Abstract
Emerging evidence suggests a role for purinergic signaling in the activation of multiprotein intracellular complexes called inflammasomes, which control the release of potent inflammatory cytokines, such as interleukin (IL) -1β and -18. Porphyromonas gingivalis is intimately associated with periodontitis and is currently considered one of the pathogens that can subvert the immune system by limiting the activation of the NLRP3 inflammasome. We recently showed that P. gingivalis can dampen eATP-induced IL-1β secretion by means of its fimbriae in a purinergic P2X7 receptor–dependent manner. Here, we further explore the role of this purinergic receptor during eATP-induced IL-1β processing and secretion by P. gingivalis–infected macrophages. We found that NLRP3 was necessary for eATP-induced IL-1β secretion as well as for caspase 1 activation irrespective of P. gingivalis fimbriae. Additionally, although the secretion of IL-1β from P. gingivalis–infected macrophages was dependent on NLRP3, its adaptor protein ASC, or caspase 1, the cleavage of intracellular pro-IL-1β to the mature form was found to occur independently of NLRP3, its adaptor protein ASC, or caspase 1. Our in vitro findings revealed that P2X7 receptor has a dual role, being critical not only for eATP-induced IL-1β secretion but also for intracellular pro-IL-1β processing. These results were relevant in vivo since P2X7 receptor expression was upregulated in a P. gingivalis oral infection model, and reduced IFN-γ and IL-17 were detected in draining lymph node cells from P2rx7-/- mice. Furthermore, we demonstrated that P2X7 receptor and NLRP3 transcription were modulated in human chronic periodontitis. Overall, we conclude that the P2X7 receptor has a role in periodontal immunopathogenesis and suggest that targeting of the P2X7/NLRP3 pathway should be considered in future therapeutic interventions in periodontitis.
Keywords: ATP, inflammasome, IL-1β, macrophage, cytokines, periodontitis
Introduction
Periodontitis is a common infection-associated oral disease in humans, which can lead to alveolar bone destruction and tooth loss (Kassebaum et al. 2014). Many studies have associated periodontitis with systemic diseases such as diabetes (Zhu et al. 2014), atherosclerosis (Slocum et al. 2014), and rheumatoid arthritis (Maresz et al. 2013). In general, periodontitis is due to a shift (dysbiosis) in the oral microflora from resident commensals to the overgrowth of pathogenic bacteria that leads to the breakdown of homeostasis between the host immune response and the dental biofilm in oral microenvironment (Hajishengallis and Lamont 2012).
Porphyromonas gingivalis is a common member of the polymicrobial dental biofilm community that is involved in the immunopathogenesis of periodontitis. Recently characterized as a proinflammatory anaerobic bacterium (Hajishengallis 2014), P. gingivalis employs multiple strategies to subvert the immune response (Taxman et al. 2012; Belibasakis et al. 2013; Maekawa et al. 2014; Huck et al. 2015). This periodontopathogen is recognized by pattern recognition receptors via conserved motifs known as pathogen-associated molecular patterns, which results in the induction of inflammatory signaling pathways that stimulate NF-κB activation and cytokine production.
Toll-like receptors (TLRs) are pattern recognition receptors that recognize P. gingivalis and induce synthesis of pro–interleukin 1β (pro-IL-1β)—an inactive precursor that must be cleaved and secreted to exert its biological function. Executed by activated caspase 1, the proteolytic processing of pro-IL-1β requires a second signal, conventionally referred as a danger-associated molecular pattern (DAMP), which triggers the formation of intracellular multiprotein complexes called inflammasomes (Gombault et al. 2012). Inflammasomes are protein complexes composed of a scaffold component, such as apoptosis protease activating factor (APAF), NOD-like receptor-pyrin-containing proteins (NLRP) or absent in melanoma 2 (AIM2), an apoptosis-associated speck-like protein containing CARD (ASC), and pro-caspase-1 which regulate the cleavage of pro-IL-1β and pro-IL-18 into their respective secreted mature forms (Di Virgilio 2013). One of the most extensively studied inflammasomes contains NLRP3. Multiple triggers for NLRP3 inflammasome activation have been described, including microbial components, pore-forming toxins, and particulate materials (Gombault et al. 2012; Latz et al. 2013).
Recently, it was demonstrated that P. gingivalis inhibits NLRP3 inflammasome activation (Taxman et al. 2012; Belibasakis et al. 2013; Huck et al. 2015). Although the exact mechanism of NLRP3 inflammasome activation remains uncertain, there is evidence linking this event to lysosomal rupture and the release of cathepsins (Lopez-Castejon et al. 2010), K+ efflux (Morandini et al. 2014; Pelegrin et al. 2008), endoplasmic reticulum stress (Shenderov et al. 2014), mitochondrial reactive oxygen species, and purinergic receptor P2X, ligand-gated ion channel 7 (P2X7) activation (Hung et al. 2013). P2X7 is an ion-gated channel that can participate in apoptotic cell death, and its ligand, extracellular adenosine triphosphate (eATP), is a prototypic DAMP, being implicated in IL-1β cleavage and secretion from proinflammatory cells (Pelegrin et al. 2008). A large body of evidence indicates that stimulation by eATP, adenosine, and related compounds can affect many additional facets of oral pathophysiology, as recently reviewed (Lim and Mitchell 2012). Yilmaz et al. (2008) showed that P. gingivalis stimulates synthesis of pro-IL-1β, but this first signal is not able to activate the NLRP3 inflammasome in gingival epithelial cells; however, addition of an exogenous source of ATP promoted secretion of the mature IL-1β. Using bone marrow macrophages infected with P. gingivalis, we also recently demonstrated that P. gingivalis fimbriae inhibited eATP-induced IL-1β secretion via P2X7 receptor (Morandini et al. 2014). Here, we tested the hypothesis that the NLRP3 inflammasome is required for eATP-mediated IL-1β processing and release during P. gingivalis infection of macrophages in vitro. Complementing these studies, we demonstrate in this work that P2X7 modulates adaptive immunity in an oral model of P. gingivalis infection, and we have initiated studies to evaluate the expression of mediators of the inflammasome pathway in human chronic periodontitis.
Materials and Methods
P. gingivalis Culture
P. gingivalis strain 381 and the major fimbriae mutant (DPG3) were cultivated as previously described (Morandini et al. 2014). Description of culture conditions is provided in the Appendix.
Experimental Animals
C57BL/6 (WT) and P2X7-/- mice were obtained from the animal facility of the Instituto de Biofísica Carlos Chagas Filho, Universidade Federal do Rio de Janeiro. Nlrp3-/-, Asc-/-, and caspase 1-/- (Casp1-/-) mice were obtained from the animal facility at the University of São Paulo (Ribeirão Preto Medical School). All experiments followed guidelines of the institutional ethical committee and underwent its approval at the Universidade Federal do Rio de Janeiro.
Bone Marrow–derived Macrophages and P. gingivalis Infection
Macrophages were derived from bone marrow of WT (C57BL/6), NLRP3-/-, ASC-/-, Casp1-/-, and P2X7-/- mice as previously described (Morandini et al. 2014). Detailed information of P. gingivalis infection and subsequent experiments are provided in the Appendix. For P2X7 antagonism experiments, cells were pretreated 30 min before infection with 500µM oxidized ATP (oATP), 500nM A-740003, or 10 U/mL of apyrase.
Total RNA Extraction and Quantitative Reverse Transcription Polymerase Chain Reaction
Total RNA was extracted from bone marrow–derived macrophages (BMDMs) with PureLink RNA Mini Kit (Life Technologies, Carlsbad, CA, USA) reagent following the manufacturer’s recommendations. Quantitative reverse transcription polymerase chain reaction is described in the Appendix.
ELISA
Mouse IL-1β, tumor necrosis factor-α (TNF-α), and IL-6 in culture supernatant were measured by enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) after 6 h of P. gingivalis stimulation, followed by 30 min of incubation with or without 5mM eATP. Interferon γ (IFNγ) and IL-17 were detected following 72 h of recall stimulation in vitro in cells from submandibular lymph nodes.
Western Blot Analysis
Western blot analysis was performed as previously described (Morandini et al. 2014) and is detailed in the Appendix. Statistical analysis of densitometric data of pro-IL-1β and mature IL-1β is shown in each corresponding figure or in Appendix Figure 2 where appropriate (n = 3).
In Vivo Studies
P. gingivalis infection was performed in a group of mice as previously described (Baker et al. 2000). Detailed description is provided in the Appendix.
Patient Information
Data from a selection of patients and periodontal clinical parameters included in quantitative reverse transcription polymerase chain reaction analyses of this study were described previously (Franco et al. 2014) and are provided in the Appendix. Patient selection and samples collection were performed after written informed consent, approved, and conducted according to the ethics committee of the Catholic University of Brasília (no. 52/2010).
Statistics
Data were evaluated by 1-way analysis of variance with Tukey’s posttest using GraphPad Prism 5. Where appropriate (comparison of 2 groups only), 2-tailed t tests were also performed. Statistical differences were considered significant at P < 0.05.
Results
NLRP3 Is Necessary for eATP-induced IL-1β Secretion and Caspase 1 Activation Irrespective of the Presence of P. gingivalis Fimbriae
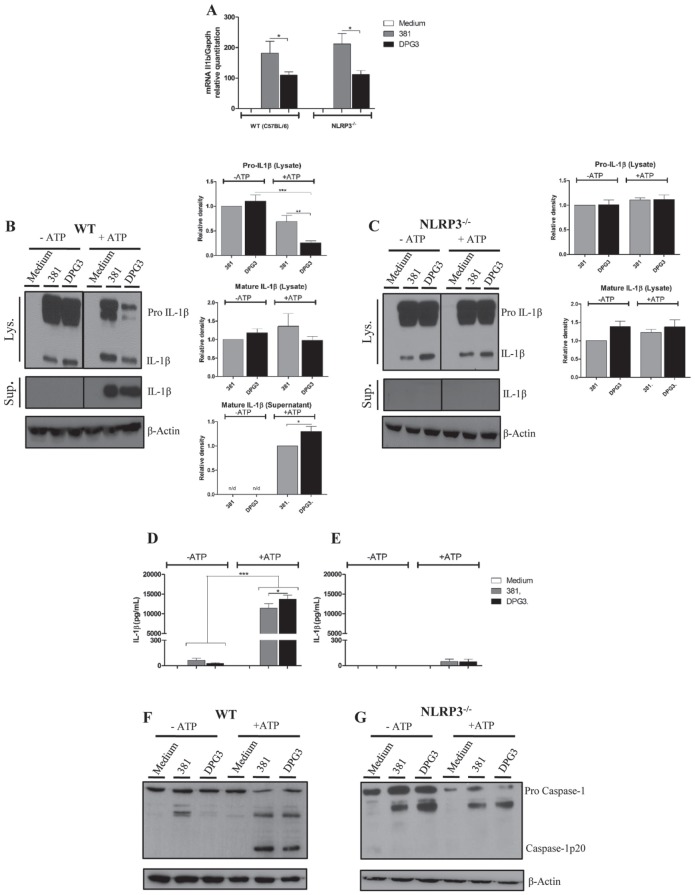
In a previous study, we demonstrated that eATP stimulates IL-1β secretion in P. gingivalis–infected BMDMs, which is dampened by its fimbriae (Morandini et al. 2014). To gain further insights into the role of NLRP3 inflammasome regarding P. gingivalis fimbriae induction of IL-1β secretion, we used BMDM derived from wild-type C57BL/6 (WT) and NLRP3 knockout (NLRP3-/-) mice for comparative analyses of IL-1β processing and release. Quantitative polymerase chain reaction analysis of IL1b levels showed that this gene was transcriptionally induced by P. gingivalis wild type (strain 381) in BMDMs from both strains of mice (Fig. 1A). However, this induction was significantly decreased in BMDMs challenged with the fimbriae-deficient P. gingivalis strain DPG3. Because IL-1β processing requires 2 signals, we next investigated the influence of eATP on IL-1β release from WT and NLRP3-/- BMDMs. Western blot analyses showed that levels of pro-IL-1β were similar between strains 381 and DPG3 in whole cells lysates for both WT-derived BMDMs and NLRP3-/--derived cells (Fig. 1B, C). In addition, mature IL-1β was produced in whole cell lysates even in the absence of eATP, but none were secreted into the culture medium. However, when eATP was added, significant production of secreted IL-1β appeared in the supernatant from WT-derived BMDMs infected with both P. gingivalis strains; with a concomitant reduction in pro-IL-1β, form was observed in the corresponding lysate (Fig. 1B, D). The reduction in the cell lysate shown by Western blot (Fig. 1B) was confirmed statistically by densitometric analysis. NLRP3 deficiency did not affect the production of pro-IL-1β in cell lysates induced by both P. gingivalis strains; however, in contrast to WT-derived BMDMs, no secretion of IL-1β was detected from NLRP3-/--derived cells in the presence of eATP (Fig. 1C, E). Curiously, even with no changes in pro-IL-1β levels, NLRP3-/-cells showed the mature form (17 kDa) in cell lysates, indicating that pro-IL-1β was processed at a low level to its mature form in an NLRP3-independent manner. Because the NLRP3 inflammasome was reported to bridge microbial signals to caspase 1 activation resulting in IL-1β release (Said-Sadier and Ojcius 2012), we next investigated the impact of NLRP3 deficiency in eATP-induced caspase 1 activation during infection with both strains of P. gingivalis. The active form of caspase 1 (20 kDa) was enhanced in cell lysates of 381- and DPG3-infected WT BMDMs after addition of eATP as a second signal (Fig. 1F), and it was undetectable in unstimulated cells, as confirmed by densitometric analysis (Appendix Fig. 2). However, in the presence of eATP, NLRP3-deficient BMDMs infected by either P. gingivalis strain could not activate caspase 1, represented by the absence of the 20-kDa cleaved form (Fig. 1G). Taken together, these results suggested that NLRP3 is essential for IL-1β secretion and caspase 1 activation by P. gingivalis–infected macrophages in response to eATP. In addition, intracellular mature IL-1β (present in cell lysates) was formed in a NLRP3-independent manner regardless of the presence of P. gingivalis fimbriae.
Figure 1.
NLRP3 is necessary to extracellular adenosine triphosphate (eATP)–induced interleukin 1β (IL-1β) secretion and caspase 1 activation irrespective of Porphyromonas gingivalis fimbriae. (A) Expression of Il1b mRNA in bone marrow–derived macrophages (BMDMs) from wild-type (WT; C57BL/6) or NLRP3-/- mice stimulated by P. gingivalis strains 381 or DPG3 (multiplicity of infection [MOI], 100) for 3 h. Total RNA from each group was extracted and reverse transcribed, and expression levels were determined by quantitative reverse transcription polymerase chain reaction. Results were normalized to the expression of Gapdh and expressed as fold change relative to noninfected cells (medium). (B, C) Western blot of pro-IL-1β and mature IL-1β detected in cell lysates (upper panels) and cell supernatants (lower panels) after 6 h of P. gingivalis infection (381 or DPG3; MOI of 100) with or without eATP as described in Materials and Methods. Protein levels were determined by densitometric quantification relative to β-actin as a loading control. IL-1β secretion was determined by ELISA in BMDMs from (D) WT or (E) NLRP3-/- mice in response to eATP stimulation. Culture supernatants were collected after 6 h of P. gingivalis infection with strains 381 or DPG3 (MOI of 100) and subsequent incubation with or without 5mM eATP for 30 min. Data are shown as mean ± SD and are representative of 3 independent experiments. *P < 0.05, ***P < 0.001. (F, G) Whole cell lysates from WT or NLRP3-/- BMDMs were analyzed for caspase 1 activation (p-20) after 6 h of 381 or DPG3infection (MOI of 100) with or without ATP addition for 30 min. Protein levels were determined by densitometric quantification relative to β-actin as a loading control and are shown in Appendix Figure 2.
Pro-IL-1β Can Be Cleaved Independently of ASC and Caspase 1 in P. gingivalis–infected Macrophages Followed by eATP Stimulation
ASC is a well-known adaptor molecule known to have a role in inflammasome complex activation, and it has been described to be required mainly for IL-1β processing during infection (Gomes et al. 2013). To directly assess the participation of ASC and caspase 1 in IL-1β processing by BMDMs infected by either P. gingivalis strains (followed by subsequent eATP stimulation), we used BMDMs derived from ASC-/- and Casp1-/- mice. There was no difference in Il1b mRNA expression in WT, ASC-/-, or Casp1-/- macrophages. All of the cells showed higher mRNA expression of this cytokine when infected by wild-type P. gingivalis 381 and were consistently lower during DPG3 infection (Fig. 2A). Similar to NLRP3-deficient cells, a considerable amount of pro-IL-1β cleavage to its mature form (17 kDa) was observed in both ASC-/-- and Casp1-/--derived cell lysates for both 381- and DPG3-infected BMDMs, as confirmed by densitometric analysis (Fig. 2B).
Figure 2.
Pro–interleukin 1β (pro-IL-1β) can be cleaved independently of apoptosis-associated speck-like protein containing CARD (ASC) and caspase 1 in Porphyromonas gingivalis–infected macrophages followed by extracellular adenosine triphosphate (eATP) stimulation. (A) Il1b mRNA expression in bone marrow–derived macrophages (BMDMs) from wild-type (WT; C57BL/6), ASC-/-, or Casp1-/- stimulated by P. gingivalis strains 381 or DPG3 at a multiplicity of infection (MOI) of 100 for 3 h. Total RNA from each group was extracted, reverse transcribed, and quantified by quantitative reverse transcription polymerase chain reaction. (B) Pro-IL-1β and mature IL-1β detected by Western blot in cell lysates (upper panels) and cell supernatants (lower panels) after 6 h of P. gingivalis infection with or without eATP as described in Materials and Methods. Protein levels were determined by densitometric quantification relative to β-actin as a loading control. (C) IL-1β secretion was analyzed by ELISA in BMDMs infected with P. gingivalis 381 or DPG3 from ASC-/- or Casp1-/- mice in response to eATP stimulation. Culture supernatants were collected after 6 h of infection with P. gingivalis strains 381 or DPG3 (MOI of 100) and subsequent incubation with or without 5mM eATP for 30 min. Data are shown as mean ± SD and are representative of 3 independent experiments. *P < 0.05, **P < 0.01. (D) Whole cell lysates from ASC-/- BMDMs were analyzed by Western blot for caspase 1 activation (p-20) after 6 h of 381 or DPG3 infection (MOI of 100) with or without eATP addition for 30 min. Protein levels were determined by densitometric quantification relative to β-actin as a loading control and are shown in Appendix Figure 2.
Moreover, in the presence of eATP as a second signal (Fig. 2B), there was intracellular IL-1β cleavage during infection with the 381 or DPG3 strain, although not statistically different in densitometric analysis (Fig. 2B). Nonetheless, no detectable levels of the mature secreted form were found by Western blot, and we barely detected the secreted form of IL-1β by ELISA (<40 pg/mL; Fig. 2C). ASC-/--derived cells did not show any caspase 1 activation with or without eATP stimulation (Fig. 2D), as expected. Combined, these data demonstrate that pro-IL-1β (33 kDa) can be cleaved independently of the ASC and caspase 1 pathway in P. gingivalis–infected BMDMs followed by eATP stimulation.
P2X7 Receptor Is Critical Not Only for eATP-induced IL-1β Secretion but Also for Intracellular Pro-IL-1β Processing in P. gingivalis–infected Macrophages
Previous studies demonstrated that the P2X7 receptor is necessary for IL-1β release during infection, followed by eATP stimulation (Hung et al. 2013; Morandini et al. 2014). Consistent with our previous work, P2X7-/--derived cells did not show any intracellular production of mature IL-1β when compared with similarly challenged WT macrophages (Fig. 3A), as confirmed by densitometric analysis of Western blots. To address the role of this purinergic receptor in IL-1β processing/cleavage, we pretreated WT BMDMs with the P2X7 antagonist, oATP (500 µM), which irreversibly blocks the P2X7 receptor. Neither strain showed considerable production of intracellular mature IL-1β in the presence of oATP (Fig. 3B), as confirmed statistically by densitometric analysis. oATP inhibited eATP-induced IL-1β secretion as expected (Western blot of supernatants in Fig. 3B; ELISA from supernatants in Fig. 3C). These results were confirmed by densitometric analysis of the Western blot results. Likewise, we confirmed these results by using a selective P2X7 receptor antagonist (A-740003), as shown in Appendix Figure 1. These lower pro-IL-1β levels can be associated with a contribution of P2X7 receptor for the first signal, with the formation of signaling platforms called lipid rafts, which can facilitate the integration between TLRs and P2X7 receptors (as revised by Vieira et al. 2010). These experiments were also performed using WT BMDMs with apyrase (Appendix Fig. 1). We showed that apyrase did not affect the amount of uncleaved or cleaved IL-1β in lysates without eATP. However, the amount of cleaved IL-1β in lysates and supernatant was clearly reduced after eATP treatment. These results demonstrate that purinergic signaling is important not only for eATP-induced IL-1β secretion but also for intracellular processing of its immature form (33 kDa) to the cleaved cytokine during P. gingivalis infection in vitro.
Figure 3.
P2X7 receptor is critical not only for extracellular adenosine triphosphate (eATP)–induced interleukin 1β (IL-1β) secretion but also for intracellular pro-IL-1β processing in Porphyromonas gingivalis–infected macrophages. (A) Pro-IL-1β and mature IL-1β detected by Western blot in cell lysates from wild-type (WT) or P2X7-/- bone marrow–derived macrophages (BMDMs) after 6 h of P. gingivalis infection with or without eATP as described in Materials and Methods. (B) Oxidized adenosine triphosphate (oATP; 500 μM) was added to WT BMDM culture for 30 min before and maintained during P. gingivalis infection for a total of 6 h. Cell lysates (upper panel) and cell supernatants (lower panel) with or without eATP as described in Materials and Methods. β-actin was used as a loading control. (C) IL-1β secretion was analyzed by ELISA in BMDMs pretreated with oATP (500 µM) and then infected with P. gingivalis 381 or DPG3 from WT mice. Culture supernatants were collected after 6 h of infection with P. gingivalis (multiplicity of infection, 100) and subsequent incubation with or without 5mM eATP for 30 min. Data are shown as mean ± SD and are representative of 3 independent experiments. *P < 0.05, ***P < 0.001. Protein levels were determined by densitometric quantification relative to β-actin as a loading control.
NLRP3, ASC, and Caspase 1 Are Dispensable for TNF-α and IL-6, Which Are Secreted Independently of Inflammasomes
We next investigated whether the deficiency of NLRP3 inflammasome components could interfere with secretion of cytokines such as TNF-α and IL-6, which are secreted through the Golgi complex. Both TNF-α and IL-6 were significantly upregulated in BMDMs challenged with wild-type P. gingivalis 381 as compared with cells challenged with the fimbria-deficient P. gingivalis DPG3 strain. However, no differences were observed in similarly challenged BMDMs from NLRP3-/-, ASC-/-, or Casp1-/- as compared with WT (C57BL/6) mice (Fig. 4). This result further shows that the NLPR3 inflammasome is involved only in IL-1β secretion, confirming the specificity of the NLRP3 deletion.
Figure 4.
NLRP3, apoptosis-associated speck-like protein containing CARD (ASC), and caspase 1 are dispensable for tumor necrosis factor α (TNF-α) and interleukin 6 (IL-6), which are secreted in a canonical pathway. (A, B) Tnfa and Il6 mRNA expression in bone marrow–derived macrophages (BMDMs) from wild-type (WT; C57BL/6), NLRP3-/-, ASC-/-, or Casp1-/- stimulated by Porphyromonas gingivalis strains 381 or DPG3 at a multiplicity of infection of 100 for 3 h. Total RNA from each group was extracted, reverse transcribed, and quantified by quantitative reverse transcription polymerase chain reaction. (C, D) TNFα and IL-6 secretion was analyzed by ELISA in supernatants from BMDMs from WT (C57BL/6), NLRP3-/-, ASC-/-, or Casp1-/-. Culture supernatants were collected after 6 h of infection with P. gingivalis strains 381 or DPG3 (multiplicity of infection of 100). Data are shown as mean ± SD and are representative of 3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
The P2X7 Receptor Has an Effect on P. gingivalis Infection In Vivo and Is Modulated in Human Chronic Periodontitis
In view of the results that showed the importance of the P2X7 receptor not only for IL-1β secretion but also for pro-IL-1β cleavage after eATP challenge in P. gingivalis–infected BMDMs, we investigated P2X7 receptor expression in an in vivo oral infection model. After 7 d of P. gingivalis infection, P2rx7 transcription increased 1.5-fold (Fig. 5A), with a corresponding increase in P2X7 receptor protein in the hemimaxilla tissue as compared with sham-infected mice (Fig. 5B), which increased 1.8-fold (Appendix Fig. 2). Therefore, explanted submandibular lymph node cells, which drain the oral region, were restimulated with P. gingivalis fimbriae in vitro (Fig. 5C, D). A significant amount of IL-17 and IFN-γ secretion was seen from infected WT cells and minimal production from P2X7-/- cells (Fig. 5C, D). Regarding IL-1β, no detectable levels were found in hemimaxilla tissue after 7 d of P. gingivalis infection (data not shown). When we investigated IL-1β mRNA in human gingival tissues from periodontitis patients before and after conventional periodontal treatment, no differences were detected between the groups (Fig. 5E). However, P2X7R (Fig. 5F) as well as NLRP3 (Fig. 5G) mRNA transcription in periodontal patients decreased after treatment as expected, since these patients were in a significantly better periodontal clinical condition, as previously described (Franco et al. 2014). These findings are consistent with a potential role for P2X7 receptor in the development of both murine and human periodontal disease.
Figure 5.
P2X7 receptor expression is relevant for Porphyromonas gingivalis infection in vivo as well as in human chronic periodontitis. Wild-type (WT) and P2X7-/- mice were orally infected with P. gingivalis or vehicle alone (sham infected). Hemimaxillae as well as submandibular lymph node cells were collected 7 d after the last inoculation. (A) P2rx7 mRNA expression and (B) P2X7 protein expression in hemimaxilla tissue from sham- and P. gingivalis–infected WT mice. mRNA was measured by quantitative polymerase chain reaction, and protein levels were visualized by Western blot. Protein levels were determined by densitometric quantification relative to β-actin as a loading control and are shown in Appendix Figure 2. (C) Interleukin 17 (IL-17) and (D) interferon γ (IFNγ) secretion detected by ELISA from explanted lymph node cells after 72 h of restimulation with 10 µg/mL of fimbriae in vitro. Data are shown as mean ± SD and are representative of 3 independent experiments. (E) IL1B, (F) NLRP3, and (G) P2RX7 mRNA expression in gingival tissue from 15 chronic periodontitis patients before and after 30 d of treatment were measured by quantitative polymerase chain reaction. *P < 0.05, **P < 0.01, ***P < 0.001. (H) Schematic representation of possible mechanisms of extracellular adenosine triphosphate (eATP)–P2X7 receptor axis in canonical and noncanonical inflammasome activation and IL-1β release during P. gingivalis infection. oATP, oxidized adenosine triphosphate.
Discussion
P2X7 receptor is an ionotropic eATP-gated channel that is expressed on a variety of immune cells, including macrophages (Coutinho-Silva and Persechini 1997; Coutinho-Silva et al. 2001). When primed cells encounter a DAMP such as eATP, activation of this purinergic receptor functions as a second signal for assembly of the NLRP3 inflammasome, which in turn initiates and amplifies the innate immune and IL-1β-dependent proinflammatory responses (Mariathasan et al. 2006). We and others previously demonstrated that purinergic signaling is an essential player in the release of IL-1β from P. gingivalis–infected cells (Morandini et al. 2014), and in this study, we further confirm the essential participation of eATP as a danger signal. Data from patients (Orozco et al. 2006; Zhong et al. 2007) and animal models (Delima et al. 2001; Zhang et al. 2004) indicate that excessive production of IL-1β plays a key role in the pathogenesis of periodontitis. Here, we provide evidence that the P2X7 receptor has a crucial role in IL-1β intracellular processing and release from P. gingivalis–infected macrophages in the presence of eATP as a second signal. Furthermore, the present study demonstrated that intracellular processing of this cytokine (unlike its secretion) was NLRP3, ASC, and caspase 1 independent.
Not surprisingly, we found that NLRP3-deficiency significantly impaired the robust IL-1β secretion from P. gingivalis–infected cells induced by eATP. This occurred independently of the presence of fimbriae. Nonetheless, NLRP3-, ASC-, and caspase 1-deficient macrophages were able to cleave intracellular pro-IL-1β. Growing evidence has shown that NLRP3 inflammasomes can be activated not only in a canonical pathway involving caspase 1 but also through a noncanonical pathway involving caspase 11 (Case et al. 2013; Casson et al. 2013) and caspase 8 (Shenderov et al. 2014), as illustrated in Figure 5H. In fact, it was recently reported that NLRP3/ASC/Casp1-independent cleavage of IL-1β occurred by means of TLR4 activation via caspase 8 (Shenderov et al. 2014), which is able to cleave pro-IL-1β at the same site as caspase 1 in response to TLR activation (Maelfait et al. 2008). Caspase 8 can also be activated downstream from the P2X7 receptor, since eATP/P2X7 receptor ligation was reported to connect functionally with other receptors involved in cell death, such as FasL, and pannexin 1 hemichannels in a caspase 8–dependent pathway (Aguirre et al. 2013). Although our results suggest that P. gingivalis is able to cleave pro-IL-1β in cell lysates from ASC-/- BMDM, its well known that this periodontopathogen produces abundant proteases called gingipains (de Diego et al. 2014), which could also be responsible for enzymatic cleavage of pro-IL-1β observed here.
Our findings revealed the importance of the P2X7 receptor in the context of P. gingivalis infection in vivo, since mRNA expression and protein production of this receptor were upregulated in hemimaxilla tissue derived from P. gingivalis orally infected mice. Furthermore, although we could not detect IL-1β production 7 d postchallenge with P. gingivalis (probably due to low levels of the cytokine), we observed that P2X7 deficiency has a large consequence for the adaptive immune response. Thus, we detected a significant reduction in IL-17 and IFN-γ secretion by draining lymph node cells restimulated in vitro. IL-17- and IFN-γ-coproducing Th cells were previously shown to be associated with multiple inflammatory diseases, and they display unique functional and gene expression profiles mediated by IL-1β (Duhen and Campbell 2014).
Finally, when we investigated IL1B, NLRP3, and P2RX7 mRNA expression in human chronic periodontitis, we found higher IL1B levels in periodontitis cases, irrespective of treatment. Of further interest, the expression levels of both NLRP3 and P2RX7 were higher in patients with periodontitis and significantly downregulated after conventional periodontal treatment. In agreement with our data, higher NLRP3 mRNA was found in subjects with periodontitis and gingivitis, compared with healthy tissues (Bostanci et al. 2009). In contrast, NLRP3 expression was downregulated by subgingival biofilm in primary gingival fibroblasts, partly due to P. gingivalis infection (Belibasakis et al. 2013).
In summary, our results highlight the contribution of the P2X7 receptor and NLRP3 inflammasome not only for IL-1β secretion but also for its cleavage in P. gingivalis–infected cells. Figure 5H illustrates a schematic representation encompassing possible mechanisms of the eATP-P2X7 receptor axis in canonical and noncanonical inflammasome activation and IL-1β release in the context of P. gingivalis infection. Moreover, we provide evidence for an involvement of the P2X7 receptor in vivo and show its modulation in human periodontitis. These studies suggest that the P2X7 purinergic receptor should be considered as a potential target for future therapeutic strategies in treatment of periodontitis.
Author Contributions
E.S. Ramos-Junior, A.C. Morandini, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; C.L.C. Almeida-da-Silva, contributed to data acquisition and analysis, critically revised the manuscript; E.J. Franco, D.M. Ojcius, contributed to data analysis and interpretation, critically revised the manuscript; J. Potempa, K.A. Nguyen, contributed to conception and data interpretation, critically revised the manuscript; A.C. Oliveira, D.S. Zamboni, contributed to design and data interpretation, critically revised the manuscript; J. Scharfstein, R. Coutinho-Silva, contributed to conception, design, and data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by funds from the Conselho Nacional de Desenvolvimento Cientifico e Tecnológico do Brasil, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, the Programa de Núcleos de Excelência, Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro, and the Instituto Nacional de Ciência e Tecnologia para Pesquisa Translacional em Saúde e Ambiente na Região Amazônica. E.S.R.-J. was supported by a Conselho Nacional de Desenvolvimento Cientifico e Tecnológico do Brasil PhD scholarship. This work was also partially supported by the National Institutes of Health (grant R01DE019444 to D.M.O. and NIDCR/R21DE023207 to J.P.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aguirre A, Shoji KF, Saez JC, Henriquez M, Quest AF. 2013. FasL-triggered death of Jurkat cells requires caspase 8–induced, ATP-dependent cross-talk between Fas and the purinergic receptor P2X(7). J Cell Physiol. 228(2):485–493. [DOI] [PubMed] [Google Scholar]
- Baker PJ, Dixon M, Evans RT, Roopenian DC. 2000. Heterogeneity of Porphyromonas gingivalis strains in the induction of alveolar bone loss in mice. Oral Microbiol Immunol. 15(1):27–32. [DOI] [PubMed] [Google Scholar]
- Belibasakis GN, Guggenheim B, Bostanci N. 2013. Down-regulation of NLRP3 inflammasome in gingival fibroblasts by subgingival biofilms: involvement of Porphyromonas gingivalis. Innate Immun. 19(1):3–9. [DOI] [PubMed] [Google Scholar]
- Bostanci N, Emingil G, Saygan B, Turkoglu O, Atilla G, Curtis MA, Belibasakis GN. 2009. Expression and regulation of the NALP3 inflammasome complex in periodontal diseases. Clin Exp Immunol. 157(3):415–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case CL, Kohler LJ, Lima JB, Strowig T, de Zoete MR, Flavell RA, Zamboni DS, Roy CR. 2013. Caspase-11 stimulates rapid flagellin-independent pyroptosis in response to Legionella pneumophila. Proc Natl Acad Sci U S A. 110(5):1851–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casson CN, Copenhaver AM, Zwack EE, Nguyen HT, Strowig T, Javdan B, Bradley WP, Fung TC, Flavell RA, Brodsky IE, et al. 2013. Caspase-11 activation in response to bacterial secretion systems that access the host cytosol. PLoS Pathog. 9(6):e1003400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Silva R, Perfettini JL, Persechini PM, Dautry-Varsat A, Ojcius DM. 2001. Modulation of P2Z/P2X(7) receptor activity in macrophages infected with Chlamydia psittaci. Am J Physiol Cell Physiol. 280(1):C81–C89. [DOI] [PubMed] [Google Scholar]
- Coutinho-Silva R, Persechini PM. 1997. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. Am J Physiol. 273(6 Pt 1):C1793–C1800. [DOI] [PubMed] [Google Scholar]
- de Diego I, Veillard F, Sztukowska MN, Guevara T, Potempa B, Pomowski A, Huntington JA, Potempa J, Gomis-Ruth FX. 2014. Structure and mechanism of cysteine peptidase gingipain K (Kgp), a major virulence factor of Porphyromonas gingivalis in periodontitis. J Biol Chem. 289(46):32291–32302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delima AJ, Oates T, Assuma R, Schwartz Z, Cochran D, Amar S, Graves DT. 2001. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J Clin Periodontol. 28(3):233–240. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. 2013. The therapeutic potential of modifying inflammasomes and NOD-like receptors. Pharmacol Rev. 65(3):872–905. [DOI] [PubMed] [Google Scholar]
- Duhen T, Campbell DJ. 2014. IL-1beta promotes the differentiation of polyfunctional human CCR6+CXCR3+ Th1/17 cells that are specific for pathogenic and commensal microbes. J Immunol. 193(1):120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco EJ, Pogue RE, Sakamoto LH, Cavalcante LL, Carvalho DR, de Andrade RV. 2014. Increased expression of genes after periodontal treatment with photodynamic therapy. Photodiagnosis Photodyn Ther. 11(1):41–47. [DOI] [PubMed] [Google Scholar]
- Gombault A, Baron L, Couillin I. 2012. ATP release and purinergic signaling in NLRP3 inflammasome activation. Front Immunol. 3:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes MT, Campos PC, Oliveira FS, Corsetti PP, Bortoluci KR, Cunha LD, Zamboni DS, Oliveira SC. 2013. Critical role of ASC inflammasomes and bacterial type IV secretion system in caspase-1 activation and host innate resistance to Brucella abortus infection. J Immunol. 190(7):3629–3638. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G. 2014. The inflammophilic character of the periodontitis-associated microbiota. Mol Oral Microbiol. 29(6):248–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Lamont RJ. 2012. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol. 27(6):409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huck O, Elkaim R, Davideau JL, Tenenbaum H. 2015. Porphyromonas gingivalis–impaired innate immune response via NLRP3 proteolysis in endothelial cells. Innate Immun. 21(1):65–72. [DOI] [PubMed] [Google Scholar]
- Hung SC, Choi CH, Said-Sadier N, Johnson L, Atanasova KR, Sellami H, Yilmaz O, Ojcius DM. 2013. P2X4 assembles with P2X7 and pannexin-1 in gingival epithelial cells and modulates ATP-induced reactive oxygen species production and inflammasome activation. PLoS One. 8(7):e70210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassebaum NJ, Bernabé E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. 2014. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 93(11):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. 2013. Activation and regulation of the inflammasomes. Nat Rev Immunol. 13(6):397–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JC, Mitchell CH. 2012. Inflammation, pain, and pressure: purinergic signaling in oral tissues. J Dent Res. 91(12):1103–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Castejon G, Theaker J, Pelegrin P, Clifton AD, Braddock M, Surprenant A. 2010. P2X(7) receptor-mediated release of cathepsins from macrophages is a cytokine-independent mechanism potentially involved in joint diseases. J Immunol. 185(4):2611–2619. [DOI] [PubMed] [Google Scholar]
- Maekawa T, Krauss JL, Abe T, Jotwani R, Triantafilou M, Triantafilou K, Hashim A, Hoch S, Curtis MA, Nussbaum G, et al. 2014. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 15(6):768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R. 2008. Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med. 205(9):1967–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maresz KJ, Hellvard A, Sroka A, Adamowicz K, Bielecka E, Koziel J, Gawron K, Mizgalska D, Marcinska KA, Benedyk M, et al. 2013. Porphyromonas gingivalis facilitates the development and progression of destructive arthritis through its unique bacterial peptidylarginine deiminase (PAD). PLoS Pathog. 9(9):e1003627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O’Rourke K, Roose-Girma M, Lee WP, Weinrauch Y, Monack DM, Dixit VM. 2006. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 440(7081):228–232. [DOI] [PubMed] [Google Scholar]
- Morandini AC, Ramos-Junior ES, Potempa J, Nguyen KA, Oliveira AC, Bellio M, Ojcius DM, Scharfstein J, Coutinho-Silva R. 2014. Porphyromonas gingivalis fimbriae dampen P2X7–dependent interleukin-1beta secretion. J Innate Immun. 6(6):831–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco A, Gemmell E, Bickel M, Seymour GJ. 2006. Interleukin-1beta, interleukin-12 and interleukin-18 levels in gingival fluid and serum of patients with gingivitis and periodontitis. Oral Microbiol Immunol. 21(4):256–260. [DOI] [PubMed] [Google Scholar]
- Pelegrin P, Barroso-Gutierrez C, Surprenant A. 2008. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J Immunol. 180(11):7147–7157. [DOI] [PubMed] [Google Scholar]
- Said-Sadier N, Ojcius DM. 2012. Alarmins, inflammasomes and immunity. Biomed J. 35(6):437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenderov K, Riteau N, Yip R, Mayer-Barber KD, Oland S, Hieny S, Fitzgerald P, Oberst A, Dillon CP, Green DR, et al. 2014. Cutting edge: endoplasmic reticulum stress licenses macrophages to produce mature IL-1beta in response to TLR4 stimulation through a caspase-8- and TRIF-dependent pathway. J Immunol. 192(5):2029–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum C, Coats SR, Hua N, Kramer C, Papadopoulos G, Weinberg EO, Gudino CV, Hamilton JA, Darveau RP, Genco CA. 2014. Distinct lipid a moieties contribute to pathogen-induced site-specific vascular inflammation. PLoS Pathog. 10(7):e1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taxman DJ, Swanson KV, Broglie PM, Wen H, Holley-Guthrie E, Huang MT, Callaway JB, Eitas TK, Duncan JA, Ting JP. 2012. Porphyromonas gingivalis mediates inflammasome repression in polymicrobial cultures through a novel mechanism involving reduced endocytosis. J Biol Chem. 287(39):32791–32799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira FS, Corrêa G, Einicker-Lamas M, Coutinho-Silva R. 2010. Host-cell lipid rafts: a safe door for micro-organisms? Biol Cell. 102(7): 391–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O, Yao L, Maeda K, Rose TM, Lewis EL, Duman M, Lamont RJ, Ojcius DM. 2008. ATP scavenging by the intracellular pathogen Porphyromonas gingivalis inhibits P2X7-mediated host-cell apoptosis. Cell Microbiol. 10(4):863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kohli M, Zhou Q, Graves DT, Amar S. 2004. Short- and long-term effects of IL-1 and TNF antagonists on periodontal wound healing. J Immunol. 173(5):3514–3523. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Slade GD, Beck JD, Offenbacher S. 2007. Gingival crevicular fluid interleukin-1beta, prostaglandin E2 and periodontal status in a community population. J Clin Periodontol. 34(4):285–293. [DOI] [PubMed] [Google Scholar]
- Zhu M, Belkina AC, DeFuria J, Carr JD, Van Dyke TE, Gyurko R, Nikolajczyk BS. 2014. B cells promote obesity-associated periodontitis and oral pathogen-associated inflammation. J Leukoc Biol. 96(2):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.