Abstract
Background
Surgical quality improvement requires well-defined benchmarks and accurate reporting of postoperative adverse events, which have not been well defined for total gastrectomy.
Study Design
Detailed post-operative outcomes on 238 patients who underwent curative intent total gastrectomy from 2003 – 2012 were reviewed by a dedicated surgeon chart reviewer to establish the 90-day patterns of adverse events.
Results
Of the 238 patients with stage I – III gastric adenocarcinoma who underwent curative intent total gastrectomy, the median age was 66, and 68% were male. Median body mass index was 28, and 68% of patients had at least one medical comorbidity. Forty three percent of our patients received neoadjuvant chemotherapy, and 34% received post-operative adjuvant chemotherapy. Over the 90 day study period, 30-day mortality was 2.5% (6/238), and 90-day mortality was 2.9% (7/238). At least one post-operative adverse event was documented in 62% of patients, with 28% of patients experiencing a major adverse event requiring invasive intervention. The readmission rate was 20%. Anemia was the most common adverse event (20%), followed by wound complications (18%). The most common major adverse event was esophageal anastomotic leak, which required invasive intervention in 10% of patients.
Conclusion
This analysis has defined comprehensive 90-day patterns in post-operative adverse events following curative intent total gastrectomy in a Western population. This benchmark allows surgeons to measure, compare, and improve outcomes and informed consent for this surgical procedure.
Keywords: Gastric Cancer, Total Gastrectomy, Surgical Secondary Events, Surgical Adverse Events, Surgical Quality Improvement
Introduction
Gastric cancer is the second leading cause of cancer related deaths globally (1), with over 21,000 new cases diagnosed annually in the United States (2). Advanced gastric cancer is often treated with a combination of chemotherapy and surgery. In recent trials of adjuvant chemotherapy for advanced gastric cancer only 48 – 67% of patients intended to receive post-operative chemotherapy based upon pathologic stage received this therapy(3–5), in part due to the occurrence of surgical complications. Esophageal anastomotic leak, a serious complication, has been shown to significantly decrease disease-specific survival following curative intent total gastrectomy (6).
Total gastrectomy (TG) is frequently used for tumors of the proximal one-third of the stomach or patients with a tumor diffusely involving the stomach (7), and accounts for approximately 30% of all gastric cancer resections in the United States (8, 9). The post-operative adverse event profile remains poorly described. Published series’ differ widely on post-operative morbidity (9 – 46%) and mortality rates (1.1 – 10.8%) (3, 8–16). The definition of specific adverse events vary widely between the series’ as there are no standardized definitions for the events, and neither the timing of the events nor their severity are consistently reported, hampering accurate comparison between studies.
There is no uniform system to report specific post-operative outcomes following total gastrectomy. Despite its initial publication in 1992 (17), and modification in 2004 (18, 19), the Clavien-Dindo classification remains incompletely adopted in the surgical literature. Numerous studies define post-operative adverse events as “minor or major” or “significant or insignificant” without providing the criteria upon how an AE is classified. In 2002 Martin et al (20) proposed ten critical elements of accurate and comprehensive reporting of surgical adverse events. The aim of this study is to accurately define the post-operative morbidity and mortality of TG using a formal, well defined and validated measure of post-operative adverse events to provide a benchmark for further reports in this area.
Methods
In 2001 a prospective surgical secondary events database was established at Memorial Sloan Kettering Cancer Center to track post-operative adverse events. Data collection methods have been described elsewhere (21, 22). Briefly, adverse events, graded on a modification of the Clavien – Dindo classification, are added at the point of care by residents, fellows, and attending surgeons, and reviewed for completeness and accuracy at service morbidity and mortality conferences. The database uses a growing list of standardized definitions, 190 at the onset of the database and over 220 currently, and adverse events are graded on a 1–5 scale of increasing severity (Table 1). Events are defined as major if they are Grade 3 or higher. A full list of definitions and their corresponding grades is available for review and download at www.mskcc.org/sse.
Table 1.
Definitions of common adverse events following total gastrectomy.
| Common Adverse Event | Definition | Grades |
|---|---|---|
| Esophageal Anastomotic Leak | Clinical signs and symptoms or radiologic confirmation of esophageal anastomotic leak, without development of fistula | 1: Bedside Care or Oral Medication 2: Intervenous Medications or Transfusion 3: Radiologic, endoscopic, or operative intervention required 4: Resulting in chronic disability or organ resection 5: Death |
| Intra-Abdominal Abscess | Clinical signs and symptoms or radiologic diagnosis of intraabdominal abscess or peritonitis without evidence of an anastomotic leak | |
| Duodenal Stump Leak | Clinical signs and symptoms or radiologic confirmation of duodenal stump leak, without development of fistula |
After obtaining IRB approval, our prospectively maintained gastric cancer database was queried for all patients who underwent a curative intent total gastrectomy over ten years, from January 1, 2003 through December 31, 2012. Post-operative adverse events on these patients were collected from our departmental database. Patients were excluded if they underwent completion total gastrectomy for recurrent gastric cancer. Medical records for all 238 patients in our cohort were reviewed by a dedicated surgeon reviewer, and no patients were lost to follow-up within 90 days. Adverse events that occurred within 90 days of operation were recorded according to the definitions and grades of our SSE database. All statistical analyses were conducted on Stata12 (StataCorp, College Station, TX).
Results
Patient demographics
Between January 1, 2003 and December 31, 2012, 238 patients underwent total gastrectomy with curative intent at our institution. The majority of these gastrectomies (229, 95%) we performed for sporadic primary gastric cancer, the remainder (13, 5%) were performed for hereditary diffuse gastric cancer, and all of these patients had foci of gastric adenocarcinoma in their final pathologic specimens. Patient and operative demographics are shown in Table 2, and final pathology is shown in Table 3.
Table 2.
Clinical and operative demographics on all patients in the cohort.
| Age | 65 (53, 72) |
| Pre-Operative BMI | 27 (24.4, 30.5) |
| Male Gender | 162 (68%) |
| Diabetic | 39 (17%) |
| Diabetic on Oral Medicine | 33 (14% of cohort) |
| Diabetic on Insulin | 6 (2.5% of cohort) |
| Hypertension requiring medication | 102 (43%) |
| Hypercholesterolemia requiring medication | 71 (30%) |
| Active Smoker | 25 (11%) |
| Previous Cardiac Event | 30 (13%) |
| Received Neoadjuvant Chemotherapy | 103 (43%) |
| Received Adjuvant Chemotherapy | 80 (34%) |
| Operative Approach | |
| Open | 219 (92%) |
| Minimally Invasive | 19 (7.9%) |
| Reconstruction Method | |
| Circular Stapler | 115 (48.3%) |
| Linear Stapler | 104 (44%) |
| Hand Sewn Anastomosis | 19 (8%) |
Continuous variables are expressed as median (IQR) and categorical variables as N (%).
Table 3.
Final pathologic staging for all patients in the cohort.
| Final Pathological Stage |
Entire Cohort (%) |
Received Neoadjuvant Chemotherapy (N = 103, 43%) |
No neoadjuvant chemotherapy (N = 135, 57%) |
|---|---|---|---|
| T Stage | |||
| T0 | 10 (4.2%) | 9 (8.7%)* | 1 (0.7%)** |
| T1 | 63 (26%) | 7 (6.8%) | 56 (41%) |
| T2 | 26 (11%) | 13 (13%) | 13 (10%) |
| T3 | 68 (29%) | 43 (42%) | 25 (19%) |
| T4 | 71 (30%) | 31 (30%) | 40 (30%) |
| N Stage | |||
| N0 | 116 (49%) | 46 (45%) | 70 (52%) |
| N1 | 49 (21%) | 24 (23%) | 25 (19%) |
| N2 | 28 (12%) | 13 (13%) | 15 (11%) |
| N3 | 45 (19%) | 20 (19%) | 25 (19%) |
| M Stage | |||
| M0 | 237 (100%) | 102 (99%) | 135 (100%) |
| M1 | 1 (0.4%) | 1 (1.0%)*** | 0 (0%) |
All patients received neoadjuvant chemotherapy and had a complete pathological response
Patient had signet cell adenocarcinoma on specimen from pre-operative endoscopic biopsy, but no identifiable adenocarcinoma on final pathology, including at the biopsy site.
One patient had a previously undetected omental implant detected in the surgical specimen after curative intent total gastrectomy.
Post-Operative Course
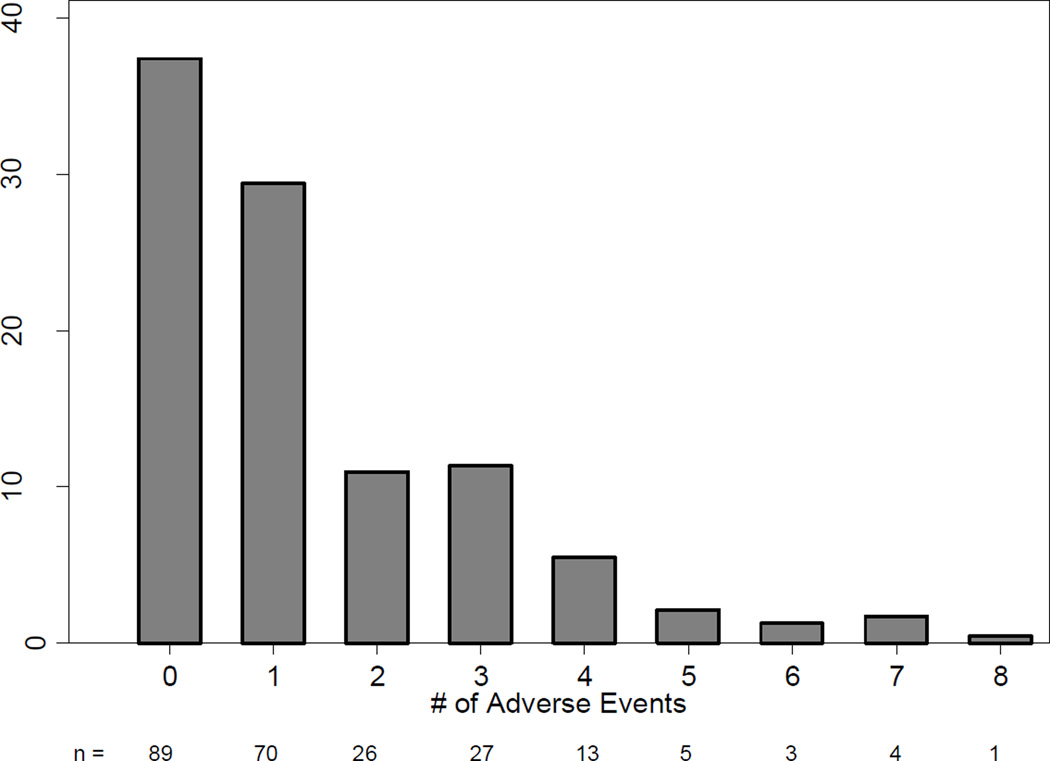
The majority of patients (52%, 123/238) were discharged without an AE. Of these patients, 33 (27%) had a post-discharge adverse event, leaving 37% of our patients (92/238) without a documented adverse event for the 90-day study period. Another 69 patients (28%) suffered a single adverse event. Twenty eight percent of our patients suffered a major adverse event. Figure 1 shows the distribution of the number of post-operative AEs per patient in the 90-day study period, and Figure 2 shows the distribution of maximum grade of AE per patient for this same period.
Figure 1.
Number of adverse events / patient in 90-day follow-up following Total Gastrectomy. Bar legends indicate the number of patients in each bar.
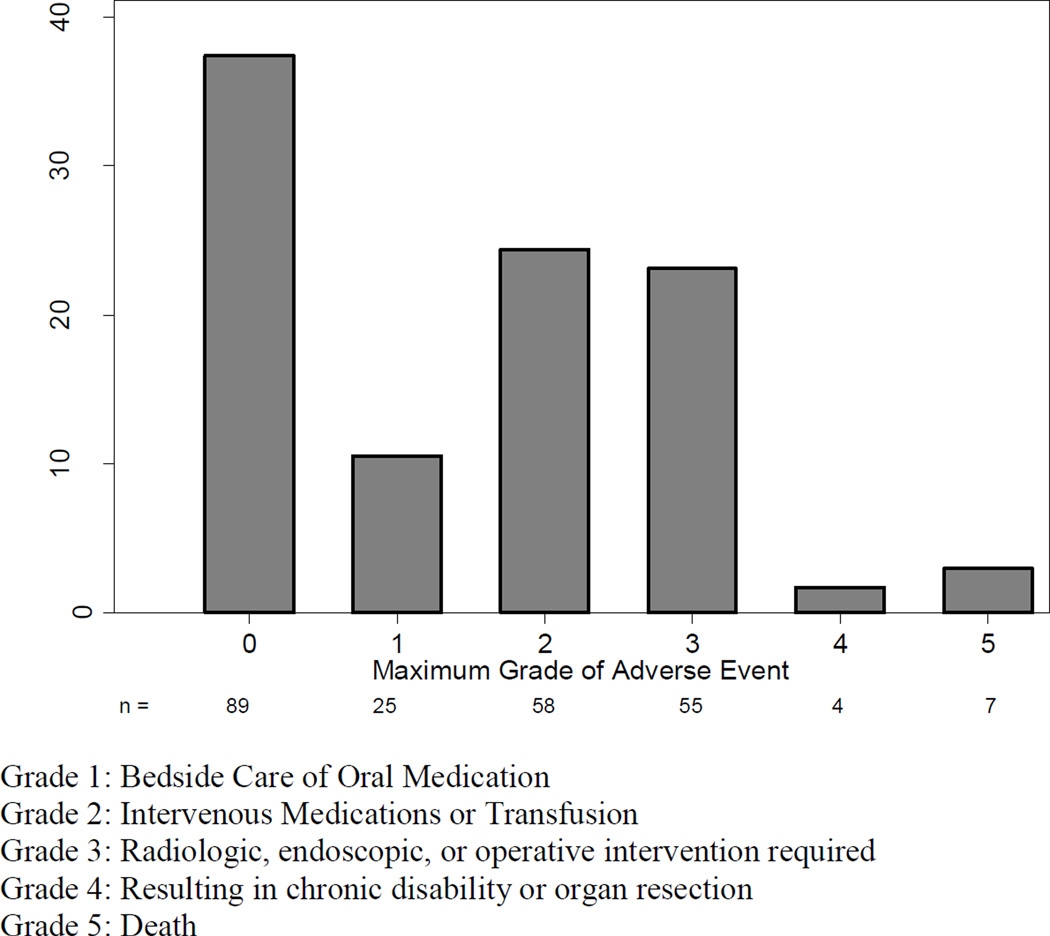
Figure 2.
Maximum Adverse Event grade in 90-day follow-up following Total Gastrectomy.
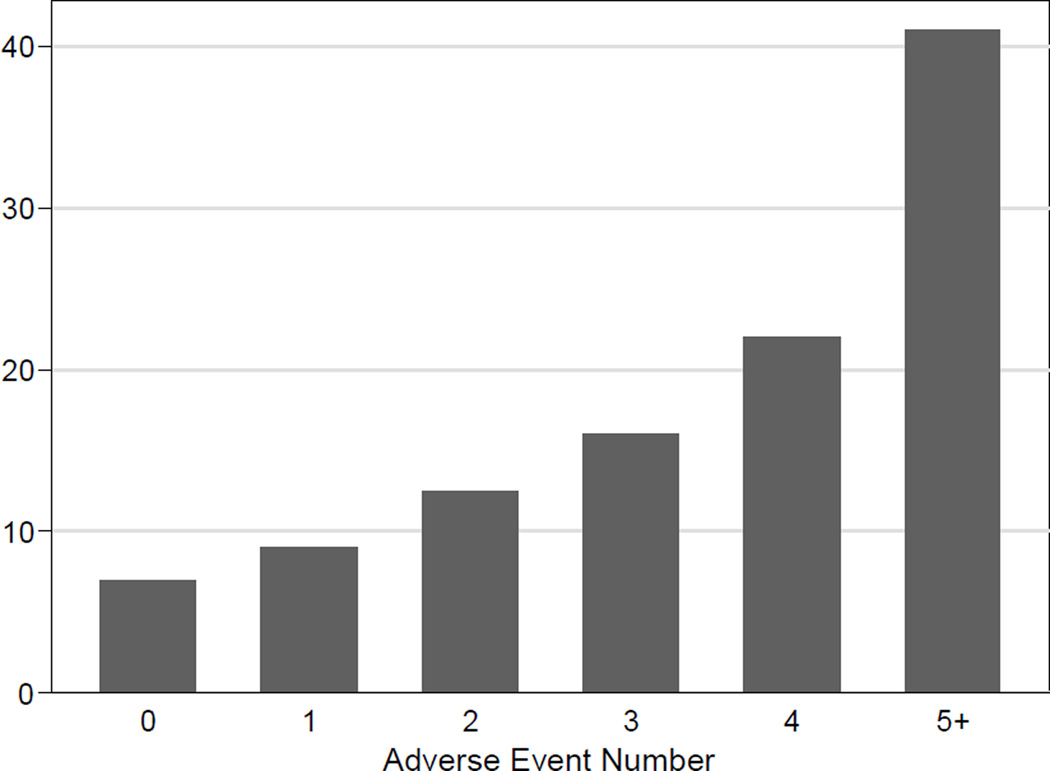
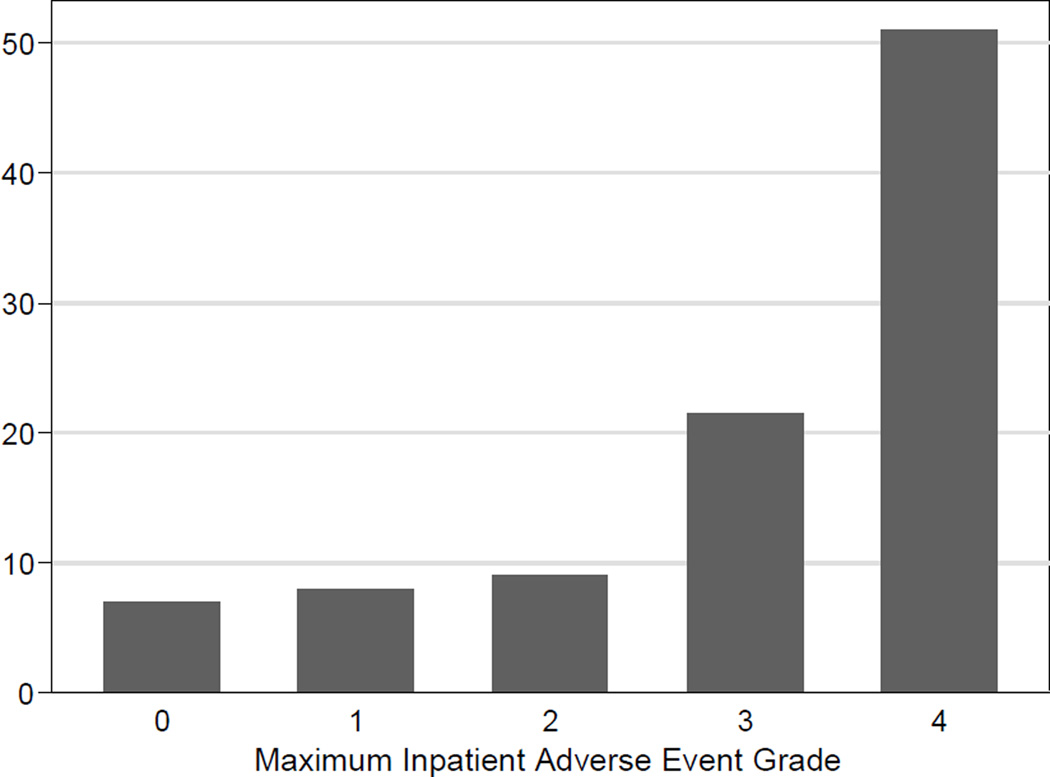
The median length of stay for the entire cohort was nine days, (range 4 – 71 days) (Figure 2). The 30-day mortality was 2.5% (6/238), and 90-day mortality was 2.9% (7/238). Six of the seven mortalities were directly related to the operative procedure: three patients succumbed to sepsis caused by duodenal stump leaks, two from sepsis after esophagojejunal anastomotic leaks, and the sixth to intraabdominal sepsis from an unclear source. The seventh patient had massive carotid artery bleed related to a prior oropharyngeal cancer and died from this event. Figure 3 shows length of stay (LOS) associated with maximum inpatient adverse event number, and Figure 4 shows LOS associated with maximum inpatient adverse event grade.
Figure 3.
Length of Stay associated with adverse event number during index hospitalization.
Figure 4.
Length of Stay associated with maximum adverse event grade during initial hospitalization
Specific Adverse Events
The most common AE was anemia requiring a blood transfusion in the absence of clinically apparent active bleeding (20% of patients), followed by a composite adverse event of wound infection, cellulitis, wound breakdown, seroma, and skin abscess that occurred in 18% of our patients. Table 4 shows the most common categorized adverse events recorded for our cohort; the full list is available as an appendix. Definitions for common intra-abdominal adverse events are listed in Table 1.
Table 4.
Ten most common categorized post-operative adverse event following Total Gastrectomy. Select definitions are listed in Table 1; a full list of adverse event definitions and grades is available at www.mskcc.org/sse
| Categorized Event | Frequency | % of Patients | % Grade 3+ |
|---|---|---|---|
| Anemia | 48 | 20.2% | 0.0% |
| Wound Complication* | 44 | 18.5% | 2.3% |
| Anastomotic Leak, Esophagus | 35 | 14.7% | 71.4% |
| Grade 1 – 2 Anastomotic Leak | 10 | 4.2% | 0% |
| Grade 3 – 5 Anastomotic Leak | 25 | 10.5% | 100% |
| Pulmonary Complication** | 34 | 14.3% | 26.5% |
| Cardiac Arrhythmia*** | 23 | 9.7% | 0.0% |
| DVT / PE | 15 | 6.3% | 13.3% |
| Intra-Abdominal Infection Or Abscess | 10 | 4.2% | 80.0% |
| Altered Level Of Consciousness**** | 10 | 4.2% | 0.0% |
| Duodenal Stump Leak | 9 | 3.8% | 77.8% |
| Hemorrhage | 8 | 3.4% | 62.5% |
Wound Complication includes Wound Infection, Cellulitis, Wound Breakdown, Seroma, Skin Abscess
Pulmonary Complication includes Pleural Effusion, Hypoxia, Pneumonia, Atelectasis, and Pneumonitis
Cardiac Arrhythmia includes Supraventricular Arrhythmia, Afib, Bradycardia, Ventricular Arrhythmia, and Tachycardia
Altered Level of Consciousness includes Depressed Level of Consciousness and Psychosis, Confusion, or Depression.
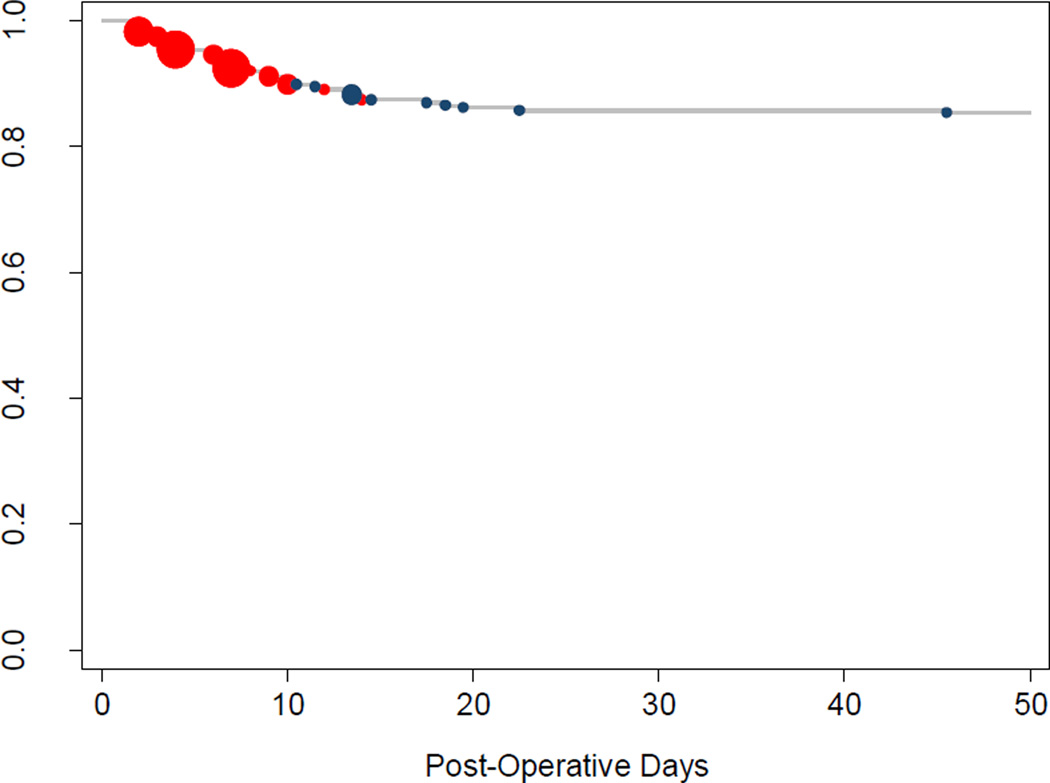
There were 35 esophageal anastomotic leaks (14% total), ten of which (5% overall incidence, 29% of leaks) were Grade 1 or 2 adverse events, meaning they did not require invasive treatment. The remaining 25 leaks (10% overall incidence, 71% of all leaks) were Grade 3 or higher. The majority of the esophageal leaks (25 / 35, 71%) were diagnosed prior to the patient’s initial discharge; ten (29% of all leaks) were diagnosed after discharge (Figure 5). With the exception of a single anastomotic leak diagnosed at post-operative day (POD) #45, all anastomotic leaks were detected within the first 22 post-operative days (median POD# 7.5; figure 3). The overall median LOS for patients whose leak was diagnosed prior to discharge was 29 days (range 5 – 71); the median LOS for minor leaks (Grade 1 – 2) diagnosed prior to discharge was 17 days, and the median LOS for major leaks (Grade 3+) diagnosed prior to discharge was 41 days. There was no statistically significant difference in the rate of esophageal leak between patients who received pre-operative chemotherapy (12%, 12 / 103) and those who did not receive pre-operative chemotherapy (17%, 23 / 135) (5% absolute difference; 95% CI −3.5% – 14%, p = 0.2), nor was there a statistically significant leak rate based on method of constructing the esophageal-jejunal anastomosis (p = 0.8).
Figure 5.
Timing of Esophageal Anastomotic Leaks. Red circles denote leaks that were diagnosed during the initial hospitalization (25 / 35) and blue circles denote leaks that were diagnosed after discharge (10/35). The size of individual circles is proportional to the number of leaks diagnosed on that particular day. All but one leak occurred by POD #22.
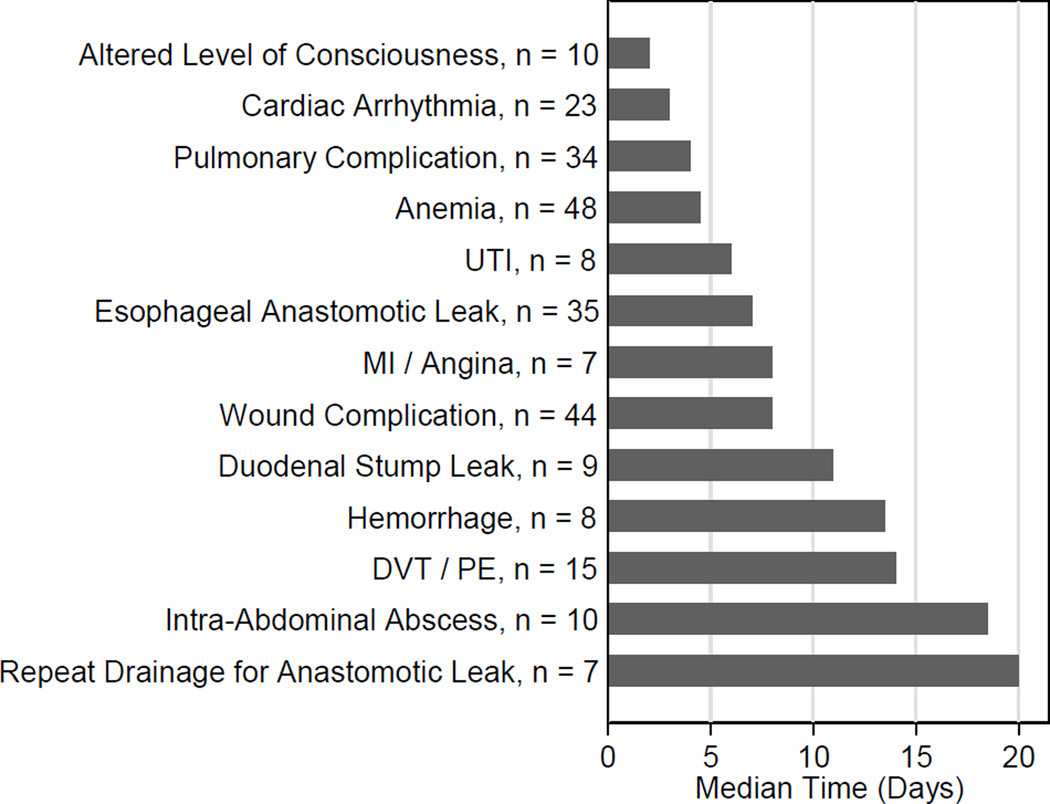
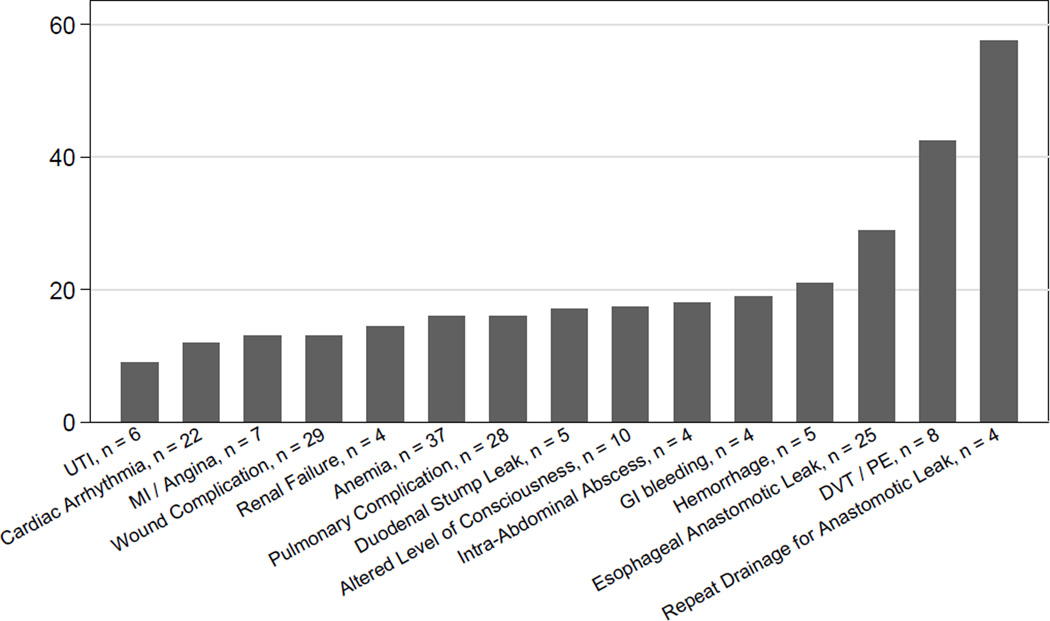
Nine patients (4%) suffered a duodenal stump leak; the median date of detection was the 11th post-operative day (range 4 – 15). There was no association between oversewing the duodenal stump and duodenal stump leak. An additional 10 patients (4%) had an intra-abdominal collection without evidence of an anastomotic breakdown; the median date of diagnosis was POD #19 (range: 3 – 81). Of the 40 patients who required invasive intervention for an intraabdominal process (25 for esophageal anastomotic leak, seven for duodenal stump leak, eight for an intra-abdominal collection), seven (17.5%) required a second invasive procedure as part of their treatment, which occurred at a median POD # 19.5 (range 13 – 30). We were unable to find significant clinical prediction of post-operative intra-abdominal adverse events. Figure 6 shows the median time to diagnosis of the most common adverse events and procedures, and figure 7 shows the median length of stay associated with common adverse events and procedures that occurred during the initial hospitalization.
Figure 6.
Median time to occurance of the most common post-operative adverse events and procedures.
Figure 7.
Median Length of Stay associated with common adverse events and procedures occuring during initial hospitalization.
There were 342 recorded adverse events during our 90-day study period. Of these, 37 (11%) occurred between POD 30 and 90. The most common adverse event during this time period, as with our entire cohort, was anemia, which occurred in 7 patients (19% of all AEs between POD 30 and 90). Anastomotic stricture and small bowel obstruction were the next most commonly diagnosed adverse event after POD 30; each diagnosed three times (8% of all events after POD 30). Both occurred more frequently after 30 days than before. The median time to diagnosis of anastomotic stricture in our cohort was 52 days (IQR: 32.5 – 61), two of the five total strictures were diagnosed in the first 30 post-operative days (days 18 and 25), Neither radiation (either pre- or post-operatively) or reconstruction technique was associated with a diagnosed anastomotic stricture. The median time to diagnosis of small bowel obstruction was 40 days (IQR: 9 – 75); three of the seven small bowel obstructions were diagnosed within the first 30 days (days 6, 9, and 15).
Almost 30% of all adverse events occurred after discharge. As with our overall frequency, wound complications and anemia were the most frequent post-discharge adverse events, and esophageal anastomotic leak was the most frequent major adverse event. While hemorrhage and gastrointestinal bleeding, both technical complications, were diagnosed most frequently during the initial hospitalization (62.5% and 66% of all diagnosed events, respectively), anastomotic stricture was predominantly a post-discharge events (80% of all diagnosed events). Taken together, deep venous thrombosis (DVT) and pulmonary embolism (PE) had almost equal incidence in both periods (54% pre-discharge and 46% post-discharge). However, the majority of DVTs (67%) were diagnosed prior to discharge, while the majority of PEs (55%) were diagnosed as outpatients. Patients diagnosed with either DVT or PE prior to discharge (Figure 7) had a median length of stay of over 40 days, reflecting the fact that patients with increased length of stay are at high risk of developing a DVT or PE.
There was no statistically significant difference in total number of adverse events, the maximum grade of adverse event (none vs minor vs major), or mortality based on receiving neoadjuvant chemotherapy, adjuvant radiation, pre-operative albumin, reconstruction technique, operating surgeon, or type of pathology.
Discussion
Accurate reporting of post-operative adverse events requires a strong institutional culture of reporting as well as clear, prospectively defined definitions and grades. At our institution we have maintained, since 2001, a department wide adverse event database that prospectively tracks and records all post-operative adverse events. Our study results were generated by a rigorous physician review of each chart to identify all post-operative adverse events within 90 days of operation, and detailing the severity and timing of these events. We reviewed all charts for 90 days rather than only the 30 days more commonly reported to collect a comprehensive picture of post-operative adverse events following total gastrectomy.
There are interesting differences between esophageal anastomotic leak and duodenal stump leak. A similar percentage of both types of leak were major adverse events (77% of duodenal leaks, 71% of esophageal leaks), and both were diagnosed prior to discharge a majority of the time (71% of all esophageal anastomotic leaks and 61% of all duodenal stump leaks) yet duodenal stump leak was much more frequently fatal (33% of all leaks) than esophageal anastomotic leak (6% of all leaks). Patients diagnosed with esophageal leak prior to discharge had a median length of stay of 29 days, compared to 17 days for patients diagnosed with a duodenal stump leak prior to discharge, a difference at least partially explained by the higher mortality rate of duodenal stump leaks. We believe the difference in effluent through the two sites as well as the difficulty in accessing duodenal stump leaks to obtain adequate drainage contributed to the higher mortality seen following duodenal stump leaks.
Treatment of patients with an intraabdominal adverse event is primarily supportive, and obtaining adequate drainage and source control is a priority. In the hemodynamically stable patient drainage can be attempted by interventional radiology while the unstable patient is taken to the operating room for intra-abdominal washout and drainage. Our institutional routine at the time of total gastrectomy does not include placement of a jejunal feeding tube for supplemental enteral feeding. Jejunal feeding tubes are sometimes placed following an esophageal anastomotic leak to allow patients to receive enteral nutrition while their anastomotic leak heals. This is done in selected cases either percutaneously or via endoscopy.
Our data also revealed that several minor adverse events anticipated the subsequent development of later more serious adverse events. Patients with asymptomatic anemia had a 15% chance of developing an intra-abdominal abscess, compared to only a 4% chance in patients who were never anemic (p = 0.008). In patients who developed a cardiac arrhythmia, 36% were diagnosed with a leak (either at the esophageal anastomosis or duodenal stump) subsequent to the arrhythmia, compared to 14% of patients who never developed an arrhythmia (p = 0.007). These minor adverse events did not cause the later development of major adverse events, rather they foretold the hospital course to follow.
Our rates of adverse events are similar to those published by other authors. Two recent studies have examined the NSQIP total gastrectomy experience (8, 11). Examining 999 total gastrectomies from 2005 – 2010, Papenfuss et al found a median length of stay of 13 days, a “serious morbidity” rate of 29.3%, and a 30 day mortality rate of 5.4%. These rates are almost identical to the 2005 – 2011 results reported by Bartlett et al, who found a median length of stay of ten days, a “serious morbidity rate” of 36%, and a 30-day mortality rate of 4.7%. In comparison, our median length of stay is 9 days, and our 30 and 90-day mortality was 2.5% and 2.9%, respectively. The NSQIP category of “serious morbidity” includes superficial and deep organ space infection, dehiscence, re-intubation, prolonged ventilation, pulmonary embolism, acute renal failure requiring dialysis, urinary tract infection, cerebral vascular accident, cardiac arrest, myocardial infarction (MI), need for transfusion greater than 4 units postoperatively, sepsis, septic shock, or return to the operating room, but does not stratify these events by their individual clinical severity. Unfortunately NSQIP does not specifically capture esophageal anastomotic leaks, so we are unable to compare these rates directly. In 2008 Pacelli and colleagues published their institutions experience with 400 total gastrectomies, 312 of which were for curative intent, over a twenty three and a half year period (10). They report an anastomotic leak rate of 8.6%, and that 30% mortality rate associated with anastomotic leak. This contrasts to our 10% major anastomotic leak rate and 6% mortality rate associated with anastomotic leaks. Table 5 shows characteristics of recent Western reports on post-operative adverse events following curative intent TG.
Table 5.
Characteristics of recent Western reports of post-operative adverse events in Total Gastrectomy.
| Series | Year | Number of TGs |
% Morbidity |
% Major Morbidity |
% Mortality |
% Leak | Time Period |
Type | Severity Graded |
Timing Reported |
|---|---|---|---|---|---|---|---|---|---|---|
| Pacelli (10) | 2008 | 400 | 24% | --- | 3.5% | 8.6% | --- | Retrospective review of prospective database | No | No |
| Papenfuss (8) | 2014 | 999 | --- | 23.6% | 5.4% | --- | 30-Day | Retrospective review of prospective database | No | No |
| Bartlett (11) | 2014 | 1,165 | --- | 36% | 4.7% | --- | 30-Day | Retrospective review of prospective database | No | No |
| Current | 2014 | 238 | 62% | 28% | 2.9% | 14% 10% major |
90-Day | Retrospective review of prospective database and chart review | Yes | Yes |
Recently, Lee et al published their institution’s results after all gastrectomies, including 126 total gastrectomies (23). Dividing adverse events into local or systemic they detail the post-operative experience typical of a high volume Eastern referral center for gastric cancer. While they do not break out their adverse events for total gastrectomy specifically, they do demonstrate that extended gastrectomy, total gastrectomy, and age greater than 60 were significant predictors of local adverse events while extended gastrectomy, ASA score of 3 or 4, total gastrectomy, age greater than 60, and moderate to severe malnutrition were significant predictors of systemic adverse events. They did not find a difference in esophageal anastomotic leak between total or subtotal gastrectomy, and they do not report leak rates for completion gastrectomies.
Comparisons of adverse event rates at our institution to rates at other institutions are subject to significant differences in methodology that may explain the differences. Over the last ten years our average annual gastrectomy volume, including palliative and curative operations, is 128 cases per year. This places us in the highest quintile of volume in New York State (24) and the United States (25). Numerous studies have demonstrated the correlation between high surgical volume and lower mortality (13, 24–26). This phenomenon is reflected by comparing our mortality (2.9%) to the other studies in the group: it is lower than the 4.7% (11) or 5.4% (8) reported in the NSQIP series’, or the 3.5% reported by Pacelli (10). Yet, our esophageal anastomotic leak rate is higher. This project was initiated to describe the typical post-operative adverse event profile following total gastrectomy. As such, the diligence with which we surveyed for adverse events was high, and a possible explanation for our rate. Evidence for this is the difference between our overall rate of all esophageal anastomotic leaks (14.7%) and our rate of major leaks requiring invasive treatment (10%), which is in keeping with other published results, including a previous study from our institution focusing on a specific reconstruction technique (27). The data suggest that high volume institutions have lower complication rates and post-operative mortality rates, which may be attributed to earlier recognition and rescue from AEs (13, 25, 26, 31), a phenomenon likely related to experience in dealing with the post-operative AEs when they do occur.
Multiple groups have investigated novel techniques to constructing the esophageal anastomosis, none of which have resulted in widespread adoption (27). We have yet to identify risk factors that predispose patients to esophageal anastomotic leaks (28). We recently published our institution’s initial experience using a biological scaffold to provide extra-luminal reinforcement of the esophageal anastomosis; 37 patients with the reinforcement had a leak rate of 2.7%, compared to a leak rate of 12% in 32 historical control patients who did not receive esophageal reinforcement (29). We are currently enrolling patients in an prospective, open-label, phase II study to further evaluate the utility of esophageal anastomotic reinforcement in patients undergoing total gastrectomy or esophagectomy (30).
Of the published series we reviewed, ours is the only series fulfilling all ten of the criteria proposed by Martin et al in 2002 (20). The most frequent omissions were the absence of a grading scale and a lack of reporting of the timing of the adverse events. Since 2001, our Department’s adverse events database has used a modification of the Clavien-Dindo classification to evaluate all post-operative adverse events at our institution (21).
We have comprehensively described the adverse event profile following curative-intent total gastrectomy, which can be used to educate our trainees and non-surgical colleagues as well as improving the informed consent process with our patients. Esophageal anastomotic leak remains the dominant post-operative event, but the finding that esophageal and duodenal leaks occur almost entirely within the first three weeks is a valuable guide to post-operative followup and post-discharge care. We are reminded that seemingly innocuous minor adverse events often precede more serious adverse events. Surgical performance improves with diligent focus on quality improvement in all phases of care. This study has comprehensively described the post-operative course following total gastrectomy, with the goal of improving the education and informed consent process and providing a benchmark for further advancements in care.
Acknowledgments
Sources of Funding: EV and DS are supported in part by funds from a P50-CA92629 SPORE grant from the National Cancer Institute to Dr. H Scher and R01AT006794 to Dr. A Vickers.
Abbreviations
- LOS
Length of Stay
- POD
Post-operative Day
- AE
Adverse Event
- TG
Total Gastrectomy
- DVT
Deep Venous Thrombosis
- PE
Pulmonary Embolism
Appendix 1: All post-operative adverse events following curative intent Total Gastrectomy
| Categorized Event | Frequency | % of Patients | % Grade 3+ |
|---|---|---|---|
| Anemia | 48 | 20.2% | 0.0% |
| Wound Complication* | 44 | 18.5% | 2.3% |
| Anastomotic Leak, Esophagus | 35 | 14.7% | 71.4% |
| Grade 1 – 2 Anastomotic Leak | 10 | 4.2% | 0% |
| Grade 3 – 5 Anastomotic Leak | 25 | 10.5% | 100% |
| Pulmonary Complication** | 34 | 14.3% | 26.5% |
| Cardiac Arrhythmia*** | 23 | 9.7% | 0.0% |
| DVT / PE | 15 | 6.3% | 13.3% |
| Intra-Abdominal Infection Or Abscess | 10 | 4.2% | 80.0% |
| Altered Level Of Consciousness**** | 10 | 4.2% | 0.0% |
| Duodenal Stump Leak | 9 | 3.8% | 77.8% |
| Hemorrhage | 8 | 3.4% | 62.5% |
| Repeat Drainage For Anastomotic Leak | 7 | 2.9% | 100.0% |
| MI / Angina | 7 | 2.9% | 28.6% |
| Urinary Tract Infection | 7 | 2.9% | 0.0% |
| Small Bowel Obstruction | 6 | 2.5% | 100.0% |
| Gastrointestinal Bleeding | 6 | 2.5% | 66.7% |
| Renal Failure | 6 | 2.5% | 16.7% |
| Gastrointestinal Anastomotic Stricture | 5 | 2.1% | 60.0% |
| Fever | 4 | 1.7% | 0.0% |
| Clostridium Difficile | 4 | 1.7% | 0.0% |
| Ileus, Paralytic | 4 | 1.7% | 0.0% |
| Hypotension, Shock | 3 | 1.3% | 33.3% |
| Liver Dysfunction / Failure | 3 | 1.3% | 33.3% |
| Hematoma | 2 | 0.8% | 100.0% |
| Anastomotic Leak, Pancreas | 2 | 0.8% | 100.0% |
| Aspiration | 2 | 0.8% | 50.0% |
| Dehydration | 2 | 0.8% | 0.0% |
| Ascites | 2 | 0.8% | 0.0% |
| Fascial Dehiscence Or Evisceration | 2 | 0.8% | 0.0% |
| Seizure | 2 | 0.8% | 0.0% |
| Pain Syndrome, Acute | 2 | 0.8% | 0.0% |
| Failure To Thrive | 1 | 0.4% | 100.0% |
| Bowel Perforation, Necrosis | 1 | 0.4% | 100.0% |
| Major Venous Bleed Or Rupture | 1 | 0.4% | 100.0% |
| Cholecystitis | 1 | 0.4% | 100.0% |
| Foreign Body | 1 | 0.4% | 100.0% |
| Carotid Artery Bleed Or Rupture | 1 | 0.4% | 100.0% |
| Intrathoracic Abscess | 1 | 0.4% | 100.0% |
| Sepsis | 1 | 0.4% | 100.0% |
| Non-Infected Intra-Abdominal / Intra-Thoracic Fluid Collection | 1 | 0.4% | 100.0% |
| Dysphagia | 1 | 0.4% | 0.0% |
| Pericardial Effusion, Pericarditis | 1 | 0.4% | 0.0% |
| Bile Reflux Gastritis | 1 | 0.4% | 0.0% |
| Fistula, Pancreatic | 1 | 0.4% | 0.0% |
| Empyema | 1 | 0.4% | 0.0% |
| Urinary Retention | 1 | 0.4% | 0.0% |
| Catheter Related Infection | 1 | 0.4% | 0.0% |
| Medication Toxicity | 1 | 0.4% | 0.0% |
| Skin Breakdown, Decubitus Ulcer | 1 | 0.4% | 0.0% |
| Iatrogenic Nerve Injury | 1 | 0.4% | 0.0% |
| Pharyngitis | 1 | 0.4% | 0.0% |
Wound Complication includes Wound Infection, Cellulitis, Wound Breakdown, Seroma, Skin Abscess
Pulmonary Complication includes Pleural Effusion, Hypoxia, Pneumonia, Atelectasis, and Pneumonitis
Cardiac Arrhythmia includes Supraventricular Arrhythmia, Afib, Bradycardia, Ventricular Arrhythmia, and Tachycardia
Altered Level of Consciousness includes Depressed Level of Consciousness and Psychosis, Confusion, or Depression.
Footnotes
Conflict of Interest: No conflicts of interest.
References
- 1.World Health Organization. [Accessed July 14, 2014];Estimated Cancer Incidence, Mortality, and Prevalence Worldwide in 2012. Available at: http://globocan.iarc.fr/Pages/fact_sheets_population.aspx.
- 2.The American Cancer Society. [Accessed July 14, 2014];Cancer Facts & Figures 2013. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036845.pdf.
- 3.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. The New England journal of medicine. 2006 Jul 6;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 4.Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012 Jan 28;379(9813):315–321. doi: 10.1016/S0140-6736(11)61873-4. [DOI] [PubMed] [Google Scholar]
- 5.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. The New England journal of medicine. 2001 Sep 6;345(10):725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 6.Sierzega M, Kolodziejczyk P, Kulig J Polish Gastric Cancer Study G. Impact of anastomotic leakage on long-term survival after total gastrectomy for carcinoma of the stomach. The British journal of surgery. 2010 Jul;97(7):1035–1042. doi: 10.1002/bjs.7038. [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network. NCCN Clinical Practice Guideline in Oncology - Gastric Cancer - Version 1.2014. [Google Scholar]
- 8.Papenfuss WA, Kukar M, Oxenberg J, et al. Morbidity and mortality associated with gastrectomy for gastric cancer. Ann Surg Oncol. 2014 Sep;21(9):3008–3014. doi: 10.1245/s10434-014-3664-z. [DOI] [PubMed] [Google Scholar]
- 9.Dhir M, Smith LM, Ullrich F, et al. A preoperative nomogram to predict the risk of perioperative mortality following gastric resections for malignancy. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2012 Nov;16(11):2026–2036. doi: 10.1007/s11605-012-2010-7. [DOI] [PubMed] [Google Scholar]
- 10.Pacelli F, Papa V, Rosa F, et al. Four hundred consecutive total gastrectomies for gastric cancer - A single-institution experience. Archives of Surgery. 2008 Aug;143(8):769–775. doi: 10.1001/archsurg.143.8.769. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett EK, Roses RE, Kelz RR, Drebin JA, Fraker DL, Karakousis GC. Morbidity and mortality after total gastrectomy for gastric malignancy using the American College of Surgeons National Surgical Quality Improvement Program database. Surgery. 2014 Aug;156(2):298–304. doi: 10.1016/j.surg.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Edwards P, Blackshaw GR, Lewis WG, Barry JD, Allison MC, Jones DR. Prospective comparison of D1 vs modified D2 gastrectomy for carcinoma. British journal of cancer. 2004 May 17;90(10):1888–1892. doi: 10.1038/sj.bjc.6601790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Finlayson EV, Goodney PP, Birkmeyer JD. Hospital volume and operative mortality in cancer surgery: a national study. Arch Surg. 2003 Jul;138(7):721–725. doi: 10.1001/archsurg.138.7.721. discussion 6. [DOI] [PubMed] [Google Scholar]
- 14.Smith JW, Shiu MH, Kelsey L, Brennan MF. Morbidity of radical lymphadenectomy in the curative resection of gastric carcinoma. Arch Surg. 1991 Dec;126(12):1469–1473. doi: 10.1001/archsurg.1991.01410360039007. [DOI] [PubMed] [Google Scholar]
- 15.Harrison LE, Karpeh MS, Brennan MF. Proximal gastric cancers resected via a transabdominal-only approach. Results and comparisons to distal adenocarcinoma of the stomach. Annals of surgery. 1997 Jun;225(6):678–683. doi: 10.1097/00000658-199706000-00005. discussion 83-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Noguchi Y, Yoshikawa T, Tsuburaya A, Motohashi H, Karpeh MS, Brennan MF. Is gastric carcinoma different between Japan and the United States? Cancer. 2000;89(11):2237–2246. [PubMed] [Google Scholar]
- 17.Clavien PA, JR S, SM S. Proposed Classification of Complications of surgery with Examples of Utility in Cholecystecomy. Surgery. 1992;111(5):518–526. [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Annals of surgery. 2004 Aug;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Annals of surgery. 2009 Aug;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 20.Martin RC, 2nd, Brennan MF, Jaques DP. Quality of complication reporting in the surgical literature. Annals of surgery. 2002 Jun;235(6):803–813. doi: 10.1097/00000658-200206000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strong VE, Selby LV, Sovel M, et al. Development and Assessment of Memorial Sloan Kettering Cancer Center's Surgical Secondary Events Grading System. Ann Surg Oncol. 2014 Oct 16; doi: 10.1245/s10434-014-4141-4. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grobmyer SR, Pieracci FM, Allen PJ, Brennan MF, Jaques DP. Defining morbidity after pancreaticoduodenectomy: use of a prospective complication grading system. Journal of the American College of Surgeons. 2007 Mar;204(3):356–364. doi: 10.1016/j.jamcollsurg.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 23.Lee KG, Lee HJ, Yang JY, et al. Risk factors associated with complication following gastrectomy for gastric cancer: retrospective analysis of prospectively collected data based on the Clavien-Dindo system. Journal of gastrointestinal surgery : official journal of the Society for Surgery of the Alimentary Tract. 2014 Jul;18(7):1269–1277. doi: 10.1007/s11605-014-2525-1. [DOI] [PubMed] [Google Scholar]
- 24.Hannan EL, Radzyner M, Rubin D, Dougherty J, Brennan MF. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery. 2002 Jan;131(1):6–15. doi: 10.1067/msy.2002.120238. [DOI] [PubMed] [Google Scholar]
- 25.Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. The New England journal of medicine. 2002 Apr 11;346(15):1128–1137. doi: 10.1056/NEJMsa012337. [DOI] [PubMed] [Google Scholar]
- 26.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011 Dec;49(12):1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 27.LaFemina J, Vinuela EF, Schattner MA, Gerdes H, Strong VE. Esophagojejunal reconstruction after total gastrectomy for gastric cancer using a transorally inserted anvil delivery system. Ann Surg Oncol. 2013 Sep;20(9):2975–2983. doi: 10.1245/s10434-013-2978-6. [DOI] [PubMed] [Google Scholar]
- 28.Rizk NP, Bach PB, Schrag D, et al. The impact of complications on outcomes after resection for esophageal and gastroesophageal junction carcinoma. Journal of the American College of Surgeons. 2004 Jan;198(1):42–50. doi: 10.1016/j.jamcollsurg.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 29.Afaneh C, Abelson J, Schattner M, et al. Esophageal Reinforcement with an Extracellular Scaffold During Total Gastrectomy for Gastric Cancer. Ann Surg Oncol. 2014 Oct 16; doi: 10.1245/s10434-014-4125-4. [DOI] [PubMed] [Google Scholar]
- 30.Evaluate Esophageal Reinforcement With ACell MatriStem Surgical Matrix: A Degradable Biologic Scaffold Material. [Accessed July 10th, 2014];ClinicalTrials.gov. Available at: http://clinicaltrials.gov/ct2/show/NCT01970306.
- 31.Spolverato G, Ejaz A, Hyder O, Kim Y, Pawlik TM. Failure to rescue as a source of variation in hospital mortality after hepatic surgery. The British journal of surgery. 2014 Jun;101(7):836–846. doi: 10.1002/bjs.9492. [DOI] [PubMed] [Google Scholar]