Abstract
Objectives
In children with traumatic brain injury (TBI), to describe between-hospital and patient-level variation in intracranial pressure (ICP) monitoring, and to evaluate ICP monitoring in association with hospital features and outcome
Design
Retrospective cohort study
Setting
Children’s hospitals participating in the Pediatric Health Information System database, January, 2001 to June, 2011
Participants
Children (age < 18 years) with TBI and head/neck Abbreviated Injury Scale (AIS) score ≥ 3 who were ventilated for ≥ 96 consecutive hours or died in the first 4 days after admission
Interventions
None
Outcome Measures
ICP monitoring
Results
4,667 children met study criteria. Hospital mortality was 41% (1,919/4,667). Overall, 55% (2,586/4,667) of patients received ICP monitoring. Expected hospital ICP monitoring rates after adjustment for patient age, cardiac arrest, inflicted injury, craniotomy or craniectomy, head/neck AIS, and injury severity score (ISS) were 47-60%. Observed hospital ICP monitoring rates were 14-83%. Hospitals with more observed ICP monitoring, relative to expected, and hospitals with higher patient volumes had lower rates of mortality or severe disability. After adjustment for between-hospital variation and patient severity of injury, ICP monitoring was independently associated with age ≥ 1 year (odds ratio [OR] 3.1, 95% confidence interval 2.5-3.8) versus age < 1 year.
Conclusions
There was significant between-hospital variation in ICP monitoring that cannot be attributed solely to differences in case mix. Hospitals that monitor ICP more often and hospitals with higher patient volumes had better patient outcomes. Infants with TBI are less likely to receive ICP monitoring than older children.
Keywords: Pediatrics, Craniocerebral Trauma, Brain Edema, Intracranial Hypertension
Introduction
Pediatric traumatic brain injury (TBI) is estimated to cause approximately 2,300 deaths, 42,000 hospitalizations, and 404,000 Emergency Department visits annually among children 0-14 years old.1,2 TBI is also a major cause of disability in children.3 Elevated intracranial pressure (ICP), a marker of severe injury, may develop after TBI via several mechanisms including intracranial hemorrhage, cellular swelling, and blood-brain barrier disruption.4
ICP monitoring is used to detect elevated ICP and is required for goal-directed treatment of intracranial hypertension. Treatment of intracranial hypertension is known to improve outcomes in adults5; however, the pediatric evidence linking ICP monitoring and improved outcomes is less robust.6
Wide variation in treatment methods not explained by patient-level factors is a marker for opportunities for care improvement.7,8 The between-hospital variation in ICP monitoring for children with TBI in the United States (U.S.) is unknown. We suspected that variation in ICP monitoring might be present based on previous reports of significant between-hospital variation in ICP monitoring in Great Britain9 and wide hospital variation in ICP monitoring for meningitis in the U.S.10 We reported significant between-hospital variation in the use of a medical treatment, osmolar therapy, for children with TBI.11
Additionally, it is not known whether hospital pediatric TBI volume, hospital American College of Surgeons (ACS) pediatric trauma designation, and hospital ICP monitoring rate are associated with improved outcome in children with TBI. We evaluated these hospital factors because adults with severe injuries have better outcomes at large or level I trauma centers.12,13 Hospital experience and ACS certification requirements are factors that logically might be associated with both decreased variation in ICP monitoring and patient outcome.
This study describes between-hospital variation in ICP monitoring, evaluates whether hospital factors and ICP monitoring practices are associated with outcomes, and describes patient-level variation in ICP monitoring using a large, retrospective, severely head-injured cohort from the Pediatric Health Information System (PHIS) database.
Patients and Methods
Study Design
The PHIS database was developed by the Child Health Corporation of America (CHCA)14 (Shawnee Mission, KS). In order to define a retrospective cohort with severe TBI, we identified children who received care for TBI at a PHIS hospital and were mechanically ventilated for ≥ 96 consecutive hours or died in the first 4 days of hospitalization.
Setting
CHCA is a business alliance of 43 children’s hospitals, and PHIS contains administrative data including demographics, diagnoses, procedures, and charges. In addition, most PHIS hospitals submit “Level II” data including billing information for pharmacy, imaging, laboratory, supply, nursing, and therapy services.15 Inpatient data on 36 PHIS hospitals have been published previously.15 Conway et al described the extensive data reliability and quality monitoring processes for PHIS data.16
Selection of Participants
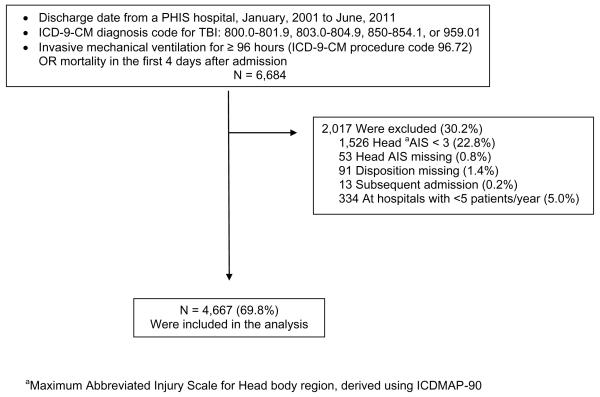
We obtained data from PHIS regarding patients who met our inclusion criteria and had supplemental billing (level II) data recorded (Figure 1). We identified children < 18 years of age discharged from a PHIS hospital between January, 2001 and June, 2011 with an International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) discharge diagnosis code for TBI (Figure 1). This set of ICD-9-CM diagnosis codes is used by the Centers for Disease Control to track hospitalization and Emergency Department visits for TBI rates nationally.17 To be included, patients were required to have either ICD-9-CM procedure code 96.72 (“continuous invasive mechanical ventilation for 96 consecutive hours or more”)18 or mortality in the first 4 days after admission.
Figure 1.
Patient selection method for children < 18 years old with traumatic brain injury (TBI)
We calculated injury severity score (ISS, or specifically, ICD/ISS) and maximum abbreviated injury scale (AIS) body region scores from ICD-9-CM diagnosis codes using ICDMAP-90 software (Johns Hopkins University and Tri-Analytics, Inc., Baltimore, MD).19 In order to refine a cohort with severe TBI, we excluded patients with maximum head body region AIS scores of less than 3 (“Serious”), patients with missing head AIS scores, patients with missing disposition, and subsequent admissions (Figure 1). We excluded hospitals with fewer than 5 patients per year (Figure 1).
We analyzed a less restrictive subset of the same cohort in earlier work.11
Covariates and Outcomes
We analyzed the study population by demographic characteristics, insurance status, injury characteristics and severity, inflicted injury, and cardiac arrest (Table 1). We dichotomized the admission date variable as before or after July, 2003, when the first guidelines for the care of severe pediatric TBI (which endorsed ICP monitor use in children) were published, to test the hypothesis that the guidelines would decrease care variation.20 These guidelines have recently been updated, and continue to endorse ICP monitor use.21 We also analyzed patients by the admission hospital’s ACS Pediatric Trauma designation22 and by hospital volume.
Table 1.
Select demographic and clinical features of pediatric traumatic brain injury cohort, by outcome
| Death or tracheostomy and gastrostomy, n(col%) N = 2,114(45) |
Survival without tracheostomy and gastrostomy, n(col%) N = 2,553(55) |
|
|---|---|---|
| Age | ||
| 0 to 364 days | 512(24) | 723(28) |
| 1 to <5 years | 662(31) | 595(23) |
| 5 to <13 years | 565(27) | 761(30) |
| 13 to <18 years | 375(18) | 474(19) |
| Gender | ||
| Male | 1,322(63) | 1,606(63) |
| Missing | 2(0) | 8(0) |
| Insurance status | ||
| Government | 1,166(55) | 1,416(55) |
| Private | 520(25) | 699(27) |
| Other | 404(19) | 407(16) |
| Missing | 24(1) | 31(1) |
| Admission Date | ||
| 10/2000-7/2003 | 482(23) | 590(22) |
| 8/2003-6/2011 | 1,632(77) | 1,993(78) |
| Cardiac Arresta | ||
| Any | 404(19) | 36(1) |
| Inflicted Injuryb | ||
| Yes | 658(31) | 715(28) |
| EDHc, no fracture | ||
| Yes | 24(1) | 50(2) |
| SAHd, no fracture | ||
| Yes | 317(15) | 253(10) |
| SDHe, no fracture | ||
| Yes | 575(27) | 617(24) |
| Craniotomy/Craniectomyf | ||
| Yes | 122(6) | 288(11) |
| Head AISg | ||
| 3 (“Serious”) | 278(13) | 1,249(49) |
| 4 (“Severe”) | 277(13) | 914(36) |
| 5 (“Critical”) | 1,559(74) | 390(15) |
| ICDISSh | ||
| < 15 | 184(9) | 855(33) |
| ≥ 15 | 1,930(91) | 1,698(67) |
| ACSi Trauma Level | ||
| I (15 hospitals) | 1,023(48) | 1,318(52) |
| II (2 hospitals) | 138(7) | 128(5) |
| None (14 hospitals) | 953(45) | 1,107(43) |
| Patients per hospital | ||
| <100 (8 hospitals) | 248(12) | 308(12) |
| 100-200 (15 hospitals) | 1,018(48) | 1,067(42) |
| >200 (8 hospitals) | 848(40) | 1,178(46) |
Column percentages may not add to 100% because of rounding
ICD-9-Cm discharge diagnosis code 427.5 or 997.1
Any of ICD-9-CM discharge diagnosis codes 995.5, E960-96843
EDH = epidural hematoma without skull fracture (ICD-9-CM diagnosis code 852.4 or 852.5)
SAH = subarachnoid hemorrhage without skull fracture (ICD-9-CM diagnosis code 852.0 or 852.1)
SDH = subdural hemorrhage without skull fracture (ICD-9-CM diagnosis code 852.2 or 852.3)
ICD-9-CM procedure code 01.24 or 01.25
Maximum Abbreviated Injury Scale score, Head body region, derived using ICDMAP-90
Injury Severity Score, derived using ICDMAP-90
American College of Surgeons Pediatric Trauma Designation: Level I, Level II, or None
The primary outcome was ICP monitoring, defined using Clinical Transaction Classification™ (CTC) codes or ICD-9-CM procedure codes (legend of Table 2).18,23,24 CTC codes reflect hospital billing, and can be used to identify services received by patients.15,16,25
Table 2.
Select demographic and clinical features of pediatric traumatic brain injury cohort, by intracranial pressure (ICP) monitoring
| ICP monitor, n(col%) N = 2,586(55) |
No ICP monitor, n(col%) N = 2,081(45) |
|
|---|---|---|
| Age | ||
| 0 to 364 days | 420(16) | 815(39) |
| 1 to <5 years | 751(29) | 506(24) |
| 5 to <13 years | 868(34) | 458(22) |
| 13 to <18 years | 547(21) | 302(15) |
| Gender | ||
| Male | 1,623(63) | 1,305(63) |
| Missing | 7(0) | 3(0) |
| Insurance status | ||
| Government | 1,341(52) | 1,241(60) |
| Private | 782(30) | 437(21) |
| Other | 437(17) | 374(18) |
| Missing | 26(1) | 29(1) |
| Admission Date | ||
| 10/2000-7/2003 | 601(23) | 441(21) |
| 8/2003-6/2011 | 1,985(77) | 1,640(79) |
| Cardiac Arresta | ||
| Any | 121(5) | 319(15) |
| Inflicted Injuryb | ||
| Yes | 571(22) | 802(39) |
| EDHc, no fracture | ||
| Yes | 47(2) | 27(1) |
| SAHd, no fracture | ||
| Yes | 258(10) | 312(15) |
| SDHe, no fracture | ||
| Yes | 576(22) | 616(30) |
| Craniotomy/Craniectomyf | ||
| Yes | 350(14) | 60(3) |
| Head AISg | ||
| 3 (“Serious”) | 961(37) | 566(27) |
| 4 (“Severe”) | 680(26) | 511(25) |
| 5 (“Critical”) | 945(37) | 1,004(48) |
| ICDISSh | ||
| < 15 | 683(26) | 356(17) |
| ≥ 15 | 1,903(74) | 1,725(83) |
| ACS Trauma Leveli | ||
| I (15 hospitals) | 1,369(53) | 972(47) |
| II (2 hospitals) | 126(5) | 140(7) |
| None (14 hospitals) | 1,091(42) | 969(47) |
| Patients per hospital | ||
| <100 (8 hospitals) | 226(9) | 330(16) |
| 100-200 (15 hospitals) | 1,144(44) | 941(45) |
| >200 (8 hospitals) | 1,216(47) | 810(39) |
Column percentages may not add to 100% because of rounding
ICP monitors were coded using any of ICD-9-CM procedure codes 01.18, 01.10, 02.2, and 02.39 or CTC codes for “ICP monitor supply” or “intracranial pressure monitoring”
ICD-9-CM discharge diagnosis code 427.5 or 997.1
Any of ICD-9-CM discharge diagnosis codes 995.5, E960-96843
EDH = epidural hematoma without skull fracture (ICD-9-CM diagnosis code 852.4 or 852.5)
SAH = subarachnoid hemorrhage without skull fracture (ICD-9-CM diagnosis code 852.0 or 852.1)
SDH = subdural hemorrhage without skull fracture (ICD-9-CM diagnosis code 852.2 or 852.3)
ICD-9-CM procedure code 01.24 or 01.25
Maximum Abbreviated Injury Scale score, Head body region, derived using ICDMAP-90
Injury Severity Score, derived using ICDMAP-90
American College of Surgeons Pediatric Trauma Designation: Level I, Level II, or None
We defined poor outcome as hospital mortality or placement of a new tracheostomy (ICD-9-CM procedure codes 31.1, 31.2x, or 31.74) and a new gastrostomy tube (ICD-9-CM procedure codes 43.1x, 46.32, or 46.39) during the hospitalization. Children receiving a tracheostomy and gastrostomy after TBI are likely severely disabled when discharged.
The patient-level factors in the multivariate models were specified a priori: patient age at admission (years, continuous), head AIS (categorical), ISS (continuous), inflicted injury, cardiac arrest, and craniotomy or craniectomy. We included age because children < 1 year old with TBI receive ICP monitoring less often than older children9,26, AIS as the best measure of head injury severity in this dataset and a factor associated with ICP monitoring27, ISS as a measure of global injury severity and likelihood of patient viability28 which is also associated with ICP monitoring27, inflicted injury to separate that effect from any age effect, cardiac arrest because it has been inversely associated with ICP monitoring26, and craniotomy/craniectomy because it may be associated with ICP monitor placement (as part of a single operating room course) and because it is recommended in children with intracranial mass lesions.29
Primary Data Analyses
We used the chi-square test for categorical variables and the Wilcoxon rank-sum test for continuous variables (patients per hospital).
In order to understand how much of the observed variation in ICP monitor use between hospitals could be attributed to patient factors (case mix at each hospital) versus hospital factors, we first standardized hospital-level rates of ICP monitoring using a population-averaged logistic model with the pre-specified covariates described above. From this model, we estimated a predicted probability of ICP monitoring for each patient, and then used these probabilities to calculate expected hospital ICP monitoring rates. We then calculated standardized ICP monitoring rates by comparing observed and expected ICP monitoring rates for each hospital in a manner similar to Weiss et al.15
We compared between-hospital variation by ACS trauma designation and hospital patient volume to the proportion of patients at each hospital with a poor outcome. Linear regression with robust standard errors was used to test the slopes of the poor outcome/time, ICP monitoring/time, outcome/variation, and outcome/volume relationships.
We then used a random-intercept logistic regression model including the same pre-specified covariates15,16 and the intraclass correlation coefficient to estimate variation in ICP monitoring between hospitals not related to patient factors.
To determine variation by patient-level characteristics, we used a multivariate logistic regression model with clustering by hospital, an exchangeable correlation structure, and robust standard errors calculated using generalized estimating equations (GEE). This model was populated with the same pre-specified covariates described above.
Statistical significance was defined as p < 0.05, and all analyses were performed using STATA™, versions 10 and 11 (StataCorp LP, College Station, TX). This study was reviewed and informed consent was not required by the University of Utah School of Medicine Institutional Review Board.
Results
Patients and Hospitals
We identified 6,684 patients with TBI and early mortality or at least 96 hours of mechanical ventilation, and 4,667 remained in the dataset after exclusions (Figure 1). In each month, a median of 37 (interquartile range (IQR) 32-42) patients were discharged from 31 PHIS hospitals. In-hospital mortality was 41.1% (1,919/4,667). The combined poor outcome rate was 45.3% (Table 1), and decreased slowly over time (highest 48.6% in 2002, lowest 42.1% in 2004, overall monthly decrease 0.014%, 95% CI 0.008 to 0.021%, p < 0.001). ACS level I hospitals (43.7%) had a similar poor outcome rate to hospitals without ACS designation (46.3%), Χ2 p = 0.088. Approximately 33% of the patients (1,555/4,667) died in the first four days of admission, and the remaining patients (3,112/4,667) were candidates for the study because they were ventilated for ≥ 96 consecutive hours.
Approximately 26% of the patients were < one year old, and the majority were male (Table 1). Nearly 10% (440/4,667) had a diagnosis of cardiac arrest, which was concentrated among infants (12.4%) and 1-5 year olds (11.4%) versus older age groups (6.9% school-age, 6.1% teenagers, Χ2 p < 0.001 across age groups). Approximately 29% of all patients and 71% of infants had a diagnosis of inflicted injury. Cardiac arrest occurred in 12.9% of the patients with inflicted injury versus 8.0% without inflicted injury, Χ2 p < 0.001. Most patients (77%) had an intracranial hemorrhage, and 51% had a skull fracture (not shown). Nearly 9% had a craniotomy or craniectomy, with 78% of each patient’s first such operation occurring on hospital day 0 (in PHIS, from the admission time until midnight that night) or hospital day 1 (the 24 hours after the first midnight of the hospitalization). Small percentages of those operations occurred on hospital day 2 (7%), day 3 (2%), and day 4 (2%), with 93% by the end of hospital day 7.
The median ISS score was 22 (IQR 16-26). All of the 11 patients with the maximum ISS score of 75 (“unsurvivable”) had the maximum Spine AIS score of 6, and 10/11 died. Most hospitals did not have an ACS Pediatric Trauma designation, and the overall median number of patients per hospital was 139 (range 57-350, IQR 85-201). The number of patients per hospital was not different between ACS level I hospitals (median 140, IQR 111-208) and hospitals without an ACS designation (median 137, IQR 72-149), Wilcoxon p = 0.432.
ICP Monitoring
Overall, 55% (2,586/4,667) of patients had documented ICP monitoring (Table 2). The ICP monitoring rate decreased slowly (monthly decrease 0.014%, 95% CI 0.007 to 0.021%, p < 0.001) over the 10.5 years of the study; annual ICP monitoring rates were lowest in 2011 (52.2%) and highest in 2002 (59.1%), with little change from guideline publication in 2003 (not shown). Most (88%) ICP monitors were placed on hospital day 0 or hospital day 1, but small fractions were placed on hospital days 2 (3.9%), 3 (2.1%), and 4 (1.0%). Of patients with ICP monitoring, 62% (1,597/2,586) had both an ICD-9-CM procedure code and a CTC code for a monitor, 25% (638/2,586) had only a procedure code, and 14% (351/2,586) had only a CTC code.
The relationships between patient demographic, injury, and treatment facility characteristics and ICP monitoring are shown in Table 2. Infants were significantly less likely to have ICP monitoring than older children (Χ2 p < 0.001).
Between-hospital variation
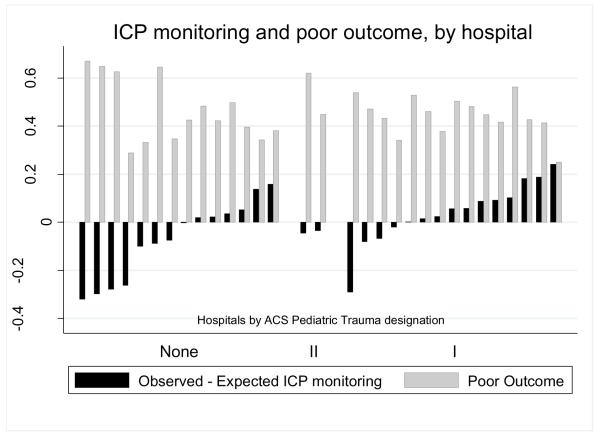
Expected rates of ICP monitoring by hospital after adjustment for age, cardiac arrest, inflicted injury, head AIS, ISS, and craniotomy/craniectomy were 47-60%, while observed rates varied from 14-83%. ICP monitoring occurred significantly more often at ACS pediatric trauma level I hospitals and hospitals with higher patient volumes (Χ2 p < 0.001 for differences across ACS trauma levels and hospital volume categories). Hospitals with higher standardized ICP monitoring rates had better patient outcomes (slope p < 0.001 overall and within ACS levels I and none, with too few ACS level II hospitals to test the within-level slope) (Figure 2). Hospitals without an ACS designation had the most variation in ICP monitoring and four of the five hospitals with the lowest standardized ICP monitoring rates had no ACS designation.
Figure 2.
Observed and expected intracranial pressure (ICP) monitoring rates and poor outcome (death or tracheostomy and gastrostomy), by hospital trauma designation
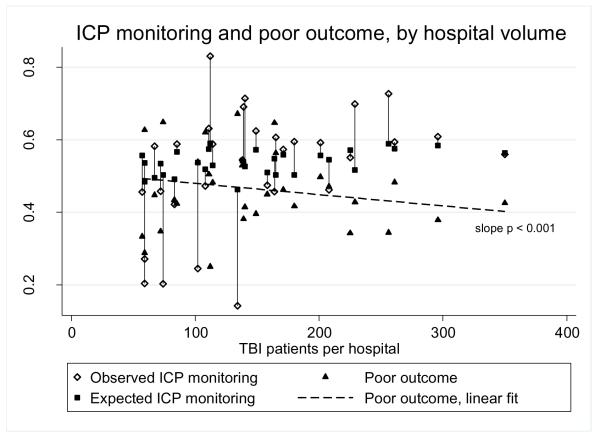
Hospitals with higher patient volume had less variation in standardized rates of ICP monitoring and modestly better patient outcomes (estimated decrease in poor outcomes 0.31% for each additional 10 patients, 95% CI 0.28% to 0.34%, p < 0.001) than hospitals with lower patient volume (Figure 3). These relationships did not change when the analysis was restricted to 2004-2011 (not shown), suggesting that the guidelines published in July, 2003 were not associated with changes in ICP monitoring variation.
Figure 3.
Observed and expected intracranial pressure (ICP) monitoring rates and poor outcome (death or tracheostomy and gastrostomy), by hospital volume
Using a random-intercept logistic model adjusted for the same pre-specified covariates, we estimated from the intraclass correlation coefficient that 12.7% (95% CI 7.7% to 20.4%) of the total variance in ICP monitoring is between-hospital variance not explained by identified patient factors.
Patient-level variation
Using GEE models, we found that age ≥ 1 year, the absence of a cardiac arrest, receipt of a craniotomy or craniectomy, head/neck AIS score of 4 versus 3, and a lower ISS were independently associated with ICP monitoring (Table 3). In an otherwise identical model with age dichotomized at one year, the adjusted OR for ICP monitoring in children ≥ one year versus less than one was 3.10 (95% CI 2.53 – 3.80).
Table 3.
Logistic regression model for intracranial pressure (ICP) monitoring, adjusted for clustering by hospital using generalized estimated equations
| ICP Monitoring aORa (95% CI) |
|
|---|---|
| Age | |
| 0 to 364 days | 1.00 (ref.) |
| 1 to <5 years | 2.95 (2.38-3.66) |
| 5 to <13 years | 3.38 (2.72-4.21) |
| 13 to <18 years | 3.21 (2.50-4.13) |
| Cardiac Arrest | |
| None | 1.00 (ref.) |
| Any | 0.36 (0.29-0.46) |
| Inflicted Injury | |
| No | 1.00 (ref.) |
| Yes | 0.86 (0.71-1.04) |
| Craniotomy/Craniectomy | |
| No | 1.00 (ref.) |
| Yes | 3.71 (2.65-5.19) |
| Head AISb | |
| 3 | 1.00 (ref.) |
| 4 | 1.37 (1.11-1.70) |
| 5 | 0.91 (0.72-1.16) |
| ICDISSc | |
| < 15 | 1.00 (ref.) |
| ≥ 15 | 0.54 (0.43-0.67) |
adjusted Odds Ratio
Maximum Abbreviated Injury Scale score, Head body region, derived using ICDMAP-90
Injury Severity Score, derived using ICDMAP-90
Discussion
In this large, multi-center database, we found significant between-hospital variation in ICP monitoring among children with TBI. Hospitals with higher standardized ICP monitoring rates and hospitals with higher patient volumes had better outcomes. ACS designation was not associated with better outcomes but was associated with less variation in monitoring. Infants (age < 1 year) were less likely to have ICP monitoring than older children.
After adjustment for patient factors, we estimated that 13% of the ICP monitoring variation was attributable to between-hospital variation. Publication of the 2003 pediatric TBI treatment guidelines did not appear to change ICP monitoring rates or variation in ICP monitoring, as we found little change over time. Similar to our findings, Bulger et al found broad between-hospital variation in ICP monitoring of adults in the U.S with TBI, and better outcomes at centers with greater ICP monitor use.30 Substantial between-hospital variation in ICP monitoring rates in children with TBI has also been reported in Great Britain; however, the variation-outcome relationship was not reported.9 Although the exact relationship between variation in hospital ICP monitoring rates and outcomes in children with TBI needs further study, it is likely that there are opportunities for improvement in the care of children with TBI.7
We found less variation in ICP monitoring at ACS level I hospitals and better outcomes at hospitals with less variation. ACS level, however, was not associated with better outcomes in a bivariate analysis. Similar to studies of adult TBI, we found better outcomes at hospitals with larger TBI patient volumes.12,13 Larger hospital volume is associated with better outcomes in children receiving critical care31 and has been associated with adherence to some pediatric quality of care guidelines.32 A recent British report did not find a significant relationship between hospital volume and outcome in pediatric TBI, although there was concerning variation in outcome according to pediatric neurosurgical availability.33 Pediatric neurosurgical availability was not present in our dataset but is required for ACS level 1 designation.
In our analysis of patient-level variation, we found that ICP monitoring was used less often in infants than in older children, after adjustment for all other independent predictors and between-hospital variation. The 2003 guideline recommendation for ICP monitoring includes infants, as open fontanelles or sutures “[do] not preclude the development of intracranial hypertension or negate the utility of ICP monitoring.”6 The factors contributing to lower use of ICP monitoring in infants are not known. Technical feasibility may be a factor, as the infant skull may not be structurally able to support some monitoring devices. Providers may place monitoring devices less frequently because of a perceived poor prognosis, as many infants have suffered inflicted injury and/or a cardiac arrest, both associated with worse outcomes.26,34
Our study has several limitations. Post-resuscitation Glasgow Coma Scale (GCS) score, pupillary exam, and head CT results, the most predictive measures of TBI severity, are not present in the PHIS database. We restricted our analysis using AIS scores and mechanical ventilation for ≥ 96 hours as proxies for severity, but they may not completely represent GCS-based severity of TBI; however, given the high mortality in our study cohort (41% versus 16-24% in studies selected by GCS), we were likely overly restrictive.35-38 The patients in our study were severely injured, representing the type of patients potentially eligible for ICP monitoring. In order to ensure that between-hospital variation in ICP monitoring was not a result of patient factors related to poor prognosis, we adjusted for head injury severity, overall injury severity, inflicted injury, and cardiac arrest as known predictors of poor outcome.
ICDMAP-90, the software package used to calculate AIS and ISS scores from ICD-9-CM diagnosis codes, has been validated in adults39 and in children40 for its ability to determine injury severity, and has been used in several pediatric TBI studies, including two from the PHIS database.11,41-43 Coates et al and Di Gennaro et al also defined their study populations using head AIS scores ≥3 in analyses of children with severe TBI.44,45 Relatively low correlation coefficients of approximately +/− 0.3 have been reported between AIS, ISS, and GCS in adults with TBI46,47, but each add independently and significantly to functional outcome prediction.47 Other limitations include that ICD-9-CM diagnosis codes for TBI do not allow ideal categorization of intracranial hemorrhages48, and that changes in hospital ACS trauma designation are not shown in the publicly available trauma center list.22
In conclusion, there is significant between-hospital variation in ICP monitoring that is unlikely to be due solely to differences in case mix. Hospitals that monitor ICP more often and hospitals with higher patient volumes had better patient outcomes, although a causal relationship between monitoring and improved outcome cannot be inferred from this analysis. Infants are less likely to receive ICP monitoring than older children. The between-hospital variation suggests opportunities to improve the quality of pediatric TBI neurocritical care.
Acknowledgements
We would like to thank Lawrence J. Cook, PhD, Intermountain Injury Control and Research Center, University of Utah, for his assistance with ICDMAP-90. Dr. Bennett had full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis. Funding: Dr. Bennett is partially supported by the Mentored Scholars Program for Translational Comparative Effectiveness Research, NIH/NCI Grant Number 1KM1CA156723.
Footnotes
Conflict of Interest: None
References
- 1.Rutland-Brown W, Langlois JA, Thomas KE, Xi YL. Incidence of traumatic brain injury in the United States, 2003. J Head Trauma Rehabil. 2006;21(6):544–8. doi: 10.1097/00001199-200611000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21(5):375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Anderson VA, Catroppa C, Haritou F, Morse S, Rosenfeld JV. Identifying factors contributing to child and family outcome 30 months after traumatic brain injury in children. J Neurol Neurosurg Psychiatry. 2005;76(3):401–8. doi: 10.1136/jnnp.2003.019174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kochanek PM, et al. Severe Traumatic Brain Injury in Infants and Children. In: Fuhrman BP, Zimmerman Jerry, editors. Pediatric Critical Care. 3rd ed Mosby Elsevier; Philadelphia, PA: 2006. pp. 1596–617. [Google Scholar]
- 5.Bratton SL, Chestnut RM, Ghajar J, McConnell Hammond FF, Harris OA, Hartl R, et al. Guidelines for the management of severe traumatic brain injury. VI. Indications for intracranial pressure monitoring. J Neurotrauma. 2007;24(Suppl 1):S37–44. doi: 10.1089/neu.2007.9990. [DOI] [PubMed] [Google Scholar]
- 6.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 5. Indications for intracranial pressure monitoring in pediatric patients with severe traumatic brain injury. Pediatr Crit Care Med. 2003;4(3 Suppl):S19–24. [PubMed] [Google Scholar]
- 7.Berwick DM. Controlling variation in health care: a consultation from Walter Shewhart. Med Care. 1991;29(12):1212–25. doi: 10.1097/00005650-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Goodman DC. Unwarranted variation in pediatric medical care. Pediatr Clin North Am. 2009;56(4):745–55. doi: 10.1016/j.pcl.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morris KP, Forsyth RJ, Parslow RC, Tasker RC, Hawley CA. Intracranial pressure complicating severe traumatic brain injury in children: monitoring and management. Intensive Care Med. 2006;32(10):1606–12. doi: 10.1007/s00134-006-0285-4. [DOI] [PubMed] [Google Scholar]
- 10.Odetola FO, Tilford JM, Davis MM. Variation in the use of intracranial-pressure monitoring and mortality in critically ill children with meningitis in the United States. Pediatrics. 2006;117(6):1893–900. doi: 10.1542/peds.2005-2179. [DOI] [PubMed] [Google Scholar]
- 11.Bennett TD, Statler KD, Korgenski EK, Bratton SL. Osmolar therapy in pediatric traumatic brain injury. Crit Care Med. 2012;40(1):208–15. doi: 10.1097/CCM.0b013e31822e9d31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demetriades D, Martin M, Salim A, Rhee P, Brown C, Chan L. The effect of trauma center designation and trauma volume on outcome in specific severe injuries. Ann Surg. 2005;242(4):512–7. doi: 10.1097/01.sla.0000184169.73614.09. discussion 17-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nathens AB, Jurkovich GJ, Maier RV, Grossman DC, MacKenzie EJ, Moore M, et al. Relationship between trauma center volume and outcomes. JAMA. 2001;285(9):1164–71. doi: 10.1001/jama.285.9.1164. [DOI] [PubMed] [Google Scholar]
- 14.Child Health Corporation of America [Accessed January 4th, 2011]; Available at http://www.chca.com/index_no_flash.html.
- 15.Weiss PF, Klink AJ, Hexem K, Burnham JM, Leonard MB, Keren R, et al. Variation in inpatient therapy and diagnostic evaluation of children with Henoch Schonlein purpura. J Pediatr. 2009;155(6):812–18. e1. doi: 10.1016/j.jpeds.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conway PH, Keren R. Factors associated with variability in outcomes for children hospitalized with urinary tract infection. J Pediatr. 2009;154(6):789–96. doi: 10.1016/j.jpeds.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Langlois JA, Rutland-Brown W, Thomas KE. In: Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations, and Deaths. Division of Injury Response Center for Disease Control and Prevention, editor. U.S. Department of Health and Human Services; 2006. [Google Scholar]
- 18.Child Health Corporation of America [Accessed: December 10, 2010];ICD-9-CM Tabular List of Procedures (FY2010) Available at https://sharepoint.chca.com/CHCAForums/PerformanceImprovement/PHIS/ReferenceLibrary/Lists/ICD9 Code Books/AllItems.aspx.
- 19.Tri-Analytics, Inc. Johns Hopkins University . ICDMAP-90 Software User’s Guide. 1997. [Google Scholar]
- 20.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 1: Introduction. Pediatr Crit Care Med. 2003;4(3 Suppl):S2–4. doi: 10.1097/01.CCM.0000066600.71233.01. [DOI] [PubMed] [Google Scholar]
- 21.Kochanek PM, Carney N, Adelson PD, Ashwal S, Bell MJ, Bratton S, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents--second edition. Pediatr Crit Care Med. 2012;13(Suppl 1):S1–82. doi: 10.1097/PCC.0b013e31823f435c. [DOI] [PubMed] [Google Scholar]
- 22.American College of Surgeons Committee on Trauma, Verified Trauma Centers [Accessed February 23, 2012]; Available at http://www.facs.org/trauma/verified.html.
- 23.Child Health Corporation of America: CTC™ [Accessed December 10, 2010];Medical Supplies, Tabular Index. 2010 Available at https://sharepoint.chca.com/CHCAForums/PerformanceImprovement/PHIS/ReferenceLibrary/CTC Resources/Forms/AllItems.aspx.
- 24.Child Health Corporation of America [Accessed: December 10, 2010];Conversion Table of New ICD-9-CM Codes, October 2009. Available at https://sharepoint.chca.com/CHCAForums/PerformanceImprovement/PHIS/ReferenceLibrary/Lists/ICD9 Code Books/AllItems.aspx.
- 25.Goldin AB, Sawin RS, Garrison MM, Zerr DM, Christakis DA. Aminoglycoside-based triple-antibiotic therapy versus monotherapy for children with ruptured appendicitis. Pediatrics. 2007;119(5):905–11. doi: 10.1542/peds.2006-2040. [DOI] [PubMed] [Google Scholar]
- 26.Keenan HT, Nocera M, Bratton SL. Frequency of intracranial pressure monitoring in infants and young toddlers with traumatic brain injury. Pediatr Crit Care Med. 2005;6(5):537–41. doi: 10.1097/01.PCC.0000164638.44600.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tilford JM, Simpson PM, Yeh TS, Lensing S, Aitken ME, Green JW, et al. Variation in therapy and outcome for pediatric head trauma patients. Crit Care Med. 2001;29(5):1056–61. doi: 10.1097/00003246-200105000-00037. [DOI] [PubMed] [Google Scholar]
- 28.Tepas JJ, 3rd, Celso BG, Leaphart CL, Graham D. Application of International Classification Injury Severity Score to National Surgical Quality Improvement Program defines pediatric trauma performance standards and drives performance improvement. J Trauma. 2009;67(1):185–8. doi: 10.1097/TA.0b013e3181a5f03c. discussion 88-9. [DOI] [PubMed] [Google Scholar]
- 29.Adelson PD, Bratton SL, Carney NA, Chesnut RM, du Coudray HE, Goldstein B, et al. Guidelines for the acute medical management of severe traumatic brain injury in infants, children, and adolescents. Chapter 17. Critical pathway for the treatment of established intracranial hypertension in pediatric traumatic brain injury. Pediatr Crit Care Med. 2003;4(3 Suppl):S65–7. [PubMed] [Google Scholar]
- 30.Bulger EM, Nathens AB, Rivara FP, Moore M, MacKenzie EJ, Jurkovich GJ. Management of severe head injury: institutional variations in care and effect on outcome. Crit Care Med. 2002;30(8):1870–6. doi: 10.1097/00003246-200208000-00033. [DOI] [PubMed] [Google Scholar]
- 31.Tilford JM, Simpson PM, Green JW, Lensing S, Fiser DH. Volume-outcome relationships in pediatric intensive care units. Pediatrics. 2000;106(2 Pt 1):289–94. doi: 10.1542/peds.106.2.289. [DOI] [PubMed] [Google Scholar]
- 32.McLeod L, French B, Dai D, Localio R, Keren R. Patient volume and quality of care for young children hospitalized with acute gastroenteritis. Arch Pediatr Adolesc Med. 2011;165(9):857–63. doi: 10.1001/archpediatrics.2011.132. [DOI] [PubMed] [Google Scholar]
- 33.Tasker RC, Fleming TJ, Young AE, Morris KP, Parslow RC. Severe head injury in children: intensive care unit activity and mortality in England and Wales. Br J Neurosurg. 2011;25(1):68–77. doi: 10.3109/02688697.2010.538770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keenan HT, Runyan DK, Marshall SW, Nocera MA, Merten DF. A population-based comparison of clinical and outcome characteristics of young children with serious inflicted and noninflicted traumatic brain injury. Pediatrics. 2004;114(3):633–9. doi: 10.1542/peds.2003-1020-L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zebrack M, Dandoy C, Hansen K, Scaife E, Mann NC, Bratton SL. Early resuscitation of children with moderate-to-severe traumatic brain injury. Pediatrics. 2009;124(1):56–64. doi: 10.1542/peds.2008-1006. [DOI] [PubMed] [Google Scholar]
- 36.Ducrocq SC, Meyer PG, Orliaguet GA, Blanot S, Laurent-Vannier A, Renier D, et al. Epidemiology and early predictive factors of mortality and outcome in children with traumatic severe brain injury: experience of a French pediatric trauma center. Pediatr Crit Care Med. 2006;7(5):461–7. doi: 10.1097/01.PCC.0000235245.49129.27. [DOI] [PubMed] [Google Scholar]
- 37.White JR, Farukhi Z, Bull C, Christensen J, Gordon T, Paidas C, et al. Predictors of outcome in severely head-injured children. Crit Care Med. 2001;29(3):534–40. doi: 10.1097/00003246-200103000-00011. [DOI] [PubMed] [Google Scholar]
- 38.Hutchison JS, Ward RE, Lacroix J, Hebert PC, Barnes MA, Bohn DJ, et al. Hypothermia therapy after traumatic brain injury in children. N Engl J Med. 2008;358(23):2447–56. doi: 10.1056/NEJMoa0706930. [DOI] [PubMed] [Google Scholar]
- 39.Expert Group on Injury Severity Measurement . Discussion document on injury severity measurement in administrative datasets. Center for Disease Control and Prevention, U.S. Department of Health and Human Services; [Accessed January 5th, 2011]. Available at http://www.cdc.gov/nchs/data/injury/DicussionDocu.pdf. [Google Scholar]
- 40.Durbin DR, Localio AR, MacKenzie EJ. Validation of the ICD/AIS MAP for pediatric use. Inj Prev. 2001;7(2):96–9. doi: 10.1136/ip.7.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tilford JM, Aitken ME, Anand KJ, Green JW, Goodman AC, Parker JG, et al. Hospitalizations for critically ill children with traumatic brain injuries: a longitudinal analysis. Crit Care Med. 2005;33(9):2074–81. doi: 10.1097/01.ccm.0000171839.65687.f5. [DOI] [PubMed] [Google Scholar]
- 42.Bowman SM, Bird TM, Aitken ME, Tilford JM. Trends in hospitalizations associated with pediatric traumatic brain injuries. Pediatrics. 2008;122(5):988–93. doi: 10.1542/peds.2007-3511. [DOI] [PubMed] [Google Scholar]
- 43.Wood JN, Hall M, Schilling S, Keren R, Mitra N, Rubin DM. Disparities in the evaluation and diagnosis of abuse among infants with traumatic brain injury. Pediatrics. 2010;126(3):408–14. doi: 10.1542/peds.2010-0031. [DOI] [PubMed] [Google Scholar]
- 44.Coates BM, Vavilala MS, Mack CD, Muangman S, Suz P, Sharar SR, et al. Influence of definition and location of hypotension on outcome following severe pediatric traumatic brain injury. Crit Care Med. 2005;33(11):2645–50. doi: 10.1097/01.ccm.0000186417.19199.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Gennaro JLMC, Malakouti A, Zimmerman JJ, Armstead W, Monica S. Vavilala. Use and Effect of Vasopressors after Pediatric Traumatic Brain Injury. Developmental Neuroscience. 2010;32(5-6):420–30. doi: 10.1159/000322083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demetriades D, Kuncir E, Murray J, Velmahos GC, Rhee P, Chan L. Mortality prediction of head Abbreviated Injury Score and Glasgow Coma Scale: analysis of 7,764 head injuries. J Am Coll Surg. 2004;199(2):216–22. doi: 10.1016/j.jamcollsurg.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 47.Foreman BP, Caesar RR, Parks J, Madden C, Gentilello LM, Shafi S, et al. Usefulness of the abbreviated injury score and the injury severity score in comparison to the Glasgow Coma Scale in predicting outcome after traumatic brain injury. J Trauma. 2007;62(4):946–50. doi: 10.1097/01.ta.0000229796.14717.3a. [DOI] [PubMed] [Google Scholar]
- 48.United States Health Care Financing Administration . The International Classification of Diseases, 9th revision, clinical modification: ICD-9-CM. 7th ed U.S. Dept. of Health and Human Services, Public Health Service; Washington, D.C.: 2007. [Google Scholar]