Abstract
Background
International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding for cytomegalovirus (CMV) has been used as a proxy for active CMV infection or disease occurring in the inpatient setting in retrospective studies of kidney transplant recipients using large administrative data. However, the accuracy of inpatient CMV coding has not been determined.
Methods
We identified 393 kidney transplant recipients who were readmitted to Barnes-Jewish Hospital in St. Louis, Missouri from January 1, 2007 to December 31, 2011 to determine the accuracy of the ICD-9-CM diagnosis code for CMV (078.5) in identifying active CMV infection or disease (asymptomatic viremia, CMV syndrome or tissue-invasive CMV disease) in the inpatient setting, using microbiologic, histopathologic or ophthalmologic evidence for CMV as the gold standard.
Results
The sensitivity and positive predictive value of CMV coding in identifying active CMV infection or disease were 0.77 and 0.71 respectively. The specificity and negative predictive value were both 0.98. The sensitivity of CMV coding in identifying CMV syndrome or tissue-invasive CMV disease was 0.93.
Conclusions
CMV coding had good accuracy in identifying active CMV infection or disease among readmitted kidney transplant recipients in our hospital. Further validation studies of CMV coding in other hospitals are needed to obtain more generalizable estimates of the accuracy of CMV coding.
Keywords: cytomegalovirus, administrative data, kidney transplantation
INTRODUCTION
Active CMV infection and disease are preventable after kidney transplantation (1,2). Active CMV infection can be asymptomatic, or manifest as CMV syndrome or tissue-invasive CMV disease (2,3). CMV prophylaxis administered for 3 to 6 months post-transplant to CMV-seronegative recipients of kidneys from CMV-seropositive donors and CMV-seropositive recipients has decreased the incidence of active CMV infection and disease early after transplantation (4–6). However, delayed-onset CMV disease can occur after stopping prophylaxis (7–13), and is associated with an increased risk of death (7,13–15). No national surveillance system for active CMV infection or disease exists, thereby limiting our ability to compare the real-world effectiveness of different CMV prevention strategies used by kidney transplant centers in the United States.
The United States Renal Database System (USRDS) Standard Analysis Files and other large administrative databases can be used to retrospectively assemble large and nationally representative cohorts of kidney transplant recipients with prolonged follow-up (14). The recent inclusion of Medicare Part D outpatient prescription drug claims to USRDS data enables the use of anti-CMV medications prescribed at treatment doses as proxies for active CMV infection or disease in the outpatient setting. However, drugs administered in the inpatient setting are not available, thereby necessitating the use of International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes assigned at hospital discharge as proxies for active CMV infection or disease in the inpatient setting (14,15). To determine the accuracy of ICD-9-CM coding in identifying active CMV infection or disease in the inpatient setting, we identified kidney transplant recipients who were readmitted to our institution over a 5-year period, and validated inpatient coding for CMV using microbiologic, histopathologic or ophthalmologic evidence for CMV as the gold standard.
METHODS
Study design and patient population
We identified a cohort of patients > 18 years of age who underwent kidney transplantation, identified using ICD-9-CM procedure code 55.69, at Barnes-Jewish Hospital (BJH), a 1,251-bed tertiary care hospital affiliated with Washington University School of Medicine in St. Louis, Missouri, from January 1, 2007 to December 31, 2010. We excluded patients who received another solid organ transplant during the same hospitalization as the kidney transplant, and censored patients on the date of any subsequent solid organ transplant. We identified all readmissions of individuals in the cohort to BJH from January 1, 2007 to December 31, 2011. All administrative data, vital signs, laboratory results, and most microbiologic data were obtained from the BJH Medical Informatics Database. Histopathologic and ophthalmologic evidence of CMV disease were obtained by medical record review. This study was approved by the Washington University Institutional Review Board.
Accuracy of ICD-9-CM coding for CMV
CMV coding during hospital readmission was identified using ICD-9-CM diagnosis code 078.5. We validated CMV coding using microbiologic, histopathologic or ophthalmologic proof of CMV replication or disease as the gold standard (Figure 1). Microbiologic evidence of CMV consisted of the detection of CMV by polymerase chain reaction (PCR) testing of blood, cerebrospinal fluid or ocular fluid; growth of CMV in standard or shell vial viral culture of bronchoalveolar lavage specimens or tissue; or detection of pp65 antigen in peripheral blood leukocytes. If there was no microbiological evidence for CMV in the electronic database, medical record review of all admissions coded for CMV and a random sample of 200 admissions that were not coded for CMV was performed to identify histopathologic or ophthalmologic evidence of CMV disease, and any microbiologic data not recorded in the BJH electronic database (e.g. results from other hospitals). Histopathologic evidence of CMV disease consisted of immunohistochemical or in situ hybridization evidence of tissue-invasive CMV disease in the lung, gastrointestinal tract, liver, kidney or other organs. Ophthalmologic proof consisted of eye findings consistent with CMV retinitis as reported by an ophthalmologist. The medical records of all patients with evidence of active CMV infection or disease during hospital readmission were reviewed, and categorized as asymptomatic viremia, CMV syndrome or tissue-invasive CMV disease using established clinical guidelines (3). The number of false negative and true negative admissions in the random sample of 200 patients was corrected for the fraction sampled. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of inpatient ICD-9-CM coding for CMV was calculated. All data management and analyses were done using SAS version 9.3 (Cary, NC) and SPSS version 20.0 (Chicago, IL).
Figure 1.
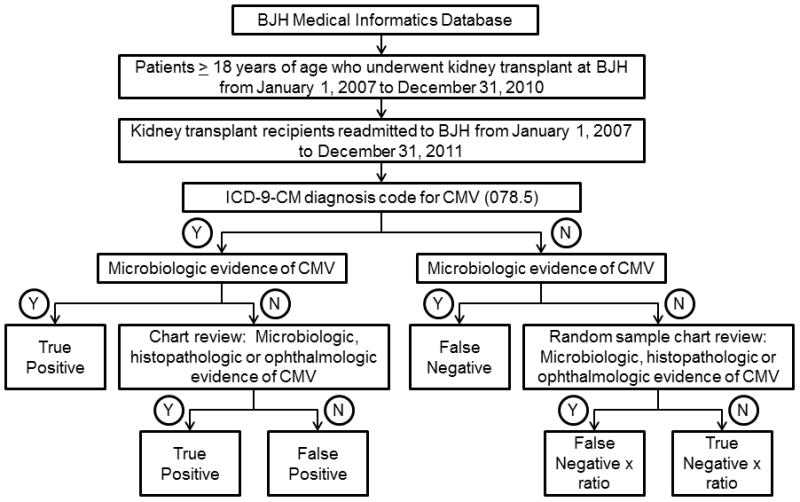
Determining the accuracy of ICD-9-CM coding for CMV in identifying active CMV infection or disease in the inpatient setting.
RESULTS
ICD-9-CM coding for CMV
We identified 692 adult kidney transplant recipients of whom 393 were readmitted to BJH. Of 28 patients with CMV coding during hospital readmission, 20 had microbiologic, histopathologic or ophthalmologic evidence of active CMV infection or disease. Of 365 patients with no CMV coding during hospital readmission, 6 had microbiologic evidence of active CMV infection in the electronic record. None of the 200 randomly sampled patients with no CMV coding or microbiologic evidence of active CMV infection in the electronic record were found to have active CMV infection or disease by medical record review (Table 1).
Table 1.
Accuracy of ICD-9-CM coding for CMV
| Microbiologic, histologic or ophthalmologic evidence of active CMV infection or disease
| |||
|---|---|---|---|
| CMV coding | Positive | Negative | Total |
| Positive | 20 | 8 | 28 |
| Negative | 6 | 359 | 365 |
| Total | 26 | 367 | 393 |
| Sensitivity = 0.77 | Specificity = 0.98 | ||
| PPV = 0.71 | NPV = 0.98 | ||
| CMV syndrome or tissue-invasive CMV disease
| |||
|---|---|---|---|
| CMV coding | Positive | Negative | Total |
| Positive | 14 | 14 | 28 |
| Negative | 1 | 364 | 365 |
| Total | 15 | 378 | 393 |
| Sensitivity = 0.93 | Specificity = 0.96 | ||
| PPV = 0.50 | NPV = 0.99 | ||
Proven cases of active CMV infection or disease
Of 26 patients with active CMV infection or disease, 11 had asymptomatic viremia, 3 had CMV syndrome and 12 had tissue-invasive CMV disease. Of 12 patients with tissue-invasive CMV disease, 2 had biopsy-proven tissue invasion (1 gastritis, 1 nephritis), 9 patients had gastrointestinal symptoms and hepatitis accompanying CMV viremia, and 1 patient had isolated hepatitis accompanying CMV viremia. There were no cases of CMV pneumonitis, meningoencephalitis or retinitis. CMV syndrome and tissue-invasive disease occurred at a median of 377 days post-transplant (range 136 – 944 days). Of 15 patients with CMV syndrome or tissue-invasive CMV disease, 14 were coded for CMV during hospital readmission (Table 1).
Accuracy of ICD-9-CM coding for CMV
The sensitivity and PPV of CMV coding in identifying active CMV infection or disease was 0.77 and 0.71 respectively. The specificity and NPV both equaled 0.98. The sensitivity and PPV of CMV coding in identifying CMV syndrome or tissue-invasive CMV disease was 0.93 and 0.50 respectively (Table 1).
False positive ICD-9-CM coding for CMV
Of 8 patients with CMV coding but no evidence of active CMV infection or disease during the hospital readmission, 1 had a blood CMV PCR result of 403,850 copies/mL that was revised 20 days after hospital discharge to < 200 copies/mL, 2 were on valganciclovir prophylaxis with no evidence of current or prior active CMV infection or disease, 1 had a negative blood CMV PCR test performed during a fever work-up, and 4 had CMV donor or recipient seropositivity mentioned in the hospitalization admission note (Table 2).
Table 2.
Possible reasons for false positive or false negative ICD-9-CM coding for CMV on medical chart review
| Patient | Reason for Admission | Possible Reason for CMV False Positive Coding |
|---|---|---|
| 1 | Disseminated histoplasmosis | CMV PCR reported at 403,850 copies/mL that was in error; revised 20 days after hospital discharge to < 200 copies/mL |
|
| ||
| 2 | Acute cellular rejection | Valganciclovir prophylaxis prescribed with no evidence of active CMV infection or disease |
| 3 | Abdominal pain | |
|
| ||
| 4 | Transplant pyelonephritis | CMV PCR test was checked and negative |
|
| ||
| 5 | Weakness, anemia | CMV mentioned in history and physical as part of donor-recipient description, no CMV testing was performed |
| 6 | Elective bariatric surgery | |
| 7 | Herpes zoster | |
| 8 | Acute cellular rejection | |
| Patient | Reason for Admission | Possible Reason for CMV False Negative Coding |
|---|---|---|
| 1 | Tissue-invasive gastrointestinal CMV disease | No identifiable reason; documented in chart; length of stay 7 days; CMV test result returned 6 days prior to discharge |
| 2 | Acute renal failure | No documentation in chart; length of stay 1 day; CMV test result returned on the day of discharge |
| 3 | Urinary tract infection | No documentation in chart; length of stay 5 days; CMV test result returned 3 days prior to discharge |
| 4 | Upper respiratory tract infection | No documentation in chart; length of stay 2 days; CMV test result returned on the day of discharge |
| 5 | Metastatic primary lung cancer | No documentation in chart; length of stay 25 days (until expiration); CMV test result returned 15 days prior to expiration |
| 6 | Acute renal failure | No documentation in chart; length of stay 5 days; CMV test result returned 4 days prior to discharge |
False negative ICD-9-CM coding for CMV
Of 6 patients with no CMV coding but had evidence of active CMV infection or disease during the hospital readmission, 1 had gastrointestinal CMV disease and 5 had asymptomatic CMV viremia. The patient with gastrointestinal CMV disease was admitted with fevers, chills, nausea, abdominal pain and hepatitis and had a blood CMV PCR level of 1,350,000 copies/mL noted in the medical record. The patient was treated with intravenous ganciclovir during the hospitalization and discharged on valganciclovir 7 days after admission. Asymptomatic CMV viremia was not documented in the medical record in the remaining 5 patients (Table 2).
DISCUSSION
We found that ICD-9-CM coding for CMV in our hospital had moderate sensitivity and PPV of 0.77 and 0.71 respectively in identifying active CMV infection or disease in the inpatient setting. False negative CMV coding occurred primarily in patients with asymptomatic CMV viremia that was not documented in the medical record. Physicians may not have documented the asymptomatic viremia due to the low severity of illness, leading to the absence of CMV coding. Potential reasons for false positive CMV coding included mistaking CMV donor or recipient seropositivity and anti-CMV medication administration for active CMV infection or disease.
We found that CMV coding in our hospital had high sensitivity of 0.93 in identifying CMV syndrome or tissue-invasive disease in the inpatient setting. Physicians may be more likely to document tissue-invasive CMV disease and CMV syndrome in the medical record due to the severity of CMV disease and its deleterious impact on allograft and patient survival (7,13).
Researchers have used the CMV ICD-9-CM diagnosis code to identify active CMV infection or disease after kidney transplantation in inpatient billing and claims data, and have reported that CMV coded during hospitalization was associated with death (14,15). Our data suggest that misclassification due to coding of latent CMV infection as active CMV infection or disease, and failure to code for all active CMV infection or disease likely led to more conservative estimates of the effect of CMV on mortality in epidemiologic studies that used administrative data.
Our study was limited to examining only inpatient coding for CMV from one hospital, thereby reducing the generalizability of our findings. Variations in coding and documentation practices across hospitals nationally may lead to different levels of accuracy in CMV coding. However, our study contributes to the literature by providing an estimate of the accuracy of CMV coding in a tertiary care center, and provides a systematic method to determine the accuracy of inpatient CMV coding that can be replicated in other hospitals. The strengths of our approach were the complete review of the medical record for all cases of active CMV infection or disease, and the consistent application of case definitions based on published guidelines (3).
In conclusion, we found that CMV coding in our hospital had moderate sensitivity and PPV in identifying active CMV infection or disease in the inpatient setting, and that CMV syndrome and tissue-invasive CMV disease were identified with high sensitivity. Further validation of CMV coding in other hospitals must be done to obtain more generalizable estimates of its accuracy. Validating inpatient ICD-9-CM coding for CMV as a proxy for active CMV infection or disease may facilitate comparative effectiveness studies of CMV prevention practices using administrative data, and help determine optimal anti-CMV strategies post-transplant.
Acknowledgments
Grant information: Carlos Santos is supported by the Washington University Institute of Clinical and Translational Sciences Multidisciplinary Clinical Research Career Development Program funded by National Institutes of Health grant KL2 TR000450.
We would like to acknowledge Joshua Doherty and Cherie Hill for help with database management. Carlos Santos is supported by the Washington University Institute of Clinical and Translational Sciences Multidisciplinary Clinical Research Career Development Program funded by National Institutes of Health grant KL2 TR000450.
Abbreviations
- BJH
Barnes-Jewish Hospital
- CMV
cytomegalovirus
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- NPV
negative predictive value
- PCR
polymerase chain reaction
- PPV
positive predictive value
- USRDS
United States Renal Database System
Contributor Information
Carlos A. Q. Santos, Email: csantos@dom.wustl.edu.
Daniel C. Brennan, Email: dbrennan@dom.wustl.edu.
Margaret A. Olsen, Email: molsen@dom.wustl.edu.
References
- 1.Eid AJ, Razonable RR. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs. 2010;70:965–981. doi: 10.2165/10898540-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 2.Razonable RR, Humar A. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013;13 (Suppl 4):93–106. doi: 10.1111/ajt.12103. [DOI] [PubMed] [Google Scholar]
- 3.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34:1094–1097. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 4.Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611–620. doi: 10.1111/j.1600-6143.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- 5.Doyle AM, Warburton KM, Goral S, Blumberg E, Grossman RA, Bloom RD. 24-week oral ganciclovir prophylaxis in kidney recipients is associated with reduced symptomatic cytomegalovirus disease compared to a 12-week course. Transplantation. 2006;81:1106–1111. doi: 10.1097/01.tp.0000204048.90367.97. [DOI] [PubMed] [Google Scholar]
- 6.Humar A, Lebranchu Y, Vincenti F, et al. The efficacy and safety of 200 days valganciclovir cytomegalovirus prophylaxis in high-risk kidney transplant recipients. Am J Transplant. 2010;10:1228–1237. doi: 10.1111/j.1600-6143.2010.03074.x. [DOI] [PubMed] [Google Scholar]
- 7.Arthurs SK, Eid AJ, Pedersen RA, et al. Delayed-onset primary cytomegalovirus disease and the risk of allograft failure and mortality after kidney transplantation. Clin Infect Dis. 2008;46:840–846. doi: 10.1086/528718. [DOI] [PubMed] [Google Scholar]
- 8.Helantera I, Lautenschlager I, Koskinen P. Prospective follow-up of primary CMV infections after 6 months of valganciclovir prophylaxis in renal transplant recipients. Nephrol Dial Transplant. 2009;24:316–320. doi: 10.1093/ndt/gfn558. [DOI] [PubMed] [Google Scholar]
- 9.Helantera I, Kyllonen L, Lautenschlager I, Salmela K, Koskinen P. Primary CMV infections are common in kidney transplant recipients after 6 months valganciclovir prophylaxis. Am J Transplant. 2010;10:2026–2032. doi: 10.1111/j.1600-6143.2010.03225.x. [DOI] [PubMed] [Google Scholar]
- 10.Cervera C, Fernandez-Ruiz M, Valledor A, et al. Epidemiology and risk factors for late infection in solid organ transplant recipients. Transpl Infect Dis. 2011;13:598–607. doi: 10.1111/j.1399-3062.2011.00646.x. [DOI] [PubMed] [Google Scholar]
- 11.Boudreault AA, Xie H, Rakita RM, et al. Risk factors for late-onset cytomegalovirus disease in donor seropositive/recipient seronegative kidney transplant recipients who receive antiviral prophylaxis. Transpl Infect Dis. 2011;13:244–249. doi: 10.1111/j.1399-3062.2011.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boobes Y, Al HM, Dastoor H, Bernieh B, Abdulkhalik S. Late cytomegalovirus disease with atypical presentation in renal transplant patients: case reports. Transplant Proc. 2004;36:1841–1843. doi: 10.1016/j.transproceed.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 13.Blyth D, Lee I, Sims KD, et al. Risk factors and clinical outcomes of cytomegalovirus disease occurring more than one year post solid organ transplantation. Transpl Infect Dis. 2012;14:149–155. doi: 10.1111/j.1399-3062.2011.00705.x. [DOI] [PubMed] [Google Scholar]
- 14.Abbott KC, Hypolite IO, Viola R, et al. Hospitalizations for cytomegalovirus disease after renal transplantation in the United States. Ann Epidemiol. 2002;12:402–409. doi: 10.1016/s1047-2797(01)00283-6. [DOI] [PubMed] [Google Scholar]
- 15.Santos CA, Brennan DC, Fraser VJ, Olsen MA. Delayed-onset cytomegalovirus disease coded during hospital readmission after kidney transplantation. Transplantation. 2014;98:187–194. doi: 10.1097/TP.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]


