Significance
Preventing extinction of small populations in rapidly changing environments is crucial to long-term preservation of diversity, because the creation of large reserves is often not feasible. An option immediately available to managers is bringing migrants in to increase size or improve genetic composition of populations at risk. We experimentally manipulate different types and combinations of migrants to evaluate which will be most effective in rescuing populations from extinction. We find that migration of numerous individuals can reduce the probability of extinction. However, migration of just a few genetically distinct individuals both reduces probability of extinction and dramatically increases fitness and population size. We suggest managers with limited conservation resources should prioritize genetic rescue over increasing demographic size for small populations.
Keywords: genetic rescue, extinction, migration, evolutionary rescue, adaptation
Abstract
Setting aside high-quality large areas of habitat to protect threatened populations is becoming increasingly difficult as humans fragment and degrade the environment. Biologists and managers therefore must determine the best way to shepherd small populations through the dual challenges of reductions in both the number of individuals and genetic variability. By bringing in additional individuals, threatened populations can be increased in size (demographic rescue) or provided with variation to facilitate adaptation and reduce inbreeding (genetic rescue). The relative strengths of demographic and genetic rescue for reducing extinction and increasing growth of threatened populations are untested, and which type of rescue is effective may vary with population size. Using the flour beetle (Tribolium castaneum) in a microcosm experiment, we disentangled the genetic and demographic components of rescue, and compared them with adaptation from standing genetic variation (evolutionary rescue in the strictest sense) using 244 experimental populations founded at either a smaller (50 individuals) or larger (150 individuals) size. Both types of rescue reduced extinction, and those effects were additive. Over the course of six generations, genetic rescue increased population sizes and intrinsic fitness substantially. Both large and small populations showed evidence of being able to adapt from standing genetic variation. Our results support the practice of genetic rescue in facilitating adaptation and reducing inbreeding depression, and suggest that demographic rescue alone may suffice in larger populations even if only moderately inbred individuals are available for addition.
Human activities, climate change, and habitat loss are putting thousands of species at risk for extinction (1). Traditional conservation approaches that concentrate on improving habitat quality and size are becoming challenging to implement as human populations expand and degrade natural resources at an ever increasing rate. Thus, conservation efforts that are constrained by the availability of habitat may instead need to focus on improving a species’ prospect of survival by maintaining sufficient population sizes and supporting the ability of populations to adapt to changing conditions. The most successful approaches are likely to be eco-evolutionary in focus, and thus aim to manipulate evolutionary processes such as inbreeding and the potential for adaptation, to influence ecological dynamics and, ultimately, population persistence.
Eco-evolutionary approaches often rely on facilitating movement of individuals among small, threatened populations (2). Brown and Kodric-Brown (3) showed theoretically that immigration in natural systems can save small populations from extinction and referred to this process as the “rescue effect.” This phenomenon has since been well documented (4–8), and human-facilitated immigration has been used to rescue populations threatened by degraded habitat or inbreeding depression, and to re-establish populations where they have been locally extirpated.
Immigration can rescue a population from extinction by either increasing its size or increasing population fitness (3). The term “demographic rescue” refers to increases in numbers of individuals that buffer a population against stochastic fluctuations and reduce Allee effects, which are processes that small populations often face (3, 9, 10). A larger population size may also have long-term effects on population fitness. For instance, the increase in numbers may give a declining population time to adapt to a challenging environment, even if migrants do not immediately bring about an appreciable genetic change (11). On the other hand, if migrants are not adapted, they may slow the process of adaptation via swamping (12–14).
The term “genetic rescue” is defined as an increase in population fitness due to the genetic contributions of immigrants (15) via reducing inbreeding depression or facilitating adaptation by enhancing genetic variation (2, 4, 6, 7, 15, 16). Some authors use genetic rescue to refer only to the reduction in inbreeding depression with outcrossing, excluding adaptive processes (10, 17). Our definition above follows the broader sense (6, 15).
Both demographic rescue and genetic rescue can be potent, but their relative strengths and effects are completely unknown. Moreover, rescue via managed movement of individuals may not always be needed. In some cases, populations could simply adapt to the changed environment, leading to increased population growth rates and population sizes. This process has been called “evolutionary rescue” (10, 17–21). Research on evolutionary rescue initially focused on adaptation to a challenging environment from standing variation (18, 21), but, clearly, populations might adapt more quickly if migrants arrive carrying alleles that facilitate adaptation to the degraded habitat (22). The concept of evolutionary rescue can include migration as well (10, 23). Thus, there is an area of overlap in the use of the terms “genetic” and “evolutionary” rescue. For our purposes here, we use the term evolutionary rescue in the strictest sense of adaptation to a challenging environment from standing variation. Regardless of the exact process, the defining feature of any rescue effect is higher population size and/or an increase in intrinsic fitness in a given habitat.
In an ideal situation, when trying to improve the prospects of a population that is at risk for extinction, a manager would bring in many genetically variable individuals that are adapted to the environment. Such individuals would solve both demographic and genetic challenges faced by the population that is at risk (24). However, such individuals typically are a limiting resource. To aid in effective management, it is therefore critical to disentangle the relative importance of demographic and genetic processes. Because greater numbers of migrants harbor greater genetic diversity in most natural populations (25, 26), the best way to separate these processes is with experiments in which numbers of individuals and the genetic variation they harbor do not covary.
Furthermore, the relative importance of demographic and genetic processes may be context-dependent. For instance, the role of purely demographic processes will depend upon population size, potentially being more important when populations are smaller (25, 27). Understanding the relative importance of genetic and demographic processes and how they compare with adaptation from standing variation will fill a fundamental knowledge gap that only experimental eco-evolutionary research can fully resolve.
Here, we manipulate immigration to small failing populations to evaluate their eco-evolutionary dynamics and understand better how to rescue populations from extinction. We experimentally partitioned the demographic and genetic effects of immigration using microcosms of the red flour beetle (Tribolium castaneum) (28). We exposed replicate experimental populations to a challenging novel environment (an alternative carbohydrate source and lower nutrient availability) to simulate a degraded habitat pushing a small population toward extinction. Our choice of environment was such that the expected density-independent, finite rate of increase of populations without rescue (i.e., intrinsic fitness) would be less than 1, and thus those populations would decline to extinction without adaptation. We studied experimental populations founded at two sizes: 50 or 150 individuals. These sizes are both small enough to represent situations that would be of immediate concern to natural resource managers but large enough that extinction should take more than one generation in most cases. Below, we refer to these different sets of experimental populations as “small” and “large.” We expected these differences in population size to affect the feasibility of evolutionary rescue and the relative importance of demographic and genetic mechanisms contributing to rescue from extinction.
Each experimental population received one of four treatments: evolutionary rescue, in which no immigrants were added; demographic rescue, in which population size was increased; genetic rescue, in which genetic variation was added; and a combination of demographic and genetic rescue. All treatments were implemented one time, in the second generation following exposure to the novel environment, when populations had declined below their initial founding sizes. All migrants had spent a single generation on the challenging experimental medium to minimize carryover of maternal effects from the standard medium. Populations assigned to the demographic rescue treatment received an addition of immigrants to stabilize population size (∼20% increase in numbers) using individuals from the same source population as the receiving population. Thus, the genetic composition of the populations was altered minimally. Populations in the genetic rescue treatment had one (small populations) or three (large populations) beetles replaced one-for-one with individuals from an alternate source population with a different genetic background. This treatment thus had no effect on population size, but the immigrants could both reduce inbreeding and supply adaptive variation because they came from a distinct population that was less maladapted to the experimental environment than the receiving population (SI Methods and Table S1). We chose one migrant for small populations for two reasons: to keep the migration rate lower than one migrant per generation (29) and because individuals adapted to a challenging environment are likely to be limiting. We tripled that number for large populations to keep the treatments proportional. The combination of the demographic and genetic rescue treatments entailed increasing population size by adding multiple individuals from the same source population and one (small populations) or three (large populations) migrants from the alternate source population. We tracked populations for six generations (including two generations before adding immigrants) and evaluated extinction, population size, and changes in intrinsic fitness following rescue.
Table S1.
Ninety-five percent confidence interval for the density independent finite growth rates of experimental and migrant populations on standard medium and 97% corn medium
| Confidence interval | |||
| Strain | Medium | Lower | Upper |
| RR | Standard | 2.53 | 4.06 |
| Corn | 0.57 | 0.93 | |
| SF | Standard | 2.00 | 3.23 |
| Corn | 1.33 | 2.29 | |
SI Methods
The growth rate of the experimental strains was evaluated on a standard medium (in 30 mL containing 28.5 mL wheat flour and 1.5 mL brewer's yeast) and an experimental medium (in patches of 30 mL containing 29.1 mL wheat flour, 0.885 mL corn flour, and 0.045 mL brewer's yeast) at a single founding size (32 individuals) (Table S1). Adult beetles were allowed 24 h to lay eggs; they were then removed, and their offspring were allowed 35 d to develop. Data were collected in the second generation on the two medium types so that maternal environment would not skew results. The natural log of growth rate (Nt/Nt−1) was analyzed as a function of strain (SF or RR), medium (standard or experimental), block (as a random effect), and interactions between those factors.
Results
Extinction.
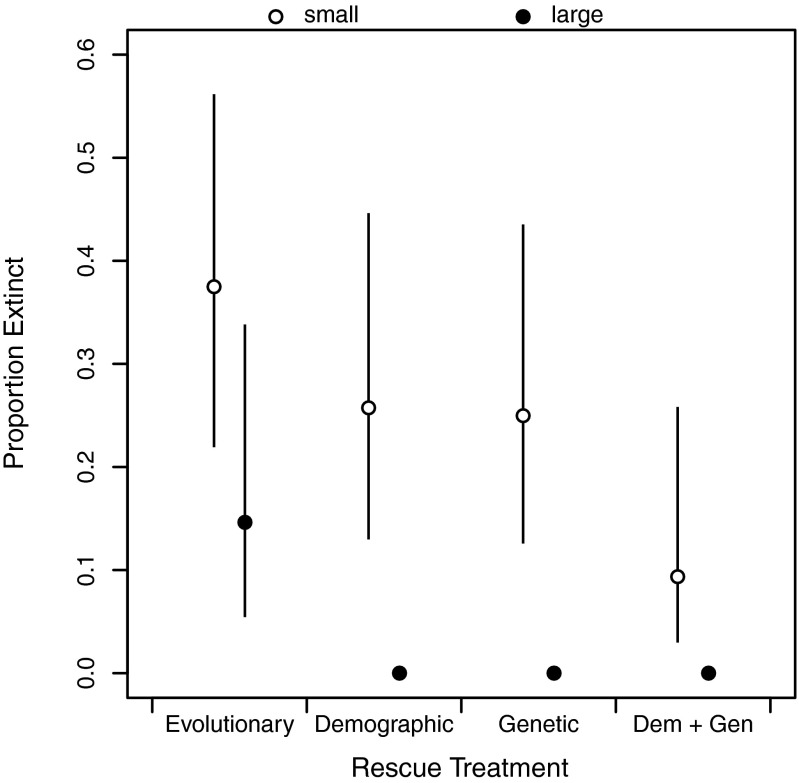
Large populations only went extinct without immigration (evolutionary rescue). All three of the rescue treatments that included migration prevented extinction of large populations entirely (Fig. 1). Small populations experienced more extinctions than large populations (F1, 238 = 14.4, P = 0.0002), with the most extinctions occurring in the evolutionary rescue treatment (Fig. 1). Demographic and genetic rescue both reduced extinction (Fig. 1; demographic: F1, 238 = 5.11, P = 0.025; genetic: F1, 238 = 4.05, P = 0.045), and their effects were additive (F1, 238 = 0.07, P = 0.792; Table S2), giving the overall lowest extinction rate for populations that received combined demographic and genetic rescue.
Fig. 1.
Proportion of initially small (founding size = 50, ○) or large (founding size = 150, ●) experimental populations that went extinct during the experiment in the evolutionary rescue (no immigration), demographic rescue, genetic rescue, and combined demographic and genetic (Dem + Gen) rescue treatments. Error bars show 95% confidence intervals for the estimated proportion. In large populations, there were no extinctions in treatments that received any immigration; extinctions occurred only in the evolutionary rescue treatment.
Table S2.
Results from analysis of extinction
| Source | F | df | P |
| Size | 14.36 | 1, 238 | 0.0002 |
| Demographic rescue | 5.11 | 1, 238 | 0.025 |
| Genetic rescue | 4.05 | 1, 238 | 0.045 |
| Demographic × genetic | 0.07 | 1, 238 | 0.792 |
Population Size.
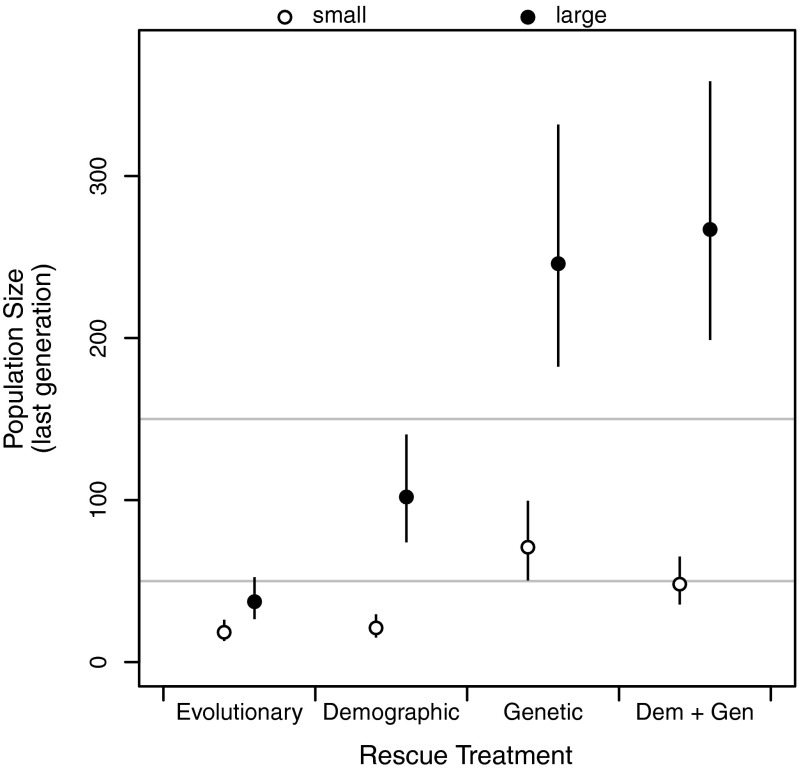
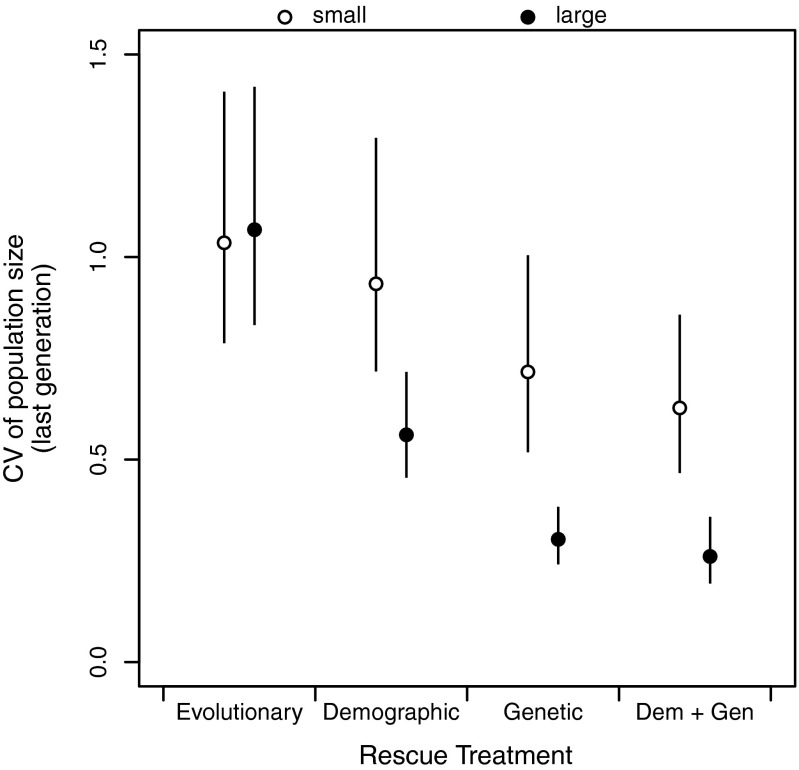
Following introduction into the challenging novel environment, population sizes initially increased (due to carryover effects from the high-quality natal environment) and then dropped dramatically (Fig. 2 A and B). By the last generation, the extant populations that had grown above the initial founding size were almost entirely those populations that received genetic rescue (Fig. S1 and Tables S3 and S4). Variability in final population sizes was comparable across all four treatments in small populations. In contrast, large populations receiving genetic rescue either alone or in combination with demographic rescue were the least variable, and demographic rescue reduced variation in population size relative to no immigrants (Fig. S2). There was more variation in population size among small populations than among large populations, except for large populations in the evolutionary rescue treatment. Large populations in the evolutionary rescue treatment were small, on average, and varied considerably in size (Figs. S1 and S2).
Fig. 2.
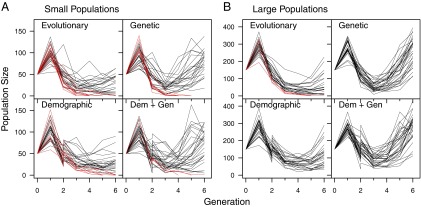
Population sizes of initially small (A; founding size = 50) or large (B; founding size = 150) populations through time. Due to environmental carryover effects, populations first increased before declining sharply in the novel environment. One-time rescue treatments were initiated at the beginning of generation 2 (note the population size increase for treatments receiving demographic inputs). Red lines indicate populations that went extinct, or had only two individuals in generation 6.
Fig. S1.
Final population size of initially small (founding size = 50, ○) or large (founding size = 150, ●) experimental populations. Horizontal gray lines show starting population sizes, and error bars are 95% confidence intervals. Dem + Gen, Demographic + Genetic.
Table S3.
Mean final sizes of extant populations in the different treatments, with 95% confidence intervals
| Starting population size | Rescue type | Final population size | Confidence interval |
| Large (150) | No migration | 37.3 | 26.5–52.4 |
| Demographic | 101.9 | 73.9–140.5 | |
| Genetic | 245.9 | 182.3–331.8 | |
| Demographic + genetic | 267.0 | 198.8–358.5 | |
| Small (50) | No migration | 18.4 | 13.0–26.1 |
| Demographic | 21.1 | 15.1–29.5 | |
| Genetic | 70.9 | 50.4–99.6 | |
| Demographic + genetic | 48.1 | 35.5–65.2 |
Table S4.
Results from analysis of population size of extant populations in the final generation
| Source | F | df | P |
| Size | 126.0 | 1, 207 | <0.0001 |
| Demographic rescue | 3.21 | 1, 207 | 0.07 |
| Genetic rescue | 115.7 | 1, 207 | <0.0001 |
| Size × demographic rescue | 8.25 | 1, 207 | 0.0045 |
| Size × genetic rescue | 2.13 | 1, 207 | 0.15 |
| Demographic × genetic rescue | 9.62 | 1, 207 | 0.0022 |
| Size × demographic × genetic rescue | 0.7 | 1, 207 | 0.39 |
Fig. S2.
Coefficients of variation (CV) of population size of initially small (founding size = 50, ○) or large (founding size = 150, ●) experimental populations in the final generation. Error bars are 95% confidence intervals.
Intrinsic Fitness.
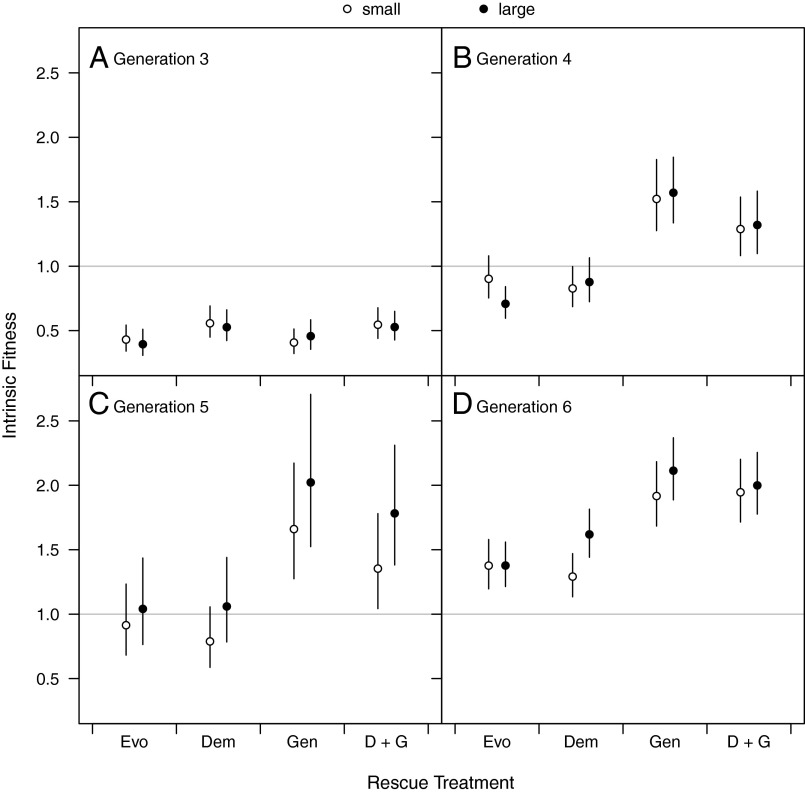
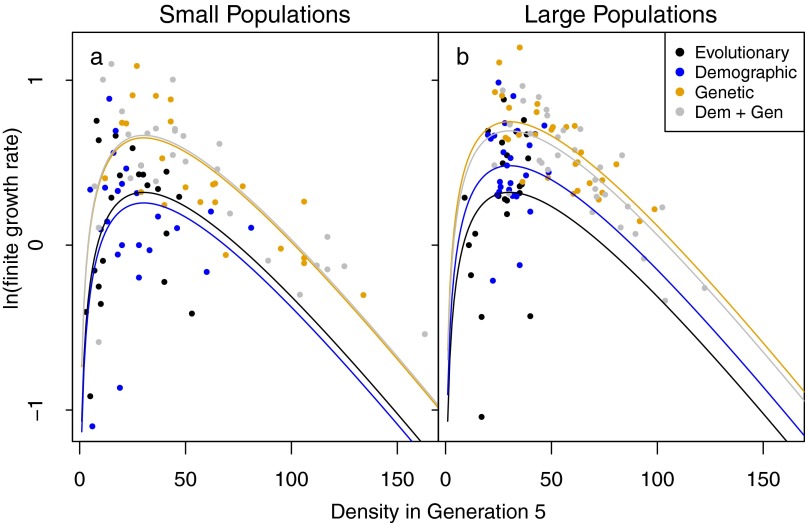
The course of changes in intrinsic fitness following rescue shows the difference in importance of different types of migrants through time. Immediately following rescue (generation 3), intrinsic fitness was below 1; populations were declining. However, demographic rescue slowed that decline (Fig. 3A; change in deviance = 12.3, P = 0.002, Table S5). Our estimate of intrinsic fitness is based on the growth rate from the rescued population size to the subsequent census size, so higher fitness is not a direct consequence of the increase in size but rather a reduction in positive density dependence (e.g., Allee effects) leading to a slower decline. In generation 3, genetic rescue was already more potent than demographic rescue (Fig. 3A; change in deviance = 73.0, P = 0.002, Table S5) and populations that received genetic rescue grew. An interaction between demographic and genetic rescue (Fig. 3B; change in deviance = 4.1, P = 0.05, Table S6), shows that in generation 4, the combination of genetic and demographic rescue reduced intrinsic fitness relative to genetic rescue alone. In generation 5, this pattern persisted but the influence of demographic rescue began to lessen as fitness in genetic rescue treatments continued to increase. Populations that had not received genetic rescue continued to decline or only to increase slightly (Fig. 3C; change in deviance = 67.0, P = 0.002, Table S7). In the sixth and final generation, intrinsic fitness of the extant populations was above 1 for all treatments (Fig. 3D and Fig. S3), indicating that populations were sufficiently adapted to the novel environment to persist. Populations that received genetic rescue had the highest intrinsic fitness (change in deviance = 44.3, P = 0.002, Table S8). Demographic rescue increased fitness of large, but not small, populations when alone, but not when combined with genetic rescue (Fig. 3D; weak three-way interaction between size, demographic rescue, and genetic rescue; change in deviance = 3.19, P = 0.06, Table S8).
Fig. 3.
Intrinsic fitness (maximum achievable fitness, in units of surviving offspring per individual) of initially small (founding size = 50, ○) or large (founding size = 150, ●) experimental populations. The horizontal gray line shows a fitness of 1, corresponding to the population replacement rate. Error bars show 95% confidence intervals. (A) In generation 3, demographic rescue significantly increased intrinsic fitness (change in deviance = 12.3, P = 0.002, Table S5). (B) In generation 4, genetic rescue increased fitness substantially (change in deviance = 73.0, P = 0.002, Table S6), and an interaction between genetic and demographic rescue highlights that fitness is improved most when genetic rescue is implemented alone (change in deviance = 4.1, P = 0.05, Table S6). (C) In generation 5, genetic rescue remained potent (change in deviance = 67.0, P = 0.002, Table S7). (D) In generation 6, all populations were increasing, and those populations that experienced genetic rescue were increasing the fastest (change in deviance = 44.3, P = 0.002, Table S8). D + G, demographic + genetic.
Table S5.
Results from analysis of intrinsic fitness in generation 3 following rescue
| Source | Change in deviance | P |
| Size | 0.06 | 0.84 |
| Demographic rescue | 12.26 | 0.002 |
| Genetic rescue | 0.08 | 0.81 |
| Size × demographic rescue | 0.23 | 0.66 |
| Size × genetic rescue | 0.77 | 0.40 |
| Demographic × genetic rescue | 0.17 | 0.67 |
| Size × demographic × genetic rescue | 0.50 | 0.53 |
P values were determined by parametric bootstrap.
Table S6.
Results from analysis of intrinsic fitness in generation 4 following rescue
| Source | Change in deviance | P |
| Size | 0.23 | 0.66 |
| Demographic rescue | 1.08 | 0.29 |
| Genetic rescue | 73.00 | 0.002 |
| Size × demographic rescue | 1.43 | 0.21 |
| Size × genetic rescue | 0.93 | 0.39 |
| Demographic × genetic rescue | 4.13 | 0.05 |
| Size × demographic × genetic rescue | 1.78 | 0.18 |
P values were determined by parametric bootstrap.
Table S7.
Results from analysis of intrinsic fitness in generation 5 following rescue
| Source | Change in deviance | P |
| Size | 14.27 | 0.002 |
| Demographic rescue | 3.92 | 0.06 |
| Genetic rescue | 67.02 | 0.002 |
| Size × demographic rescue | 0.93 | 0.34 |
| Size × genetic rescue | 0.03 | 0.87 |
| Demographic × genetic rescue | 0.66 | 0.41 |
| Size × demographic × genetic rescue | 0.10 | 0.73 |
P values were determined by parametric bootstrap.
Fig. S3.
Fit of the generalized Ricker model to data from the last two generations. The finite growth rate is density in generation 6 divided by density in generation 5 for initially small (founding size = 50) or large (founding size = 150) experimental populations. Each point is one population. For large populations split across three boxes, the density in generation 5 is the total abundance divided by 3.
Table S8.
Results from analysis of intrinsic fitness in generation 6 (final) following rescue
| Source | Change in deviance | P |
| Size | 3.83 | 0.06 |
| Demographic rescue | 0.09 | 0.78 |
| Genetic rescue | 44.30 | 0.002 |
| Size × demographic rescue | 0.61 | 0.46 |
| Size × genetic rescue | 0.53 | 0.53 |
| Demographic × genetic rescue | 0.89 | 0.33 |
| Size × demographic × genetic rescue | 3.19 | 0.06 |
P values were determined by parametric bootstrap.
Discussion
Recent research shows clearly that evolutionary processes affect ecological dynamics even over the course of a single generation (30–32). Our results for the effect of immigration on the dynamics of small populations support these findings and their application to rescuing vulnerable populations from extinction. We found that in both small and large populations, any immigration reduced extinction rates and a combination of multiple individuals (demographic rescue) with genetically distinct ones (genetic rescue) reduced extinction the most. Genetic rescue both alone and in combination with demographic rescue improved the outlook for long-term survival similarly in small and large populations. We discuss the mechanisms at play for each rescue type and population size combination below.
Evolutionary Rescue.
Most experimental research on evolutionary rescue from standing genetic variation has focused on organisms that can reproduce asexually (e.g., yeast, bacteria), and much, although not all, has focused on quite large populations (e.g., ≥105 yeast cells) (19, 23). Adaptation from standing genetic variation (along with additional variation entering via mutation) is common in such large populations without the input of adaptive variation from migration (10). Here, we tested whether small populations of a diploid sexual species were able to adapt from standing genetic variation. Whereas extinction was highest for populations that did not receive migrants (20% overall), extant populations successfully evolved an intrinsic fitness above 1, which is sufficient for persistence in the challenging novel environment. The simplest explanation for the increase in intrinsic fitness is adaptation to the novel habitat. The surviving populations largely exhibited the predicted U-shaped curve (10, 21) proposed as indicative of evolutionary rescue (e.g., Figs. 2 and 3), where populations initially decline when faced with a challenging environment and then rebound. Interestingly, the final sizes of large and small populations in the evolutionary rescue treatment were not significantly different from each other (Fig. S1), and estimates of intrinsic fitness were also comparable (Fig. 3), indicating that smaller and larger populations adapted to the same extent. This similarity is somewhat unexpected, given that larger populations should harbor greater genetic diversity, and thus selection should be more efficient, leading to higher fitness (33).
Demographic Rescue.
Populations that received only the demographic rescue treatment had a reduced extinction probability compared with those populations without migration. This finding agrees with theory, which shows that stochastic fluctuations in birth and death rates make smaller populations more vulnerable to extinction compared with larger ones. The effect of demographic rescue on fitness was apparent just after migration, when it significantly slowed the decline in the following generation. Additionally, demographic rescue had a long-term positive effect (relative to evolutionary rescue) on the size of large populations but not on the size of small populations (Fig. S1). The lack of response of small populations to demographic rescue might be fundamentally a matter of the founding size. We hypothesize that demographic rescue did not increase population size enough to reduce the influence of stochasticity in vital rates. Indeed, in large populations, demographic rescue led to less variation in population sizes, but in small populations, stochasticity was not reduced (Fig. S2). Nevertheless, population growth was positive in both small and large populations in the novel environment by the end of six generations, suggesting that the populations were adapting.
Genetic Rescue.
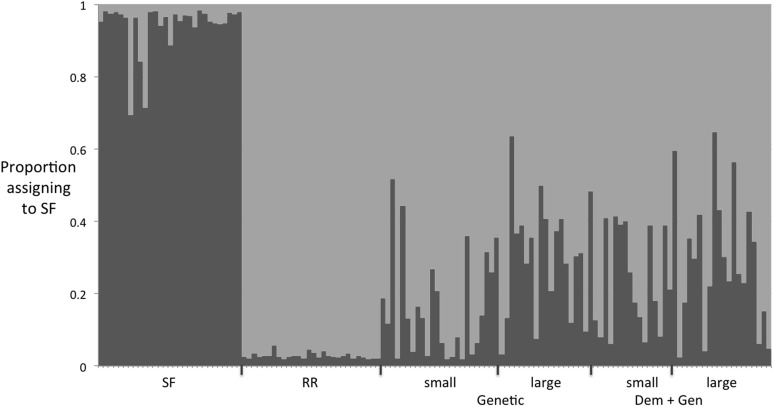
Populations in the genetic rescue treatment had both lower extinction risk and substantially higher intrinsic fitness in the final generation. The reduction in extinction in this case was not a direct demographic effect, but a reduction in the risk of extinction as the population fitness increased, enabling population growth. Microsatellite data confirmed that migrants’ alleles were incorporated into experimental populations (SI Microsatellites, Fig. S4). Two mechanisms underlie the potency of genetic rescue in this experiment. First, as genetic migrants bred with individuals from the experimental populations, inbreeding depression likely would be reduced. The experimental populations are from a long-term laboratory lineage (Methods) similar to model organisms used in other experimental evolution studies (34, 35). The fact that adaptation from standing genetic variation (e.g., evolutionary rescue) was common shows that they are by no means genetically depauperate, but genetic variation is limited and inbreeding in the form of sib-mating is known to reduce fitness (36). The rapid increase in fitness following rescue supports the idea that a reduction in inbreeding depression contributed to genetic rescue. Second, the immigrants also provided genetic variation that could enhance adaptation to the novel environment. As noted above (also SI Methods), the genetic rescue population experienced a moderate reduction in fitness in the challenging experimental environment, but maintained positive population growth. With both potential reductions in inbreeding depression and increases in adaptive variation combined, genetic rescue was quite dramatic, increasing final population sizes of both small and large populations relative to the evolutionary rescue control. In practice, individuals to use for rescue that are adapted to degraded environmental conditions are likely to be rare. However, genetic rescue that alleviates inbreeding depression alone can be beneficial (6). Future research should address the relative potency of alleviating inbreeding depression vs. adding adaptive variation under different contexts. Another issue to consider in implementing genetic rescue is that in some populations, outbreeding depression (7) or migration of individuals adapted to different environments (22, 23) may reduce rather than increase fitness. Understanding the relative balance of all these factors using both mathematical and biological models should improve the success rates of genetic rescue.
Fig. S4.
Results from STRUCTURE admixture analysis, with K = 2. The x axis shows individuals in each of the six populations summarized in Table S1. Dark gray indicates alleles and allele combinations associated with the SF migrants, and light gray indicates alleles and allele combinations associated with the RR experimental populations. These results suggest that at the end of the experiment, most individuals were of hybrid origin, although some retained a nearly pure RR genotype.
Demographic and Genetic Rescue.
Combining demographic and genetic rescue reduced extinction risk more than the other treatments, but there was no apparent benefit to combining these treatments with respect to final population sizes and intrinsic fitness of surviving populations. Indeed, generation 4 (Fig. 3B) provides evidence of a cost of combining the two types of rescue, in that intrinsic fitness of populations given the combination was not as high as intrinsic fitness of populations given just genetic rescue. This finding suggests that demographic rescue swamped out the beneficial effects of genetic rescue. However, this pattern was lost in the subsequent generations, as the positive effects of genetic rescue fully emerged. Small and large populations responded similarly whether genetic rescue was alone or combined with demographic rescue. These results suggest that when populations are small enough, the risk of imminent extinction is high, but that combining demographic and genetic rescue can reduce extinction risk without sacrificing the long-term benefits of genetic rescue.
Minimizing extinction risk of small populations while enhancing their ability to adapt to a changing environment will depend on a number of factors, including population size, standing genetic variation, numbers and identity of migrants available for rescue, the strength of selection, and stochasticity inherent to the system. Our model system cannot address all of these factors, but we believe experiments like ours are an important step in understanding how to reduce extinction risk and promote adaptation. In cases where maintaining the distinct genetic characteristics of a population is vital, despite inbreeding depression, implementing demographic rescue can at least reduce the risk of imminent extinction, giving a population time to adapt from standing genetic variation. If management aims at a viable population to fulfill ecological functions rather than a locally pure one, implementing genetic rescue is preferable. Infusion of genetic material from even a few individuals can provide the necessary variation to reduce inbreeding depression and increase adaptation, greatly improving the outlook for long-term persistence. Our findings contribute to the growing evidence of the benefits of genetic rescue in conservation (7).
SI Microsatellites
We collected data on variation at 12 microsatellite loci following the method of Szűcs et al. (36) on individuals from four example populations that had experienced genetic rescue. Sampling occurred in generation 5. We evaluated admixture using the program STRUCTURE, version 2.3 (43). Results are visualized in Fig. S4.
Methods
Model System.
Our experiments used T. castaneum from laboratory populations maintained in 4 × 4 × 6-cm enclosures (patches) partly filled with 30 mL of flour and yeast medium by volume, at 31 °C and 54 ± 14% relative humidity. Stock populations were maintained at a large size (2,000 individuals) in a standard medium of 95% wheat flour with 5% brewer's yeast (in 20 containers holding 30 mL media, comprised of 28.5 mL wheat flour and 1.5 mL brewer’s yeast, mixed every generation). Experimental populations came from a long-term laboratory strain named “RR” used in other studies (28, 36, 37). Migrants were from a strain more recently collected from the wild (∼15 generations before use), called “SF,” also used in another study (36). We controlled the life history of the populations to mimic a seasonally breeding organism with discrete generations (28), a life history widespread among animals and plants. At the start of each generation, adults were placed in patches with fresh medium and allowed to lay eggs for 24 h. Adults were then removed and discarded, and eggs were allowed to develop through larval and pupal stages to adulthood over 35 d. At that point, we sifted the medium to remove and census adults, which were then used to initiate the following generation.
Experiment.
We challenged experimental populations of T. castaneum with a harsh medium that consisted of only 2.85% wheat flour, 97% corn flour, and 0.15% brewers yeast (28.1 mL corn flour, 0.855 mL wheat flour, 0.045 mL brewer's yeast in each 30 mL patch) to mimic quick environmental deterioration. This diet results in a growth rate of the RR strain that would lead to extinction, whereas the growth rate of the SF strain on this challenging medium was less than the growth rate on the standard medium but would not cause deterministic extinction (SI Methods and Table S1). Thus, the migrants could provide genetic variation relevant to adaptation to the environment. Lower population growth rates in the harsh environment are likely due to both the novel carbohydrate source and the reduced nutrient availability. This combination leads to slower development time, preventing some individuals from contributing to the next generation, or greater cannibalism rates, increasing density-dependent mortality. Adaptation to a novel carbohydrate and reduced nutrient availability can occur through the evolution of reduced body size, faster development time, or increased cannibalism, as well as potentially through physiological adaptations (38). Populations were initiated with 50 or 150 beetles (herein referred to as small and large populations), with large populations housed in three patches, so that initial density effects were comparable between the small and large population sizes, isolating differences in stochasticity from direct density effects. We implemented the experiment in two temporal blocks 2 d apart. For both blocks, at the beginning of the second generation of the experiment, the rescue treatments outlined below were imposed a single time. Experimental populations were followed for six generations, and thus four generations following the one-time rescue event. Stock colonies to supply immigrants for rescue were reared on the experimental medium for a single generation before use, so that their effects would be directly demographic and genetic and not a consequence of their maternal environment.
We imposed a factorial rescue design crossing two levels of demographic inputs (present/absent) with two levels of genetic inputs (present/absent). Our four treatments were as follows:
-
i)
An “evolutionary rescue treatment” received no immigrants, so as to evaluate the ability of the experimental populations to adapt from standing genetic variation.
-
ii)
The “demographic rescue treatment” entailed immigration of multiple individuals to stabilize population size close to the original founding size. The number of individuals to be added was calculated by taking the geometric mean of population sizes in generation 2 and subtracting it from the founding population sizes. Thus, 11 and 29 individuals were added to small and large populations, respectively, in the first block, and a few more (13, 33) were added in the second block because populations were slightly smaller for that block at the time of treatment. Because we added the same number of individuals to each population experiencing demographic rescue within a block and population size combination, experimental populations could have been either below or above their founding size after rescue. Immigrants for demographic rescue were from RR, the same source population as the experimental populations; thus, this treatment altered the demography, although not markedly changing the genetic composition.
-
iii)
The “genetic rescue treatment” entailed immigration of a single (small population) or three (large population) individuals from SF, which replaced an equal number of haphazardly chosen residents. The number of genetic rescue individuals was chosen so that the migration rate would be well below the one migrant per generation, which is a theoretical lower end for maintaining genetic cohesiveness among populations (reviewed in 29). This migrant theoretically could mask genetic load, thereby reducing inbreeding depression, or it could provide variation that would facilitate adaptation to the environment without altering the demographics of the experimental populations. The sex of the migrant was unknown, and females likely had mated already with SF males; however, due to sperm precedence, sperm from subsequent matings with beetles from the experimental populations were likely to produce outcrossed offspring (39). The unknown sex and mating status of the migrant beetles are likely to have increased variation in responses to rescue, and thus increase the robustness of our results.
-
iv)
The “demographic plus genetic rescue treatment” combined demographic rescue with genetic rescue, enabling us to test explicitly for interactions between them. For example, a small population from the first block would have received a total of 11 migrants, 10 from RR and a single migrant from SF.
We initiated from 27 to 32 populations of each of the treatment combinations for a total of 244 experimental populations. As population size changed, small populations remained in one box, whereas large populations generally remained in three boxes, but they were put in only two boxes or one box if their populations fell below 60 or below 50, respectively. Reducing the number of boxes as population size dropped allowed us to isolate the effect of population size better from the effect of density. In each generation, all individuals in large populations were mixed and individuals were split evenly between the boxes.
Statistical Analyses.
A main goal of adding migrants to a small population is to prevent extinctions; thus, we examined whether the probability of extinction varied by rescue type. Extinction was evaluated using a generalized linear mixed model (40) with a binomial distribution and a logit link. Factors in the model were initial population size (a categorical variable with two levels, small and large), demographic rescue treatment (present/absent), genetic rescue treatment (present/absent), and the interaction between demographic and genetic rescue treatments as fixed effects, with temporal block as a random effect. Three populations of size 1 and three populations of size 2 in the final generation were scored as extinct (pseudoextinct). Because the only extinctions among large founding populations were in a single treatment (evolutionary rescue), there was no variance in extinction among the other treatments; thus, the data were perfectly predicted for some treatment combinations (the complete separation phenomenon) (41), and an explicit interaction between founding size and the rescue treatments could not be included.
We compared the final size of extant populations using a linear mixed model, log-transforming population size to improve normality of the residuals. Fixed and random factors in the model were as described for extinction. Variability in population sizes might also be of interest to natural resource managers, because extreme fluctuations could increase long-term extinction risk. We calculated coefficients of variation (CV = SD/mean) for population sizes at the end of the experiment and compared variability across treatments by estimating a 95% confidence interval using the adjusted bootstrap percentile method (42).
We estimated intrinsic fitness of the experimental groups by fitting a generalized Ricker model with an Allee effect:
where Nt+1 is population density (beetles per container) in the final generation, Nt is density in the previous generation, R is low-density fitness achieved in the absence of an Allee effect, θ models the Allee effect (positive density dependence at low density), and α is the effect of negative density dependence. The Ricker model describes the biology of this system well (28). Furthermore, this model can be linearized by a logarithmic transformation as follows:
where a = ln(R), b = −α, and c = θ − 1. We fitted the linearized model as a linear mixed model, with the response ln(Nt+1/Nt) as a function of population density at generation t and ln(density at generation t). Fixed and random experimental factors in the model were as described for extinction. Intrinsic fitness was calculated as the growth rate at the maximum (i.e., stationary point) of the estimated growth rate curve for each treatment (Fig. S3). Confidence intervals for intrinsic fitness were estimated by parametric bootstrapping using the adjusted bootstrap percentile method (42).
Acknowledgments
We thank J. P. Demuth and M. J. Wade for providing the SF beetles. We also thank the excellent team of undergraduate students who helped with data collection: A. Aradi, K. Fishburn, D. Maitland, M. McClellan, D. Pastore, B. Raasch, R. Poliakon, K. Stamm, T. Wilkinson, and A. Wyatt. The manuscript was improved by comments and suggestions from J. Drew, A. Gonzalez, and an anonymous reviewer. Funding for this research was provided by the US National Science Foundation [Grant DEB-0949619, Grant DEB-0949595, Graduate Research Fellowship DGE-1321845 Amend. 3 (to M.J.K.), and two associated Research Experiences for Undergraduates supplements], and additional support came from the US Department of Agriculture via the Colorado Agricultural Experiment Station.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Dryad database (doi:10.5061/dryad.p96b7).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504732112/-/DCSupplemental.
References
- 1.Barnosky AD, et al. Has the Earth’s sixth mass extinction already arrived? Nature. 2011;471(7336):51–57. doi: 10.1038/nature09678. [DOI] [PubMed] [Google Scholar]
- 2.Hedrick PW, Fredrickson R. Genetic rescue guidelines with examples from Mexican wolves and Florida panthers. Conserv Genet. 2010;11(2):615–626. [Google Scholar]
- 3.Brown JH, Kodric-Brown A. Turnover rates in insular biogeography: Effect of immigration on extinction. Ecology. 1977;58(2):445–449. [Google Scholar]
- 4.Richards CM. Inbreeding depression and genetic rescue in a plant metapopulation. Am Nat. 2000;155(3):383–394. doi: 10.1086/303324. [DOI] [PubMed] [Google Scholar]
- 5.Drake JM, Baggenstos P, Lodge DM. Propagule pressure and persistence in experimental populations. Biol Lett. 2005;1(4):480–483. doi: 10.1098/rsbl.2005.0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tallmon DA, Luikart G, Waples RS. The alluring simplicity and complex reality of genetic rescue. Trends Ecol Evol. 2004;19(9):489–496. doi: 10.1016/j.tree.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Frankham R. Genetic rescue of small inbred populations: Meta-analysis reveals large and consistent benefits of gene flow. Mol Ecol. 2015;24(11):2610–2618. doi: 10.1111/mec.13139. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez A, Lawton JH, Gilbert FS, Blackburn TM, Evans-Freke I. Metapopulation dynamics, abundance, and distribution in a microecosystem. Science. 1998;281(5385):2045–2047. doi: 10.1126/science.281.5385.2045. [DOI] [PubMed] [Google Scholar]
- 9.Freckleton RP, Gill JA, Noble D, Watkinson AR. Large-scale population dynamics, abundance-occupancy relationships and the scaling from local to regional population size. J Anim Ecol. 2005;74(2):353–364. [Google Scholar]
- 10.Carlson SM, Cunningham CJ, Westley PAH. Evolutionary rescue in a changing world. Trends Ecol Evol. 2014;29(9):521–530. doi: 10.1016/j.tree.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 11.Holt RD, Gomulkiewicz R. How does immigration influence local adaptation? A reexamination of a familiar paradigm. Am Nat. 1997;149(3):563–572. [Google Scholar]
- 12.Boulding EG, Hay T. Genetic and demographic parameters determining population persistence after a discrete change in the environment. Heredity (Edinb) 2001;86(Pt 3):313–324. doi: 10.1046/j.1365-2540.2001.00829.x. [DOI] [PubMed] [Google Scholar]
- 13.Aitken SN, Whitlock MC. Assisted gene flow to facilitate local adaptation to climate change. Annu Rev Ecol Evol Syst. 2013;44(44):367–388. [Google Scholar]
- 14.Hufford KM, Mazer SJ. Plant ecotypes: Genetic differentiation in the age of ecological restoration. Trends Ecol Evol. 2003;18(3):147–155. [Google Scholar]
- 15.Whiteley AR, Fitzpatrick SW, Funk WC, Tallmon DA. Genetic rescue to the rescue. Trends Ecol Evol. 2015;30(1):42–49. doi: 10.1016/j.tree.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Ingvarsson PK. Restoration of genetic variation lost—The genetic rescue hypothesis. Trends Ecol Evol. 2001;16(2):62–63. doi: 10.1016/s0169-5347(00)02065-6. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez A, Ronce O, Ferriere R, Hochberg ME. Evolutionary rescue: An emerging focus at the intersection between ecology and evolution. Philos Trans R Soc Lond B Biol Sci. 2013;368(1610):20120404. doi: 10.1098/rstb.2012.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bell G, Collins S. Adaptation, extinction and global change. Evol Appl. 2008;1(1):3–16. doi: 10.1111/j.1752-4571.2007.00011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bell G, Gonzalez A. Evolutionary rescue can prevent extinction following environmental change. Ecol Lett. 2009;12(9):942–948. doi: 10.1111/j.1461-0248.2009.01350.x. [DOI] [PubMed] [Google Scholar]
- 20.Cameron TC, O’Sullivan D, Reynolds A, Piertney SB, Benton TG. Eco-evolutionary dynamics in response to selection on life-history. Ecol Lett. 2013;16(6):754–763. doi: 10.1111/ele.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomulkiewicz R, Holt RD. When does evolution by natural selection prevent extinction? Evolution. 1995;49(1):201–207. doi: 10.1111/j.1558-5646.1995.tb05971.x. [DOI] [PubMed] [Google Scholar]
- 22.Sexton JP, Strauss SY, Rice KJ. Gene flow increases fitness at the warm edge of a species’ range. Proc Natl Acad Sci USA. 2011;108(28):11704–11709. doi: 10.1073/pnas.1100404108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bell G, Gonzalez A. Adaptation and evolutionary rescue in metapopulations experiencing environmental deterioration. Science. 2011;332(6035):1327–1330. doi: 10.1126/science.1203105. [DOI] [PubMed] [Google Scholar]
- 24.Pickup M, Field DL, Rowell DM, Young AG. Source population characteristics affect heterosis following genetic rescue of fragmented plant populations. Proc Biol Sci. 2013;280(1750):20122058. doi: 10.1098/rspb.2012.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Willi Y, Fischer M. Genetic rescue in interconnected populations of small and large size of the self-incompatible Ranunculus reptans. Heredity (Edinb) 2005;95(6):437–443. doi: 10.1038/sj.hdy.6800732. [DOI] [PubMed] [Google Scholar]
- 26.Fauvergue X, Vercken E, Malausa T, Hufbauer RA. The biology of small, introduced populations, with special reference to biological control. Evol Appl. 2012;5(5):424–443. doi: 10.1111/j.1752-4571.2012.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lande R. Genetics and demography in biological conservation. Science. 1988;241(4872):1455–1460. doi: 10.1126/science.3420403. [DOI] [PubMed] [Google Scholar]
- 28.Melbourne BA, Hastings A. Extinction risk depends strongly on factors contributing to stochasticity. Nature. 2008;454(7200):100–103. doi: 10.1038/nature06922. [DOI] [PubMed] [Google Scholar]
- 29.Mills LS, Allendorf FW. The one-migrant-per-generation rule in conservation and management. Conserv Biol. 1996;10(6):1509–1518. [Google Scholar]
- 30.Turcotte MM, Reznick DN, Hare JD. The impact of rapid evolution on population dynamics in the wild: Experimental test of eco-evolutionary dynamics. Ecol Lett. 2011;14(11):1084–1092. doi: 10.1111/j.1461-0248.2011.01676.x. [DOI] [PubMed] [Google Scholar]
- 31.Schoener TW. The newest synthesis: Understanding the interplay of evolutionary and ecological dynamics. Science. 2011;331(6016):426–429. doi: 10.1126/science.1193954. [DOI] [PubMed] [Google Scholar]
- 32.Botero CA, Weissing FJ, Wright J, Rubenstein DR. Evolutionary tipping points in the capacity to adapt to environmental change. Proc Natl Acad Sci USA. 2015;112(1):184–189. doi: 10.1073/pnas.1408589111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weber KE. Increased selection response in larger populations. I. Selection for wing-tip height in Drosophila melanogaster at three population sizes. Genetics. 1990;125(3):579–584. doi: 10.1093/genetics/125.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burke MK, et al. Genome-wide analysis of a long-term evolution experiment with Drosophila. Nature. 2010;467(7315):587–590. doi: 10.1038/nature09352. [DOI] [PubMed] [Google Scholar]
- 35.Bolnick DI. Intraspecific competition favours niche width expansion in Drosophila melanogaster. Nature. 2001;410(6827):463–466. doi: 10.1038/35068555. [DOI] [PubMed] [Google Scholar]
- 36.Szűcs M, Melbourne BA, Tuff T, Hufbauer RA. The roles of demography and genetics in the early stages of colonization. Proc Biol Sci. 2014;281(1792):20141073. doi: 10.1098/rspb.2014.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melbourne BA, Hastings A. Highly variable spread rates in replicated biological invasions: Fundamental limits to predictability. Science. 2009;325(5947):1536–1539. doi: 10.1126/science.1176138. [DOI] [PubMed] [Google Scholar]
- 38.Agashe D, Falk JJ, Bolnick DI. Effects of founding genetic variation on adaptation to a novel resource. Evolution. 2011;65(9):2481–2491. doi: 10.1111/j.1558-5646.2011.01307.x. [DOI] [PubMed] [Google Scholar]
- 39.Arnaud L, Gage MJG, Haubruge E. The dynamics of second- and third-male fertilization precedence in Tribolium castaneum. Entomol Exp Appl. 2001;99(1):55–64. [Google Scholar]
- 40.SAS Institute . SAS/STAT Software: Changes and Enhancements Through Release 9.2. SAS Institute, Inc.; Cary, NC: 2010. [Google Scholar]
- 41.Albert A, Anderson JA. On the existence of maximum-likelihood estimates in logistic regression models. Biometrika. 1984;71(1):1–10. [Google Scholar]
- 42.Davidson AC, Hinkley DV. Bootstrap Methods and Their Application. Cambridge Univ Press; Cambridge, UK: 1997. [Google Scholar]
- 43.Pritchard JK, Stephens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155(2):945–959. doi: 10.1093/genetics/155.2.945. [DOI] [PMC free article] [PubMed] [Google Scholar]