Abstract
During the last two decades our understanding of human B cell differentiation has developed considerably. Our understanding of the human B cell compartment has advanced from a point where essentially all assays were based on the presence or not of class-switched antibodies to a level where a substantial diversity is appreciated among the cells involved. Several consecutive transitional stages that newly formed IgM expressing B cells go through after they leave the bone marrow, but before they are fully mature, have been described, and a significant complexity is also acknowledged within the IgM expressing and class-switched memory B cell compartments. It is possible to isolate plasma blasts in blood to follow the formation of plasma cells during immune responses, and the importance and uniqueness of the mucosal IgA system is now much more appreciated. Current data suggest the presence of at least one lineage of human innate-like B cells akin to B1 and/or marginal zone B cells in mice. In addition, regulatory B cells with the ability to produce IL-10 have been identified. Clinically, B cell depletion therapy is used for a broad range of conditions. The ability to define different human B cell subtypes using flow cytometry has therefore started to come into clinical use, but as our understanding of human B cell development further progresses, B cell subtype analysis will be of increasing importance in diagnosis, to measure the effect of immune therapy and to understand the underlying causes for diseases. In this review the diversity of human B cells will be discussed, with special focus on current data regarding their phenotypes and functions.
Introduction
The existence of a distinct cell lineage responsible for the production of antibodies was first appreciated in birds. When the Bursa of Fabricius, a lymphoid structure in contact with the gut, was removed from newly hatched chickens, no antibodies were produced, which demonstrated that this organ played an essential role in the development of antibody producing cells[1]. This led to a search for a Bursa equivalent in other species, a largely unsuccessful task as early B cell development mainly occurs in fetal spleen and bone marrow in mammalians. However, recent studies have highlighted that gut associated lymphoid tissues (GALT) may in fact have an important role in the maturation of mammalian B cells as well[2-5].
Early B cell development can be divided into stages based on genetic modifications of the antibody genes and the expression of cell surface markers (Fig. 1A)[6]. Functional antibody genes are created in the bone marrow and the fetal liver through random recombination of V, D and J segments in pro- and pre-B cells. Cells that have successfully recombined their antibody genes leave the bone marrow, but are still not fully mature and are termed transitional B cells. Eventually, they develop into fully mature naïve cells that can be activated by antigen recognized by antibodies present on their cell surface as B cell receptors (BCR). During the maturation process, most B cells that carry self-reactive BCR are deleted, largely dependent on interactions between auto-antigens and the BCR during immature and transitional stages[7]. In addition, at least in some animals, cells are selected for development into different B cell lineages at this stage[8].
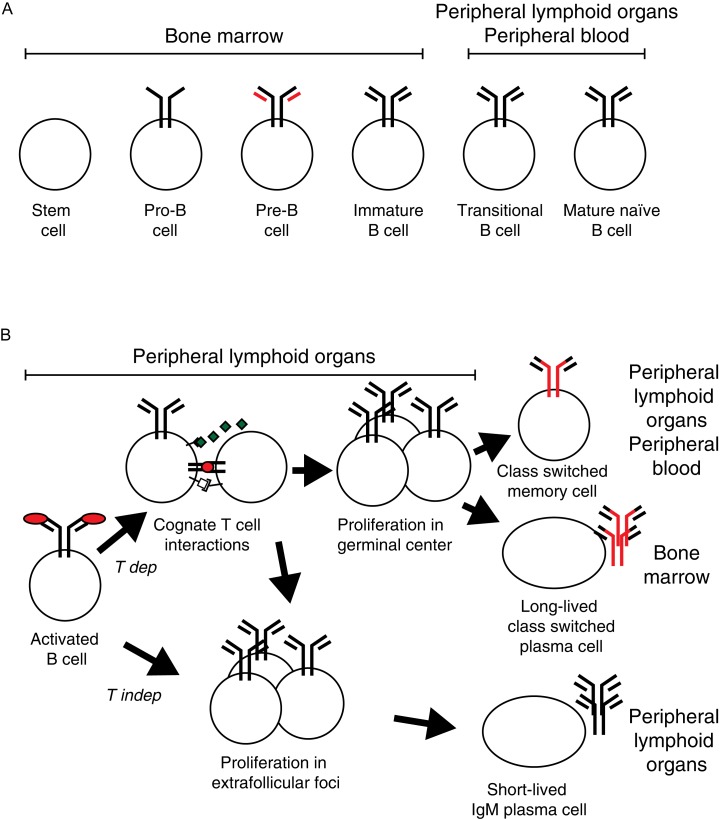
Fig. 1. Antigen independent and dependent B lymphoid development.
B cell development can be divided into an antigen independent phase (A) that generates vast diversity while still avoiding the formation of autoreactive cells and an antigen dependent phase (B) that allows for the activation, expansion and differentiation of antigen-specific B cells into effector and memory cells. A: During the antigen independent phase, hematopoietic stem cells in the bone marrow are selected to become B cells. During the pre- and pro-B cell stages the cells create a functional heavy chain gene through random recombination of V, D and J segments, a process that is monitored through pairing of the heavy chain gene with surrogate light chains (red). When a functional heavy chain gene has formed, the cell creates functional light chain genes through recombination of V and J segments, first in the κ light chain locus and then, if κ is unsuccessful, the λ light chain locus. When productive rearrangements have been achieved for both a heavy and a light chain, the mature antigen receptor (the B cell receptor, BCR) of the IgM class as is expressed on the cell surface on immature B cell. After the immature B cell leave the bone marrow and enter into blood stream and the peripheral lymphoid organs, it goes through a transitional stage before becoming a fully mature naïve B cell. If the cell encounters antigen that bind to the BCR on the cell surface during the immature or transitional stages, the cell will be deleted, a mechanism that hinders autoreactive B cells to reach the mature stage. B: An external antigen will be recognized by BCRs on a small number of mature naïve B cells that will be activated. This leads to that the B cells start to proliferate, take up antigen and present peptides derived from it on MHC II molecules on the cell surface. If the B cells encounter T cells that recognize peptides from the same antigen presented on MHC II through their T cell receptors (TCR) a T dependent (T dep) response is initiated. B cells will revive additional signals from the T cells, in particular from secreted cytokines and CD40L biding to CD40, and proliferating cells will enter B cell follicles to form germinal centers of rapidly proliferating cells. Here, the B cells change antibody class to other classes than IgM (i.e. IgG, IgE and IgA) through class switch recombination. Furthermore, the antigen binding V regions of the antibody genes goes through targeted somatic hypermutation followed by a selection for cells that express high affinity BCR, a process known as affinity maturation. Subsequently, cells leave the germinal center to form long-lived class-switched memory B cells that can rapidly respond if we reencounter the same antigen and long-lived plasma cells that home to bone marrow and produce secreted antigen-specific class-switched antibodies for extended periods. B cells that recognize antigens in the absence of T cell interactions (T independent responses; T indep) and some B cells that respond to T dependent antigen swill form extrafollicular foci and form relatively short-lived plasma cells secreting IgM that are maintained in peripheral lymphoid organs.
Only a small proportion of mature naïve B cells will ever be activated by antigen. When this happens, it leads to clonal expansion and differentiation (Fig. 1B)[9]. Antigen binding to the BCR activates the B cell in the follicle and induces it to leave the follicle. After extra-follicular proliferation, comparatively short-lived plasma cells form within the lymphoid organ producing soluble antibodies, primarily of the IgM class. If antigen-activated B cells make cognate MHC-TCR interactions with CD4+ T follicular helper cells recognizing peptides from the same antigen, the cells will exchange signals and some B cells will re-enter B cell lymphoid follicles where they proliferate and form a germinal center (GC). Here, they fine-tune their antigen specificity through antibody V region somatic hypermutation (SHM) and change the functional characteristics of the antibody heavy chain constant regions through class switch recombination (CSR) to other classes than IgM. B cells expanded through follicular clonal expansion from the original B cell and then leave the GC to differentiate into antigen-specific class-switched high-affinity long-lived plasma cells and memory B cells.
Advances have been done in defining human B cell subtypes through the use of multi-parameter flow cytometry and in vitro culturing. This has led to the identification of different stages that B cells go through when they transition from early bone marrow stages to fully mature naïve B cells, the description of human B cells similar to mouse marginal zone (MZ) and B1 B cells, the division of the human memory B cell compartment into sub-compartments and the characterization of B cells with regulatory properties. In this review, I will discuss different types of human B cells encountered outside of the bone marrow with the aim of casting light on their relationship to each other.
Transitional B cells
Only a small proportion of B cells that leave the bone marrow will become fully mature naïve B cells[10]. BCR interactions with self-antigens will inactivate or deplete immature and transitional B cells before they become mature, thus preventing the formation of auto-reactive mature B cells[7,11]. At the same time, BCR signals can determine the relative proportion of cells within different B cell lineages and are crucial for cell survival[12-14]. Thus, BCR signals select B cells through positive as well as negative selection during this stage. B cells that have recently left the bone marrow are termed transitional B cells[15]. There has been an interest in defining where and how B cells are selected during the transitional stage, as they then are at a critical stage that ensures that auto-reactive immune responses are not initiated. In mice several stages on the way to become mature B cells have been defined (Fig. 2A)[8]. Recently, substantial progress has also been made to understand stages during transitional B cell development in humans (Fig. 2B).
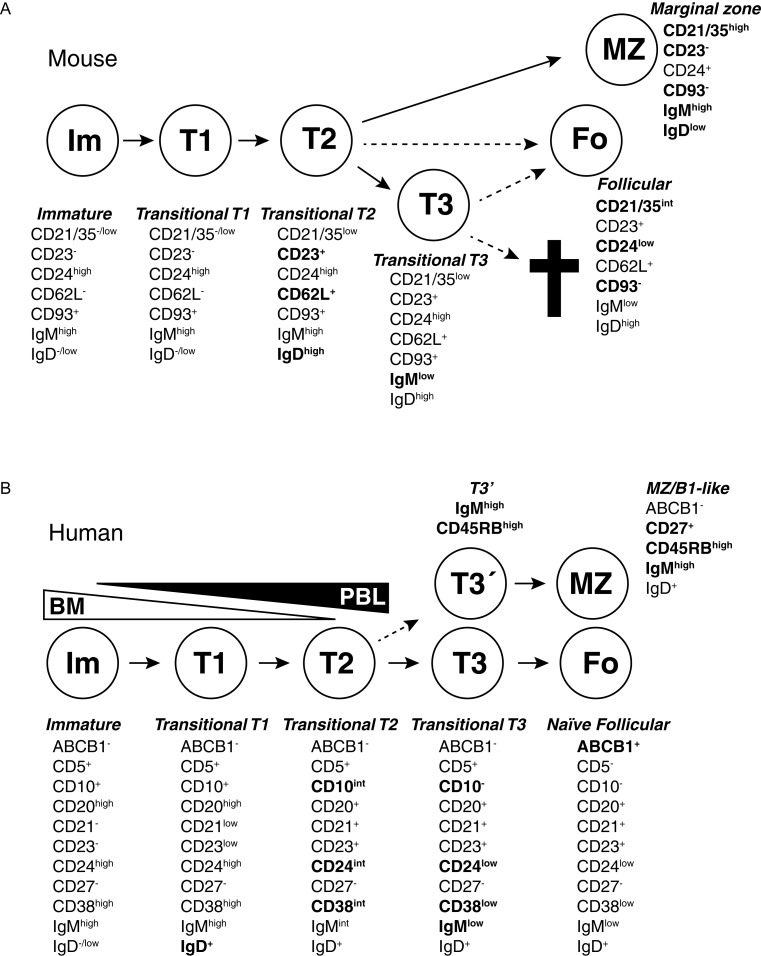
Fig. 2. Transitional B cells in mouse and humans.
B cells go through several stages after leaving the bone marrow but before becoming fully mature. During these stages, self-reactive cells are depleted due to interactions with self-antigens and are also selected into different lineages. The different stages can be defined based on expression of cell surface antigens. Changes important for defining different stages have been marked in bold. A: Several stages that mouse transitional T cells go through after leaving the bone marrow have been defined based on cell surface markers and transfer experiments. T1 cells in spleen are similar to immature B cells in the bone marrow. CD23 and IgD are upregulated when the cells become T2 cells, and T1/T2 cells are also precursors to MZ B cells. A T3 stage has also been defined. T3 cells were first suggested to be a pre-stage to mature follicular cells, but later analysis revealed that this population may be enriched for autoreactive cells and may be targeted for deletion through apoptosis due to interactions with self antigens. Cell phenotypes adopted from Allman and Pillai, 2008[8] in which the relationship between different transitional and more mature stages is discussed. B: Also in humans several transitional stages have been defined, going from immature B cells leaving bone marrow to naïve follicular B cells and circulating MZ-like/B1-like lineage B cells. Although transitional cells are termed T1, T2 and T3 in both humans and mice, there is not necessarily a direct correlation between them. Many expression changes during human transitional development are continuous, making it hard to set clear gates between different populations. The T3 population can be divided into two (T3 and T3′) based on expression of the CD45RBMEM55glyco-epitope and IgM, and CD45RBMEM55/IgMhigh T3 cells appear to be precursors to innate MZ/B1-like B cells. Cells with T1, T2 or T3 phenotypes have been identified both in peripheral blood and in the bone marrow. It is unknown if this is due to circulation of cells or if some cells go through these developmental stages within the bone marrow before release. Cell phenotypes are adopted from Bemark et al., 2012[17].
Expression of CD10 is a sign of early B cell stages, but in humans CD10 is also present on antigen-activated cells in GC[16,17]. This initially led to some confusion, as a circulating B cell population with a phenotype overlapping with that later described for transitional B cells was suggested to be circulating GC founder cells[18]. However, GC B cells are not normally found in peripheral blood and it is now clear that CD10 is a relatively specific marker for early B cell stages in blood[19]. In line with that these cells are at an early stage, CD10-expressing B cells have not been fully negatively selected to avoid self-antigens, and antibodies from them more frequently bind cytoplasmic or nuclear antigens than antibodies from more mature B cells do[20]. A more refined phenotype of transitional B cells was subsequently defined and it was found that less than 5% of all B cells in adult human blood are typical CD24highCD38highCD43+CD10+CD5+IgMhighIgD+ transitional cells do[21-23]. Consistent with the suggestion that these cells are at an early stage of development, they make up a higher proportion of all B cells in children and after hematopoietic stem cell transplantation (HSCT)[23-25], and are also found at a higher frequency among B cells in bone marrow than in peripheral blood[26].
The transitional markers CD24, CD38, CD10 and IgM are gradually lost as B cells develop towards a more mature phenotype, and based on this human transitional B cells have been divided into two consecutive stages, T1 and T2 (intermediate) B cells[21,27]. CD5 expression is lowered on T1 cells in the bone marrow which are likely the earliest transitional cells, and its expression is lost later than other transitional markers in blood[26,27]. Differential expression of CD21 has also been observed on T1/T2 transitional cells, with the majority of the transitional cells being CD21low in spleen whereas CD21high transitional cells dominate in peripheral blood[19]. Based on their characteristics it was suggested that CD21high transitional B cells were at a more mature stage, a suggestion that got support from the observation that low CD21 expression is associated with immature B cells and less mature transitional B cells in the bone marrow[19,21,26]. However, the division of transitional cells into CD21low and CD21high compartments do not fully correlate with stages based on gradual loss of other transitional markers or CD5 expression[19,21], and an alternative explanation of differences in CD21 expression could be selection for deletion or anergy, as low CD21 expression on human B cells have been associated with anergy of auto-reactive cells and exhaustion of memory-like cells[28-30].
Early stages of peripheral B cell development have alternatively been defined based on expression of the ABCB1 transporter protein, with transitional B cells having an ABCB1−IgMhighIgD+CD27− phenotype[31]. The presence, or not, of the ABCB1 transporter can be detected by the ability of cells to extrude the dye Rhodamine. The expression of the transporter is restricted to naïve B cells but it is not present on CD27+ memory cells, class-switched CD27− cells or CD24highCD38highIgMhighIgD+ transitional B cells[31]. When the expression of the ABCB1 transporter and other transitional markers were compared in patients after treatment with the B cell depleting antibody-based drug Rituximab, a third population, human T3 transitional cells were identified. These cells did not express the ABCB1 transporter but were otherwise phenotypically similar to naïve cells[32]. During in vitro culture in the presence of anti-BCR antibodies, CpG and IL-2, these T3 cells developed into apparently normal naïve cells, and it was suggested that T3 B cells were at a stage between T2 and naïve B cells. However, later data demonstrated that the T3 population is not homogenous and can be subdivided into two populations based on expression of IgM and the glycosylation-dependent epitope CD45RBMEM55[25,33]. This division of the T3 population does not seem to represent a linear developmental relationship but two separate pathways of differentiation, an observation that suggests that human B cells, similar to their mouse counterparts, may be selected into different lineages during the transitional stage (see below).
An important question is where and how transitional cells are selected for further survival and differentiation. In mice the spleen has been suggested to play a dominant role[11,34,35], but splenectomized and spleen-less Hox11-deficient mice still develop apparently normal peripheral B cells, only lacking the relatively minor B1 lineage of B cells[36]. Low CD21 expression (which may suggest immaturity, see above) on transitional cells in the spleen suggest that immature human transitional B cells may migrate to spleen to mature[19], but human patients that lack a spleen from birth or go through splenectomy have apparently normal mature naïve B cells. Only a single population, IgM-expressing CD27+ B cells, is lacking in these patients, and these cells may represent a separate lineage of cells (see below)[37-39]. Thus, although the presence of transitional cells in spleen both in mice and humans suggest that the organ may play a role in selecting B cells for survival, B cells can develop into mature naïve cells in the absence of a spleen, a possible exception of cells that belong to specialized lineages of B cells.
It was recently suggested that GALT may play an important role in the maturation of human transitional B cells[3]. The authors in this study found that, in the same individual, the proportions of T1 and T2 transitional B cells were not the same in peripheral venous blood and blood from the portal vein that drain the gut, with T2 transitional cells specifically lacking in portal vein blood. T2 transitional cells were instead enriched in gut Peyeŕ's patches (PP), suggesting that T2 cells are trapped in PP for selection and maturation. Tyrosine phosphorylation suggestive of BCR signals was found in many of the T2 cells in PP, and a possible interaction that could mediate such maturation signals could be interactions with gut antigens. Alternatively, transitional cells respond strongly to toll like receptor (TLR) signals, and the high number of TLR ligands in the gut may drive maturation of transitional B cells[40]. Interestingly, PP do not develop normally in patients suffering from the autoimmune disease SLE, which is associated with diminished expression of the α4β7 integrinon T2 transitional B cells in blood[3]. Since this integrin facilitates gut homing, this may suggest a role for PP in removing autoreactive B cells.
B cell lineages
The concept of lineages - that cells with different but similar characteristics develop along parallel pathways - is well established for T cells. Whereas most T cells rearrange α and β genes to form functional TCR, a separate lineage is created from cells that rearrange γ and δ TCR genes instead[41]. In addition, αβ expressing T cells are selected to become CD4+ helper, CD8+ cytotoxic or invariant natural killer (iNK) T cells in the thymus based on the ability of their rearranged TCR to interact with MHC I, MHC II and CD1d[42,43]. Some of the CD4+ cells take on suppressive functions already in the thymus and become natural regulatory T cells[44]. Later, as a consequence of signals from antigen presenting cells, CD4+ T cells can adopt stable characteristics ranging from regulatory to strongly inflammatory[45]. Thus, although that the term T cells describes similar cells, there exist several parallel pathways for T cell differentiation, making up different lineages.
B cells can recombine either of two different light chain genes (κ orλ) in the bone marrow. Although some differences in antigen specificity has been described between heavy chains paired with κ or λ chains, no clear functional differences between cells expressing one or the other have been described[46]. Regardless which chain they express, mouse B cells are selected into different lineages with distinct phenotypes and functions[8]. The dominant B cell subtype in most lymphoid organs is follicular (FO) B2 B cells, but in mice there are two additional populations that represent distinct pathways of B cell differentiation - MZ and B1 B cells (Fig. 3A). Of these, MZ B cells share their early development with B2 cells in the bone marrow, but are selected into a distinct differentiation pathway during the transitional stage based on BCR interactions and notch signals during maturation[47,48]. B1 cells instead develop from stem cells distinct from the ones that produce FO and MZ B cells[49,50]. If one restricts the term lineage to cell types that derive from different committed non-rearranged precursor cells, B1 but not MZ B cells are in a separate lineage from follicular B2 cells. However, with a less stringent definition - that cells committed to develop along functionally distinct pathways represent different lineages - both are. In this review I will use the latter definition, thus designating both B1 and MZ B cells as separate lineages from FO B cells.
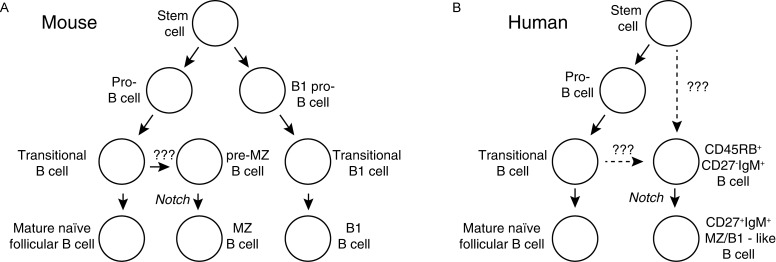
Fig. 3. B cell lineages.
Different lineages of B cells in mice, and data in humans support that this is also true in humans. A: Three mature lineages of B cells have been defined in mice – follicular, marginal zone and B1 B cells. Follicular and marginal zone B cells share early maturation stages and are formed all through life. After MZ B cells receive a yet undefined cue they interact via Notch-mediated signals with cells in the spleen to become mature MZ B cells. B1 cells form, at least partly, from distinct stem cells in bone marrow and fetal spleen. Furthermore, B1 cells formed during early life form a self-renewing pool of cells in peritoneal cavities that does not depend on bone marrow production of cells for maintenance. B: The situation in humans is less clear, but several groups have described cell populations with similarities to MZ and/or B1 cells in lymphoid organs and blood. These express high levels of IgM, CD27 and the CD45RBMEM55glyco-epitope. It is not known if they form from specific stem cells or transitional cells, but a cell population with the ability to differentiate into this lineage through Notch-mediated signals similar to the situation in mouse spleen was recently defined in the human spleen[123].
Table 1. Human memory and memory-like B cells in blood.
Several populations of memory or memory-like B cell subsets have been defined in human blood. The exact relationships between these are still unsure, but their characteristics and presence or not in immune-defect patients cast light on their origins. Their dependence on germinal centers (CD40 signals), gene modifications (the AID protein), spleen (splenectomy), bacterial signals (TLR) and notch signals (haplodeficient in some Alagille patients) and if they have proliferated (diluted KREC levels) and mutated more or less than germinal center cells have been tested. The results of these studies are presented in the table for different B memory-like populations. The data support that class switched cells are typical memory cells formed in germinal centers, and that this also is the case for IgG-expressing CD27− and IgM-only CD27+ cells, although these may leave the germinal centers before class switched cells. IgA+CD27− cells and IgM+IgD+CD27+ MZ-like B cells form in the absence of germinal centers and may not to be “true” memory cells.
| Dependent on | |||||||
|---|---|---|---|---|---|---|---|
| CD40 [92] | AID [67] | TLR [94] | Notch [123] | Spleen [67] | Mutation levels [92] | Number of divisions [92] | |
| Class switched CD27+ B cells | Yes | Yes | No | Normal | Small Reduction | Higher than GC | Much higher than GC |
| IgG CD27- B cells | Yes | Yes | No | ? | ? | Similar to GC | Higher than GC |
| IgA CD27- B cells | No | Yes | ? | ? | ? | Lower than GC | Lower than GC |
| IgM only CD27+ B cells | Yes | No | ? | ? | ? | Higher than GC | Higher than GC |
| IgM/IgD CD27+ MZ-like B cells | No | No | Yes* | Large Reduction | Large Reduction | Similar to GC | Higher than GC |
Whereas mature naïve FOB2 B cells recirculate between B cell follicles in lymphoid organs in mice, MZ B cells are predominantly situated within the MZ of the spleen and B1 cells in peritoneal and pleural cavities[49,51]. However, non-FO B cells are not placid. Substantial numbers of B1 cells appear to transfer between spleen and pleural cavities, and MZ B cells shuttle between the MZ and the B cell follicle within the spleen[52,53]. Although they do not share developmental pathways, B1 and MZ B cells are sometimes together referred to as innate like B cells based on their similarities[54].
Follicular B2 cells are typically involved in the response to T dependent antigens, whereas B1 and MZ B cells have been ascribed other functions, including production of “natural” IgM in the absence of an immune response, responses to T independent (TI) antigens, secretion of cytokines, antigen presentation to NKT cells, and complement-mediated transport of antigen to follicular dendritic cells (FDCs) within B cell follicles[43,53,54]. In addition, MZ and B1 B cells share expression of a small subset of genes normally not present in FO B cells[55]. Some studies have come to the conclusion that B1 B cells may substantially contribute to IgA production in mice through T independent activation of cells[5,56]. However, most recent studies give little support for this notion[57]. For example, most IgA transcripts have mutations in their V regions, suggesting that they had been part of a GC response involving T cell interactions[58-60]. Furthermore, a study where mice were engineered so that B1 B cell in the peritoneal cavity produced antibodies of an allo type distinct from that produced by bone-marrow derived B cells, found that B1 B cells produced natural IgM but made no significant contribution to IgA in the gut[61].
Mouse B1 and MZ B cells can be identified based on if they express a number of cell surface markers that differentiate them from FO B cells. There are at least two phenotypically different types of B1 B cells (B1a and B1b), which appear to represent differentiation pathways rather than distinct stages of development[49]. In addition, there are developmental stages specific for MZ B cells and B1 B cells that differ from the ones described for follicular B2 cells[8,50]. Immature MZ precursors, presumably derived from transitional cells, have been defined in mouse spleen[62,63], and B1 B cells that lack expression of CD11b (Mac-1) has been suggested to be at an earlier stage of differentiation than cells that do express it[64]. Thus, the lineage concept in mice is not only supported by differences between mature B cells, but also the definition of distinct developmental stages on the way to full maturation of the cells.
Human follicular B cells
Many early studies of human B cells did not divide the cells into subpopulations based on if they are transitional, naïve or memory B cells or if they belong to distinct lineages, as the large diversity within the compartment was not appreciated. Although the intention was to study the in vitro function of mature naïve follicular B2 cells, the B cell fraction in many studies contained phenotypically distinct cell types. In particular, as many as 40% of all B cells isolated from human adult blood have mutations in their antibody genes, suggesting that they are memory cells or belong to a lineage distinct from FO B cells[65]. In tonsils, lymph nodes and spleen there is also considerable diversity among the B cells, and GC, memory and MZ-like B cells make up substantial numbers[16,66,67]. Sometimes B cells were separated on Percoll gradients based on relative density to distinguish between resting and activated cells to get a more defined population[68]. However, many mutated CD27+ B cells separate together with naïve cells when this approach is used, making it hard to judge the relative importance of naïve follicular and memory B cells responses in subsequent in vitro cultures[69]. This has to be taken into account when interpreting data from human functional studies performed in vitro without separation of B cells into subtypes.
The most straight forward way to purify naïve follicular B cells from human blood is to remove cells that express CD27[65]. However, this approach will not differentiate naive B cells from cells at transitional stages or class-switched cells that do not express CD27[31,70]. Subsequently, the addition of alternative markers offers advantages over CD27 alone in defining naïve cells. Among markers that can be used are the CD45RBMEM55glyco-epitope that is expressed on most class-switched CD27− cells, cells at pre-stages for MZ B cells and on all CD27+ cells, and expression of the ABCB1 transporter that is largely restricted to naïve cells[25,31,33].
Studies using CD27− purified naïve B cells have shown that resting FO B cells need stronger signals for activation than CD27+memory B cells. Naïve human B cells express low levels of TLRs and do not respond with proliferation or differentiation after TLR stimulation unless they are first stimulated through antigen receptor crosslinking which differentiate them from memory cells[71,72]. In line with this, there are many differences between CD27-expressing and naïve human B cells with regard to expression of activation markers, co-stimulation molecules and survival factors[73].
Human MZ B cells
Like mice, humans have MZ in spleen that contain IgM-expressing B cell distinct from FO B cells. There are differences between MZ B cells in humans and mice[51,67,74]. Whereas mouse MZ B cells have unmutated antibody genes, B cells in human MZ have mutations in the V regions and usually express the memory marker CD27[75,76]. CD27-expression is lowered or even missing on B cells in the MZ of young children, suggesting that the MZ and the cells within it gradually develop during infancy[77]. Nevertheless, CD27+ splenic MZ B cells of polyclonal origin are present in children less than 2 years old, whereas GC and class-switched memory B cells are highly oligoclonal at this stage[67,78]. This suggests that human MZ B cells are part of a specific lineage that develops in parallel with FO B cells in a polyclonal fashion, whereas GC and memory B cells form as a consequence of antigen activation and selection. There is data to indicate that marginal zone B cells in humans, as in mice, are involved in T independent responses to blood-borne antigens[51,74,79-81], and it has been suggested that the reason that humans without a functional spleen are vulnerable to infections with encapsulated bacteriais that they have an inability to form protective MZ B cell derived antibody responses against T independent antigens[38,82].
Humans have areas in PP, tonsils and activated lymph nodes that contain B cells similar to splenic MZ B cells[66]. These are situated mainly towards regions containing a high antigenic load, such as the subepithelial domes of PP, subepithelial regions in the tonsils and subcapsular regions of lymph nodes[83-85]. Like splenic MZ B cells, the majority of MZ-like cells have mutations in their V regions[69,86,87]. However, it is uncertain if these MZ-like cells are identical to splenic MZ cells, or if the cells have distinct local functions. An interesting possibility is that splenic MZ B cells and MZ-like B cells from other organs are involved in local transport of antigens into B cell follicles, a role that MZ B cells appear to have in mouse spleens[53,88,89]. Alternatively, MZ-like cells may be at a pre-stage where they are selected into the mature MZ B cell lineage[2,3,67]. Whichever the function of MZ-like cells, it is clear that cells in the spleen has some non-redundant, as other localities cannot functionally substitute for the spleen in asplenic individuals[51,82].
A link between human blood mutated CD27+IgM+IgD+ cells and MZ cells in spleen was suggested based on that two cell types are highly similar to each other[37,67]. For example, hyper-IgM patients that lack CD40L or CD40, a receptor/ligand pair critical for cognate B-T cell interactions, have significant numbers of circulating CD27+IgM+IgD+B cells, demonstrating that CD27+IgM+IgD+ B cells can form in the absence of GC reactions[90,91]. Furthermore, following splenectomy circulating IgM+IgD+CD27+B cells largely disappear[37,38,78,90,92,93], and MZ B cells in spleen and circulating CD27+IgM+IgD+B cells develop in parallel during infancy[78,94].
Thus, there are several lines of evidence to suggest that MZ B cells and circulating CD27+IgM+IgD+B cells are linked and may belong to the same compartment, a notion that has many implications. It, for example, suggests that human MZ B cells and memory cells are almost identical to each other with regard to mRNA expression and in their responses to stimuli, although they do not share developmental pathways or GC dependence[73]. Furthermore, it implies that human and mouse MZ B cells differ in their migration patterns; human MZ B cells re-circulate in blood whereas their mouse counterpart are maintained in the spleen and only move between the MZ and the follicle[53].
A recent study of patients with known inherited defects in TLR signaling pathways made interesting observations regarding the requirements for the formation of CD27+IgM+IgD+ B cells. Despite the fact that both circulating MZ and memory CD27+ human B cells in blood express a similar set of TLRs, it was found that that patients that lacked certain proteins involved in TLR signaling showed a specific lack of CD27+IgM+IgD+B cells whereas they had normal numbers of class-switched CD27+memory cells[72,94,95]. Only certain TLR gene defects (MyD88, IRAK4, TIRAP) were associated with this lack whereas others (TLR3, TRIF, UNC93B) were not. This indicated that extracellular but not endosomal TLR signals were involved in selecting or maintaining CD27+IgM+IgD+cells.The paper also demonstrated that TLR signals are not necessary to maintain human class-switched memory cells through polyclonal activation, a notion that had been previously suggested based on their expression of TLRs[96-99].
Human B1 B cells
In contrast to mice, human adults have few B cells in the peritoneum[100,101] or the omentum that is situated close to it[102]. Thus, if there are human B1 cells their tissue distribution differs from that in mice. Studies designed to identify human B1 have therefore focused on cells circulating in blood, assuming that B1 cells would be circulating in a similar fashion as FO B cells. In humans, chronic lymphatic leukemia cells often express CD5, and the identification of CD5-expressing mouse B1 cells was, at least in part, the result of an attempt to define the normal counterpart of this neoplasm[103]. As a consequence, it was for a long time assumed that CD5 expressing human B cells also represented a B1 lineage[104,105]. However, it is now clear that the majority of CD5-expressing human B cells are at early transitional stages of development and do not belong to a B1 lineage[27].
Instead, it was recently suggested that co-expression of CD27 and CD43 can be used to identify human B1 cells in blood[106]. The authors found that such cells displayed characteristics associated with mouse B1 cells, namely that they were present already in cord blood, expressed unmutated antibody genes, spontaneously secreted IgM, were efficient antigen presenters and displayed an antigen reactivity similar to mouse B1 cells. Most of these B1-like cells also expressed CD5, although they made up a small proportion of all CD5-expressing B cells in blood. A later study found that CD11b, the human equivalent to the mouse Mac-1 antigen that is expressed on a population of mouse B1 cells, could be used to subgroup cells belonging to the CD43-expressing human B1 populations, and that these subgroups differed in their spontaneous secretion of IgM, production of IL-10 and their ability to stimulate T cells[107,108]. Subsequent studies suggested that the number of CD27+CD43+B cells is increased in lupus patients and after HSCT and decreased in patients with common variable immunodeficiency or multiple sclerosis (MS)[107,109-112], and that they are involved in the response to capsular polysaccharides from Streptococcus pneumonia[113,114]. In addition, CD27+CD43+ B cells were found in immunodeficient mice reconstituted with human hematopoietic stem cells, both in spleen and in the peritoneum[115].
The role of CD27+CD43+B1 B cells in human blood has, however, been questioned by several groups that argued that the cells were early plasmablasts and/or flow cytometry artifacts[116-118]. A comparison of published gene expression profiles of proposed human B1 cells and mouse B1 cells found very few similarities between them[55]. Many researchers have also failed to identify a substantial population of B cells expressing CD27 and CD43, and instead find that the majority of B cells that express CD43 are CD27− transitional cells[21]. The correct gating strategy to identify the cells has been discussed, and it has been argued that whereas T cells are CD43high, the B1 cells are CD43low[111,119]. A recent study supported the view that CD27+CD43+ B cells in blood where at a preplasmablast stage of differentiating B cells in blood instead of a B1 subset[120]. This was based on a number of observations. The number of antigen-specific cells having this phenotype increased after immunization with a T cell-dependent tetanus toxoid-based vaccine, and the cells had an RNA expression profile between memory B cells and plasmablasts. In addition, other B cell subsets could differentiate in vitro to the proposed B1 phenotype, and cells with the B1 phenotype could in turn differentiate to a plasmablast/plasma cells. Nevertheless, the group that originally identified the cells have strongly contested any suggestions that they are not human B1 B cells[121].
It can be concluded that there is currently no consensus regarding the existence and functional importance of CD27+CD43+ B cells, and whether they are the human equivalent of mouse B1 B cells or not. Even if the majority of these cells are plasmablasts, it is still hard to exclude that a proportion of them represents a distinct lineage[122]. Notably, the proposed phenotype of the cells partly overlap with that proposed for circulating CD27+IgM+IgD+ MZ B cells, and differences between the two subsets are subsequently to some extent a question of semantics. There are indeed many functional similarities between B1 and MZ mouse B cells, and it may be a single population of IgM+IgD+CD27+ B cells in humans that makes up all innate-like B cells. Interestingly, mouse B1a B and human CD27+IgM+IgD+B cells both depend on a spleen for their development, linking these populations[36-39].
Human precursors to non-FO B cell lineages
B cell reconstitution after pediatric HSCT has provided novel insights into human non-FO B cell lineages. Recently, we found that the majority of all B cells had a CD27−IgMhighphenotype for more than one year after pediatric HSCT[25]. Based on this phenotype alone, it would seem that the cells were T1/T2 transitional B cells. However, the number of CD27−IgMhigh cells was much higher than the number of transitional T1/T2 B cells. We found that CD45RBMEM55, a glycosylation-dependent epitope that is normally expressed on human memory B cells[33], was expressed on 35% of all CD27−IgMhigh B cells and that CD45RBMEM55high B cells did not express transitional markers. Expression of the CD45RBMEM55epitope divided the T3 transitional B cell population into two similarly sized subpopulations, CD45RBMEM55highIgMhigh and CD45RBMEM55lowIgMlow T3 cells. In addition, we found high expression of CD45RBMEM55 on a population of IgMhigh B cells that unlike T3 expressed the ABCB1 transporter. Notably, all cell populations defined using this marker - T1, T2, CD45RBhighIgMhighT3, CD45RBlowIgMlow T3 and CD45RBhighIgMhigh non-T1/T2/T3 B cells - were found in increased numbers after HSCT, in cord blood and in healthy children compared to adults, consistent with them being early B cell developmental stages[25].
It seems unlikely that all these B cell subtypes represent consecutive stages of development of a single lineage, as this would require the cells to change CD45 glycosylation patterns and IgM expression levels several times during their early antigen-independent development. An intriguing alterative is that cells that express high CD45RBMEM55 levels have been selected to develop into a separate lineage. Instead of a gradually diminishing expression of transitional markers as is observed along the T1/T2/CD45RBlowIgMlow T3/FO naïve B cell axis, the development of this lineage would be characterized by a relatively rapid loss of transitional markers, continued expression of high IgM levels, and a change in CD45RB glycosylation. Finally, CD27 expression is induced, resulting in the formation of human MZ B cells.
Interestingly, in mice, the selection of MZ B cells is regulated through glycosylation changes. If transitional B cells receive a so far ill-defined cue to enter the MZ lineage, they will change their Notch 2 glycosylation pattern through activation of the glycosyltranferases Manic and Lunatic Fringe. This subsequently allows Notch 2 to engage with Delta Like (DL) 1 expressed on vascular endothelial cells in the MZ of the spleen, triggering a signal that will select the cells to develop into MZ B cells. Thus, CD45RBMEM55 expression changes due to glycosylation changes on human B cells may indicate differentiation into an innate-like B cell lineage. It is unclear if the CD27− CD45RBMEM55high B cells would then correspond to T1/T2 cells that have received a developmental cue but have not been selected based on Notch 2 signals, or cells that have already been selected based on Notch 2 signals or even a lineage developing from distinct stem cells akin to B1 B cells in mice. Interestingly, whereas MZ B cells in adults are CD27+, no or little expression of CD27 has been observed in splenic MZ from children[77], suggesting that CD27−CD45RBMEM55high B cells may home to the marginal zone even before expressing CD27.
The suggestions that MZ precursor cells can be identified based on the expression of CD45RBMEM55 on non-switched CD27− B cells was recently supported from a study from another research group[123]. The authors of this study found that patients with Alagille syndrome as a consequence of Notch 2 haplo-insufficiency had lowered numbers of circulating MZ B cells in blood, a situation that is similar to that observed in Notch 2 haplodeficient mice. They then demonstrated that non-switched CD27− CD45RBMEM55high B cells isolated from spleens of healthy donors were induced to express CD 27 when they were cultured on feeder cells expressing the Notch 2 ligand DL1, and then differentiated to cells that were highly similar to mature MZ B cells. Before stimulation, CD27−CD45RBMEM55high B cells had a gene expression profile in between naïve FO B cells and MZ B cells and this was altered towards a profile more similar to MZ B cells during DL1 feeder cultures. Similarly to B cells in blood discussed above, splenic CD27−CD45RBMEM55high B cells could be divided into two populations based on expression of the ABCB1 transporter, with ABCB1-expressing cells being more similar to naïve cells and cells lacking ABCB1 more similar to MZ B cells with regard to gene expression profiles, mutations in their V regions and tendency to upregulate CD27. Thus, these two populations of B cells in human spleen may represent consecutive steps in the maturation towards mature MZ B cells. Alternatively, it is an effect of plasticity (where both transitional and FO B cells can be selected to become MZ B cells; something that indeed has been suggested in mice[8]). Furthermore, whereas the study demonstrate that these cells can become MZ B cells, it is still not known what triggers them to enter into this pathway during the transitional stage.
Activation of B cells in lymphoid tissues
Activation of antigen-specific B cells in lymphoid tissues has been studied extensively in mice. Recent data suggest that mature naïve FO B cells primarily encounter antigen within B cell follicles on FDCs. In lymph nodes, low molecular antigens are transported through lymphoid conduits whereas larger antigens are trapped on macrophages at the subcapsular sinuses that facilitate further transport into the B cell follicles that is mediated through antigen-independent interactions with complement receptors on B cells[124,125]. MZ B cells play a similar role in spleen where they transport of antigen from marginal zones into B cell follicles based on interactions with complement receptors[53]. Antigen-activated FO B cells travel from the B cell follicle towards the T cell area where cognate interactions occur between B and T cells recognizing the same antigen[126]. Following this encounter, B cells either differentiate into early short-lived plasma cells or return to B cell follicles where they proliferate to form GC[127,128]. In the GC, B cells continue to rapidly proliferate, and during this process they undergo CSR to antibody classes other than IgM, undergo random mutagenesis of their antibody V regions through SHM and are selected based on antigen affinity[129]. Finally, the B cells leave the GC to become memory cells or long-lived plasma cells homing to the bone marrow[127]. As a consequence of the processes in the GC, the cells that leave carry mutations in their V regions, are of high affinity, and often express other class-switched antibody classes.
It has been hard to study the faith of antigen-specific B cells from human GC, since lymphoid organs are rarely available from recently vaccinated and/or infected individuals. Most studies have instead focused on GC B cells that respond to unknown antigens. An important source of human GC B cells has been tonsils that are removed due to chronic inflammation and/or snoring. In judging tonsil data, it has to be remembered that inflamed tonsils may not be representative of non-inflamed tissues. In addition, tonsils are to some extent more similar to GALT than to lymph nodes, with constant contact with microbiota and with a universal presence of GC.
Nevertheless, tonsil analysis has given a good overview of the phenotype of human B cells inside and outside of GC. The expression of two cell-surface markers has been used extensively to define their differentiation stages, CD38 and IgD[16]. In humans, high CD38 expression is primarily found on B cells in GC and IgD is expressed at high levels on naïve pre-GC B cells but at low levels in the GC B cells, post-GC plasmablasts as well as in class-switched and IgM memory cells (see below). Thus, during a GC reaction, B cells differentiate from being CD38lowIgD+(naïve), via CD38highIgD+ (pre-GC) and CD38highIgD− (GC) to finally become CD38−IgD− memory cells.
Cell types defined by CD38 and IgD expression are often further divided corresponding to the so-called Bm system[130,131]. According to this scheme, naïve cells in the CD38lowIgD+ quadrant is made up of two populations, CD23+ Bm2 cells that can develop into CD23− Bm1 cells. Following activation these become pre-GC CD38highIgD+ Bm2′, and then differentiate to become Bm3 CD38highIgD− (GC) cells that can be defined as Bm3 CD77+ centroblasts and Bm4 CD77− centrocytes. After leaving the GC cells differentiate to long-lived Bm5 CD38−IgD− memory cells, or plasmablasts destined for final plasma cell differentiation.
Still, there are weaknesses to the Bm system. For example, transitional cells in tissue would be classified as Bm2′ cells, a problem that is further accentuated by that both GC cells and transitional cells express CD10 and led to the false identification of circulating pre-GC cells in blood[18]. A possible way around this is to include CD24 that is expressed at high levels on transitional cells but lower levels on GC cells. Another weakness is that IgD−CD38highplasmablasts would be included among the GC cells. In addition, we recently described a population of CD38high cells in tonsils that express high levels of CD45RBMEM55 that does not localize to GC[33]. Thus, although the Bm system has advantages and has been extensively used to define B cells subtypes in tissues, there are some caveats to using this system if additional markers are not used to identify cells.
GC can be divided into two regions, the dark and light zones. Proliferating B cells are primarily found in the dark zone, whereas B cells in the light zone are at a stage where they are selected based on interactions with antigens presented as immune complexes on FDCs[129]. Traditionally, centroblasts have been thought to populate the dark zones and centrocytes the light zone. In mice, B cells in these zones can be distinguished from each other based on their expression of CXCR4, CD83 and CD86, and these divided cells into more stringent subpopulations than the classical centroblast/centrocyte division[132]. More recently, it was demonstrated that the same markers could be used to identify human GC B cells dependent on localization, with dark zone GC B cells being CXCR4highCD83lowCD86lowand light zone cells being CXCR4lowCD83highCD86high[133]. Remarkably, the gene expression profiles differed between light and dark zone cells similarly in humans and mice, and indicated differences in proliferation and activation programs[133].
Regulatory B cells
The existence of lymphoid cells with the ability to suppress immune responses was suggested already in the 60s. Many studies during the 70s aimed to define and characterize these so called suppressor cells, and pointed to the existence of thymus derived suppressor T cells[134]. However, further characterization proved difficult, and it was not until 1995 that regulatory CD25-expressing CD4+ T (Treg) cells that could hinder the development of autoimmune disease were defined[135]. Subsequent studies have proven their importance in humans and mice, and several subtypes of Treg are now acknowledged[44].
Shortly after the description of Treg, mouse studies suggested that B cells also can take on regulatory roles[136]. IL-10 production from B cells was identified as an important mechanism to suppress immune induced morbidity in mouse models of MS arthritis and gut inflammation[137-139]. This suggested the existence of IL-10 producing regulatory B cells akin to regulatory T cells that could suppress immune responses. However, a definitive phenotype of regulatory B cells has been elusive[140,141]. IL-10 expression in B cells can usually only be detected after in vitro stimulation, and different research groups have designated different phenotypes to B cells that can be induced to produce IL-10. It has even been argued that cells defined as regulatory B cells only should be considered precursors, with plasma cells being the effector producers of IL-10 as well as IL-35 in vivo[142,143]. Thus, whereas B cell IL-10 and possibly IL-35 production play important roles in vivo, it has been very hard to elucidate which B cells that produce it and where, when and how it regulate immune response.
With the identification of the importance of IL-10 producing regulatory B cells in mice, a search for similar cells in humans followed. It was demonstrated that IL-10 production could be induced in CD27− non-memory cells B cells from blood upon in vitro stimulation, and that the numbers of these cells were lowered in MS patients compared to healthy controls[144]. Later it was found that helminthic infection decreased disease in MS patients and that this parasitic infection was associated with increased number of CD1d+ naïve B cells that produced IL-10[145,146]. It was also established that cells with a phenotype indistinguishable from transitional B cells (i.e. being CD24highCD38highCD10+CD27−) produced IL-10 after in vitro stimulation, and that these cells inhibited T cell activation in vitro[147,148]. Furthermore, IL-10 induction and the ability to inhibit T cells were defective in SLE patients, although the number of transitional B cells was normal or increased[147,148]. Contrary, but probably with a similar outcome, in RA patients the number of B cells with a transitional phenotype was lowered, resulting in diminished IL-10 production[149]. Similarly, the number of CD24highCD38high B cells was lowered in non-splenectomized patients with immune thrombocytopenia with low platelet counts, and immune thrombocytopenia patients showed diminished numbers of B cells that produced IL-10 and inhibited monocyte activation regardless of platelet count and if they were splenectomized or not[150]. Interestingly, treatment of thrombocytopenia patients with dexamethasone resulted in increased levels of IL-10 in B cells[151].
Transitional type human B regulatory cells have not only been studied in the light of autoimmune diseases. For example, IL-10 production from transitional B cells was associated with graft tolerance in kidney transplant patients[152]. Furthermore, IL-10 production from CD24highCD38high B cells has been linked to diminished CD8+ T cell responses and more active viral disease in both chronic hepatitis B and HIV patients[153,154].
However, as in mice, the phenotype of IL-10 producing B cells in humans is to some extent controversial. IL-10 production was observed in most B cell populations after in vitro stimulation of the cells, although the relative number of cells that produced IL-10 was increased among CD27+ memory and CD24highCD38high transitional populations[155]. Iwata et al. observed that IL-10 expressing B cells expressed the memory marker CD27[156]. Unexpectedly, and in contrast to the studies mentioned above, it was found that the number of B cells that could be induced to IL-10 expression was increased in many autoimmune conditions[156]. However, active Graves’ disease was later associated with a lowered number of IL-10 producing B cells that expressed CD27 that could inhibit CD4 proliferation and cytokine secretion in vitro[157]. In addition, CD11b-expressing B cells from the controversial CD27+CD43+ human B1 population discussed aboveappear to spontaneously secrete IL-10, possibly linking them to plasmablasts/plasma cells that secrete IL-10 and IL-35 in mice[108,143].
It has been suggested that all B cells may be able to produce IL-10[141,155]. In this case, normal naïve B cells may play a more important role than observed in the studies above - even though a relatively lower proportion of them respond with IL-10 expression, they would still dominate in numbers in vivo due to the large size of the compartment. Alternatively, different B cell populations may respond with IL-10 production to different stimuli, and may be involved in different responses[140]. It should be noted that regulatory B cells that spontaneously secrete IL-10 are very rare, at least in blood[156], and where and when they conduct suppressive functions in vivo is largely unknown. Furthermore, even after stimulation, a relatively small proportion of B cells secrete IL-10, and it is possible that they do not do so until final differentiation to the plasma cell stage[142]. Thus, although that mouse in vivo data clearly demonstrate that IL-10 producing cells from the B cell lineage play important regulatory roles, it is currently hard to predict which human B cell subtypes that are important in vivo and where and how they conduct their functions. Thus, unlike Treg that can be reproducibly sub-classified by phenotype and function, regulatory B cells with the Breg tag are not a clearly defined entity.
Memory B cells
Memory B cells are mature cells that have previously encountered antigen and as a consequence have changed their functional characteristics so that they have become long-lived and more apt to respond to subsequent antigen encounters. In humans it is hard to know if a given cell has previously encountered antigen, and memory cells have instead been defined based on secondary changes induced during immune responses, for example signs of that the cells have gone through the germinal center process, such as that CSR has taken place or that their antibody chains have been mutated through SHM. An important step in the study of human memory B cells was the realization that essentially all B cells that carry V region mutations express CD27[65]. Subsequently, CD27 expression has been used extensively as a marker to identify human memory B cells. Other phenotypic markers have been identified, such as that memory B cell are unable to extrude the dye Rhodamine 123 due to a lack of the ABCB1 transporter, or that they carry a specific glycosylated epitope on the RB exon of CD45[31,33]. Importantly, the expression of these markers show a slightly different profile than CD27, and this have demonstrate that CD27 expression is not as closely linked to memory B cells as first thought. Rather, a combination of markers may be needed when identifying memory B cells.
Class switched memory cells
Whereas the nature of IgM+CD27+ B cells has been debated (see below), few have questioned that class switched CD27+ B cells represent memory cells. Their antibody V regions are heavily mutated as a consequence of SHM, and they are almost totally absent in hyper-IgM patients that lack expression of CD40L or CD40, demonstrating the importance of T cell interactions and GC reactions in their formation[90]. Nevertheless, recent data demonstrate that substantial numbers of class-switched cells do not express CD27, and that class-switched cells can form in the absence of CD40 interactions.
Class-switched human memory B cells that do not express CD27 were first described when the expression of the ABCB1 transporter protein on human B cells was studied[31]. CD27− class-switched cells, like CD27+ B cell, lack ABCB1 and were found to express IgA, IgG1 and IgG3 but rarely IgG2. Like CD27+ class-switched cells, they produce antibodies reactive to antigens such as tetanus toxoid or influenza, and make up as much as one quarter of all IgG-expressing B cells[31,70]. Similar to their CD27+ counterparts, CD 27− IgG+ and IgA+ memory cells have mutated antibody genes, although in the case of IgG expressing cells the frequency of mutations is lower in CD27− than CD27+ cells[70,92]. Thus, humans possess a sizeable fraction of class-switched cells, hypermutated memory B cells reactive to previously encountered antigens that do not express CD27.
In addition to their expression of switched antibody classes and ABCB1, CD27− class-switched cells can be identified using expression of CD45RBMEM55[31,33]. This marker can also aid in the identification of other B cell subtypes, such as transitional cells and MZ precursor cells (see above). Notably, this means that a lack of IgM-negative CD27+ B cells is not a proof for that class-switched memory B cells are not present as such cells may lack CD27. Thus, care needs to be taken when analyzing the presence of class-switched memory B cells, for example by using markers alongside CD27 and/or antibodies specifically recognizing IgG and IgA.
As an example, CD40L-deficient hyper-IgM patients maintain normal or even slightly increased numbers of CD27−IgA+ cells whereas there is an almost complete absence of all other class-switched B cells[92,158]. Furthermore, CD27−IgA+ B cells have gone through fewer cell divisions than typical GC cells in healthy adults, suggesting that many of them do not form in GC even when CD40 signals are present[92]. Noteworthy, IgA production in gut as well as serum has been observed in both humans and mice that lack CD40 signaling, demonstrating that substantial CSR can occur in the absence of GC[57,59,90,159,160]. It has been suggested that CD40-independet IgA CSR may occur in the lamina propria of mucosal membranes[160,161]. However, other groups, including our, have not been able to detect any signs of ongoing CSR outside of organized tissues, either in humans or mice, and there are therefore reasons to believe that CD40-independent, and therefore GC-independent, IgA-CSR is almost totally restricted to organized lymphoid tissues[57,59,159,162-165].
There has been a long-standing interest to study the specificities of the memory B cell compartment, but these efforts have been plagued by the detection of cells with a single specificity due to their low frequencies. The approach used most successfully has been to activate memory cells in an antigen-independent manner using polyclonal activators, either rather ill defined mitogens or defined TLR ligands[166,167]. These methods have been used to define the development of memory B cells over time and compare it to antibody titres in serum. Whereas there for some antigens, in particular natural infections, there is such a correlation, for other, notably vaccine antigens, there is not[97,98].
IgM+ memory cells
The idea that there is a substantial population of IgM-expressing human memory B cells originates from the observation that IgM transcripts isolated from peripheral blood often carry V region mutations, suggesting that these are IgM+ post-GC B cells[168,169]. It was later demonstrated that mutated IgM-expressing cells express higher levels of IgM transcripts than other IgM expressing cells, and that their relative numbers therefore cannot be directly inferred from the relative proportion of mutated transcripts[170]. Nevertheless, single cell cloning demonstrated that approximately 10-20% of all B cells in blood are mutated IgM-expressing cells[170]. These IgM-expressing cells with V region mutations were later found to express CD27, whereas IgM+CD27− B cells were very rarely mutated[65].
Following the demonstration that CD27+IgM+ B cells are present in peripheral blood of CD40L and CD40 deficient individuals, it was concluded that at least some formed outside of GC[90,91]. Based on multiple similarities between cells found in the marginal zone of the spleen and circulating CD27+IgM+ cells, it was suggested that these were not memory cells but rather the human equivalent to mouse marginal zone B cells (see above). Mass sequencing of antibody genes indeed revealed little clonal overlap between IgM and class-switched blood CD27+ B cells, suggesting that they were not descendants from the same proliferating GC clones[93].
However, long-lived IgM-expressing memory cells can form in mice[171,172], and there are arguments in favor of “true” human IgM-expressing memory cells[173]. As noted above, the majority of IgM+CD27+ B cells are not clonally related to class-switched B cells[93]. However, identical V(D)J rearrangements and shared mutations from IgM and IgG have been detected in blood using targeted PCR amplification of selected antibody genes[174]. A possible explanation is that IgM+CD27+ B cells can be divided into two subpopulations, a dominating one expressing IgD and IgM and a smaller one made up of IgM-only cells[65,67,92]. IgM-only CD27+ B cells have gone through more cell divisions and carry more mutations than GC B cells demonstrating that they may be GC decedents, whereas IgM+IgD+ CD27+ B cells have gone through less cell divisions and carry less mutations and are likely not generated in GC[92]. Interestingly, the presumably post-GC IgM-only population is enlarged in hyper-IgM patients that lack AID, a protein critical for somatic hypermutation of V regions and CSR[37,67]. Patients with this mutation have enlarged GC filled with non-switched IgM-expressing B cells, and thus appear to form increased numbers of post-GC IgM-only memory cells.
Taken together, current data support the view that whereas the relatively larger IgM+IgD+ CD27+ B cell population is not made up of memory B cells but circulating MZ B cells (or possibly human B1 cells), the smaller IgM-only CD27+ B cell population is composed of post-GC IgM-expressing memory B cells.
Plasmablasts and plasma cells
None of the cells discussed above are directly involved in the major effector functions of the B lymphoid lineage, the production of secreted antibodies. Antibodies play important roles in resolving ongoing infections, are critical for sterile immunity following contact with pathogens or vaccines, and represent a memory that can be transferred into the unborn child from the mother. In addition to being beneficial, pathogenic antibodies play roles in the development of autoimmune diseases, allergies and some types of rejection of transplanted organs.
For the production of secreted antibodies to start, cells need to differentiate into a plasma cell. This differentiation goes via an intermediate stage known as plasmablasts that form in lymphoid organs and then travel via blood to home to their final destination. The final differentiation can be triggered in different ways depending on B cell subtype involved, but in most cases involve activation of B cells through binding of antigen to the BCR (i.e. antibodies on the cell surface of B cells) to ensure antigen specificity. Studies using model antigens in mice have divided B cell antigens into two major subtypes, thymus dependent (TD) and independent (TI) antigens[175]. In general, TD antigens require interactions between B and T cells whereas TI antigens do not. A typical example of a TD antigen is a non-repeating protein that trigger BCR signals and at the same time can be proteolytically processed and presented to T cells as peptide on MHC II molecules on dendritic cells and B cells. This induces GC responses, followed by the formation of memory B cells and plasma cells. TI antigens can be divided into TI-1 antigens that consist of epitopes linked to TLR ligands that give simultaneous activation of the BCR and TLR, and TI-2 antigens that are multivalent antigens that result in extensive BCR crosslinking. In general, these will not trigger GC formation, and result in relatively little production of antibody classes than IgM and diminished development of memory B cells. Notably, most infections will result in a mixture TD, TI-1 and TI-2 antigens that will trigger multiple B cell responses. Still, in the development of vaccines, differences between antigens need to be taken into account.
Some plasma cells, in particular the ones situated in bone marrow, are very long-lived, and do not depend on continuous differentiation of other B cells to maintain specific antibody titres in serum. Others, often formed and maintained within lymphoid organs, are short lived and die within days after their formation. Long-lived plasma cells have been associated with TD antigens and short-lived with TI antigens, although recent data argue that this division may not be absolute[176]. The ability of the bone marrow to support long-lived plasma cells is dependent on that supporting cells, including stromal cells, macrophages and eosinophils[177]. In addition, signals to B cells during the response may induce intrinsic signals to the B cells that promote long-term survival[178-180]. Thus, some antigens, in particular natural infections, result in responses that do not decline for the life-time of the individual, whereas other, in particular in some vaccine, induce responses with antibody half-lives around 10 years[97]. It was recently observed that fully mature IgM and IgA but not IgG expressing plasma cells not only secrete antibodies but also express cell surface antibodies[181,182]. This allows for purification of living antigen-specific plasma cells, but since these also can transmit BCR signals, also raises the possibility that antigen may influence the homing and/or longevity of plasma cells[181,182].
In humans, in vivo studies of the final differentiation stages have been done mainly using peripheral blood samples. The titres of specific antibodies, in particular IgG class antibodies, have been used as a proxy for long-lived plasma cells, in particular some time after the initial response[97]. Measurements of antigen-specific antibodies of different classes in blood has been done extensively over the years, and are clinically used both to determine if the titres are sufficient for protection after vaccination or infection, and to determine if an individual has previously been infected with a certain pathogen
In addition, the presence of antibody producing plasma blasts in peripheral blood has been determined using ELISPOT[183]. This approach forms the basis for identifying antigen-specific plasmablasts in blood, often in combination with cloning of antibody genes from single B cells. Using these techniques, it was shown that a recall response to influenza vaccine is dominatedby CD38highCD27highCD19+CD20-/low antigen-specific plasma blasts that form within seven days after immunization and produce high affinity antibodies[184]. Such cells make up as many as 6% of all CD19+ B cells seven days after influenza vaccination and then disappeared from the circulation during the next week. After infection with the novel H1N1 influenza strain, the response was instead dominated by cells producing broadly neutralizing antibodies recognizing atypical epitopes on the viral particle, suggesting that the plasma blasts derived from memory cells against these epitopes rather than naïve strain-specific B cells[185]. Similarly, early after infection with dengue fever, large numbers of antigen-specific plasmablasts were encountered in blood[186]. An increase in the number of IgG expressing plasma blasts of a similar magnitude was observed after revaccination against tetanus/diphtheria, whereas plasma blasts observed in the absence of vaccination were primarily IgA producing cells expressing β7 integrin and CCR 10 indicative of gut homing[187]. The steady state IgA plasma blast levels were maintained during CD20-depleting Rituximab therapy, suggesting that gut B cell responses are not sensitive to this treatment[188]. However, IgA producing plasma blasts in blood also can derive from specific immunizations[57], and both infection with V. cholerae and oral vaccination with the drinkable cholera vaccine Dukoral induced circulating IgA and IgG plasma blasts specific for the pathogen[189].
Notably, most studies of circulating plasma blasts have been done in subjects that may have encountered the immunizing antigen before, and there are differences in memory responses vs. primary or repeated boost responses[189,190]. Also, it has to be remembered that plasmablasts in blood primarily consists of IgG and IgA cells generated from GC responses destined to become long-lived plasma cells, whereas the relatively large numbers of primarily short-lived plasmablasts/plasma cells that form within lymphoid tissues may never enter the bloodstream. Thus, there are still many questions to be answered about how different B cell subpopulations contribute to the formation of effector plasma cells in humans.
Conclusion
There has been considerable development in our understanding of the human B cell compartment during the last years. To only measure the number of CD19 expressing B cells in blood gives very little information, as there are now a large number of functionally distinct B cell subpopulations that have now been defined in human blood and lymphoid tissues using multicolor follow cytometry and in vitro assays. As in all rapidly evolving areas, there are still many unanswered questions. Thus, some of the defined phenotypes overlap with each other, and may contain the same cells. It is also still unclear exactly how the different subpopulations relate to each other, and how they are selected for final plasma cell differentiation to become part of humoral antibody responses. Although human B cell subtyping has started to be used clinically, it will play an ever-increasing role as the understanding of subtypes develops further and disturbances are linked to specific diseases. In particular, as B cell depletion therapies are used more and more, our understanding will increase regarding the role that pathogenic and regulatory B cells play in the development disease.
References
- [1].Ribatti D, Crivellato E, Vacca A. The contribution of Bruce Glick to the definition of the role played by the bursa of Fabricius in the development of the B cell lineage[J] Clin Exp Immunol. 2006;145(1):1–4. doi: 10.1111/j.1365-2249.2006.03131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tarlinton D. Sheepish B cells: evidence for antigen-independent antibody diversification in humans and mice[J] J Exp Med. 2008;205(6):1251–1254. doi: 10.1084/jem.20081057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vossenkamper A, Blair PA, Safinia N, et al. A role for gut-associated lymphoid tissue in shaping the human B cell repertoire[J] J Exp Med. 2013;210(9):1665–1674. doi: 10.1084/jem.20122465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wesemann DR, Portuguese AJ, Meyers RM, et al. Microbial colonization influences early B-lineage development in the gut lamina propria[J] Nature. 2013;501(7465):112–115. doi: 10.1038/nature12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rosado MM, Aranburu A, Capolunghi F, et al. From the fetal liver to spleen and gut: the highway to natural antibody[J] Mucosal Immunol. 2009;2(4):351–361. doi: 10.1038/mi.2009.15. [DOI] [PubMed] [Google Scholar]
- [6].Jung D, Giallourakis C, Mostoslavsky R, et al. Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus[J] Annu Rev Immunol. 2006;24:541–570. doi: 10.1146/annurev.immunol.23.021704.115830. [DOI] [PubMed] [Google Scholar]
- [7].Meffre E, Wardemann H. B-cell tolerance checkpoints in health and autoimmunity[J] Curr Opin Immunol. 2008;20(6):632–638. doi: 10.1016/j.coi.2008.09.001. [DOI] [PubMed] [Google Scholar]
- [8].Allman D, Pillai S. Peripheral B cell subsets[J] Curr Opin Immunol. 2008;20(2):149–157. doi: 10.1016/j.coi.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Tarlinton D, Good-Jacobson K. Diversity among memory B cells: origin, consequences, and utility[J] Science. 2013;341(6151):1205–1211. doi: 10.1126/science.1241146. [DOI] [PubMed] [Google Scholar]
- [10].Srivastava B, Lindsley RC, Nikbakht N, et al. Models for peripheral B cell development and homeostasis[J] Semin Immunol. 2005;17(3):175–182. doi: 10.1016/j.smim.2005.02.008. [DOI] [PubMed] [Google Scholar]
- [11].Melchers F. Anergic B cells caught in the act[J] Immunity. 2006;25(6):864–567. doi: 10.1016/j.immuni.2006.11.003. [DOI] [PubMed] [Google Scholar]
- [12].Casola S, Otipoby KL, Alimzhanov M, et al. B cell receptor signal strength determines B cell fate[J] Nat Immunol. 2004;5(3):317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- [13].Lam KP, Kuhn R, Rajewsky K. In vivo ablation of surface immunoglobulin on mature B cells by inducible gene targeting results in rapid cell death[J] Cell. 1997;90(6):1073–1083. doi: 10.1016/s0092-8674(00)80373-6. [DOI] [PubMed] [Google Scholar]
- [14].Kraus M, Alimzhanov MB, Rajewsky N, et al. Survival of resting mature B lymphocytes depends on BCR signaling via the Igalpha/beta heterodimer[J] Cell. 2004;117(6):787–800. doi: 10.1016/j.cell.2004.05.014. [DOI] [PubMed] [Google Scholar]
- [15].Carsetti R, Köhler G, Lamers MC. Transitional B cells are the target of negative selection in the B cell compartment[J] J Exp Med. 1995;181(6):2129–2140. doi: 10.1084/jem.181.6.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Jackson SM, Wilson PC, James JA, et al. Human B cell subsets[J] AdvImmunol. 2008;98:151–224. doi: 10.1016/S0065-2776(08)00405-7. [DOI] [PubMed] [Google Scholar]
- [17].Bemark M, Holmqvist J, Abrahamsson J, et al. Translational Mini-Review Series on B cell subsets in disease. Reconstitution after haematopoietic stem cell transplantation - revelation of B cell developmental pathways and lineage phenotypes[J] Clin Exp Immunol. 2012;167(1):15–25. doi: 10.1111/j.1365-2249.2011.04469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bohnhorst Jø, Bjørgan MB, Thoen JE, et al. Bm1-Bm5 classification of peripheral blood B cells reveals circulating germinal center founder cells in healthy individuals and disturbance in the B cell subpopulations in patients with primary Sjögren's syndrome[J] J Immunol. 2001;167(7):3610–3618. doi: 10.4049/jimmunol.167.7.3610. [DOI] [PubMed] [Google Scholar]
- [19].Suryani S, Fulcher DA, Santner-Nanan B, et al. Differential expression of CD21 identifies developmentally and functionally distinct subsets of human transitional B cells[J] Blood. 2010;115(3):519–529. doi: 10.1182/blood-2009-07-234799. [DOI] [PubMed] [Google Scholar]
- [20].Wardemann H, Yurasov S, Schaefer A, et al. Predominant autoantibody production by early human B cell precursors[J] Science. 2003;301(5638):1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- [21].Sims GP, Ettinger R, Shirota Y, et al. Identification and characterization of circulating human transitional B cells[J] Blood. 2005;105(11):4390–4398. doi: 10.1182/blood-2004-11-4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Carsetti R, Rosado MM, Wardmann H. Peripheral development of B cells in mouse and man[J] Immunol Rev. 2004;197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- [23].Cuss AK, Avery DT, Cannons JL, et al. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity[J] J Immunol. 2006;176(3):1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- [24].Marie-Cardine A, Divay F, Dutot I, et al. Transitional B cells in humans: characterization and insight from B lymphocyte reconstitution after hematopoietic stem cell transplantation[J] Clin Immunol. 2008;127(1):14–25. doi: 10.1016/j.clim.2007.11.013. [DOI] [PubMed] [Google Scholar]
- [25].Bemark M, Friskopp L, Saghafian-Hedengren S, et al. A glycosylation-dependent CD45RB epitope defines previously unacknowledged CD27−IgMhigh B cell subpopulations enriched in young children and after hematopoietic stem cell transplantation[J] Clin Immunol. 2013;149(3):421–431. doi: 10.1016/j.clim.2013.08.011. [DOI] [PubMed] [Google Scholar]
- [26].Agrawal S, Smith SABC, Tangye SG, et al. Transitional B cell subsets in human bone marrow[J] Clin Exp Immunol. 2013;174(1):53–59. doi: 10.1111/cei.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lee J, Kuchen S, Fischer R, et al. Identification and characterization of a human CD5+ pre-naive B cell population[J] J Immunol. 2009;182(7):4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- [28].Terrier B, Joly F, Vazquez T, et al. Expansion of functionally anergic CD21−/low marginal zone-like B cell clones in hepatitis C virus infection-related autoimmunity[J] J Immunol. 2011;187(12):6550–6563. doi: 10.4049/jimmunol.1102022. [DOI] [PubMed] [Google Scholar]
- [29].Isnardi I, Ng Y-S, Menard L, et al. Complement receptor 2/CD21-negative human naive B cells mostly contain autoreactive unresponsive clones[J] Blood. 2010;115(24):5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Andrews SF, Wilson PC. The anergic B cell[J] Blood. 2010;115(24):4976–4978. doi: 10.1182/blood-2010-03-276352. [DOI] [PubMed] [Google Scholar]
- [31].Wirths S, Lanzavecchia A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells[J] Eur J Immunol. 2005;35(12):3433–3441. doi: 10.1002/eji.200535364. [DOI] [PubMed] [Google Scholar]
- [32].Palanichamy A, Barnard J, Zheng B, et al. Novel human transitional B cell populations revealed by B cell depletion therapy[J] J Immunol. 2009;182(10):5982–5993. doi: 10.4049/jimmunol.0801859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Koethe S, Zander L, Köster S, et al. Pivotal Advance: CD45RB glycosylation is specifically regulated during human peripheral B cell differentiation[J] J Leukoc Biol. 2011;90(1):5–19. doi: 10.1189/jlb.0710404. [DOI] [PubMed] [Google Scholar]
- [34].Loder F, Mutschler B, Ray RJ, et al. B cell development in the spleen takes place in discrete steps and is determined by the quality of B cell receptor-derived signals[J] J Exp Med. 1999;190(1):75–89. doi: 10.1084/jem.190.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Allman DM, Ferguson SE, Lentz VM, et al. Peripheral B cell maturation. II. Heat-stable antigen splenic B cells are an immature developmental intermediate in the production of long-lived marrow-derived B cells[J] J Immunol. 1993;151(9):4431–4444. [PubMed] [Google Scholar]
- [36].Wardemann H, Boehm T, Dear N, et al. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen[J] J Exp Med. 2002;195(6):771–780. doi: 10.1084/jem.20011140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Weller S, Braun MC, Tan BK, et al. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire[J] Blood. 2004;104(12):3647–3654. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Kruetzmann S, Rosado MM, Weber H, et al. Human immunoglobulin M memory B cells controlling. Streptococcus pneumoniae infections are generated in the spleen[J] J Exp Med. 2003;197(7):939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Cameron PU, Jones P, Gorniak M, et al. Splenectomy associated changes in IgM memory B cells in an adult spleen registry cohort[J] PLoS ONE. 2011;6(8):e23164. doi: 10.1371/journal.pone.0023164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Capolunghi F, Cascioli S, Giorda E, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies[J] J Immunol. 2008;180(2):800–808. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- [41].Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology[J] Nat Rev Immunol. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Singer A, Adoro S, Park J-H. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice[J] Nat Rev Immunol. 2008;8(10):788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Brennan PJ, Brigl M, Brenner MB. Invariant natural killer T cells: an innate activation scheme linked to diverse effector functions[J] Nat Rev Immunol. 2013;13(2):101–117. doi: 10.1038/nri3369. [DOI] [PubMed] [Google Scholar]
- [44].Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity[J] Nat Immunol. 2010;11(1):7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- [45].Coomes SM, Pelly VS, Wilson MS. Plasticity within the αβ+CD4+ T-cell lineage: when, how and what for?[J] Open Biol. 2013;3(1):120157. doi: 10.1098/rsob.120157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wardemann H, Hammersen J, Nussenzweig MC. Human autoantibody silencing by immunoglobulin light chains[J] J Exp Med. 2004;200(2):191–199. doi: 10.1084/jem.20040818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Pillai S, Cariappa A. The follicular versus marginal zone B lymphocyte cell fate decision[J] Nat Rev Immunol. 2009;9(11):767–777. doi: 10.1038/nri2656. [DOI] [PubMed] [Google Scholar]
- [48].Allman D, Calamito M. Instructing B cell fates on the fringe[J] Immunity. 2009;30(2):175–177. doi: 10.1016/j.immuni.2009.01.003. [DOI] [PubMed] [Google Scholar]
- [49].Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions[J] Nat Rev Immunol. 2010;11(1):34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- [50].Montecino-Rodriguez E, Dorshkind K. B-1 B cell development in the fetus and adult[J] Immunity. 2012;36(1):13–21. doi: 10.1016/j.immuni.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Garraud O, Borhis G, Badr G, et al. Revisiting the B-cell compartment in mouse and humans: more than one B-cell subset exists in the marginal zone and beyond[J] BMC Immunol. 2012;13(1):63. doi: 10.1186/1471-2172-13-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Hayakawa K, Hardy RR, Herzenberg LA, et al. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells[J] J Exp Med. 1985;161(6):1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Arnon TI, Horton RM, Grigorova IL, et al. Visualization of splenic marginal zone B-cell shuttling and follicular B-cell egress[J] Nature. 2013;493(7434):684–688. doi: 10.1038/nature11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zhang X. Regulatory functions of innate-like B cells[J] Cell Mol Immunol. 2013;10(2):113–121. doi: 10.1038/cmi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Mabbott NA, Gray D. Identification of co-expressed gene signatures in mouse B1, marginal zone and B2 B-cell populations[J] Immunology. 2014;141(1):79–95. doi: 10.1111/imm.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kroese FG, Cebra JJ, van der Cammen MJ, et al. Contribution of B-1 cells to intestinal IgA production in the mouse[J] Methods. 1995;8(1):37–43. [Google Scholar]
- [57].Bemark M, Boysen P, Lycke NY. Induction of gut IgA production through T cell-dependent and T cell-independent pathways[J] Ann N Y Acad Sci. 2012;1247(1):97–116. doi: 10.1111/j.1749-6632.2011.06378.x. [DOI] [PubMed] [Google Scholar]
- [58].Lycke N, Bemark M. Mucosal adjuvants and long-term memory development with special focus on CTA1-DD and other ADP-ribosylating toxins[J] Mucosal Immunol. 2010;3(6):556–566. doi: 10.1038/mi.2010.54. [DOI] [PubMed] [Google Scholar]
- [59].Bergqvist P, Stensson A, Lycke NY, et al. T cell-independent IgA class switch recombination is restricted to the GALT and occurs prior to manifest germinal center formation[J] J Immunol. 2010;184(7):3545–3553. doi: 10.4049/jimmunol.0901895. [DOI] [PubMed] [Google Scholar]
- [60].Lindner C, Wahl B, Föhse L, et al. Age, microbiota, and T cells shape diverse individual IgA repertoires in the intestine[J] J Exp Med. 2012;209(2):365–377. doi: 10.1084/jem.20111980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Thurnheer MC, Zuercher AW, Cebra JJ, et al. B1 cells contribute to serum IgM, but not to intestinal IgA, production in gnotobiotic Ig allotype chimeric mice[J] J Immunol. 2003;170(9):4564–4571. doi: 10.4049/jimmunol.170.9.4564. [DOI] [PubMed] [Google Scholar]
- [62].Srivastava B, Quinn WJ, Hazard K, et al. Characterization of marginal zone B cell precursors[J] J Exp Med. 2005;202(9):1225–1234. doi: 10.1084/jem.20051038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Carey JB, Moffatt-Blue CS, Watson LC, et al. Repertoire-based selection into the marginal zone compartment during B cell development[J] J Exp Med. 2008;205(9):2043–2052. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ghosn EEB, Yang Y, Tung J, et al. CD11b expression distinguishes sequential stages of peritoneal B-1 development[J] Proc Natl Acad Sci USA. 2008;105(13):5195–5200. doi: 10.1073/pnas.0712350105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Klein U, Rajewsky K, Küppers R. Human immunoglobulin (Ig)M+IgD+ peripheral blood B cells expressing the CD27 cell surface antigen carry somatically mutated variable region genes: CD27 as a general marker for somatically mutated (memory) B cells[J] J Exp Med. 1998;188(9):1679–1689. doi: 10.1084/jem.188.9.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Spencer J, Perry ME, Dunn-Walters DK. Human marginal-zone B cells[J] Immunol Today. 1998;19(9):421–426. doi: 10.1016/s0167-5699(98)01308-5. [DOI] [PubMed] [Google Scholar]
- [67].Weill J-C, Weller S, Reynaud C-A. Human marginal zone B cells[J] Annu Rev Immunol. 2009;27:267–285. doi: 10.1146/annurev.immunol.021908.132607. [DOI] [PubMed] [Google Scholar]
- [68].Dagg MK, Levitt D. Human B-lymphocyte subpopulations. I. Differentiation of density-separated B lymphocytes[J] Clin Immunol Immunopathol. 1981;21(1):39–49. doi: 10.1016/0090-1229(81)90193-8. [DOI] [PubMed] [Google Scholar]
- [69].Dono M, Zupo S, Leanza N, et al. Heterogeneity of tonsillarsubepithelial B lymphocytes, the splenic marginal zone equivalents[J] J Immunol. 2000;164(11):5596–5604. doi: 10.4049/jimmunol.164.11.5596. [DOI] [PubMed] [Google Scholar]
- [70].Fecteau JF, Côté G, Néron S. A new memory CD27−IgG+ B cell population in peripheral blood expressing VH genes with low frequency of somatic mutation[J] J Immunol. 2006;177(6):3728–3736. doi: 10.4049/jimmunol.177.6.3728. [DOI] [PubMed] [Google Scholar]
- [71].Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells[J] Eur J Immunol. 2006;36(4):810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- [72].Bernasconi NL, Onai N, Lanzavecchia A. A role for Toll-like receptors in acquired immunity: up-regulation of TLR9 by BCR triggering in naive B cells and constitutive expression in memory B cells[J] Blood. 2003;101(11):4500–1504. doi: 10.1182/blood-2002-11-3569. [DOI] [PubMed] [Google Scholar]
- [73].Good KL, Avery DT, Tangye SG. Resting human memory B cells are intrinsically programmed for enhanced survival and responsiveness to diverse stimuli compared to naive B cells[J] J Immunol. 2009;182(2):890–901. doi: 10.4049/jimmunol.182.2.890. [DOI] [PubMed] [Google Scholar]
- [74].Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes[J] Nat Rev Immunol. 2013;13(2):118–132. doi: 10.1038/nri3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Dunn-Walters DK, Isaacson PG, Spencer J. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells[J] J Exp Med. 1995;182(2):559–566. doi: 10.1084/jem.182.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Tangye SG, Liu YJ, Aversa G, et al. Identification of functional human splenic memory B cells by expression of CD148 and CD27[J] J Exp Med. 1998;188(9):1691–1703. doi: 10.1084/jem.188.9.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Zandvoort A, Lodewijk ME, de Boer NK, et al. CD27 expression in the human splenic marginal zone: the infant marginal zone is populated by naive B cells[J] Tissue Antigens. 2001;58(4):234–242. doi: 10.1034/j.1399-0039.2001.580403.x. [DOI] [PubMed] [Google Scholar]
- [78].Weller S, Mamani-Matsuda M, Picard C, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+IgD+ CD27+ B cell repertoire in infants[J] J Exp Med. 2008;205(6):1331–1342. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Amlot PL, Hayes AE. Impaired human antibody response to the thymus-independent antigen, DNP-Ficoll, after splenectomy[J]. Implications for post-splenectomy infections. Lancet. 1985;1(8436):1008–1011. doi: 10.1016/s0140-6736(85)91613-7. [DOI] [PubMed] [Google Scholar]
- [80].Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”[J] Immunol Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- [81].Amlot PL, Hayes AE, Gray D, et al. Human immune responses in vivo to protein (KLH) and polysaccharide (DNP-Ficoll) neoantigens: normal subjects compared with bone marrow transplant patients on cyclosporine[J] Clin Exp Immunol. 1986;64(1):125–135. [PMC free article] [PubMed] [Google Scholar]
- [82].Di Sabatino A, Carsetti R, Corazza GR. Post-splenectomy and hyposplenic states[J] Lancet. 2011;378(9785):86–97. doi: 10.1016/S0140-6736(10)61493-6. [DOI] [PubMed] [Google Scholar]
- [83].Spencer J, Finn T, Pulford KA, et al. The human gut contains a novel population of B lymphocytes which resemble marginal zone cells[J] Clin Exp Immunol. 1985;62(3):607–612. [PMC free article] [PubMed] [Google Scholar]
- [84].Morente M, Piris MA, Orradre JL, et al. Human tonsil intraepithelial B cells: a marginal zone-related subpopulation[J] J Clin Pathol. 1992;45(8):668–672. doi: 10.1136/jcp.45.8.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].van den Oord JJ, de Wolf-Peeters C, Desmet VJ. The marginal zone in the human reactive lymph node[J] Am J Clin Pathol. 1986;86(4):475–479. doi: 10.1093/ajcp/86.4.475. [DOI] [PubMed] [Google Scholar]
- [86].Tierens A, Delabie J, Michiels L, et al. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion[J] Blood. 1999;93(1):226–234. [PubMed] [Google Scholar]
- [87].Dunn-Walters DK, Isaacson PG, Spencer J. Sequence analysis of rearranged IgVH genes from microdissected human Peyer's patch marginal zone B cells[J] Immunology. 1996;88(4):618–624. [PMC free article] [PubMed] [Google Scholar]
- [88].Cinamon G, Zachariah MA, Lam OM, et al. Follicular shuttling of marginal zone B cells facilitates antigen transport[J] Nat Immunol. 2008;9(1):54–62. doi: 10.1038/ni1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Ferguson AR, Youd ME, Corley RB. Marginal zone B cells transport and deposit IgM-containing immune complexes onto follicular dendritic cells[J] IntImmunol. 2004;16(10):1411–1422. doi: 10.1093/intimm/dxh142. [DOI] [PubMed] [Google Scholar]
- [90].Weller S, Faili A, Garcia C, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans[J] Proc Natl Acad Sci USA. 2001;98(3):1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Agematsu K, Nagumo H, Shinozaki K, et al. Absence of IgD−CD27+ memory B cell population in X-linked hyper-IgM syndrome[J] J Clin Invest. 1998;102(4):853–860. doi: 10.1172/JCI3409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Berkowska MA, Driessen GJA, Bikos V, et al. Human memory B cells originate from three distinct germinal center-dependent and -independent maturation pathways[J] Blood. 2011;118(8):2150–2158. doi: 10.1182/blood-2011-04-345579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Wu Y-C, Kipling D, Leong HS, et al. High-throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B-cell populations[J] Blood. 2010;116(7):1070–1078. doi: 10.1182/blood-2010-03-275859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Weller S, Bonnet M, Delagreverie H, et al. IgM+IgD+CD27+ B cells are markedly reduced in IRAK-4-, MyD88-, and TIRAP- but not UNC-93B-deficient patients[J] Blood. 2012;120(25):4992–5001. doi: 10.1182/blood-2012-07-440776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Bourke E, Bosisio D, Golay J, et al. The toll-like receptor repertoire of human B lymphocytes: inducible and selective expression of TLR9 and TLR10 in normal and transformed cells[J] Blood. 2003;102(3):956–963. doi: 10.1182/blood-2002-11-3355. [DOI] [PubMed] [Google Scholar]
- [96].Bernasconi NL, Traggiai E, Lanzavecchia A. Maintenance of serological memory by polyclonal activation of human memory B cells[J] Science. 2002;298(5601):2199–2202. doi: 10.1126/science.1076071. [DOI] [PubMed] [Google Scholar]
- [97].Amanna IJ, Carlson NE, Slifka MK. Duration of humoral immunity to common viral and vaccine antigens[J] N Engl J Med. 2007;357(19):1903–1915. doi: 10.1056/NEJMoa066092. [DOI] [PubMed] [Google Scholar]
- [98].Rosado MM, Gesualdo F, Marcellini V, et al. Preserved antibody levels and loss of memory B cells against pneumococcus and tetanus after splenectomy: Tailoring better vaccination strategies[J] Eur J Immunol. 2013;43(10):2659–2670. doi: 10.1002/eji.201343577. [DOI] [PubMed] [Google Scholar]
- [99].Rosado MM, Scarsella M, Pandolfi E, et al. Switched memory B cells maintain specific memory independently of serum antibodies: the hepatitis B example[J] Eur J Immunol. 2011;41(6):1800–1808. doi: 10.1002/eji.201041187. [DOI] [PubMed] [Google Scholar]
- [100].Kubicka U, Olszewski WL, Tarnowski W, et al. Normal human immune peritoneal cells: subpopulations and functional characteristics[J] Scand J Immunol. 1996;44(2):157–163. doi: 10.1046/j.1365-3083.1996.d01-297.x. [DOI] [PubMed] [Google Scholar]
- [101].Donze HH, Lue C, Julian BA, et al. Human peritoneal B-1 cells and the influence of continuous ambulatory peritoneal dialysis on peritoneal and peripheral blood mononuclear cell (PBMC) composition and immunoglobulin levels[J] Clin Exp Immunol. 1997;109(2):356–561. doi: 10.1046/j.1365-2249.1997.4541352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Boursier L, Montalto SA, Raju S, et al. Characterization of cells of the B lineage in the human adult greater omentum[J] Immunology. 2006;119(1):90–97. doi: 10.1111/j.1365-2567.2006.02411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Ledbetter JA, Evans RL, Lipinski M, et al. Evolutionary conservation of surface molecules that distinguish T lymphocyte helper/inducer and cytotoxic/suppressor subpopulations in mouse and man[J] J Exp Med. 1981;153(2):310–323. doi: 10.1084/jem.153.2.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Bhat NM, Kantor AB, Bieber MM, et al. The ontogeny and functional characteristics of human B-1 (CD5+ B) cells[J] Int Immunol. 1992;4(2):243–252. doi: 10.1093/intimm/4.2.243. [DOI] [PubMed] [Google Scholar]
- [105].Dono M, Cerruti G, Zupo S. The CD5+ B-cell. Int J Biochem Cell Biol. 2004;36(11):2105–2111. doi: 10.1016/j.biocel.2004.05.017. [DOI] [PubMed] [Google Scholar]
- [106].Griffin DO, Holodick NE, Rothstein TL. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−[J] J Exp Med. 2011;208(1):67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Griffin DO, Rothstein TL. A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus[J] J Exp Med. 2011;208(13):2591–2598. doi: 10.1084/jem.20110978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Griffin DO, Rothstein TL. Human “orchestrator” CD11b+ B1 cells spontaneously secrete interleukin-10 and regulate T-cell activity[J] Mol Med. 2012;18:1003–1008. doi: 10.2119/molmed.2012.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Tørring C, Petersen CC, Bjerg L, et al. The B1-cell subpopulation is diminished in patients with relapsing-remitting multiple sclerosis[J] J Neuroimmunol. 2013;262(1-2):92–99. doi: 10.1016/j.jneuroim.2013.06.002. [DOI] [PubMed] [Google Scholar]
- [110].Kraljevic K, Wong S, Fulcher DA. Circulating phenotypic B-1 cells are decreased in common variable immunodeficiency and correlate with immunoglobulin M levels[J] Clin Exp Immunol. 2013;171(3):278–282. doi: 10.1111/cei.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Suchanek O, Sadler R, Bateman EA, et al. Immunophenotyping of putative human B1 B cells in healthy controls and common variable immunodeficiency (CVID) patients[J] Clin Exp Immunol. 2012;170(3):333–341. doi: 10.1111/j.1365-2249.2012.04656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Moins-Teisserenc H, Busson M, Herda A, et al. CD19+CD5+ B cells and B1-like cells following allogeneic hematopoietic stem cell transplantation[J] Biol Blood Marrow Transplant. 2013;19(6):988–991. doi: 10.1016/j.bbmt.2013.03.006. [DOI] [PubMed] [Google Scholar]
- [113].Verbinnen B, Covens K, Moens L, et al. Human CD20+CD43+CD27+CD5− B cells generate antibodies to capsular polysaccharides of Streptococcus pneumoniae[J] J Allergy Clin Immunol. 2012;130(1):272–275. doi: 10.1016/j.jaci.2012.04.040. [DOI] [PubMed] [Google Scholar]
- [114].Leggat DJ, Khaskhely NM, Iyer AS, et al. Pneumococcal polysaccharide vaccination induces polysaccharide-specific B cells in adult peripheral blood expressing CD19++</emph>CD3−CD70−CD27+IgM+CD43+CD5+/−[J] Vaccine. 2013;31(41):4632–4640. doi: 10.1016/j.vaccine.2013.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Vuyyuru R, Liu H, Manser T, et al. Characteristics of Borreliahermsii infection in human hematopoietic stem cell-engrafted mice mirror those of human relapsing fever[J] Proc Natl Acad Sci USA. 2011;108(51):20707–20712. doi: 10.1073/pnas.1108776109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Reynaud C-A, Weill J-C. Gene profiling of CD11b+ and CD11b− B1 cell subsets reveals potential cell sorting artifacts[J] J Exp Med. 2012;209(3):433–434. doi: 10.1084/jem.20120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Descatoire M, Weill J-C, Reynaud C-A, et al. A human equivalent of mouse B-1 cells? J Exp Med. 2011;208(13):2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Perez-Andres M, Grosserichter-Wagener C, Teodosio C, et al. The nature of circulating CD27+CD43+ B cells. J Exp Med. 2011;208(13):2565–2566. doi: 10.1084/jem.20112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Griffin DO, Rothstein TL. Human B1 cell frequency: isolation and analysis of human B1 cells[J] Front Immun. 2012;3:122. doi: 10.3389/fimmu.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Covens K, Verbinnen B, Geukens N, et al. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype[J] Blood. 2013;121(26):5176–5183. doi: 10.1182/blood-2012-12-471953. [DOI] [PubMed] [Google Scholar]
- [121].Rothstein TL, Griffin DO, Holodick NE, et al. Human B-1 cells take the stage[J] Ann N Y Acad Sci. 2013;1285:97–114. doi: 10.1111/nyas.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Tangye SG. To B1 or not to B1: that really is still the question! Blood. 2013;121(26):5109–5110. doi: 10.1182/blood-2013-05-500074. [DOI] [PubMed] [Google Scholar]
- [123].Descatoire M, Weller S, Irtan S, et al. Identification of a human splenic marginal zone B cell precursor with NOTCH2-dependent differentiation properties[J] J Exp Med. 2014;211(5):987–1000. doi: 10.1084/jem.20132203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Harwood NE, Batista FD. The antigen expressway: follicular conduits carry antigen to B cells[J] Immunity. 2009;30(2):177–179. doi: 10.1016/j.immuni.2009.01.004. [DOI] [PubMed] [Google Scholar]
- [125].Roozendaal R, Mempel TR, Pitcher LA, et al. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles[J] Immunity. 2009;30(2):264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Garside P, Ingulli E, Merica RR, et al. Visualization of specific B and T lymphocyte interactions in the lymph node[J] Science. 1998;281(5373):96–99. doi: 10.1126/science.281.5373.96. [DOI] [PubMed] [Google Scholar]
- [127].Goodnow CC, Vinuesa CG, Randall KL, et al. Control systems and decision making for antibody production[J] Nat Immunol. 2010;11(8):681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- [128].MacLennan ICM, Toellner K-M, Cunningham AF, et al. Extrafollicular antibody responses[J] Immunol Rev. 2003;194:8–18. doi: 10.1034/j.1600-065x.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- [129].Victora GD, Nussenzweig MC. Germinal centers[J] Annu Rev Immunol. 2012;30:429–457. doi: 10.1146/annurev-immunol-020711-075032. [DOI] [PubMed] [Google Scholar]
- [130].Pascual V, Liu YJ, Magalski A, et al. Analysis of somatic mutation in five B cell subsets of human tonsil[J] J Exp Med. 1994;180(1):329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Liu YJ, Barthélémy C, De Bouteiller O, et al. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2[J] Immunity. 1995;2(3):239–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- [132].Victora GD, Schwickert TA, Fooksman DR, et al. Germinal center dynamics revealed by multiphoton microscopy with a photoactivatable fluorescent reporter[J] Cell. 2010;143(4):592–605. doi: 10.1016/j.cell.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Victora GD, Dominguez-Sola D, Holmes AB, et al. Identification of human germinal center light and dark zone cells and their relationship to human B-cell lymphomas[J] Blood. 2012;120(11):2240–2248. doi: 10.1182/blood-2012-03-415380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Gershon RK, Kondo K. Cell interactions in the induction of tolerance: the role of thymic lymphocytes[J] Immunology. 1970;18(5):723–737. [PMC free article] [PubMed] [Google Scholar]
- [135].Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases[J] J Immunol. 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- [136].Wolf SD, Dittel BN, Hardardottir F, et al. Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice[J] J Exp Med. 1996;184(6):2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Mauri C, Gray D, Mushtaq N, et al. Prevention of arthritis by interleukin 10-producing B cells[J] J Exp Med. 2003;197(4):489–501. doi: 10.1084/jem.20021293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Fillatreau S, Sweenie CH, McGeachy MJ, et al. B cells regulate autoimmunity by provision of IL-10[J] Nat Immunol. 2002;3(10):944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- [139].Mizoguchi A, Mizoguchi E, Takedatsu H, et al. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation[J] Immunity. 2002;16(2):219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- [140].Mauri C, Bosma A. Immune regulatory function of B cells[J] Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- [141].Gray D, Gray M. What are regulatory B cells? Eur J Immunol. 2010;40(10):2677–2679. doi: 10.1002/eji.201040961. [DOI] [PubMed] [Google Scholar]
- [142].Ries S, Hilgenberg E, Lampropoulou V, et al. B-type suppression: A role played by "regulatory B cells" or "regulatory plasma cells"?[J] Eur J Immunol. 2014;44(5):1251–1257. doi: 10.1002/eji.201343683. [DOI] [PubMed] [Google Scholar]
- [143].Shen P, Roch T, Lampropoulou V, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases[J] Nature. 2014;507(7492):366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Duddy M, Niino M, Adatia F, et al. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis[J] J Immunol. 2007;178(10):6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- [145].Correale J, Farez M. Association between parasite infection and immune responses in multiple sclerosis[J] Ann Neurol. 2007;61(2):97–108. doi: 10.1002/ana.21067. [DOI] [PubMed] [Google Scholar]
- [146].Correale J, Farez M, Razzitte G. Helminth infections associated with multiple sclerosis induce regulatory B cells[J] Ann Neurol. 2008;64(2):187–199. doi: 10.1002/ana.21438. [DOI] [PubMed] [Google Scholar]
- [147].Blair PA, Noreña LY, Flores-Borja F, et al. CD19+CD24hiCD38hi B Cells exhibit regulatory capacity in healthy individuals but are functionally impaired in systemic lupus erythematosus patients[J] Immunity. 2010;32(1):129–140. doi: 10.1016/j.immuni.2009.11.009. [DOI] [PubMed] [Google Scholar]
- [148].Lemoine S, Morva A, Youinou P, et al. Human T cells induce their own regulation through activation of B cells[J] J Autoimmun. 2011;36(3-4):228–238. doi: 10.1016/j.jaut.2011.01.005. [DOI] [PubMed] [Google Scholar]
- [149].Flores-Borja F, Bosma A, Ng D, et al. CD19+CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation[J] Sci Transl Med. 2013;5(173):173ra23. doi: 10.1126/scitranslmed.3005407. [DOI] [PubMed] [Google Scholar]
- [150].Li X, Zhong H, Bao W, et al. Defective regulatory B-cell compartment in patients with immune thrombocytopenia[J] Blood. 2012;120(16):3318–3325. doi: 10.1182/blood-2012-05-432575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Hua F, Ji L, Zhan Y, et al. Pulsed high-dose dexamethasone improves interleukin 10 secretion by CD5+ B cells in patients with primary immune thrombocytopenia[J] J Clin Immunol. 2012;32(6):1233–1242. doi: 10.1007/s10875-012-9714-z. [DOI] [PubMed] [Google Scholar]
- [152].Newell KA, Asare A, Kirk AD, et al. Identification of a B cell signature associated with renal transplant tolerance in humans[J] J Clin Invest. 2010;120(6):1836–1847. doi: 10.1172/JCI39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Das A, Ellis G, Pallant C, et al. IL-10-producing regulatory B cells in the pathogenesis of chronic hepatitis B virus infection[J] J Immunol. 2012;189(8):3925–3935. doi: 10.4049/jimmunol.1103139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Siewe B, Stapleton JT, Martinson J, et al. Regulatory B cell frequency correlates with markers of HIV disease progression and attenuates anti-HIV CD8+ T cell function in vitro[J] J Leukoc Biol. 2013;93(5):811–818. doi: 10.1189/jlb.0912436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Bouaziz J-D, Calbo S, Maho-Vaillant M, et al. IL-10 produced by activated human B cells regulates CD4(+) T-cell activation in vitro[J] Eur J Immunol. 2010;40(10):2686–2691. doi: 10.1002/eji.201040673. [DOI] [PubMed] [Google Scholar]
- [156].Iwata Y, Matsushita T, Horikawa M, et al. Characterization of a rare IL-10-competent B-cell subset in humans that parallels mouse regulatory B10 cells[J] Blood. 2011;117(2):530–541. doi: 10.1182/blood-2010-07-294249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Zha B, Wang L, Liu X, et al. Decrease in proportion of CD19+ CD24hi CD27+ B cells and impairment of their suppressive function in Graves' disease[J] PLoS ONE. 2012;7(11):e49835. doi: 10.1371/journal.pone.0049835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].vanZelm MC, Bartol SJW, Driessen GJ, et al. Human CD19 and CD40L deficiencies impair antibody selection and differentially affect somatic hypermutation[J] J Allergy Clin Immunol. 2014;134(1):135–144. doi: 10.1016/j.jaci.2013.11.015. [DOI] [PubMed] [Google Scholar]
- [159].Bergqvist P, Gärdby E, Stensson A, et al. Gut IgA class switch recombination in the absence of CD40 does not occur in the lamina propria and is independent of germinal centers[J] J Immunol. 2006;177(11):7772–7783. doi: 10.4049/jimmunol.177.11.7772. [DOI] [PubMed] [Google Scholar]
- [160].He B, Xu W, Santini PA, et al. Intestinal bacteria trigger T cell-independent immunoglobulin A(2) class switching by inducing epithelial-cell secretion of the cytokine APRIL[J] Immunity. 2007;26(6):812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- [161].Cerutti A, Chen K, Chorny A. Immunoglobulin responses at the mucosal interface[J] Annu Rev Immunol. 2011;29:273–293. doi: 10.1146/annurev-immunol-031210-101317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Lin M, Du L, Brandtzaeg P, et al. IgA subclass switch recombination in human mucosal and systemic immune compartments[J] Mucosal Immunol. 2014;7(3):511–520. doi: 10.1038/mi.2013.68. [DOI] [PubMed] [Google Scholar]
- [163].Barone F, Patel P, Sanderson JD, et al. Gut-associated lymphoid tissue contains the molecular machinery to support T-cell-dependent and T-cell-independent class switch recombination[J] Mucosal Immunol. 2009;2(6):495–503. doi: 10.1038/mi.2009.106. [DOI] [PubMed] [Google Scholar]
- [164].Shikina T, Hiroi T, Iwatani K, et al. IgA class switch occurs in the organized nasopharynx- and gut-associated lymphoid tissue, but not in the diffuse lamina propria of airways and gut[J] J Immunol. 2004;172(10):6259–6264. doi: 10.4049/jimmunol.172.10.6259. [DOI] [PubMed] [Google Scholar]
- [165].Barone F, Vossenkamper A, Boursier L, et al. IgA-producing plasma cells originate from germinal centers that are induced by B-cell receptor engagement in humans[J] Gastroenterology. 2011;140(3):947–956. doi: 10.1053/j.gastro.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Amanna IJ, Slifka MK. Quantitation of rare memory B cell populations by two independent and complementary approaches[J] J Immunol Methods. 2006;317(1-2):175–185. doi: 10.1016/j.jim.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Pinna D, Corti D, Jarrossay D, et al. Clonal dissection of the human memory B-cell repertoire following infection and vaccination[J] Eur J Immunol. 2009;39(5):1260–1270. doi: 10.1002/eji.200839129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].vanEs JH, Meyling FH, Logtenberg T. High frequency of somatically mutated IgM molecules in the human adult blood B cell repertoire[J] Eur J Immunol. 1992;22(10):2761–2764. doi: 10.1002/eji.1830221046. [DOI] [PubMed] [Google Scholar]
- [169].Huang C, Stewart AK, Schwartz RS, et al. Immunoglobulin heavy chain gene expression in peripheral blood B lymphocytes[J] J Clin Invest. 1992;89(4):1331–1343. doi: 10.1172/JCI115719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Klein U, Küppers R, Rajewsky K. Evidence for a large compartment of IgM-expressing memory B cells in humans[J] Blood. 1997;89(4):1288–1298. [PubMed] [Google Scholar]
- [171].Dogan I, Bertocci B, Vilmont V, et al. Multiple layers of B cell memory with different effector functions[J] Nat Immunol. 2009;10(12):1292–1299. doi: 10.1038/ni.1814. [DOI] [PubMed] [Google Scholar]
- [172].Pape KA, Taylor JJ, Maul RW, et al. Different B cell populations mediate early and late memory during an endogenous immune response[J] Science. 2011;331(6021):1203–1207. doi: 10.1126/science.1201730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or “memory” B cells?[J] J Immunol. 2007;179(1):13–19. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- [174].Seifert M, Küppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation[J] J Exp Med. 2009;206(12):2659–2669. doi: 10.1084/jem.20091087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Vinuesa CG, Chang P-P. Innate B cell helpers reveal novel types of antibody responses[J] Nat Immunol. 2013;14(2):119–126. doi: 10.1038/ni.2511. [DOI] [PubMed] [Google Scholar]
- [176].Bortnick A, Allman D. What is and what should always have been: long-lived plasma cells induced by T cell-independent antigens[J] J Immunol. 2013;190(12):5913–5918. doi: 10.4049/jimmunol.1300161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Chu VT, Berek C. The establishment of the plasma cell survival niche in the bone marrow[J] Immunol Rev. 2013;251(1):177–188. doi: 10.1111/imr.12011. [DOI] [PubMed] [Google Scholar]
- [178].Chevrier S, Emslie D, Shi W, et al. The BTB-ZF transcription factor Zbtb20 is driven by Irf4 to promote plasma cell differentiation and longevity[J] J Exp Med. 2014;211(5):827–840. doi: 10.1084/jem.20131831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Wang Y, Bhattacharya D. Adjuvant-specific regulation of long-term antibody responses by ZBTB20[J] J Exp Med. 2014;211(5):841–856. doi: 10.1084/jem.20131821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity[J] Immunol Rev. 2010;236:125–138. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Di Niro R, Mesin L, Raki M, et al. Rapid generation of rotavirus-specific human monoclonal antibodies from small-intestinal mucosa[J] J Immunol. 2010;185(9):5377–5383. doi: 10.4049/jimmunol.1001587. [DOI] [PubMed] [Google Scholar]
- [182].Pinto D, Montani E, Bolli M, et al. A functional BCR in human IgA and IgM plasma cells[J] Blood. 2013;121(20):4110–4114. doi: 10.1182/blood-2012-09-459289. [DOI] [PubMed] [Google Scholar]
- [183].Czerkinsky CC, Tarkowski A, Nilsson LA, et al. Reverse enzyme-linked immunospot assay (RELISPOT) for the detection of cells secreting immunoreactive substances[J] J Immunol Methods. 1984;72(2):489–496. doi: 10.1016/0022-1759(84)90017-6. [DOI] [PubMed] [Google Scholar]
- [184].Wrammert J, Smith K, Miller J, et al. Rapid cloning of high-affinity human monoclonal antibodies against influenza virus[J] Nature. 2008;453(7195):667–671. doi: 10.1038/nature06890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Wrammert J, Koutsonanos D, Li G-M, et al. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection[J] J Exp Med. 2011;208(1):181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Wrammert J, Onlamoon N, Akondy RS, et al. Rapid and massive virus-specific plasmablast responses during acute dengue virus infection in humans[J] J Virol. 2012;86(6):2911–2918. doi: 10.1128/JVI.06075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Mei HE, Yoshida T, Sime W, et al. Blood-borne human plasma cells in steady state are derived from mucosal immune responses[J] Blood. 2009;113(11):2461–2469. doi: 10.1182/blood-2008-04-153544. [DOI] [PubMed] [Google Scholar]
- [188].Mei HE, Frölich D, Giesecke C, et al. Steady-state generation of mucosal IgA+plasmablasts is not abrogated by B-cell depletion therapy with rituximab[J] Blood. 2010;116(24):5181–5190. doi: 10.1182/blood-2010-01-266536. [DOI] [PubMed] [Google Scholar]
- [189].Rahman A, Rashu R, Bhuiyan TR, et al. Antibody-secreting cell responses after Vibrio cholerae O1 infection and oral cholera vaccination in adults in Bangladesh[J] Clin Vaccine Immunol. 2013;20(10):1592–1598. doi: 10.1128/CVI.00347-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Czerkinsky C, Svennerholm AM, Quiding M, et al. Antibody-producing cells in peripheral blood and salivary glands after oral cholera vaccination of humans[J] Infect Immun. 1991;59(3):996–1001. doi: 10.1128/iai.59.3.996-1001.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]