Significance
Vitality form is a term that describes the manner with which actions are performed. Despite their crucial importance in interpersonal communication, vitality forms have been almost completely neglected in neuroscience. Here, using a functional MRI technique, we investigated the neural correlates of vitality forms in three tasks: action observation, imagination, and execution. We found that, in all three tasks, there is a common specific activation of the dorsocentral sector of the insula in addition to the parietofrontal network that is typically active during arm movements production and observation. Thus, the dorsocentral part of the insula seems to represent a fundamental and previously unsuspected node that modulates the cortical motor circuits, allowing individuals to express their vitality forms and understand those of others.
Keywords: vitality forms, insula, action style, mirror mechanism, fMRI
Abstract
Vitality form is a term that describes the style with which motor actions are performed (e.g., rude, gentle, etc.). They represent one characterizing element of conscious and unconscious bodily communication. Despite their importance in interpersonal behavior, vitality forms have been, until now, virtually neglected in neuroscience. Here, using the functional MRI (fMRI) technique, we investigated the neural correlates of vitality forms in three different tasks: action observation, imagination, and execution. Conjunction analysis showed that, in all three tasks, there is a common, consistent activation of the dorsocentral sector of the insula. In addition, a common activation of the parietofrontal network, typically active during arm movements production, planning, and observation, was also found. We conclude that the dorsocentral part of the insula is a key element of the system that modulates the cortical motor activity, allowing individuals to express their internal states through action vitality forms. Recent monkey anatomical data show that the dorsocentral sector of the insula is, indeed, connected with the cortical circuit involved in the control of arm movements.
The observation of actions done by another individual allows the observer to understand (typically) what that individual is doing and in some instances, why that individual is doing it. Other than goal and motor intention, observing the expressive qualities of others’ movements enables one to understand the internal psychological state of the agent on the basis of how the action is performed. This information carried by the kinematics of the observed actions has been defined by Stern as “vitality affects” or “vitality forms” (1–4).
This concept is of great interest, because it describes a primary feature of the psychological affective/communicative quality underlying the relation between the agent and the action recipient. According to Stern (1–4), the appraisal of vitality forms relies on five specific properties of the observed movements: time, space, force, trajectory, and direction. These movement aspects create a particular experience that reflects the affective/communicative state of the agent. The capacity to express and understand vitality forms represents a fundamental element of interpersonal relations and is at the basis of the sympathetic engagement in the early mother–child relationship (1–7).
Despite its psychological importance, little attention has been paid up to now to vitality forms in neuroscience. Recently, using functional MRI (fMRI), we showed that, when an individual pays attention to the action style rather than to its goal, there is a specific activation of the dorsocentral insula (8). It is unclear, however, the neural basis of the planning and production of vitality forms and the relationships between vitality form production and understanding.
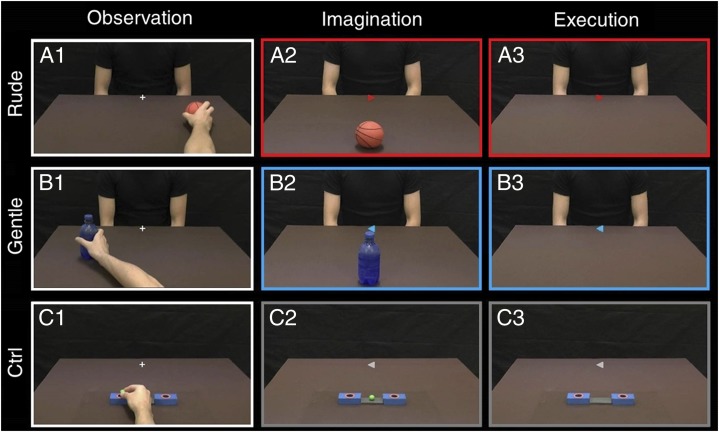
In this study, we investigated the neurological bases of vitality forms in healthy participants using fMRI. Participants were required to execute, imagine, and observe arm action performed with two different vitality forms (gentle and rude). In the first task [observation (OBS)], the participants observed video clips showing the right arm of an actor performing actions toward another actor (e.g., passing a cup) with either a gentle or a rude vitality form (Fig. 1 A1 and B1 shows examples, SI Methods, and Fig. S1). In the second task [imagination (IMA)], participants had to imagine themselves performing the actions seen during the OBS task (Fig. 1 A2 and B2), again with gentle or rude vitality forms (SI Methods and Fig. S2). In the third task [execution (EXE)], participants moved a packet of crackers located on a plane lying on their chest as if offering them to another person with a gentle or rude vitality form. Finally, in a control condition (Ctrl), the participants were requested to observe video clips showing the hand of an actor placing a small ball in a box (Fig. 1C1), imagine themselves performing the same action (Fig. 1C2), or finally, place a small ball inside one of two boxes placed inside the scanner and seen through digital visors (Fig. 1C3).
Fig. 1.
Experimental task design. (Left) Observation. The participant observed the right hand of an actor moving an object in a (A1) rightward or (B1) leftward direction. Four objects were used. The observed action could be executed with a gentle or rude vitality form, and the request was to focus attention on the style of action. (C1) As a control, the participant observed a hand placing a small ball in the right or left hole of a box randomly. (Center) Imagination. The participant was required to imagine himself giving an object to another actor sitting in front of him with either a gentle or a rude vitality form. (A2 and B2) In the central part of the screen, there was an arrow indicating the stile (blue, gentle; red, rude) and the direction of the imagined action. The contour of the screen (red or blue) reminded the participant of the requested action style. (C2) As a control, the participant had to imagine placing the ball in the box according to the direction of the arrow. (Right) Execution. The participant held a package of crackers and had to move it with (A3) rude (red) or (B3) gentle (blue) style toward the actor in front of him. As a control, the participant had to place the small ball in the box. All stimuli in OBS, IMA, and EXE tasks were viewed through digital visors (VisuaSTIM) with a 500,000px × 0.25-in2 resolution and a horizontal eye field of 30°. The digital transmission of the signal to the scanner was through optic fiber. During the EXE task, the participant saw only the scene depicted and his hand was out of his field of vision.
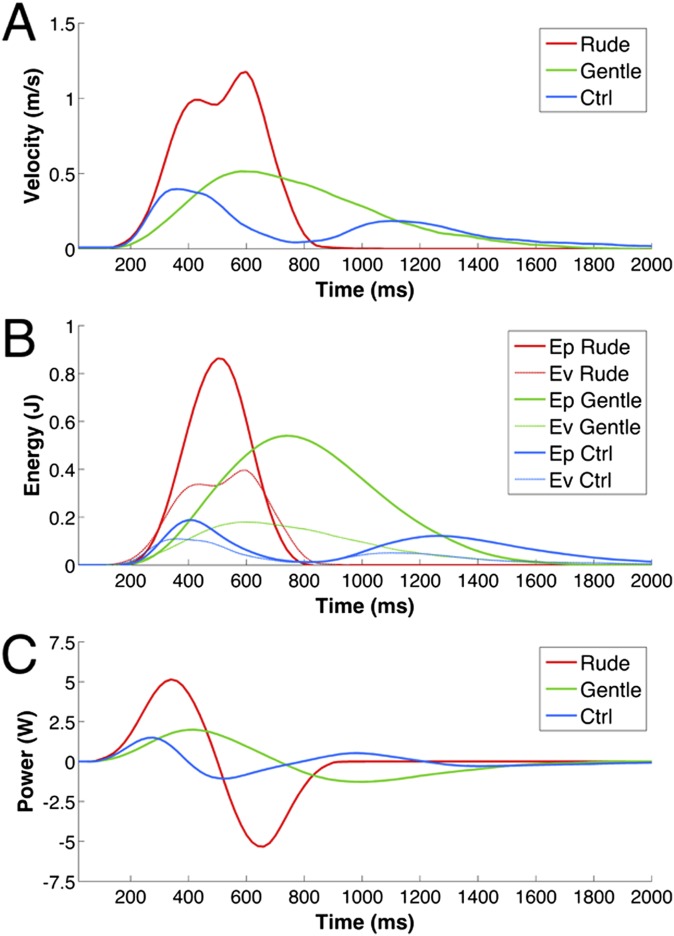
Fig. S1.
Kinematic and dynamic average profiles of the actions performed by the actor with different objects (vitality: ball, cup, bottle, and crackers; Ctrl: small ball). (A) Graph depicts the velocity profiles and duration of the hand performing the action (blue, Ctrl; green, Gentle; red, Rude). (B) Graph depicts the potential (Ep) and kinetic (Ev) energies. (C) Graph depicts the power required to perform the action on the objects.
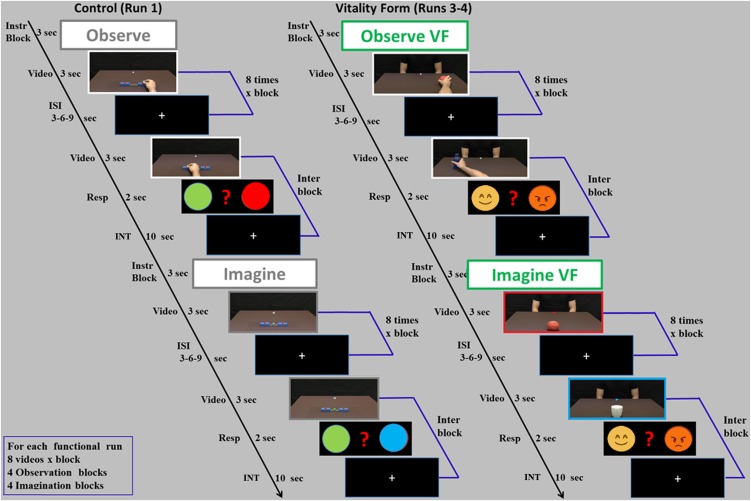
Fig. S2.
Experimental paradigm. In the OBS task, participants were asked to either observe the placing of the ball action (Ctrl) or pay attention to the style of social actions [vitality form (VF) condition]. In the IMA task, participants were asked to imagine performing the action in a neutral way (Ctrl) or a gentle and a rude way (VF condition). Within blocks, after each experimental stimulus was present an interstimulus interval (ISI) and between two sequential experimental blocks was present in an interblock interval. The interblock interval was composed of a catch trial (video stimulus of an action plus a task-related questionnamed Resp) and a 10-s interval (INT).
The main result of our study was the demonstration that the execution of actions performed with a vitality form relative to control actions determines a specific activation of the dorsocentral insula. The same region also became active during motor imagery and vitality forms observation. Note that recent anatomical evidence in monkeys shows that this sector of the insula is connected with the cortical circuit formed by the anterior intraparietal area (9), the frontal area F5 (10) and the prefrontal area F12r (11) (i.e., the circuit that controls hand/arm movements). We postulate that, during action execution, the insula may modulate this circuit, determining different vitality forms according to the internal state of the individual.
SI Methods
Experimental Design and Stimuli.
The experiment was a mixed event-related/blocked design. It was based on a 3 × 3 factorial design with tasks (OBS, IMA, and EXE) and conditions (rude vitality form, gentle vitality form, and Ctrl) as factors.
In the OBS task, participants were presented with video clips showing the right hand of an actor performing four leftward and four rightward actions toward another actor sitting in front of the first one using two different vitality forms (rude and gentle) (Fig. 1 A1 and B1). The actions were move a bottle, move a cup, give a packet of crackers, and pass a ball. Additionally, video clips also showed the actor’s hand placing a small ball of one of four different colors (red, green, yellow, or blue) randomly into the right or left box (Ctrl) (Fig. 1C1). In all video clips, the actor started the action from an initial position and reached a final one.
In the IMA task, participants were presented with video clips showing an object (ball, cup, bottle, or crackers) that appeared to be located close to the participant and an actor in front of him. Additionally, in Ctrl, video clips showed four different-colored balls that the observer had to imagine placing into one of two boxes (Ctrl) (Fig. 1C2).
In the EXE task, participants were presented with video clips showing an actor in front of them (Fig. 1 A3 and B3), and they have to move a packet of crackers toward the actor as if to offer it to him. In Ctrl, they were presented with two boxes (Fig. 1C3) and had to place a ball inside one of them.
To avoid possible repetition suppression effect in the vitality form condition, we changed randomly the direction of action, object, and action style. In Ctrl, we changed the color of the ball and the action direction. Note also that, in the EXE task, participants in the vitality form condition moved the same object (crackers) and participants in the Ctrl also moved the same object (small ball). The stronger activation during the experimental condition relative to Ctrl indicates that no repetition suppression effect was found.
Each video clip lasted 3 s. In total, 24 stimuli were shown: 16 for vitality conditions (4 objects × 2 vitality forms × 2 directions) and 8 for Ctrl (1 action × 4 colors × 2 directions). Using VirtualDubMod software v1.5, all of the original actor videos were cropped to remove the head area. This cropping was done to prevent vision of the actor’s face. The human face is generally a powerful social cue that attracts the viewer’s attention, which we wanted to focus on the performed action that participants were required to evaluate. Additionally, to focus participants’ attention on performed actions, the videos were recorded in a dark scenario, and actors wore black shirts to emphasize the forelimbs.
Kinematic and Dynamic Analyses of Vitality Forms.
After video recording, all actors’ movement profiles were analyzed using the 3D point kinematics method with MATLAB (The Mathworks). A specific point of the actor’s hand was marked for all video clips. For all actions, the origins of the x, y, and z axes were fixed in the rest position of the thumb, and the positions were tracked in the space every 20 ms until the end of action. We calculated the velocity and trajectory curves for all actions (rude, gentle, and Ctrl). The module of velocity was calculated for all actions and then, averaged. The velocity profiles are shown in Fig. S1A (red line shows the average of all rude actions, green line shows the average of all gentle actions, and blue line shows the average of the Ctrl actions).
For each action, we estimated the kinetic energy (Ek = 1/2 mV2), the potential energy (Eu = mgh), and the power [P = d(Ek + Eu)/dt] required to perform the action with the object. To this purpose, the mass of each object (bottle = 0.350 kg, cup = 0.200 kg, ball = 0.200 kg, cracker = 0.025 kg, and small ball = 0.050 kg) was measured and added to the mass of the actor’s hand (0.5 kg). The potential and kinetic energy curves related to movements performed with these objects are shown in Fig. S1B. To compare the curves of the different objects, which had different weights, the potential and kinetic energies were normalized with respect to the mass (E/kg). Finally, we calculated the power [P = d(Ek + Eu)/dt] used by the actor to move the objects with his hand in association with specific velocities and trajectories (Fig. S1C).
Paradigm.
During the experiment, participants laid in the scanner in a dimly lit environment. The stimuli were viewed through digital visors (VisuaSTIM) with a 500,000 px × 0.25-in2 resolution and horizontal eye field of 30°. The digital transmission of the signal to the scanner was through optic fiber. The software E-Prime 2 Professional (Psychology Software Tools, Inc.; www.pstnet.com) was used for both stimuli presentation and the recording of participants’ responses.
The experiment was subdivided into six runs. In three of them, participants executed motor actions; specifically, in two runs, they moved a packet of crackers, offering it to a person shown on a screen with either a rude or gentle vitality form (experimental condition), and in one run, they placed a small ball in a hole (Ctrl), viewing the real image of the boxes placed inside the scanner through digital visors. In the other three runs, the participants imagined (motor imagery) or observed the same experimental and control actions presented on a screen (Fig. 1 and details in SI Methods, Experimental Design and Stimuli). For the OBS and IMA tasks (runs 1, 3, and 4), each experimental trial presented a single video clip for 3 s followed by a jittered interval (fixation cross) ranging from 3 to 6 s. In total, participants viewed 71 video clips (64 experimental trials and 7 catch trials) organized in eight blocks (four blocks for OBS task and four blocks for IMA task) that were presented in a randomized order. As shown in Fig. S2, one interblock was present between two sequential experimental blocks. It was composed of a catch trial (video stimulus of an action plus a task-related question) and a 10-s interval. Within each block, the videos were presented eight times in a randomized order. Each functional run lasted about 13 min.
For the execution task (runs 2, 5, and 6), each trial lasted 3 s, during which participants had to perform the action (vitality condition: pass a packet of crackers; Ctrl: place a ball in the box). Each trial was followed by a jittered interval (fixation cross) of 6, 9, or 12 s. In total, during the execution run, participants performed 32 trials.
fMRI Data Acquisition.
Anatomical T1-weighted and functional T2*-weighted magnetic resonance images were acquired with a 3-T General Electrics Scanner equipped with an eight-channel receiver head coil. Functional images were acquired using a T2*-weighted gradient-echo, echo-planar pulse sequence (acceleration factor asset 2; 40 interleaved transverse slices; slice thickness = 3 plus interslice gap = 0.5 mm) covering the whole brain, with a repetition time (TR) of 3,000 ms [echo time (TE) = 30 ms, flip angle = 90°, field of view (FOV) = 205 × 205 mm2, in-plane resolution = 2.5 × 2.5 mm2]. The scanning sequence comprised 287 interleaved volumes for run 1; 147 volumes for runs 2, 5, and 6; and 266 volumes for runs 3 and 4. After the third functional run, a T1-weighted structural image was acquired for each participant [acceleration factor arc 2; 156 sagittal slices; matrix = 256 × 256; isotropic resolution = 1 × 1 × 1 mm3; inversion time (TI) = 450 ms; TR = 8,100 ms; TE = 3.2 ms; flip angle = 12°].
Statistical Analysis.
Data analysis was performed with SPM8 (Statistical Parametric Mapping software; The Wellcome Department of Imaging Neuroscience; www.fil.ion.ucl.ac.uk) running on MATLAB R2013 (The Mathworks). The first four volumes of each run were discarded to allow for T1 equilibration effects. For each participant, all volumes were spatially realigned to the first volume of the first session and unwarped to correct for between-scan motion, and a mean image from the realigned volumes was created. T1-weighted images were realigned to create a mean image; then segmented into gray, white, and cerebrospinal fluid; and spatially normalized to the Montreal Neurological Institute coordinates. The derived spatial transformation by T1 normalization was applied to the realigned echo planar imaging (EPI) volumes, which after normalization, were resampled in 2 × 2 × 2-mm3 voxels using trilinear interpolation in space. All functional volumes were then spatially smoothed with a 6-mm FWHM isotropic Gaussian kernel for group analysis.
Data were analyzed using a random effects model (30) implemented in a two-level procedure. In the first level, single-subject fMRI BOLD (blood oxygen level dependent) signal was modeled in a general linear model by a design matrix comprising the onsets and the durations of each event according to the experimental task for each functional run. In the experiment, at the first level, four different general linear models were used: OBS and IMA control model (run 1: OBS and IMA tasks), EXE control model (run 2: EXE task), OBS and IMA vitality model (runs 3 and 4: VF-OBS task and VF-IMA task), and EXE vitality model (runs 5 and 6: vitality form EXE task). In OBS and IMA control model (run 1), the Ctrl condition was modeled using four regressors as follows: OBS Ctrl, IMA Ctrl, instruction, and response. Differently, the vitality condition (runs 3 and 4) was modeled using six regressors as follows: OBS rude, OBS gentle, IMA rude, IMA gentle, instruction, and response. Within each block, the videos were modeled as a single event lasting 3 s. The instruction and response were modeled with durations of 3 and 2 s, respectively. For the EXE task, Ctrl (run 2) was modeled using one regressor (EXE Ctrl), whereas vitality condition (runs 5 and 6) was modeled using two regressors (EXE rude and EXE gentle). In the first-level analysis, for EXE and vitality form EXE tasks, we used motion regressors to control possible artifacts related to head motion. For all participants, we also checked possible head motion. Head motion never exceeded one voxel (3 mm).
In the second-level analysis (group analysis), corresponding contrast images of the first level for each participant were entered into a flexible ANOVA with sphericity correction for repeated measures (31). This model was composed of nine regressors (OBS rude, OBS gentle, IMA rude, IMA gentle, OBS Ctrl, IMA Ctrl, EXE rude, EXE gentle, and EXE Ctrl) and considered the activation pattern obtained for different tasks (OBS, IMA, and EXE) in three different conditions (gentle, rude, and Ctrl) vs. implicit baseline. Within this model, we assessed activations associated with each task vs. implicit baseline and activations resulting from the direct contrast between conditions (OBS rude vs. OBS Ctrl, OBS gentle vs. OBS Ctrl, IMA rude vs. IMA Ctrl, IMA gentle vs. IMA Ctrl, EXE rude vs. EXE Ctrl, and EXE gentle vs. EXE Ctrl; PFWE < 0.05 corrected at the cluster level).
The location of the activation foci was determined in the stereotaxic space of the Montreal Neurological Institute coordinates system. Those cerebral regions for which maps are provided were also localized with reference to cytoarchitectonical probabilistic maps of the human brain using the SPM Anatomy Toolbox v1.7 (32).
Results
Cortical Activations During OBS, IMA, and EXE Tasks.
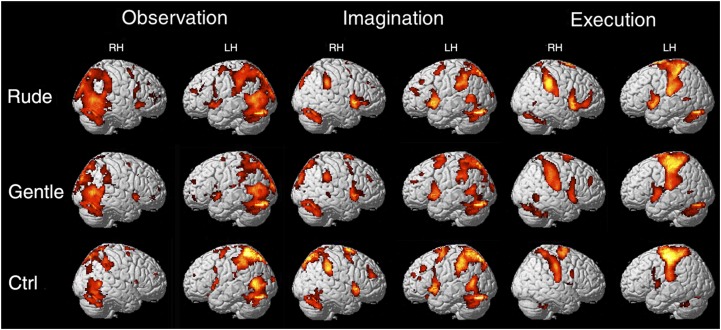
The data showed that, during the OBS task, there was a bilateral activation of the occipital lobe, the dorsal premotor cortex, and the superior parietal lobule extending laterally into the rostral inferior parietal lobule. A similar activation pattern was observed during the IMA task but with a much less extended activation of the occipital areas. During the EXE task, activations were found in the same regions of the parietal and premotor cortices as in the other two tasks, and there was a strong activation of the left somatosensory and motor cortices (PFWE < 0.05 at voxel level) (SI Methods and Fig. S3).
Fig. S3.
Brain activations resulting from rude, gentle, and Ctrl vs. implicit baseline during three different tasks (OBS, IMA, and EXE). These activations are rendered into a standard Montreal Neurological Institute brain template (PFWE < 0.05 at voxel level). LH, left hemisphere; RH, right hemisphere.
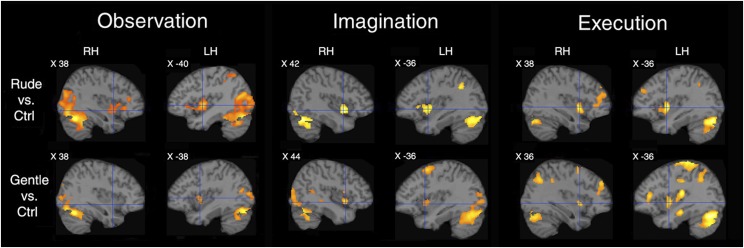
The main aim of our study was to assess the activation of the insula during the three tasks (OBS, IMA, and EXE). The contrasts vitality forms relative to control are shown in Fig. 2. In all three tasks, there was a consistent activation of the dorsocentral sector of the insula (PFWE < 0.05 at cluster level). The effect was present in all contrasts, except for gentle vs. control in the right hemisphere.
Fig. 2.
Brain activations during vitality form processing. Parasagittal sections showing the insular activations in the two hemispheres during the contrasts rude vs. Ctrl and gentle vs. Ctrl in OBS, IMA, and EXE tasks. LH, left hemisphere; RH, right hemisphere.
Conjunction Analysis of OBS, IMA, and EXE Tasks.
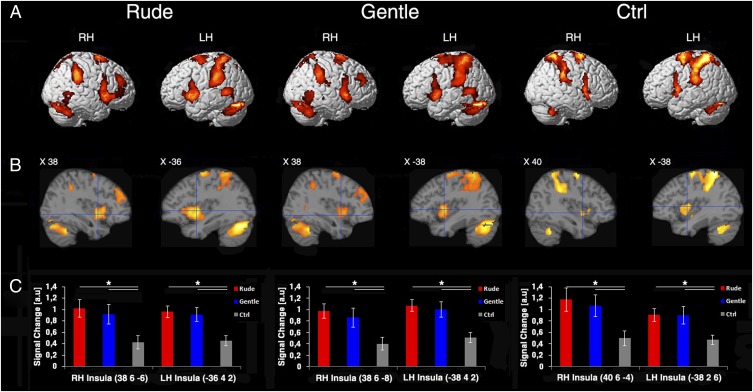
Fig. 3 shows the results of the conjunction analysis (PFWE < 0.05 at cluster level) of the activations found in the three tasks (coordinates and statistical values are in Table S1). As far as the cortical convexity is concerned (Fig. 3A), in both rude and gentle conditions, there was a bilateral signal increase near the posterior end of the middle temporal gyrus (possibly corresponding to hMT/V5+) (12), a bilateral activation of the posterior parietal cortex (larger in the left hemisphere than in the right hemisphere), and a bilateral activation of the dorsal premotor cortex. Signal increase was also present in the dorsal part of the cerebellum bilaterally. In the Ctrl condition, the activation pattern was similar to that observed for the vitality conditions, except for the lack of activation of visual area hMT/V5+.
Fig. 3.
Overlapping of areas active during the three different tasks (OBS, IMA, and EXE). (A) Lateral views of the right and left hemispheres. The activations in the three conditions (rude, gentle, and Ctrl) obtained with a conjunction analysis are rendered on a standard Montreal Neurological Institute brain template (PFWE < 0.05 at cluster level). (B) Parasagittal sections showing the insular activations in two hemispheres in three conditions. (C) Signal changes in six ROIs created on the central insula. All ROIs were defined centering the sphere (radium 10 mm) around the maxima of the functional maps resulting from conjunction analysis of OBS, IMA, and EXE tasks. The horizontal lines above the columns indicate the comparisons between the conditions. LH, left hemisphere; RH, right hemisphere. *Significant differences at P < 0.05 (Bonferroni corrected).
Table S1.
Cerebral activity obtained with overlapping of three different tasks (OBS, IMA, and EXE) for each condition: Rude, Gentle, and Ctrl
| Anatomical region | Left hemisphere | Right hemisphere | ||||||||
| x | y | z | Z score | ATB | x | y | z | Z score | ATB | |
| Rude | ||||||||||
| Calcarine gyrus | −2 | −82 | 12 | Inf | 60% Area 17* | |||||
| Lingual gyrus | 4 | −74 | −4 | Inf | 70% Area 17* | |||||
| Inferior parietal lobule | 52 | −42 | 48 | 5.74 | 40% IPC(PF)* | |||||
| Supra marginal gyrus | 62 | −36 | 38 | 6.07 | 100% IPC(PF)* | |||||
| Temporal gyrus | −56 | −52 | 4 | 5.33 | ||||||
| Insula | −36 | 4 | 2 | 6.12 | 38 | 6 | −6 | 5.35 | ||
| Superior frontal gyrus | −30 | −4 | 68 | 5.69 | 30% Area 6 | |||||
| Inferior frontal gyrus | 62 | 12 | 8 | 5.10 | 50% Area 44* | |||||
| Middle frontal gyrus | 50 | 40 | 24 | 4.30 | ||||||
| Middle cingulate cortex | −2 | 12 | 42 | 5.18 | 4 | 16 | 38 | 5.48 | ||
| Cerebellum | −32 | −70 | −22 | Inf | Inf | |||||
| Gentle | ||||||||||
| Calcarine gyrus | −2 | −82 | 12 | Inf | 60% Area 17* | 8 | −72 | 14 | Inf | 50% Area 17* |
| Lingual gyrus | 4 | −74 | −4 | Inf | 70% Area 17* | |||||
| Superior parietal lobule | −28 | −52 | 66 | 5.37 | 30% Area 2* | |||||
| Inferior parietal lobule | 52 | −42 | 50 | 4.90 | 40% IPC(PF)* | |||||
| Supra marginal gyrus | 60 | −40 | 32 | 5.71 | 100% IPC(PF)* | |||||
| Insula | −38 | 4 | 2 | 5.81 | 38 | 6 | −8 | 4.53 | ||
| Inferior frontal gyrus | 40 | 32 | 28 | 3.51 | ||||||
| Middle frontal gyrus | 40 | 44 | 34 | 4.34 | ||||||
| Cerebellum | −32 | −70 | −22 | Inf | 71% VIIa CrusI* | 22 | −70 | −18 | 7.25 | 59% Lob VI (Hem)* |
| Control | ||||||||||
| Precuneus | −12 | −56 | 66 | 7.06 | 50% SPL (7A) | 10 | −54 | 68 | 6.23 | 50% SPL (5L)* |
| Lingual gyrus | 6 | −70 | −8 | 6.55 | ||||||
| Superior parietal lobule | −38 | −46 | 64 | 6.59 | ||||||
| Inferior parietal lobule | −46 | −38 | 54 | 6.66 | 60% Area 2* | |||||
| Supra marginal gyrus | 44 | −34 | 42 | 5.64 | 30% hIP2 | |||||
| Postcentral gyrus | −36 | −36 | 42 | 5.68 | ||||||
| Precentral gyrus | 62 | 10 | 22 | 4.02 | ||||||
| Insula | −38 | 2 | 6 | 4.46 | 40 | 6 | −4 | 4.04 | ||
| Inferior frontal gyrus | −58 | 8 | 14 | 5.28 | 60% Area 44* | 50 | 12 | 8 | 4.38 | 60% Area 44* |
| Cerebellum | −16 | −76 | −20 | 5.78 | 83% Lob VI (Hem)* | 18 | −74 | −20 | 5.35 | 87% Lob VI (Hem)* |
Local maxima, as shown in Fig. 3, are given in Montreal Neurological Institute standard brain coordinates at cluster-level PFWE < 0.05. ATB, most probable anatomical region in the Anatomy Toolbox 1.7 (30). hIP2, intraparietal area 2; Inf, infinite; IPC, inferior parietal cortex; Lob VI Hem, cerebellum lobe; SPL, superior parietal lobule; VIIa CrusI, cerebellum lobe.
Assigned areas.
Fig. 3B shows the results of conjunction analysis in the insula. The most interesting result concerned the dorsocentral insula, where the conjunction analysis revealed activation in all three tasks (OBS, IMA, and EXE). This finding implies that the same sector of insula is involved in planning and producing actions endowed with specific vitality forms as well as during vitality form recognition.
Fig. 3C illustrates the signal changes in six regions of interest (ROIs) created on the central insula (details in Methods). A significant difference was present among the three conditions for all ROIs (ROI 1: F2,28 = 24.62, P < 0.05; ROI 2: F2,28 = 29.24, P < 0.05; ROI 3: F2,28 = 23.13, P < 0.05; ROI 4: F2,28 = 32.43, P < 0.05; ROI 5: F2,28 = 25.58, P < 0.05; and ROI 6: F2,28 = 23.55, P < 0.05). Posthoc analysis revealed that, for all ROIs, rude and gentle conditions produced higher signal change relative to Ctrl [rude > Ctrl (P < 0.05); gentle > Ctrl (P < 0.05); Bonferroni corrected]. No difference was present between rude and gentle conditions (P > 0.05).
Discussion
The vitality forms are a psychological construct that deals with the style of actions and in particular, those directed toward others (1–7). In everyday life, people produce vitality forms in voluntary actions to communicate to others their internal state as well as automatically, even in the absence of another person, on the basis of their mood as a motor agent. However, despite their well-recognized importance in social life (1–7), very little attention has been paid to the neural bases of this behavior.
In a previous study (8), we examined the neural substrate of vitality form recognition. We asked participants to observe two consecutive actions with the instruction to decide whether the action goal was the same or different (what task) or whether their vitality form was the same or different (how task). The contrast in how vs. what tasks revealed a specific activation of the dorsocentral insula during vitality forms recognition. Planning and execution of action with specific vitality forms were not examined in that experiment.
The main result of this study has been the demonstration that the dorsocentral part of the insula is involved in not only the observation of vitality forms but also, their planning (motor imagery) and execution. A weak insular activation was also present during the apparently neutral Ctrl (Fig. 3B). The most likely explanation of this finding is that, even when there is no request to perform an action with a specific style, its execution automatically elicits a vitality form, suggesting that all motor actions are bound to a specific vitality form.
Our findings that the dorsocentral part of the insula plays an important role in vitality forms are in agreement with previous findings on the general functional organization of the insula in monkeys and humans.
Experiments in monkeys showed that electrical stimulation of the dorsocentral part of the insula determines body parts movements, with a rich representation of the movements of the upper limb. These movements are radically different from the complex motor behaviors obtained by the stimulation of the rostral insula. In fact, the stimulation of the latter sector elicits complex positive ingestive behavior dorsally and negative ingestive behavior (e.g., disgust) ventrally (13).
A similar organization pattern has been reported by Kurth et al. (14) in humans. In a meta-analysis based on 1,768 functional neuroimaging experiments, Kurth et al. (14) described four distinct functional fields in the human insula: the sensorimotor, the socioemotional, the olfactory–gustatory, and the cognitive fields. The sensorimotor field corresponds to the insula sector involved in vitality form production and the analogous sensorimotor functional field of the monkey.
The finding that the dorsocentral insula is involved in both vitality form execution and recognition suggests that neurons of this sector of the insula might be endowed with the mirror mechanism transforming visual and possibly, auditory representations of the perceived vitality forms in their motor representations. This view is in line with the fMRI findings showing that the anterior sector of the insula is active during both expression and recognition of disgust in others (15, 16). A similar matching mechanism is likely involved in feeling pain and recognizing it in others (17). Thus, anterior and dorsocentral sectors of the insula, although underlying different functions, both seem to be endowed with the mirror mechanism.
An interesting question is how the dorsocentral insula may modulate the cortical circuits mostly responsible for voluntary movements. Is there an insulocortical circuit that may transmit vitality form information to the cortex? Anatomical data in the monkey support this possibility by showing that the dorsocentral sector of the insula has rich connections with areas AIP (9), F5 (10), and 12r (11) [that is, with the parietal and frontal areas that form the circuit involved in the organization of arm movements in the monkey (18, 19) as well as humans (20–22)] (Fig. 3A). In agreement with these findings, there are also results of intracortical electrical stimulation of the middle and posterior short gyri of the insula in humans. These stimulation data showed that these insular sectors determine evoked potential in the precentral gyrus and the superior and inferior parietal lobules (23), thus confirming the strict connections between these areas and the insula anatomically shown in the monkey.
It has been proposed that the general functional role of the insula is to integrate information coming from the external context with that encoding the internal state of the individual (24–26). The importance of the subcortical centers for affective self-regulation and emotional communication of other individuals has been stressed by Trevarthen and coworkers (27, 28). We propose that the dorsocentral sector of the insula plays a fundamental role in determining the overt expression of the internal state of the individuals and more specifically, that this sector sets the physical parameters of the performed movements through modulation of the cortical circuit controlling motor actions. The strong activation of the cerebellum during OBS, IMA, and EXE tasks suggests that this structure might play role in modulating the motor timing of the vitality forms.
As mentioned in the Introduction, vitality forms are a core element in social interactions (1–7). Behavioral studies show that children with autism are markedly impaired in vitality form recognition relative to children with typical development (29), thus implying that vitality form recognition deficit may play a role in determining the social impairments characterizing this disorder. These data indicating that the same sector of the insula is involved in both vitality form recognition and vitality form production suggest that children with autism might have deficits in executing actions with vitality forms appropriate to their internal state. If this hypothesis is correct, this deficit may play an important role in the genesis of early communication deficits in children with autism, with subsequent cascading effects in the development of their social abilities.
Methods
Participants.
The experiment was carried out on 15 healthy right-handed volunteers (six females: mean age = 23.1 y, SD age = 2.1 y and nine males: mean age = 26 y, SD age = 3.9 y). All participants had normal or corrected-to-normal visual acuity. None reported a history of psychiatric or neurological disorders or current use of any psychoactive medications. They gave their written informed consent to the experimental procedure, which was approved by the Local Ethics Committee of Parma.
Paradigm and Task.
The experiment was composed of six functional runs (two runs for Ctrl and four runs for vitality conditions: rude and gentle). To avoid possible biases elicited by the vitality conditions on Ctrl, we decided to present Ctrl before the vitality conditions. This decision was motivated by the concern that instruction to pay attention on the vitality form may also bias Ctrl.
In the first run, we presented participants with video clips in two different tasks: OBS and IMA (Ctrl). The two tasks were presented in independent miniblocks (OBS block and IMA block) in a sequential order. The OBS task started with the instruction to observe and required the participants to pay attention to the action (Fig. 1C1). The IMA task started with the instruction to imagine and required the participants to imagine themselves performing the action (Fig. 1C2). During the IMA task, in the central part of the screen, a cue indicated the direction (left side or right side) toward which they were requested to imagine the performance of the action. In 10% of the trials in both tasks, participants had to provide an explicit response on a response box placed inside the scanner concerning the color of the small ball observed in the video clip.
In the second run, we presented a static image of two boxes (Fig. 1C3) and asked participants to place a small ball in the box (EXE task). In the central part of the screen, a cue indicated to which box to perform the action (left box or right box).
In the third and fourth runs, we presented participants with video clips in two different tasks: vitality form OBS (VF-OBS) and vitality form IMA (VF-IMA). For each run, the two tasks were presented in independent blocks in sequential order. The VF-OBS task started with the instruction to observe and subsequent presentation of the video clip (Fig. 1 A1 and B1). During this task, participants had to pay attention to the style of the action (vitality form). The VF-IMA task started with the instruction to imagine and required the participants to imagine themselves performing the action in a gentle or rude way (Fig. 1 A2 and B2). During the VF-IMA task, the color of the edge screen indicated the action style in which to imagine the performance of the action (red, rude; blue, gentle). Finally, in the central part of the screen, a cue indicated the direction toward which to imagine the performance of the action (left side or right side). In 10% of the trials, in both tasks, participants had to indicate whether VF-OBS and VF-IMA were rude or gentle (SI Methods and Fig. S2).
In the fifth and sixth runs, we presented a static image of an actor seated opposite the observer and asked participants to move a packet of crackers toward the opposite actor in a rude or gentle way by simply rotating the wrist (Fig. 1 A3 and B3). A cue indicated the direction (left side or right side) in which to perform the action, whereas the color of the edge screen indicated the style to use during the execution of action (red, rude; blue, gentle). In each video, a fixation cross was introduced to control for restrained eye movements.
fMRI Data Acquisition and Analysis.
Anatomical T1-weighted and functional T2*-weighted magnetic resonance images were acquired with a 3-T General Electrics Scanner (details in SI Methods). After standard preprocessing steps, a series of within-subject, whole-brain general linear models was conducted using SPM8 (The Wellcome Department of Imaging Neuroscience) to examine effects of conditions (rude, gentle, and Ctrl) in the three different tasks (OBS, IMA, and EXE) (details in SI Methods). On the basis of the functional maps (group analysis) resulting from the overlapping of the OBS, IMA, and EXE tasks obtained for rude, gentle, and Ctrl conditions (Fig. 3C), six regions of interest (ROIs) were created. Using MarsBaR ROI Toolbox for SPM (release 0.42), all ROIs were defined on the central insula, centering the sphere (radium 10 mm) around the maxima (rude, right hemisphere ROI 1: x = 38, y = 6, and z = −6; left hemisphere ROI 2: x = −36, y = 4, and z = 2; gentle, right hemisphere ROI 3: x = 38, y = 6, and z = −8; left hemisphere ROI 4: x = −38, y = 4, and z = 2; Ctrl, right hemisphere ROI 5: x = 40, y = 6, and z = −4; left hemisphere ROI 6: x = −38, y = 2, and z = 6). Signal change for each subject was extracted using REX Toolbox. In the ROIs, signal change values associated with rude, gentle, and Ctrl were calculated for all three task (OBS, IMA, and EXE) for each subject on the basis of contrast images (second-level analysis) (SI Methods, Statistical Analysis). Finally, the signal change values were averaged among tasks, and a general linear model analysis was carried out on the time course in the ROIs.
Acknowledgments
This study was supported by Advanced European Research Grant COGSYSTEM and Inter-University Attraction Pole (to G.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512133112/-/DCSupplemental.
References
- 1.Stern DN. The Interpersonal World of the Infant. Basic Books; New York: 1985. [Google Scholar]
- 2.Stern DN. Affect attunement. In: Call JD, Galenson E, Tyson R, editors. Frontiers of Infant Psychiatry. Vol 2. Basic Books; New York: 1984. pp. 3–14. [Google Scholar]
- 3.Stern DN. The Present Moment in Psychotherapy and Everyday Life. Norton; New York: 2004. [Google Scholar]
- 4.Stern DN. Forms of vitality exploring dynamic experience in psychology, arts, psychotherapy, and development. Oxford Univ Press; Oxford: 2010. [Google Scholar]
- 5.Trevarthen C. The concept and foundations of infant intersubjectivity. In: Braten S, editor. Intersubjective Communication and Emotion in Early Ontogeny. Cambridge Univ Press; New York: 1998. pp 15–46. [Google Scholar]
- 6.Trevarthen C, Aitken KJ. Infant intersubjectivity: Research, theory, and clinical applications. J Child Psychol Psychiatry. 2001;42(1):3–48. [PubMed] [Google Scholar]
- 7.Rochat P. Others in Mind: The Origins of Self-Consciousness. Cambridge Univ Press; New York: 2009. [Google Scholar]
- 8.Di Cesare G, et al. The neural correlates of ‘vitality form’ recognition: An fMRI study: This work is dedicated to Daniel Stern, whose immeasurable contribution to science has inspired our research. Soc Cogn Affect Neurosci. 2014;9(7):951–960. doi: 10.1093/scan/nst068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borra E, et al. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb Cortex. 2008;18(5):1094–1111. doi: 10.1093/cercor/bhm146. [DOI] [PubMed] [Google Scholar]
- 10.Gerbella M, Belmalih A, Borra E, Rozzi S, Luppino G. Connections of the anterior (F5a) subdivision of the macaque ventral premotor area F5. Brain Struct Funct. 2011;216(1):43–65. doi: 10.1007/s00429-010-0293-6. [DOI] [PubMed] [Google Scholar]
- 11.Borra E, Gerbella M, Rozzi S, Luppino G. Anatomical evidence for the involvement of the macaque ventrolateral prefrontal area 12r in controlling goal-directed actions. J Neurosci. 2011;31(34):12351–12363. doi: 10.1523/JNEUROSCI.1745-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferri S, Kolster H, Jastorff J, Orban GA. The overlap of the EBA and the MT/V5 cluster. Neuroimage. 2012;1:412–425. doi: 10.1016/j.neuroimage.2012.10.060. [DOI] [PubMed] [Google Scholar]
- 13.Jezzini A, Caruana F, Stoianov I, Gallese V, Rizzolatti G. The functional organization of the insula and of inner perisylvian regions: An intracortical microstimulation study. Proc Natl Acad Sci USA. 2012;109(25):10077–10082. doi: 10.1073/pnas.1200143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: Functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214(5-6):519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wicker B, et al. Both of us disgusted in My insula: The common neural basis of seeing and feeling disgust. Neuron. 2003;40(3):655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- 16.Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends Cogn Sci. 2004;8(9):396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 18.Jeannerod M, Arbib MA, Rizzolatti G, Sakata H. Grasping objects: The cortical mechanisms of visuomotor transformation. Trends Neurosci. 1995;18(7):314–320. [PubMed] [Google Scholar]
- 19.Nelissen K, Vanduffel W. Grasping-related functional magnetic resonance imaging brain responses in the macaque monkey. J Neurosci. 2011;31(22):8220–8229. doi: 10.1523/JNEUROSCI.0623-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keysers C, Fadiga L. The mirror neuron system: New frontiers. Soc Neurosci. 2008;3(3-4):193–198. doi: 10.1080/17470910802408513. [DOI] [PubMed] [Google Scholar]
- 21.Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 22.Rizzolatti G, Cattaneo L, Fabbri-Destro M, Rozzi S. Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol Rev. 2014;94(2):655–706. doi: 10.1152/physrev.00009.2013. [DOI] [PubMed] [Google Scholar]
- 23.Almashaikhi T, et al. Functional connectivity of insular efferences. Hum Brain Mapp. 2014;35(10):5279–5294. doi: 10.1002/hbm.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craig AD. How do you feel? Interoception: The sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3(8):655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 25.Damasio AR, et al. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nat Neurosci. 2000;3(10):1049–1056. doi: 10.1038/79871. [DOI] [PubMed] [Google Scholar]
- 26.Gothard K, Hoffman K. Circuits of emotion in the primate brain. In: Platt M, Ghazanfar A, editors. Primate Neuroethology. Oxford Univ Press; New York: 2010. pp. 292–315. [Google Scholar]
- 27.Trevarthen C, Delafield-Butt JT. Autism as a developmental disorder in intentional movement and affective engagement. Front Integr Neurosci. 2013;7:49. doi: 10.3389/fnint.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trevarthen C, et al. 2006. Collaborative Regulations of Vitality in Early Childhood: Stress in Intimate Relationships and Postnatal Psychopathology, Developmental Psychopathology, eds Cicchetti D, Cohen DJ (Wiley, New York), 2nd Ed, Vol 2.
- 29.Rochat MJ, et al. Impaired vitality form recognition in autism. Neuropsychologia. 2013;51(10):1918–1924. doi: 10.1016/j.neuropsychologia.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Eickhoff SB, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 31.Friston KJ, et al. Classical and Bayesian inference in neuroimaging: Applications. Neuroimage. 2002;16(2):484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- 32.Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage. 1999;10(1):1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]