Abstract
When measured once, psychological stress predicts development of painful temporomandibular disorder (TMD). However, a single measurement fails to characterize the dynamic nature of stress over time. Moreover, effects of stress on pain likely vary according to biological susceptibility. We hypothesized that temporal escalation in stress exacerbates risk for TMD, and the effect is amplified by allelic variants in a gene, catechol-O-methyltransferase (COMT), regulating catechol neurotransmitter catabolism. We used data from the Orofacial Pain: Prospective Evaluation and Risk Assessment prospective cohort study of 2,707 community-dwelling adults with no lifetime history of TMD on enrollment. At baseline and quarterly periods thereafter, the Perceived Stress Scale (PSS) measured psychological stress. Genotyped DNA from blood samples determined COMT diplotypes. During follow-up of 0.25 to 5.2 y, 248 adults developed examiner-verified incident TMD. PSS scores at baseline were 20% greater (P < 0.001) in adults who developed incident TMD compared with TMD-free controls. Baseline PSS scores increased by 9% (P = 0.003) during follow-up in cases but remained stable in controls. This stress escalation was limited to incident cases with COMT diplotypes coding for low-activity COMT, signifying impaired catabolism of catecholamines. Cox regression models confirmed significant effects on TMD hazard of both baseline PSS (P < 0.001), modeled as a time-constant covariate, and change in PSS (P < 0.001), modeled as a time-varying covariate. Furthermore, a significant (P = 0.04) interaction of COMT diplotype and time-varying stress showed that a postbaseline increase of 1.0 standard deviation in PSS more than doubled risk of TMD incidence in subjects with low-activity COMT diplotypes (hazard ratio = 2.35; 95% confidence limits: 1.66, 3.32), an effect not found in subjects with high-activity COMT diplotypes (hazard ratio = 1.42; 95% confidence limits: 0.96, 2.09). Findings provide novel insights into dynamic effects of psychological stress on TMD pain, highlighting that effects are most pronounced in individuals whose genetic susceptibility increases responsiveness to catecholamine neurotransmitters.
Keywords: temporomandibular joint dysfunction syndrome, human COMT protein, psychological stress, gene-environment interaction, cohort studies, proportional hazards models
Introduction
In 1969, Laskin theorized that myofascial pain-dysfunction syndrome was a stress-induced psychophysiological disorder (Laskin 1969). Cross-sectional research over ensuing decades provided empirical support for this theoretical model, demonstrating a strong association between psychological factors and painful temporomandibular disorders (TMDs). More recent prospective studies confirmed the temporal sequence of this relationship, showing that increases in several dimensions of psychological distress predicted greater risk of TMD development (Slade et al. 2007; Aggarwal et al. 2010; Kindler et al. 2012; Fillingim et al. 2013; Sipila et al. 2013).
However, psychological stress (hereafter “stress”) is temporally dynamic. When measured repeatedly at intervals ranging from 2 to 20 wk, stress scores fluctuate considerably (Birmingham et al. 2006; Thornton et al. 2007; Lix et al. 2008; Schliep et al. 2015) in response to new stressors or changing appraisals of stress. Effects of these temporal dynamics on TMD have yet to be investigated and can be classified into 3 conceptually meaningful patterns. Persistently elevated stress, signifying a lack of adaptation, may increase susceptibility to TMD onset. Alternatively, a sudden “spike” in stress, representing newly heightened vulnerability, might trigger TMD onset. Third, a continuous gradient of increasing stress may have additive effects that induce TMD symptoms at a critical person-specific threshold.
These temporal dynamics may be modified by genetic susceptibility, representing gene-environment interactions on TMD risk. Because the adrenergic system plays a prominent role in the stress response, genes regulating this system are candidates for such interactions. For example, polymorphisms in the gene encoding catechol-O-methyltransferase (COMT), an enzyme that metabolizes catecholamine neurotransmitters, alter activity of the COMT enzyme (Diatchenko et al. 2005; Nackley et al. 2007). Variants of the COMT gene that reduce the enzyme’s activity are associated with greater experimental pain sensitivity (Zubieta et al. 2003; Diatchenko et al. 2006), heightened clinical pain ratings (George et al. 2008), and increased incidence of TMD (Diatchenko et al. 2005). COMT genetic variation also mediates responses to stress (Hernaus et al. 2013).
This study evaluates effects of dynamic patterns of stress on incident TMD. In a community-based sample of adults with no history of TMD at enrollment, we first describe temporal patterns of change in stress. We then quantify the association between these temporal patterns of stress and TMD incidence. Finally, we evaluate whether COMT gene variation modifies the association between stress and TMD incidence.
Methods
This article complies with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (von Elm et al. 2008) in reporting findings from the project, Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA). Participants provided written consent, and relevant institutional review boards approved the study. Methods are described in detail elsewhere (Bair et al. 2013; Slade, Sanders, et al. 2013; Slade et al. 2014) and summarized here.
Study Design and Setting
Data are from 2 study designs used in OPPERA: 1) a prospective cohort study of TMD incidence and 2) a nested case-control study of TMD incidence in the same sample.
Study Participant Selection Criteria
The cohort of 3,263 community-based volunteers was enrolled between May 2006 and November 2008 at 4 US study sites: Baltimore, Maryland; Buffalo, New York; Chapel Hill, North Carolina; and Gainesville, Florida. Screening eligibility criteria were as follows: aged 18 to 44 y, no significant history of TMD symptoms, no significant medical illnesses or recent history of facial injury or surgery, not pregnant or nursing, ≤4 headaches per month within the preceding 3 mo, not receiving orthodontic treatment, never diagnosed with TMD, and no use of a night-guard occlusal splint.
Baseline Data Collection
The 10-item Perceived Stress Scale (PSS) (Cohen et al. 1983) evaluated perceptions of distress and coping to daily stress with responses recorded using a 5-point ordinal scale. Trained examiners applied the Research Diagnostic Criteria for TMD (RDC/TMD) (Dworkin and LeResche 1992) to classify clinical TMD and exclude anyone with the condition. The 2 criteria required for TMD classification were 1) history of pain in masticatory tissues on ≥5 d of the preceding 30 d and 2) pain in masticatory tissues evoked by standardized jaw movements or examiner-palpation of the masticatory muscles and temporomandibular joints.
Genotyping
DNA extracted from a blood sample was genotyped using an Affymetrix (Santa Clara, CA, USA) MegAllele platform (Smith et al. 2011). The platform assessed single-nucleotide polymorphisms (SNPs) from 358 genes that influence biological systems relevant to pain perception. This analysis examined 2 SNPs from the COMT gene: rs6269 and rs4633.
Follow-up Data Collection
At quarterly (i.e., 3-monthly) postenrollment intervals, participants completed PSS questionnaires and TMD screening questions. Participants with TMD symptoms returned for a follow-up examination in which study examiners determined TMD case classification using the same RDC/TMD criteria. Follow-up data collection continued for each participant until clinical TMD was classified or until censoring (i.e., the study closeout date of May 31, 2011, or, for people lost to follow-up, the date of the final follow-up questionnaire).
Nested Case-Control Study of TMD Incidence
As each incident TMD case was identified, 1 TMD-free control was sampled at random from the cohort and examined. Controls were matched to cases according to study site, sex, and time in study (Slade et al. 2014).
Variables Used in This Analysis
The binary outcome variable was the clinical classification of incident TMD. For time-to-event analysis, time in study was the number of days from enrollment to censoring or the visit when incident TMD was classified. The main predictor variable was the PSS summary score (Cohen et al. 1983) measured at baseline and in quarterly follow-up questionnaires. It had a potential range of 0 to 40, with higher values signifying greater stress.
COMT SNPs were used to create 3 previously reported haplotypes, labeled low-, average-, and high-pain sensitivity (LPS, APS, and HPS, respectively), that influence catabolic efficacy of the COMT enzyme (Diatchenko et al. 2005). Two groups were created: 1) participants with LPS-LPS and LPS-APS diplotypes were labeled “high-activity COMT” consistent with reduced pain sensitivity, and 2) remaining diplotypes (HPS-APS, HPS-HPS, APS-APS, or HPS-LPS) were labeled “low-activity COMT,” consistent with increased pain sensitivity. This classification assumed that the LPS haplotype is recessive to HPS, although not to APS, in determining COMT activity. Two previously reported (Diatchenko et al. 2013) classifications for COMT diplotype were used for sensitivity analysis.
Age in years, sex, race/ethnicity (white, African American, Hispanic, Asian, or other), and a binary indicator (yes, no) of lifetime US residence were included in multivariable models because of their association with TMD incidence in this cohort (Bair et al. 2013). In Cox models that used COMT diplotypes, adjustment was made for population stratification using the first 3 dimensions of variance (eigenvectors) from multidimensional scaling analysis of all genotyped SNPs (Smith et al. 2011). Study site was a covariate because of the multisite study design.
Statistical Analysis
Data from participants in the nested case-control study were plotted to show the distribution of PSS change scores. Temporal trends in mean PSS scores were plotted at: 1) the baseline visit; 2) intermediate quarters, defined as follow-up questionnaires completed before the penultimate quarter; 3) the penultimate quarter, defined as the follow-up questionnaire completed 3 mo before the final quarter; and 4) the final quarter, terminated by the follow-up examination. Adjusted means were calculated according to case classification and COMT diplotype using a generalized estimating equation regression model in which the PSS score was the dependent variable. Predictor variables were time (4 categories), COMT diplotype (2 categories), and incident case classification (2 categories) along with all 2-way and 3-way interactions of those predictor variables. Covariates were study site and the demographic characteristics described above.
For aim 2, data from the prospective cohort study were analyzed using Cox proportional hazards models, which are appropriate when participants have censored observations and different follow-up periods. In Cox models, repeated measurements were analyzed as time-varying covariates, meaning that each participant’s data contribute multiple times to the model. Separate contributions came from each quarterly follow-up when 3 variables were calculated: a) the participant’s duration of follow-up at the time the questionnaire was completed, b) case classification at that time, and c) the time-varying, PSS change score. At each follow-up, 4 methods used widely in Cox models evaluated conceptually distinct effects of time on the outcome (Allison and SAS Institute 1995):
The “concurrent” method used the PSS score from the questionnaire completed at the quarterly follow-up, and the baseline score was subtracted to create a PSS change score at each follow-up. For the last quarter among incident cases, the 3-mo reference period of the PSS overlapped the 30-d reference period used to determine clinical TMD at that follow-up. This creates potential for reverse causation (i.e., clinical TMD causing stress).
The “lagged” method addresses that problem by using the questionnaire that preceded the concurrent quarter to compute the change score at each follow-up. Because all participants, including incident cases, were TMD free in the lagged quarter, this variable precludes the possibility of reverse causation, permitting evaluation of the concept that a “single spike” in stress contributes to risk of TMD.
The “average” PSS change score was the mean of PSS change scores between the first quarterly questionnaire through the lagged quarter (inclusive). This is an indicator of the overall change in postenrollment stress, not merely a single spike in stress at the lagged quarter.
The “gradient” of PSS change was calculated for each participant from the slope of the linear regression line created by regressing PSS from each follow-up through the lagged questionnaire against time. This evaluates both direction and rate of change in stress since enrollment, not merely the overall change in stress.
Separate Cox models were created for each method, and each model included time-constant covariates of baseline PSS score, demographics, and study site. Overall model fit was judged using the likelihood ratio test, while effects of individual predictors were quantified as hazard ratios and their 95% confidence limits (95% CLs). In these models, the time-varying PSS change scores were transformed to z scores so that hazard ratios were interpreted consistently as the relative effect on TMD incidence rate associated with a 1–standard deviation (SD) increase in the PSS score. Because lagged quarters could be computed only for participants who completed at least 2 follow-up PSS questionnaires, all models were limited to participants who completed ≥2 follow-up questionnaires to maintain a consistent sample size.
For aim 3, the optimal Cox model from aim 2 was extended to evaluate interactions of the COMT diplotype and PSS score. When statistically significant interactions were found, hazard ratios and 95% CLs were estimated separately for each stratum of COMT diplotype. By necessity, these models were further restricted to genotyped participants.
Sample Size Justification
In the OPPERA prospective cohort study, the target sample size of 3,200 enrollees was expected to yield 196 first-onset TMD cases during a 3-y follow-up period. Calculations made when designing the study indicated that those numbers would provide 80% statistical power to detect risk ratios of at least 1.8 for risk predictors with as few as 15% of people in the high-risk category (Bair et al. 2013).
Results
Of 3,263 enrollees in the prospective cohort study, 2,707 participants provided follow-up data, completing a median of 10 quarterly follow-up questionnaires during follow-up periods ranging from 0.25 to 5.2 y. The incidence rate of TMD was 3.5% per annum (Table 1). Although the incidence rate varied among demographic groups, it did not differ appreciably according to COMT diplotype. Based on nonoverlap of 95% CLs, mean PSS scores at baseline differed significantly according to sex and race/ethnicity but not other characteristics.
Table 1.
Descriptive Findings from the OPPERA Prospective Cohort Study of TMD Incidence.
| No. (%) of Participants | Median No. of QHUs per Person | Baseline PSS Score: Mean (95% CL) | TMD Incidence Rate: % of People per Annum (95% CL) | |
|---|---|---|---|---|
| All subjects | 2,707 (100.0) | 10 | 14.5 (14.2, 14.7) | 3.5 (3.1, 4.0) |
| Age, y | ||||
| 18–24 | 1,409 (52.1) | 10 | 14.4 (14.1, 14.7) | 2.5 (2.0, 3.1) |
| 25–34 | 733 (27.1) | 11 | 14.3 (13.9, 14.8) | 3.7 (3.0, 4.7) |
| 35–44 | 565 (20.9) | 9 | 14.7 (14.1, 15.2) | 4.5 (3.5, 5.7) |
| Sex | ||||
| Female | 1,617 (59.7) | 10 | 14.8 (14.5, 15.1) | 3.6 (3.0, 4.2) |
| Male | 1,090 (40.3) | 9 | 13.9 (13.5, 14.3) | 2.8 (2.3, 3.5) |
| Race/ethnicity | ||||
| White | 1,439 (53.2) | 11 | 13.7 (13.3, 14.0) | 3.0 (2.5, 3.6) |
| African American | 747 (27.6) | 7 | 15.7 (15.2, 16.2) | 4.7 (3.7, 5.9) |
| Hispanic | 177 (6.5) | 10 | 14.1 (13.3, 14.9) | 2.9 (1.8, 4.6) |
| Asian | 256 (9.5) | 10 | 15.9 (15.1, 16.7) | 1.2 (0.6, 2.3) |
| Other | 88 (3.3) | 11 | 13.0 (11.6, 14.3) | 2.6 (1.2, 5.5) |
| Lifetime US resident | ||||
| Yes | 2,212 (81.7) | 10 | 14.4 (14.1, 14.6) | 3.7 (3.2, 4.2) |
| No | 495 (18.3) | 11 | 14.8 (14.2, 15.3) | 1.4 (0.9, 2.1) |
| COMT dipotype | ||||
| Low activity | 1,208 (44.6) | 10 | 14.9 (14.2, 15.6) | 3.3 (2.8, 4.0) |
| High activity | 1,174 (43.4) | 10 | 14.5 (14.2, 14.9) | 3.1 (2.5, 3.8) |
| Not genotyped | 325 (12.0) | 9 | 14.2 (13.9, 14.6) | 3.6 (2.6, 5.1) |
Low-activity COMT diplotypes are HPS-APS, HPS-HPS, APS-APS, or HPS-LPS; high-activity COMT diplotypes are LPS-LPS or LPS-APS. APS, average-pain sensitivity; CL, confidence limit; COMT, catechol-O-methyltransferase; HPS, high-pain sensitivity; LPS, low-pain sensitivity; OPPERA, Orofacial Pain: Prospective Evaluation and Risk Assessment; PSS, Perceived Stress Scale; QHU, Quarterly Health Update questionnaire; TMD, temporomandibular disorder.
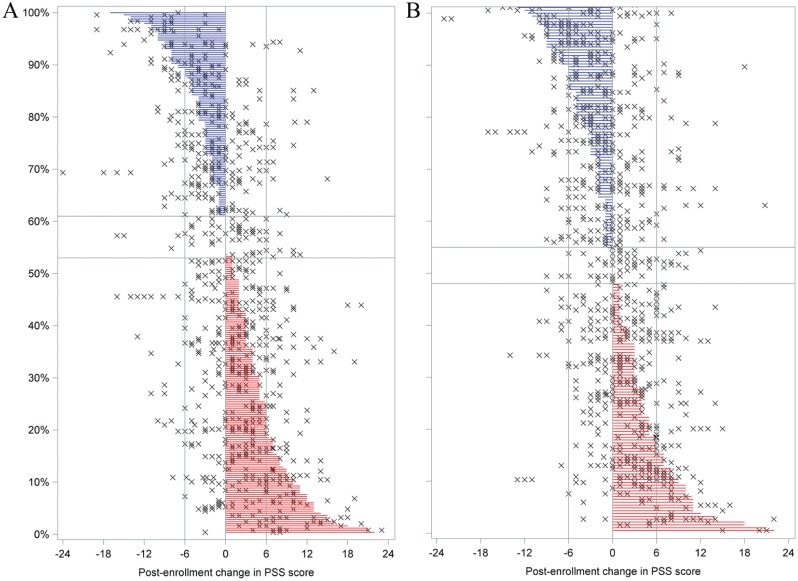
Fifty-three percent of incident cases experienced a net increase in mean PSS between baseline and final follow-up questionnaires (i.e., percentage of red lines in Fig. 1A). For 18%, the increase was at least 6 units (i.e., percentage of red lines extending at least to the right reference line in Fig. 1A). Conversely, 39% of incident cases experienced a net decrease, and for 12%, it was ≥6 units (see blue lines in Fig. 1A). For the 184 matched controls, the corresponding values were 48%, 18%, 45%, and 15% (Fig. 1B). PSS scores also fluctuated considerably at other time points, as indicated by the horizontal scatter of plus symbols in Figure 1. For many participants, changes at other follow-up intervals were more pronounced than the change from baseline to final follow-up, and in many instances, those other changes were in the opposite direction. Overall, the scatterplots show a pattern of more increases and fewer decreases in PSS scores among TMD cases than controls.
Figure 1.
Post-baseline change in Perceived Stress Scale (PSS) scores at each quarterly (i.e., 3-mo) follow-up period for incident temporomandibular disorder (TMD) cases (A) and controls (B) in the Orofacial Pain: Prospective Evaluation and Risk Assessment (OPPERA) nested case-control study. Each horizontal line depicts a study participant’s net decrease (blue lines) or increase (red lines) between enrollment and the final follow-up period; symbols (×) in the same row depict change scores at other follow-up periods for the same individual. Study participants are ranked from largest net decrease (top of plot) to largest net increase (bottom of plot). Horizontal reference lines delimit study participants with identical PSS scores at enrollment and final follow-up period. Vertical reference lines depict changes of ±6 units, equivalent to ±1 standard deviation of the distribution of all PSS scores. The vertical axis reports the percentage of study participants.
At baseline, the mean PSS score was 20% greater in participants who later developed incident TMD compared with TMD-free controls (Table 2). For incident cases, the mean PSS scores increased monotonically during follow-up to become 9% greater in the final quarter relative to the baseline visit (Table 2). However, there was no such trend for controls. Furthermore, when the findings were stratified according to COMT diplotype, the increase was confined to incident cases that had low activity COMT diplotypes (Fig. 2A). In contrast, there was no significant trend among controls with low-activity COMT diplotypes (Fig. 2A) or among cases or controls that had high-activity COMT diplotypes (Fig. 2B).
Table 2.
Adjusted Mean Perceived Stress Scale (PSS) Scores at 4 Time Points in the OPPERA Nested Case-Control Study of TMD.
| Follow-up |
||||
|---|---|---|---|---|
| Baseline Visit | Intermediate Quarter | Penultimate Quarter | Final Quarter | |
| TMD incident cases (n = 211) | ||||
| Adjusted PSS score, mean (SE) | 16.3 (0.5) | 16.7 (0.5) | 17.3 (0.5) | 17.7 (0.6) |
| % Change relative to baseline | Referent | 2 | 6 | 9 |
| P value for change | 0.319 | 0.029 | 0.003 | |
| TMD-free controls (n = 173) | ||||
| Adjusted PSS score, mean (SE) | 13.6 (0.5) | 14.1 (0.5) | 14.4 (0.6) | 14.0 (0.5) |
| % Change relative to baseline | Referent | 4 | 6 | 3 |
| P value for change | 0.181 | 0.064 | 0.458 | |
| Contrast: cases versus controls | ||||
| % Difference | 20 | 18 | 20 | 27 |
| P value for contrast | <0.001 | <0.001 | <0.001 | <0.001 |
Adjusted means were calculated from a generalized estimating equation regression model in which the PSS score was the dependent variable; predictor variables were time of data collection (5 categories) and incident TMD case classification (2 categories) along with 2-way and 3-interactions of those predictor variables. Covariates were study site (4 categories), age (continuous measure), sex (2 categories), and race/ethnicity (5 categories). PSS scores were computed at 4 time points: the day of the baseline visit, when all subjects were TMD free; intermediate follow-up quarters were 3-mo periods after enrollment but before the penultimate quarter; the penultimate follow-up quarter was the 3-mo period preceding the final quarter; and the final follow-up quarter was the 3-mo period that coincided with the follow-up clinical visit at which incident TMD was determined. OPPERA, Orofacial Pain: Prospective Evaluation and Risk Assessment; QHU, Quarterly Health Update questionnaire; TMD, temporomandibular disorder.
Figure 2.
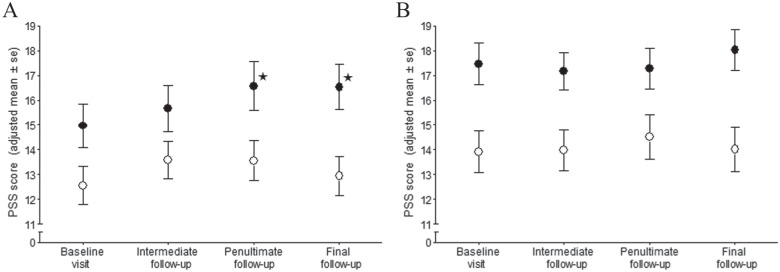
Adjusted mean Perceived Stress Scale (PSS) scores at 4 time points for incident cases of first-onset temporomandibular disorder (TMD) (•) and TMD-free controls (○), stratified according to diplotypes of the gene encoding catechol-O-methyltransferase (COMT). Limited to study participants who completed the PSS in 2 or more follow-up periods: (A) n = 96 incident cases and 90 TMD-free controls with low-activity COMT diplotypes (HPS-APS, HPS-HPS, APS-APS, or HPS-LPS). (B) n = 84 incident cases and 63 TMD-free controls with high-activity COMT diplotypes (LPS-LPS or LPS-APS). The 4 follow-up periods were the day of the baseline visit, when all participants were TMD free; intermediate follow-up represents quarterly periods after enrollment but before the penultimate quarter; the penultimate follow-up was the quarterly period preceding the final quarter; and the final follow-up was the quarterly period that coincided with the clinical visit at which incident TMD was determined. Adjusted means were calculated from a generalized estimating equation regression model in which the PSS score was the dependent variable; predictor variables were time of data collection (4 categories), COMT diplotype (2 categories), and incident case classification (2 categories) along with all 2-way and 3-way interactions of those predictor variables; covariates were study site (4 categories), age (continuous measure), sex (2 categories), and race/ethnicity (5 categories). Error bars represent ± 1 standard error (SE) of the adjusted mean. Data points denoted by asterisk (✶) represent PSS scores that differ significantly (P < 0.05) from baseline for participants with the same case classification within the same stratum of the COMT diplotype. APS, average-pain sensitivity; HPS, high-pain sensitivity; LPS, low-pain sensitivity.
Two or more follow-up questionnaires were completed by 2,481 participants in the prospective cohort study, and multivariable modeling began by replicating the finding (Fillingim et al. 2013) that baseline PSS score was positively associated with increased incidence of TMD (Table 3, model 1). The time-varying PSS change score, calculated using the concurrent quarter, was also a significant predictor of TMD incidence (HR = 1.55; 95% CL: 1.34, 1.79; model 2). When the lagged PSS change score was instead added to model 1, it too was a significant predictor (model 3), although overall model fit (χ2 = 110.0) was less than for model 2 (χ2 = 125.2). Likewise, the average change in PSS was a significant time-varying predictor of TMD incidence (HR = 1.84; 95% CL: 1.42, 2.37; model 4), and its model fit was similar to model 3. However, the PSS score gradient was a weak predictor of TMD incidence, yielding a poorer overall model fit (model 5).
Table 3.
Cox Regression Models of Time-Constant and Time-Varying Influences of Psychological Stress on Hazard of First-Onset TMD: OPPERA Prospective Cohort Study.
| Likelihood Ratio Test |
|||||
|---|---|---|---|---|---|
| Model | n | χ2 | df | Predictor | Hazard Ratio (95% CL) |
| 1. Baseline stress | 2,481 | 89.4 | 11 | Time-constant baseline PSS score (per 6.4 units) | 1.32 (1.16, 1.50) |
| 2. Model 1 + concurrent PSS change score | 2,481 | 125.2 | 12 | Time-constant baseline PSS score (per 6.4 units) | 1.66 (1.43, 1.93) |
| Time-varying concurrent quarter PSS score minus baseline (per 6.4 units) | 1.55 (1.34, 1.79) | ||||
| 3. Model 1 + lagged PSS change score | 2,481 | 110.0 | 12 | Time-constant baseline PSS score (per 6.4 units) | 1.56 (1.34, 1.81) |
| Time-varying lagged quarter PSS score minus baseline (per 6.4 units) | 1.42 (1.22, 1.65) | ||||
| 4. Model 1 + average PSS change score | 2,481 | 111.6 | 12 | Time-constant baseline PSS score (per 6.4 units) | 1.63 (1.39, 1.90) |
| Time-varying average quarterly PSS score minus baseline (per 6.4 units) | 1.84 (1.42, 2.37) | ||||
| 5. Model 1 + PSS score gradient | 2,481 | 95.8 | 12 | Time-constant baseline PSS score (per 6.4 units) | 1.40 (1.22, 1.62) |
| Time-varying regression slope of quarterly PSS scores (per 1.0 units) | 1.07 (1.01, 1.13) | ||||
| 6. Model 4, limited to genotyped subjects | 2,186 | 97.9 | 11 | Time-constant baseline PSS score (per 6.4 units) | 1.71 (1.44, 2.02) |
| Time-varying average quarterly PSS score minus baseline (per 6.4 units) | 1.86 (1.40, 2.46) | ||||
| 7. Model 6, with Time-varying PSS × COMT diplotype interaction | 2,186 | 104.8 | 13 | Time-constant baseline PSS score (per 6.4 units) | 1.72 (1.46, 2.04) |
| Low-activity COMT stratum: Time-varying average quarterly PSS score minus baseline (per 6.4 units) | 2.34 (1.65, 3.31) | ||||
| High-activity COMT stratum: Time-varying average quarterly PSS score minus baseline (per 6.4 units) | 1.41 (0.96, 2.07) | ||||
| Decreasing stress stratum (net reduction of 6.4 PSS units): effect of low-activity relative to high-activity COMT diplotype | 0.75 (0.42, 1.34) | ||||
| Increasing stress stratum (net increase of 6.4 PSS units): effect of low-activity relative to high-activity COMT diplotype | 2.07 (1.20, 3.57) | ||||
For the interaction, χ2 = 4.32, df = 1, P = 0.038. All models include time-constant covariates of study site (4 categories), age (continuous measure), and sex (2 categories). Models 1 through 5 also adjusted for self-reported race/ethnicity (5 categories). Models 6 and 7 did not use self-reported race/ethnicity but instead adjusted for population stratification using the first 3 eigenvectors from multidimensional scaling analysis. APS, average-pain sensitivity; CL, confidence limit; COMT, catechol-O-methyltransferase; OPPERA, Orofacial Pain: Prospective Evaluation and Risk Assessment; PSS, Perceived Stress Scale; TMD, temporomandibular disorder.
Model 4 formed the basis for investigating interactions because it had the best overall model fit aside from model 2, in which reverse causation was problematic. Model 6 first replicated the effect of average PSS change among the 2,186 genotyped participants. Model 7 added the COMT diplotype and its interaction with average PSS change, yielding a statistically significant interaction (Table 3). Hazard ratios were therefore estimated in each stratum of the COMT diplotype, revealing a stronger effect of average PSS change in participants who had low-activity COMT diplotypes (HR = 2.34; 95% CL: 1.65, 3.31) compared with participants with high-activity COMT diplotypes (HR = 1.41; 95% CL: 0.96, 2.07). Stated another way, the low-activity COMT diplotype significantly increased risk of TMD in participants whose stress increased (HR = 2.07; 95% CL = 1.20, 3.57), whereas it was weakly protective in participants whose stress decreased (HR = 0.75; 95% CL = 0.42, 1.34).
Models using alternative groupings of COMT diplotypes revealed generally similar findings (Appendix Table): the effect of average PSS change was amplified for participants with low-activity COMT diplotypes defined either as no copies of LPS (model A1) or ≥1 copy of HPS (model A2). Meanwhile, a model with an interaction of COMT diplotype and baseline PSS score yielded very similar effects of baseline stress in the low-activity COMT diplotype (HR = 1.57; 95% CL = 1.26, 1.95) and high-activity COMT diplotype (HR = 1.89; 95% CL: 1.52, 2.36; model A3).
Discussion
In this community-based sample, a temporal increase in psychological stress was associated with elevated risk of developing painful TMD. The magnitude of elevated risk varied according to the genotype. Risk was amplified for participants with low-activity COMT diplotypes and was attenuated in those with high-activity COMT diplotypes. These effects of temporally increasing stress were in addition to the positive association between baseline stress and risk of TMD. We interpret the combination of low-activity COMT diplotype and increased stress to constitute a gene-environment interaction that increases the nociceptive effects of catecholamine neurotransmitters, thereby elevating risk of clinical TMD.
The observed interaction is a novel finding. Previous prospective cohort studies of TMD either did not investigate genes (Von Korff et al. 1993; Aggarwal et al. 2010; Kindler et al. 2012; Plesh et al. 2012) or lacked statistical power to detect interactions (Slade et al. 2007). The finding is consistent with animal experimental and in vitro studies demonstrating that hyperalgesic effects of COMT inhibition are mediated through adrenergic pathways (Nackley et al. 2006; Nackley et al. 2007). Adrenergic pathways likewise mediate responses to psychological stress. The interaction is consistent with findings from a randomized controlled trial of TMD patients in whom propranolol, a nonselective beta-adrenergic antagonist, was efficacious in reducing pain, but only in patients whose genotype encoded low-activity COMT (Tchivileva et al. 2010). The interaction also has parallels in a cross-sectional epidemiologic study in Germany that found significant additive interactions between COMT genetic variants and depression—another psychological characteristic—in prevalence of TMD (Schwahn et al. 2012). Taken together, these precedents and the biological evidence support plausibility of our conclusion that the gene-stress interaction contributes causally to risk of TMD onset.
The interaction was significant for the PSS change score but not for the baseline PSS score. This might be because the multiple questionnaires used to compute each participant’s change increased fidelity of the measure compared with the single, baseline score. However, it may be due to different effects of dynamic versus static stress. Experimental studies that manipulate environmental stressors are needed to investigate such effects.
These findings expand on previous prospective cohort studies where stress was measured only once at baseline. We used repeated measures of stress to evaluate several theories of its effects on pain (Dunn 2010), restricting follow-up through the lagged period to eliminate reverse causation. Model fit was similar using the single, lagged PSS change score and the average PSS change score, suggesting comparable effects on TMD of a “single spike” and overall amount of stress, respectively. In contrast, model fit appeared poor using the gradient in PSS change, suggesting that a gradual change in stress did not contribute substantially to risk of TMD. However, there was substantial within-person variability in PSS scores over time, which probably added noise to the gradient measure. In this community, we therefore conclude that a continuous gradient in stress is less critical in the etiology of TMD than overall amount of stress.
A potential limitation of this study is bias created by loss to follow-up. In our previous analysis of the problem (Bair et al. 2013), we found that the rate of loss was greater among participants with higher baseline PSS scores, although the association of baseline PSS score and TMD incidence did not change appreciably using multiple imputation to account for the loss, suggesting little bias. Despite the large sample size, there were small numbers of incident cases for some diplotypes, forcing us to group diplotypes. However, the findings were generally similar whether participants were combined into 2- or 3-diplotype groups. Finally, while this analysis focuses on psychological stress and the COMT gene, we recognize that other phenotypic and genetic characteristics also influence risk of TMD (Slade, Fillingim, et al. 2013).
This study builds on a long legacy of research to understand the relationship between psychological stress and TMD pain. It shows that stress is dynamic and that patterns of fluctuating stress differentially influence the risk of developing painful TMD. The COMT gene-by-stress interaction represents an example of a biopsychosocial explanation as to why stress can have dramatically different effects on health for different patients. The interaction also highlights potential of a personalized medicine strategy that could target stress reduction according to biologic susceptibility to pain.
Author Contributions
G.D. Slade, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript; A. Sanders, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; R. Ohrbach, W. Maixner, J. Greenspan, R. Fillingim, contributed to data acquisition, critically revised the manuscript; E. Bair, S. Smith, contributed to data analysis, critically revised the manuscript; L. Diatchenko, contributed to conception, data acquisition, and interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplementary Material
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This work was supported by the National Institutes of Health (NIH) and National Institutes of Dental and Cranial Research (NIDCR) (grant numbers U01-DE17018 and R03-DE022595).
Drs. Slade, Fillingim, Smith, Maixner, and Diatchenko have equity ownership in Algynomics, Inc., the commercial provider of the genotyping platform used in this article. Drs. Slade, Maixner, and Diatchenko are consultants to the company; Drs. Maixner and Diatchenko are on the Board of Directors. Drs. Maixner and Diatchenko are inventors on a patent application related to COMT diplotypes and pain sensitivity that has been licensed to Proove Biosciences. These relationships have been reviewed in conjunction with this research and are under management by UNC–Chapel Hill. The authors declare no other potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Aggarwal VR, Macfarlane GJ, Farragher TM, McBeth J. 2010. Risk factors for onset of chronic oro-facial pain—results of the North Cheshire oro-facial pain prospective population study. Pain. 149(2):354–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD; SAS Institute. 1995. Survival analysis using SAS: a practical guide. Cary (NC): SAS Institute. [Google Scholar]
- Bair E, Brownstein NC, Ohrbach R, Greenspan JD, Dubner R, Fillingim RB, Maixner W, Smith SB, Diatchenko L, Gonzalez Y, et al. 2013. Study protocol, sample characteristics, and loss to follow-up: the OPPERA prospective cohort study. J Pain. 14(12 Suppl):T2–T19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham DJ, Nagaraja HN, Rovin BH, Spetie L, Zhao Y, Li X, Hackshaw KV, Yu CY, Malarkey WB, Hebert LA. 2006. Fluctuation in self-perceived stress and increased risk of flare in patients with lupus nephritis carrying the serotonin receptor 1A-1019 G allele. Arthritis Rheum. 54(10):3291–3299. [DOI] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. 1983. A global measure of perceived stress. J Health Soc Behav. 24(4):385–396. [PubMed] [Google Scholar]
- Diatchenko L, Fillingim RB, Smith SB, Maixner W. 2013. The phenotypic and genetic signatures of common musculoskeletal pain conditions. Nat Rev Rheumatol. 9(6):340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diatchenko L, Nackley AG, Slade GD, Bhalang K, Belfer I, Max MB, Goldman D, Maixner W. 2006. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain. 125(3):216–224. [DOI] [PubMed] [Google Scholar]
- Diatchenko L, Slade GD, Nackley AG, Bhalang K, Sigurdsson A, Belfer I, Goldman D, Xu K, Shabalina SA, Shagin D, et al. 2005. Genetic basis for individual variations in pain perception and the development of a chronic pain condition. Hum Mol Genet. 14(1):135–143. [DOI] [PubMed] [Google Scholar]
- Dunn KM. 2010. Extending conceptual frameworks: life course epidemiology for the study of back pain. BMC Musculoskelet Disord. 11:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin S, LeResche L. 1992. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 6(4):301–355. [PubMed] [Google Scholar]
- Fillingim RB, Ohrbach R, Greenspan JD, Knott C, Diatchenko L, Dubner R, Bair E, Baraian C, Mack N, Slade GD, et al. 2013. Psychological factors associated with development of TMD: the OPPERA prospective cohort study. J Pain. 14(12 Suppl):T75–T90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SZ, Wallace MR, Wright TW, Moser MW, Greenfield WH, III, Sack BK, Herbstman DM, Fillingim RB. 2008. Evidence for a biopsychosocial influence on shoulder pain: pain catastrophizing and catechol-O-methyltransferase (COMT) diplotype predict clinical pain ratings. Pain. 136(1–2):53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernaus D, Collip D, Lataster J, Ceccarini J, Kenis G, Booij L, Pruessner J, Van Laere K, van Winkel R, van Os J, et al. 2013. COMT Val158Met genotype selectively alters prefrontal [18F]fallypride displacement and subjective feelings of stress in response to a psychosocial stress challenge. PLoS One. 8(6):e65662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler S, Samietz S, Houshmand M, Grabe HJ, Bernhardt O, Biffar R, Kocher T, Meyer G, Volzke H, Metelmann HR, et al. 2012. Depressive and anxiety symptoms as risk factors for temporomandibular joint pain: a prospective cohort study in the general population. J Pain. 13(12):1188–1197. [DOI] [PubMed] [Google Scholar]
- Laskin DM. 1969. Etiology of the pain-dysfunction syndrome. J Am Dent Assoc. 79(1):147–153. [DOI] [PubMed] [Google Scholar]
- Lix LM, Graff LA, Walker JR, Clara I, Rawsthorne P, Rogala L, Miller N, Ediger J, Pretorius T, Bernstein CN. 2008. Longitudinal study of quality of life and psychological functioning for active, fluctuating, and inactive disease patterns in inflammatory bowel disease. Inflamm Bowel Dis. 14(11):1575–1584. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Shabalina SA, Tchivileva IE, Satterfield K, Korchynskyi O, Makarov SS, Maixner W, Diatchenko L. 2006. Human catechol-O-methyltransferase haplotypes modulate protein expression by altering mRNA secondary structure. Science. 314(5807):1930–1933. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Tan KS, Fecho K, Flood P, Diatchenko L, Maixner W. 2007. Catechol-O-methyltransferase inhibition increases pain sensitivity through activation of both beta2- and beta3-adrenergic receptors. Pain. 128(3):199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plesh O, Gansky S, Curtis DO. 2012. Chronic pain in a biracial cohort of young women. Open Pain J. 5:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliep KC, Mumford SL, Vladutiu CJ, Ahrens KA, Perkins NJ, Sjaarda LA, Kissell KA, Prasad A, Wactawski-Wende J, Schisterman EF. 2015. Perceived stress, reproductive hormones, and ovulatory function: a prospective cohort study. Epidemiology. 26(2):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwahn C, Grabe HJ, Meyer zu, Schwabedissen H, Teumer A, Schmidt CO, Brinkman C, Kocher T, Nauck M, Volzke H, Biffar R, et al. 2012. The effect of catechol-O-methyltransferase polymorphisms on pain is modified by depressive symptoms. Eur J Pain. 16(6):878–889. [DOI] [PubMed] [Google Scholar]
- Sipila K, Maki P, Laajala A, Taanila A, Joukamaa M, Veijola J. 2013. Association of depressiveness with chronic facial pain: a longitudinal study. Acta Odontol Scand. 71(3–4):644–649. [DOI] [PubMed] [Google Scholar]
- Slade GD, Diatchenko L, Bhalang K, Sigurdsson A, Fillingim RB, Belfer I, Max MB, Goldman D, Maixner W. 2007. Influence of psychological factors on risk of temporomandibular disorders. J Dent Res. 86(11):1120–1125. [DOI] [PubMed] [Google Scholar]
- Slade GD, Fillingim RB, Sanders AE, Bair E, Greenspan JD, Ohrbach R, Dubner R, Diatchenko L, Smith SB, Knott C, et al. 2013. Summary of findings from the OPPERA prospective cohort study of incidence of first-onset temporomandibular disorder: implications and future directions. J Pain. 14(12 Suppl):T116–T124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Sanders AE, Bair E, Brownstein N, Dampier D, Knott C, Fillingim R, Maixner WO, Smith S, Greenspan J, et al. 2013. Preclinical episodes of orofacial pain symptoms and their association with health care behaviors in the OPPERA prospective cohort study. Pain. 154(5):750–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade GD, Sanders AE, Ohrbach R, Fillingim RB, Dubner R, Gracely RH, Bair E, Maixner W, Greenspan JD. 2014. Pressure pain thresholds fluctuate with, but do not usefully predict, the clinical course of painful temporomandibular disorder. Pain. 155(10):2134–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SB, Maixner DW, Greenspan JD, Dubner R, Fillingim RB, Ohrbach R, Knott C, Slade GD, Bair E, Gibson DG, et al. 2011. Potential genetic risk factors for chronic TMD: genetic associations from the OPPERA case control study. J Pain. 12(11 Suppl):T92–T101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchivileva IE, Lim PF, Smith SB, Slade GD, Diatchenko L, McLean SA, Maixner W. 2010. Effect of catechol-O-methyltransferase polymorphism on response to propranolol therapy in chronic musculoskeletal pain: a randomized, double-blind, placebo-controlled, crossover pilot study. Pharmacogenet Genomics. 20(4):239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton LM, Andersen BL, Crespin TR, Carson WE. 2007. Individual trajectories in stress covary with immunity during recovery from cancer diagnosis and treatments. Brain Behav Immun. 21(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. 2008. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 61(4):344–349. [DOI] [PubMed] [Google Scholar]
- Von Korff M, Le Resche L, Dworkin SF. 1993. First onset of common pain symptoms: a prospective study of depression as a risk factor. Pain. 55(2):251–258. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Heitzeg MM, Smith YR, Bueller JA, Xu K, Xu Y, Koeppe RA, Stohler CS, Goldman D. 2003. COMT val158met genotype affects mu-opioid neurotransmitter responses to a pain stressor. Science. 299(5610):1240–1243. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.