Significance
This article applies a range of protein ligation methods at the level of chromatin to understand the cross-talk mechanism between the well-established biomedical target the human Dot1 (hDot1L) methyltransferase and the ubiquitylation of H2B at lysine 120. Through a systematic structure–activity relationship study of the ubiquitin surface in regard to hDot1L-mediated H3K79 methylation and further investigation with precisely engineered chromatin substrates, a functional hotspot within ubiquitin was identified that is essential to the stimulation of hDot1L activity. More broadly, this work shows how chemical synthesis approaches can be used to precisely tailor protein posttranslational modifications to afford mechanistic insights that would be impossible by other methods.
Keywords: chromatin, ubiquitin, Dot1L, epigenetics, protein chemistry
Abstract
Ubiquitylation of histone H2B at lysine 120 (H2B-Ub) plays a critical role in transcriptional elongation, chromatin conformation, as well as the regulation of specific histone H3 methylations. Herein, we report a strategy for the site-specific chemical attachment of ubiquitin to preassembled nucleosomes. This allowed expedited structure–activity studies into how H2B-Ub regulates H3K79 methylation by the methyltransferase human Dot1. Through an alanine scan of the ubiquitin surface, we identified a functional hotspot on ubiquitin that is required for the stimulation of human Dot1 in vitro. Importantly, this result was validated in chromatin from isolated nuclei by using a synthetic biology strategy that allowed selective incorporation of the hotspot-deficient ubiquitin mutant into H2B. The ubiquitin hotspot additionally impacted the regulation of ySet1-mediated H3K4 methylation but was not required for H2B-Ub–induced impairment of chromatin fiber compaction. These data demonstrate the utility of applying chemical ligation technologies to preassembled chromatin and delineate the multifunctionality of ubiquitin as a histone posttranslational modification.
Histone posttranslational modifications (PTMs) modulate chromatin structure and function either by directly altering the intrinsic physical properties of the chromatin fiber or by nucleating the recruitment and activity of a host of transacting nuclear factors (1–3). The chemical diversity, differential dynamics, and sheer number (currently over 100) (4, 5) of these PTMs, along with their combinatorial occurrence at the level of the nucleosome, create a complex and nonstatic molecular architecture in which all chromatin-related processes function. A central challenge in the field of epigenetics is to disentangle how distinct chromatin states control biochemical outputs, which requires the elucidation of the critical determinants governing histone PTM readout.
A particularly fascinating histone PTM is the ubiquitylation of H2B at lysine 120 (H2B-Ub). H2B-Ub is enriched near the 5′ end of highly expressed genes and has been implicated in transcriptional elongation, as well as chromatin structure definition (6–9). Moreover, H2B-Ub directly regulates the H3K4 and H3K79 methyltransferases Set1 and Dot1, respectively (10–13). The mechanistic principles underlying these various phenomena remain poorly understood. At 8.5 kDa, ubiquitin is nearly as large as the histone to which it is linked (13.8 kDa in the case of H2B), increasing the nucleosome surface by as much as 4,800 A2 (14). Thus, compared with smaller PTMs such as acetylation and methylation, ubiquitin is “information rich” in that it alters the steric and electrostatic properties around its attachment site, as well as presenting a large surface area for the recruitment of binding factors. Structural and biochemical studies of ubiquitin–ligand complexes, including the ubiquitylation of histone H2A at lysine 15, have revealed a canonical binding hotspot on ubiquitin involving a hydrophobic patch centered on Leu8/Ile44 (15, 16), however additional interaction surfaces have been extensively characterized (17, 18). It is currently unclear whether the chromatin-associated functions of ubiquitin—for instance, the stimulation of Set1 and Dot1 activities—operate through a single functional epitope or whether discrete surfaces of ubiquitin are involved.
Understanding the biochemical cross-talk between ubiquitin and histone methylation has important biomedical implications. For instance, H3K79 hypermethylation, by deregulated human Dot1 (hDot1L), is tightly linked to the progression of certain hematopoietic malignancies (19–21). The molecular features of ubiquitin needed to directly stimulate hDot1L activity remain undefined, although a direct physical interaction between ubiquitin and the hDot1L yeast homolog Dot1p has been reported (22). The inability of some ubiquitin-like proteins (Ubls) to substitute for ubiquitin indicates that a specific region on the ubiquitin surface is required for hDot1L stimulation, rather than the effect being simply due to steric bulk (23, 24). However, the observation that the double mutation of Leu8 and Ile44 to alanine does not affect H3K79 methylation by hDot1L implicates a noncanonical surface in the stimulation (24). Ubiquitylation of H2B has also been shown to directly inhibit chromatin compaction (25). Again, this property requires a specific surface feature on ubiquitin, rather than its size per se, but as before the precise region involved is not known.
In this study, we use chemical procedures to identify functional epitopes on ubiquitylated chromatin. Using a facile library screening method, we identify two leucine residues near the C terminus of ubiquitin critical for stimulation of hDot1L activity in vitro. We confirmed the importance of these residues for endogenous hDot1L activity in isolated nuclei through monitoring H3K79 methylation levels subsequent to the site-specific incorporation of a ubiquitin mutant, lacking these residues, into chromatin (26). Extending our findings to other well-established H2B-Ub processes, we find that this ubiquitin hotspot is also necessary for the stimulation of ySet1-mediated H3K4 methylation. Surprisingly, the mutation of the hotspot has no impact on the ability of H2B-Ub to inhibit chromatin fiber compaction. Our work reveals multifunctionality in the ubiquitin surface with respect to H2B-Ub–dependent functions with an approach that can be easily extended to study other histone ubiquitylations.
Results
Direct Chemical Ubiquitylation of Mononucleosomes.
To determine which residues in ubiquitin are critical for H3K79 methylation cross-talk, we set out to develop a streamlined methodology for the rapid preparation of ubiquitylated nucleosomes. Current synthetic routes for histone ubiquitylation involve multistep protocols and are performed at the histone level, making the preparation of multiple ubiquitin nucleosomal substrates a formidable challenge (10, 23, 24, 27, 28).
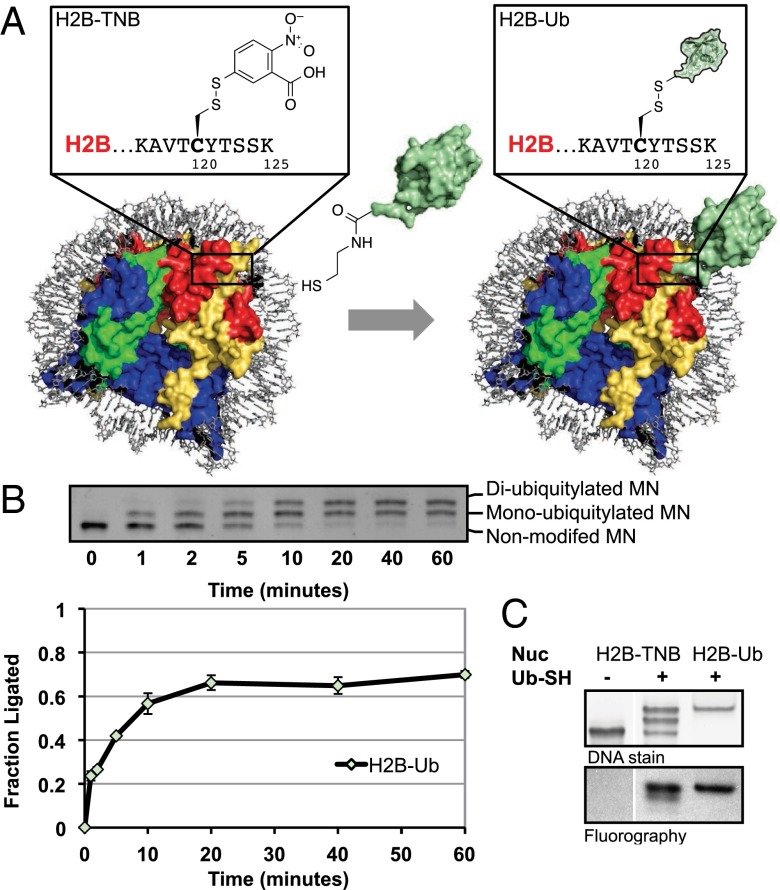
We envisioned that the synthesis of H2B-Ub–containing nucleosomes could be expedited via ligation of ubiquitin directly to the premade nucleosome using an asymmetric disulfide ligation strategy (23, 29). With this in mind, we generated a mutant H2B in which residue Lys120 was changed to a cysteine—importantly, this is the only cysteine in any of the four histones used in this study. This protein was reacted with 5,5′-dithiobis-(2-nitrobenzoic acid) (Di-TNB) to give an activated asymmetric disulfide (H2B-TNB), which was subsequently incorporated into mononucleosomes. Addition of ubiquitin engineered with a C-terminal aminoethanethiol moiety (Ub-SH) to the activated nucleosomes resulted in ubiquitylated H2B nucleosomes, as observed via gel shift and confirmed by mass spectrometry (Fig. 1 A and B and SI Appendix, Fig. S1). The reaction went to 60% completion over the course of 1 h (see note in SI Appendix for quantification details), resulting in approximately an equimolar ratio of diubiquitylated to monoubiquitylated nucleosomes, as each nucleosome contains two copies of H2B-TNB (Fig. 1B).
Fig. 1.
Preparation of H2B-Ub nucleosomes by on-nucleosomal asymmetric disulfide formation. (A) Scheme of nucleosomal ligation strategy. Site-specific ligation of ubiquitin (Ub) directly to the nucleosome is achieved upon addition of Ub-SH [unmodified ubiquitin shown in green; Protein Data Bank (PDB) ID code 1UBQ3] to H2B-TNB nucleosomes (wild-type nucleosome shown; PDB ID code 3LZ0). The H2B-Ub nucleosome is modeled as a composite of Ub and nucleosome structures. (Inset) Close-up of the H2B-TNB and H2B-Ub modifications. (B) Nucleosome ligations were monitored via native PAGE followed by ethidium bromide staining (Top panel) and quantified using ImageJ software (Bottom panel; see SI Appendix for quantification details). The reaction progressed to about 60% completion over the course of 1 h, resulting in a mix of non-, mono-, and diubiquitylated nucleosome species. Error bars, s.e.m. (n = 3). (C) hDot1L activity on H2B-Ub substrates prepared by nucleosomal ligations compared with fully reconstituted H2B-Ub substrates. hDot1L methyltransferase assays using 3H-SAM were performed on H2B-TNB nucleosomes (lane 1) and H2B-Ub nucleosomes, either prepared by direct nucleosomal ligations (lane 2) or fully reconstituted H2B-Ub (lane 3). Nucleosomes used in this assay were visualized via ethidium bromide staining of native PAGE gels (Top panel), and 3H-methyl incorporation was monitored by fluorography (Bottom panel). hDot1L was active on both types of H2B-Ub–containing nucleosomes but not H2B-TNB nucleosomes.
We next investigated the activity of the catalytic domain of the H3K79 methyltransferase hDot1L toward these ubiquitylated nucleosomes in comparison with H2B-Ub synthesized to contain the identical disulfide H2B-Ub linkage and reconstituted into nucleosomal substrates at the histone octamer level. H2B-Ub is required for the stimulation of hDot1L in vitro, as unmodified H2B nucleosomes are poor hDot1L substrates (10). H2B-Ub substrates formed via direct nucleosomal ligation stimulated hDot1L-mediated H3K79 methylation and showed an expected 1:1 ratio of H2B-Ub to H3K79 methylation, as evidenced by the twofold difference in fluorography between the diubiquitylated and monoubiquitylated species (Fig. 1C, lane 2 Lower panel). No methylation was observed on the nonubiquitylated species (Fig. 1C, lane 1 Lower panel).
Importantly, hDot1L-mediated H3K79 methylation was not stimulated in the presence of H2B-TNB, free TNB, or unligated Ub (SI Appendix, Fig. S2). Thus, hDot1L methyltransferase assays could be performed subsequent to H2B-Ub ligation without further purification. This on-nucleosomal ligation method worked well for the attachment of ubiquitin to other regions of the nucleosome surface and hence will be of practical use for accessing other histone ubiquitylations in a rapid, high-throughput manner (SI Appendix, Fig. S3).
A C-Terminal Ubiquitin Patch Is Critical for hDot1L Stimulation in Vitro.
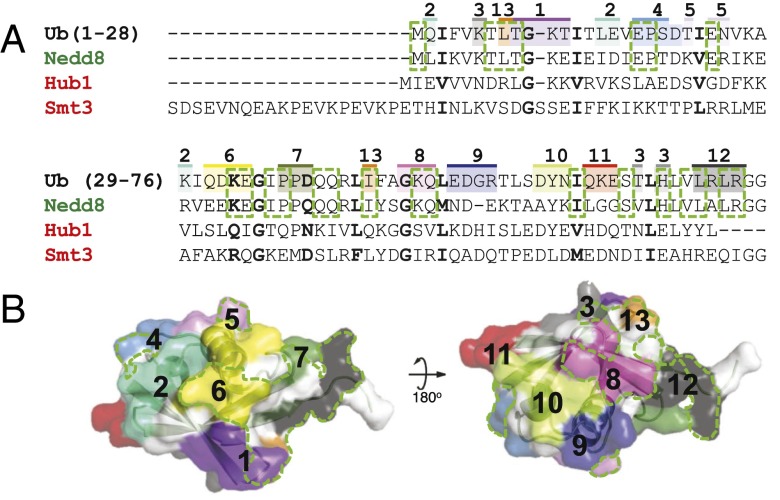
Having developed a protocol for the rapid generation of H2B-Ub nucleosomes, we moved on to determine which part of the ubiquitin surface contributed to hDot1L-mediated H3K79 methylation. Previously, it was shown that mutation of the canonical hydrophobic patch (L8/I44) in ubiquitin to alanine did not affect hDot1L stimulation (24). A complementary study, involving the attachment of Ubls to H2B at K120, revealed that ubiquitin functions in a residue-specific manner (23, 24). Specifically, hDot1L stimulation was found to be dependent on the primary sequence of the Ubl, as Nedd8 stimulated hDot1L, whereas two other Ubls—Smt3 and Hub1—did not. These Ubl proteins share the same tertiary structure but have between 23% and 55% sequence identity to ubiquitin (Fig. 2A), leading us to hypothesize a specific surface shared between ubiquitin and Nedd8 was critical for hDot1L-mediated H3K79 methylation (Fig. 2B).
Fig. 2.
Design of the ubiquitin surface mutant library. (A) Ubiquitin and Ubl homology alignment. Ubiquitin, Nedd8, Hub1, and Smt3 were aligned using the Lalign multiple sequence server. Similar residues shared between all Ubls and ubiquitin are bolded. Conserved residues between only ubiquitin and Nedd8 are outlined in a green dashed box. The surface residues in ubiquitin that were mutated to alanine are shaded. The colors, and the numbers above, indicate the groupings in the 13 surface mutants. Individual sequences are annotated in SI Appendix, Fig. S4. (B) Visual representation of the ubiquitin surface mutants mapped on to the ubiquitin structure (PDB ID code 1UBQ3) in their respective color. Homologous regions between ubiquitin and Nedd8, but not Hub1 and Smt3, are shown in a dashed green line.
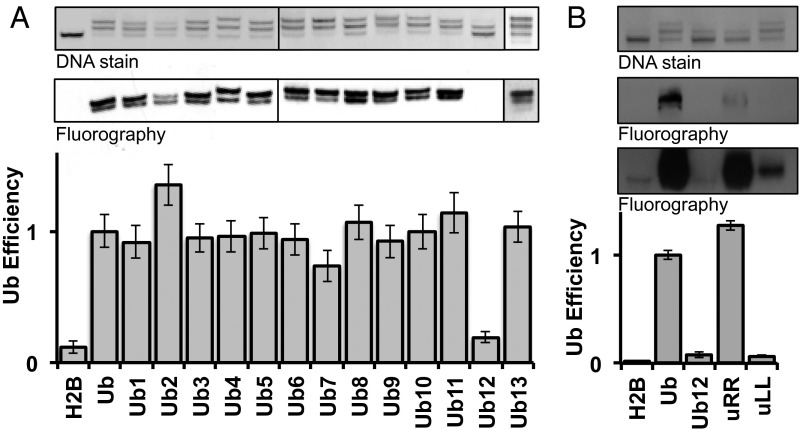
To test this hypothesis, we generated a library of ubiquitin mutants to use in hDot1L methyltransferase assays. Surface-exposed residues were subdivided into 13 distinct patches consisting of two to four residues, and each individual patch was mutated to alanine (Fig. 2 and SI Appendix, Fig. S4). The L8/I44A mutant of ubiquitin (Ub13) was included in our library and served as an additional positive control. This alanine mutant library was prepared in a similar manner to that used for Ub-SH and ligated to the H2B-TNB nucleosome (Fig. 3A, Top panel). All ligations, except for the Ub12 mutant, proceeded similarly to the Ub-SH ligation, with ligation efficiencies between 50% and 67%. hDot1L methyltransferase activity was subsequently assayed using this ubiquitin mutant nucleosome library (SI Appendix, Fig. S5). After adjusting for differences in nucleosomal ubiquitylation (see SI Appendix for details), it was evident that Ub12, and to a lesser extent Ub7, disrupted hDot1L-mediated H3K79 methylation, whereas all other mutants, including the Ub13 mutant, showed activity comparable to that of wild-type ubiquitin (Fig. 3A, Middle and Bottom panels). Consistent with our initial hypothesis, the residues collectively mutated in Ub7 and Ub12 have 71% sequence identity to Nedd8 and only 14% sequence identity to both Hub1 and Smt3 (Fig. 2). Note that the Ub2 mutant nucleosome was observed to be less stable than the other H2B-Ub nucleosomes, resulting in an overestimation of its efficiency as a substrate for hDot1L (Fig. 3A and SI Appendix, Fig. S5, lane 4).
Fig. 3.
Surface features on ubiquitin critical for hDot1L stimulation. For all methyltransferase assays, nucleosomes were visualized by native PAGE followed by ethidium bromide staining (Top panels), and 3H-methyl incorporation was probed by fluorography (Middle panels). Quantification of methylation was performed by filter binding assays followed by liquid scintillation counting and was adjusted to include the extent of Ub-SH ligation, termed Ub efficiency (Bottom panels; see note in SI Appendix for details). Error bars, s.e.m. (n = 3–6). (A) hDot1L activity on each of the Ub surface mutants, 1–13. Nonubiquitylated nucleosomes (H2B) and wild-type ubiquitylated H2B nucleosomes (Ub) were included as negative and positive controls, respectively. Only the Ub7 and Ub12 mutants led to a significant reduction in hDot1L stimulation. The Ub13 mutant, centered on the canonical hydrophobic hotspot, did not lead to reduction in hDot1L activity, which is consistent with a previous study (see the Introduction). (B) Ub12 was split into two alanine submutants, uLL and uRR, and hDot1L activity was assayed via 3H-SAM methyltransferase assays. Middle panels show two different exposures of 12 h (Top Middle panel) and 5 d (Bottom Middle panel).
To confirm the results of this initial screen, we prepared H2B-Ub nucleosomes containing the Ub7 and Ub12 mutants via the standard asymmetric disulfide reconstitution protocol, which affords homogeneous substrates. Methyltransferase assays with hDot1L established that both mutants have reduced ability to activate the enzyme, with Ub12 having the most profound effect, again consistent with the initial screen (SI Appendix, Fig. S6). Interestingly, although not contiguous in primary sequence, the residues mutated in Ub7 and Ub12 formed a continuous surface patch near the C terminus of ubiquitin (Fig. 2B).
Both L71 and L73 Residues in Ubiquitin Are Critical for hDot1L Activity.
As Ub12 returned H3K79 methylation levels to those exhibited by unmodified H2B nucleosomes, we chose to investigate this mutant further. Ub12 is a quadruple alanine mutant where the leucine–arginine–leucine–arginine motif near the C terminus of ubiquitin has been changed. This region is critical for ubiquitin recognition by the ubiquitylation machinery, displays structural plasticity, and has been implicated in many known ubiquitin interactions (17, 18, 30, 31).
We reasoned that the proximity of these mutations to the ligation junction could be responsible for the slower ligation kinetics of Ub12 compared with wild-type ubiquitin (SI Appendix, Fig. S7). Although it has been shown that the ligation junction can be varied substantially with little effect on hDot1L-mediated H3K79 methylation (23), we wondered whether the inability of Ub12 to promote H3K79 methylation and the sluggish ligation kinetics of this mutant were in some way linked. We chose to subdivide Ub12 into two further mutants retaining either both arginines (UbL71/73A or uLL) or both leucines (UbR72/74A or uRR), potentially narrowing this ligation/H3K79me defect into either a charge-based or hydrophobic-based effect. The two Ub12-derived mutants were prepared, ligated to the H2B-TNB nucleosome, and hDot1L methyltransferase assays were performed (Fig. 3B). uRR showed a ligation defect similar to Ub12 but still promoted robust H3K79 methylation considering the extent of ligation of this mutant (Fig. 3B, lane 4). Conversely, uLL ligated to the nucleosome to a similar extent as wild-type ubiquitin but was unable to stimulate hDot1L-mediated H3K79 methylation (Fig. 3B, lane 5). Additional mutagenesis studies indicated that both leucine residues in Ub contribute to the stimulation of hDot1L activity (SI Appendix, Fig. S8).
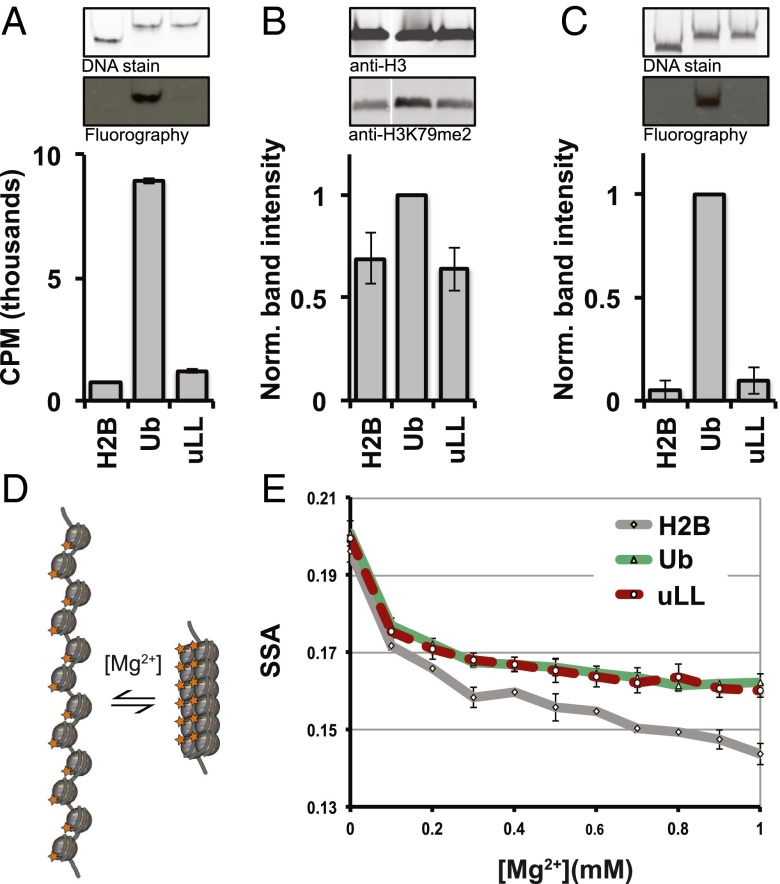
To further confirm the importance of the Leu71/Leu73 patch for hDot1L stimulation, we synthesized uLL-containing nucleosomes with an isopeptide linkage. This allowed us to rule out that the nonnative conjugation chemistry used in the initial analysis in some way contributed to disruption of the cross-talk. Importantly, this version of H2B-uLL was also unable to stimulate hDot1L (both the catalytic domain and full-length enzyme) in either a mononucleosomal or chromatinized plasmid context (Fig. 4A and SI Appendix, Fig. S9). Analysis of both the circular dichroism and (15N, 1H) heteronuclear single quantum coherence NMR spectrum of the uLL mutant revealed that it retained the ubiquitin fold, ruling out the possibility that the mutant lacks activity through indirect means by globally disrupting the ubiquitin tertiary structure (SI Appendix, Fig. S10). Collectively, these data indicate that stimulation of hDot1L activity is directly mediated by this hydrophobic surface patch on ubiquitin.
Fig. 4.
H2B-uLL abrogates H3K79 and H3K4 methyltransferase activity but not H2B-Ub–dependent chromatin compaction. (A) 3H-SAM hDot1L methyltransferase assays using unmodified (lane 1), H2B-Ub (lane 2), and H2B-uLL (lane 3) nucleosomes. H2B-Ub and H2B-uLL were prepared semisynthetically and contained isopeptide linkages between H2B-K120 and the ubiquitin. Nucleosomes were visualized by native PAGE followed by Sybr Gold staining (Top panel), and 3H-methyl incorporation was probed by fluorography (Middle panel). Quantification of methylation was performed by filter binding assays followed by liquid scintillation counting and reported as the direct readout (in cpm). Error bars, s.e.m. (n = 3). (B) In nucleo H3K79 methylation levels subsequent to semisynthetic incorporation of an H2B-Ub or H2B-uLL construct into native chromatin. Ub and uLL were incorporated into chromatin via protein transsplicing, and H3K79me2 levels were monitored (Middle panel) by Western blotting. For each sample, H3K79me2 levels were first adjusted relative to the total H3 signal within the sample (Top panel). All adjusted H3K79me2 signals were then normalized to the adjusted H3K79me2 level of the H2B-Ub sample (Bottom panel). Note that lane 1 (labeled H2B) refers to nuclei from nontransfected control cells. Error bars, s.e.m. (n = 3). (C) 3H-SAM methyltransferase assays using ySet1C. Methyltransferase assays using H2B- (lane 1), H2B-Ub– (lane 2), and H2B-uLL– (lane 3) containing nucleosomes were performed. Nucleosomes were visualized by native PAGE followed by Sybr Gold staining (Top panel), and 3H-methyl incorporation was probed by fluorography (Middle panel). The extent of 3H-methyl incorporation was quantified by densitometry (Bottom panel). Error bars, s.e.m. (n = 3). (D) Homo-FRET assay schematic to probe chromatin compaction. The 12-mer nucleosomal arrays containing a fluorescein labeled H2A compact upon addition of Mg2+. Compaction correlates with a lower SSA signal due to homo-FRET. (E) Nucleosomal array compaction as a function of Mg2+ for H2B- (gray line), H2B-Ub– (green line), and H2B-uLL– (red dashed line) containing 12-mer arrays. Error bars, s.e.m. (n = 3).
The Leu71/Leu73 Patch Is Required for hDot1L Stimulation on Chromatin in Isolated Nuclei.
We next asked whether the Leu71/Leu73 surface patch on ubiquitin is required to stimulate endogenous hDot1L activity in the context of native chromatin. Ubiquitin is a highly conserved protein, abundant in cells, and is essential in a range of activities such as protein degradation, localization, and signaling (15). An alanine scan of ubiquitin in yeast identified all nonviable ubiquitin mutants, which importantly included both L71 and L73 residues (30). The toxicity of uLL complicates cellular studies; simply expressing the ubiquitin mutant in mammalian cells is not a viable approach for validating our in vitro findings. This problem could be solved were it possible to selectively target the mutant ubiquitin to H2B within chromatin. Recently, van Leeuwen and coworkers reported a clever approach to mimic H2B-Ub in yeast by fusing ubiquitin to the N terminus of H2A, which positions ubiquitin near its normal attachment site on the nucleosome surface (32). In principle, this strategy could be adapted to mammalian cells, allowing targeted introduction of uLL. However, this study also showed that hDot1L is stimulated by these Ub-H2A fusion constructs to a much lower level than yDot1 in vitro (32). In light of this observation, we elected to use an alternative approach that allows for the direct semisynthesis of an isopeptide-linked H2B-Ub within mammalian chromatin. This approach uses naturally split inteins [referred to herein as the N-terminal intein (IntN) and C-terminal intein (IntC)] to assemble the desired H2B-Ub construct through an in nucleo protein ligation procedure (SI Appendix, Fig. S11A) (26). This reaction results in attachment of ubiquitin to H2BK120 through an isopeptide linkage that is identical to that used in our in vitro studies and, importantly, without mutating any of the other histones.
Nuclei were isolated from HEK293 cells transfected with a plasmid encoding a truncated version of H2B fused to the IntN [H2B(1–116)-IntN]. These were then treated with synthetically prepared constructs comprising the IntC fragment linked to the remaining residues of H2B conjugated, via an isopeptide linkage, to either wild-type Ub or uLL [IntC-H2B(117–125)-Ub or IntC-H2B(117–125)-uLL]. Both in nucleo reactions were successful, and the semisynthetic products, H2B-Ub and H2B-uLL, were generated within the chromatin fraction as indicated by Western blotting (SI Appendix, Fig. S11B). As expected based on the known cross-talk mechanism, in nucleo production of H2B-Ub led to an increase in overall H3K79 dimethylation, compared with nuclei from nontransfected cells (Fig. 4B). Importantly, we observed no stimulation of H3K79 dimethylation as a consequence of generating H2B-uLL on chromatin, despite there being comparable levels of the H2B-Ub and H2B-uLL semisynthetic products (Fig. 4B and SI Appendix, Fig. S11B). This result validates our biochemical data, confirming the essentiality of these two residues for endogenous hDot1L-mediated H3K79 methylation.
The Leu71/Leu73 Patch Is Important for ySet1C-Mediated H3K4 Methylation.
We wondered whether the L71 and L73 patch on ubiquitin is important for other H2B-Ub–associated functions. To this end, we asked whether H2B-uLL nucleosomes are able to stimulate methylation of H3K4 by Set1 in vitro. H3K4 methylation in mammalian cells is catalyzed by multiple Set1/MLL family histone methyltransferases, which play nonredundant, conditionally ubiquitin-independent roles (33). Thus, we turned to the Set1 methyltransferase complex from Saccharomyces cerevisiae, ySet1C, which is stimulated by H2B-Ub in vitro (34). Methyltransferase assays using 3H-S-adenosyl methionine (3H-SAM) were performed on semisynthetic H2B-Ub and H2B-uLL–containing nucleosomes using a ySet1C complex affinity-purified from Sf9 cells coinfected with baculoviruses that express FLAG-Set1 and the seven other untagged ySet1C subunits (SI Appendix, Fig. S12). As expected (34), ySet1C was stimulated by H2B-Ub nucleosomes and showed no activity toward unmodified H2B nucleosomes (Fig. 4C, lane 1 compared with lane 2). Importantly, and analogous to hDot1L, ySet1C was not stimulated by H2B-uLL–containing nucleosomes, highlighting the importance of L71/73 residues for both H3K4 and H3K79 methylation (Fig. 4C, lane 3).
The Leu71/Leu73 Patch Is Not Involved in H2B-Ub–Induced Impairment of Chromatin Fiber Compaction.
Finally, we investigated whether the L71/73 surface patch was important for the intrinsic ability of ubiquitin to inhibit nucleosomal array compaction. Previously, we showed that ubiquitin is able to impede the Mg2+-induced compaction of nucleosome arrays (25). Because this intrinsic function did not extend to the structurally related Ubl Hub1, we concluded that H2B-Ub prevents nucleosomal array compaction through sequence-specific mechanisms, analogous to the regulation of H3K79 methylation. We adopted the homo-fluorescence resonance energy transfer (homo-FRET) steady-state anisotropy (SSA) approach developed in this earlier study and directly compared the folding properties of unmodified, H2B-Ub, and H2B-uLL 12-mer nucleosome arrays (Fig. 4 D and E and SI Appendix, Fig. S13). As expected, the presence of wild-type ubiquitin on H2B impeded Mg2+-induced compaction of the array compared with unmodified H2B arrays (Fig. 4E). Surprisingly, given the profound effect on methyltransferase activity, mutation of the L71/73 surface patch had no impact on this intrinsic compaction property of ubiquitin; that is, the H2B-Ub and H2B-uLL 12-mer nucleosome arrays behaved identically in the SSA assay (Fig. 4E). This result indicates that different surface features on ubiquitin are involved in this intrinsic chromatin folding property than for the cross-stimulation of H3K4/H3K79 methylation.
Discussion
The H2B-Ub modification is intimately involved in transcription elongation, both acting as a positive regulator of H3K4 and H3K79 methylation as well as contributing to the maintenance of a less compact local chromatin state (10, 13, 25, 35). This is achieved through specific properties of the ubiquitin modification and is not simply due to the steric bulk of adding an 8.5-kDa protein to the nucleosomal surface. For instance, replacement of ubiquitin with Smt3 and Hub1, Ubls that share the same fold as ubiquitin but have substantially different surface residues (Fig. 2A), leads to a loss of H2B-Ub–dependent transactivation functions (11, 23, 24). By contrast, Nedd8, a Ubl with higher sequence homology to Ub than either Smt or Hub1, phenocopies the transactivation function of the native modification when incorporated into nucleosomes (23). In the present study, we set out to narrow down the region(s) on ubiquitin that is sensed by the enzymes responsible for H3K4 and H3K79 methylation: Set1 and Dot1, respectively. Development of a convenient nucleosome ligation approach allowed a comprehensive structure–activity relationship study of ubiquitin in regard to H2B-Ub function, revealing a functional hotspot on the Ub surface essential for the stimulation of hDot1L and ySet1C activities. Surprisingly, we found that this surface is not required for the H2B-Ub–dependent impairment of chromatin compaction. Collectively, these studies paint a picture of ubiquitin as an information-rich modification able to orchestrate distinct biochemical functions on chromatin by using nonoverlapping surface epitopes.
Several methods for the preparation of H2B-Ub substrates have been described, making biochemical reconstitution of homogenous H2B-Ub–containing chromatin accessible (10, 23, 24). A caveat to these existing methods is the need to prepare the H2B-Ub before incorporation into chromatin, resulting in a multistep process that is both time-consuming and sample-intensive. Here, we greatly simplify the process by showing that the ubiquitylation PTM can be chemically introduced in a site-specific fashion to a preassembled nucleosome. The approach involves an asymmetric disulfide formation reaction, compatible with nucleosomal reconstitution protocols, and achieves regioselectivity by exploiting the ability to introduce a unique cysteine residue into recombinant chromatin. As this method relies on the late stage diversification of the nucleosome substrate, systematic structure–activity analysis of the system becomes tractable, as demonstrated herein. This approach will be of use to study other histone ubiquitylations and their associated functions in a high-throughput manner. Additionally, the disulfide attachment chemistry used has the attractive feature that the modification can be “erased” through simple reduction, thereby offering the potential to study, in a time-resolved way, the functional and structural consequences of PTM removal. Moreover, it may be possible to adapt the strategy to give nonreducible thioether linkages through use of alternate thiol-directed chemistries (36, 37). Indeed, analogs of other PTMs can, in principle, be introduced chemically into preassembled chromatin using known cysteine derivatization routes—for example, lysine methylation (2) and acetylation (38). Conceivably, other chemical functionalities used in bio-orthogonal reactions could be used instead of, or in addition to, cysteine, thereby further expanding the palette of PTMs that can be introduced into chromatin templates.
Our structure–activity studies identify a hydrophobic surface on ubiquitin, comprising L71 and L73, required for the stimulation of hDot1L-mediated H3K79 methylation as well as ySet1C-mediated H3K4 methylation. This epitope could promote productive enzyme–nucleosome complex formation and/or allosterically activate these enzymes or the H2B-Ub nucleosome for H3K4/K79 methylation. As these enzymes are not structurally homologous, or enzymatically analogous (they belong to different branches of the methyltransferase family) (39), a conserved Ub recognition element or allosteric mechanism seems unlikely (24, 34, 40). In light of this, we favor a mechanism in which the L71/L73 patch on ubiquitin engages the nucleosome surface near the H2B-K120 attachment site, effectively locking ubiquitin down on the nucleosome. Interestingly, hDot1L can bind both unmodified and ubiquitylated nucleosomes (23), with only the latter interaction being conducive to H3K79 methylation. The role of H2B-Ub might, therefore, be to prevent nonproductive hDot1L–nucleosome interactions, which dominate in unmodified nucleosomes, through a physical “corralling” effect resulting from ubiquitin positioning. We note that a similar Dot1 activation model has recently been suggested by van Leeuwen and coworkers, based on the results of Ub-H2A fusion experiments in yeast (32). Because we found only one surface epitope on ubiquitin required for hDot1L stimulation, our data would argue against there being a specific interaction between ubiquitin and this enzyme. In the case of ySet1C, a similar activation mechanism could be operable, although we cannot rule out that additional surface epitopes on ubiquitin might engage this protein complex. Further biochemical and structural experiments will be needed to test the proposed corralling mechanism as well as to pinpoint which surface residues on ubiquitin are needed for the ability of H2B-Ub to impede the compaction of chromatin fibers. Nonetheless, our observations highlight that Ub is an information-rich PTM, able to drive different biochemical/biophysical outputs using nonoverlapping mechanisms, which in the context of chromatin adds yet another layer of complexity that must be considered when correlating histone PTMs to chromatin states.
Materials and Methods
In a typical reaction, reconstituted H2BK120C containing octamers (5 nmol, 50 μL) in octamer formation buffer (10 mM Tris, 2 M NaCl, 1 mM EDTA, pH 7.5) were mixed 1:1 with octamer formation buffer containing Di-TNB (50 μmol, 50 μL) and nutated at room temperature for 10 min to yield activated (H2B-TNB) octamers. H2B-TNB nucleosomes were formed by combining octamers and “601” DNA in high-salt buffer followed by dialysis into a low-salt buffer as described previously (10). The quality of the reconstitution was assessed by separation on a Criterion 5% (vol/vol) Tris-borate-EDTA (TBE) gel run in 0.5× TBE buffer, followed by staining with ethidium bromide or Sybr Gold. Ub-SH or Ub-SH mutants (4.25 μg, 0.5 nmol) were added to 50 pmol of H2B-TNB nucleosomes in a final reaction volume of 10 μL and incubated at 55 °C for 1 h. The degree of Ub-SH ligation was determined by separation on a Criterion 5% TBE gel run in 0.5× TBE buffer, followed by staining with ethidium bromide. These H2B-Ub nucleosomes were used in methyltransferase assays without subsequent purification.
Supplementary Material
Acknowledgments
The authors thank the current and former members of the T.W.M. laboratory, in particular Beat Fierz, Champak Chatterjee, Galia Delelouchina, Neel Shah, and Uyen Nguyen, for many valuable discussions. This work was supported by National Institutes of Health Grants R37-GM086868, R01 GM107047, CA 129325, and DK071900.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1504483112/-/DCSupplemental.
References
- 1.Zentner GE, Henikoff S. Regulation of nucleosome dynamics by histone modifications. Nat Struct Mol Biol. 2013;20(3):259–266. doi: 10.1038/nsmb.2470. [DOI] [PubMed] [Google Scholar]
- 2.Lu X, et al. The effect of H3K79 dimethylation and H4K20 trimethylation on nucleosome and chromatin structure. Nat Struct Mol Biol. 2008;15(10):1122–1124. doi: 10.1038/nsmb.1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patel DJ, Wang Z. Readout of epigenetic modifications. Annu Rev Biochem. 2013;82:81–118. doi: 10.1146/annurev-biochem-072711-165700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan M, et al. Identification of 67 histone marks and histone lysine crotonylation as a new type of histone modification. Cell. 2011;146(6):1016–1028. doi: 10.1016/j.cell.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128(4):693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Minsky N, et al. Monoubiquitinated H2B is associated with the transcribed region of highly expressed genes in human cells. Nat Cell Biol. 2008;10(4):483–488. doi: 10.1038/ncb1712. [DOI] [PubMed] [Google Scholar]
- 7.Jung I, et al. H2B monoubiquitylation is a 5′-enriched active transcription mark and correlates with exon-intron structure in human cells. Genome Res. 2012;22(6):1026–1035. doi: 10.1101/gr.120634.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma MK, Heath C, Hair A, West AG. Histone crosstalk directed by H2B ubiquitination is required for chromatin boundary integrity. PLoS Genet. 2011;7(7):e1002175. doi: 10.1371/journal.pgen.1002175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batta K, Zhang Z, Yen K, Goffman DB, Pugh BF. Genome-wide function of H2B ubiquitylation in promoter and genic regions. Genes Dev. 2011;25(21):2254–2265. doi: 10.1101/gad.177238.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGinty RK, Kim J, Chatterjee C, Roeder RG, Muir TW. Chemically ubiquitylated histone H2B stimulates hDot1L-mediated intranucleosomal methylation. Nature. 2008;453(7196):812–816. doi: 10.1038/nature06906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chandrasekharan MB, Huang F, Sun ZW. Ubiquitination of histone H2B regulates chromatin dynamics by enhancing nucleosome stability. Proc Natl Acad Sci USA. 2009;106(39):16686–16691. doi: 10.1073/pnas.0907862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ng HH, Xu RM, Zhang Y, Struhl K. Ubiquitination of histone H2B by Rad6 is required for efficient Dot1-mediated methylation of histone H3 lysine 79. J Biol Chem. 2002;277(38):34655–34657. doi: 10.1074/jbc.C200433200. [DOI] [PubMed] [Google Scholar]
- 13.Kim J, et al. RAD6-mediated transcription-coupled H2B ubiquitylation directly stimulates H3K4 methylation in human cells. Cell. 2009;137(3):459–471. doi: 10.1016/j.cell.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krissinel E, Henrick K. Inference of macromolecular assemblies from crystalline state. J Mol Biol. 2007;372(3):774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 16.Fradet-Turcotte A, et al. 53BP1 is a reader of the DNA-damage-induced H2A Lys 15 ubiquitin mark. Nature. 2013;499(7456):50–54. doi: 10.1038/nature12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winget JM, Mayor T. The diversity of ubiquitin recognition: Hot spots and varied specificity. Mol Cell. 2010;38(5):627–635. doi: 10.1016/j.molcel.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Lange OF, et al. Recognition dynamics up to microseconds revealed from an RDC-derived ubiquitin ensemble in solution. Science. 2008;320(5882):1471–1475. doi: 10.1126/science.1157092. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen AT, Taranova O, He J, Zhang Y. DOT1L, the H3K79 methyltransferase, is required for MLL-AF9-mediated leukemogenesis. Blood. 2011;117(25):6912–6922. doi: 10.1182/blood-2011-02-334359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suvà ML, Riggi N, Bernstein BE. Epigenetic reprogramming in cancer. Science. 2013;339(6127):1567–1570. doi: 10.1126/science.1230184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okada Y, et al. hDOT1L links histone methylation to leukemogenesis. Cell. 2005;121(2):167–178. doi: 10.1016/j.cell.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Oh S, Jeong K, Kim H, Kwon CS, Lee D. A lysine-rich region in Dot1p is crucial for direct interaction with H2B ubiquitylation and high level methylation of H3K79. Biochem Biophys Res Commun. 2010;399(4):512–517. doi: 10.1016/j.bbrc.2010.07.100. [DOI] [PubMed] [Google Scholar]
- 23.Chatterjee C, McGinty RK, Fierz B, Muir TW. Disulfide-directed histone ubiquitylation reveals plasticity in hDot1L activation. Nat Chem Biol. 2010;6(4):267–269. doi: 10.1038/nchembio.315. [DOI] [PubMed] [Google Scholar]
- 24.McGinty RK, et al. Structure-activity analysis of semisynthetic nucleosomes: Mechanistic insights into the stimulation of Dot1L by ubiquitylated histone H2B. ACS Chem Biol. 2009;4(11):958–968. doi: 10.1021/cb9002255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fierz B, et al. Histone H2B ubiquitylation disrupts local and higher-order chromatin compaction. Nat Chem Biol. 2011;7(2):113–119. doi: 10.1038/nchembio.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.David Y, Vila-Perelló M, Verma S, Muir TW. Chemical tagging and customizing of cellular chromatin states using ultrafast trans-splicing inteins. Nat Chem. 2015;7(5):394–402. doi: 10.1038/nchem.2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Siman P, Karthikeyan SV, Nikolov M, Fischle W, Brik A. Convergent chemical synthesis of histone H2B protein for the site-specific ubiquitination at Lys34. Angew Chem Int Ed Engl. 2013;52(31):8059–8063. doi: 10.1002/anie.201303844. [DOI] [PubMed] [Google Scholar]
- 28.Ajish Kumar KS, Haj-Yahya M, Olschewski D, Lashuel HA, Brik A. Highly efficient and chemoselective peptide ubiquitylation. Angew Chem Int Ed Engl. 2009;48(43):8090–8094. doi: 10.1002/anie.200902936. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Ai Y, Wang J, Haracska L, Zhuang Z. Chemically ubiquitylated PCNA as a probe for eukaryotic translesion DNA synthesis. Nat Chem Biol. 2010;6(4):270–272. doi: 10.1038/nchembio.316. [DOI] [PubMed] [Google Scholar]
- 30.Sloper-Mould KE, Jemc JC, Pickart CM, Hicke L. Distinct functional surface regions on ubiquitin. J Biol Chem. 2001;276(32):30483–30489. doi: 10.1074/jbc.M103248200. [DOI] [PubMed] [Google Scholar]
- 31.Kiel C, Serrano L. The ubiquitin domain superfold: Structure-based sequence alignments and characterization of binding epitopes. J Mol Biol. 2006;355(4):821–844. doi: 10.1016/j.jmb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 32.Vlaming H, et al. Flexibility in crosstalk between H2B ubiquitination and H3 methylation in vivo. EMBO Rep. 2014;15(10):1077–1084. doi: 10.15252/embr.201438793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tang Z, et al. SET1 and p300 act synergistically, through coupled histone modifications, in transcriptional activation by p53. Cell. 2013;154(2):297–310. doi: 10.1016/j.cell.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, et al. The n-SET domain of Set1 regulates H2B ubiquitylation-dependent H3K4 methylation. Mol Cell. 2013;49(6):1121–1133. doi: 10.1016/j.molcel.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weake VM, Workman JL. Histone ubiquitination: Triggering gene activity. Mol Cell. 2008;29(6):653–663. doi: 10.1016/j.molcel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 36.Chalker JM, Lercher L, Rose NR, Schofield CJ, Davis BG. Conversion of cysteine into dehydroalanine enables access to synthetic histones bearing diverse post-translational modifications. Angew Chem Int Ed Engl. 2012;51(8):1835–1839. doi: 10.1002/anie.201106432. [DOI] [PubMed] [Google Scholar]
- 37.Valkevich EM, et al. Forging isopeptide bonds using thiol-ene chemistry: Site-specific coupling of ubiquitin molecules for studying the activity of isopeptidases. J Am Chem Soc. 2012;134(16):6916–6919. doi: 10.1021/ja300500a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li F, et al. A direct method for site-specific protein acetylation. Angew Chem Int Ed Engl. 2011;50(41):9611–9614. doi: 10.1002/anie.201103754. [DOI] [PubMed] [Google Scholar]
- 39.Copeland RA, Solomon ME, Richon VM. Protein methyltransferases as a target class for drug discovery. Nat Rev Drug Discov. 2009;8(9):724–732. doi: 10.1038/nrd2974. [DOI] [PubMed] [Google Scholar]
- 40.Wu L, et al. ASH2L regulates ubiquitylation signaling to MLL: Trans-regulation of H3 K4 methylation in higher eukaryotes. Mol Cell. 2013;49(6):1108–1120. doi: 10.1016/j.molcel.2013.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.