Abstract
Objectives
There is currently no information on whether products evaluated in HIV microbicide trials affect the detection of the semen biomarkers prostate-specific antigen (PSA) or Y chromosome DNA.
Study Design
We tested (in vitro) dilutions of tenofovir (TFV), UC781 and the hydroxyethylcellulose (HEC) placebo gels using the Abacus ABAcard and the quantitative (Abbott Architect total PSA) assays for PSA and Y chromosome DNA by real-time polymerase chain reaction.
Results
TFV gel and the HEC placebo adversely affected PSA detection using the ABAcard but not the Abbott Architect total PSA assay. UC781 adversely affected both the ABAcard and Abbott Architect total PSA assays. While there were some quantitative changes in the magnitude of the signal, none of the products affected positivity of the Y chromosome assay.
Conclusions
The presence of TFV or HEC gels did not affect quantitative PSA or Y chromosome detection in vitro. Confirmation of these findings is recommended using specimens obtained following use of these gels in vivo.
Implications
Researchers should consider the potential for specific microbicides or any products to affect the particular assay used for semen biomarker detection. The ABAcard assay for PSA detection should not be used with TFV UC781, or HEC.
Keywords: Biomarkers, Semen, Microbicides, Gels, Prostate-specific antigen, Y chromosome, Tenofovir, UC781, Hydroxyethylcellulose, HEC placebo
1. Introduction
Biomarkers of sexual behavior have been identified as critically needed for microbicide trials by many researchers (http://www.mtnstophiv.org/) [1–4]. The use of semen biomarkers to detect or confirm recent sexual behavior and potential exposure to HIV is desirable in clinical trials evaluating topical microbicides such as tenofovir (TFV) gel. Prostate-specific antigen (PSA) and Y chromosome DNA have been used as biomarkers of semen exposure in forensic settings [5–7], and more recently, in reproductive health research, as indicators of semen exposure from unprotected sex or incorrect condom use [8–12]. For example, Y chromosome DNA has been used in studies evaluating teen's self-reported condom use [10], and PSA has been used to validate self-reports of recent sexual activity [11] and as an indicator of condom failure [12].
While both PSA and Y chromosome DNA are indicators of semen exposure, these two markers have unique characteristics, which researchers must take into consideration when choosing the appropriate marker for their study. PSA reliably indicates very recent semen exposure (up to 24 h), while Y chromosome DNA can reliably detect semen12 h post exposure up to 1–2 weeks [13]. To our knowledge, there is no information as to whether use of microbicides or the hydroxyethylcellulose (HEC) placebo may affect the performance of the PSA or Y chromosome DNA assays for detection of such markers.
Given the important role that biomarkers of semen exposure can play in microbicide trials and future contraception research, as well as the lack of information on this topic, we undertook a series of laboratory investigations to determine whether TFV gel, UC781 or the HEC placebo affect PSA or Y chromosome DNA detection in vitro. TFV is an antiretroviral (nucleotide reverse transcriptase inhibitor) microbicide and has demonstrated efficacy when used as a gel vaginally for preexposure prophylaxis for HIV infection [14]. UC781 is an antiretroviral (nonnucleoside reverse transcriptase inhibitor) gel, which was evaluated as a microbicide in clinical trials for the prevention of sexual transmission of HIV [15]. Although UC781 is no longer in development as a single microbicide gel product, there are efforts under way to develop a combined UC781 and TFV microbicide product, which may make it relevant in the future. The HEC placebo is a gel that contains no active microbicide; it has been adopted as the placebo in many clinical trials of microbicides (Microbicides Trial Network, available at: http://www.mtnstopshiv.org/studies, accessed 5/25/2012)) [16,17]. TFV's gel base is identical to HEC (2.5% hydroxyethylcellulose) (Table 1).
Table 1. Gels tested in vitro for their effects on PSA detection by the Abbott Architect and ABAcard assays and Y chromosome DNA detection by real-time PCR.
| Product | Brand name (manufacturer) | Main ingredient | Type |
|---|---|---|---|
| TFV | Gilead Sciences | 1% TFV with 2.5% hydroxyethylcellulose | Microbicide |
| UC781 | Cellegy Pharmaceuticals | 0.1% in methycellulose and carbomer 974P | Microbicide |
| HEC | ReProtect | 2.5% hydroxyethylcellulose | Placebo |
2. Methods
2.1. PSA detection
There are several methods available for PSA detection. For our investigation, we used the following two PSA assays; the Abacus One Step ABAcard [18] is a rapid qualitative or semiquantitative assay with a lower limit of PSA detection of 4 ng/ml [3,18,19]. The total PSA assay [20] used on the Abbott Architect system is a chemiluminescent immunoassay that yields quantitative results [20], with a readable range of 0 to 100 ng/mL.
2.2. Y chromosome PCR assay
Real-time polymerase chain reaction (PCR) was used for detection of Y chromosome DNA as previously described [21]. The Y chromosome assay offers a qualitative indication of the presence of Y chromosome DNA (positive at >0 ng/mL); the assay also yields quantitative results that have been shown to decrease with time since exposure (detectable up to 14 days) [5–7]. The specificity of the Yc PCR assay for sperm-derived Yc sequences is based on a series of centrifugation steps to separate semen components (as well as other cells) from sperm cells. Briefly, semen samples with and without gels were centrifuged for 3 min at 12,500 rpm, and 100 μL of the supernatant was removed. The sample was extracted for DNA using a multistep extraction protocol. First, a proteinase-K solution (10 mg/mL) was added to the specimens to lyse nonsperm cells and incubated for 30 min at 56°C. The specimen was then centrifuged for 3 min at 12,500 rpm, and DNA from the cell pellet (containing sperm cells and product, if present) was extracted. Two hundred microliters of a proteinase-K (10 mg/mL)/DTT (40 mmol) solution were added to the specimen, vortexed and incubated in a sonicator bath at 56°C for 30 min. The resulting sample was used for PCR-based detection of Yc DNA sequences.
2.3. In vitro experiments
The following products were tested (Table 1): TFV 1% vaginal gel formulated in 2.5% hydroxyethylcellulose (Gilead Sciences), UC781 gel 0.1% formulated in methylcellulose and carbomer 974P (Cellegy Pharmaceuticals, now merged with Adamis Pharmaceutical Corporation) [15] and the HEC (2.5% hydroxyethylcellulose) placebo (ReProtect) [16,17].
First, semen controls (positive controls) were prepared by serially diluting [with phosphate buffered saline (PBS)] pooled semen stock which had been stored at −80°C at a 1:50 dilution and then thawed. Twofold serial dilutions were created, and then, select dilutions were tested (without the products), using the ABAcard [18] and Abbott Architect total PSA assay [20] (for PSA assessment) and real-time PCR (for Y chromosome DNA assessment) [22].
Next, each of the gels was mixed with semen to achieve final gel dilutions of 1:10, 1:20 and 1:40 (and 1:80 for Y chromosome DNA only). These dilutions were estimated to represent a plausible range for the amount of product that would be present following typical vaginal use. Specifically, each of the products was diluted in PBS and then added to the series of semen dilutions in equal volumes; the final semen dilutions were twofold 1:1600 through 1:1,638,400. Selected dilutions were tested with and without the products for each assay. Finally, products mixed with PBS only, without semen, were tested as negative controls for the ABAcard PSA assay. Even though no negative controls (no semen) were used for the total PSA and Yc DNA assays, very high semen dilutions (up to 1:409,600 for the quantitative PSA assay, and 1:12,800 for Y chromosome DNA assay) were used, with or without product. For each of the samples tested for PSA with the ABAcard, 200 μL were placed in the test device, and all test results were read at 10 min. For each of the samples tested for PSA with Abbott Architect, 200 μL were placed in the machine's sample cup, and quantitative results were produced upon completion. For each of the samples tested for Y chromosome DNA, 5 μL were tested by real-time PCR [22].
All PSA testing was performed by a single lab technician at the Centers for Disease Control and Prevention laboratory. The quantitative total PSA assay testing was performed in triplicate. All Y chromosome DNA real-time PCR testing was done in quadruplicate by a female technician (to avoid accidental Y chromosome contamination) at the Johns Hopkins University laboratory.
3. Results
3.1. Detection of PSA by ABAcard
Results of a representative experiment of PSA testing using the ABAcard are shown in Table 2. Using the ABAcards, TFV and the HEC placebo gave invalid results at the 1:10 dilution. TFV and the HEC placebo at 1:20 and UC781 at 1:10 dilution showed false positive results (positive at all semen dilutions and even when no semen was present). TFV and UC781 at 1:40 gave a strong positive result at a semen dilution of 1:204,800. UC781 at 1:20 and the HEC placebo at 1:40 also gave this type of result, as well as a weakly positive result at 1:409,600.
Table 2. In vitro effects of TFV 1% gel, UC 781, and the HEC placebo on PSA detection by ABAcard.
| Semen dilutions | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 1: 3,200 | 1: 51,200 | 1:102,400 | 1:204,800 | 1: 409,600 | 1: 819,200 | No semen* | |
| Semen alone* | + | + | + | +/− | − | − | n/a |
| TFV 1:10 | i | i | i | i | i | i | i |
| TFV 1:20 | + | + | + | + | + | + | + |
| TFV 1:20 | + | + | + | + | + | + | + |
| TFV 1:40 | + | + | + | + | − | − | − |
| UC 781 1:10 | + | + | + | + | + | + | + |
| UC 781 1:20 | + | + | + | + | +/− | − | − |
| UC 781 1:40 | + | + | + | + | − | − | − |
| HEC 1:10 | i | i | i | i | i | i | i |
| HEC 1:20 | + | + | + | + | + | + | + |
| HEC 1:40 | + | + | + | + | +/− | − | − |
+, positive; +/−, weakly positive; −, negative; i, invalid; n/a, not applicable.
Positive control: semen alone. Semen dilutions were tested without mixing with products. Negative control: no semen/product only. Products were tested without mixing with semen.
3.2. Detection of PSA by the Abbott Architect total PSA assay
Results of PSA testing using the Abbott Architect total PSA assay are presented in Figs. 1 and 2. The figures depict the mean results (and, in parentheses, standard deviations) from the three experiments. Comparisons of the means for each product dilution with the corresponding control for each semen dilution were performed using student's t test, and statistical significance is reported at the 0.05 level.
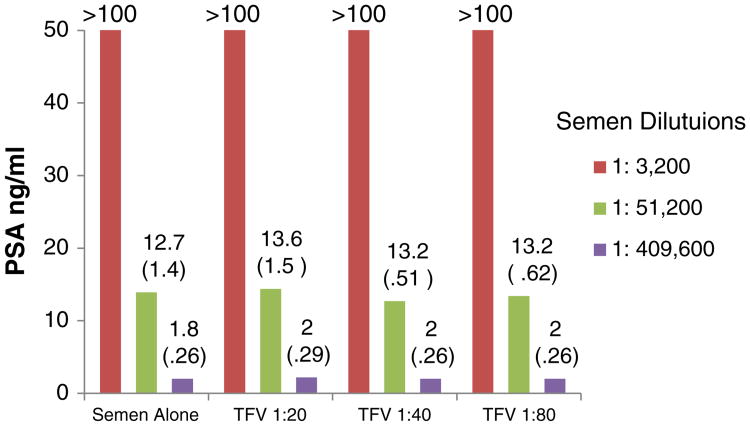
Fig. 1.
In vitro effects of TFV 1% gel on PSA detection by the Abbott Architect total PSA assay. The experiments were run in triplicate (on three different dates). The values presented in the graph are the means (in parentheses, standard deviations).
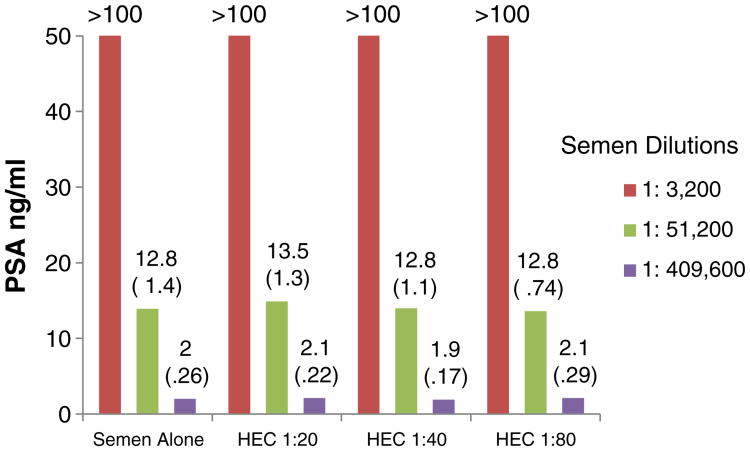
Fig. 2.
In vitro effects of HEC on PSA detection by the Abbott Architect total PSA assay. The experiments were run in triplicate (on three different dates). The values presented in the graph are the means (standard deviations).
As seen in Figs. 1 and 2, TFV and the HEC placebo did not affect PSA detection using the quantitative Abbott Architect total PSA assay. Results were not different with or without the two gels at each of the dilutions tested (p>.05 for all comparisons). All concentrations of UC781 caused invalid results (results not shown).
3.3. Detection of Y chromosome DNA using real-time PCR
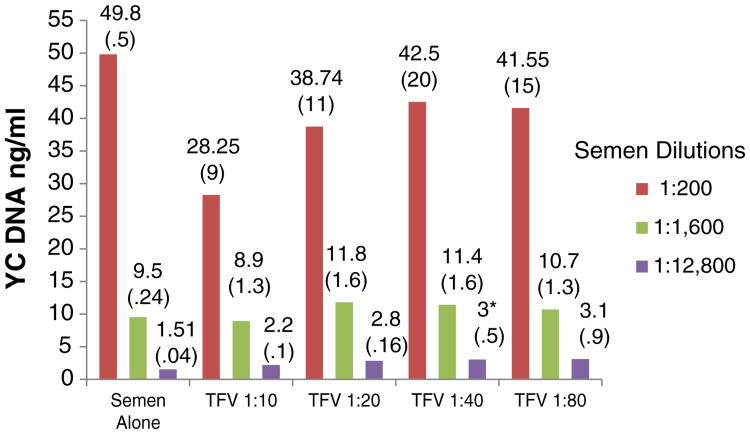
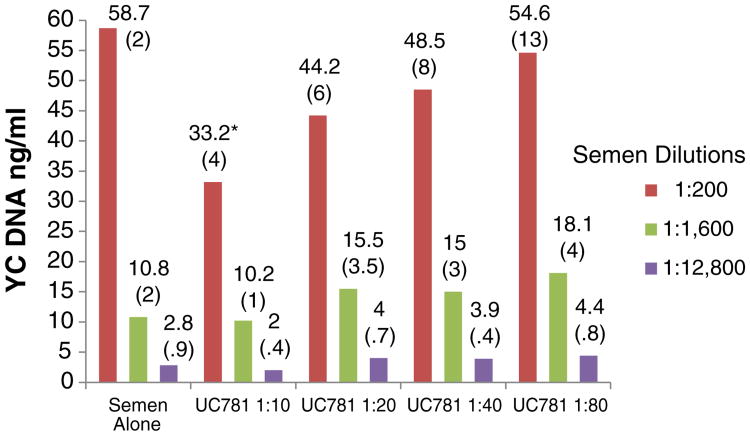
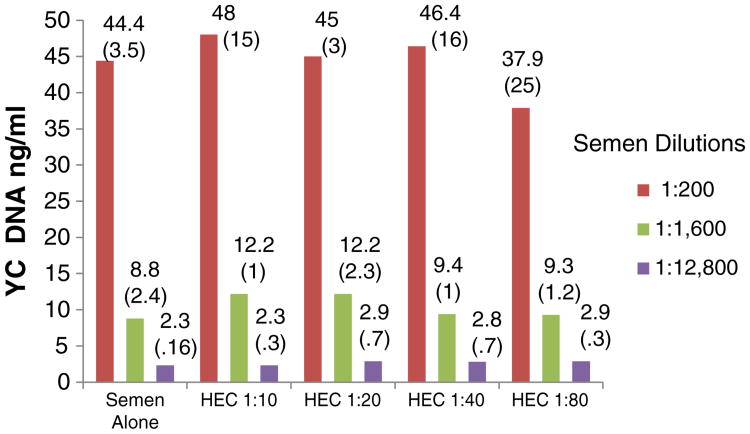
None of the products in the concentrations tested altered the qualitative indication of the presence of Y chromosome DNA by PCR (interpretation of a positive result) (Fig. 3). However, the quantitative assessment was slightly (but statistically significantly) affected in some cases. For example, the levels of Y chromosome DNA in 1:12 800 semen samples mixed with 1:40 TFV were higher than in semen controls (p=.04). Lower levels of Y chromosome DNA were detected when 1:200 semen samples were mixed with 1:10 UC781 (p=.03) (Fig. 4). For both TFV and UC781, a tendency toward inhibition of Y chromosome detection with progressively higher concentrations was observed (even though the values were not significantly different than the semen-only control, unless as noted above), suggesting a dose-dependent effect. The HEC placebo did not affect the qualitative or quantitative detection of Y chromosome DNA (p>.05 for all comparisons).
Fig. 3.
In vitro effects of TFV 1% gel on Y chromosome DNA detection by real-time PCR. Semen dilutions. The experiments were run in quadruplicate. The values presented in the graph are the means (standard deviations). Asterisks denote statistically significant difference from control (p<.05).
Fig. 4.
In vitro effects of UC 781 on Y chromosome DNA detection by realtime PCR. The experiments were run in quadruplicate. The values presented in the graph are the means (standard deviations). Asterisks denote statistically significant difference from control (p<.05).
4. Discussion
Our findings indicate that specific concentrations of TFV, UC781 and the HEC placebo affect PSA detection in vitro when using the ABAcard (Fig. 5). The ABAcard's invalid results for TFV and the HEC placebo at the 1:10 dilution are probably due to the solution being too viscous to migrate through the membrane in the 10-min interval. TFV gel is formulated with HEC as its base, which may explain the similar results. On the other hand, TFV and the HEC placebo did not affect results of the Abbott Architect assay. No results could be obtained when UC781 was present probably because of the color or other intrinsic properties of the UC781 gel, which could have affected the Abbott Architect's chemiluminescent immunoassay, rendering all quantitative PSA results invalid when this product was present. Although UC781 is no longer being developed as a microbicide [15], the results were included as an illustrative example of individual differences of topical microbicide products in their effects on the performance of two different PSA assays. Further, as efforts are in process to combine TFV with UC781 in combination microbicide gels, testing its effects could be relevant in the near future [34]. None of the microbicides tested in vitro inhibited detection of the presence of Y chromosome DNA by real-time PCR, but in some cases, the products affected the amount of Y chromosome DNA detected. In most instances, this effect was not statistically significant (p was greater than .05). But, for some concentrations of TFV (1:40) and UC781 (1:10) gels, the amount of Y chromosome DNA detected by realtime PCR was statistically significantly higher or lower than what was present in the corresponding semen control. The reasons for this are unknown. A possibility could be that, since the experimental protocol required several centrifugation steps for the proper separation of sperm cells from other semen components, the particular gels could have formed a physical barrier between the sperm cells and the other semen components during centrifugation and prevented the removal of sperms cells, resulting in higher levels of Y chromosome DNA. However, as noted above, one of the differences observed between the control and the products would not affect the interpretation of the presence of Y chromosome DNA, since its presence would be considered positive [5]; in turn, the quantitative results may not be accurate or reliable. In previous work [28] we have indeed demonstrated that other products, such as Replens, completely inhibited qualitative detection of Y chromosome sequences.
Fig. 5.
In vitro effects of HEC on Y chromosome DNA detection by realtime PCR. The experiments were run in quadruplicate. The values presented in the graph are the means (standard deviations).
To our knowledge, no previous information is available on the effect of the specific microbicide products evaluated on PSA or Y chromosome PCR detection, either in vitro or in vivo. There is some evidence on the effect of spermicides, other products (such as detergents) and proteins from body fluids on PSA detection, using various assays, with contradictory results [23–31]. Our laboratories have also evaluated the effects of spermicides and lubricants, and results are presented elsewhere [28,31]. It is important to know if specific products or gels affect semen biomarker detection, rather than assuming that they are all universally reliable because specific products can affect specific assays differently, ultimately affecting the objective assessment of recent semen exposure.
There are some limitations to our study. The amount or dose of a product potentially affects the assays differently. We estimated the range of product amounts that we thought would approximate the presence of products intravaginally, but we do not know if these estimates are accurate. We did not test the gel base of UC781 (methycellulose and carbomer 974P). We only tested one product at a time in the lab, but multiple products may be present in clinically obtained specimens. The characteristics of individual or multiple products theoretically can affect assays in different ways. How the results of a controlled laboratory experiment (in vitro) translate to actual clinical samples (in vivo) remains uncertain. For example, PBS, used as the diluting medium, could be quite different from vaginal fluids in pH or other physicochemical characteristics. In vivo factors such as phase of the menstrual cycle, local inflammation, vaginal infection, or hormonal contraception may affect some of the local parameters and thus lead to different results for actual clinical samples.
Future microbicide studies in which a semen biomarker is used should consider the potential for microbicides or other local products to affect the particular biomarker assay. Similar considerations should be made for rectal microbicides. Given the important role that TFV gel can play in preexposure prophylaxis against sexual HIV acquisition [1,31–33], it is particularly important to know whether its use may affect detection of such markers. While our results provide reassurance about the lack of effect of TFV gel on the quantitative Abbott Architect's total PSA assay and the qualitative findings of the real-time PCR assay for Y chromosome DNA, more studies are warranted, especially with clinically obtained specimens. Additional testing on the effects of lubricants or other products used concurrently with microbicides and confirmation of these results with in vivo studies are also needed.
Acknowledgments
The authors acknowledge Teresa M. Brown, Laurie Howard Jones and Edmund Gumisiriza for expert technical assistance as well as the CONRAD Clinical Working Group on Evaluation of Markers of Intercourse in Trials of Vaginal Barriers for helpful discussions. We thank Dr. Jonathan Zenilman for all of his support and expertise.
Footnotes
Conflicts of Interest and Source of Funding: For all authors none were declared.
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Use of trade names is for identification only and does not imply endorsement by the US Department of Health and Human Services.
References
- 1.Mauck CK, va der Straten A. Using objective markers to assess participant behavior in HIV prevention trials of vaginal microbicides. J Acquir Immune Defic Syndr. 2008;49(1):64–9. doi: 10.1097/QAI.0b013e318183a917. [DOI] [PubMed] [Google Scholar]
- 2.Mauck CK, Doncel GF. Biomarkers of semen in the vagina: applications in clinical trials of contraception and prevention of sexually transmitted pathogens including HIV. Contraception. 2007;75(6):407–19. doi: 10.1016/j.contraception.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Macaluso M, Lawson L, Akers R, Valappil T, Hammond K, Blackwell R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999;59(3):195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 4.Gallo MF, Steiner MJ, Hobbs MM, Warner L, Jamieson DJ, Macaluso M. Biological markers of semen exposure: tools for improving the assessment of sexual behavior in HIV/STI prevention research. Sex Transm Dis. 2013;40(6):447–52. doi: 10.1097/OLQ.0b013e31828b2f77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Culhane JF, Nyirjesy P, McCollum K, Casabellata G, Di Santolo M, Cauci S. Evaluation of semen detection in vaginal secretions: comparison of four methods. Am J Reprod Immunol. 2008;60(3):274–81. doi: 10.1111/j.1600-0897.2008.00632.x. [DOI] [PubMed] [Google Scholar]
- 6.Graves H, Sensabaugh G, Blake E. Postcoital detection of a male-specific semen protein. Application to the investigation of rape. N Engl J Med. 1985;312(6):338–43. doi: 10.1056/NEJM198502073120603. [DOI] [PubMed] [Google Scholar]
- 7.Hochmeister MN, Budowle B, Rudin O, Gehrig C, Borer U, Thali M, et al. Evaluation of prostate-specific antigen (PSA) membrane test assays for the forensic identification of seminal fluid. J Forensic Sci. 1999;44(5):1057–60. [PubMed] [Google Scholar]
- 8.Zenilman JM, Yuenger J, Galai N, Turner CF, Rogers SM. Polymerase chain reaction detection of Y chromosome sequences in vaginal fluid: preliminary studies of a potential biomarker for sexual behavior. Sex Transm Dis. 2005;32(2):90–4. doi: 10.1097/01.olq.0000149668.08740.91. [DOI] [PubMed] [Google Scholar]
- 9.Jadack RA, Yuenger J, Ghanem KG, Zenilman J. Polymerase chain reaction detection of Y-chromosome sequences in vaginal fluid of women accessing a sexually transmitted disease clinic. Sex Transm Dis. 2006;33(1):22–5. doi: 10.1097/01.olq.0000194600.83825.81. [DOI] [PubMed] [Google Scholar]
- 10.Rose E, Diclemente RJ, Wingood GM, Sales JM, Latham TP, Crosby RA, et al. The validity of teens' and young adults' self-reported condom use. Arch Pediatr Adolesc Med. 2009;163(1):61–4. doi: 10.1001/archpediatrics.2008.509. [DOI] [PubMed] [Google Scholar]
- 11.Minnis AM, Steiner MJ, Gallo MF, Warner L, Hobbs MM, van der Straten A, et al. Biomarker validation of reports of recent sexual activity: results of a randomized controlled study in Zimbabwe. Am J Epidemiol. 2009;170(7):918–24. doi: 10.1093/aje/kwp219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghanem KG, Melendez JH, McNeil-Solis C, Giles JA, Yuenger J, Smith TD, et al. Condom use and vaginal Y-chromosome detection: the specificity of a potential biomarker. Sex Transm Dis. 2007;34(8):620–3. doi: 10.1097/01.olq.0000258318.99606.d9. [DOI] [PubMed] [Google Scholar]
- 13.Jamshidi R, Penman-Aguilar A, Wiener J, Gallo MF, Zenilman JM, Melendez JH, et al. Detection of two biological markers of intercourse:prostate-specific antigen and Y-chromosomal DNA. Contraception. 2013;88(6):749–57. doi: 10.1016/j.contraception.2013.08.003. http://dx.doi.org/10.1016/j.contraception.2013.08.003. ( http://www.sciencedirect.com/science/article/pii/S0010782413005490) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abdool Karim QA, Karim SSA, Frohlich JA, et al. Effectiveness and safety of TFVgel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anton Peter A, Saunders Terry, Elliott Julie, Khanukhova Elena, Dennis Robert, Adler Amy, et al. First phase 1 double-blind, placebo-controlled, randomized rectal microbicide trial using UC781 gel with a novel index of ex vivo efficacy. PLOS ONE. 2011;6(9):e23243. doi: 10.1371/journal.pone.0023243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz JL, Ballagh SA, Kwok C, Mauck CK, Weiner DH, Rencher WF, et al. Fourteen-day safety and acceptability study of the universal placebo gel. Contraception. 2007;75(2):136–41. doi: 10.1016/j.contraception.2006.09.003. Epub 2006 Oct 25. [DOI] [PubMed] [Google Scholar]
- 17.Tien D, Schnaare RL, Kang F, Cohl G, et al. In vitro and in vivo characterization of a potential universal placebo designed for use in vaginal microbicide clinical trials. AIDS Res Hum Retroviruses. 2005;21(10):845–53. doi: 10.1089/aid.2005.21.845. [DOI] [PubMed] [Google Scholar]
- 18.Abacus Diagnostics. ABAcard. West Hills, CA: cited 2012 April 27; Available from: http://www.abacusdiagnostics.com/semen.htm. [Google Scholar]
- 19.Hobbs MM, Steiner MJ, Rich KD, et al. Good performance of rapid prostate-specific antigen test for detection of semen exposure in women: implications for qualitative research. Sex Transm Dis. 2009;36(8):501–6. doi: 10.1097/OLQ.0b013e3181a2b4bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abbott Diagnostics. ARCHITECT i2000SR. Abbott Park, IL: cited 2012 April 27; Available from: http://www.abbottdiagnostics.com/Products/Instruments_by_Platform/default.cfm?system=ARCHITECT&suffix=i2000sr®=us. [Google Scholar]
- 21.Walsh T, Warner L, Macaluso M, Frezieres R, Snead MC, Wraxall B. Prostate-specific antigen as a biomarker of condom failure: comparison of three laboratory assays and self-reported condom use problems in a randomized trial of female condom performance. Contraception. 2012;86(1):55–61. doi: 10.1016/j.contraception.2011.10.018. Epub March 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melendez JH, Giles JA, Yuenger JD, et al. Detection and quantification of Y-chromosomal sequences by real-time PCR using the light cycler system. Sex Transm Dis. 2007;34(8):617–9. doi: 10.1097/01.olq.0000258336.65285.31. [DOI] [PubMed] [Google Scholar]
- 23.Pang BC, Cheung BK. Identification of human semenogelin in membrane strip test as an alternative method for the detection of semen. Forensic Sci Int. 2007;169(1):27–31. doi: 10.1016/j.forsciint.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 24.Laffan A, Sawyer I, Quinones I, Daniel B. Evaluation of semen presumptive tests for use at crime scenes. Med Sci Law. 2011;51(1):11–7. doi: 10.1258/msl.2010.010040. [DOI] [PubMed] [Google Scholar]
- 25.Maher J, Vintiner S, Elliot D, Melia L. Evaluation of the BioSign PSA membrane test for the identification of semen stains in forensic casework. N Z Med J. 2002;115(1147):48–9. [PubMed] [Google Scholar]
- 26.Bitner SE. False positives observed on the Seratec(®) PSA semiquant cassette test with condom lubricants. J Forensic Sci. 2012;57(6):1545–8. doi: 10.1111/j.1556-4029.2012.02141.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 27.Sutton JG, Bosley C, Rands A. The detection by enzyme linked immunosorbent assay of P30 and 19-OH prostaglandin F1/F2, in the presence of a range of possible contaminants. Sci Justice. 1998;38(3):157–64. doi: 10.1016/S1355-0306(98)72100-8. [DOI] [PubMed] [Google Scholar]
- 28.Melendez JH, Doncel GF, Chaney D, Schwartz JL, Snead MC, Mauck CK, et al. Inhibitory effect of lubricants and a vaginal contraceptive gel on detection of biomarkers of semen exposure. London: International Society for STD Research; 2009. abstract. [Google Scholar]
- 29.Johnson ED, Kotowski TM. Detection of prostate specific antigen by ELISA. J Forensic Sci. 1993;38(2):250–8. [PubMed] [Google Scholar]
- 30.Stowell LI, Sharman LE, Hamel K. An enzyme-linked immunosorbent assay (ELISA) for prostate-specific antigen. Forensic Sci Int. 1991;50(1):125–38. doi: 10.1016/0379-0738(91)90141-5. [DOI] [PubMed] [Google Scholar]
- 31.Snead MC, Kourtis AP, Black CM, et al. Effect of topical vaginal products on the detection of prostate specific antigen, a biomarker of semen exposure, using ABAcards. Contraception. 2013;88(3):382–6. doi: 10.1016/j.contraception.2012.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Eng J Med. 2012;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiser PF, Mahalingam A, Fabian J, et al. Design of Tenofovir-UC781 combination microbicide vaginal gels. J Pharm Sci. 2012;101:1852–4. doi: 10.1002/jps.23089. [DOI] [PubMed] [Google Scholar]