the C-terminal Flag-tagged proteins. cDNAs for Gbps were inserted into the pMRX-Blasticidin vector for the N-terminal HA-tagged proteins. Gbp2 cDNA was also cloned into a pcDNA vector for the N-terminal HA-tagged proteins. The series of RabGDIα fragments containing point mutations were generated using the following primers: RabGDIα_F and RabGDIα_Y39V_R for Y39V; RabGDIα_R218A/Y219A_F and RabGDIα_R218A/Y219A_R for R218A/Y219A; RabGDIα_E233S/R240A_F and RabGDIα_E233S/R240A_R for E233S/ R240A; and RabGDIα_M132I_F and RabGDIα_M132I_R for M132I (Table S1). The series of Gbp2 mutants containing point mutations were generated using the following primers: Gbp2_F and Gbp2_C586S_R for C586S; and Gbp2_F and Gbp2_K51A_R for K51A (Table S1). cDNA for RabGDIαY39V, R218A/Y219A, E233S/ R240A, and M132I were ligated into the pcDNA and pMRX-Puromycin vector for the C-terminal Flag-tagged proteins. cDNA for Gbp2 mutants were inserted into the pMRX-Blasticidin vector for the N-terminal HA-tagged proteins. Finally, Rab10 cDNA were amplified using Rab10_F and Rab10_R primers and cloned into the pcDNA vector for the N-terminal HA-tagged proteins. The sequences of all constructs were confirmed by sequencing using ABI PRISM Genetic Analyzer 3130xl (PE Applied Biosystems). Flag-Gbp1 expression vector has been described previously (48). We could not detect recruitment of HA-tagged Gbp1 to T. gondii for unknown reasons. Therefore, Flag-tagged Gbp1 was used in this study.
In Vivo Measurement of Parasites by Imaging.
Female mice (8–12 wk old) were used in experiments. Mice were intraperitoneally infected with 5 × 103 freshly egressed ME49 tachyzoites expressing luciferase that were resuspended in 100 μL of PBS, and bioluminescence was assessed on the indicated days after infection. For the detection of bioluminescence emission, mice were intraperitoneally injected with 3 mg of d-luciferin in 200 μL of PBS (Promega), maintained for 5 min to allow adequate dissemination of the luciferin, and then anesthetized with isoflurane (Dainippon Sumitomo Pharma). At 10 min postinjection of d-luciferin, abdominal photon emission was assessed during a 60-s exposure by an in vivo imaging system (IVIS-Spectrum; Xenogen) and analyzed by Living Image software (Xenogen).
Analysis of Phagocytic Activity and Phagosomal Acidification.
Thioglycolate-elicited mouse macrophages (5.0 × 105 cells per condition) were added in 20 µg/mL FITC-conjugated dextran or 10 µg/mL pH sensor-conjugated dextran. The cells immediately collected after adding those reagents were used as a 0-min sample. The rest of the samples were incubated at 37 °C at the indicated time points. The cells were washed three times with cold PBS and analyzed on FACSVerse (Becton Dickinson).
Immunofluorescence.
MEFs or macrophages stimulated with 10 ng/mL IFN-γ for 24 h were infected with T. gondii (moi = 4 or 0.5, respectively). Cells were fixed for 10 min in PBS containing 3.7% (vol/vol) formaldehyde, permeabilized with PBS containing 0.1% Triton X-100, and then blocked with 8% (vol/vol) FCS in PBS. Subsequently, cells were incubated with anti-GAP45 rabbit antibody (1:500), anti-T. gondii goat antibody (1:100), anti-Irga6 mouse antibody (1:100), anti-Irgb6 goat antibody (1:100), anti-Gbp2 rabbit antibody (1:100), anti-Flag mouse antibody (1:500), anti-HA rabbit antibody (1:100), anti-HA mouse antibody (1:500), anti-CD11b rat antibody (1:500) for 1 h at 37 °C, followed by incubation with Alexa Fluor 594- or Alexa Fluor 647-conjugated anti-mouse IgG, Alexa Fluor 488- or Alexa Fluor 594-conjugated anti-rabbit IgG, or Alexa Fluor 594-conjugated anti-goat IgG (Invitrogen) for 1 h at room temperature in the dark. Nuclei were counterstained with DAPI (Wako). Finally, the immunostained cells were mounted with PermaFluor (Thermo Scientific) on glass slides and analyzed by confocal laser microscopy (FV1200 IX-83, Olympus); the images were analyzed with Fluoview (Olympus).
In Situ Proximity-Ligation Assay.
A Duolink In Situ PLA Kit was used according to the manufacturer's instructions (Olink Bioscience). In brief, MEFs untreated or treated with 10 ng/mL IFN-γ for 24 h were infected with the YFP-expressing T. gondii (moi = 4), fixed, and permeabilized as performed in immunofluorescence. Slides were incubated with primary Abs [anti-Gbp2 rabbit antibody (1:100), anti-Irga6 mouse antibody (1:100), anti-Flag mouse antibody (1:500), or anti-Flag rabbit antibody (1:500)] in antibody buffer for 1 h at 37 °C and then washed in wash buffer A with shaking. Thereafter, slides were incubated with PLA Probes from Duolink anti-mouse PLA MINUS and anti-rabbit PLA PLUS diluted 1:5 in antibody buffer for 1 h at 37 °C and washed in wash buffer A with shaking. For the ligation, slides were incubated with the ligation reagent [Ligation Stock 1:5 in antibody buffer plus ligase (1:40)] for 30 min at 37 °C and washed in wash buffer A. Next, Amplification-Polymerase solution [amplification stock 1:5 in antibody buffer and polymerase (1:80)] was added to the slides and incubated for 100 min at 37 °C, followed by washings in wash buffer B. After the final washing in 0.01× wash buffer B for 1 min, slides were mounted with Duolink In Situ Mounting Medium with DAPI. Finally, glass slides were analyzed by confocal laser microscopy.
Quantitative Real-Time PCR.
Total RNA was extracted, and cDNA was synthesized using Verso Reverse Transcriptase (Thermo). Real-time PCR was performed with a CFX connect real-time PCR system (BIO-RAD) using the Go-Taq Real-Time PCR system (Promega). The values were normalized to the amount of β-actin in each sample. The following primer sets were used: RabGDIα_qpF and RabGDIα_qpR for Gdi1; and Actin_qpF and Actin_qpR for Actb. The primer sequences are listed in Table S1.
Western Blot Analysis.
MEFs and macrophages untreated or treated with 10 ng/mL IFN-γ for 24 h were lysed in a lysis buffer containing 1% Nonidet P-40, 150 mM NaCl, and 20 mM Tris⋅HCl (pH 7.5), 1 mM EDTA, and protease inhibitor mixture (Nacalai Tesque).
The cell lysates were separated by SDS/PAGE, transferred to polyvinyl difluoride membrane, and subjected to Western blotting using the indicated antibodies.
Immunoprecipitation.
MEFs or 293T cells were lysed in lysis buffer (0.5% Nonidet P-40, 150 mM NaCl, 1 mM EDTA, and 20 mM Tris⋅HCl, pH 7.5) containing protease inhibitor mixture (Nacalai Tesque) and phosphatase inhibitor mixture (Nacalai Tesque). For transient protein expression, 1 d before lysis, HA-tagged Rab10 and Flag-tagged RabGDIα plasmids were cotransfected into 293T cells using Lipofectamine 2000 reagent (Invitrogen) (Fig. 5C). Cell lysates were precleared with protein G-Sepharose (GE Healthcare) for 1 h and then incubated with protein G-Sepharose containing 1 μg of the indicated antibodies for 12 h with rotation at 4 °C. The immunoprecipitants were washed three times with lysis buffer, eluted by boiling with Laemmli sample buffer, and subjected to Western blotting using the indicated antibodies, as described above.
Measurement of T. gondii Numbers by Luciferase Assay.
MEFs (5 × 105) or peritoneal macrophages (1 × 106) were untreated or treated with 10 ng/mL IFN-γ and/or 10 ng/mL TNF-α for 24 h, and followed by the infection of the luciferase-expressing T. gondii (moi = 0.5) for indicated periods. To measure the T. gondii titers, the infected cells were harvested and lysed with 100 μL of lysis buffer (Promega) with sonication. After centrifugation at 20,000 × g at 4 °C, the luciferase activity of 5 μL of the supernatants was measured using the Dual-Luciferase Reporter Assay System (Promega) using a GLOMAX 20/20 luminometer (Promega). The percentages of the activities in IFN-γ–stimulated cells over those in unstimulated cells were shown as “Relative T. gondii numbers” in figures.
Calculation of Parasite Numbers in Organs by Luciferase Assay.
To measure the T. gondii titers in organs, organs were removed 8 dpi (spleens) or 20 dpi (spleens, lungs, brains, kidneys, and livers). Brains, lungs, spleens, and kidneys were homogenized and lysed in 1 mL of Passive Lysis Buffer (Promega) with sonication. Livers were homogenized in 2 mL of lysis buffer and sonicated. After centrifugation at 20,000 × g at 4 °C, the luciferase activity of 5 μL of the supernatants was measured using the Dual-Luciferase Reporter Assay System (Promega) as described previously (21). To calculate the parasite number according to the luciferase units, serial dilutions of ME49 parasites expressing luciferase (and YFP) (104 to 107 parasites per each log10 point) were prepared and lysed, and the luciferase activities were measured as described above, resulting in an almost linear standard curve (Fig. S6C).
Cell Death Assay.
Macrophages were cultured in six-well plates (1 × 106 cells per well) with 10 ng/mL IFN-γ for 24 h and/or infected with ME49 tachyzoites (moi = 0.5). At 1 or 36 h postinfection, the concentrations of lactate dehydrogenase (LDH) in the culture supernatants were measured by CytoTox 96 Non-Radioactive Cytotoxity Assay (Promega). The percentage of LDH release was calculated in the following formula: (LDH infected − LDH uninfected)/(LDH total lysis − LDH uninfected) × 100.
Measurement of the Production of NO2−.
Peritoneal macrophages were cultured in six-well plates (1 × 106 cells per well) with 10 ng/mL TNF-α and/or IFN-γ for 24 h. The concentrations of NO in the culture supernatant were measured by NO2/NO3 Assay Kit-FX (Dojindo) according to the manufacturer’s instructions.
Measurement of Cytokines.
The concentrations of proinflammatory cytokines in the sera taken from mice infected with ME49 T. gondii at day 3, 6, and 9 were measured by ELISA using antibodies for IFN-γ and IL-12 p40 obtained from eBioscience.
In Silico RabGDIα Structural Modeling.
We submitted the mouse RabGDIα sequence to the HHPred server, and the top-ranked templates and alignments were selected for 3D modeling (66). The top two templates were lipid-bound forms so we selected the third-best template (PDB identifier 2bcg). The sequence identity to the mouse RabGDIα was 53%, which was high enough to build an accurate model using the fragment-assembly modeling tool spanner in combination with OSCAR-star side-chain modeling (67). The protein surface was generated and rendered by the ef-surf and jV proGrams, respectively (68).
Acknowledgments
We thank M. Enomoto for secretarial and technical assistance. We thank Dr. Akiko Iwasaki for suggestions and discussion of this study. We also thank Dr. Dominique Soldati-Favre for providing us with the anti-T. gondii antibody. This work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Homeostatic regulation by various types of cell death, 15H01377; and Matoryoshka-type evolution, 26117713) from the Ministry of Education, Culture, Sports, Science and Technology; The Inoue Research Award; the Takeda Science Foundation; the Naito Foundation; the Daiichi-Sankyo Foundation of Life Science; and the Sumitomo Foundation and Research Foundation for Microbial Diseases of Osaka University. E.-M.F. is supported by a Wellcome Trust Career Development Fellowship and by Medical Research Council Grant MC_UP_1202/12. J.O. is the recipient of a scholarship from the Iwadare Scholarship Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1510031112/-/DCSupplemental.
References
- 1.Boehm U, Klamp T, Groot M, Howard JC. Cellular responses to interferon-gamma. Annu Rev Immunol. 1997;15:749–795. doi: 10.1146/annurev.immunol.15.1.749. [DOI] [PubMed] [Google Scholar]
- 2.MacMicking JD. Interferon-inducible effector mechanisms in cell-autonomous immunity. Nat Rev Immunol. 2012;12(5):367–382. doi: 10.1038/nri3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sadler AJ, Williams BR. Interferon-inducible antiviral effectors. Nat Rev Immunol. 2008;8(7):559–568. doi: 10.1038/nri2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim BH, Shenoy AR, Kumar P, Bradfield CJ, MacMicking JD. IFN-inducible GTPases in host cell defense. Cell Host Microbe. 2012;12(4):432–444. doi: 10.1016/j.chom.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deretic V. Autophagy as an immune defense mechanism. Curr Opin Immunol. 2006;18(4):375–382. doi: 10.1016/j.coi.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Shenoy AR, et al. GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science. 2012;336(6080):481–485. doi: 10.1126/science.1217141. [DOI] [PubMed] [Google Scholar]
- 7.Montoya JG, Remington JS. Management of Toxoplasma gondii infection during pregnancy. Clin Infect Dis. 2008;47(4):554–566. doi: 10.1086/590149. [DOI] [PubMed] [Google Scholar]
- 8.Boothroyd JC. Toxoplasma gondii: 25 years and 25 major advances for the field. Int J Parasitol. 2009;39(8):935–946. doi: 10.1016/j.ijpara.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sibley LD. Invasion and intracellular survival by protozoan parasites. Immunol Rev. 2011;240(1):72–91. doi: 10.1111/j.1600-065X.2010.00990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yarovinsky F, Sher A. Toll-like receptor recognition of Toxoplasma gondii. Int J Parasitol. 2006;36(3):255–259. doi: 10.1016/j.ijpara.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Hunter CA, Remington JS. The role of IL12 in toxoplasmosis. Res Immunol. 1995;146(7-8):546–552. doi: 10.1016/0923-2494(96)83030-6. [DOI] [PubMed] [Google Scholar]
- 12.Koblansky AA, et al. Recognition of profilin by Toll-like receptor 12 is critical for host resistance to Toxoplasma gondii. Immunity. 2013;38(1):119–130. doi: 10.1016/j.immuni.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrade WA, et al. Combined action of nucleic acid-sensing Toll-like receptors and TLR11/TLR12 heterodimers imparts resistance to Toxoplasma gondii in mice. Cell Host Microbe. 2013;13(1):42–53. doi: 10.1016/j.chom.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aliberti J, et al. CCR5 provides a signal for microbial induced production of IL-12 by CD8 alpha+ dendritic cells. Nat Immunol. 2000;1(1):83–87. doi: 10.1038/76957. [DOI] [PubMed] [Google Scholar]
- 15.Aliberti J, et al. Molecular mimicry of a CCR5 binding-domain in the microbial activation of dendritic cells. Nat Immunol. 2003;4(5):485–490. doi: 10.1038/ni915. [DOI] [PubMed] [Google Scholar]
- 16.Langermans JA, et al. IFN-gamma-induced L-arginine-dependent toxoplasmastatic activity in murine peritoneal macrophages is mediated by endogenous tumor necrosis factor-alpha. J Immunol. 1992;148(2):568–574. [PubMed] [Google Scholar]
- 17.Zhao Y, et al. Virulent Toxoplasma gondii evade immunity-related GTPase-mediated parasite vacuole disruption within primed macrophages. J Immunol. 2009;182(6):3775–3781. doi: 10.4049/jimmunol.0804190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter CA, Sibley LD. Modulation of innate immunity by Toxoplasma gondii virulence effectors. Nat Rev Microbiol. 2012;10(11):766–778. doi: 10.1038/nrmicro2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taylor GA, Feng CG, Sher A. p47 GTPases: Regulators of immunity to intracellular pathogens. Nat Rev Immunol. 2004;4(2):100–109. doi: 10.1038/nri1270. [DOI] [PubMed] [Google Scholar]
- 20.Selleck EM, et al. Guanylate-binding protein 1 (Gbp1) contributes to cell-autonomous immunity against Toxoplasma gondii. PLoS Pathog. 2013;9(4):e1003320. doi: 10.1371/journal.ppat.1003320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, et al. A cluster of interferon-γ-inducible p65 GTPases plays a critical role in host defense against Toxoplasma gondii. Immunity. 2012;37(2):302–313. doi: 10.1016/j.immuni.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Degrandi D, et al. Murine guanylate binding protein 2 (mGBP2) controls Toxoplasma gondii replication. Proc Natl Acad Sci USA. 2013;110(1):294–299. doi: 10.1073/pnas.1205635110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liesenfeld O, et al. The IFN-γ-inducible GTPase, Irga6, protects mice against Toxoplasma gondii but not against Plasmodium berghei and some other intracellular pathogens. PLoS One. 2011;6(6):e20568. doi: 10.1371/journal.pone.0020568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor GA, et al. Pathogen-specific loss of host resistance in mice lacking the IFN-gamma-inducible gene IGTP. Proc Natl Acad Sci USA. 2000;97(2):751–755. doi: 10.1073/pnas.97.2.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melzer T, Duffy A, Weiss LM, Halonen SK. The gamma interferon (IFN-gamma)-inducible GTP-binding protein IGTP is necessary for toxoplasma vacuolar disruption and induces parasite egression in IFN-gamma-stimulated astrocytes. Infect Immun. 2008;76(11):4883–4894. doi: 10.1128/IAI.01288-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papic N, Hunn JP, Pawlowski N, Zerrahn J, Howard JC. Inactive and active states of the interferon-inducible resistance GTPase, Irga6, in vivo. J Biol Chem. 2008;283(46):32143–32151. doi: 10.1074/jbc.M804846200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunn JP, et al. Regulatory interactions between IRG resistance GTPases in the cellular response to Toxoplasma gondii. EMBO J. 2008;27(19):2495–2509. doi: 10.1038/emboj.2008.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ling YM, et al. Vacuolar and plasma membrane stripping and autophagic elimination of Toxoplasma gondii in primed effector macrophages. J Exp Med. 2006;203(9):2063–2071. doi: 10.1084/jem.20061318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Howe DK, Sibley LD. Toxoplasma gondii comprises three clonal lineages: Correlation of parasite genotype with human disease. J Infect Dis. 1995;172(6):1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 30.Niedelman W, et al. The rhoptry proteins ROP18 and ROP5 mediate Toxoplasma gondii evasion of the murine, but not the human, interferon-gamma response. PLoS Pathog. 2012;8(6):e1002784. doi: 10.1371/journal.ppat.1002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behnke MS, et al. Virulence differences in Toxoplasma mediated by amplification of a family of polymorphic pseudokinases. Proc Natl Acad Sci USA. 2011;108(23):9631–9636. doi: 10.1073/pnas.1015338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reese ML, Zeiner GM, Saeij JP, Boothroyd JC, Boyle JP. Polymorphic family of injected pseudokinases is paramount in Toxoplasma virulence. Proc Natl Acad Sci USA. 2011;108(23):9625–9630. doi: 10.1073/pnas.1015980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinfeldt T, et al. Phosphorylation of mouse immunity-related GTPase (IRG) resistance proteins is an evasion strategy for virulent Toxoplasma gondii. PLoS Biol. 2010;8(12):e1000576. doi: 10.1371/journal.pbio.1000576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fentress SJ, et al. Phosphorylation of immunity-related GTPases by a Toxoplasma gondii-secreted kinase promotes macrophage survival and virulence. Cell Host Microbe. 2010;8(6):484–495. doi: 10.1016/j.chom.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi J, et al. The parasitophorous vacuole membrane of Toxoplasma gondii is targeted for disruption by ubiquitin-like conjugation systems of autophagy. Immunity. 2014;40(6):924–935. doi: 10.1016/j.immuni.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haldar AK, Piro AS, Pilla DM, Yamamoto M, Coers J. The E2-like conjugation enzyme Atg3 promotes binding of IRG and Gbp proteins to Chlamydia- and Toxoplasma-containing vacuoles and host resistance. PLoS One. 2014;9(1):e86684. doi: 10.1371/journal.pone.0086684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohshima J, et al. Role of mouse and human autophagy proteins in IFN-γ-induced cell-autonomous responses against Toxoplasma gondii. J Immunol. 2014;192(7):3328–3335. doi: 10.4049/jimmunol.1302822. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Z, et al. Autophagosome-independent essential function for the autophagy protein Atg5 in cellular immunity to intracellular pathogens. Cell Host Microbe. 2008;4(5):458–469. doi: 10.1016/j.chom.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haldar AK, et al. IRG and GBP host resistance factors target aberrant, “non-self” vacuoles characterized by the missing of “self” IRGM proteins. PLoS Pathog. 2013;9(6):e1003414. doi: 10.1371/journal.ppat.1003414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsui Y, et al. Molecular cloning and characterization of a novel type of regulatory protein (GDI) for smg p25A, a ras p21-like GTP-binding protein. Mol Cell Biol. 1990;10(8):4116–4122. doi: 10.1128/mcb.10.8.4116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sasaki T, et al. Purification and characterization from bovine brain cytosol of a protein that inhibits the dissociation of GDP from and the subsequent binding of GTP to smg p25A, a ras p21-like GTP-binding protein. J Biol Chem. 1990;265(4):2333–2337. [PubMed] [Google Scholar]
- 42.Nishimura N, Nakamura H, Takai Y, Sano K. Molecular cloning and characterization of two rab GDI species from rat brain: Brain-specific and ubiquitous types. J Biol Chem. 1994;269(19):14191–14198. [PubMed] [Google Scholar]
- 43.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. Interferon-gamma: The major mediator of resistance against Toxoplasma gondii. Science. 1988;240(4851):516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 44.Fairn GD, Grinstein S. How nascent phagosomes mature to become phagolysosomes. Trends Immunol. 2012;33(8):397–405. doi: 10.1016/j.it.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Zhao YO, Khaminets A, Hunn JP, Howard JC. Disruption of the Toxoplasma gondii parasitophorous vacuole by IFNgamma-inducible immunity-related GTPases (IRG proteins) triggers necrotic cell death. PLoS Pathog. 2009;5(2):e1000288. doi: 10.1371/journal.ppat.1000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sibley LD, Adams LB, Fukutomi Y, Krahenbuhl JL. Tumor necrosis factor-alpha triggers antitoxoplasmal activity of IFN-gamma primed macrophages. J Immunol. 1991;147(7):2340–2345. [PubMed] [Google Scholar]
- 47.Weibrecht I, et al. Proximity ligation assays: A recent addition to the proteomics toolbox. Expert Rev Proteomics. 2010;7(3):401–409. doi: 10.1586/epr.10.10. [DOI] [PubMed] [Google Scholar]
- 48.Virreira Winter S, et al. Determinants of GBP recruitment to Toxoplasma gondii vacuoles and the parasitic factors that control it. PLoS One. 2011;6(9):e24434. doi: 10.1371/journal.pone.0024434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prakash B, Praefcke GJ, Renault L, Wittinghofer A, Herrmann C. Structure of human guanylate-binding protein 1 representing a unique class of GTP-binding proteins. Nature. 2000;403(6769):567–571. doi: 10.1038/35000617. [DOI] [PubMed] [Google Scholar]
- 50.Praefcke GJ, et al. Identification of residues in the human guanylate-binding protein 1 critical for nucleotide binding and cooperative GTP hydrolysis. J Mol Biol. 2004;344(1):257–269. doi: 10.1016/j.jmb.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 51.Sinensky M, Lutz RJ. The prenylation of proteins. BioEssays. 1992;14(1):25–31. doi: 10.1002/bies.950140106. [DOI] [PubMed] [Google Scholar]
- 52.Stickney JT, Buss JE. Murine guanylate-binding protein: Incomplete geranylgeranyl isoprenoid modification of an interferon-gamma-inducible guanosine triphosphate-binding protein. Mol Biol Cell. 2000;11(7):2191–2200. doi: 10.1091/mbc.11.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alory C, Balch WE. Organization of the Rab-GDI/CHM superfamily: The functional basis for choroideremia disease. Traffic. 2001;2(8):532–543. doi: 10.1034/j.1600-0854.2001.20803.x. [DOI] [PubMed] [Google Scholar]
- 54.Rak A, et al. Structure of Rab GDP-dissociation inhibitor in complex with prenylated YPT1 GTPase. Science. 2003;302(5645):646–650. doi: 10.1126/science.1087761. [DOI] [PubMed] [Google Scholar]
- 55.Ignatev A, Kravchenko S, Rak A, Goody RS, Pylypenko O. A structural model of the GDP dissociation inhibitor rab membrane extraction mechanism. J Biol Chem. 2008;283(26):18377–18384. doi: 10.1074/jbc.M709718200. [DOI] [PubMed] [Google Scholar]
- 56.Schalk I, et al. Structure and mutational analysis of Rab GDP-dissociation inhibitor. Nature. 1996;381(6577):42–48. doi: 10.1038/381042a0. [DOI] [PubMed] [Google Scholar]
- 57.Garrett MD, Zahner JE, Cheney CM, Novick PJ. GDI1 encodes a GDP dissociation inhibitor that plays an essential role in the yeast secretory pathway. EMBO J. 1994;13(7):1718–1728. doi: 10.1002/j.1460-2075.1994.tb06436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nuoffer C, Balch WE. GTPases: Multifunctional molecular switches regulating vesicular traffic. Annu Rev Biochem. 1994;63:949–990. doi: 10.1146/annurev.bi.63.070194.004505. [DOI] [PubMed] [Google Scholar]
- 59.Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of Rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368(6467):157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- 60.D’Adamo P, et al. Deletion of the mental retardation gene Gdi1 impairs associative memory and alters social behavior in mice. Hum Mol Genet. 2002;11(21):2567–2580. doi: 10.1093/hmg/11.21.2567. [DOI] [PubMed] [Google Scholar]
- 61.Shisheva A, Chinni SR, DeMarco C. General role of GDP dissociation inhibitor 2 in membrane release of Rab proteins: Modulations of its functional interactions by in vitro and in vivo structural modifications. Biochemistry. 1999;38(36):11711–11721. doi: 10.1021/bi990200r. [DOI] [PubMed] [Google Scholar]
- 62.Kresse A, et al. Analyses of murine GBP homology clusters based on in silico, in vitro and in vivo studies. BMC Genomics. 2008;9:158. doi: 10.1186/1471-2164-9-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yamamoto M, et al. ATF6beta is a host cellular target of the Toxoplasma gondii virulence factor ROP18. J Exp Med. 2011;208(7):1533–1546. doi: 10.1084/jem.20101660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang H, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ma JS, et al. Selective and strain-specific NFAT4 activation by the Toxoplasma gondii polymorphic dense granule protein GRA6. J Exp Med. 2014;211(10):2013–2032. doi: 10.1084/jem.20131272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soding J, Biegert A, Lupas AN. The HHpred interactive server for protein homology detection and structure prediction. Nucleic Acids Res. 2005;33(Web Server issue):W244–W248. doi: 10.1093/nar/gki408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang S, Zheng D, Zhang C, Standley DM. Fast and accurate prediction of protein side-chain conformations. Bioinformatics. 2011;27(20):2913–2914. doi: 10.1093/bioinformatics/btr482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kinoshita K, Nakamura H. eF-site and PDBjViewer: Database and viewer for protein functional sites. Bioinformatics. 2004;20(8):1329–1330. doi: 10.1093/bioinformatics/bth073. [DOI] [PubMed] [Google Scholar]